化工进展 ›› 2021, Vol. 40 ›› Issue (2): 1035-1047.DOI: 10.16085/j.issn.1000-6613.2020-0744
金属有机骨架混合基质水处理分离膜研究进展
- 南阳师范学院土木建筑工程学院,河南 南阳 473061
-
收稿日期:2020-05-06修回日期:2020-08-18出版日期:2021-02-05发布日期:2021-02-09 -
作者简介:赵东升(1984—),男,博士,讲师,研究方向为膜分离技术。E-mail:zds123123@yeah.net 。 -
基金资助:河南省科技攻关计划(202102310260);河南省高等学校重点科研项目(20B610005)
Recent advances in metal organic frameworks mixed matrix membranes for water treatment
- College of Civil Engineering and Architecture, Nanyang Normal University, Nanyang 473061, Henan, China
-
Received:2020-05-06Revised:2020-08-18Online:2021-02-05Published:2021-02-09
摘要:
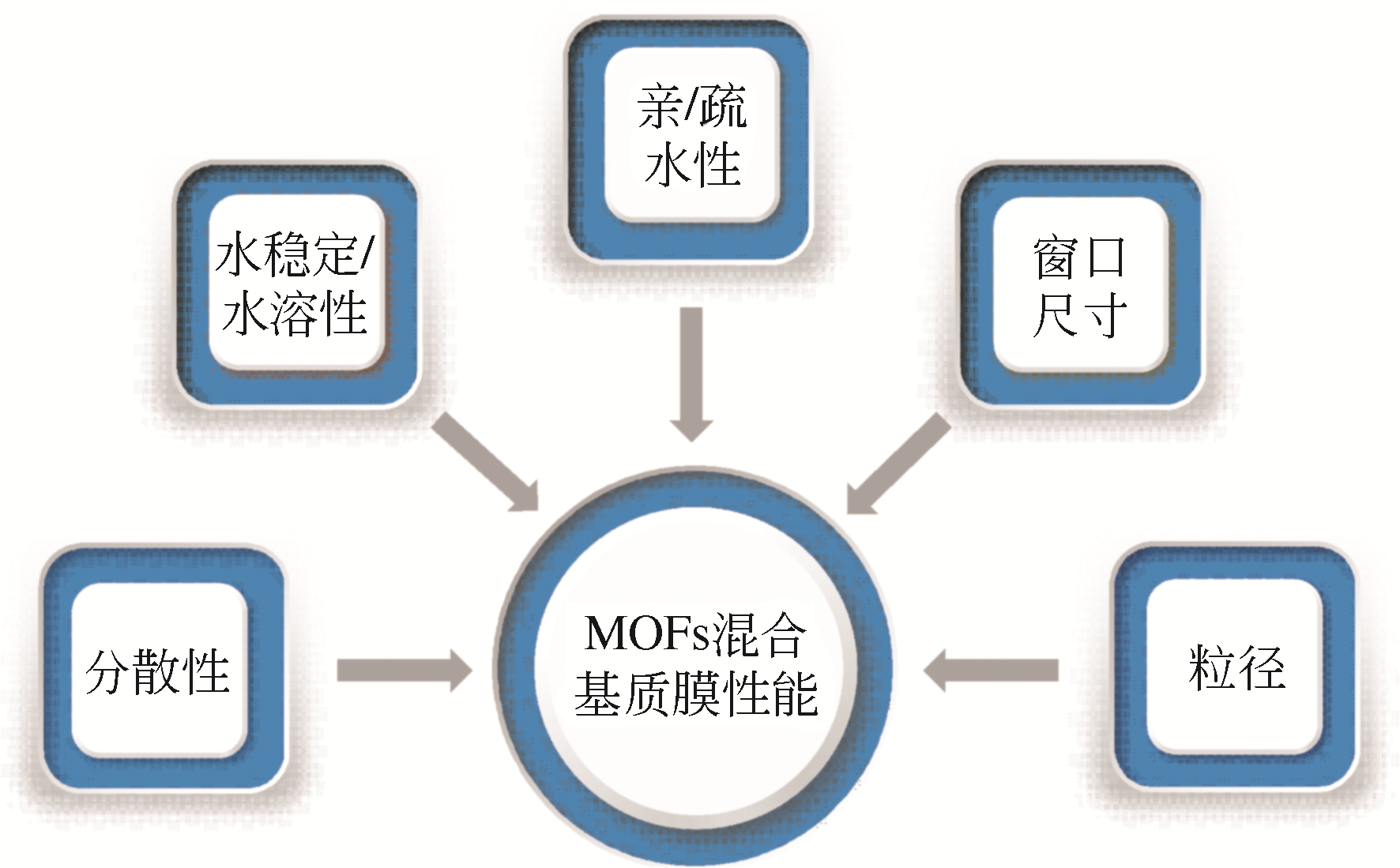
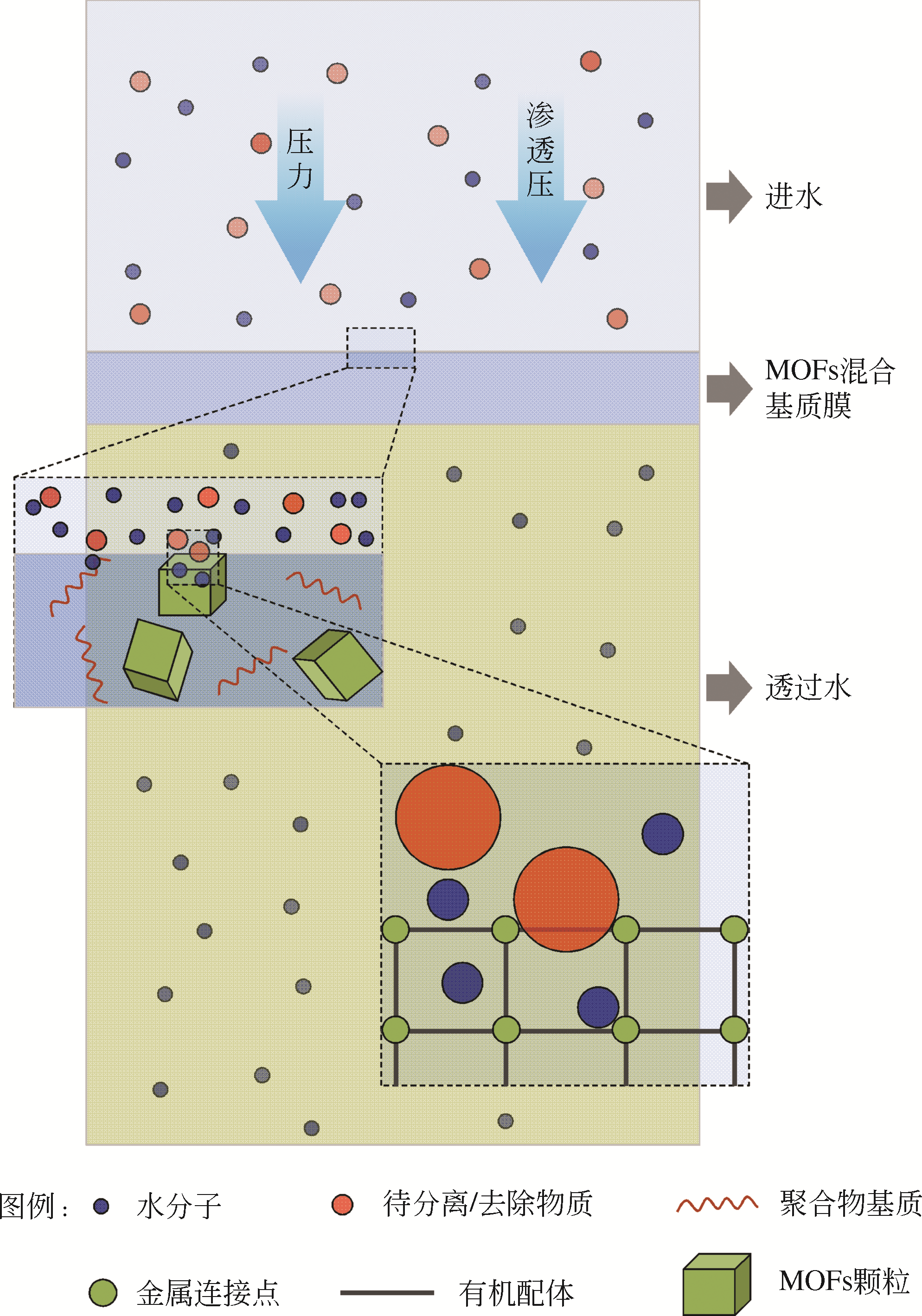
金属有机骨架(MOFs)晶体由无机金属离子和有机配体通过自组装合成,具有高的孔隙率和可调节的窗口尺寸,可使MOFs混合基质膜在水处理时同步获得高通量和高截留率,有望突破传统分离膜的渗透性和选择性之间此消彼长的trade-off效应。本文综述了MOFs的典型构造、影响MOFs混合基质膜性能的关键因素、MOFs混合基质膜的制备方法、MOFs颗粒改善混合基质膜水传输和溶质分离性能的原理以及MOFs混合基质膜在水处理微滤/超滤、纳滤/反渗透和正渗透领域的最新研究进展。最后总结了MOFs混合基质膜在水处理领域的未来发展亟待解决的关键问题,主要包括高性能、低成本膜的可控制备、膜结构和性能之间定量构效关系的深入探索以及如何拓宽其应用范围等,对加快MOFs混合基质膜的产业化进程具有指导意义。
中图分类号:
引用本文
赵东升. 金属有机骨架混合基质水处理分离膜研究进展[J]. 化工进展, 2021, 40(2): 1035-1047.
Dongsheng ZHAO. Recent advances in metal organic frameworks mixed matrix membranes for water treatment[J]. Chemical Industry and Engineering Progress, 2021, 40(2): 1035-1047.
| 膜的分类 | MOFs混合基质膜 | 进料液 | 渗透性 /L·m-2·h-1·bar-1 | 分离的溶质, 分离性能 | 改善水传输和溶质分离性能的原理 | 参考文献 |
|---|---|---|---|---|---|---|
| 微滤 | ZIF-8/聚四氟乙烯膜 | 纯水 | 54800 | 黄体酮,约95% | 水分子与疏水性ZIF-8内壁的摩擦低,膜孔隙率增大,ZIF-8表面N-H与水分子通过氢键结合形成水的快速传输通道;黄体酮与ZIF-8的特异性相互作用和氢键形成 | [ |
| 超滤 | 聚磺基甲基丙烯酸酯功能化UiO-66/聚砜膜 | 纯水 | 301 | 牛血清白蛋白,>98% | 膜面亲水性改善,膜孔隙率增大,功能化UiO-66的多孔结构及其与聚砜基质之间的微间隙为水分子的通过提供额外的传输通道 | [ |
| 超滤 | 亲水性中空ZIF-8/ 聚砜膜 | 纯水 | 298.5 | 牛血清白蛋白,>98% | 膜面亲水性改善,膜结构均匀,亲水中空ZIF-8的多孔结构为水分子提供额外的优先传输通道 | [ |
| 超滤 | 聚丙烯酸/ZIF-8/ 聚偏氟乙烯膜 | 纯水 | 460 | [Ni2+]0=2mg/L, <0.1mg/L | Ni2+与ZIF-8的羟基及聚丙烯酸层的羧基之间的静电吸引和氢键作用 | [ |
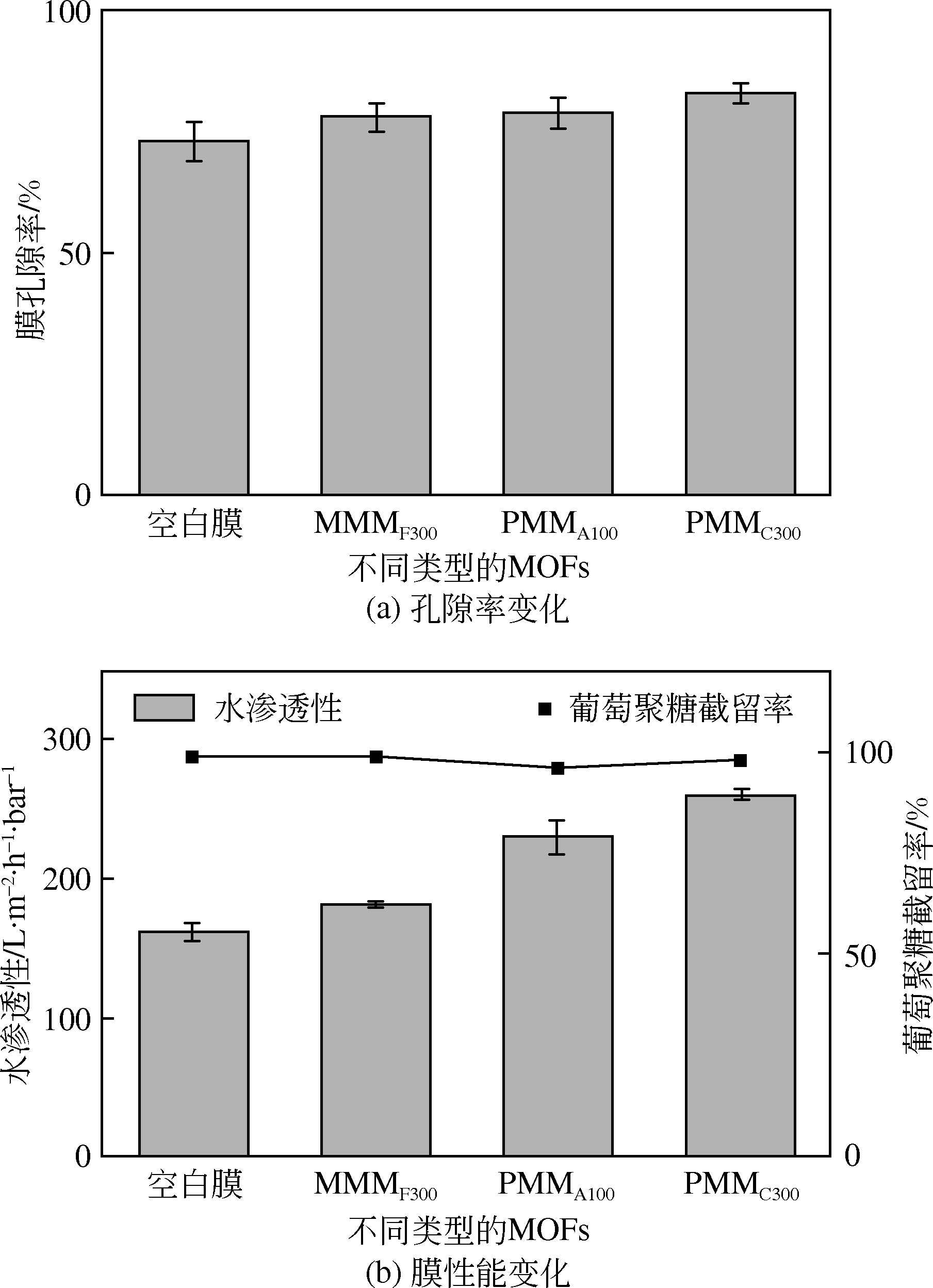
| 超滤 | F300、A100和C300/ 聚丙烯腈膜 | 纯水 | 182、230和261 | 葡萄聚糖,>98% | 水溶性去除部分MOFs增强膜孔隙的连通性,提高膜的孔隙率 | [ |
| 超滤 | HKUST-1/聚醚砜膜 | 纯水 | 490 | 牛血清白蛋白,约96% | 水溶性去除HKUST-1,提高膜的孔隙率,为水分子创造额外的传输通道,降低传输阻力;膜的尺寸筛分效应及与牛血清白蛋白之间强静电排斥作用,使其保持较高的截留性能 | [ |
表1 MOFs混合基质微滤/超滤膜在水处理中的应用
| 膜的分类 | MOFs混合基质膜 | 进料液 | 渗透性 /L·m-2·h-1·bar-1 | 分离的溶质, 分离性能 | 改善水传输和溶质分离性能的原理 | 参考文献 |
|---|---|---|---|---|---|---|
| 微滤 | ZIF-8/聚四氟乙烯膜 | 纯水 | 54800 | 黄体酮,约95% | 水分子与疏水性ZIF-8内壁的摩擦低,膜孔隙率增大,ZIF-8表面N-H与水分子通过氢键结合形成水的快速传输通道;黄体酮与ZIF-8的特异性相互作用和氢键形成 | [ |
| 超滤 | 聚磺基甲基丙烯酸酯功能化UiO-66/聚砜膜 | 纯水 | 301 | 牛血清白蛋白,>98% | 膜面亲水性改善,膜孔隙率增大,功能化UiO-66的多孔结构及其与聚砜基质之间的微间隙为水分子的通过提供额外的传输通道 | [ |
| 超滤 | 亲水性中空ZIF-8/ 聚砜膜 | 纯水 | 298.5 | 牛血清白蛋白,>98% | 膜面亲水性改善,膜结构均匀,亲水中空ZIF-8的多孔结构为水分子提供额外的优先传输通道 | [ |
| 超滤 | 聚丙烯酸/ZIF-8/ 聚偏氟乙烯膜 | 纯水 | 460 | [Ni2+]0=2mg/L, <0.1mg/L | Ni2+与ZIF-8的羟基及聚丙烯酸层的羧基之间的静电吸引和氢键作用 | [ |
| 超滤 | F300、A100和C300/ 聚丙烯腈膜 | 纯水 | 182、230和261 | 葡萄聚糖,>98% | 水溶性去除部分MOFs增强膜孔隙的连通性,提高膜的孔隙率 | [ |
| 超滤 | HKUST-1/聚醚砜膜 | 纯水 | 490 | 牛血清白蛋白,约96% | 水溶性去除HKUST-1,提高膜的孔隙率,为水分子创造额外的传输通道,降低传输阻力;膜的尺寸筛分效应及与牛血清白蛋白之间强静电排斥作用,使其保持较高的截留性能 | [ |
膜的 分类 | MOFs混合基质膜 | 进料液 | 渗透性 /L·m-2·h-1·bar-1 | 分离的溶质,分离性能 | 改善水传输和溶质分离性能的原理 | 参考 文献 |
