Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (10): 5956-5974.DOI: 10.16085/j.issn.1000-6613.2024-1408
• Resources and environmental engineering • Previous Articles
Recent progress on synthesis strategies of covalent organic framework materials and their adsorption application for heavy metal ions
HE Bianyan( ), WANG Yubing, LI Shanshan(
), WANG Yubing, LI Shanshan( ), YAN Wei(
), YAN Wei( )
)
- Xi’an Key Laboratory of Solid Waste Recycling and Resource Recovery, Department of Environmental Science and Engineering, Xi’an Jiaotong University, Xi’an 710049, Shaanxi, China
-
Received:2024-08-28Revised:2024-10-14Online:2025-11-10Published:2025-10-25 -
Contact:LI Shanshan, YAN Wei
共价有机框架材料的合成策略及其在重金属离子吸附中的研究进展
- 西安市固体废物资源再生与循环利用重点实验室,西安交通大学环境科学与工程系,陕西 西安 710049
-
通讯作者:李珊珊,延卫 -
作者简介:何边燕(2000—),女,博士研究生,研究方向为环境吸附材料设计。E-mail:hby3626@stu.xjtu.edu.cn。 -
基金资助:国家自然科学基金(51978569)
CLC Number:
Cite this article
HE Bianyan, WANG Yubing, LI Shanshan, YAN Wei. Recent progress on synthesis strategies of covalent organic framework materials and their adsorption application for heavy metal ions[J]. Chemical Industry and Engineering Progress, 2025, 44(10): 5956-5974.
何边燕, 王玉冰, 李珊珊, 延卫. 共价有机框架材料的合成策略及其在重金属离子吸附中的研究进展[J]. 化工进展, 2025, 44(10): 5956-5974.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-1408
| 制备方法 | 优势 | 劣势 |
|---|---|---|
| 溶剂热法合成 | 结晶度、孔隙率和形貌控制良好 | 反应时间长,反应速度慢 |
