| 1 |
Yunho LEE , Daniel GERRITY , Minju LEE , et al . Prediction of micropollutant elimination during ozonation of municipal wastewater effluents: use of kinetic and water specific information[J]. Environmental Science & Technology, 2013, 47(11): 5872-5881.
|
| 2 |
BOKARE Alok D , Wonyong CHOI . Zero-valent aluminum for oxidative degradation of aqueous organic pollutants[J]. Environ. Sci. Technol., 2009, 43: 7130-7135.
|
| 3 |
朱永昌, 李剑锋, 刘妍君, 等 . 绿色合成纳米铁类芬顿氧化处理水中苯酚[J]. 水处理技术, 2019, 45(5): 63-67.
|
|
ZHU Yongchang , LI Jianfeng , LIU Yanjun , et al . Fenton-like oxidation of phenol in water by green synthetic iron nanoparticles[J]. Technology of Water Treatment, 2019, 45(5): 63-67.
|
| 4 |
DONG Guohui , AI Zhihui , ZHANG Lizhi . Total aerobic destruction of azo contaminants with nanoscale zero-valent copper at neutral pH:promotion effect of in-situ generated carbon center radicals[J].Water Research, 2014(66): 22-30.
|
| 5 |
任逸, 施梦琦, 尹越, 等 . Fe2O3/MIL-53(Al)催化类芬顿氧化性能及其作用机制研究[J]. 环境科学学报, 2019, 39(8): 2508-2516.
|
|
REN Yi , SHI Mengqi , YIN Yue , et al . Study on efficiency and mechanism of Fe2O3/MIL-53(Al) catalytic Fenton-like oxidation[J]. Acta Scientiae Circum Stantiae, 2019, 39(8): 2508-2516.
|
| 6 |
宿程远, 郑鹏, 廖黎明, 等 . Fe3O4 NPs 类芬顿预处理对活性污泥法处理阿莫西林废水的影响[J]. 化工学报, 2018, 69(12): 5237-5245.
|
|
SU Chengyuan , ZHENG Peng , LIAO Liming , et al . Influence of Fe3O4 NPs heterogeneous Fenton-like pre-treatment on activated sludge technology for treatment amoxicillin wastewater[J].CIESC Journal, 2018, 69(12): 5237-5245.
|
| 7 |
LIU Yuyang , JIN Wei , ZHAO Yaping , et al . Enhanced catalytic degradation of methylene blue by α-Fe2O3/graphene oxide via heterogeneous photo-Fenton reactions[J]. Applied Catalysis B: Environmental, 2017, 206(5): 642-652.
|
| 8 |
HAMMOUDA Samia Ben, Nafaâ ADHOUM , Lotfi MONSER , et al . Synthesis of magnetic alginate beads based on Fe3O4 nanoparticles for the removal of 3-methylindole from aqueous solution using Fenton process[J]. Journal of Hazardous Materials, 2015, 294(30): 128-136.
|
| 9 |
YAO Yunjin , CAI Yunmu , LU Fang , et al . Magnetic ZnFe2O4-C3N4 hybrid for photocatalytic degradation of aqueous organic pollutants by visible light[J]. Ind. Eng. Chem. Res., 2014, 53 (44): 17294-17302.
|
| 10 |
ZHANG Liping , LIU Zhuang , Yousef FARAJ , et al . High-flux efficient catalytic membranes incorporated with iron-based Fenton-like catalysts for degradation of organic pollutants[J]. Journal of Membrane Science, 2019, 573(1): 493-503.
|
| 11 |
吕来, 胡春 . 多相芬顿催化水处理技术与原理[J]. 化学进展, 2017, 29(9): 981-999.
|
|
LÜ Lai, HU Chun . Heterogeneous Fenton catalytic water treatment technology and mechanism[J].Progress Chemistry, 2017, 29(9) : 981- 999.