|---|---|---|---|---|---|---|
| 纳滤 | UiO-66/聚酰胺膜 | 纯水 | 11.5 | SeO | 膜面亲水性改善,引入多孔性的水分子通道,分离层厚度减小;膜的平均孔径减小,孔径分布变窄,表面负电荷改善增强 | [ |
| 纳滤 | 聚苯乙烯磺酸钠 改性ZIF/聚酰胺膜 | 纯水 | 14.9 | 活性黑5和活性蓝2, >99% | 膜面亲水性提高,聚酰胺层交联度下降,纳米颗粒自身的孔隙及其与聚酰胺之间的间隙为水分子快速传输提供通道;空间位阻和对荷负电染料分子的静电排斥作用增强 | [ |
| 纳滤 | 氨基化UiO-66/ 聚酰胺膜 | 1500mg·L-1Na2SO4 | 30.8 | Na2SO4,97.5% | 改善膜的亲水性,形成渔网状褶皱结构增大有效过滤面积,增加水分子的传输通道 | [ |
| 纳滤 | 聚苯乙烯磺酸钠/HKUST-1膜 | — | — | Li+、Na+、K+和Mg2+,Li+/Na+=35、Li+/K+=67和Li+/Mg2+=1815 | 水合离子直径的不同及其与磺酸基团亲合性的差异 | [ |
| 纳滤 | MIL-101(Cr) /聚酰胺膜 | 纯水 | 39.5 | 内分泌干扰物:羟苯甲酯、羟苯丙酯、羟苯甲酯和双酚A,47.4%、45.9%、51.1%和79.8% | MIL-101(Cr)的孔窗作为亲水选择性纳米通道增强水分子的传输速率;通过抑制疏水相互作用增强对疏水内分泌干扰物的去除 | [ |
| 纳滤 | MIL-53(Al)、NH2-UiO-66、 ZIF-8/聚酰胺膜 | 10mmol·L-1NaCl和50μg·L-1药物 | 7.2 | 药物:萘啶酸、磺胺甲 唑、舒必利,>80%、 >80%、>90% 唑、舒必利,>80%、 >80%、>90% | MOFs独特的通道促进水分子的传输;MOFs大小孔窗的选择性屏障作用,维持膜的选择性;降低聚酰胺层的交联度,膜面羧基含量增大,增强对荷负电溶质的静电排斥作用 | [ |
| 纳滤 | PD/ZIF-8/聚酰胺膜 | 1000mg·L-1Na2SO4 | 53.5 | Na2SO4,>95% | 通过控制模板MOFs的负载量和粒径,对聚酰胺层的表面形貌和表面积进行微调,形成具有褶皱形貌的聚酰胺层,增大高渗透性有效过滤面积 | [ |
| 反渗透 | ZIF-8/聚酰胺膜 | 2000mg·L-1NaCl | 3.35 | NaCl,98.5% | 降低聚酰胺层的交联度,水在疏水性ZIF-8纳米受限通道内的摩擦阻力低,有利于其快速传输;ZIF-8的咪唑酯连接增强与聚酰胺基质的相容性,保证高的截留率 | [ |
| 反渗透 | MIL-101(Cr)/ 聚酰胺膜 | 2000mg·L-1NaCl | 2.2 | NaCl,>99% | MIL-101(Cr)的多孔结构在致密的聚酰胺层中建立直接的水通道;MIL-101(Cr)与聚酰胺层的相容性好,两者紧密结合,从而表现出高的截留率 | [ |
表2 MOFs混合基质纳滤/反渗透膜在水处理中的应用
膜的 分类 | MOFs混合基质膜 | 进料液 | 渗透性 /L·m-2·h-1·bar-1 | 分离的溶质,分离性能 | 改善水传输和溶质分离性能的原理 | 参考 文献 |
|---|---|---|---|---|---|---|
| 纳滤 | UiO-66/聚酰胺膜 | 纯水 | 11.5 | SeO | 膜面亲水性改善,引入多孔性的水分子通道,分离层厚度减小;膜的平均孔径减小,孔径分布变窄,表面负电荷改善增强 | [ |
| 纳滤 | 聚苯乙烯磺酸钠 改性ZIF/聚酰胺膜 | 纯水 | 14.9 | 活性黑5和活性蓝2, >99% | 膜面亲水性提高,聚酰胺层交联度下降,纳米颗粒自身的孔隙及其与聚酰胺之间的间隙为水分子快速传输提供通道;空间位阻和对荷负电染料分子的静电排斥作用增强 | [ |
| 纳滤 | 氨基化UiO-66/ 聚酰胺膜 | 1500mg·L-1Na2SO4 | 30.8 | Na2SO4,97.5% | 改善膜的亲水性,形成渔网状褶皱结构增大有效过滤面积,增加水分子的传输通道 | [ |
| 纳滤 | 聚苯乙烯磺酸钠/HKUST-1膜 | — | — | Li+、Na+、K+和Mg2+,Li+/Na+=35、Li+/K+=67和Li+/Mg2+=1815 | 水合离子直径的不同及其与磺酸基团亲合性的差异 | [ |
| 纳滤 | MIL-101(Cr) /聚酰胺膜 | 纯水 | 39.5 | 内分泌干扰物:羟苯甲酯、羟苯丙酯、羟苯甲酯和双酚A,47.4%、45.9%、51.1%和79.8% | MIL-101(Cr)的孔窗作为亲水选择性纳米通道增强水分子的传输速率;通过抑制疏水相互作用增强对疏水内分泌干扰物的去除 | [ |
| 纳滤 | MIL-53(Al)、NH2-UiO-66、 ZIF-8/聚酰胺膜 | 10mmol·L-1NaCl和50μg·L-1药物 | 7.2 | 药物:萘啶酸、磺胺甲 唑、舒必利,>80%、 >80%、>90% 唑、舒必利,>80%、 >80%、>90% | MOFs独特的通道促进水分子的传输;MOFs大小孔窗的选择性屏障作用,维持膜的选择性;降低聚酰胺层的交联度,膜面羧基含量增大,增强对荷负电溶质的静电排斥作用 | [ |
| 纳滤 | PD/ZIF-8/聚酰胺膜 | 1000mg·L-1Na2SO4 | 53.5 | Na2SO4,>95% | 通过控制模板MOFs的负载量和粒径,对聚酰胺层的表面形貌和表面积进行微调,形成具有褶皱形貌的聚酰胺层,增大高渗透性有效过滤面积 | [ |
| 反渗透 | ZIF-8/聚酰胺膜 | 2000mg·L-1NaCl | 3.35 | NaCl,98.5% | 降低聚酰胺层的交联度,水在疏水性ZIF-8纳米受限通道内的摩擦阻力低,有利于其快速传输;ZIF-8的咪唑酯连接增强与聚酰胺基质的相容性,保证高的截留率 | [ |
| 反渗透 | MIL-101(Cr)/ 聚酰胺膜 | 2000mg·L-1NaCl | 2.2 | NaCl,>99% | MIL-101(Cr)的多孔结构在致密的聚酰胺层中建立直接的水通道;MIL-101(Cr)与聚酰胺层的相容性好,两者紧密结合,从而表现出高的截留率 | [ |
| 膜的类型 | 渗透性/L·m-2·h-1·bar-1 | Na2SO4截留率/% | 参考文献 |
|---|---|---|---|
| PD/ZIF-8模板纳滤膜(PIP/PD/ZIF-8-TMC) | 53.5 | >95.0 | [ |
| TFN(PIP/MoS2-TMC) | 7.8 | 94.4 | [ |
| NFM-15(PIP-TMC) | 23.7 | 99.4 | [ |
| NFM-4(PIP-TMC/TAC) | 13.2 | 97.6 | [ |
| CS(PIP-TMC) | 10.1 | 89.1 | [ |
| M-50(PIP-TMC) | 26.2 | 97.7 | [ |
| CNC-TFC-M2(PIP-CNCs/TMC) | 16.2 | 98.8 | [ |
