化工进展 ›› 2021, Vol. 40 ›› Issue (5): 2917-2927.DOI: 10.16085/j.issn.1000-6613.2020-1272
不同类型土壤胡敏酸提取物环境持久性自由基特征及影响因素
鲁遥1( ), 王朋2(
), 王朋2( ), 尹梦楠1, 杨名毅1, 张凰3,4(
), 尹梦楠1, 杨名毅1, 张凰3,4( )
)
- 1.昆明理工大学环境科学与工程学院,云南 昆明 650500
2.成都理工大学生态环境学院,四川 成都 610059
3.昆明理工大学农业与食品学院,云南 昆明 650500
4.云南省土壤固碳与污染控制重点实验室,云南 昆明 650500
-
收稿日期:2020-07-06出版日期:2021-05-06发布日期:2021-05-24 -
通讯作者:张凰 -
作者简介:鲁遥(1995—),女,硕士研究生,研究方向为土壤中持久性自由基的稳定机制。E-mail:847119422@qq.com |王朋(1987—),男,工学博士,硕士生导师,研究方向为环境土壤科学。E-mail:wpeng0815@163.com 。 -
基金资助:国家自然科学基金(41663014);云南省中青年学术和技术带头人后备人才项目(2018HB008);云南省万人计划青年拔尖人才项目
Characteristics and influencing factors of environmental persistent free radicals of humic acid extracts from different types of soil
