Chemical Industry and Engineering Progress ›› 2021, Vol. 40 ›› Issue (8): 4600-4609.DOI: 10.16085/j.issn.1000-6613.2020-2303
• Resources and environmental engineering • Previous Articles Next Articles
Co3O4/TiO2/porous carbon for organic wastewater treatment
ZHANG Shuquan( ), XU Min, WANG Guotao
), XU Min, WANG Guotao
- College of Zhicheng, Fuzhou University, Fuzhou 350002, Fujian, China
-
Received:2020-11-18Online:2021-08-12Published:2021-08-05 -
Contact:ZHANG Shuquan
用于水中有机污染物处理的Co3O4/TiO2/多孔炭复合材料
- 福州大学至诚学院,福建 福州 350002
-
通讯作者:张书泉 -
作者简介:张书泉(1984—),男,博士,副教授,主要从事功能复合材料在能源与环境方面应用的研究。E-mail:sqzhang@fzu.edu.cn 。 -
基金资助:福建省自然科学基金(2018J01431);福建省教育厅中青年教师教育科研项目(JT180814);国家级大学生创新训练计划(202013470002);福建省高校杰出青年科研人才培育计划(ZJ1751)
CLC Number:
Cite this article
ZHANG Shuquan, XU Min, WANG Guotao. Co3O4/TiO2/porous carbon for organic wastewater treatment[J]. Chemical Industry and Engineering Progress, 2021, 40(8): 4600-4609.
张书泉, 徐敏, 王国滔. 用于水中有机污染物处理的Co3O4/TiO2/多孔炭复合材料[J]. 化工进展, 2021, 40(8): 4600-4609.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2020-2303
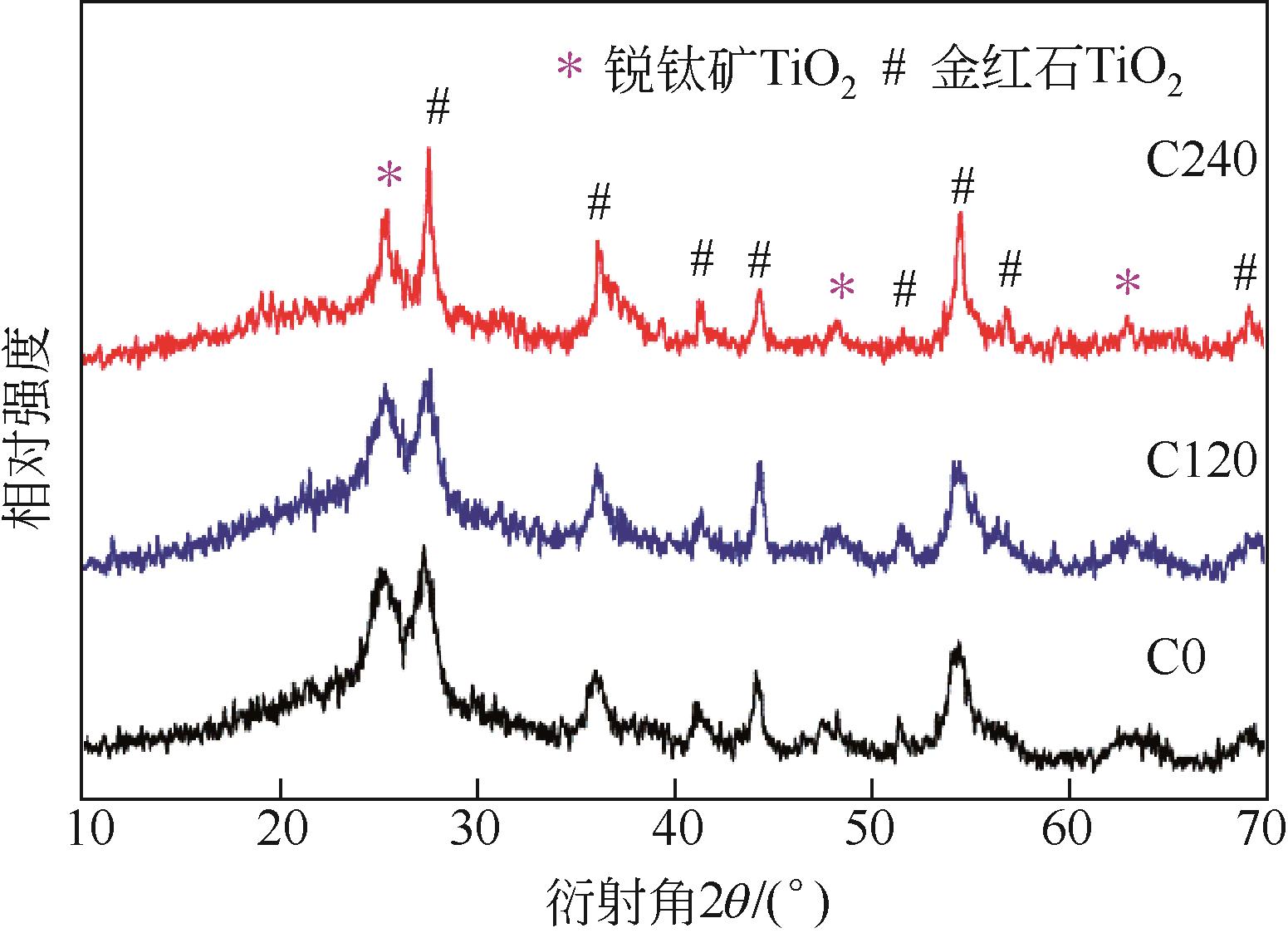
| 样品 | 合成时加入的NH2-MIL-125(Ti) 质量/mg | 合成时加入的Co(NO3)2·6H2O质量/mg | 实测样品中TiO2的 质量分数/% | 实测样品中Co3O4的 质量分数/% |
|---|---|---|---|---|
| C0 | 800 | 0 | 52.48 | 0 |
| C80 | 800 | 80 | 49.66 | 3.49 |
| C120 | 800 | 120 | 48.72 | 5.23 |
| C160 | 800 | 160 | 48.87 | 6.91 |
| C200 | 800 | 200 | 46..69 | 8.56 |
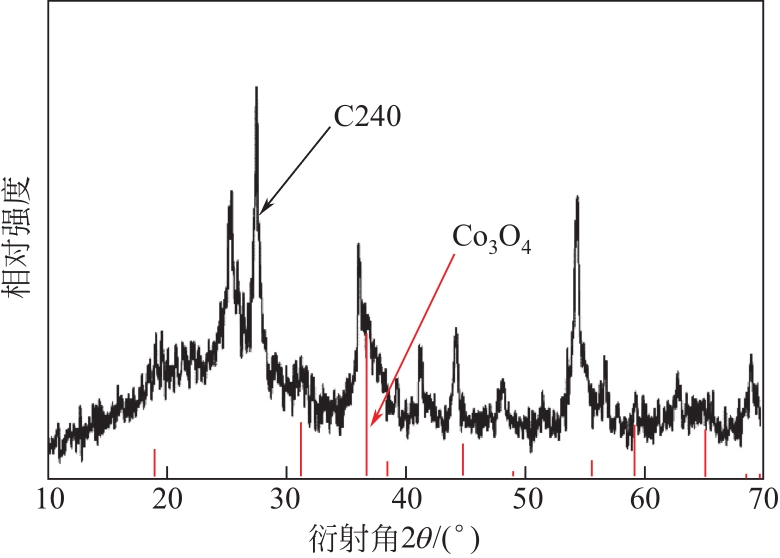
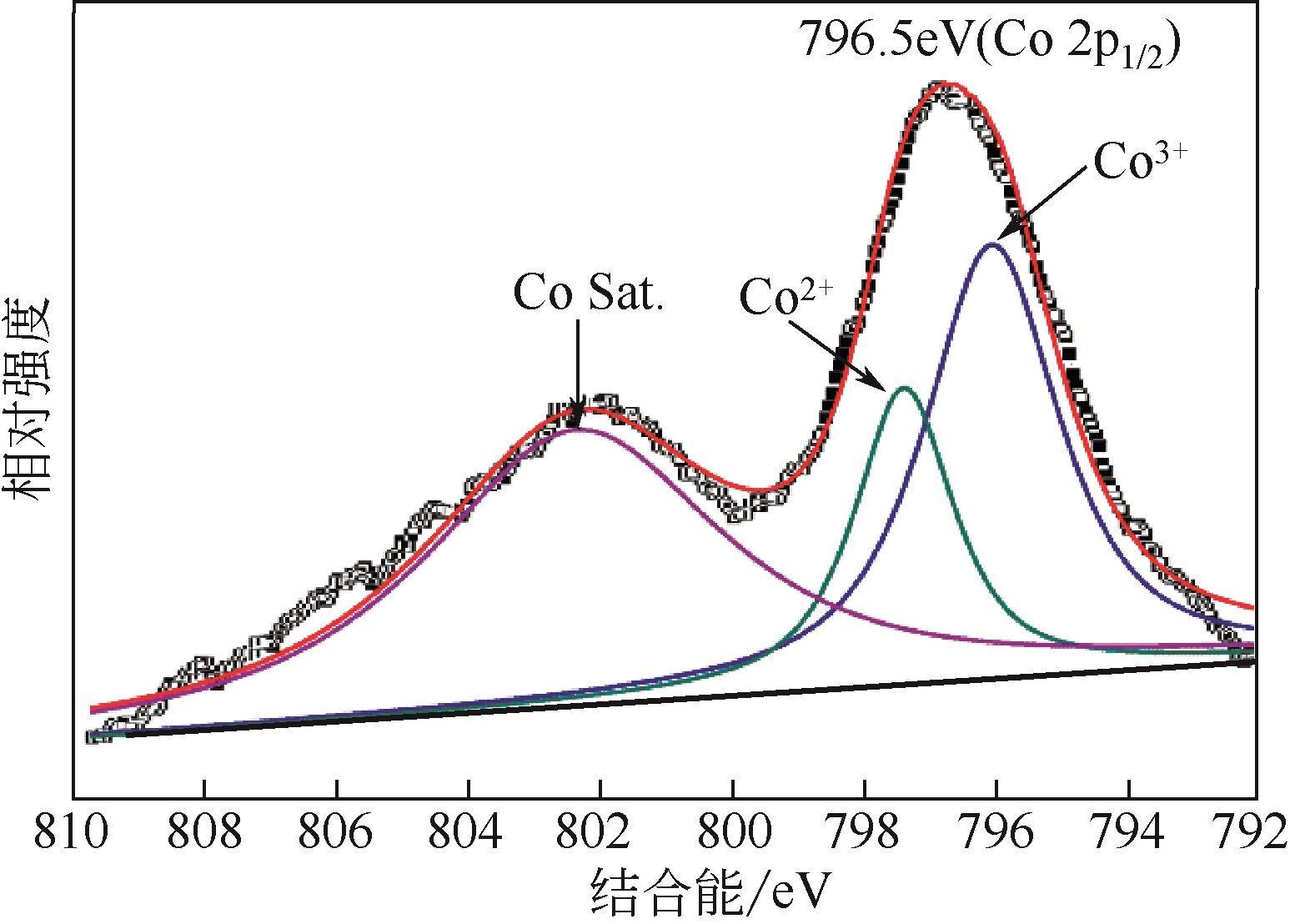
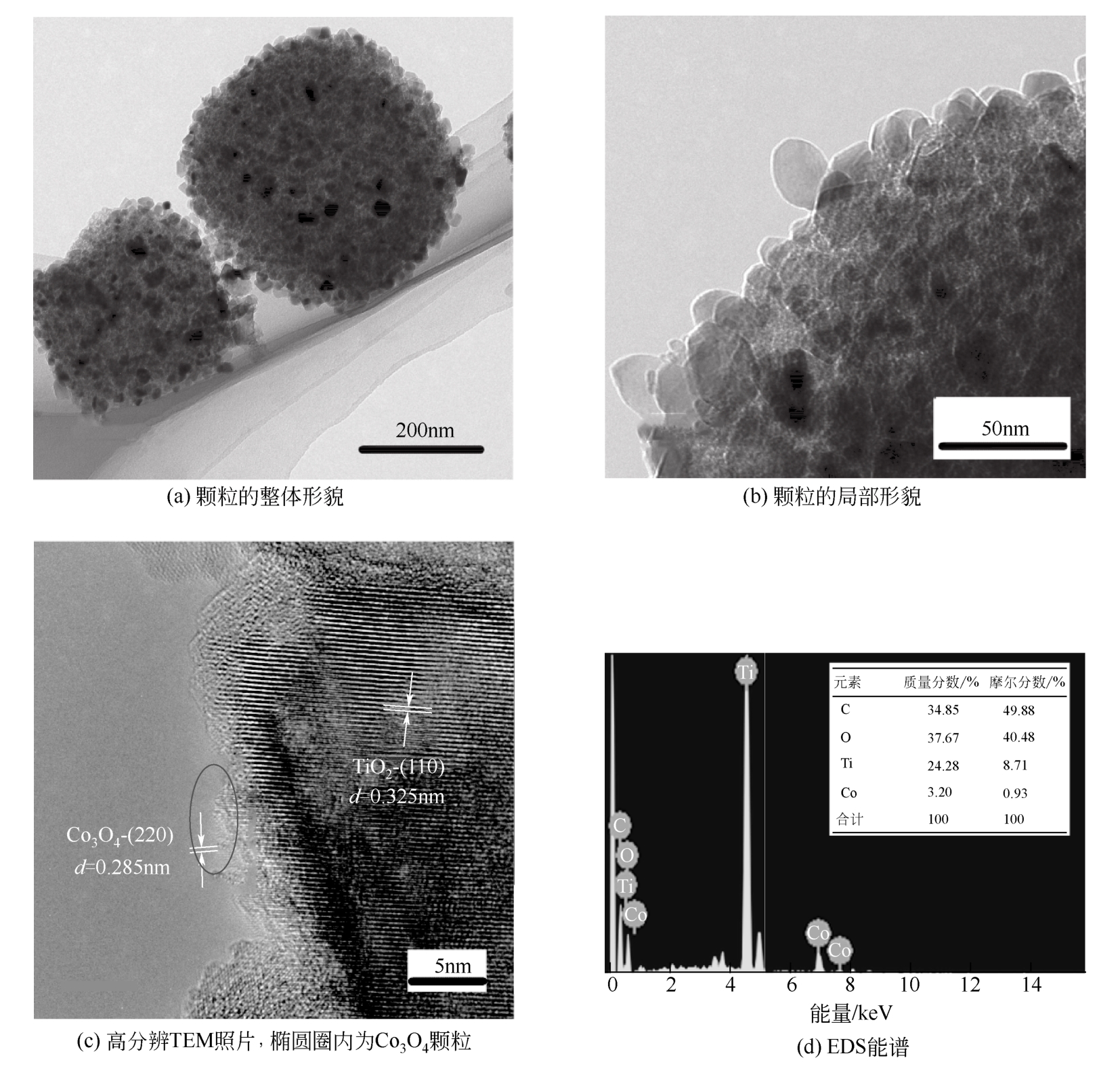
| C240 | 800 | 240 | 45.73 | 9.87 |
| 样品 | 合成时加入的NH2-MIL-125(Ti) 质量/mg | 合成时加入的Co(NO3)2·6H2O质量/mg | 实测样品中TiO2的 质量分数/% | 实测样品中Co3O4的 质量分数/% |
|---|---|---|---|---|
| C0 | 800 | 0 | 52.48 | 0 |
| C80 | 800 | 80 | 49.66 | 3.49 |
| C120 | 800 | 120 | 48.72 | 5.23 |
| C160 | 800 | 160 | 48.87 | 6.91 |
| C200 | 800 | 200 | 46..69 | 8.56 |
| C240 | 800 | 240 | 45.73 | 9.87 |
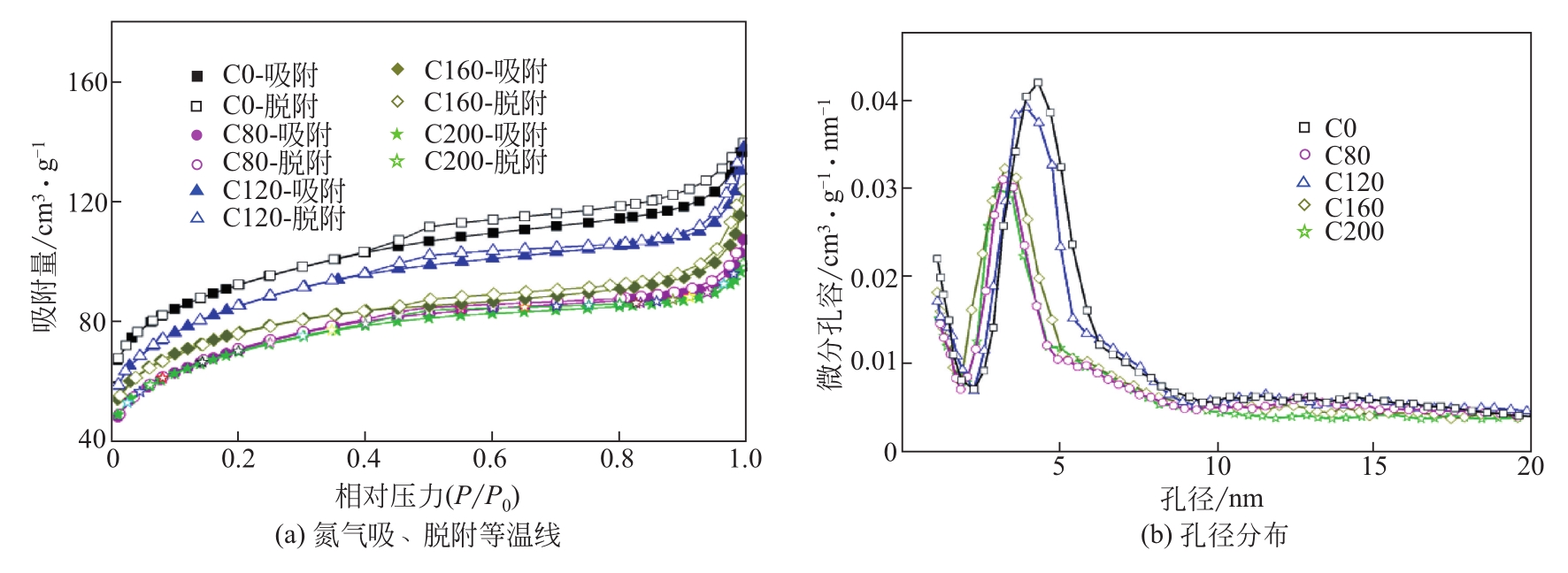
| 样品 | 比表面积/m2?g-1 | 孔容/cm3?g-1 |
|---|---|---|
| C0 | 298 | 0.217 |
| C80 | 238 | 0.172 |
| C120 | 279 | 0.213 |