| 离子热法合成 | 热稳定性高 | 反应温度高,结晶度差,特殊反应溶剂 |
| 机械化学合成 | 操作方便,生产成本低,生态友好 | 结晶度和孔隙率有限,重现性差 |
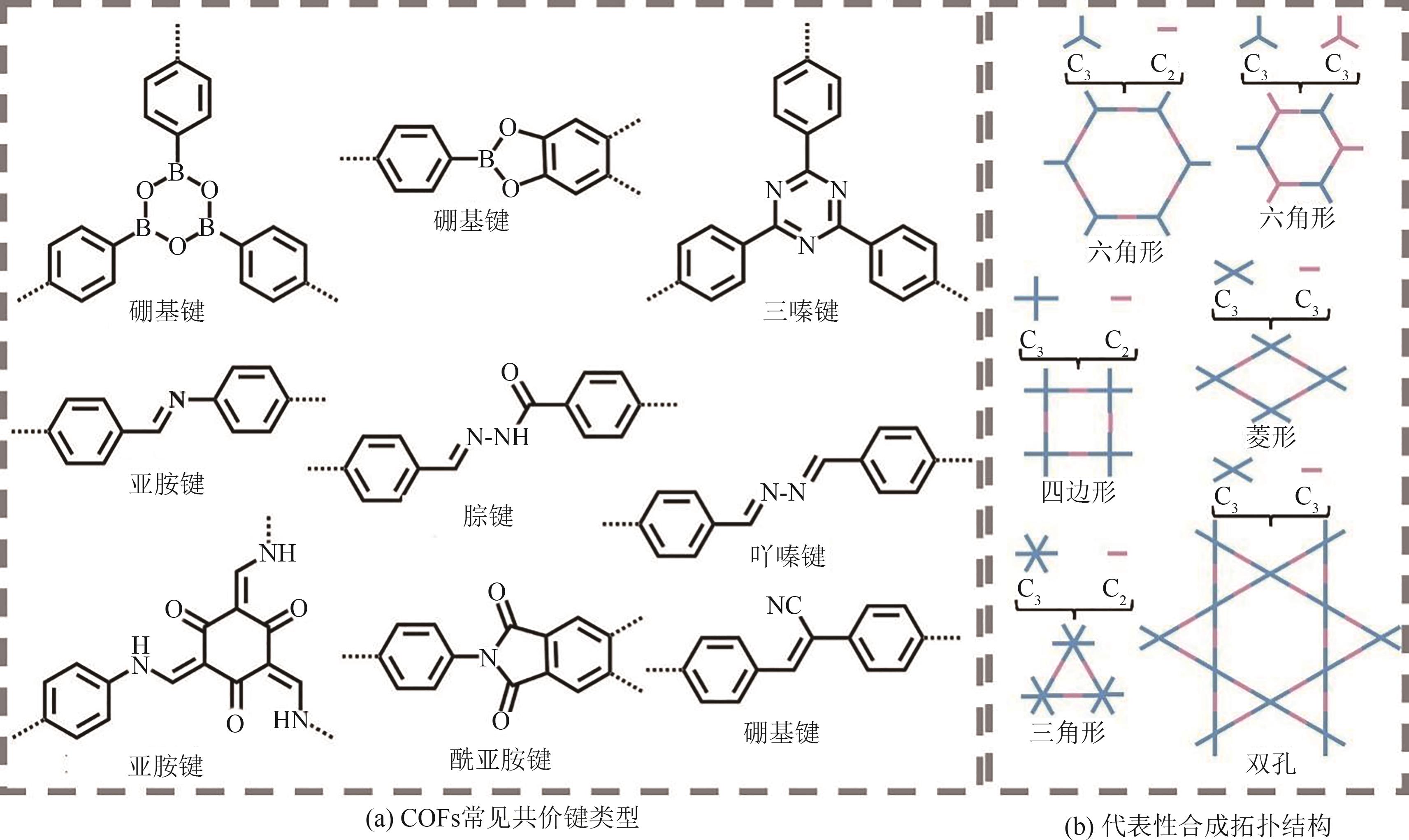
| 微波辅助合成 | 反应时间短,产率高 | 密封容器 |
| 声化学合成 | 产率高,操作方便 | 结构多样,纯度低 |
| 界面合成 | 二维薄膜产物 | 结晶度差 |
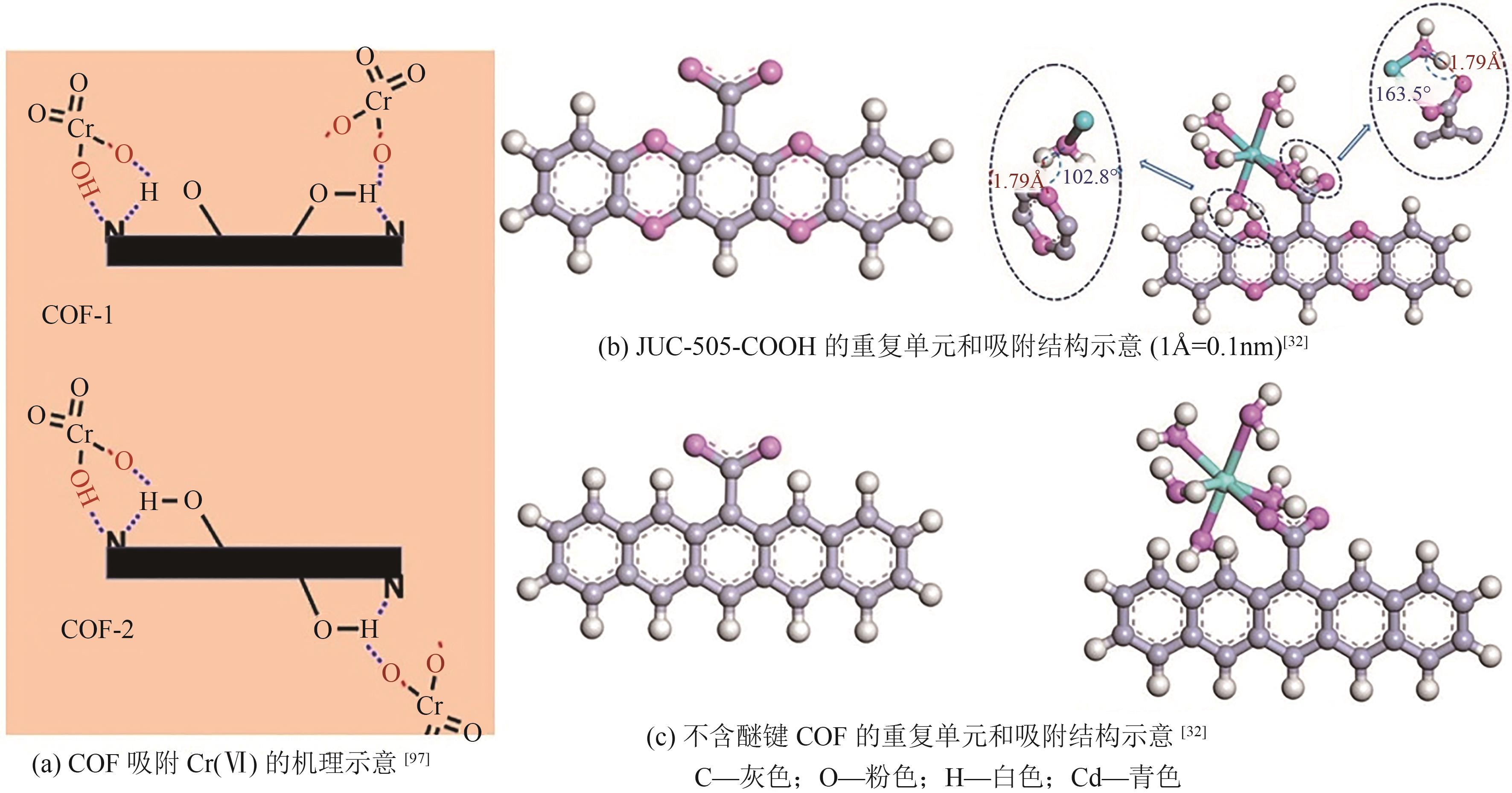
| 制备方法 | 优势 | 劣势 |
|---|---|---|
| 溶剂热法合成 | 结晶度、孔隙率和形貌控制良好 | 反应时间长,反应速度慢 |
| 离子热法合成 | 热稳定性高 | 反应温度高,结晶度差,特殊反应溶剂 |
| 机械化学合成 | 操作方便,生产成本低,生态友好 | 结晶度和孔隙率有限,重现性差 |
| 微波辅助合成 | 反应时间短,产率高 | 密封容器 |
| 声化学合成 | 产率高,操作方便 | 结构多样,纯度低 |
| 界面合成 | 二维薄膜产物 | 结晶度差 |
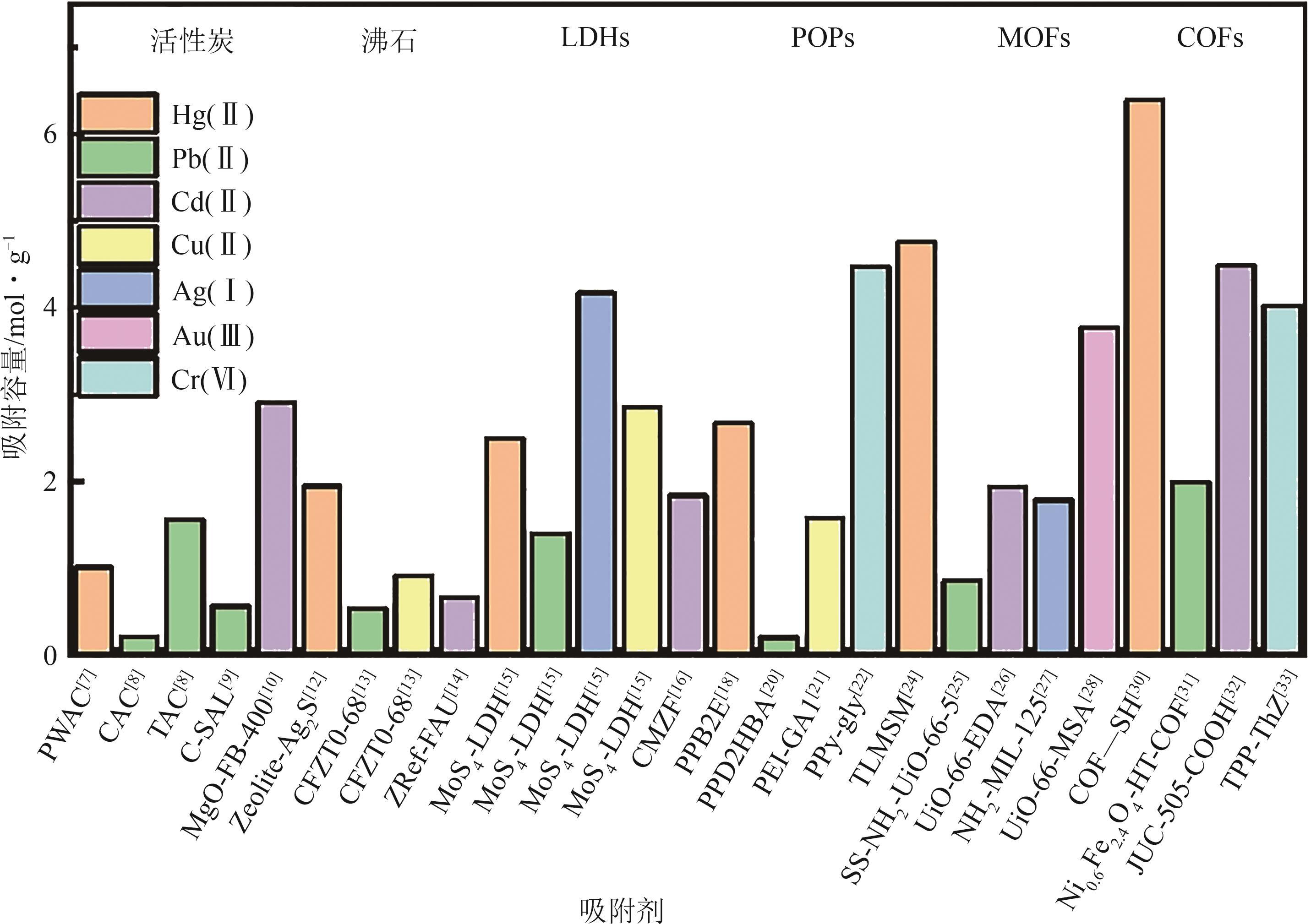
| 目标污染物 | 反应类型 | 吸附剂 | C∶N∶O∶S(摩尔比) | 比表面积/ m2·g-1 | 吸附容量/ mg·g-1 | 参考文献 |
|---|---|---|---|---|---|---|
| Hg(Ⅱ) | Schiff 碱 | 1∶0.07∶0∶0 | 282.2 | <40 | [ | |
| Hg(Ⅱ) | Schiff 碱 | COF-1 | 1∶0.17∶0.17∶0 | 417.4 | 53.1 | [ |
| Hg(Ⅱ) | Schiff 碱 | COF-V | 1∶0.07∶0∶0 | 1152 | 147 | [ |
| Pb(Ⅱ) | Schiff 碱 | 1∶0.07∶0∶0 | 282.2 | <40 | [ | |
| Pb(Ⅱ) | Schiff 碱 | TAVA | 1∶0.15∶0∶0 | 1046.59 | 105 | [ |
| Pb(Ⅱ) | 1∶0.1∶0.2∶0 | 189.824 | 133.92 | [ | ||
| Cd(Ⅱ) | 1∶0.25∶0∶0 | 490 | 29.2 | [ | ||
| Cd(Ⅱ) | Schiff 碱 | 1∶0.11∶0.11∶0 | 885 | 29.6 | [ | |
| Cu(Ⅱ) | Schiff 碱 | TFBODH | 1∶0.5∶0.25∶0 | 94 | 23 | [ |
| Cu(Ⅱ) | Schiff 碱 | TpTc | 1∶0.43∶0.29∶0.14 | 63.5 | 73.5 | [ |
| 目标污染物 | 反应类型 | 吸附剂 | C∶N∶O∶S(摩尔比) | 比表面积/ m2·g-1 | 吸附容量/ mg·g-1 | 参考文献 |
|---|---|---|---|---|---|---|
| Hg(Ⅱ) | Schiff 碱 | 1∶0.07∶0∶0 | 282.2 | <40 | [ | |