| Dow NF-270 | 11.6 | 94.0 | [ |
表3 PD/ZIF-8模板纳滤膜与最新报道纳滤膜的总体性能比较
| 膜的类型 | 渗透性/L·m-2·h-1·bar-1 | Na2SO4截留率/% | 参考文献 |
|---|---|---|---|
| PD/ZIF-8模板纳滤膜(PIP/PD/ZIF-8-TMC) | 53.5 | >95.0 | [ |
| TFN(PIP/MoS2-TMC) | 7.8 | 94.4 | [ |
| NFM-15(PIP-TMC) | 23.7 | 99.4 | [ |
| NFM-4(PIP-TMC/TAC) | 13.2 | 97.6 | [ |
| CS(PIP-TMC) | 10.1 | 89.1 | [ |
| M-50(PIP-TMC) | 26.2 | 97.7 | [ |
| CNC-TFC-M2(PIP-CNCs/TMC) | 16.2 | 98.8 | [ |
| Dow NF-270 | 11.6 | 94.0 | [ |
| MOFs混合基质膜 | 进料液 | 汲取液 | 运行模式 | 渗透通量 /L·m-2·h-1 | 比盐通量 /g·L-1 | 改善膜渗透选择性的原理 | 参考文献 |
|---|---|---|---|---|---|---|---|
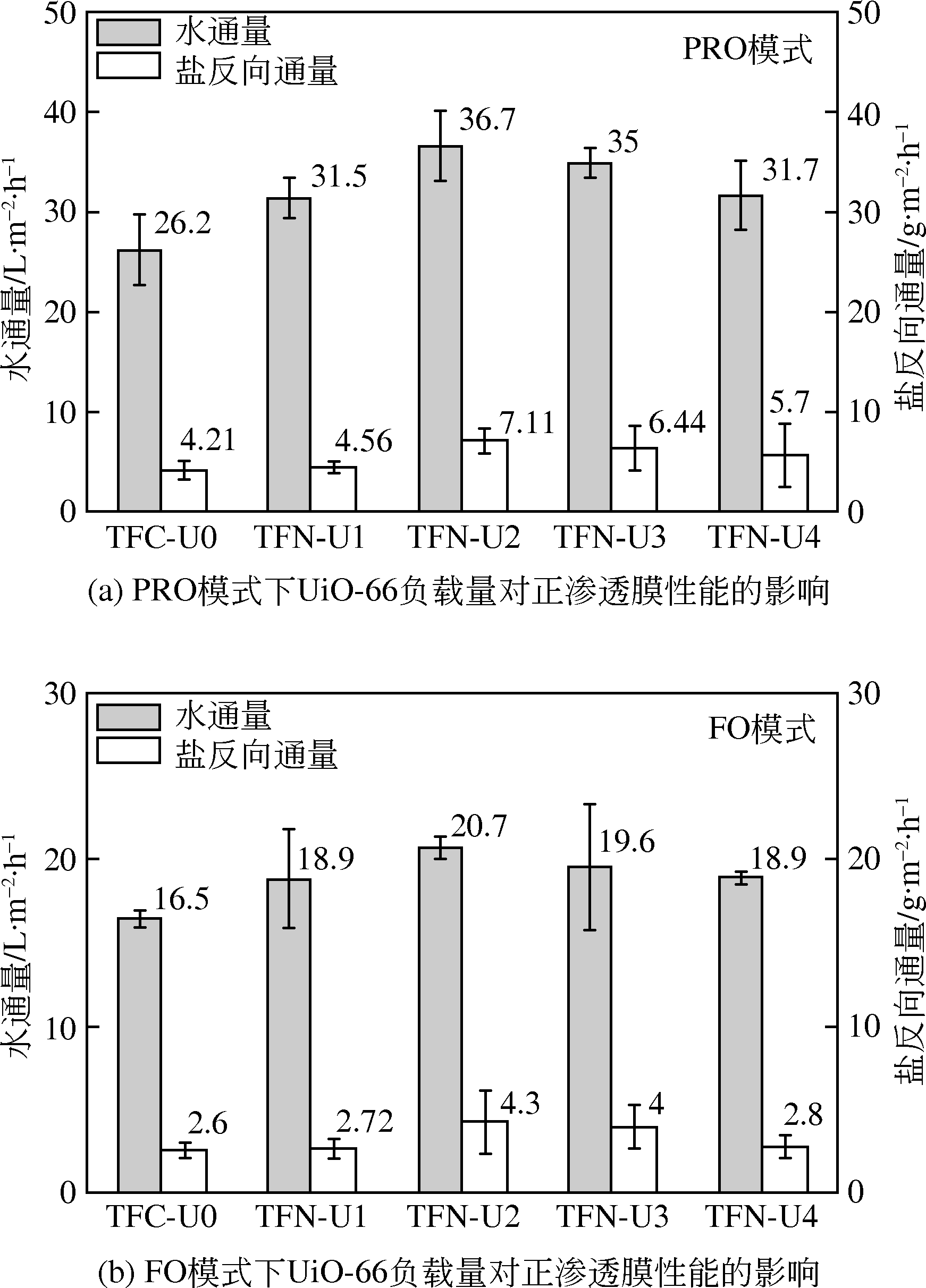
| UiO-66/聚酰胺膜 | 纯水 | 1mol·L-1 NaCl | 活性层-汲取液 | 36.7 | 0.20 | 膜面亲水性提高,UiO-66的小孔径限制高浓度汲取液的溶质反向通量,缓解内部浓差极化,提高净渗透驱动力 | [ |
| 3HBTC/聚酰胺膜 | 纯水 | 2mol·L-1 NaCl | 活性层-汲取液 | 82 | 0.13 | 3HBTC的嵌入使聚合物链填充中断,降低聚酰胺层的交联度,增强膜面亲水性,吸引水分子并降低其传输阻力 | [ |
| UiO-66/磺化聚砜/聚砜膜 | 纯水 | 1.25mol·L-1 Na2SO4 | — | 88.3 | 0.12 | 亚纳米孔径的增强微孔,使UiO-66基吸附和离子筛分特性直接转化,消除渗透压驱动下的内部浓度极化 | [ |
| 以F300、A100、C300/聚丙烯腈混合基质膜为基底的聚酰胺膜 | 纯水 | 0.5mol·L-1 MgCl2 | 活性层-汲取液 | 93、 100、 107 | 0.08、 0.13、 0.17 | 水溶性去除部分F300、A100和C300,提高基底的孔隙率和亲水性,降低孔曲折程度,正渗透膜的内部浓差极化得到有效改善,膜通量增加;基底孔隙率的增大导致溶质截留率略有下降 | [ |
表4 MOFs混合基质正渗透膜在水处理中的应用
| MOFs混合基质膜 | 进料液 | 汲取液 | 运行模式 | 渗透通量 /L·m-2·h-1 | 比盐通量 /g·L-1 | 改善膜渗透选择性的原理 | 参考文献 |
|---|---|---|---|---|---|---|---|
| UiO-66/聚酰胺膜 | 纯水 | 1mol·L-1 NaCl | 活性层-汲取液 | 36.7 | 0.20 | 膜面亲水性提高,UiO-66的小孔径限制高浓度汲取液的溶质反向通量,缓解内部浓差极化,提高净渗透驱动力 | [ |
| 3HBTC/聚酰胺膜 | 纯水 | 2mol·L-1 NaCl | 活性层-汲取液 | 82 | 0.13 | 3HBTC的嵌入使聚合物链填充中断,降低聚酰胺层的交联度,增强膜面亲水性,吸引水分子并降低其传输阻力 | [ |
| UiO-66/磺化聚砜/聚砜膜 | 纯水 | 1.25mol·L-1 Na2SO4 | — | 88.3 | 0.12 | 亚纳米孔径的增强微孔,使UiO-66基吸附和离子筛分特性直接转化,消除渗透压驱动下的内部浓度极化 | [ |
| 以F300、A100、C300/聚丙烯腈混合基质膜为基底的聚酰胺膜 | 纯水 | 0.5mol·L-1 MgCl2 | 活性层-汲取液 | 93、 100、 107 | 0.08、 0.13、 0.17 | 水溶性去除部分F300、A100和C300,提高基底的孔隙率和亲水性,降低孔曲折程度,正渗透膜的内部浓差极化得到有效改善,膜通量增加;基底孔隙率的增大导致溶质截留率略有下降 | [ |
| 1 | SHANNON M A, BOHN P W, ELIMELECH M, et al. Science and technology for water purification in the coming decades[J]. Nature, 2008, 452(7185): 301-310. |
| 2 | LAU W J, GRAY S, MATSUURA T, et al. A review on polyamide thin film nanocomposite (TFN) membranes: history, applications, challenges and approaches[J]. Water Research, 2015, 80: 306-324. |
| 3 | YANG H-C, HOU J, CHEN V, et al. Surface and interface engineering for organic-inorganic composite membranes[J]. Journal of Materials Chemistry A, 2016, 4(25): 9716-9729. |
| 4 | LONG J R, YAGHI O M. The pervasive chemistry of metal-organic frameworks[J]. Chemical Society Reviews, 2009, 38(5): 1213-1214. |
| 5 | 姜蕾, 张大鹏, 朱桂茹, 等. Zr-MOF改性聚酰胺正渗透复合膜的制备与表征[J]. 功能材料, 2017, 3(48): 3102-3107. |
| JIANG L, ZHANG D P, ZHU G R, et al. Fabrication and characterization of Zr-MOF incorporated thin-film nanocomposite membrane for forward osmosis[J]. Functional Materials, 2017, 3(48): 3102-3107. | |
| 6 | FARHA O K, YAZAYDIN A O, ERYAZICI I, et al. De novo synthesis of a metal-organic framework material featuring ultrahigh surface area and gas storage capacities[J]. Nature Chemistry, 2010, 2(11): 944-948. |