| C160 | 247 | 0.190 |
| C200 | 230 | 0.154 |
| 样品 | 比表面积/m2?g-1 | 孔容/cm3?g-1 |
|---|---|---|
| C0 | 298 | 0.217 |
| C80 | 238 | 0.172 |
| C120 | 279 | 0.213 |
| C160 | 247 | 0.190 |
| C200 | 230 | 0.154 |
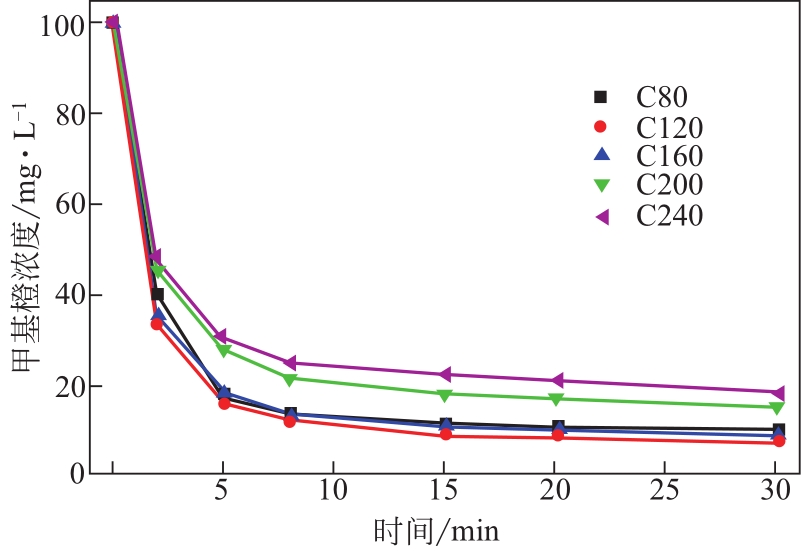
| 样品 | 甲基橙的平衡吸附量qe/mg·g-1sample | 吸附速率常数k2/g sample·mg-1·min-1 | 相关系数R2/% |
|---|---|---|---|
| C80 | 252.53 | 2.91×10-3 | 99.7 |
| C120 | 273.22 | 2.55×10-3 | 99.9 |
| C160 | 253.81 | 2.91×10-3 | 99.9 |
| C200 | 164.47 | 6.50×10-3 | 99.5 |
| C240 | 144.09 | 8.16×10-3 | 99.7 |
| 样品 | 甲基橙的平衡吸附量qe/mg·g-1sample | 吸附速率常数k2/g sample·mg-1·min-1 | 相关系数R2/% |
|---|---|---|---|
| C80 | 252.53 | 2.91×10-3 | 99.7 |
| C120 | 273.22 | 2.55×10-3 | 99.9 |
| C160 | 253.81 | 2.91×10-3 | 99.9 |
| C200 | 164.47 | 6.50×10-3 | 99.5 |
| C240 | 144.09 | 8.16×10-3 | 99.7 |
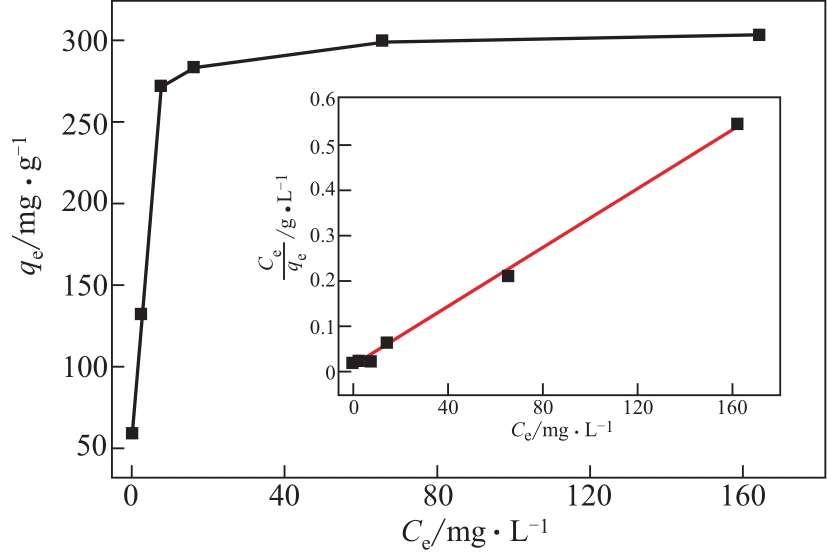
| 吸附剂 | 甲基橙的平衡吸附量qe/mg·g-1sample | 吸附条件 | 参考文献 |
|---|---|---|---|
| Ni/多孔炭/碳纳米管 | 271 | 5mg吸附剂于10mL MO水溶液(20~350mg·L-1);吸附时间60min;吸附温度室温 | [ |
| (Fe2O3,Fe3C,Fe)@多孔炭 | 183 | 10mg吸附剂于20mL MO水溶液(12.5~400mg·L-1);吸附时间90min;吸附温度室温 | [ |
| ZnFe2O4/木质素衍生炭 | 113 | 20mg吸附剂于20mL MO水溶液(20~180mg·L-1);吸附时间160min;吸附温度293K;pH=5 | [ |
| Fe负载多孔炭 | 187 | 20mg吸附剂于50mL MO水溶液(10~100mg·L-1);吸附时间60min;吸附温度室温;pH=7 | [ |
| Fe2O3/Mn3O4 | 333 | 200mg吸附剂于200mL MO水溶液(60~1000mg·L-1);吸附时间45min;吸附温度室温;pH=2 | [ |
| SiO2@Fe3O4 | 240 | 5mg吸附剂于10mL MO水溶液(0~100mg·L-1);吸附时间180min;吸附温度293K;pH=5.5 | [ |
| NiFe2O4/活性炭 | 183 | 300mg吸附剂于100mL MO水溶液(0~200mg·L-1);吸附时间30min;吸附温度303K;pH=3 | [ |
| Fe3O4/季铵壳聚糖衍生物 | 267 | 100mg吸附剂于100mL MO水溶液(50~300mg·L-1);吸附时间60min;吸附温度293K | [ |
| Fe3O4/PPY | 149 | 10mg吸附剂于10mL MO水溶液(60~320mg·L-1);吸附时间90min;吸附温度298K | [ |
| LnBDC/活性炭 | 125 | 5mg吸附剂于10mL MO水溶液(0~200mg·L-1);吸附时间1440min;吸附温度303K;pH=5 | [ |
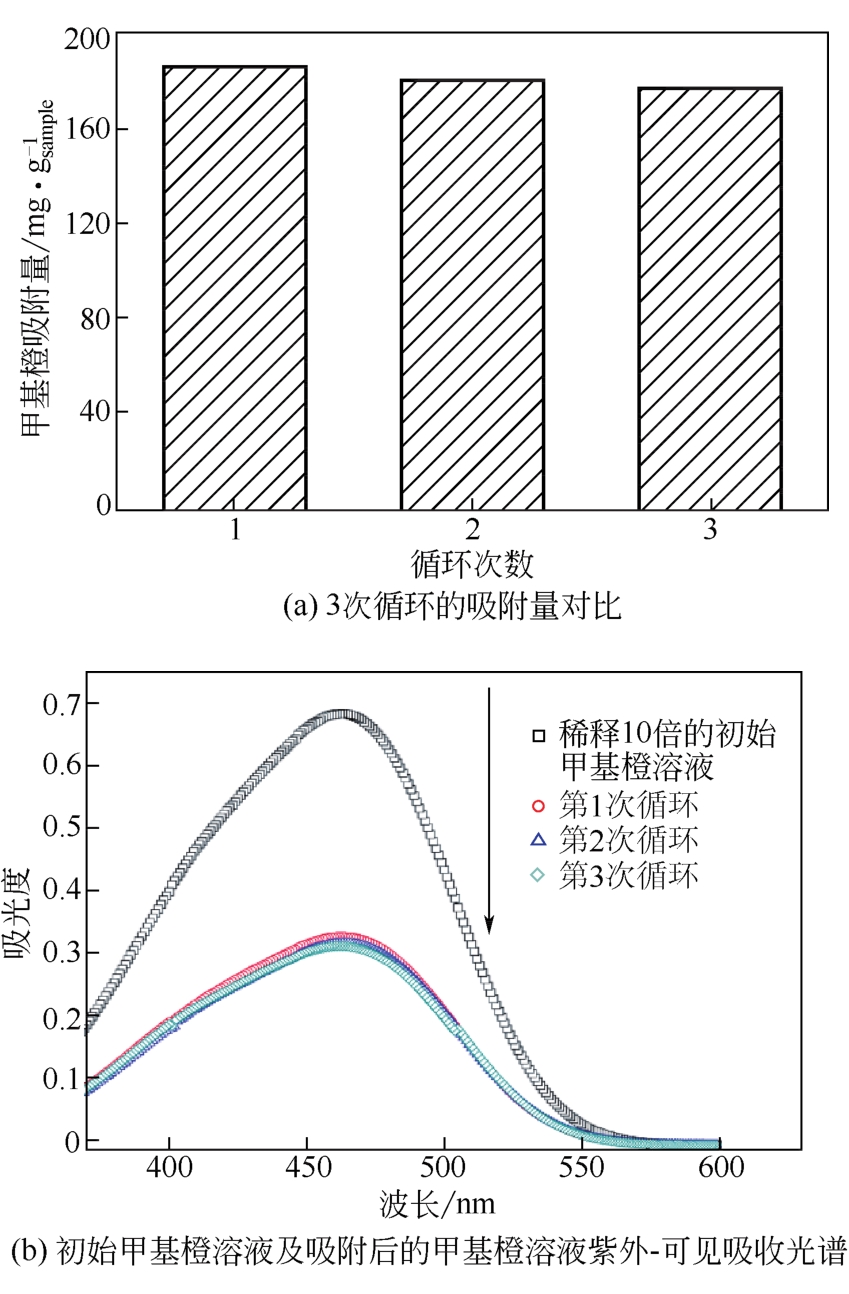
| Co3O4/TiO2/多孔炭 | 273 | 50mg吸附剂于100mL MO水溶液(30~300mg·L-1);吸附时间30min;吸附温度室温 | 本工作 |
| 吸附剂 | 甲基橙的平衡吸附量qe/mg·g-1sample | 吸附条件 | 参考文献 |
|---|---|---|---|
| Ni/多孔炭/碳纳米管 | 271 | 5mg吸附剂于10mL MO水溶液(20~350mg·L-1);吸附时间60min;吸附温度室温 | [ |
| (Fe2O3,Fe3C,Fe)@多孔炭 | 183 | 10mg吸附剂于20mL MO水溶液(12.5~400mg·L-1);吸附时间90min;吸附温度室温 | [ |