|
| 12 |
Babuponnusami ARJUNAN , Muthukumar KARUPPAN . Advanced oxidation of phenol: a comparison between fenton, electro-fenton, sono electro-Fenton and photo-electro-Fenton processes[J]. Chemical Engineering Journal, 2012, 183: 1-9.
|
| 13 |
WANG Nan , ZHU Lihua , LEI Ming , et al . LigandInduced drastic enhancement of catalytic activity of nano-BiFeO3 for oxidative degradation of bisphenol A[J]. ACS Catal., 2011, 1: 1193-1202.
|
| 14 |
CHEN Liwei , MA Jun , LI Xuchun , et al . Strong enhancement on Fenton oxidation by addition of hydroxylamine to accelerate the ferric and ferrous iron cycles[J]. Environ. Sci. Technol., 2011, 45: 3925-3930.
|
| 15 |
MA Zichuan , WEI Xiaoyu , XING Shengtao , et al .Hydrothermal synthesis and characterization of surface modified δ-MnO2 with high Fenton-like catalytic activity[J]. Catalysis Communications, 2015, 67: 68-71.
|
| 16 |
KOMBAIAH K , Judith VIJAYA J , John KENNEDY L , et al . Catalytic studies of NiFe2O4 nanoparticles prepared by conventional and microwave combustion method[J].Materials Chemistry and Physics, 2019, 221: 11-28.
|
| 17 |
JIANG Linqin , GAO Lian , SUN Jing . Production of aqueous colloidal dispersions of carbon nanotubes[J]. Journal of Colloid and Interface Science, 2003, 260(1): 89-94.
|
| 18 |
DE BOER J H , IPPENS B C . Studies on pore systems in catalysts Ⅱ. The shapes of pores in aluminum oxide systems[J]. Journal of Catalysis, 1964, 3(1): 38-43.
|
| 19 |
景青锋 . 氯化十六烷基吡啶改性纳米材料吸附水中典型芳香污染物的作用及机制[D]. 杭州: 浙江大学, 2016.
|
|
JING Qingfeng . Aqueous sorption of typical aromatic pollutants on nanomaterials modified with cetyl pvridinium chloride[D].Hangzhou: Zhejiang University, 2016.
|
| 20 |
张霄, 张静, 闫春晖, 等 . CuO@C催化过二硫酸盐降解盐酸四环素研究[J]. 水处理技术, 2019, 45(4): 17-20.
|
|
ZHANG Xiao , ZHANG Jing , YAN Chunhui , et al . Study on degradation of tetracycline hydrochloride by CuO@C catalyzed persulfate[J]. Technology of Water Treatment, 2019, 45(4): 17-20.
|
| 21 |
PARK Joo Yang, KIM Jung Hwan . Role of sol with oxyhydroxide/sodium dodecyl sulfate composites on Fenton oxidation of sorbed phenanthrene in sand[J]. Environ. Manage., 2013,126: 72-78.
|
| 22 |
Ottó HORVATH , Róbert HUSZANK . Degradation of surfactants by hydroxyl radicals photogenerated from hydroxoiron(Ⅲ) complexes[J]. Photochem. Photobio., 2003, 2: 960-966.
|
| 23 |
Chedly TIZAOUL , Nazira KARODIA , Mohsen ABUROWAIS . Kinetic study of the manganese-based catalytic hydrogen peroxide oxidation of a persistent azo-dye[J]. Chem. Technol. Biotechnol., 2010, 85: 234-242.