LU Yao1( ), WANG Peng2(
), WANG Peng2( ), YIN Mengnan1, YANG Mingyi1, ZHANG Huang3,4(
), YIN Mengnan1, YANG Mingyi1, ZHANG Huang3,4( )
)
- 1.Faculty of Environmental Science and Engineering, Kunming University of Science and Technology, Kunming 650500, Yunnan, China
2.College of Ecology and Environment, Chengdu University of Technology, Chengdu 610059, Sichuan, China
3.Faculty of Agriculture and Food, Kunming University of Science and Technology, Kunming 650500, Yunnan, China
4.Yunnan Provincial Key Laboratory of Soil Carbon Sequestration and Pollution Control, Kunming 650500, Yunnan, China
-
Received:2020-07-06Online:2021-05-06Published:2021-05-24 -
Contact:ZHANG Huang
摘要:
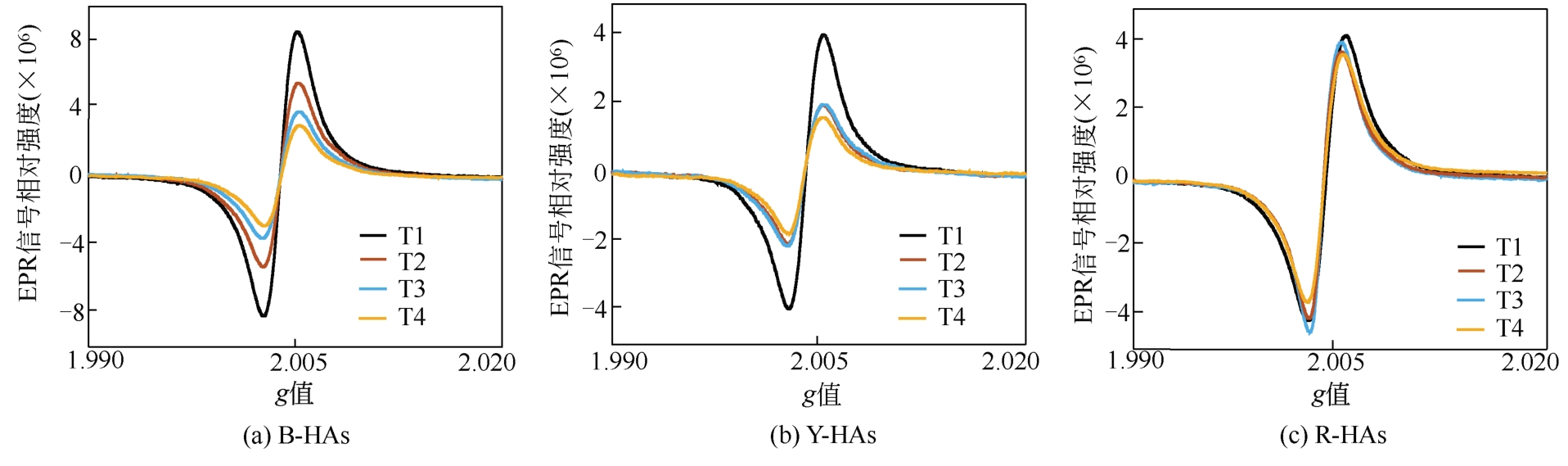
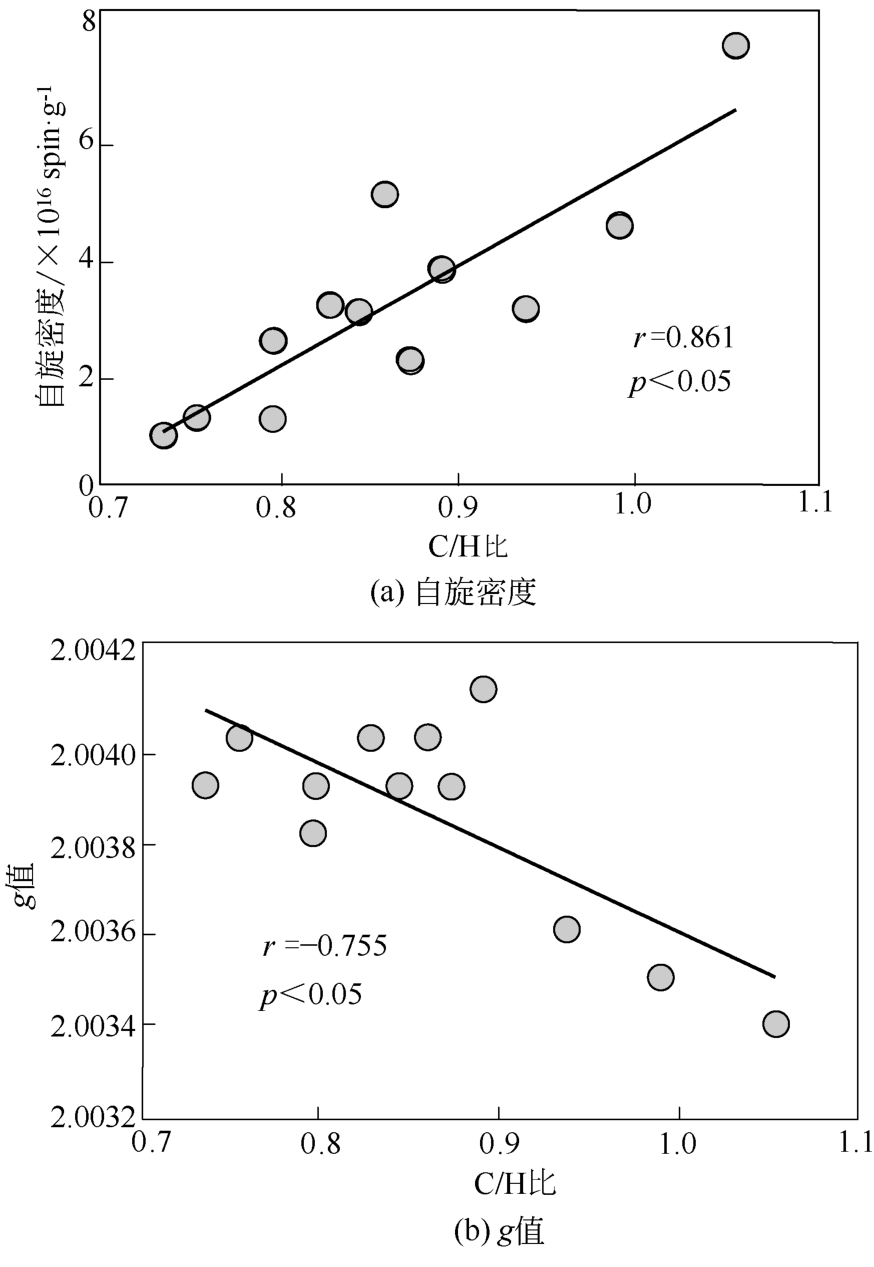
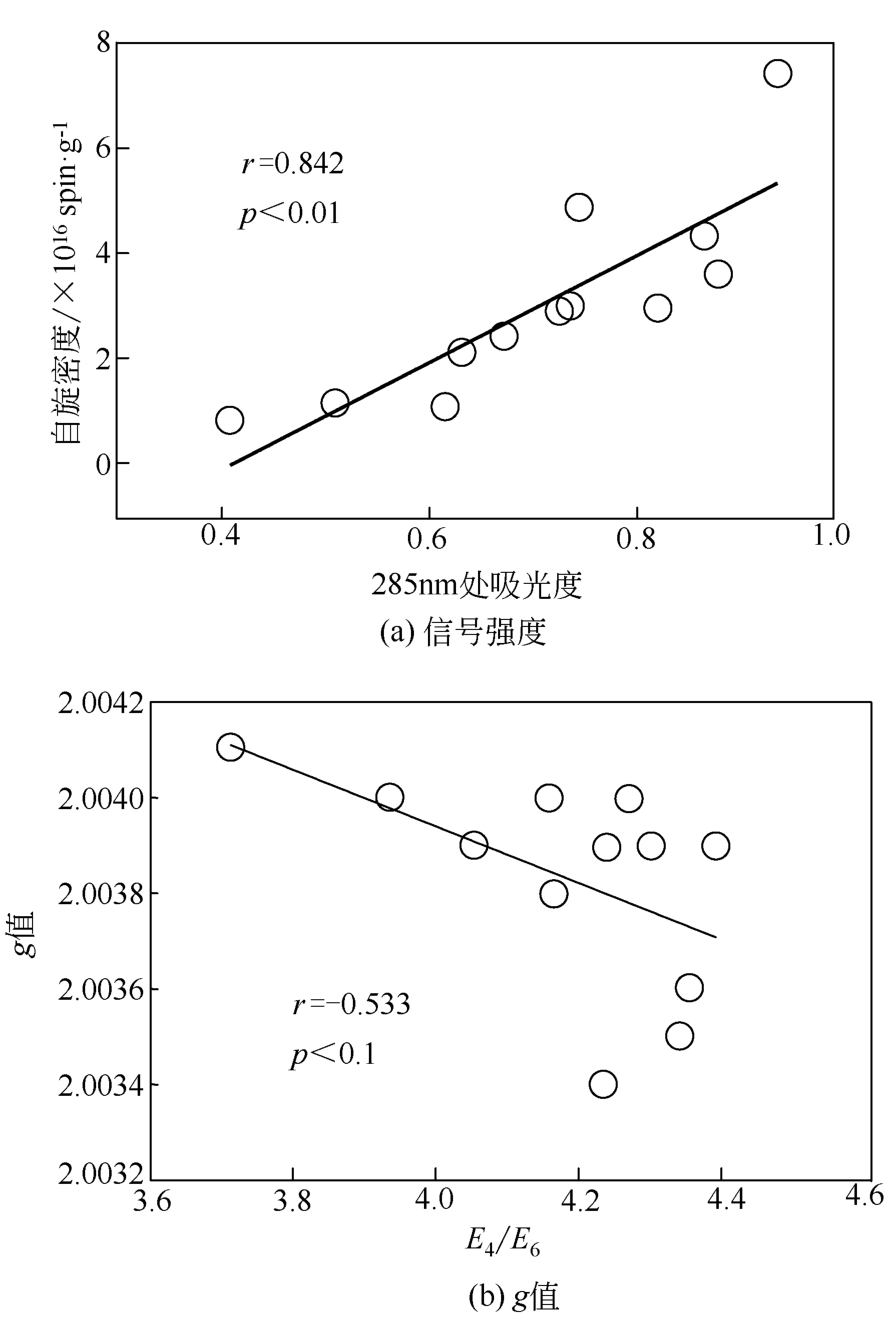
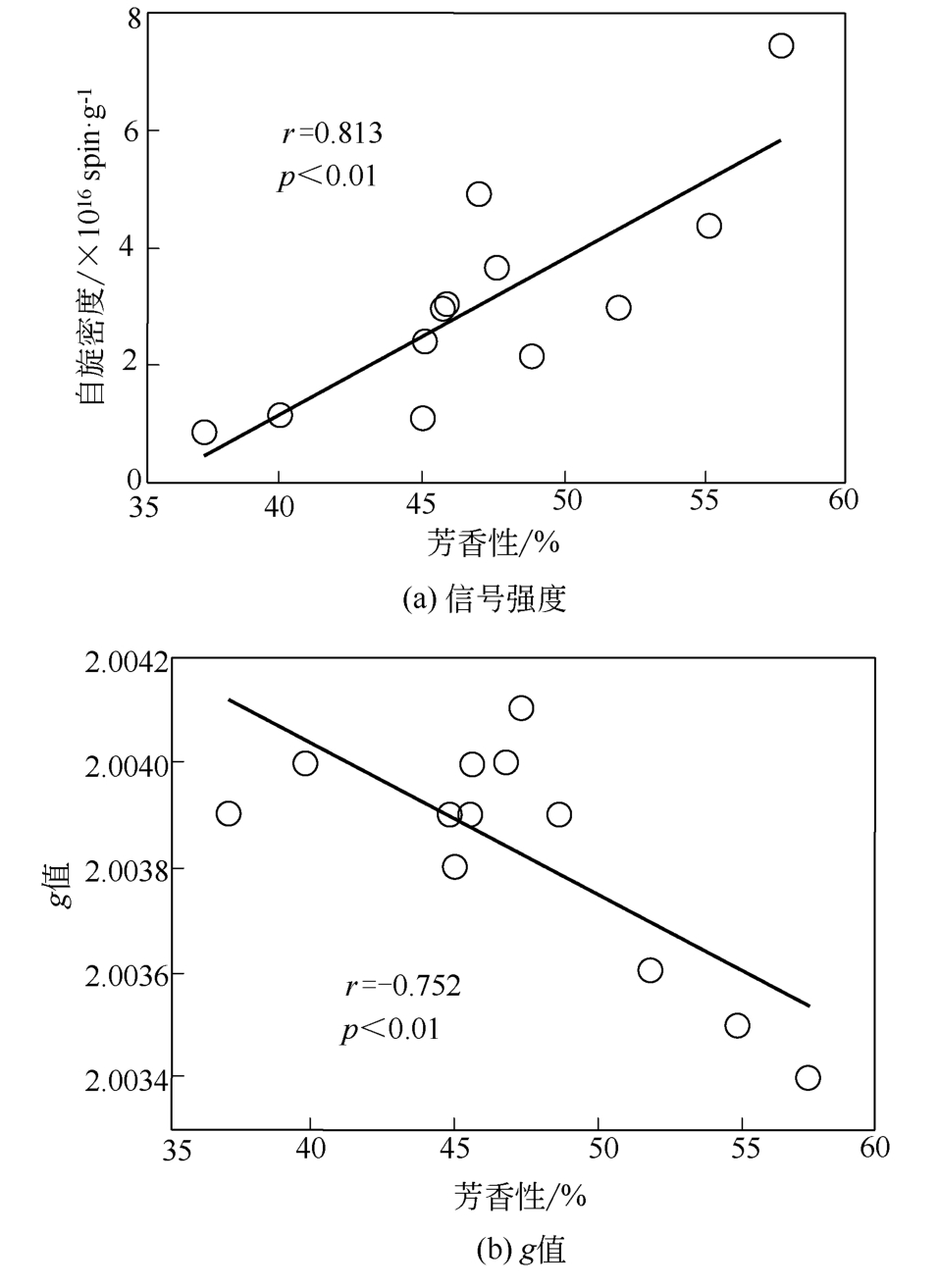
土壤有机质中存在环境持久性自由基(EPFRs),其特征(信号强度、g值和线宽)与天然有机质组分及其结构之间关联的研究还存在不足。本文采用黑龙江黑土、云南红土和山东黄土提取的胡敏酸(HAs)作为实验样品,通过电子顺磁共振波谱技术(EPR)分析测定HAs中自由基的信号特征,利用元素分析、紫外分光光度法、13C NMR核磁、高效液相等方法测定HAs提取物的结构、组分和分子量分布等。结果表明,HAs中EPFRs的 量(0.84×1016 ~7.42×1016 spin/g)随着分子量的增加而减少,且与HAs的芳香性有显著的正相关关系(r=0.813, p<0.01),这归因于芳香族化合物自由电子能够部分离域形成EPFRs。EPFRs的g值(2.0034~2.0041)随芳香性的增加而减少(r=-0.752,p<0.01),表明芳香性组分对碳中心自由基有主要的贡献。相比于其他两个地区的土壤,红土HAs中EPFRs信号最稳定,主要归因于云南土壤中Fe3+含量丰富,在强紫外线的辐射下更有利于形成稳定的EPFRs。
中图分类号:
引用本文
鲁遥, 王朋, 尹梦楠, 杨名毅, 张凰. 不同类型土壤胡敏酸提取物环境持久性自由基特征及影响因素[J]. 化工进展, 2021, 40(5): 2917-2927.