| ZnFe2O4/木质素衍生炭 | 113 | 20mg吸附剂于20mL MO水溶液(20~180mg·L-1);吸附时间160min;吸附温度293K;pH=5 | [ |
| Fe负载多孔炭 | 187 | 20mg吸附剂于50mL MO水溶液(10~100mg·L-1);吸附时间60min;吸附温度室温;pH=7 | [ |
| Fe2O3/Mn3O4 | 333 | 200mg吸附剂于200mL MO水溶液(60~1000mg·L-1);吸附时间45min;吸附温度室温;pH=2 | [ |
| SiO2@Fe3O4 | 240 | 5mg吸附剂于10mL MO水溶液(0~100mg·L-1);吸附时间180min;吸附温度293K;pH=5.5 | [ |
| NiFe2O4/活性炭 | 183 | 300mg吸附剂于100mL MO水溶液(0~200mg·L-1);吸附时间30min;吸附温度303K;pH=3 | [ |
| Fe3O4/季铵壳聚糖衍生物 | 267 | 100mg吸附剂于100mL MO水溶液(50~300mg·L-1);吸附时间60min;吸附温度293K | [ |
| Fe3O4/PPY | 149 | 10mg吸附剂于10mL MO水溶液(60~320mg·L-1);吸附时间90min;吸附温度298K | [ |
| LnBDC/活性炭 | 125 | 5mg吸附剂于10mL MO水溶液(0~200mg·L-1);吸附时间1440min;吸附温度303K;pH=5 | [ |
| Co3O4/TiO2/多孔炭 | 273 | 50mg吸附剂于100mL MO水溶液(30~300mg·L-1);吸附时间30min;吸附温度室温 | 本工作 |
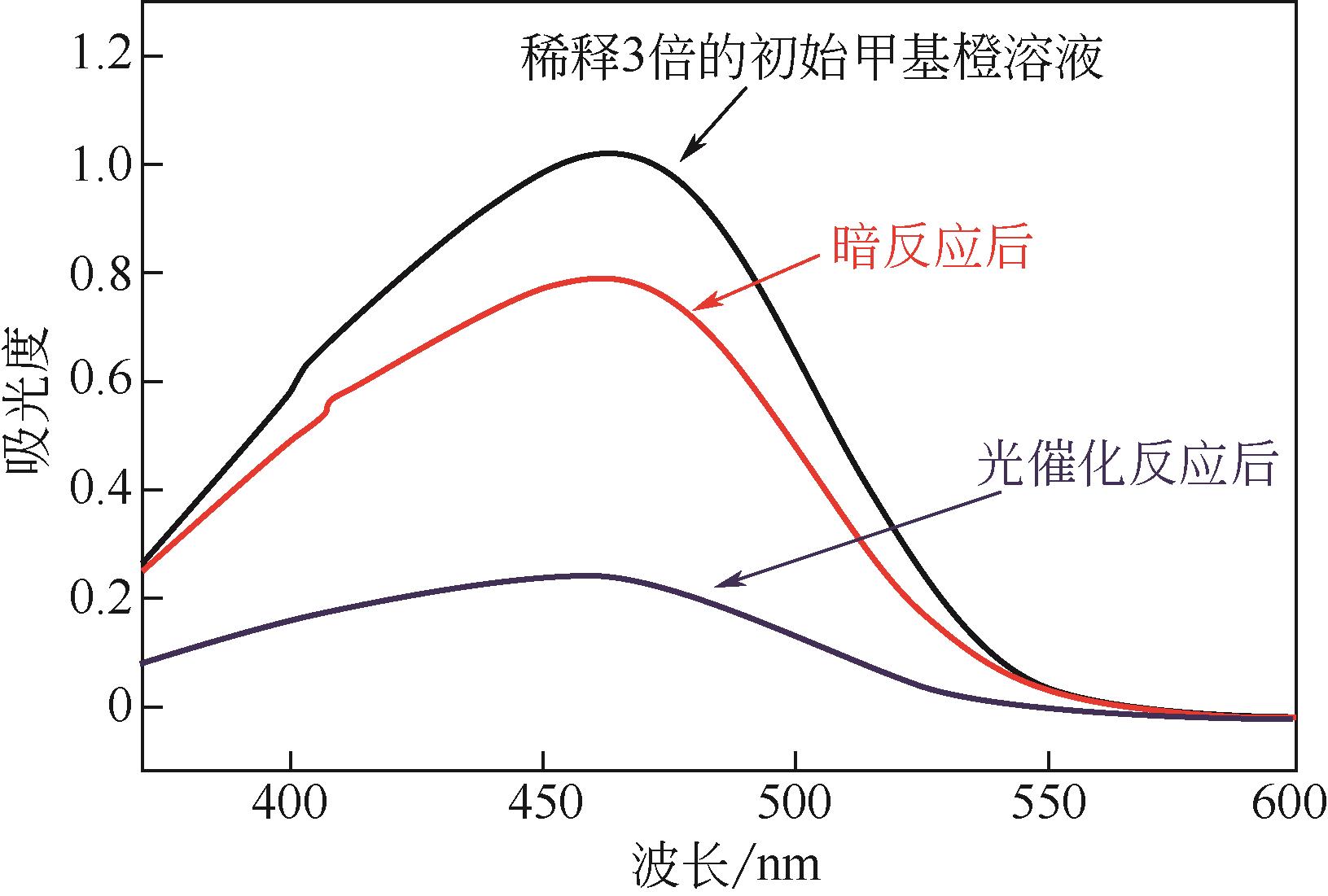
| 样品 | 降解率/% |
|---|---|
| C80 | 70 |
| C120 | 68 |
| C160 | 72 |
| C200 | 61 |
| C240 | 58 |
| 样品 | 降解率/% |
|---|---|
| C80 | 70 |
| C120 | 68 |
| C160 | 72 |
| C200 | 61 |
| C240 | 58 |
| 1 | KUMAR REDDY D H, LEE S M. Water pollution and treatment technologies[J]. Journal of Environmental & Analytical Toxicology, 2012, 2(5): 1000e103. |
| 2 | NIU L J, WEI T, LI Q G, et al. Ce-based catalysts used in advanced oxidation processes for organic wastewater treatment: a review[J]. Journal of Environmental Sciences, 2020, 96: 109-116. |
| 3 | DONG L J, WU S Y, LI S B, et al. Sorption behaviors and mechanisms of Eu(III) on rice straw-derived biochar[J]. Journal of Inorganic Materials, 2020, 35(3): 390-398.. |
| 4 | HAN H W, RAFIQ M K, ZHOU T Y, et al. A critical review of clay-based composites with enhanced adsorption performance for metal and organic pollutants[J]. Journal of Hazardous Materials, 2019, 369: 780-796. |
| 5 | OUKIL S, BALI F, HALLICHE D. Adsorption and kinetic studies of methylene blue on modified HUSY zeolite and an amorphous mixture of γ-alumina and silica[J]. Separation Science and Technology, 2020, 55(15): 2642-2658. |
