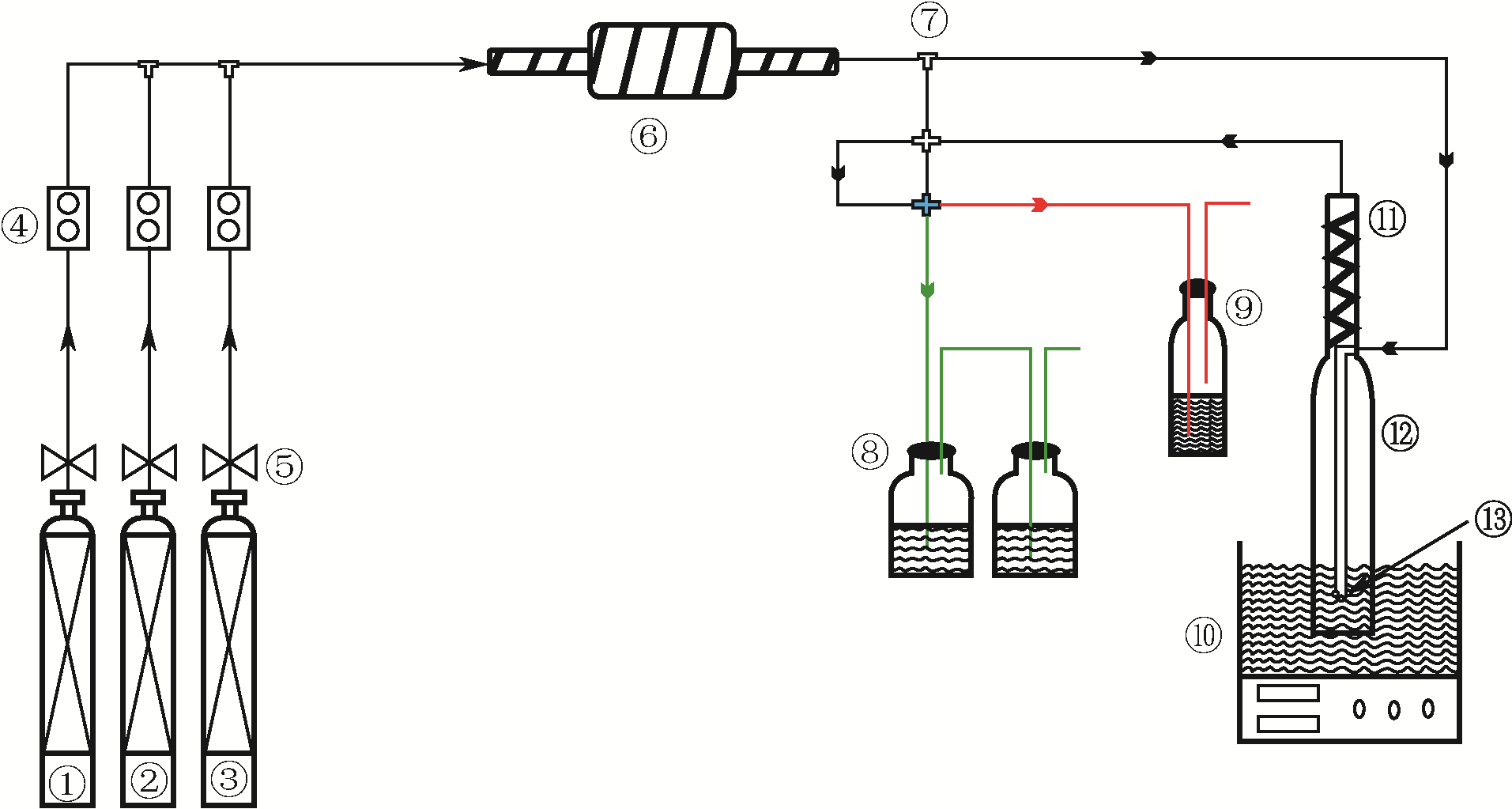
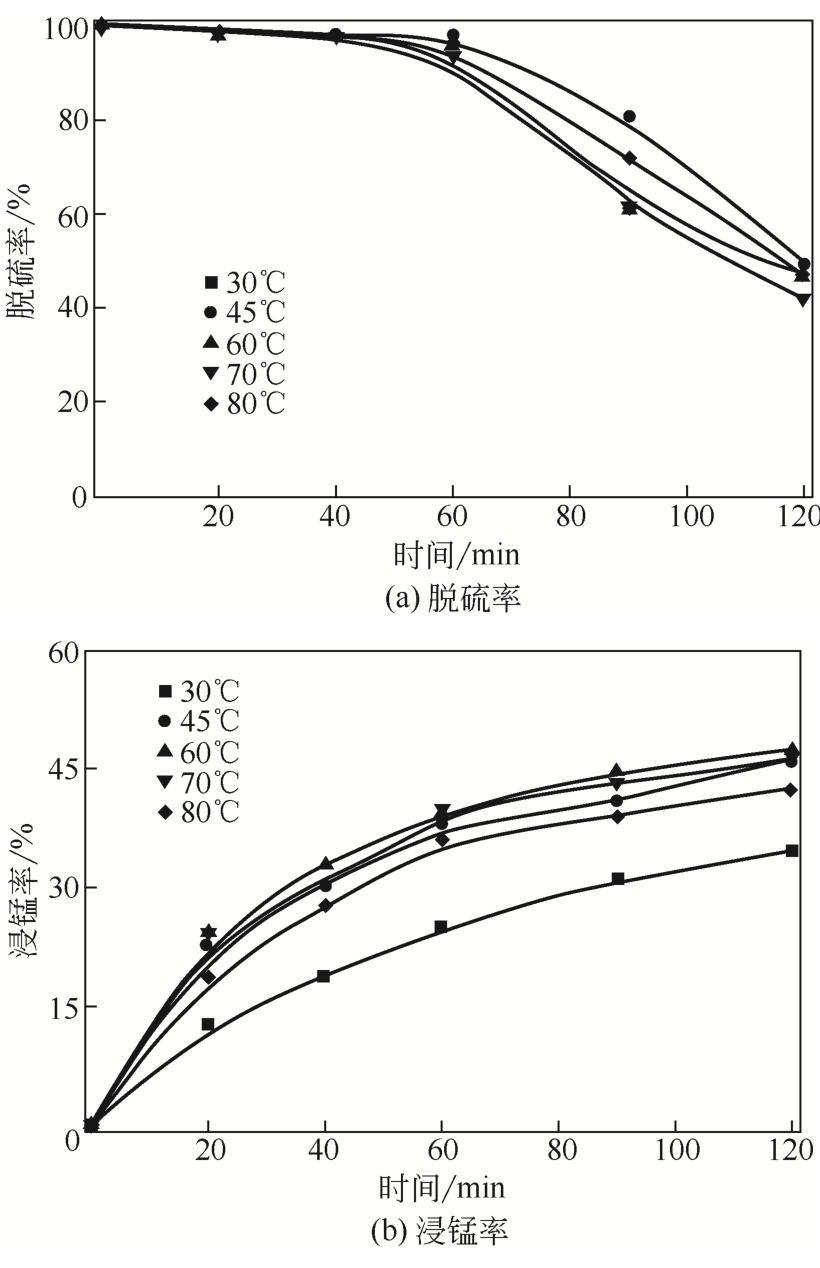