LU Yao, WANG Peng, YIN Mengnan, YANG Mingyi, ZHANG Huang. Characteristics and influencing factors of environmental persistent free radicals of humic acid extracts from different types of soil[J]. Chemical Industry and Engineering Progress, 2021, 40(5): 2917-2927.
| 样品 | g值 | 线宽ΔH | 自旋密度/spin·g-1 |
|---|---|---|---|
| B-HA-1 | 2.0034 | 4.36 | 7.42×1016 |
| B-HA-2 | 2.0035 | 4.52 | 4.37×1016 |
| B-HA-3 | 2.0036 | 4.58 | 2.99×1016 |
| B-HA-4 | 2.0039 | 4.66 | 2.13×1016 |
| R-HA-1 | 2.0041 | 4.66 | 3.66××1016 |
| R-HA-2 | 2.0040 | 4.35 | 4.91××1016 |
| R-HA-3 | 2.0039 | 4.38 | 2.94××1016 |
| R-HA-4 | 2.0038 | 4.66 | 2.43××1016 |
| Y-HA-1 | 2.0040 | 4.36 | 3.03×1016 |
| Y-HA-2 | 2.0039 | 4.51 | 1.11×1016 |
| Y-HA-3 | 2.0040 | 4.58 | 1.14×1016 |
| Y-HA-4 | 2.0039 | 4.66 | 0.84×1016 |
表1 不同来源HAs中EPR信号特征参数
| 样品 | g值 | 线宽ΔH | 自旋密度/spin·g-1 |
|---|---|---|---|
| B-HA-1 | 2.0034 | 4.36 | 7.42×1016 |
| B-HA-2 | 2.0035 | 4.52 | 4.37×1016 |
| B-HA-3 | 2.0036 | 4.58 | 2.99×1016 |
| B-HA-4 | 2.0039 | 4.66 | 2.13×1016 |
| R-HA-1 | 2.0041 | 4.66 | 3.66××1016 |
| R-HA-2 | 2.0040 | 4.35 | 4.91××1016 |
| R-HA-3 | 2.0039 | 4.38 | 2.94××1016 |
| R-HA-4 | 2.0038 | 4.66 | 2.43××1016 |
| Y-HA-1 | 2.0040 | 4.36 | 3.03×1016 |
| Y-HA-2 | 2.0039 | 4.51 | 1.11×1016 |
| Y-HA-3 | 2.0040 | 4.58 | 1.14×1016 |
| Y-HA-4 | 2.0039 | 4.66 | 0.84×1016 |
| 样品 | 元素分析(质量分数) | 灰分 /% | E285 | E4/E6 | |||||
|---|---|---|---|---|---|---|---|---|---|
| C/% | H/% | O/% | N/% | C/H比 | (N+O)/C比 | ||||
| B-HA-1 | 54.39 | 4.26 | 37.36 | 3.06 | 1.04 | 0.57 | 0.9 | 0.95 | 4.23 |
| B-HA-2 | 54.63 | 4.53 | 36.99 | 3.24 | 1.00 | 0.56 | 1.1 | 0.87 | 4.33 |
| B-HA-3 | 54.70 | 4.87 | 36.40 | 3.59 | 0.94 | 0.55 | 1.1 | 0.83 | 4.35 |
| B-HA-4 | 54.81 | 5.24 | 35.72 | 3.82 | 0.87 | 0.54 | 1.2 | 0.64 | 4.38 |
| R-HA-1 | 53.75 | 5.03 | 37.44 | 3.20 | 0.89 | 0.57 | 2.6 | 0.89 | 3.71 |
| R-HA-2 | 53.88 | 5.22 | 37.12 | 3.22 | 0.86 | 0.56 | 1.4 | 0.75 | 3.93 |
| R-HA-3 | 54.57 | 5.39 | 36.23 | 3.27 | 0.84 | 0.54 | 2.2 | 0.73 | 4.05 |
| R-HA-4 | 55.34 | 5.79 | 34.93 | 3.32 | 0.80 | 0.52 | 2.0 | 0.68 | 4.16 |
| Y-HA-1 | 51.13 | 5.14 | 39.83 | 3.57 | 0.83 | 0.64 | 2.7 | 0.74 | 4.15 |
| Y-HA-2 | 51.30 | 5.34 | 38.31 | 4.42 | 0.80 | 0.63 | 1.7 | 0.62 | 4.23 |
| Y-HA-3 | 51.59 | 5.70 | 37.82 | 4.26 | 0.75 | 0.62 | 2.6 | 0.51 | 4.27 |
| Y-HA-4 | 51.72 | 5.86 | 36.87 | 4.92 | 0.73 | 0.61 | 2.4 | 0.41 | 4.30 |
表2 3种土壤分批次提取的HAs的元素分析和紫外吸收光谱
| 样品 | 元素分析(质量分数) | 灰分 /% | E285 | E4/E6 | |||||
|---|---|---|---|---|---|---|---|---|---|
| C/% | H/% | O/% | N/% | C/H比 | (N+O)/C比 | ||||
| B-HA-1 | 54.39 | 4.26 | 37.36 | 3.06 | 1.04 | 0.57 | 0.9 | 0.95 | 4.23 |
| B-HA-2 | 54.63 | 4.53 | 36.99 | 3.24 | 1.00 | 0.56 | 1.1 | 0.87 | 4.33 |
| B-HA-3 | 54.70 | 4.87 | 36.40 | 3.59 | 0.94 | 0.55 | 1.1 | 0.83 | 4.35 |
| B-HA-4 | 54.81 | 5.24 | 35.72 | 3.82 | 0.87 | 0.54 | 1.2 | 0.64 | 4.38 |
| R-HA-1 | 53.75 | 5.03 | 37.44 | 3.20 | 0.89 | 0.57 | 2.6 | 0.89 | 3.71 |
| R-HA-2 | 53.88 | 5.22 | 37.12 | 3.22 | 0.86 | 0.56 | 1.4 | 0.75 | 3.93 |
| R-HA-3 | 54.57 | 5.39 | 36.23 | 3.27 | 0.84 | 0.54 | 2.2 | 0.73 | 4.05 |
| R-HA-4 | 55.34 | 5.79 | 34.93 | 3.32 | 0.80 | 0.52 | 2.0 | 0.68 | 4.16 |
| Y-HA-1 | 51.13 | 5.14 | 39.83 | 3.57 | 0.83 | 0.64 | 2.7 | 0.74 | 4.15 |
| Y-HA-2 | 51.30 | 5.34 | 38.31 | 4.42 | 0.80 | 0.63 | 1.7 | 0.62 | 4.23 |
| Y-HA-3 | 51.59 | 5.70 | 37.82 | 4.26 | 0.75 | 0.62 | 2.6 | 0.51 | 4.27 |
| Y-HA-4 | 51.72 | 5.86 | 36.87 | 4.92 | 0.73 | 0.61 | 2.4 | 0.41 | 4.30 |
| 样品 | 脂肪碳 (δ0~45) | 烷氧基碳 (δ45~110) | 芳香碳 (δ110~165) | 羧基碳 (δ165~190) | 羰基碳 (δ190~220) | 芳香性/% | 脂肪性/% |
|---|---|---|---|---|---|---|---|
| B-HA-1 | 19.95 | 13.05 | 45.02 | 13.08 | 8.90 | 57.69 | 42.31 |
| B-HA-2 | 21.14 | 13.86 | 42.95 | 16.18 | 5.87 | 55.13 | 44.87 |
| B-HA-3 | 22.45 | 15.55 | 40.96 | 17.08 | 3.96 | 51.89 | 48.10 |
| B-HA-4 | 24.66 | 17.34 | 39.96 | 11.09 | 6.95 | 48.78 | 51.22 |
| R-HA-1 | 24.75 | 16.83 | 37.62 | 12.87 | 7.93 | 47.50 | 52.50 |
| R-HA-2 | 27.27 | 15.15 | 37.37 | 16.16 | 4.05 | 46.83 | 53.16 |
| R-HA-3 | 31.63 | 12.24 | 36.73 | 17.35 | 2.05 | 45.57 | 54.43 |
| R-HA-4 | 30.00 | 19.00 | 36.36 | 11.11 | 3.53 | 41.86 | 58.14 |