| 6 | ROJAS S, HORCAJADA P. Metal-organic frameworks for the removal of emerging organic contaminants in water[J]. Chemical Reviews, 2020, 120(16): 8378-8415. |
| 7 | BŮŽEK D, ONDRUŠOVÁ S, HYNEK J, et al. Robust aluminum and iron phosphinate metal-organic frameworks for efficient removal of bisphenol A[J]. Inorganic Chemistry, 2020, 59(8): 5538-5545. |
| 8 | LIN K Y A, CHANG H A, CHEN R C. MOF-derived magnetic carbonaceous nanocomposite as a heterogeneous catalyst to activate oxone for decolorization of Rhodamine B in water[J]. Chemosphere, 2015, 130: 66-72. |
| 9 | ZHANG S Q, HAN L, LI L N, et al. A highly symmetric metal-organic framework based on a propeller-like Ru-organic metalloligand for photocatalysis and explosives detection[J]. Crystal Growth & Design, 2013, 13(12): 5466-5472. |
| 10 | ANI I J, AKPAN U G, OLUTOYE M A, et al. Photocatalytic degradation of pollutants in petroleum refinery wastewater by TiO2- and ZnO-based photocatalysts: recent development[J]. Journal of Cleaner Production, 2018, 205: 930-954. |
| 11 | LI Y F, ZHOU M H, CHENG B, et al. Recent advances in g-C3N4-based heterojunction photocatalysts[J]. Journal of Materials Science & Technology, 2020, 56: 1-17. |
| 12 | KAMEGAWA T, KUWAHARA Y, YAMASHITA H. Design of TiO2-loaded porous siliceous materials and application to photocatalytic environmental purification[J]. Journal of the Japan Petroleum Institute, 2016, 59(5): 165-173. |
| 13 | ZHANG D S, CAI H, GAO K Y, et al. Preparation and visible-light photocatalytic degradation on metronidazole of Zn2SiO4-ZnO-biochar composites[J]. Journal of Inorganic Materials, 2020, 35(8): 923-930. |
| 14 | ESRAFILI L, MORSALI A, DEHGHANI F F, et al. Development of porous cobalt-/copper-doped carbon nanohybrids derived from functionalized MOFs as efficient catalysts for the ullmann cross-coupling reaction: insights into the active centers[J]. ACS Applied Materials & Interfaces, 2020, 12(38): 43115-43124. |
| 15 | SUN T T, XU L B, WANG D S, et al. Metal organic frameworks derived single atom catalysts for electrocatalytic energy conversion[J]. Nano Research, 2019, 12(9): 2067-2080. |