| 7 | SENKOVSKA I, KASKEL S. Ultrahigh porosity in mesoporous MOFs: promises and limitations[J]. Chemical Communications, 2014, 50(54): 7089-7098. |
| 8 | LIU J, CHEN L, CUI H, et al. Applications of metal-organic frameworks in heterogeneous supramolecular catalysis[J]. Chemical Society Reviews, 2014, 43(16): 6011-6061. |
| 9 | CHENG M, LAI C, LIU Y, et al. Metal-organic frameworks for highly efficient heterogeneous Fenton-like catalysis[J]. Coordination Chemistry Reviews, 2018, 368: 80-92. |
| 10 | FURUKAWA H, CORDOVA K E, O'KEEFFE M, et al. The chemistry and applications of metal-organic frameworks[J]. Science, 2013, 341(6149): 974. |
| 11 | RUI K, WANG X, DU M, et al. Dual-function metal-organic framework-based wearable fibers for gas probing and energy storage[J]. ACS Applied Materials & Interfaces, 2018, 10(3): 2837-2842. |
| 12 | GAO C-Y, TIAN H-R, AI J, et al. A microporous Cu-MOF with optimized open metal sites and pore spaces for high gas storage and active chemical fixation of CO2[J]. Chemical Communications, 2018, 54(51): 7093-7094. |
| 13 | DESANTIS D, MASON J A, JAMES B D, et al. Techno-economic analysis of metal-organic frameworks for hydrogen and natural gas storage[J]. Energy & Fuels, 2017, 31(2): 2024-2032. |
| 14 | HORCAJADA P, GREF R, BAATI T, et al. Metal-organic frameworks in biomedicine[J]. Chemical Reviews, 2012, 112(2): 1232-1268. |
| 15 | LEI B, WANG M, JIANG Z, et al. Constructing redox-responsive metal-organic framework nanocarriers for anticancer drug delivery[J]. ACS Applied Materials & Interfaces, 2018, 10(19): 16698-16706. |
| 16 | LAZARO I A, LAZARO S A, FORGAN R S. Enhancing anticancer cytotoxicity through bimodal drug delivery from ultrasmall Zr MOF nanoparticles[J]. Chemical Communications, 2018, 54(22): 2792-2795. |
| 17 | ROTH STEFANIAK K, EPLEY C C, NOVAK J J, et al. Photo-triggered release of 5-fluorouracil from a MOF drug delivery vehicle[J]. Chemical Communications, 2018, 54(55): 7617-7620. |
| 18 | LI X, LIU Y, WANG J, et al. Metal-organic frameworks based membranes for liquid separation[J]. Chemical Society Reviews, 2017, 46(23): 7124-7144. |
| 19 | WILMER C E, LEAF M, LEE C Y, et al. Large-scale screening of hypothetical metal-organic frameworks[J]. Nature Chemistry, 2012, 4(2): 83-89. |
| 20 | KONDO A, MAEDA K. Anisotropic thermal expansion of a 3D metal-organic framework with hydrophilic and hydrophobic pores[J]. Journal of Solid State Chemistry, 2015, 221: 126-131. |
| 21 | KNEBEL A, SUNDERMANN L, MOHMEYER A, et al. Azobenzene guest molecules as light-switchable CO2 valves in an ultrathin UiO-67 membrane[J]. Chemistry of Materials, 2017, 29(7): 3111-3117. |
| 22 | HU J, SUN T, LIU X, et al. Rationally tuning the separation performances of [M3(HCOO)6] frameworks for CH4/N2 mixtures via metal substitution[J]. Microporous and Mesoporous Materials, 2016, 225: 456-464. |
| 23 | HE M, WANG L, LYU Y, et al. Novel polydopamine/metal organic framework thin film nanocomposite forward osmosis membrane for salt rejection and heavy metal removal[J]. Chemical Engineering Journal, 2020, 389: 124452 |