| Hg(Ⅱ) | Schiff 碱 | COF-1 | 1∶0.17∶0.17∶0 | 417.4 | 53.1 | [ |
| Hg(Ⅱ) | Schiff 碱 | COF-V | 1∶0.07∶0∶0 | 1152 | 147 | [ |
| Pb(Ⅱ) | Schiff 碱 | 1∶0.07∶0∶0 | 282.2 | <40 | [ | |
| Pb(Ⅱ) | Schiff 碱 | TAVA | 1∶0.15∶0∶0 | 1046.59 | 105 | [ |
| Pb(Ⅱ) | 1∶0.1∶0.2∶0 | 189.824 | 133.92 | [ | ||
| Cd(Ⅱ) | 1∶0.25∶0∶0 | 490 | 29.2 | [ | ||
| Cd(Ⅱ) | Schiff 碱 | 1∶0.11∶0.11∶0 | 885 | 29.6 | [ | |
| Cu(Ⅱ) | Schiff 碱 | TFBODH | 1∶0.5∶0.25∶0 | 94 | 23 | [ |
| Cu(Ⅱ) | Schiff 碱 | TpTc | 1∶0.43∶0.29∶0.14 | 63.5 | 73.5 | [ |
| 目标污染物 | 官能化原子 | 反应类型 | 吸附剂 | BET比表面积/m2·g-1 | 吸附容量/mg·g-1 | 等温线 | 参考文献 |
|---|---|---|---|---|---|---|---|
| Hg(Ⅱ) | N | Knoevenagel | Bpy-sp2c-COF | 567 | 718.48 | Langmuir | [ |
| Hg(Ⅱ) | N | Schiff base | Pyridine-COF | 348.5 | 719.4 | Langmuir | [ |
| Hg(Ⅱ) | N | Schiff base | Tpy-COF-2 | 1220 | 295 | Langmuir | [ |
| Hg(Ⅱ) | N | Schiff base | Tpy-COF-1 | 137 | 420 | Langmuir | [ |
| Hg(Ⅱ) | S | Schiff base | TABB-BMTTPA-COF | 1934 | 734 | Langmuir | [ |
| Hg(Ⅱ) | N | Friedel-Crafts | TPA-TPC (3/1) | 148 | 270 | Langmuir | [ |
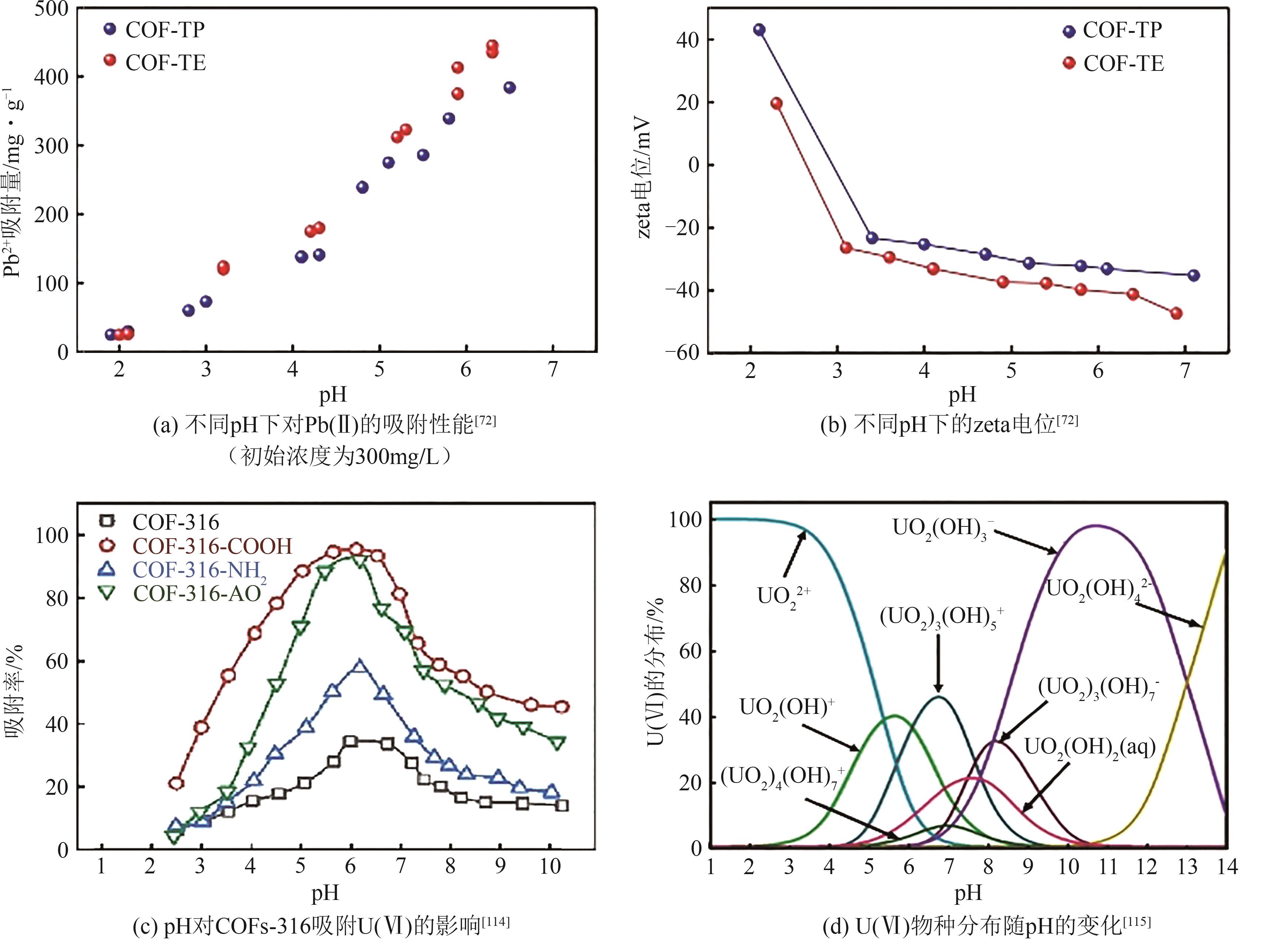
| Hg(Ⅱ) | N | Friedel-Crafts | TPA-TPC-8MA | 656 | 781 | Freundlich | [ |
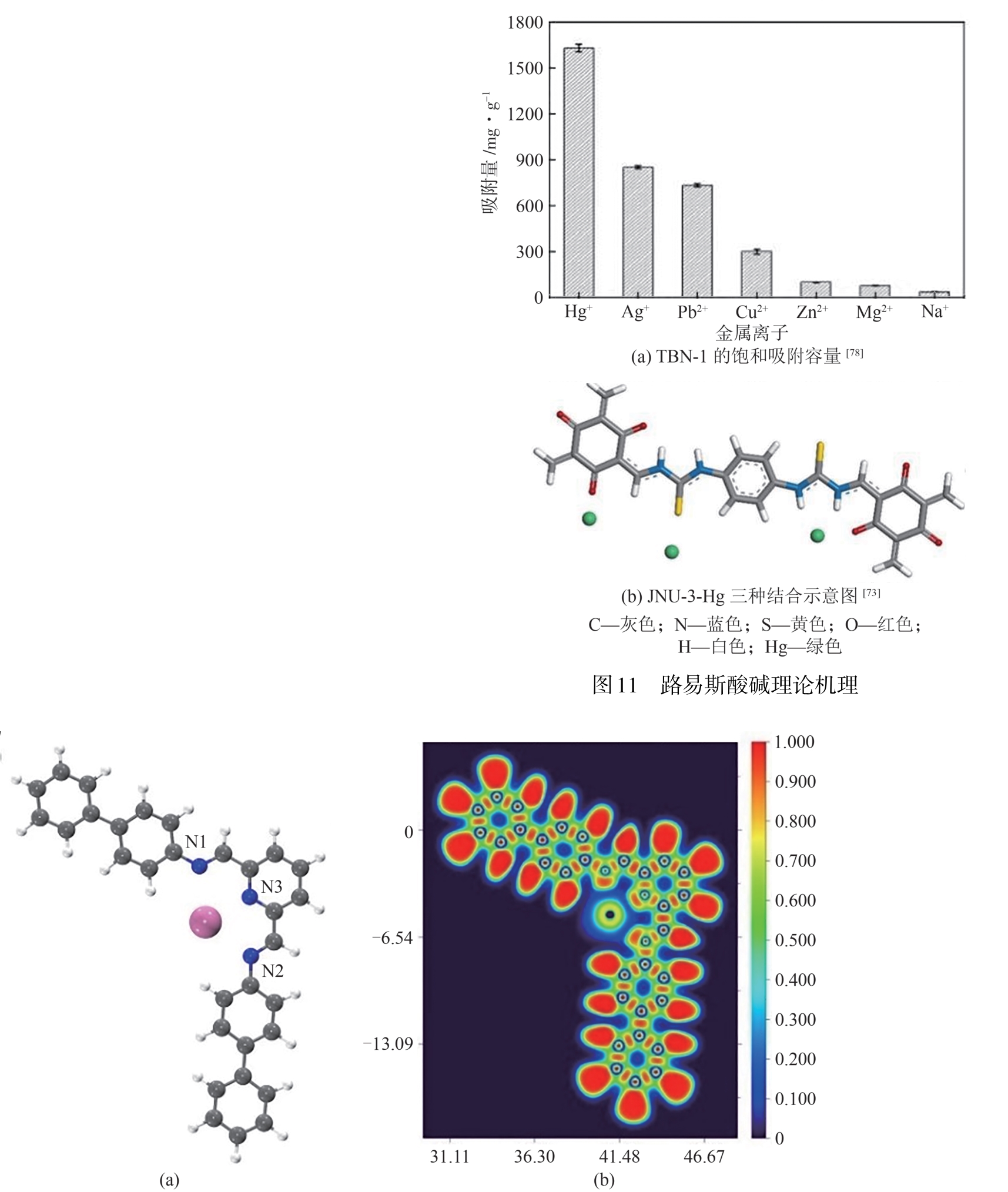
| Hg(Ⅱ) | S | Schiff base | JNU-3 | 420 | 959 | Langmuir | [ |
| Hg(Ⅱ) | Schiff base | COF-1 | 417.4 | 53.1 | Langmuir | [ | |
| Hg(Ⅱ) | S | Schiff base | COF-SH | 235.0 | 1282.6 | Langmuir | [ |