| 16 | RASHEED T, HASSAN A A, BILAL M, et al. Metal-organic frameworks based adsorbents: a review from removal perspective of various environmental contaminants from wastewater[J]. Chemosphere, 2020, 259: 127369. |
| 17 | FU Y, SUN D, CHEN Y, et al. An amine-functionalized titanium metal-organic framework photocatalyst with visible-light-induced activity for CO2 reduction[J]. Angewandte Chemie:International Edition, 2012, 51(14): 3364-3367. |
| 18 | LIU D S, LI M N, LI X C, et al. Core-shell Zn/Co MOFs derived Co3O4/CNTs as an efficient magnetic heterogeneous catalyst for persulfate activation and oxytetracycline degradation[J]. Chemical Engineering Journal, 2020, 387: 124008. |
| 19 | NIE R F, SHI J J, DU W C, et al. A sandwich N-doped graphene/Co3O4 hybrid: an efficient catalyst for selective oxidation of olefins and alcohols[J]. Journal of Materials Chemistry A, 2013, 1(32): 9037-9045. |
| 20 | DAN-HARDI M, SERRE C, FROT T, et al. A new photoactive crystalline highly porous titanium(Ⅳ) dicarboxylate[J]. Journal of the American Chemical Society, 2009, 131(31): 10857-10859. |
| 21 | ZHU Q L, XIA W, AKITA T, et al. Metal-organic framework-derived honeycomb-like open porous nanostructures as precious-metal-free catalysts for highly efficient oxygen electroreduction[J]. Advanced Materials, 2016, 28(30): 6391-6398. |
| 22 | HO Y S, MCKAY G. Pseudo-second order model for sorption processes[J]. Process Biochemistry, 1999, 34(5): 451-465. |
| 23 | JIN L N, ZHAO X S, QIAN X Y, et al. Nickel nanoparticles encapsulated in porous carbon and carbon nanotube hybrids from bimetallic metal-organic-frameworks for highly efficient adsorption of dyes[J]. Journal of Colloid and Interface Science, 2018, 509: 245-253. |
| 24 | WANG L H, KE F, ZHU J F. Metal-organic gel templated synthesis of magnetic porous carbon for highly efficient removal of organic dyes[J]. Dalton Transactions, 2016, 45(11): 4541-4547. |
| 25 | MA Y Z, ZHENG D F, MO Z Y, et al. Magnetic lignin-based carbon nanoparticles and the adsorption for removal of methyl orange[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2018, 559: 226-234. |