| Y-HA-1 | 26.26 | 17.17 | 36.63 | 11.98 | 7.98 | 45.68 | 54.32 |
| Y-HA-2 | 24.63 | 18.81 | 35.35 | 12.12 | 9.09 | 44.87 | 55.13 |
| Y-HA-3 | 21.11 | 28.29 | 32.35 | 9.43 | 8.82 | 39.76 | 60.24 |
| Y-HA-4 | 27.35 | 23.65 | 30.50 | 14.50 | 4.00 | 37.04 | 62.96 |
表3 固态13C NMR谱分析结果
| 样品 | 脂肪碳 (δ0~45) | 烷氧基碳 (δ45~110) | 芳香碳 (δ110~165) | 羧基碳 (δ165~190) | 羰基碳 (δ190~220) | 芳香性/% | 脂肪性/% |
|---|---|---|---|---|---|---|---|
| B-HA-1 | 19.95 | 13.05 | 45.02 | 13.08 | 8.90 | 57.69 | 42.31 |
| B-HA-2 | 21.14 | 13.86 | 42.95 | 16.18 | 5.87 | 55.13 | 44.87 |
| B-HA-3 | 22.45 | 15.55 | 40.96 | 17.08 | 3.96 | 51.89 | 48.10 |
| B-HA-4 | 24.66 | 17.34 | 39.96 | 11.09 | 6.95 | 48.78 | 51.22 |
| R-HA-1 | 24.75 | 16.83 | 37.62 | 12.87 | 7.93 | 47.50 | 52.50 |
| R-HA-2 | 27.27 | 15.15 | 37.37 | 16.16 | 4.05 | 46.83 | 53.16 |
| R-HA-3 | 31.63 | 12.24 | 36.73 | 17.35 | 2.05 | 45.57 | 54.43 |
| R-HA-4 | 30.00 | 19.00 | 36.36 | 11.11 | 3.53 | 41.86 | 58.14 |
| Y-HA-1 | 26.26 | 17.17 | 36.63 | 11.98 | 7.98 | 45.68 | 54.32 |
| Y-HA-2 | 24.63 | 18.81 | 35.35 | 12.12 | 9.09 | 44.87 | 55.13 |
| Y-HA-3 | 21.11 | 28.29 | 32.35 | 9.43 | 8.82 | 39.76 | 60.24 |
| Y-HA-4 | 27.35 | 23.65 | 30.50 | 14.50 | 4.00 | 37.04 | 62.96 |
| 1 | 韩林, 陈宝梁. 环境持久性自由基的产生机理及环境化学行为[J]. 化学进展, 2017, 29(9): 1008-1020. |
| HAN L, CHEN B L. Generation mechanism and fate behaviors of environmental persistent free radicals[J]. Progress in Chemistry, 2017,29(9): 1008-1020. | |
| 2 | GOMBERG M. An instance of trivalent carbon:triphenylmethyl[J]. Journal of the American Chemical Society, 1900, 22(11): 757-771. |
| 3 | CASTRANOVA V. Signaling pathways controlling the production of inflammatory mediators in response to crystalline silica exposure: role of reactive oxygen/nitrogen species[J]. Free Radical Biology and Medicine, 2004, 37(7):916-925. |
| 4 | BALAKRISHNA S, LOMNICKI S, MCAVEY K M, et al. Environmentally persistent free radicals amplify ultrafine particle mediated cellular oxidative stress and cytotoxicity[J]. Particle and Fibre Toxicology, 2009, 6(1): 11. |
| 5 | PAN B, LI H, LANG D, et al. Environmentally persistent free radicals: occurrence,formation mechanisms and implications[J]. Environmental Pollution, 2019, 248:320-331. |
| 6 | HEIMER N E. Persistent free radicals from the reaction of sulfenamides with tetracyanoethylene[J]. The Journal of Organic Chemistry, 1977, 42(23): 3767-3769. |
| 7 | DELLINGER B, LOMNICKI S, KHACHATRYAN L, et al. Formation and stabilization of persistent free radicals[J]. Proceedings of the Combustion Institute, 2007, 31(1): 521-528. |
| 8 | GEHLING W, DELLINGER B. Environmentally persistent free radicals and their lifetimes in PM2.5[J]. Environmental Science & Technology, 2013, 47(15): 8172-8178. |
| 9 | GEHLING W, KHACHATRYAN L, DELLINGER B. Hydroxyl radical generation from environmentally persistent free radicals (EPFRs) in PM2.5[J]. Environmental Science & Technology, 2014, 48(8): 4266-4272. |
| 10 | 阮秀秀, 孙万雪, 程玲, 等. 环境持久性自由基的研究进展[J]. 上海大学学报(自然科学版), 2016, 22(2): 114-121. |
| RUAN Xiuxiu, SUN Wanxue, CHENG Ling, et al. Research progress of environmental persistent free radicals [J]. Journal of Shanghai University (Natural Science Edition), 2016, 22(2): 114-121. | |
| 11 | DELA CRUZ A N, GEHLING W, LOMNICKI S, et al. Detection of environmentally persistent free radicals at a superfund wood treating site[J]. Environmental Science & Technology, 2011, 45(15): 6356-6365. |
| 12 | DELA CRUZ A L, COOK R L, LOMNICKI S M, et al. Effect of low temperature thermal treatment on soils contaminated with pentachlorophenol and environmentally persistent free radicals[J]. Environmental Science & Technology, 2012, 46(11): 5971-5978. |
| 13 | DELA CRUZ A L, COOK R L, DELLINGER B, et al. Assessment of environmentally persistent free radicals in soils and sediments from three superfund sites[J]. Environmental Science: Processes & Impacts, 2014, 16(1): 44-52. |
| 14 | REX R W. Electron paramagnetic resonance studies of stable free radicals in lignins and humic acids[J]. Nature, 1960, 188(4757): 1185-1186. |