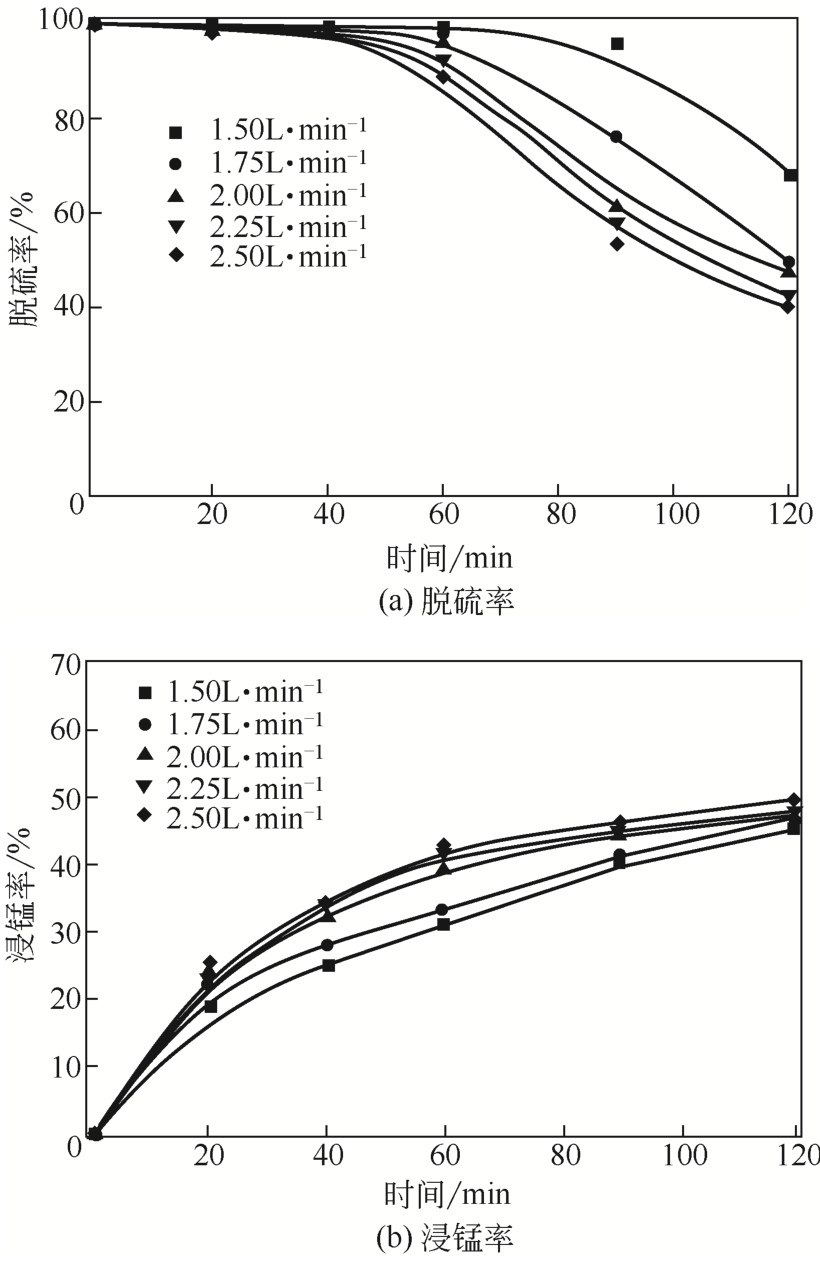
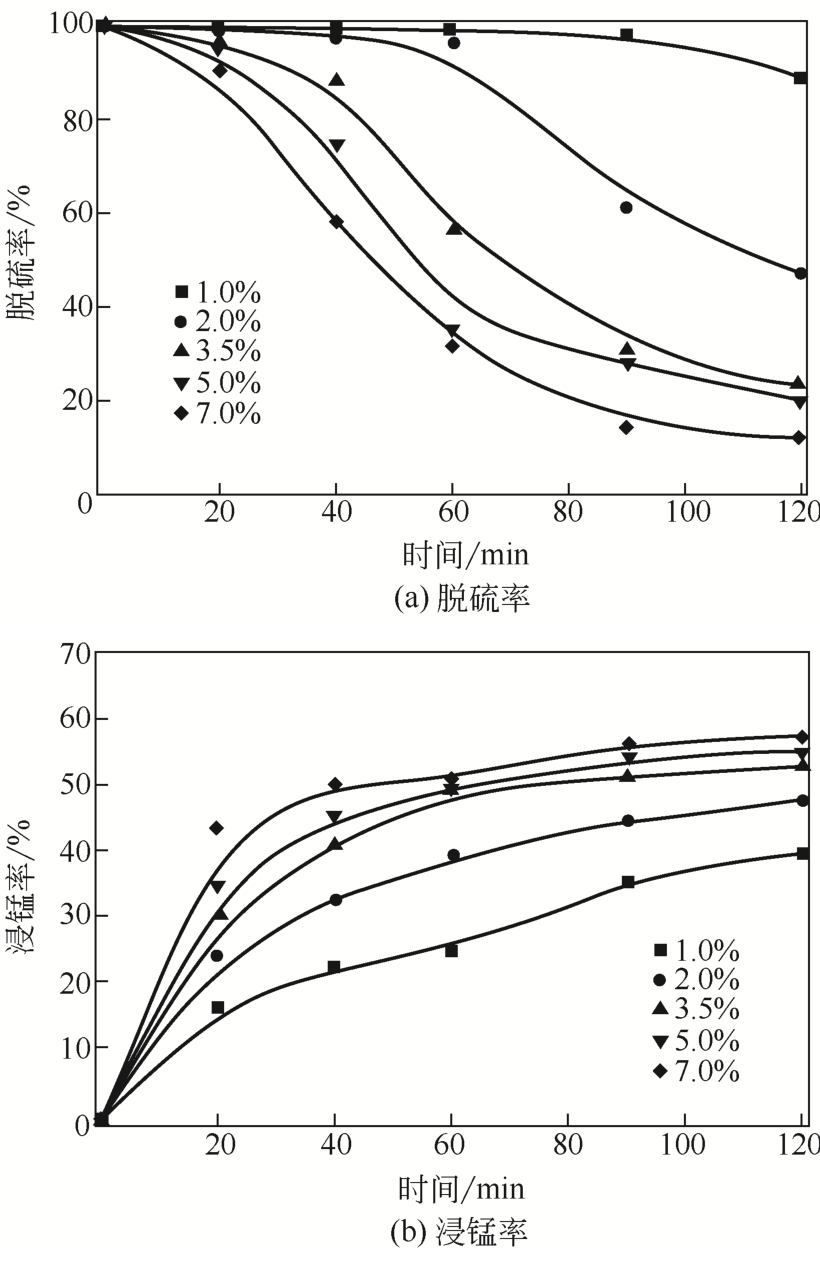