| 26 | JIANG T S, FANG W B, ZHAO Q, et al. Synthesis of Fe (Co or Ni) loaded mesoporous carbon composites and their adsorption behaviors for methyl orange[J]. Journal of Nanoscience and Nanotechnology, 2017, 17(8): 5261-5270. |
| 27 | BHOWMIK M, DEB K, DEBNATH A, et al. Mixed phase Fe2O3 /Mn3O4 magnetic nanocomposite for enhanced adsorption of methyl orange dye: neural network modeling and response surface methodology optimization[J]. Applied Organometallic Chemistry, 2018, 32(3): e4186. DOI:10.1002/aoc.4186. |
| 28 | GALLO-CORDOVA A, LEMUS J, PALOMARES F J, et al. Superparamagnetic nanosorbent for water purification: assessment of the adsorptive removal of lead and methyl orange from aqueous solutions[J]. Science of the Total Environment, 2020, 711: 134644. |
| 29 | JIANG T, LIANG Y D, HE Y J, et al. Activated carbon/NiFe2O4 magnetic composite: a magnetic adsorbent for the adsorption of methyl orange[J]. Journal of Environmental Chemical Engineering, 2015, 3(3): 1740-1751. |
| 30 | ZHAO B, SUN X J, WANG L, et al. Adsorption of methyl orange from aqueous solution by composite magnetic microspheres of chitosan and quaternary ammonium chitosan derivative[J]. Chinese Journal of Chemical Engineering, 2019, 27(8): 1973-1980. |
| 31 | ZHANG M M, YU Z H, YU H C. Adsorption of Eosin Y, methyl orange and brilliant green from aqueous solution using ferroferric oxide/polypyrrole magnetic composite[J]. Polymer Bulletin, 2020, 77(2): 1049-1066. |
| 32 | SANTOS G D C, BARROS A L, DE OLIVEIRA C A F, et al. New composites LnBDC@AC and CB[6]@AC: from design toward selective adsorption of methylene blue or methyl orange[J]. PLoS One, 2017, 12(1): e0170026. |
| 33 | SIYASUKH A, CHIMUPALA Y, TONANON N. Preparation of magnetic hierarchical porous carbon spheres with graphitic features for high methyl orange adsorption capacity[J]. Carbon, 2018, 134: 207-221. |
| 34 | ZHAO X R, CAO Y Q, CHEN J, et al. Photocatalytic properties of Co3O4-coated TiO2 powders prepared by plasma-enhanced atomic layer deposition[J]. Nanoscale Research Letters, 2017, 12(1): 1-9. |