| Hg(Ⅱ) | Schiff base | COF-V | 1152 | 147 | Langmuir | [ | |
| Hg(Ⅱ) | S | Schiff base | COF-S-SH | 546 | 1350 | Langmuir | [ |
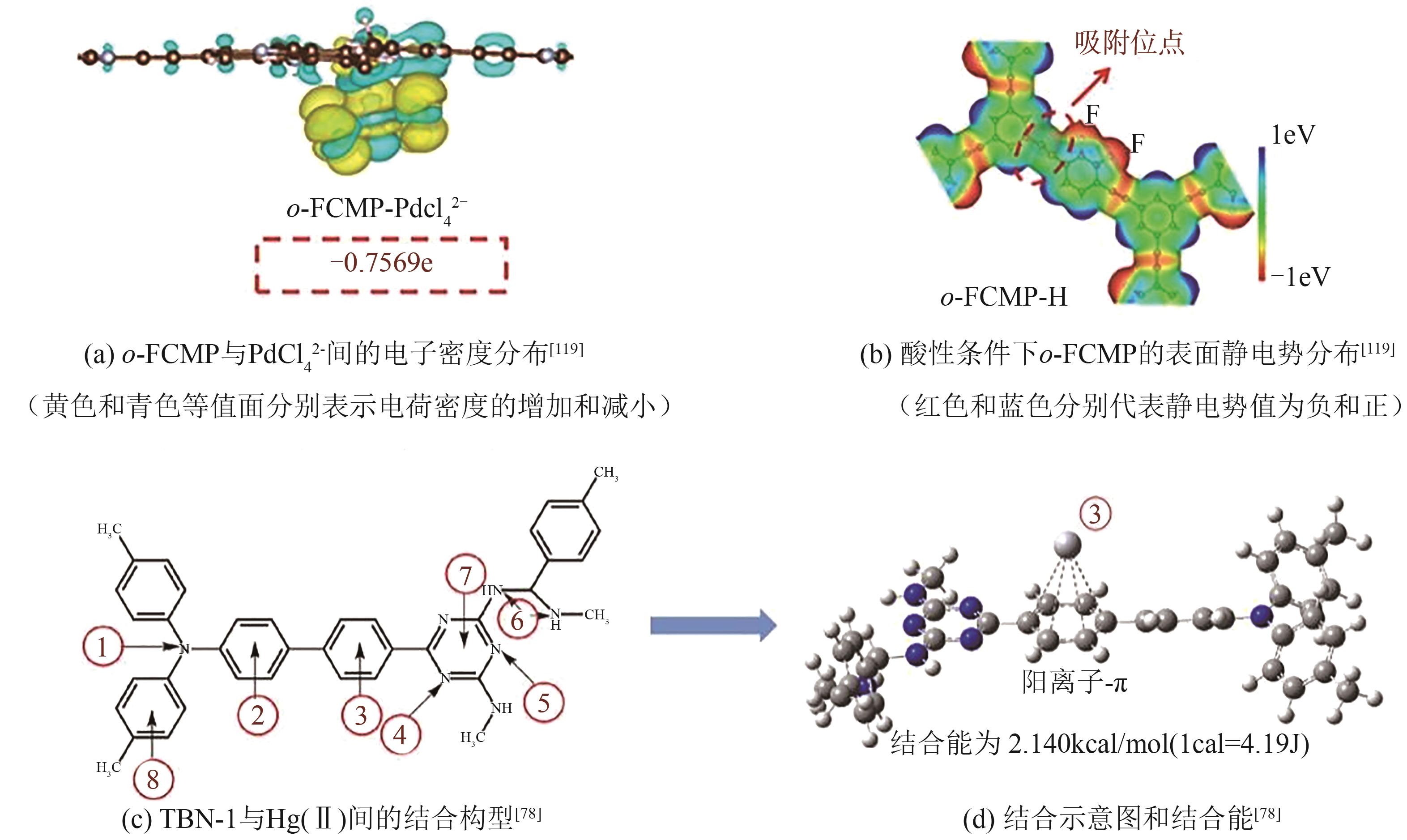
| Hg(Ⅱ) | N | Schiff base | TBN-1 | 1270.10 | 1630 | Langmuir | [ |
| Hg(Ⅱ) | N、O | Schiff base | TpODH | 835 | 1692 | Langmuir | [ |
| Hg(Ⅱ) | N、S | Schiff base | TPB-DMTP-COF-SH | 291 | 4395 | Langmuir | [ |
| Pb(Ⅱ) | Schiff base | PDA-TFPT-COF | 533.19 | 77.7 | Langmuir | [ | |
| Pb(Ⅱ) | O | Schiff base | DDA-TFPT-COF | 948.04 | 128 | Langmuir | [ |
| Pb(Ⅱ) | N、O | Schotten-Baumann | COF-TP | 11.4 | 140.0 | Freundlich | [ |
| Pb(Ⅱ) | N、O | Schotten-Baumann | COF-TE | 3.5 | 185.7 | Freundlich | [ |
| Pb(Ⅱ) | N | Schiff base | Tpy-COF-1 | 137 | 167 | Langmuir | [ |
| Pb(Ⅱ) | N | Schiff base | Tpy-COF-2 | 1220 | 200 | Langmuir | [ |
| Pb(Ⅱ) | Schiff base | COF-V | 167.3 | 38.24 | Freundlich | [ | |
| Pb(Ⅱ) | S | Schiff base | COF-SH | 40.4 | 239 | Freundlich | [ |
| Pb(Ⅱ) | Schiff base | TAVA | 1046.59 | 105 | Langmuir | [ | |
| Pb(Ⅱ) | S | Schiff base | TAVA-S-Et-SH | 145.70 | 303 | Langmuir | [ |
| Pb(Ⅱ) | SNAr | JUC-500 | 189.824 | 133.92 | Langmuir | [ | |
| Pb(Ⅱ) | N、O | SNAr | COF-NHOH | 37.665 | 368.68 | Langmuir | [ |
| Pb(Ⅱ) | O | SNAr | JUC-505-COOH | 380 | 559 | Langmuir | [ |
| Pb(Ⅱ) | N | Schiff base | TBN-1 | 1270.10 | 730 | Langmuir | [ |
| Cd(Ⅱ) | O | Schiff base | COF-ETTA-2,3-DHA | 1476.5 | 116 | — | [ |
