Chemical Industry and Engineering Progress ›› 2021, Vol. 40 ›› Issue (12): 6738-6751.DOI: 10.16085/j.issn.1000-6613.2021-0099
• Materials science and technology • Previous Articles Next Articles
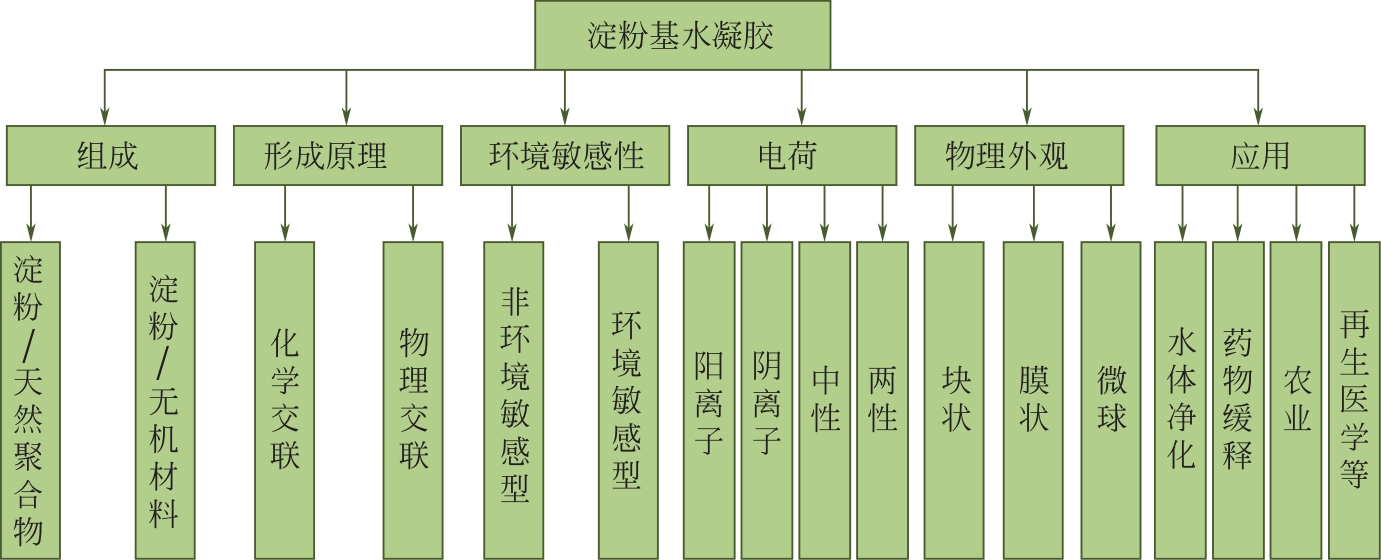
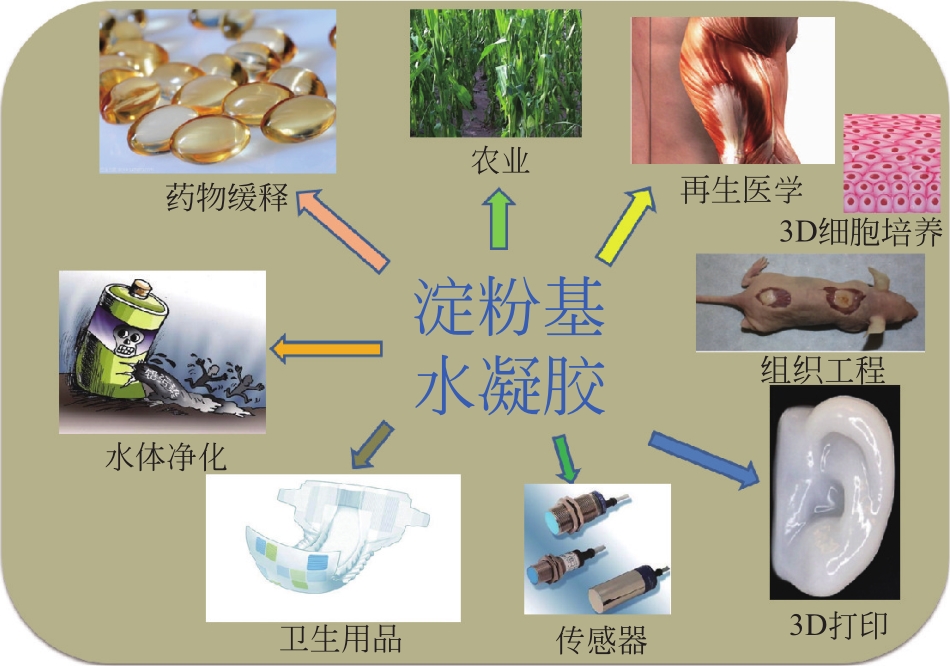
Research progress of starch - based hydrogels
LIU Yuhua( ), WEI Hongliang(
), WEI Hongliang( ), LI Songmao, LIU Zijun, LI Weikun, WANG Gang
), LI Songmao, LIU Zijun, LI Weikun, WANG Gang
- School of Chemistry and Chemical Engineering, Henan University of Technology, Zhengzhou 450001, Henan, China
-
Received:2021-01-15Revised:2021-06-07Online:2021-12-21Published:2021-12-05 -
Contact:WEI Hongliang
淀粉基水凝胶的研究进展
刘玉华( ), 魏宏亮(
), 魏宏亮( ), 李松茂, 刘子君, 李维坤, 王刚
), 李松茂, 刘子君, 李维坤, 王刚
- 河南工业大学化学化工学院,河南 郑州 450001
-
通讯作者:魏宏亮 -
作者简介:刘玉华(1995—),女,硕士研究生,研究方向为高分子凝胶材料。E-mail:3207253167@qq.com 。 -
基金资助:国家自然科学基金(U1904171);河南省科技厅科技攻关(202102310008);河南省高等学校重点科研项目(20A430008);河南工业大学校基金(HAUTZX202003)
CLC Number:
Cite this article
LIU Yuhua, WEI Hongliang, LI Songmao, LIU Zijun, LI Weikun, WANG Gang. Research progress of starch - based hydrogels[J]. Chemical Industry and Engineering Progress, 2021, 40(12): 6738-6751.