| 35 | SAEED M, USMAN M, IBRAHIM M, et al. Enhanced photo catalytic degradation of methyl orange using p-n Co3O4-TiO2 hetero-junction as catalyst[J]. International Journal of Chemical Reactor Engineering, 2020, 18(5/6). DOI: 10.1515/ijcre-2020-0004. |
| 36 | 张金龙,陈锋,田宝柱, 等. 光催化[M]. 2版. 上海: 华东理工大学出版社, 2015: 3-4. |
| ZHANG Jinlong, CHEN Feng, TIAN Baozhu, et al. Photocatalysis[M]. 2rd edition. Shanghai: East China University of Science and Technology Press, 2015: 3-4. | |
| 37 | HEIDARI-ASIL S A, ZINATLOO-AJABSHIR S, AMIRI O, et al. Amino acid assisted-synthesis and characterization of magnetically retrievable ZnCo2O4-Co3O4 nanostructures as high activity visible-light-driven photocatalyst[J]. International Journal of Hydrogen Energy, 2020, 45(43): 22761-22774. |
| [1] | MA Yi, CAO Shiwei, WANG Jiajun, LIN Liqun, XING Yan, CAO Tengliang, LU Feng, ZHAO Zhenlun, ZHANG Zhijun. Research progress in recovery of spent cathode materials for lithium-ion batteries using deep eutectic solvents [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 219-232. |
| [2] | WANG Shengyan, DENG Shuai, ZHAO Ruikai. Research progress on carbon dioxide capture technology based on electric swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 233-245. |
| [3] | ZHANG Mingyan, LIU Yan, ZHANG Xueting, LIU Yake, LI Congju, ZHANG Xiuling. Research progress of non-noble metal bifunctional catalysts in zinc-air batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 276-286. |
| [4] | HU Xi, WANG Mingshan, LI Enzhi, HUANG Siming, CHEN Junchen, GUO Bingshu, YU Bo, MA Zhiyuan, LI Xing. Research progress on preparation and sodium storage properties of tungsten disulfide composites [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 344-355. |
| [5] | CUI Shoucheng, XU Hongbo, PENG Nan. Simulation analysis of two MOFs materials for O2/He adsorption separation [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 382-390. |