|
 ),Xu LI,Linlin GUO,Xiaozhen FAN(
),Xu LI,Linlin GUO,Xiaozhen FAN( )
)
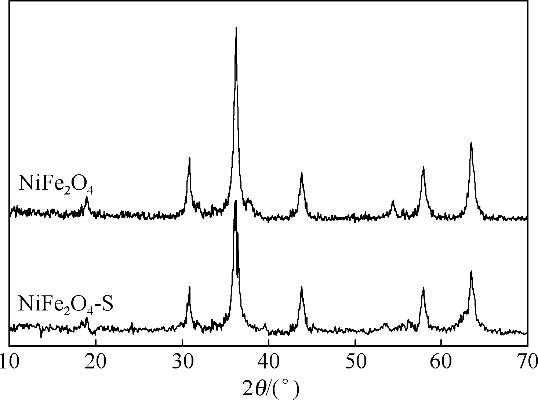
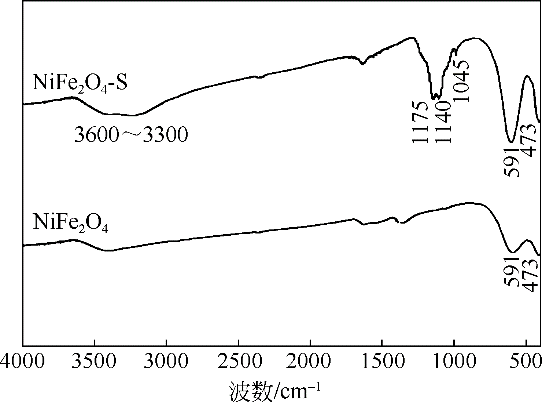
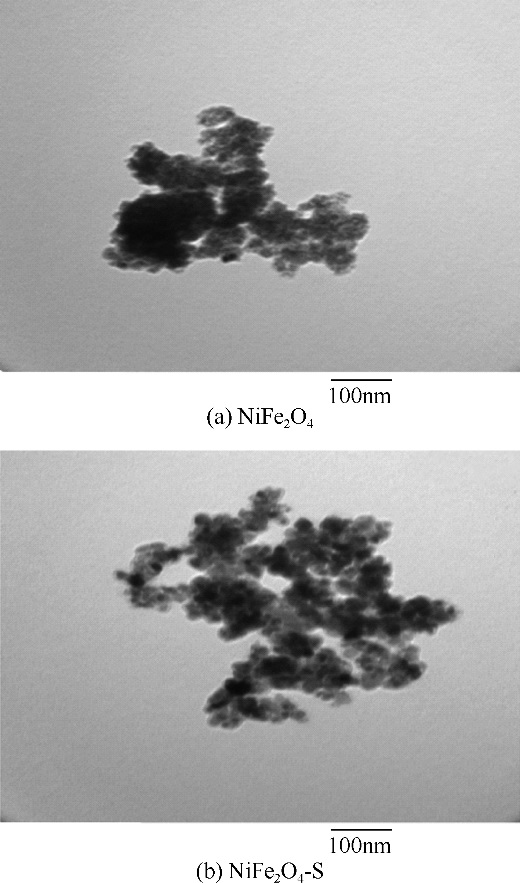
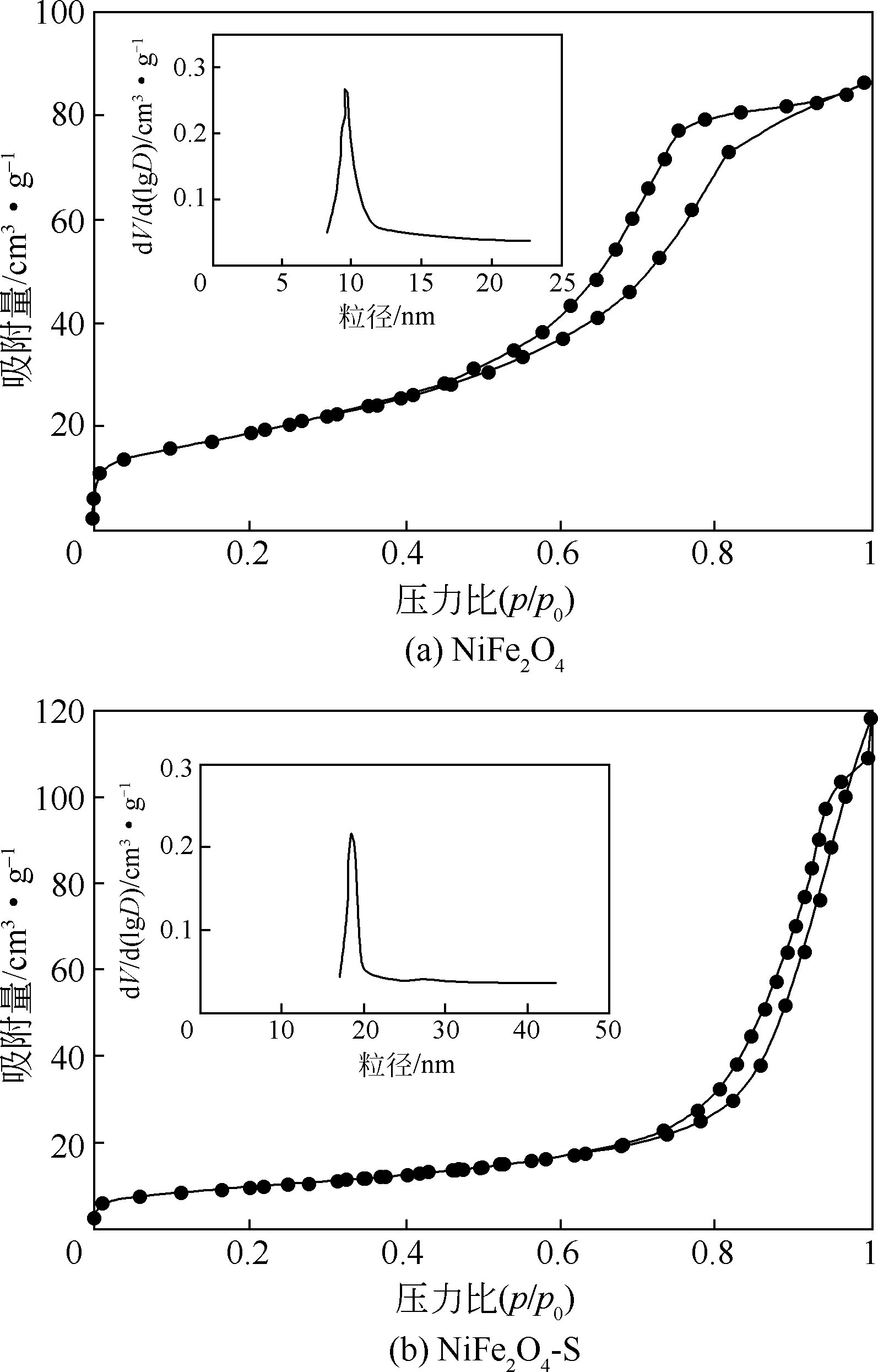
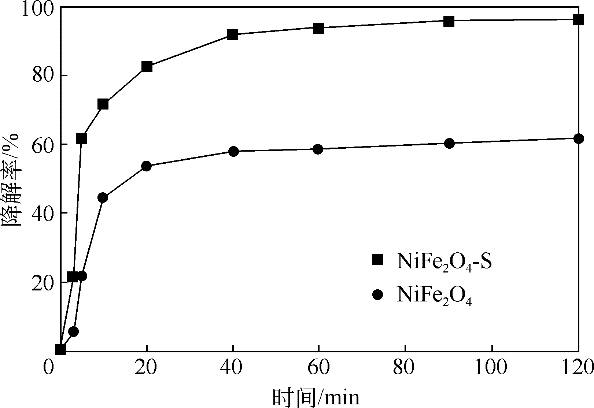
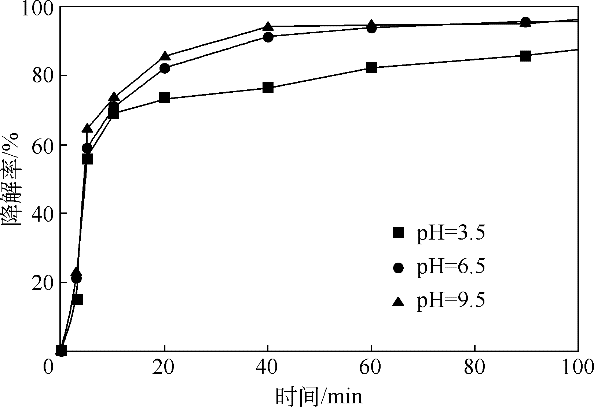
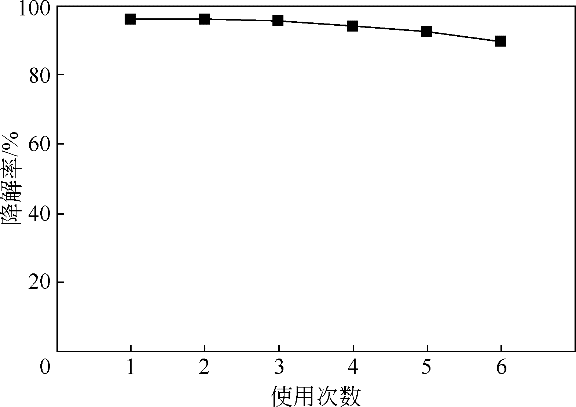