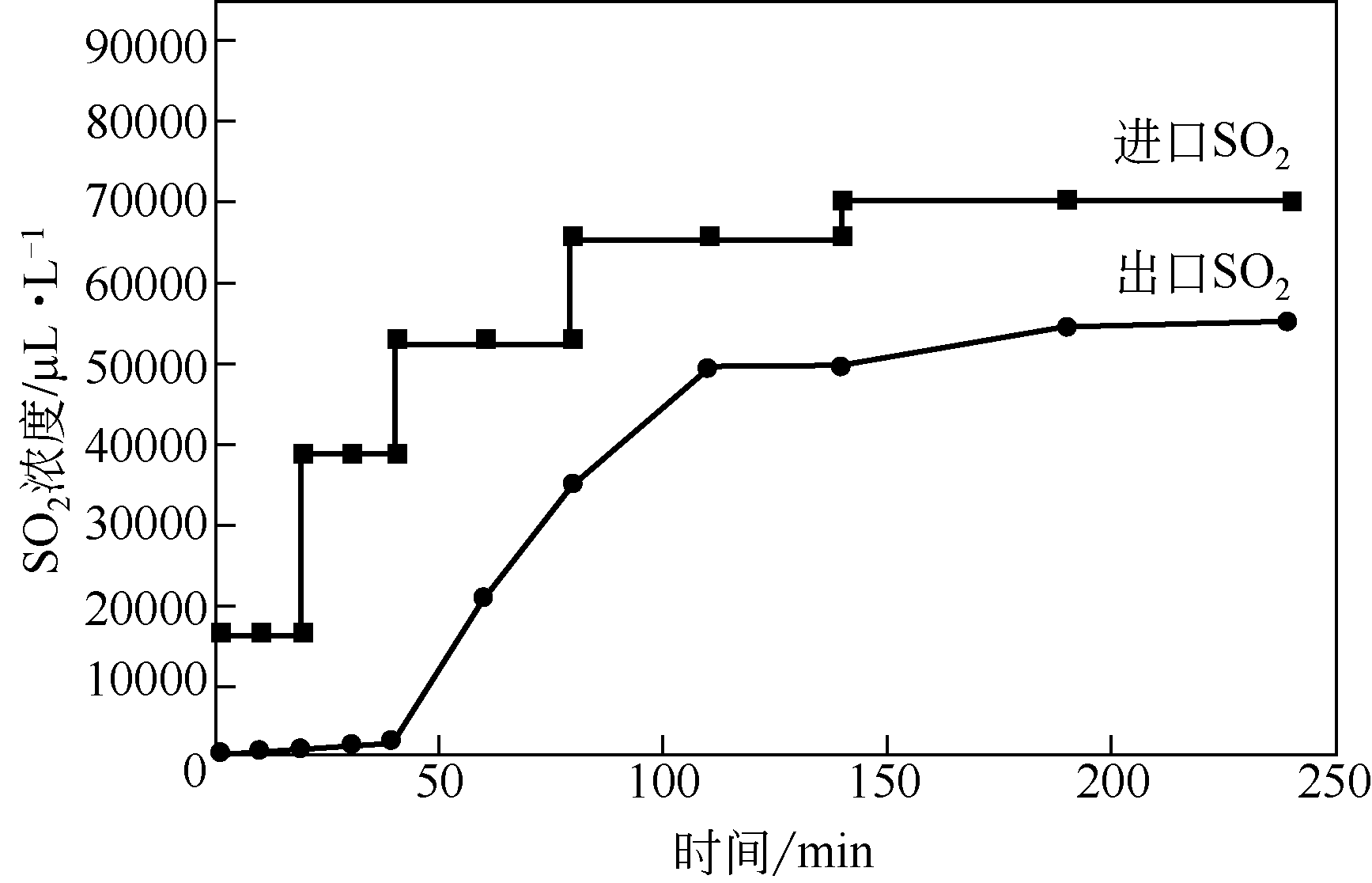
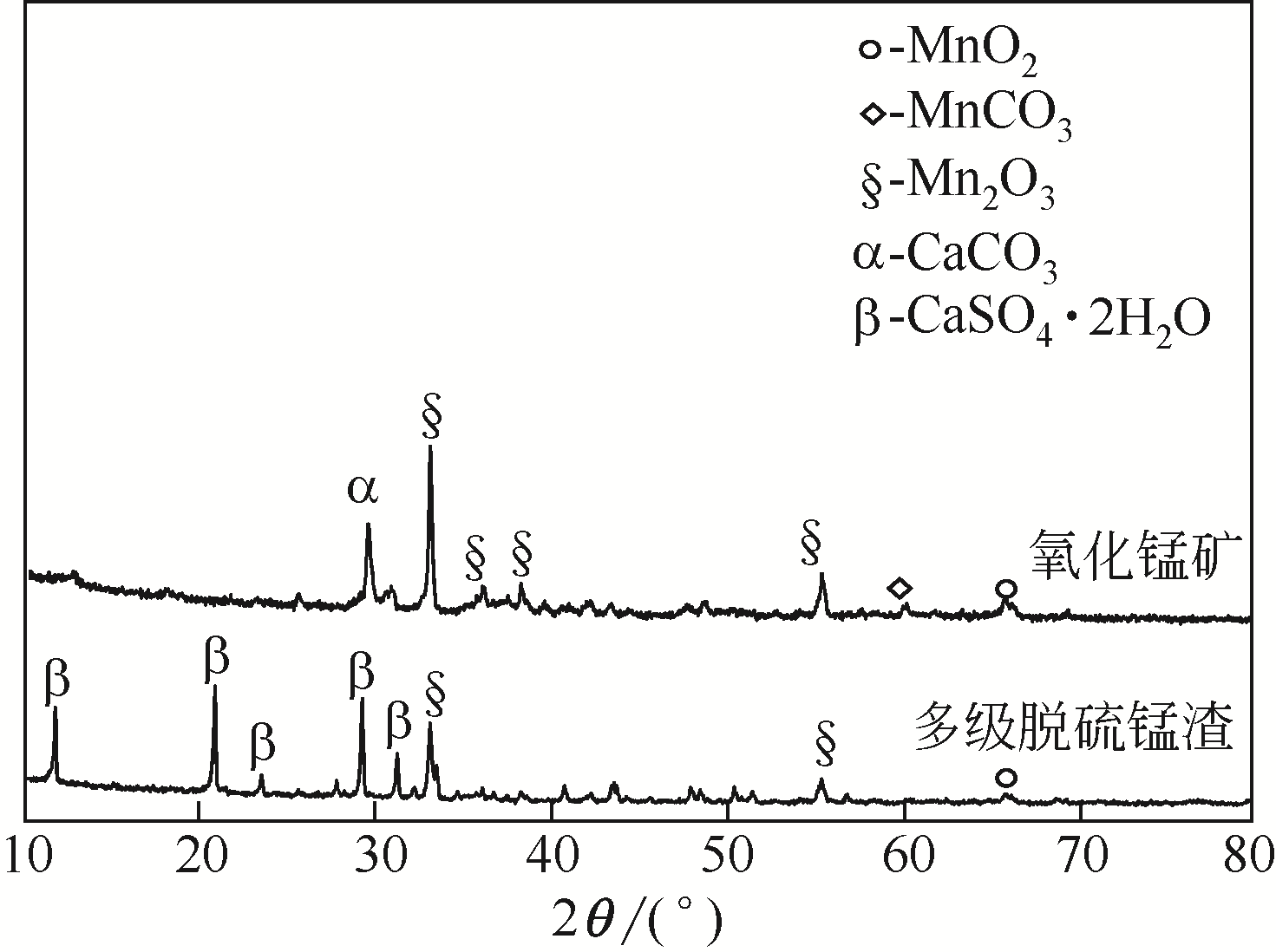
| 2 |
DU B, ZHOU C, DAN Z, et al. Preparation and characteristics of steam-autoclaved bricks produced from electrolytic manganese solid waste[J]. Construction and Building Materials, 2014, 50: 291-299.