刘玉华, 魏宏亮, 李松茂, 刘子君, 李维坤, 王刚. 淀粉基水凝胶的研究进展[J]. 化工进展, 2021, 40(12): 6738-6751.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2021-0099
| 淀粉类型 | 其他材料 | 制备方法 | 应用 | 主要结论 | 参考文献 |
|---|---|---|---|---|---|
| 可溶淀粉 | 氧化纤维素纳米纤维 | 静电作用 | 用作流变改性剂 | 纤维素的加入提高水凝胶的刚度和黏度 | [ |
| 玉米淀粉 | 纤维素纳米晶须 | 辐射交联 | 药物缓释 | 加入纳米晶须后,药物释放时间延长了2.9倍 | [ |
| 原淀粉 | 纤维素纳米纤维/丙烯酸 | 原子转移自由基聚合 | 吸附Cu2+ | 水凝胶最大吸附量为957mg/g | [ |
| 原淀粉 | 磁功能化纤维素纳米晶(MCNCs) | 酶催化 | 吸附阳离子染料 | 加入MCNCs的水凝胶对结晶紫、亚甲基蓝的最大吸附量分别为2500.0mg/g、1428.6mg/g,且表现出良好的可重复性 | [ |
| 原淀粉 | 羧甲基纤维素 | 物理交联 | 药物缓释 | 在pH为6.8和7.4的磷酸盐缓冲液中,水凝胶在12h的药物释放量最高 | [ |
| 玉米淀粉 | 壳聚糖 | 自由基聚合 | 吸附亚甲基蓝 | 淀粉/壳聚糖比例为50/50时,溶胀度为120%左右,在5h达到平衡,且对染料吸附效果最好 | [ |
| 玉米淀粉 | 化学改性天然炭纳米颗粒 | 物理、化学交联 | 高吸水性材料 | 化学改性的碳纳米材料水凝胶的吸水量是纯水凝胶的2倍,为389.9g/g,且保水性为23.1%,是纯水凝胶的3倍 | [ |
| 原淀粉 | 碳化大豆荚 | 交联剂交联 | 吸附萘普生药物和Cr(Ⅵ) | 最高溶胀度为500%,对Cr(Ⅵ)和萘普生药物的去除率分别为96.45%、78% | [ |
| 玉米淀粉 | 氧化石墨烯 | 一步法 | 染料吸附 | 在石墨烯薄片之间掺入淀粉制备的水凝胶,吸附的染料比石墨烯水凝胶多75% | [ |
| 马铃薯淀粉 | 埃洛石纳米管 | 自由基聚合 | 肥料缓释 | 水凝胶溶胀度可达5390%,对尿素缓释放在12h后达到84.6% | [ |
| 双醛淀粉 | N-琥珀酰壳聚糖(SCS) | 席夫碱反应 | 药物缓释 | 姜黄素释放在2天达到平衡,且SCS的含量显著提高了人牙龈成纤维细胞黏附在水凝胶表面的数量,使水凝胶在组织工程软骨修复等方面有应用潜力 | [ |
| 醛基淀粉 | 氨基羧甲基壳聚糖 | 席夫碱反应 | 用于软组织黏合剂、止血等 | 通过改变醛和氨基的含量,可以调节水凝胶的成胶时间、溶胀率和机械拉伸性能,其最高拉伸强度可达到(42.73±1.18)kPa | [ |
| 双醛淀粉 | 明胶 | 湿法纺丝法 | 生物医学 | 海藻酸盐水凝胶纤维和海藻酸盐/明胶混合水凝胶纤维的线密度值由5.79dtex降至4.14dtex,证明明胶的过量加入会破坏藻酸盐/明胶混合水凝胶纤维的力学性能 | [ |
| 氧化淀粉 | CuO | 原位自由基聚合 | 药物缓释 | 该水凝胶在pH为2.1时的溶胀率低于pH为7.4时的溶胀率,其控释性均随纳米氧化铜含量的增加而增加 | [ |
| 氧化淀粉 | ZnO | 交联剂交联 | 抑菌性能 | 该水凝胶在pH为7时水溶液的溶胀度最大,大约为2700%,用于抑菌时细菌零增长,抑菌圈达到11mm | [ |
戊烯酸 功能化淀粉 | 明胶 | 交联剂交联 | 组织再生 | 交联最少的明胶水凝胶具有最高的成脂分化程度,其取代度为31%,储存模量为14kPa | [ |
呋喃功能化 淀粉 | 石墨烯 | Diels-Alder反应 | 生物医学 | 加入石墨烯使纳米复合水凝胶的力学性能、抗菌活性得到了显著提高,电导率从1.23×10-4S/m增加到1.16×10-3S/m | [ |
| 肉豆蔻酸功能化淀粉 | 氧化石墨烯 | 主客体相互作用 | 水体净化 | 制备的纳米复合水凝胶具有明显的、相互连通的三维多孔网络,孔径在亚微米到几微米之间 | [ |
| 可溶淀粉 | 氧化石墨烯 | 辐射交联 | 智能电子设备胶黏剂 | 具有快速自动自愈能力,离子电导率大约为10.5mS/dm | [ |
| 淀粉纳米晶体(SNCs) | 明胶 | 物理交联 | 细胞培养 | 添加0.5%的SNCs压缩模量从(2.0±0.1)kPa增加至(3.1±0.1)kPa,溶胀率变化不大 | [ |
| 可压性淀粉 | 埃洛石 | 偶联法 | 药物缓释 | 药物装入埃洛石腔内,而不是将药物嵌入水凝胶网络,可以有效地抑制初始的爆发性释放,且在30min达到平衡 | [ |
| 淀粉类型 | 其他材料 | 制备方法 | 应用 | 主要结论 | 参考文献 |
|---|---|---|---|---|---|
| 可溶淀粉 | 氧化纤维素纳米纤维 | 静电作用 | 用作流变改性剂 | 纤维素的加入提高水凝胶的刚度和黏度 | [ |
| 玉米淀粉 | 纤维素纳米晶须 | 辐射交联 | 药物缓释 | 加入纳米晶须后,药物释放时间延长了2.9倍 | [ |
| 原淀粉 | 纤维素纳米纤维/丙烯酸 | 原子转移自由基聚合 | 吸附Cu2+ | 水凝胶最大吸附量为957mg/g | [ |
| 原淀粉 | 磁功能化纤维素纳米晶(MCNCs) | 酶催化 | 吸附阳离子染料 | 加入MCNCs的水凝胶对结晶紫、亚甲基蓝的最大吸附量分别为2500.0mg/g、1428.6mg/g,且表现出良好的可重复性 | [ |
| 原淀粉 | 羧甲基纤维素 | 物理交联 | 药物缓释 | 在pH为6.8和7.4的磷酸盐缓冲液中,水凝胶在12h的药物释放量最高 | [ |
| 玉米淀粉 | 壳聚糖 | 自由基聚合 | 吸附亚甲基蓝 | 淀粉/壳聚糖比例为50/50时,溶胀度为120%左右,在5h达到平衡,且对染料吸附效果最好 | [ |
| 玉米淀粉 | 化学改性天然炭纳米颗粒 | 物理、化学交联 | 高吸水性材料 | 化学改性的碳纳米材料水凝胶的吸水量是纯水凝胶的2倍,为389.9g/g,且保水性为23.1%,是纯水凝胶的3倍 | [ |
| 原淀粉 | 碳化大豆荚 | 交联剂交联 | 吸附萘普生药物和Cr(Ⅵ) | 最高溶胀度为500%,对Cr(Ⅵ)和萘普生药物的去除率分别为96.45%、78% | [ |
| 玉米淀粉 | 氧化石墨烯 | 一步法 | 染料吸附 | 在石墨烯薄片之间掺入淀粉制备的水凝胶,吸附的染料比石墨烯水凝胶多75% | [ |
| 马铃薯淀粉 | 埃洛石纳米管 | 自由基聚合 | 肥料缓释 | 水凝胶溶胀度可达5390%,对尿素缓释放在12h后达到84.6% | [ |
| 双醛淀粉 | N-琥珀酰壳聚糖(SCS) | 席夫碱反应 | 药物缓释 | 姜黄素释放在2天达到平衡,且SCS的含量显著提高了人牙龈成纤维细胞黏附在水凝胶表面的数量,使水凝胶在组织工程软骨修复等方面有应用潜力 | [ |
| 醛基淀粉 | 氨基羧甲基壳聚糖 | 席夫碱反应 | 用于软组织黏合剂、止血等 | 通过改变醛和氨基的含量,可以调节水凝胶的成胶时间、溶胀率和机械拉伸性能,其最高拉伸强度可达到(42.73±1.18)kPa | [ |
| 双醛淀粉 | 明胶 | 湿法纺丝法 | 生物医学 | 海藻酸盐水凝胶纤维和海藻酸盐/明胶混合水凝胶纤维的线密度值由5.79dtex降至4.14dtex,证明明胶的过量加入会破坏藻酸盐/明胶混合水凝胶纤维的力学性能 | [ |
| 氧化淀粉 | CuO | 原位自由基聚合 | 药物缓释 | 该水凝胶在pH为2.1时的溶胀率低于pH为7.4时的溶胀率,其控释性均随纳米氧化铜含量的增加而增加 | [ |
| 氧化淀粉 | ZnO | 交联剂交联 | 抑菌性能 | 该水凝胶在pH为7时水溶液的溶胀度最大,大约为2700%,用于抑菌时细菌零增长,抑菌圈达到11mm | [ |
戊烯酸 功能化淀粉 | 明胶 | 交联剂交联 | 组织再生 | 交联最少的明胶水凝胶具有最高的成脂分化程度,其取代度为31%,储存模量为14kPa | [ |
呋喃功能化 淀粉 | 石墨烯 | Diels-Alder反应 | 生物医学 | 加入石墨烯使纳米复合水凝胶的力学性能、抗菌活性得到了显著提高,电导率从1.23×10-4S/m增加到1.16×10-3S/m | [ |
| 肉豆蔻酸功能化淀粉 | 氧化石墨烯 | 主客体相互作用 | 水体净化 | 制备的纳米复合水凝胶具有明显的、相互连通的三维多孔网络,孔径在亚微米到几微米之间 | [ |
| 可溶淀粉 | 氧化石墨烯 | 辐射交联 | 智能电子设备胶黏剂 | 具有快速自动自愈能力,离子电导率大约为10.5mS/dm | [ |