| Cd(Ⅱ) | Schiff base | TpBD COF | 885 | 29.6 | Langmuir | [ | |
| Cd(Ⅱ) | O | Schiff base | TpBD-COOH COF | 339 | 150 | Langmuir | [ |
| Cd(Ⅱ) | N | Schiff base | N-riched COF | 1935 | 396.76 | Langmuir | [ |
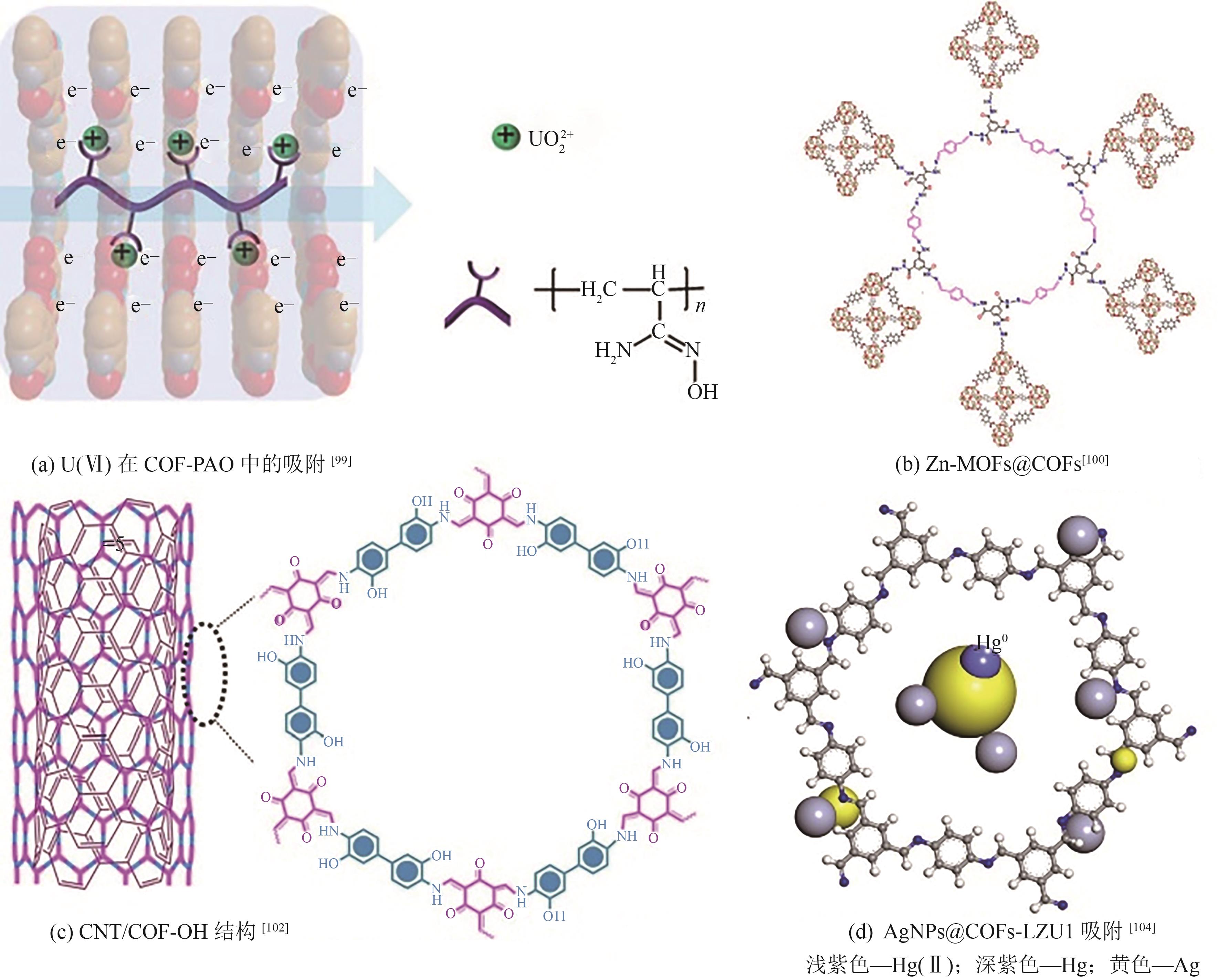
| Cd(Ⅱ) | O | SNAr | JUC-505-COOH | 380 | 504 | Langmuir | [ |
| Cu(Ⅱ) | Schiff base | TFBODH | 94 | 23 | Langmuir | [ | |
| Cu(Ⅱ) | O | Schiff base | TpODH | 835 | 324 | Langmuir | [ |
| Cr(Ⅵ) | Schiff base | COF-BTA-BZ | 1048 | 64 | — | [ | |
| Cr(Ⅵ) | O | Schiff base | COF-BTA-DHBZ | 816 | 384 | — | [ |
| Cr(Ⅵ) | O | Schiff base | COF-1 | 28.79 | 462.96 | Langmuir | [ |
| Cr(Ⅵ) | O | Schiff base | COF-2 | 26.40 | 649.35 | Langmuir | [ |
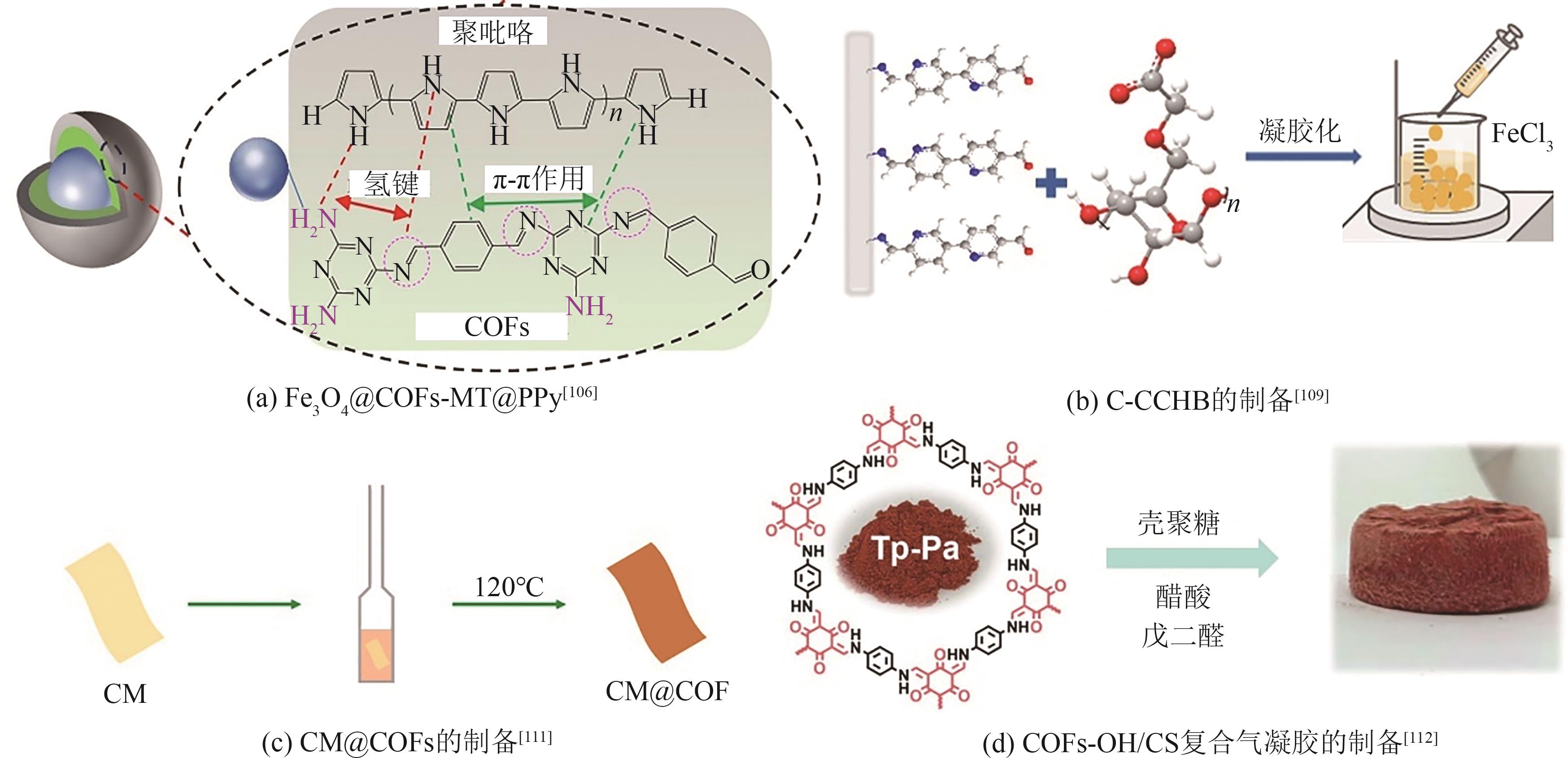
| Au(Ⅲ) | S | Schiff base | TTB-COF | 36 | 560 | — | [ |
| Au(Ⅲ) | O | Scholl | COP-TPC8 | 7.0144 | 943.4 | Langmuir | [ |
| Au(Ⅲ) | O | Scholl | COP-TPC6 | 11.9693 | 1157 | Langmuir | [ |
| Au(Ⅲ) | N | Schiff base | Ionic-COF-Cl | 452.9 | 1270.8 | Langmuir | [ |
| 目标污染物 | 官能化原子 | 反应类型 | 吸附剂 | BET比表面积/m2·g-1 | 吸附容量/mg·g-1 | 等温线 | 参考文献 |
|---|---|---|---|---|---|---|---|
| Hg(Ⅱ) | N | Knoevenagel | Bpy-sp2c-COF | 567 | 718.48 | Langmuir | [ |
| Hg(Ⅱ) | N | Schiff base | Pyridine-COF | 348.5 | 719.4 | Langmuir | [ |
| Hg(Ⅱ) | N | Schiff base | Tpy-COF-2 | 1220 | 295 | Langmuir | [ |
| Hg(Ⅱ) | N | Schiff base | Tpy-COF-1 | 137 | 420 | Langmuir | [ |