|
| 3 |
DONG S, LIN Y, NING L, et al. Sulfur resource recovery based on electrolytic manganese residue calcination and manganese oxide ore desulfurization for the clean production of electrolytic manganese[J]. Chinese Journal of Chemical Engineering, 2020, 28: 864-870.
|
| 4 |
ZHAO J D, SU S J, AI N S, et al. Modelling flue gas desulfurization using pyrolusite pulp in a jet bubbling reactor[J]. Materials Science Forum, 2009, 610/611/612/613: 85-96.
|
| 5 |
SUN D, XIN G, YAO L, et al. Manganese leaching in high concentration flue gas desulfurization process with semi-oxidized manganese ore[J]. Chinese Journal of Chemical Engineering, 2020, 28(2): 571-578.
|
| 6 |
ULRICH R K, ROCHELLE G T, PRADA R E. Enhanced oxygen absorption into bisulphite solutions containing transition metal ion catalysts[J]. Chemical Engineering Science, 1986, 41(8): 2183-2191.
|
| 7 |
YAO L, YANG L, JIANG W, et al. Removal of SO2 from flue gas on a copper-modified activated coke prepared by a novel one-step carbonization activation blending method[J]. Industrial & Engineering Chemistry Research, 2019, 58(34): 15693-15700.
|
| 8 |
GUO J X, FAN L, PENG J F, et al. Desulfurization activity of metal oxides blended into walnut shell based activated carbons[J]. Journal of Chemical Technology & Biotechnology, 2014, 89(10): 1565-1575.
|
| 9 |
刘洁岭, 汤争光, 陈杰, 等. 新型生物质活性炭烟气脱硫研究[J]. 环境科学, 2013, 34(4): 1623-1627.
|
|
LIU Jieling, TANG Zhengguang, CHEN Jie, et al. Flue gas desulferization by a novel biomass activated carbon[J] .Environmental Science, 2013, 34(4): 1623-1627.
|
| 10 |
赵珂, 宁平, 李凯, 等. Mn/Cu-BTC催化剂同时脱硫脱硝实验研究[J].化工进展, 2020, 39(5): 1784-1791.
|
|
ZHAO Ke, NING Ping, LI Kai, et al. Experimental study on simultaneous removal of SO2 and NO by Mn/Cu-BTC catalyst[J]. Chemical Industry and Engineering Progress,2020, 39(5): 1784-1791.
|
| 11 |
李万全, 张永奎, 巫延斌, 等. 微生物代谢与Fe3+催化氧化脱除烟气中SO2的研究[J]. 环境污染与防治, 2006, 28(9): 690-692.
|
|
LI Wanquan, ZHANG Yongkui, WU Yanbin, et al. Bio-enhanced catalytic oxidation process for removing sulfur dioxide from flue gas[J]. Environmental Pollution & Control, 2006, 28(9): 690-692.
|
| 12 |
DAS S C, SAHOO P K, RAO P K. Extraction of manganese from low-grade manganese ores by FeSO4 leaching[J]. Hydrometallurgy, 1982, 8(1): 35-47.
|
| 1 |
WANG J, PENG B, CHAI L Y, et al. Preparation of electrolytic manganese residue-ground granulated blastfurnace slag cement[J]. Powder Technology, 2013, 241: 12-18.