| 24 | HE Y, TANG Y P, MA D, et al. UiO-66 incorporated thin-film nanocomposite membranes for efficient selenium and arsenic removal[J]. Journal of Membrane Science, 2017, 541: 262-270. |
| 25 | LIU X-L, LI Y-S, ZHU G-Q, et al. An organophilic pervaporation membrane derived from metal-organic framework nanoparticles for efficient recovery of bio-alcohols[J]. Angewandte Chemie-International Edition, 2011, 50(45): 10636-10639. |
| 26 | REN Y, LI T, ZHANG W, et al. MIL-PVDF blend ultrafiltration membranes with ultrahigh MOF loading for simultaneous adsorption and catalytic oxidation of methylene blue[J]. Journal of Hazardous Materials, 2019, 365: 312-321. |
| 27 | ZHANG Y, WANG N, ZHAO C, et al. Co(HCOO)2-based hybrid membranes for the pervaporation separation of aromatic/aliphatic hydrocarbon mixtures[J]. Journal of Membrane Science, 2016, 520: 646-656. |
| 28 | YANG L, WANG Z, ZHANG J. Zeolite imidazolate framework hybrid nanofiltration (NF) membranes with enhanced permselectivity for dye removal[J]. Journal of Membrane Science, 2017, 532: 76-86. |
| 29 | MA X-H, YANG Z, YAO Z-K, et al. A facile preparation of novel positively charged MOF/chitosan nanofiltration membranes[J]. Journal of Membrane Science, 2017, 525: 269-276. |
| 30 | SUN H, TANG B, WU P. Development of hybrid ultrafiltration membranes with improved water separation properties using modified superhydrophilic metal-organic framework nanoparticles[J]. ACS Applied Materials & Interfaces, 2017, 9(25): 21473-21484. |
| 31 | WANG C, LIU X, DEMIR N K, et al. Applications of water stable metal-organic frameworks[J]. Chemical Society Reviews, 2016, 45(18): 5107-5134. |
| 32 | COLOMBO V, GALLI S, CHOI H J, et al. High thermal and chemical stability in pyrazolate-bridged metal-organic frameworks with exposed metal sites[J]. Chemical Science, 2011, 2(7): 1311-1319. |
| 33 | YUAN S, FENG L, WANG K, et al. Stable metal-organic frameworks: design, synthesis, and applications[J]. Advanced Materials, 2018, 30(37): 1704303. |
| 34 | YANG C, KAIPA U, MATHER Q Z, et al. Fluorous metal-organic frameworks with superior adsorption and hydrophobic properties toward oil spill cleanup and hydrocarbon storage[J]. Journal of the American Chemical Society, 2011, 133(45): 18094-18097. |
| 35 | TAYLOR J M, VAIDHYANATHAN R, IREMONGER S S, et al. Enhancing water stability of metal-organic frameworks via phosphonate monoester linkers[J]. Journal of the American Chemical Society, 2012, 134(35): 14338-14340. |
| 36 | DENNY M S, COHEN S M JR. In situ modification of metal-organic frameworks in mixed-matrix membranes[J]. Angewandte Chemie-International Edition, 2015, 54(31): 9029-9032. |
| 37 | J-Y LEE, TANG C Y, HUO F. Fabrication of porous matrix membrane (PMM) using metal-organic framework as green template for water treatment[J]. Scientific Reports, 2014, 4: 3740. |
| 38 | LIN Y, WU H C, YASUI T, et al. Development of an HKUST-1 nanofiller-templated poly(ether sulfone) mixed matrix membrane for a highly efficient ultrafiltration process[J]. ACS Applied Materials & Interfaces, 2019, 11(20): 18782-18796. |