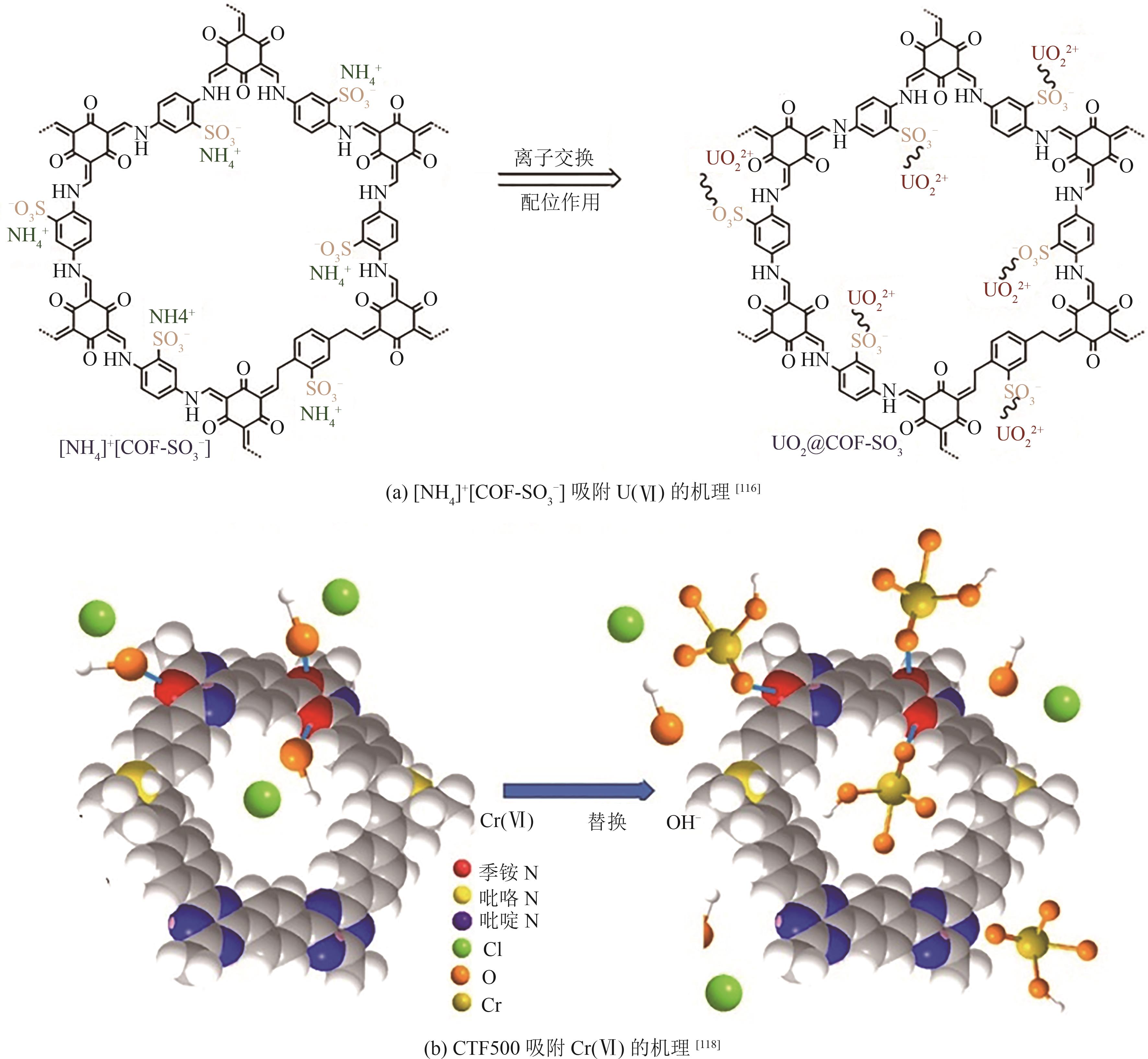
| Hg(Ⅱ) | S | Schiff base | TABB-BMTTPA-COF | 1934 | 734 | Langmuir | [ |
| Hg(Ⅱ) | N | Friedel-Crafts | TPA-TPC (3/1) | 148 | 270 | Langmuir | [ |
| Hg(Ⅱ) | N | Friedel-Crafts | TPA-TPC-8MA | 656 | 781 | Freundlich | [ |
| Hg(Ⅱ) | S | Schiff base | JNU-3 | 420 | 959 | Langmuir | [ |
| Hg(Ⅱ) | Schiff base | COF-1 | 417.4 | 53.1 | Langmuir | [ | |
| Hg(Ⅱ) | S | Schiff base | COF-SH | 235.0 | 1282.6 | Langmuir | [ |
| Hg(Ⅱ) | Schiff base | COF-V | 1152 | 147 | Langmuir | [ | |
| Hg(Ⅱ) | S | Schiff base | COF-S-SH | 546 | 1350 | Langmuir | [ |
| Hg(Ⅱ) | N | Schiff base | TBN-1 | 1270.10 | 1630 | Langmuir | [ |
| Hg(Ⅱ) | N、O | Schiff base | TpODH | 835 | 1692 | Langmuir | [ |
| Hg(Ⅱ) | N、S | Schiff base | TPB-DMTP-COF-SH | 291 | 4395 | Langmuir | [ |
| Pb(Ⅱ) | Schiff base | PDA-TFPT-COF | 533.19 | 77.7 | Langmuir | [ | |
| Pb(Ⅱ) | O | Schiff base | DDA-TFPT-COF | 948.04 | 128 | Langmuir | [ |
| Pb(Ⅱ) | N、O | Schotten-Baumann | COF-TP | 11.4 | 140.0 | Freundlich | [ |
| Pb(Ⅱ) | N、O | Schotten-Baumann | COF-TE | 3.5 | 185.7 | Freundlich | [ |
| Pb(Ⅱ) | N | Schiff base | Tpy-COF-1 | 137 | 167 | Langmuir | [ |
| Pb(Ⅱ) | N | Schiff base | Tpy-COF-2 | 1220 | 200 | Langmuir | [ |
| Pb(Ⅱ) | Schiff base | COF-V | 167.3 | 38.24 | Freundlich | [ | |
| Pb(Ⅱ) | S | Schiff base | COF-SH | 40.4 | 239 | Freundlich | [ |