| 15 | AESCHBACHER M, SANDER M, SCHWARZENBACH R P. Novel electrochemical approach to assess the redox properties of humic substances[J]. Environmental Science & Technology, 2010, 44(1):87-93. |
| 16 | PATIL S V, ARGYROPOULOS D S. Stable organic radicals in lignin: a review[J]. ChemSusChem, 2017, 10(17): 3284-3303. |
| 17 | RIFFALDI R, SCHNITZER M. Electron spin resonance spectrometry of humic substances[J]. Soil Science Society of America Journal, 1972,36(2): 301-305. |
| 18 | SAAB S C, MARTIN-NETO L. Studies of semiquinone free radicals by ESR in the whole soil, HA, FA and humin substances[J]. Journal of the Brazilian Chemical Society, 2004, 15: 34-37. |
| 19 | JIA H Z, ZHAO S, NULAJI G, et al. Environmentally persistent free radicals in soils of past coking sites: distribution and stabilization[J]. Environmental Science & Technology, 2017, 51(11): 6000-6008. |
| 20 | JIA H Z, ZHAO S, SHI Y F, et al. Formation of environmentally persistent free radicals during the transformation of anthracene in different soils: roles of soil characteristics and ambient conditions[J]. J. Hazard Mater., 2019, 362: 214-223. |
| 21 | FELD-COOK E E, BOVENKAMP-LANGLOIS L, LOMNICKI S M. Effect of particulate matter mineral composition on environmentally persistent free radical (EPFR) formation[J]. Environmental Science & Technology, 2017, 51(18): 10396-10402. |
| 22 | TIAN L W, KOSHLAND C P, YANO J, et al. Carbon-centered free radicals in particulate matter emissions from wood and coal combustion[J]. Energy & Fuels, 2009, 23(5): 2523-2526. |
| 23 | 赵力, 陈建, 李浩, 等. 裂解温度和酸处理对生物炭中持久性自由基产生的影响[J]. 环境化学, 2017, 36(11): 2472-2478. |
| ZHAO Li, CHEN Jian, LI Hao, et al. Effects of pyrolysis temperature and acid treatment on the generation of free radicals in biochars[J]. Environmental Chemistry, 2017, 36(11): 2472-2478. | |
| 24 | LIAO S H, PAN B, LI H, et al. Detecting free radicals in biochars and determining their ability to inhibit the germination and growth of corn, wheat and rice seedlings[J]. Environmental Science & Technology, 2014, 48(15): 8581-8587. |
| 25 | YANG J, PAN B, LI H, et al. Degradation of p-nitrophenol on biochars: role of persistent free radicals[J]. Environmental Science & Technology, 2016, 50(2): 694-700. |
| 26 | LIU Y, DAI Q Y, JIN X Q, et al. Negative impacts of biochars on urease activity: high pH, heavy metals, polycyclic aromatic hydrocarbons, or free radicals?[J]. Environmental Science & Technology, 2018, 52(21): 12740-12747. |
| 27 | DUGAS T R, HEBERT V Y, DELLINGER B, et al. Environmentally persistent free radicals are redox active and more cytotoxic than the average ultrafine particle[J]. Free Radical Biology and Medicine, 2009, 47: S121. |
| 28 | WHITMAN T, ZHU Z H, LEHMANN J. Carbon mineralizability determines interactive effects on mineralization of pyrogenic organic matter and soil organic carbon[J]. Environmental Science & Technology, 2014, 48(23): 13727-13734. |
| 29 | SEDLECKIJ I, BRUNOWSKIJ B. Structure of humic acid and its connection with lignin and to carbon[J].Comptes Rendus De L Academie Des Sciences De L Urss, 1935, 9: 279-281. |
| 30 | 王春霞, 许善锦, 王夔. 自由基在大骨节病病理过程中的作用—Ⅰ.活性氧自由基和大骨节病病区腐植酸对Ⅱ型胶原蛋白造成的损伤[J]. 北京医科大学学报, 1989, 21(4): 307-310. |
| WANG Chunxia, XU Shanjin, WANG Kui. The role of free radicals in the pathological process of Kashin Beck disease—Ⅰ. The damage to type Ⅱ collagen caused by active oxygen radicals and fulvic acid from epidemicdistrict of KBD[J]. Journal of Beijing Medical University, 1989, 21(4): 307-310. | |
| 31 | 张若瑄, 王朋, 张绪超, 等. 土壤中环境持久性自由基的形成与稳定及其影响因素[J].化工进展, 2020, 39(4): 1528-1538. |
| ZHANG Ruoxuan, WANG Peng, ZHANG Xuchao, et al. Formation, stability and influencing factors of environmentally persistent free radicals in soil: a review[J]. Chemical Industry and Engineering Progress, 2020, 39(4): 1528-1538. | |
| 32 | GONZÁLEZ PERÉZ M, MARTIN-NETO L, SAAB S C. Characterization of humic acids from a Brazilian oxisol under different tillage systems by EPR, 13C NMR, FTIR and fluorescence spectroscopy[J]. Geoderma, 2004, 118(3/4): 181-190. |