| 39 | WANG Z, WANG Z, LIN S, et al. Nanoparticle-templated nanofiltration membranes for ultrahigh performance desalination[J]. Nature Communication, 2018, 9(1): 2004. |
| 40 | J-Y LEE, SHE Q, HUO F, et al. Metal-organic framework-based porous matrix membranes for improving mass transfer in forward osmosis membranes[J]. Journal of Membrane Science, 2015, 492: 392-399. |
| 41 | RUAN H, GUO C, YU H, et al. Fabrication of a MIL-53(Al) nanocomposite membrane and potential application in desalination of dye solutions[J]. Industrial & Engineering Chemistry Research, 2016, 55(46): 12099-12110. |
| 42 | ZHU J, QIN L, ULIANA A, et al. Elevated performance of thin film nanocomposite membranes enabled by modified hydrophilic MOFs for nanofiltration[J]. ACS Applied Materials & Interfaces, 2017, 9(2): 1975-1986. |
| 43 | LIU T Y, YUAN H G, LIU Y Y, et al. Metal-organic framework nanocomposite thin films with interfacial bindings and self-standing robustness for high water flux and enhanced ion selectivity[J]. ACS Nano, 2018, 12(9): 9253-9265. |
| 44 | MENG Y, SHU L, LIU L, et al. A high-flux mixed matrix nanofiltration membrane with highly water-dispersible MOF crystallites as filler[J]. Journal of Membrane Science, 2019, 591: 117360. |
| 45 | BAO Y, CHEN Y, LIM T T, et al. A novel metal-organic framework (MOF)-mediated interfacial polymerization for direct deposition of polyamide layer on ceramic substrates for nanofiltration[J]. Advanced Materials Interfaces, 2019, 6(9): 1900132. |
| 46 | ZHANG R, JI S, WANG N, et al. Coordination-driven in situ self-assembly strategy for the preparation of metal-organic framework hybrid membranes[J]. Angewandte Chemie-International Edition, 2014, 53(37): 9775-9779. |
| 47 | SORRIBAS S, GORGOJO P, TELLEZ C, et al. High flux thin film nanocomposite membranes based on metal-organic frameworks for organic solvent nanofiltration[J]. Journal of the American Chemical Society, 2013, 135(40): 15201-15208. |
| 48 | ZHU J, HOU J, YUAN S, et al. MOF-positioned polyamide membranes with a fishnet-like structure for elevated nanofiltration performance[J]. Journal of Materials Chemistry A, 2019, 7(27): 16313-16322. |
| 49 | DENNY M S, MORETON J C JR, BENZ L, et al. Metal-organic frameworks for membrane-based separations[J]. Nature Reviews Materials, 2016, 1(12): 16078. |
| 50 | MA D, PEH S B, HAN G, et al. Thin-film nanocomposite (TFN) membranes incorporated with super-hydrophilic metal-organic framework (MOF) UiO-66: Toward enhancement of water flux and salt rejection[J]. ACS Applied Materials & Interfaces, 2017, 9(8): 7523-7534. |
| 51 | SUN H, TANG B, WU P. Hydrophilic hollow zeolitic imidazolate framework-8 modified ultrafiltration membranes with significantly enhanced water separation properties[J]. Journal of Membrane Science, 2018, 551: 283-293. |
| 52 | DAI R, GUO H, TANG C Y, et al. Hydrophilic selective nanochannels created by metal organic frameworks in nanofiltration membranes enhance rejection of hydrophobic endocrine-disrupting compounds[J]. Environmental Science & Technology, 2019, 53(23): 13776-13783. |