| Pb(Ⅱ) | Schiff base | TAVA | 1046.59 | 105 | Langmuir | [ | |
| Pb(Ⅱ) | S | Schiff base | TAVA-S-Et-SH | 145.70 | 303 | Langmuir | [ |
| Pb(Ⅱ) | SNAr | JUC-500 | 189.824 | 133.92 | Langmuir | [ | |
| Pb(Ⅱ) | N、O | SNAr | COF-NHOH | 37.665 | 368.68 | Langmuir | [ |
| Pb(Ⅱ) | O | SNAr | JUC-505-COOH | 380 | 559 | Langmuir | [ |
| Pb(Ⅱ) | N | Schiff base | TBN-1 | 1270.10 | 730 | Langmuir | [ |
| Cd(Ⅱ) | O | Schiff base | COF-ETTA-2,3-DHA | 1476.5 | 116 | — | [ |
| Cd(Ⅱ) | Schiff base | TpBD COF | 885 | 29.6 | Langmuir | [ | |
| Cd(Ⅱ) | O | Schiff base | TpBD-COOH COF | 339 | 150 | Langmuir | [ |
| Cd(Ⅱ) | N | Schiff base | N-riched COF | 1935 | 396.76 | Langmuir | [ |
| Cd(Ⅱ) | O | SNAr | JUC-505-COOH | 380 | 504 | Langmuir | [ |
| Cu(Ⅱ) | Schiff base | TFBODH | 94 | 23 | Langmuir | [ | |
| Cu(Ⅱ) | O | Schiff base | TpODH | 835 | 324 | Langmuir | [ |
| Cr(Ⅵ) | Schiff base | COF-BTA-BZ | 1048 | 64 | — | [ | |
| Cr(Ⅵ) | O | Schiff base | COF-BTA-DHBZ | 816 | 384 | — | [ |
| Cr(Ⅵ) | O | Schiff base | COF-1 | 28.79 | 462.96 | Langmuir | [ |
| Cr(Ⅵ) | O | Schiff base | COF-2 | 26.40 | 649.35 | Langmuir | [ |
| Au(Ⅲ) | S | Schiff base | TTB-COF | 36 | 560 | — | [ |
| Au(Ⅲ) | O | Scholl | COP-TPC8 | 7.0144 | 943.4 | Langmuir | [ |
| Au(Ⅲ) | O | Scholl | COP-TPC6 | 11.9693 | 1157 | Langmuir | [ |
| Au(Ⅲ) | N | Schiff base | Ionic-COF-Cl | 452.9 | 1270.8 | Langmuir | [ |