| 淀粉纳米晶体(SNCs) | 明胶 | 物理交联 | 细胞培养 | 添加0.5%的SNCs压缩模量从(2.0±0.1)kPa增加至(3.1±0.1)kPa,溶胀率变化不大 | [ |
| 可压性淀粉 | 埃洛石 | 偶联法 | 药物缓释 | 药物装入埃洛石腔内,而不是将药物嵌入水凝胶网络,可以有效地抑制初始的爆发性释放,且在30min达到平衡 | [ |
| 应用领域 | 制备方法 | 凝胶组成 | 污染物 | 比表面积 /m2·g-1 | 孔洞容量 /cm3·g-1 | 最大吸附量 /mg·g-1 | 参考 文献 |
|---|---|---|---|---|---|---|---|
| 染料吸附 | 电旋法 | 淀粉/聚乙烯醇 | 亚甲基蓝 | 24.72 | 0.0421 | 400 | [ |
| 原子转移自由基聚合 | 木薯淀粉/丙烯酰胺 | 亚甲基蓝 | — | — | 1917 | [ | |
| 酶催化 | 原淀粉/MCNCs | 结晶紫、亚甲基蓝 | — | — | 2500.0、1428.6 | [ | |
| 一步法 | 玉米淀粉/多孔石墨烯 | 核壳荧光 | 45 | 0.03 | 1.1069 | [ | |
| 金属离子 | 交联剂交联 | 原淀粉/生物炭 | Cr(Ⅵ) | 226.94 | 9.88 | 420.13 | [ |
| 交联剂交联 | 2-羟基-3-异丙氧基丙基淀粉/海藻酸钠 | — | — | [ | |||
| 辐射交联 | 木薯淀粉/丙烯酸 | — | — | [ | |||
| 原子转移自由基聚合 | 原淀粉/纤维素/丙烯酸 | — | — | [ |
| 应用领域 | 制备方法 | 凝胶组成 | 污染物 | 比表面积 /m2·g-1 | 孔洞容量 /cm3·g-1 | 最大吸附量 /mg·g-1 | 参考 文献 |
|---|---|---|---|---|---|---|---|
| 染料吸附 | 电旋法 | 淀粉/聚乙烯醇 | 亚甲基蓝 | 24.72 | 0.0421 | 400 | [ |
| 原子转移自由基聚合 | 木薯淀粉/丙烯酰胺 | 亚甲基蓝 | — | — | 1917 | [ | |
| 酶催化 | 原淀粉/MCNCs | 结晶紫、亚甲基蓝 | — | — | 2500.0、1428.6 | [ | |
| 一步法 | 玉米淀粉/多孔石墨烯 | 核壳荧光 | 45 | 0.03 | 1.1069 | [ | |
| 金属离子 | 交联剂交联 | 原淀粉/生物炭 | Cr(Ⅵ) | 226.94 | 9.88 | 420.13 | [ |
| 交联剂交联 | 2-羟基-3-异丙氧基丙基淀粉/海藻酸钠 | — | — | [ | |||
| 辐射交联 | 木薯淀粉/丙烯酸 | — | — | [ | |||
| 原子转移自由基聚合 | 原淀粉/纤维素/丙烯酸 | — | — | [ |
| 1 | PANDISELVAM R, MANIKANTAN M R, DIVYA V, et al. Ozone: an advanced oxidation technology for starch modification[J]. Ozone: Science & Engineering, 2019, 41(6): 491-507. |
| 2 | EL-HOSHOUDY A N, DESOUKY S M. Synthesis and evaluation of acryloylated starch-g-poly (acrylamide/vinylmethacrylate/1-vinyl-2-pyrrolidone) crosslinked terpolymer functionalized by dimethylphenylvinylsilane derivative as a novel polymer-flooding agent[J]. International Journal of Biological Macromolecules, 2018, 116: 434-442. |
| 3 | HOSSAIN K M Z, CALABRESE V, SILVA M A DA, et al. Cationic surfactants as a non-covalent linker for oxidised cellulose nanofibrils and starch-based hydrogels[J]. Carbohydrate Polymers, 2020, 233: 115816. |
| 4 | ZHU F. Encapsulation and delivery of food ingredients using starch based systems[J]. Food Chemistry, 2017, 229: 542-552. |
| 5 | DENG Z X, GUO Y, ZHAO X, et al. Multifunctional stimuli-responsive hydrogels with self-healing, high conductivity, and rapid recovery through host-guest interactions[J]. Chemistry of Materials, 2018, 30(5): 1729-1742. |
| 6 | KHAN S, AKHTAR N, MINHAS M U, et al. pH/Thermo-dual responsive tunable in situ cross-linkable depot injectable hydrogels based on poly(N-isopropylacrylamide)/carboxymethyl chitosan with potential of controlled localized and systemic drug delivery[J]. AAPS PharmSciTech, 2019, 20(3): 119. |
| 7 | WANG Y, ZHAO F, Wang J, et al. Tungsten-doped VO2/starch derivative hybrid nanothermochromic hydrogel for smart window[J]. Nanomaterials, 2019, 9(7): 970. |
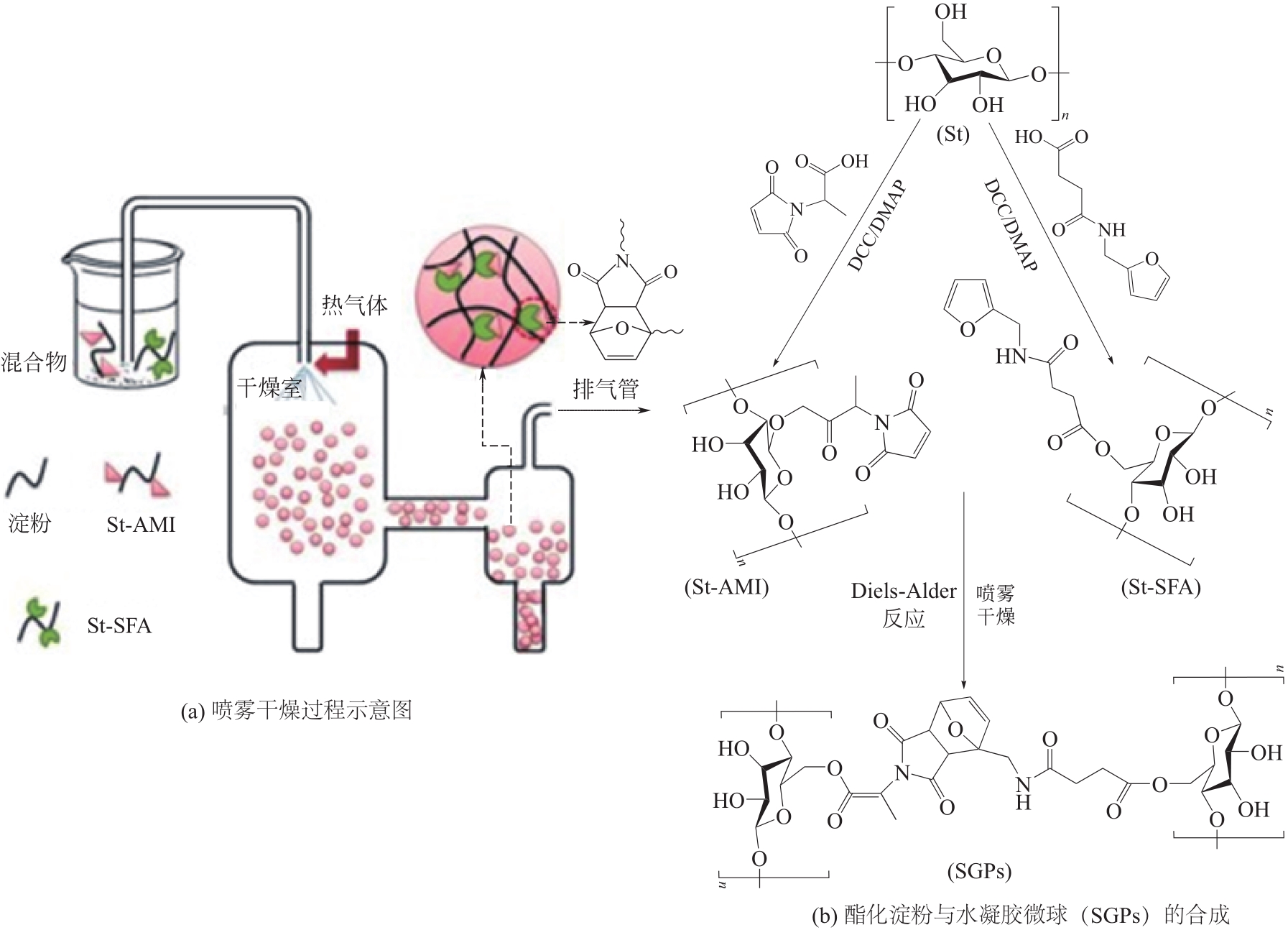
| 8 | WEI H L, YANG X Q, CHU H J, et al. Facile and green preparation of thermal and pH sensitive hydrogel microspheres based on spray drying and the diels-alder reaction[J]. Polymer Engineering and Science, 2019, 59(10): 1999-2007. |
| 9 | WANG Y W, CHEN L Y, AN F P, et al. A novel polysaccharide gel bead enabled oral enzyme delivery with sustained release in small intestine[J]. Food Hydrocolloids, 2018, 84: 68-74. |
| 10 | CHEN X, ZHAN Z X, LIU Q M, et al. Modeling the response characteristics of photo-sensitive hydrogel electrolytes in Hofmeister salt solution for the development of smart energy storage devices[J]. Sustainable Energy & Fuels, 2020, 4(12): 6112-6124. |
| 11 | MAURICIO M R, COSTA P G DA, HARAGUCHI S K, et al. Synthesis of a microhydrogel composite from cellulose nanowhiskers and starch for drug delivery[J]. Carbohydrate Polymers, 2015, 115: 715-722. |