| 53 | ZHAO Y Y, LIU Y L, WANG X M, et al. Impacts of metal-organic frameworks on structure and performance of polyamide thin-film nanocomposite membranes[J]. ACS Applied Materials & Interfaces, 2019, 11(14): 13724-13734. |
| 54 | RAGAB D, GOMAA H G, SABOUNI R, et al. Micropollutants removal from water using microfiltration membrane modified with ZIF-8 metal organic frameworks (MOFs)[J]. Chemical Engineering Journal, 2016, 300: 273-279. |
| 55 | LI T, ZHANG W, ZHAI S, et al. Efficient removal of nickel(Ⅱ) from high salinity wastewater by a novel PAA/ZIF-8/PVDF hybrid ultrafiltration membrane[J]. Water Research, 2018, 143: 87-98. |
| 56 | GUO Y, YING Y, MAO Y, et al. Polystyrene sulfonate threaded through a metal-organic framework membrane for fast and selective lithium-ion separation[J]. Angewandte Chemie: International Edition, 2016, 55(48): 15120-15124. |
| 57 | YIN N, WANG K, WANG L, et al. Amino-functionalized MOFs combining ceramic membrane ultrafiltration for Pb(Ⅱ) removal[J]. Chemical Engineering Journal, 2016, 306: 619-628. |
| 58 | MA J, GUO X, YING Y, et al. Composite ultrafiltration membrane tailored by MOF@GO with highly improved water purification performance[J]. Chemical Engineering Journal, 2017, 313: 890-898. |
| 59 | YANG S, ZOU Q, WANG T, et al. Effects of GO and MOF@GO on the permeation and antifouling properties of cellulose acetate ultrafiltration membrane[J]. Journal of Membrane Science, 2019, 569: 48-59. |
| 60 | GUTMAN J, FOX S, GILRON J. Interactions between biofilms and NF/RO flux and their implications for control—A review of recent developments[J]. Journal of Membrane Science, 2012, 421: 1-7. |
| 61 | WANG J, WANG Y, ZHANG Y, et al. Zeolitic imidazolate framework/graphene oxide hybrid nanosheets functionalized thin film nanocomposite membrane for enhanced antimicrobial performance[J]. ACS Applied Materials & Interfaces, 2016, 8(38): 25508-25519. |
| 62 | DUAN J, PAN Y, PACHECO F, et al. High-performance polyamide thin-film-nanocomposite reverse osmosis membranes containing hydrophobic zeolitic imidazolate framework-8[J]. Journal of Membrane Science, 2015, 476: 303-310. |
| 63 | XU Y, GAO X, WANG X, et al. Highly and stably water permeable thin film nanocomposite membranes doped with MIL-101 (Cr) nanoparticles for reverse osmosis application[J]. Materials, 2016, 9(11): 870. |
| 64 | YANG S, ZHANG K. Few-layers MoS2 nanosheets modified thin film composite nanofiltration membranes with improved separation performance[J]. Journal of Membrane Science, 2020, 595: 117526. |
| 65 | SUN H, LIU J, LUO X, et al. Fabrication of thin-film composite polyamide nanofiltration membrane based on polyphenol intermediate layer with enhanced desalination performance[J]. Desalination, 2020, 488: 114525. |
| 66 | ZHANG Z, KANG G, YU H, et al. Fabrication of a highly permeable composite nanofiltration membrane via interfacial polymerization by adding a novel acyl chloride monomer with an anhydride group[J]. Journal of Membrane Science, 2019, 570: 403-409. |