| 33 | PAN B, XING B, LIU W, et al. Investigating interactions of phenanthrene with dissolved organic matter: limitations of Sterne-Volmer plot[J]. Chemosphere, 2007, 69(10): 1555-1562. |
| 34 | 曹文华, 刘国维.低含量风化煤活化提取高纯度腐植酸研究[J]. 腐植酸, 2000(4): 33-34. |
| CAO Wenhua, LIU Guowei. Study on activation extraction of high purity humic acid from low content weathered coal [J]. Humic Acid, 2000(4): 33-34. | |
| 35 | ZHANG D, PAN B, COOK R L, et al. Multi-walled carbon nanotube dispersion by the adsorbed humic acids with different chemical structures[J]. Environmental Pollution, 2015, 196:292-299. |
| 36 | LI H, PAN B, LIAO S, et al. Formation of environmentally persistent free radicals as the mechanism for reduced catechol degradation on hematite-silica surface under UV irradiation[J]. Environmental Pollution, 2014, 188:153-158. |
| 37 | LOVLEY D R, COATES J D, BLUNT-HARRIS E L, et al. Humic substances as electron acceptors for microbial respiration[J]. Nature, 1996, 382(6590): 445-448. |
| 38 | 盛明, 韩晓增, 龙静泓, 等. 中国不同地区土壤有机质特征比较研究[J]. 土壤与作物, 2019, 8(3): 320-330. |
| SHENG Ming, HAN Xiaozeng, LONG Jinghong, et al. Characterization of soil organic matter in different regions of China[J]. Soils and Crops, 2019, 8(3): 320-330. | |
| 39 | 仲伟鉴, 印木泉. 废气煤烟颗粒提取物诱发DNA氧化损伤的实验研究[J]. 第二军医大学学报, 2000, 21(9): 874-876. |
| ZHONG Weijian, YIN Muquan. Oxidative DNA damage induced by cool soot[J]. Academic Journal of Second Military Medical University, 2000, 21(9): 874-876. | |
| 40 | ARANGIO A M, TONG H, SOCORRO J, et al. Quantification of environmentally persistent free radicals and reactive oxygen species in atmospheric aerosol particles[J]. Atmospheric Chemistry and Physics, 2016, 16(20): 13105-13119. |
| 41 | CRUZ A L N D, COOK R L, LOMNICKI S M, et al.Effect of low temperature thermal treatment on soils contaminated with pentachlorophenol and environmentally persistent free radicals[J]. Environmental Science & Technology, 2012, 46(11): 5971-5978. |
| 42 | SHALTOUT A A, BOMAN J, SHEHADEH Z F, et al. Spectroscopic investigation of PM2.5 collected at industrial,residential and traffic sites in Taif, Saudi Arabia[J]. Journal of Aerosol Science,2015,79:97-108. |
| 43 | STERN N, MEJIA J, HE S M, et al. Dual role of humic substances as electron donor and shuttle for dissimilatory iron reduction[J]. Environ. Sci. Technol., 2018, 52(10): 5691-5699. |
| 44 | SENESI N. Application of electron spin resonance and fluorescence spectroscopies to the study of soil humic substances[J]. Developments in Agricultural and Managed Forest Ecology, 1992, 25:11-26. |
| 45 | WATANABE A, MCPHAIL D B, MAIE N, et al. Electron spin resonance characteristics of humic acids from a wide range of soil types [J]. Organic Geochemistry, 2005, 36(7): 981-990. |
| 46 | 聂艳龙. 黑龙江省农田黑土区腐殖物质结构差异及光谱特性研究[D].哈尔滨: 东北农业大学, 2016. |
| NIE Yanlong. Research structure and spectral characteristics of humus substance from black soil region in Heilongjiang Province[D]. Harbin:Northeast Agricultural University, 2016. | |
| 47 | KIM I, BUCKAU G, LI G H, et al. Characterization of humic and fulvic acids from Gorleben groundwater[J]. Fresenius Journal of Analytical Chemistry, 1990, 338(3): 245-252. |
| 48 | LU X Q, HANNA J V, JOHNSON W D. Source indicators of humic substances: an elemental composition solid state 13C CP/MAS NMR and Py-GC/MS study[J]. Applied Geochemist, 2000, 15(7): 1019-1033. |
| 49 | SENESI N. Application of electron spin resonance (ESR) spectroscopy in soil chemistry[M]//Advances in Soil Science, 1990: 77-130. |
| 50 | HARGITAI L. Biochemical transformation of humic substances during humification related to their environmental functions[J]. Environment International, 1994, 20: 43-48. |
| 51 | BAKER A. Fluorescence excitation-emission matrix characterization of some sewage-impacted rivers[J]. Environmental Science & Technology, 2001, 35(5): 948-953. |
| 52 | SENESI N, MIANO T M, PROVENZANO M R, et al. Characterization,differentiation,and classification of humic substances by fluorescence spectroscopy [J]. Soil Science, 1991, 152: 259-271. |
| 53 | 魏自民, 席北斗,李鸣晓,等. 微生物接种堆肥胡敏酸三维荧光特性研究[J].光谱学与光谱分析, 2008, 28(12): 2895-2899. |