| 12 | BAGHBADORANI N B, BEHZAD T, ETESAMI N, et al. Removal of Cu2+ ions by cellulose nanofibers-assisted starch-g-poly(acrylic acid) superadsorbent hydrogels[J]. Composites Part B: Engineering, 2019, 176: 107084. |
| 13 | MOHARRAMI P, MOTAMRDI E. Application of cellulose nanocrystals prepared from agricultural wastes for synthesis of starch-based hydrogel nanocomposites: efficient and selective nanoadsorbent for removal of cationic dyes from water[J]. Bioresource Technology, 2020, 313: 123661. |
| 14 | GHOLAMALI I, YADOLLAHI M. Doxorubicin-loaded carboxymethyl cellulose/starch/ZnO nanocomposite hydrogel beads as an anticancer drug carrier agent[J]. International Journal of Biological Macromolecules, 2020, 160: 724-735. |
| 15 | PEIDAYESH H, AHMADI Z, KHONAKDAR H A, et al. Baked hydrogel from corn starch and chitosan blends cross-linked by citric acid: preparation and properties[J]. Polymers for Advanced Technologies, 2020, 31(6): 1256-1269. |
| 16 | MOTAMEDI E, MOTESHAREZEDEH B, SHIRINFEKR A, et al. Synthesis and swelling behavior of environmentally friendly starch-based superabsorbent hydrogels reinforced with natural char nano/micro particles[J]. Journal of Environmental Chemical Engineering, 2020, 8(1): 103583. |
| 17 | MOHAMED A K, MAHMOUD M E. Nanoscale pisum sativum pods biochar encapsulated starch hydrogel: a novel nanosorbent for efficient chromium (Ⅵ) ions and naproxen drug removal[J]. Bioresource Technology, 2020, 308: 123263. |
| 18 | SUBHI A A, KIAMAHALLEH M V, FIROUZI M, et al. Self-assembled graphene hydrogel composites for selective dye removal[J]. Advanced Sustainable Systems, 2020, 4(9): 2000055. |
| 19 | WEI H L, WANG H, CHU H J, et al. Preparation and characterization of slow-release and water-retention fertilizer based on starch and halloysite[J]. International Journal of Biological Macromolecules, 2019, 133: 1210-1218. |
| 20 | KAMOUN E A. N-succinyl chitosan-dialdehyde starch hybrid hydrogels for biomedical applications[J]. Journal of Advanced Research, 2016, 7(1): 69-77. |
| 21 | LIU J, LI J, YU F, et al. In situ forming hydrogel of natural polysaccharides through Schiff base reaction for soft tissue adhesive and hemostasis[J]. International Journal of Biological Macromolecules, 2020, 147: 653-666. |
| 22 | WANG Q Q, LIU Y, ZHANG C J, et al. Alginate/gelatin blended hydrogel fibers cross-linked by Ca2+ and oxidized starch: preparation and properties[J]. Materials Science & Engineering C: Materials for Biological Applications, 2019, 99: 1469-1476. |
| 23 | GHOLAMALI I, HOSSEINI S N, ALIPOUR E, et al. Preparation and characterization of oxidized starch/CuO nanocomposite hydrogels applicable in a drug delivery system[J]. Starch-Stärke, 2019, 71(3/4): 1800118. |
| 24 | NAMAZI H, HASANI M, YADOLLAHI M. Antibacterial oxidized starch/ZnO nanocomposite hydrogel: synthesis and evaluation of its swelling behaviours in various pHs and salt solutions[J]. International Journal of Biological Macromolecules, 2019, 126: 578-584. |
| 25 | NIEUWENHOVE I V, SALAMON A, ADAM S, et al. Gelatin- and starch-based hydrogels. part B: in vitro mesenchymal stem cell behavior on the hydrogels[J]. Carbohydrate Polymers, 2017, 161: 295-305. |
| 26 | GONZÁLEZ K, GARCÍA-ASTRAIN C, SANTAMARIA-ECHART A, et al. Starch/graphene hydrogels via click chemistry with relevant electrical and antibacterial properties[J]. Carbohydrate Polymers, 2018, 202: 372-381. |
| 27 | PARSAMANESH M, TEHRANI A D, MANSOURPANAH Y. Supramolecular hydrogel based on cyclodextrin modified GO as a potent natural organic matter absorbent[J]. European Polymer Journal, 2017, 92: 126-136. |
| 28 | WANG Y M, HUANG F R, CHEN X B, et al. Stretchable, conductive, and self-healing hydrogel with super metal adhesion[J]. Chemistry of Materials, 2018, 30(13): 4289-4297. |