| 67 | TANG Y-J, WANG L-J, XU Z-L, et al. Novel chitosan-piperazine composite nanofiltration membranes for the desalination of brackish water and seawater[J]. Journal of Polymer Research, 2018, 25(5): 118. |
| 68 | HUANG B-Q, TANG Y-J, ZENG Z-X, et al. Microwave heating assistant preparation of high permselectivity polypiperazine-amide nanofiltration membrane during the interfacial polymerization process with low monomer concentration[J]. Journal of Membrane Science, 2020, 596: 117718. |
| 69 | BAI L, LIU Y, DING A, et al. Fabrication and characterization of thin-film composite (TFC) nanofiltration membranes incorporated with cellulose nanocrystals (CNCs) for enhanced desalination performance and dye removal[J]. Chemical Engineering Journal, 2019, 358: 1519-1528. |
| 70 | ANG M B M Y, JI Y-L, HUANG S-H, et al. Incorporation of carboxylic monoamines into thin-film composite polyamide membranes to enhance nanofiltration performance[J]. Journal of Membrane Science, 2017, 539: 52-64. |
| 71 | SINGH N, PETRINIC I, HELIX-NIELSEN C, et al. Concentrating molasses distillery wastewater using biomimetic forward osmosis (FO) membranes[J]. Water Research, 2018, 130: 271-280. |
| 72 | SUN Y, TIAN J, ZHAO Z, et al. Membrane fouling of forward osmosis (FO) membrane for municipal wastewater treatment: a comparison between direct FO and OMBR[J]. Water Research, 2016, 104: 330-339. |
| 73 | CHUN Y, ZAVISKA F, KIM S-J, et al. Fouling characteristics and their implications on cleaning of a FO-RO pilot process for treating brackish surface water[J]. Desalination, 2017, 402: 185-187. |
| 74 | ZIREHPOUR A, RAHIMPOUR A, ULBRICHT M. Nano-sized metal organic framework to improve the structural properties and desalination performance of thin film composite forward osmosis membrane[J]. Journal of Membrane Science, 2017, 531: 59-67. |
| [1] | 贺美晋. 分子管理在炼油领域分离技术中的应用和发展趋势[J]. 化工进展, 2023, 42(S1): 260-266. |
| [2] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [3] | 张祚群, 高扬, 白超杰, 薛立新. 二次界面聚合同步反扩散原位生长ZIF-8纳米粒子制备聚酰胺混合基质反渗透(RO)膜[J]. 化工进展, 2023, 42(S1): 364-373. |
| [4] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [5] | 李世霖, 胡景泽, 王毅霖, 王庆吉, 邵磊. 电渗析分离提取高值组分的研究进展[J]. 化工进展, 2023, 42(S1): 420-429. |
| [6] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [7] | 李化全, 王明华, 邱贵宝. 硫酸酸解钙钛矿相精矿的行为[J]. 化工进展, 2023, 42(S1): 536-541. |
| [8] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [9] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [10] | 廖志新, 罗涛, 王红, 孔佳骏, 申海平, 管翠诗, 王翠红, 佘玉成. 溶剂脱沥青技术应用与进展[J]. 化工进展, 2023, 42(9): 4573-4586. |
| [11] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [12] | 朱传强, 茹晋波, 孙亭亭, 谢兴旺, 李长明, 高士秋. 固体高分子脱硝剂选择性非催化还原NO x 特性[J]. 化工进展, 2023, 42(9): 4939-4946. |
| [13] | 李雪佳, 李鹏, 李志霞, 晋墩尚, 郭强, 宋旭锋, 宋芃, 彭跃莲. 亲水和疏水改性膜的抗结垢和润湿能力的对比[J]. 化工进展, 2023, 42(8): 4458-4464. |
| [14] | 潘宜昌, 周荣飞, 邢卫红. 高效分离同碳数烃的先进微孔膜:现状与挑战[J]. 化工进展, 2023, 42(8): 3926-3942. |
| [15] | 徐杰, 夏隆博, 罗平, 邹栋, 仲兆祥. 面向膜蒸馏过程的全疏膜制备及其应用进展[J]. 化工进展, 2023, 42(8): 3943-3955. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||