| WEI Zimin, XI Beidou, LI Mingxiao, et al. Study on three-dimensional fluorescence spectroscopy characteristics of humic acid during composting with microbes inoculation[J]. Spectroscopy and Spectral Analysis, 2008,28(12): 2895-2899. | |
| 54 | 黄曼, 卞科. 蛋白质疏水性测定方法研究进展[J]. 粮油食品科技, 2004, 12(2): 31-32. |
| HUANG Man, BIAN Ke. Researching development on the methods of protein hydrophobicity estimation[J]. Science and Technology of Cereals,Oils and Foods, 2004, 12(2): 31-32. | |
| 55 | WILSON S A, WEBER J H. Electron spin resonance analysis of semiquinone free radicals of aquatic and soil fulvic and humic acids[J]. Analytical Letters, 1977, 10(1): 75-84. |
| 56 | 何海军, 瞿文川, 钱君龙, 等. 湖泊沉积物中腐殖酸的紫外-可见分光光度法测定[J]. 分析测试技术与仪器, 1996, 2(1): 14-18. |
| HE Haijun, QU Wenchuan, QIAN Junlong, et al. Determination of humic acid in lake sediment by UV-vis absorbance spectrophotomter[J]. Analysis and Testing Technology and Instruments, 1996, 2(1): 14-18. | |
| 57 | THOMSEN M, LASSEN P, DOBEL S, et al. Characterisation of humic materials of different origin: a multivariate approach for quantifying the latent properties of dissolved organic matter[J]. Chemosphere, 2002, 49(10): 1327-1337. |
| 58 | CHEN J, GU B H, LEBOEUF E J, et al. Spectroscopic characterization of the structural and functional properties of natural organic matter fractions[J]. Chemosphere, 2002, 48(1): 59-68. |
| 59 | KANG K H, SHIN H S, PARK H. Characterization of humic substances present in landfill leachates with different landfill ages and its implications[J]. Water Research, 2002, 36(16): 4023-4032. |
| 60 | 徐磊. 基于高分辨质谱的富里酸和腐殖酸分子组成与结构研究[D]. 南昌: 南昌大学, 2019. |
| XU Lei. Study on molecular composition and structure of fulvic acid and humic acid by high resolution mass spectrometry[D]. Nanchang: Nanchang University, 2019. | |
| 61 | 张广彩, 王雅南, 常昕, 等. 应用多元统计研究蘑菇湖水体DOM紫外光谱特征[J]. 环境科学研究, 2019, 32(2): 301-308. |
| ZHANG Guangcai, WANG Yanan, CHANG Xin, et al. Apply multivariate statistical method to study DOM ultraviolet spectral characteristics of Moguhu lake[J]. Research of Environmental Sciences, 2019, 32(2): 301-308. | |
| 62 | SONG J Z, HUANG W L, PENG P A, et al. Humic acid molecular weight estimation by high-performance size-exclusion chromatography with ultraviolet absorbance detection and refractive index detection[J]. Soil Science Society of America Journal, 2010, 74(6): 2013-2020. |
| 63 | ZHU S, HAMIELEC A E. Chain-length-dependent termination for free radical polymerization[J]. Macromolecules, 1989, 22(7): 3093-3098. |
| 64 | SCHEREN P A G M, RUSSELL G T, SANGSTER D F, et al. Chain-length-dependent termination rate processes in free-radical polymerizations. 3. Styrene polymerizations with and without added inert diluent as an experimental test of model[J]. Macromolecules, 1995, 28(10): 3637-3649. |
| 65 | PAUL A, STÖSSER R, ZEHL A, et al. Nature and abundance of organic radicals in natural organic matter: effect of pH and irradiation[J]. Environmental Science & Technology, 2006, 40(19): 5897-5903. |
| 66 | 杨颖, 孙振亚. 一类新的环境有害物质——环境持久性自由基(EPFRs)的研究进展[J]. 矿物岩石地球化学通报, 2012, 31(3): 287-290. |
| YANG Ying, SUN Zhenya. The research progress on a new type harmful matter-environmental persistent free radicals(EPFRs)[J]. Bulletin of Mineralogy,Petrology and Geochemistry, 2012, 31(3): 287-290. |
| [1] | 李昕, 杨早, 钟欣茹, 韩昊轩, 庄绪宁, 白建峰, 董滨, 徐祖信. 污泥超高温堆肥衍生胡敏酸对Pb2+的结合机制[J]. 化工进展, 2023, 42(9): 4957-4966. |
| [2] | 张若瑄,王朋,张绪超,段文焱. 土壤中环境持久性自由基的形成与稳定及其影响因素[J]. 化工进展, 2020, 39(4): 1528-1538. |
| [3] | 周敏茹,姚培,张启蒙,李树白,刘媛,夏守鑫. 磁响应型胡敏酸纳米材料的制备及其对Cu(Ⅱ)的吸附性能[J]. 化工进展, 2020, 39(3): 1145-1152. |
| [4] | 黄倩, 付美龙, 赵众从. 超临界CO2压裂液增黏剂的长管实验评价及增黏机制探讨[J]. 化工进展, 2019, 38(06): 2939-2946. |
| [5] | 姚正天, 沈本贤, 仝玉军, 孙辉. 春风减渣改质塔河减渣制取60#道路沥青效果[J]. 化工进展, 2018, 37(10): 4109-4118. |
| [6] | 王朋, 吴敏, 李浩, 郎笛, 潘波. 环境持久性自由基对有机污染物环境行为的影响研究进展[J]. 化工进展, 2017, 36(11): 4243-4249. |
| [7] | 刘向君, 罗丹序, 熊健, 梁利喜. 龙马溪组页岩干酪根平均分子结构模型的构建[J]. 化工进展, 2017, 36(02): 530-537. |
| [8] | 赵波,陆居有,毛伟,王博,吕剑. 氟代烃类发泡剂的研究进展[J]. 化工进展, 2014, 33(07): 1864-1870. |
| [9] | 郑 敏,金晓英,王清萍,陈祖亮. 胡敏酸改性膨润土同时吸附铜离子和2,4-二氯苯酚 [J]. 化工进展, 2010, 29(9): 1767-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||