| 29 | PILUSO S, LABET M, ZHOU C, et al. Engineered three-dimensional microenvironments with starch nanocrystals as cell-instructive materials[J]. Biomacromolecules, 2019, 20(10): 3819-3830. |
| 30 | LIU F, BAI L B, ZHANG H L, et al. Smart H2O2-responsive drug delivery system made by halloysite nanotubes and carbohydrate polymers[J]. ACS Applied Materials & Interfaces, 2017, 9(37): 31626-31633. |
| 31 | CHA C, SHIN S R, GAO X G, et al. Controlling mechanical properties of cell-laden hydrogels by covalent incorporation of graphene oxide[J]. Small, 2014, 10(3): 514-523. |
| 32 | CUI W, JI J, CAI Y F, et al. Robust, anti-fatigue, and self-healing graphene oxide/hydrophobically associated composite hydrogels and their use as recyclable adsorbents for dye wastewater treatment[J]. Journal of Materials Chemistry A, 2015, 3(33): 17445-17458. |
| 33 | SABBAGH N, AkBARI A, ARSALANI N, et al. Halloysite-based hybrid bionanocomposite hydrogels as potential drug delivery systems[J]. Applied Clay Science, 2017, 148: 48-55. |
| 34 | YU C, TANG X Z, LIU S W, et al. Laponite crosslinked starch/polyvinyl alcohol hydrogels by freezing/thawing process and studying their cadmium ion absorption[J]. International Journal of Biological Macromolecules, 2018: 117: 1-6. |
| 35 | REN J Y, XUAN H Y, GE L Q. Double network self-healing chitosan/dialdehyde starch-polyvinyl alcohol film for gas separation[J]. Applied Surface Science, 2019, 469: 213-219. |
| 36 | ALMEIDA F S, SATO A C K. Structure of gellan gum-hydrolyzed collagen particles: effect of starch addition and coating layer[J]. Food Research International, 2019, 121: 394-403. |
| 37 | KOOPMANS C, RITTER H. Formation of physical hydrogels via host-guest interactions of beta-cyclodextrin polymers and copolymers bearing adamantyl groups[J]. Macromolecules, 2008, 41(20): 7418-7422. |
| 38 | LI J, JI C D, LÜ B Z, et al. Dually crosslinked supramolecular hydrogel for cancer biomarker sensing[J]. ACS Applied Materials & Interfaces, 2020, 12(33): 36873-36881. |
| 39 | WANG Y F, LIN M H, DAI W Q, et al. Enhancement of Fe(Ⅲ) to electro-response of starch hydrogel[J]. Colloid and Polymer Science, 2020, 298(11): 1533-1541. |
| 40 | ASHRI A, AMALINA N, KAMIL A, et al. Modified Dioscoreahispida starch-based hydrogels and their in-vitro cytotoxicity study on small intestine cell line (FHS-74 Int)[J]. International Journal of Biological Macromolecules, 2018, 107: 2412-2421. |
| 41 | OLAD A, DOUSTDAR F, GHAREKHANI H. Starch-based semi-IPN hydrogel nanocomposite integrated with clinoptilolite: preparation and swelling kinetic study[J]. Carbohydrate Polymers, 2018, 200: 516-528. |
| 42 | CHAUDHURI S D, MANDAL A, DEY A, et al. Tuning the swelling and rheological attributes of bentonite clay modified starch grafted polyacrylic acid based hydrogel[J]. Applied Clay Science, 2020, 185: 105405. |
| 43 | ZAIN A H M, WAHAB M K A, ISMAIL H. Solid-state photo-cross-linking of cassava starch: improvement properties of thermoplastic starch[J]. Polymer Bulletin, 2018, 75(8): 3341-3356. |
| 44 | NOÈ C, TONDA-TURO C, CHIAPPONE A, et al. Light processable starch hydrogels[J]. Polymers, 2020, 12(6): 1359. |
| 45 | SENNA M M, MOSTAFA A E B, MAHDY S R, et al. Characterization of blend hydrogels based on plasticized starch/cellulose acetate/carboxymethyl cellulose synthesized by electron beam irradiation[J]. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 2016, 386: 22-29. |
| 46 | FEKETE T, BORSA J, TAKÁCS E, et al. Synthesis of carboxymethylcellulose/starch superabsorbent hydrogels by gamma-irradiation[J]. Chemistry Central Journal, 2017, 11(1): 46. |
| 47 | MOHAMED A K, MAHMOUD M E. Encapsulation of starch hydrogel and doping nanomagnetite onto metal-organic frameworks for efficient removal of fluvastatin antibiotic from water[J]. Carbohydrate Polymers, 2020, 245: 116438. |