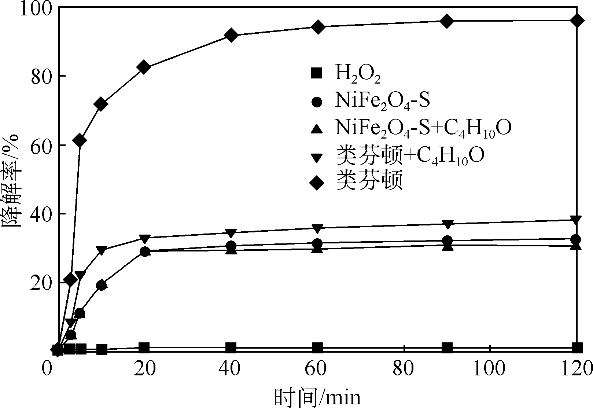
 酸镍(记为NiFe2O4-S),利用X射线衍射(XRD)、红外光谱(FTIR)、能谱(EDS)、透射电镜(TEM)、BET比表面等技术手段对样品进行了表征。采用多相芬顿氧化技术,以亚甲基蓝溶液为模拟污染物废水,研究了SDS对铁酸镍的改性效果、亚甲基蓝溶液初始pH以及催化剂循环使用等不同条件因素对样品类芬顿催化活性的影响。结果显示,经SDS改性后的NiFe2O4-S比纯相NiFe2O4表现出了更优异的催化性能,NiFe2O4-S对酸性(pH=3.5)、近中性(pH=6.5)和碱性(pH=9.5)的亚甲基蓝溶液均有着较好的催化降解效果;NiFe2O4-S具有良好的催化稳定性和重复使用性。对该催化反应体系的作用机理进行了详细探讨,NiFe2O4-S表现出优异的类芬顿催化活性归因于更强的电子转移能力,吸附的SDS能促进H2O2和
酸镍(记为NiFe2O4-S),利用X射线衍射(XRD)、红外光谱(FTIR)、能谱(EDS)、透射电镜(TEM)、BET比表面等技术手段对样品进行了表征。采用多相芬顿氧化技术,以亚甲基蓝溶液为模拟污染物废水,研究了SDS对铁酸镍的改性效果、亚甲基蓝溶液初始pH以及催化剂循环使用等不同条件因素对样品类芬顿催化活性的影响。结果显示,经SDS改性后的NiFe2O4-S比纯相NiFe2O4表现出了更优异的催化性能,NiFe2O4-S对酸性(pH=3.5)、近中性(pH=6.5)和碱性(pH=9.5)的亚甲基蓝溶液均有着较好的催化降解效果;NiFe2O4-S具有良好的催化稳定性和重复使用性。对该催化反应体系的作用机理进行了详细探讨,NiFe2O4-S表现出优异的类芬顿催化活性归因于更强的电子转移能力,吸附的SDS能促进H2O2和