| [6] | CHEN Chongming, CHEN Qiu, GONG Yunqian, CHE Kai, YU Jinxing, SUN Nannan. Research progresses on zeolite-based CO2 adsorbents [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 411-419. |
| [7] | XU Chunshu, YAO Qingda, LIANG Yongxian, ZHOU Hualong. Research progress on functionalization strategies of covalent organic frame materials and its adsorption properties for Hg(Ⅱ) and Cr(Ⅵ) [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 461-478. |
| [8] | GU Yongzheng, ZHANG Yongsheng. Dynamic behavior and kinetic model of Hg0 adsorption by HBr-modified fly ash [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 498-509. |
| [9] | GUO Qiang, ZHAO Wenkai, XIAO Yonghou. Numerical simulation of enhancing fluid perturbation to improve separation of dimethyl sulfide/nitrogen via pressure swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 64-72. |
| [10] | GE Quanqian, XU Mai, LIANG Xian, WANG Fengwu. Research progress on the application of MOFs in photoelectrocatalysis [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4692-4705. |
| [11] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [12] | YANG Ying, HOU Haojie, HUANG Rui, CUI Yu, WANG Bing, LIU Jian, BAO Weiren, CHANG Liping, WANG Jiancheng, HAN Lina. Coal tar phenol-based carbon nanosphere prepared by Stöber method for adsorption of CO2 [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 5011-5018. |
| [13] | ZHANG Zhen, LI Dan, CHEN Chen, WU Jinglan, YING Hanjie, QIAO Hao. Separation and purification of salivary acids with adsorption resin [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4153-4158. |
| [14] | WANG Baoying, WANG Huangying, YAN Junying, WANG Yaoming, XU Tongwen. Research progress of polymer inclusion membrane in metal separation and recovery [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3990-4004. |
| [15] | LYU Jie, HUANG Chong, FENG Ziping, HU Yafei, SONG Wenji. Performance and control system of gas engine heat pump based on waste heat recovery [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4182-4192. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||