| 48 | LI Y L, TAN Y, XU K, et al. A biodegradable starch hydrogel synthesized via thiol-ene click chemistry[J]. Polymer Degradation and Stability, 2017, 137: 75-82. |
| 49 | LI X M, WU Z Z, ZHANG B, et al. Fabrication of chitosan hydrochloride and carboxymethyl starch complex nanogels as potential delivery vehicles for curcumin[J]. Food Chemistry, 2019, 293: 197-203. |
| 50 | NOH G J, LIM S A, LEE E S. pH-responsive squeezing polysaccharidic nanogels for efficient docetaxel delivery[J]. Polymers for Advanced Technologies, 2019, 30(8): 2067-2074. |
| 51 | WEI H L, LI W K, CHEN H L, et al. Simultaneous Diels-Alder click reaction and starch hydrogel microsphere production via spray drying[J]. Carbohydrate Polymers, 2020, 241: 116351. |
| 52 | MORADI E, EBRAHIMZADEH H, MEHRANI Z, et al. The efficient removal of methylene blue from water samples using three-dimensional poly(vinyl alcohol)/starch nanofiber membrane as a green nanosorbent[J]. Environmental Science and Pollution Research, 2019, 26(34): 35071-35081. |
| 53 | KUCHAIYAPHUM P, CHOTICHAYAPONG C, BUTWONG N, et al. Silk fibroin/poly(vinyl alcohol) hydrogel cross-linked with dialdehyde starch for wound dressing applications[J]. Macromolecular Research, 2020, 28(9): 844-850. |
| 54 | QIN Y, WANG J P, QIU C, et al. A dual cross-linked strategy to construct moldable hydrogels with high stretchability, good self-recovery, and self-healing capability[J]. Journal of Agricultural and Food Chemistry, 2019, 67(14): 3966-3980 |
| 55 | 李玉芳, 刘君瑜, 王翔宇, 等. 智能水凝胶药物控释系统的研究进展[J]. 口腔医学, 2020, 40(10): 951-954. |
| LI Yufang,LIU Junyu,WANG Xiangyu, et al. Research progress of intelligent hydrogel drug controlled release system[J]. Stomatology, 2020, 40(10): 951-954. | |
| 56 | 刘展晴. 半互穿网络淀粉水凝胶的制备及溶胀性研究[J]. 化学研究与应用, 2019, 31(3): 516-521. |
| LIU Zhanqing. Preparation and swelling properties of semi-interpenetrating network starch hydrogel[J]. Chemical Research and Application, 2019, 31(3): 516-521. | |
| 57 | EKENSEAIR A K, BOERE K W M, TZOUANAS S N, et al. Synthesis and characterization of thermally and chemically gelling injectable hydrogels for tissue engineering[J]. Biomacromolecules, 2012, 13(6): 1908-1915. |
| 58 | HAQ M A, SU Y L, WANG D J. Mechanical properties of PNIPAM based hydrogels: a review[J]. Materials Science & Engineering C: Materials for Biological Applications, 2017, 70: 842-855. |
| 59 | JENA D K, SAHOO P K. New strategies for the construction of eggshell powder reinforced starch based fire hazard suppression biomaterials with tailorable thermal, mechanical and oxygen barrier properties[J]. International Journal of Biological Macromolecules, 2019, 140: 496-504. |
| 60 | FENG J C, DOU J P, ZHANG Y Z, et al. Thermosensitive hydrogel for encapsulation and controlled release of biocontrol agents to prevent peanut aflatoxin contamination[J]. Polymers, 2020, 12(3): 547. |
| 61 | BANERJEE S L, SWIFT T, HOSKINS R, et al. A muscle mimetic polyelectrolyte-nanoclay organic-inorganic hybrid hydrogel: its self-healing, shape-memory and actuation properties[J]. Journal of Materials Chemistry B, 2019, 7(9): 1475-1493. |
| 62 | SIYAMAK S, LUCKMAN P, LAYCOCK B. Rapid and solvent-free synthesis of pH-responsive graft-copolymers based on wheat starch and their properties as potential ammonium sorbents[J]. International Journal of Biological Macromolecules, 2020, 149: 477-486. |
| 63 | TANAN W, PANICHPAKDEE J, SAENGSUWAN S. Novel biodegradable hydrogel based on natural polymers: synthesis, characterization, swelling/reswelling and biodegradability[J]. European Polymer Journal, 2019, 112: 678-687. |
| 64 | MAHMOUDI M, SANT S, WANG B, et al. Superparamagnetic iron oxide nanoparticles (SPIONs): development, surface modification and applications in chemotherapy[J]. Advanced Drug Delivery Reviews, 2011, 63(1/2): 24-46 |