|
| 13 |
TEKIN T, BAYRAMOĜLU M. Kinetics of the reduction of MnO2 with Fe2+ ions in acidic solutions[J]. Hydrometallurgy, 1993, 32(1): 9-20.
|
| 14 |
ZAKERI A, SHAHRIARI S, BAFGHI M S. Dissolution kinetics of manganese dioxide ore in sulfuric acid in the presence of ferrous ion[J]. Iranian Journal of Materials Science & Engineering, 2007, 4(3/4): 22-27.
|
| 15 |
姚露, 杨林, 邹敏杰, 等. 氧化锰矿浆脱除电解锰渣煅烧烟气二氧化硫工艺研究[J]. 工程科学与技术, 2020, 52(5):250-256.
|
|
YAO Lu, YANG Lin, ZOU Minjie, et al. Study on Flue gas desulfurization with oxide manganese slurry for electrolytic manganese calcining[J]. Advanced Engineering Sciences, 2020, 52(5):250-256.
|
| 16 |
NAIK P K, SUKLA L B, DAS S C. Aqueous SO2 leaching studies on Nishikhal manganese ore through factorial experiment[J]. Hydrometallurgy, 2000, 54(2/3): 217-228.
|
| 17 |
MILLER J D, WAN R Y. Reaction kinetics for the leaching of MnO2 by sulfur dioxide[J]. Hydrometallurgy, 1983, 10(2): 219-242.
|
| 18 |
朱晓帆, 蒋文举, 苏仕军, 等. 软锰矿浆烟气脱硫反应机理研究[J]. 环境污染治理技术与设备, 2002(3): 44-46.
|
|
ZHU X F, JIANG W J, SU S J, et al. The study of reaction mechanism of desulfurization in flue gas with pyrolusite pulp[J]. Technigues Equipment for Enviropollcont, 2002(3): 44-46.
|
| 19 |
黄妍, 王治军, 童志权. 软锰矿浆脱除烟气中SO2的研究[J]. 环境工程, 1998, 16(4): 43-46.
|
|
HUANG Yan, WANG Zhijun, TONG Zhiquan. Research on removing SO2 from fume gas by pyrolusite sludge[J]. Environmental Engineering, 1998, 16(4): 43-46.
|
| 20 |
PASIUK-BRONIKOWSKA W, BRONIKOWSKI T. The rate equation for SO2 autoxidation in aqueous MnSO4 solutions containing H2SO4[J]. Chemical Engineering Science, 1981, 36(1): 215-219.
|
| 21 |
SUN W Y, DING S L, ZENG S S, et al. Simultaneous absorption of NOx and SO2 from flue gas with pyrolusite slurry combined with gas-phase oxidation of NO using ozone[J]. Journal of Hazardous Materials, 2011, 192(1): 124-130.
|
| 22 |
В.М.КАКАБАДЗЕ, 杨正莘. 锰泥和锰矿的二氧化硫炼制法[J]. 化学世界, 1955, 10(2): 23-29.
|
|
В.М.Какабадзе, YANG Zhengxin. Sulfur dioxide refining of manganese mud and manganese ore[J]. Chemical World, 1955, 10(2): 23-29.
|
| 23 |
WALANDA D K, LAWRANCE G A, DONNE S W. Kinetics of Mn2O3 digestion in H2SO4 solutions[J]. Journal of Solid State Chemistry, 2009, 182(6): 1336-1342.
|
| 24 |
吴复忠, 蔡九菊, 张琦, 等. 软锰矿、菱锰矿吸收烧结烟气中的SO2制取硫酸锰[J]. 钢铁, 2007, 42(4): 78-82.
|
|
WU Fuzhong, CAI Jiuju, ZHANG Qi, et al. Study on production of manganese sulfate by absorption of SO2 from sintering fume with pyrolusite and mild manganese[J]. Iron & Steel, 2007, 42(4): 78-82.
|
 ), 辛广智1, 杨林1,2, 蒲鹏燕1, 江霞1,2, 蒋文举1,2(
), 辛广智1, 杨林1,2, 蒲鹏燕1, 江霞1,2, 蒋文举1,2( )
)
 ), XIN Guangzhi1, YANG Lin1,2, PU Pengyan1, JIANG Xia1,2, JIANG Wenju1,2(
), XIN Guangzhi1, YANG Lin1,2, PU Pengyan1, JIANG Xia1,2, JIANG Wenju1,2( )
)