| 65 | MASSOUMI B, MOZAFFARI Z, JAYMAND M. A starch-based stimuli-responsive magnetite nanohydrogel as de novo drug delivery system[J]. International Journal of Biological Macromolecules, 2018, 117: 418-426. |
| 66 | NEMATOLLAHI M R, MONTAZER M. Low-temperature assembling of naturally driven copper ferrite starch nanocomposites hydrogel with magnetic and antibacterial activities[J]. Journal of Applied Polymer Science, 2020, 137(33): 48961. |
| 67 | STRACHOTA B, STRACHOTA A, ŠLOUFl M, et al. Monolithic intercalated PNIPAm/starch hydrogels with very fast and extensive one-way volume and swelling responses to temperature and pH: prospective actuators and drug release systems[J]. Soft Matter, 2019, 15(4): 752-769. |
| 68 | LIMA-TENÓRIO M K, TENÓRIO-NETO E T, GUILHERME M R, et al. Water transport properties through starch-based hydrogel nanocomposites responding to both pH and a remote magnetic field[J]. Chemical Engineering Journal, 2015, 259: 620-629. |
| 69 | SUN T B, ZHU C Z, XU J. Multiple stimuli-responsive selenium-functionalized biodegradable starch-based hydrogels[J]. Soft Matter, 2018, 14(6): 921-926. |
| 70 | JUNLAPONG K, MAIJAN P, CHAIBUNDIT C, et al. Effective adsorption of methylene blue by biodegradable superabsorbent cassava starch-based hydrogel[J]. International Journal of Biological Macromolecules, 2020, 158: 258-264. |
| 71 | DAI M Y, LUI Y, JU B Z, et al. Preparation of thermoresponsive alginate/starch ether composite hydrogel and its application to the removal of Cu(Ⅱ) from aqueous solution[J]. Bioresource Technology, 2019, 294: 122192. |
| 72 | 刘子杰, 梁兴唐, 钟书明, 等. 微波合成淀粉基水凝胶的Pb2+吸附性能[J]. 精细化工, 2016, 33(10): 1135-1140. |
| LIU Zijie, LIANG Xingtang, ZHONG Shuming, et al. Synthesis and characterization of starch-graft-acrylic acid hydrogels under microwave irradiation for adsorption of Pb2+[J]. Fine Chemicals, 2016, 33(10): 1135-1140. | |
| 73 | NEZAMI S, SADEGHI M. pH-sensitive free AgNPs composite and nanocomposite beads based on starch as drug delivery systems[J]. Polymer Bulletin, 2020, 77(3): 1255-1279. |
| 74 | LI S S, XIA Y Z, QIU Y, et al. Preparation and property of starch nanoparticles reinforced aldehyde-hydrazide covalently crosslinked PNIPAM hydrogels[J]. Journal of Applied Polymer Science, 2018, 135(5): 45761. |
| 75 | 高凤苑, 关欣, 韩良亮, 等. 木薯淀粉水凝胶负载姜黄素及缓释性能研究[J]. 食品与发酵工业, 2019, 45(11): 204-210. |
| GAO Fengyuan, GUAN Xin, HAN Liangliang, et al. Study on curcumin loading and sustained-release properties of cassava starch hydrogel[J]. Food and Fermentation Industries, 2019, 45(11): 204-210. | |
| 76 | LIU J, SUN L S, XU W Y, et al. Current advances and future perspectives of 3D printing natural-derived biopolymers[J]. Carbohydrate Polymers, 2019, 207: 297-316. |
| 77 | MANIGLIA B C, LIMA D C, JUNIOR M M D, et al. Hydrogels based on ozonated cassava starch: effect of ozone processing and gelatinization conditions on enhancing 3D-printing applications[J]. International Journal of Biological Macromolecules, 2019, 138: 1087-1097. |
| 78 | PATHAK V, AMBROSE R P K. Starch-based biodegradable hydrogel as seed coating for corn to improve early growth under water shortage[J]. Journal of Applied Polymer Science, 2020, 137(14): 48523. |
| 79 | ZAIN G, NADA A A, EL-SHEIKH M A, et al. Superabsorbent hydrogel based on sulfonated-starch for improving water and saline absorbency[J]. International Journal of Biological Macromolecules, 2018, 115: 61-68. |
| 80 | WEI X Q, BAO X Y, YU L, et al. Correlation between gel strength of starch-based hydrogel and slow release behavior of its embedded urea[J]. Journal of Polymers and the Environment, 2020, 28(3): 863-870. |
| 81 | FLORES-ARRIAGA J C, POZOS-GUILLÉN A D J, ESCOBAR-GARCÍA D M, et al. Cell viability and hemocompatibility evaluation of a starch-based hydrogel loaded with hydroxyapatite or calcium carbonate for maxillofacial bone regeneration[J]. Odontology, 2017, 105(4): 398-407. |
| 82 | NOURMOHAMMADI J, GHAEE A, LIAVALI S H. Preparation and characterization of bioactive composite scaffolds from polycaprolactone nanofibers-chitosan-oxidized starch for bone regeneration[J]. Carbohydrate Polymers, 2016, 138: 172-179. |
| [1] | CUI Shoucheng, XU Hongbo, PENG Nan. Simulation analysis of two MOFs materials for O2/He adsorption separation [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 382-390. |
| [2] | CHEN Chongming, CHEN Qiu, GONG Yunqian, CHE Kai, YU Jinxing, SUN Nannan. Research progresses on zeolite-based CO2 adsorbents [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 411-419. |
| [3] | XU Chunshu, YAO Qingda, LIANG Yongxian, ZHOU Hualong. Research progress on functionalization strategies of covalent organic frame materials and its adsorption properties for Hg(Ⅱ) and Cr(Ⅵ) [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 461-478. |
| [4] | GU Yongzheng, ZHANG Yongsheng. Dynamic behavior and kinetic model of Hg0 adsorption by HBr-modified fly ash [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 498-509. |
| [5] | GUO Qiang, ZHAO Wenkai, XIAO Yonghou. Numerical simulation of enhancing fluid perturbation to improve separation of dimethyl sulfide/nitrogen via pressure swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 64-72. |
| [6] | WANG Shengyan, DENG Shuai, ZHAO Ruikai. Research progress on carbon dioxide capture technology based on electric swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 233-245. |
| [7] | ZHU Jie, JIN Jing, DING Zhenghao, YANG Huipan, HOU Fengxiao. Modification of CaSO4 oxygen carrier by Zhundong coal ash in chemical looping gasification and its mechanism [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4628-4635. |
| [8] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [9] | YANG Ying, HOU Haojie, HUANG Rui, CUI Yu, WANG Bing, LIU Jian, BAO Weiren, CHANG Liping, WANG Jiancheng, HAN Lina. Coal tar phenol-based carbon nanosphere prepared by Stöber method for adsorption of CO2 [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 5011-5018. |
| [10] | ZHANG Zhen, LI Dan, CHEN Chen, WU Jinglan, YING Hanjie, QIAO Hao. Separation and purification of salivary acids with adsorption resin [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4153-4158. |
| [11] | WANG Baoying, WANG Huangying, YAN Junying, WANG Yaoming, XU Tongwen. Research progress of polymer inclusion membrane in metal separation and recovery [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3990-4004. |
| [12] | JIANG Jing, CHEN Xiaoyu, ZHANG Ruiyan, SHENG Guangyao. Research progress of manganese-loaded biochar preparation and its application in environmental remediation [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4385-4397. |
| [13] | YU Jingwen, SONG Luna, LIU Yanchao, LYU Ruidong, WU Mengmeng, FENG Yu, LI Zhong, MI Jie. An indole-bearing hypercrosslinked polymer In-HCP for iodine adsorption from water [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3674-3683. |
| [14] | LI Yanling, ZHUO Zhen, CHI Liang, CHEN Xi, SUN Tanglei, LIU Peng, LEI Tingzhou. Research progress on preparation and application of nitrogen-doped biochar [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3720-3735. |
| [15] | BAI Yadi, DENG Shuai, ZHAO Ruikai, ZHAO Li, YANG Yingxia. Exploration on standardized test scheme and experimental performance of temperature swing adsorption carbon capture unit [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3834-3846. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||