| [1] | LEBRON Yuri Abner Rocha, MOREIRA Victor Rezende, AMARAL Míriam Cristina Santos. Metallic ions recovery from membrane separation processes concentrate: A special look onto ion exchange resins[J]. Chemical Engineering Journal, 2021, 425: 131812. |
| [2] | XIANG Hongrui, MIN Xiaobo, TANG Chongjian, et al. Recent advances in membrane filtration for heavy metal removal from wastewater: A mini review[J]. Journal of Water Process Engineering, 2022, 49: 103023. |
| [3] | YANG Xiong, LIU Lihu, TAN Wenfeng, et al. High-performance Cu2+ adsorption of birnessite using electrochemically controlled redox reactions[J]. Journal of Hazardous Materials, 2018, 354: 107-115. |
| [4] | CHAI Wai Siong, CHEUN Jie Ying, Senthil KUMAR P, et al. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application[J]. Journal of Cleaner Production, 2021, 296: 126589. |
| [5] | QASEM Naef A A, MOHAMMED Ramy H, LAWAL Dahiru U. Removal of heavy metal ions from wastewater: A comprehensive and critical review[J]. NPJ Clean Water, 2021, 4: 36. |
| [6] | KHURSHID Hifsa, MUSTAFA Muhammad Raza Ul, Mohamed Hasnain ISA. Adsorption of chromium, copper, lead and mercury ions from aqueous solution using bio and nano adsorbents: A review of recent trends in the application of AC, BC, nZVI and MXene[J]. Environmental Research, 2022, 212: 113138. |
| [7] | SAJJADI Seyed-Ali, MOHAMMADZADEH Alireza, TRAN Hai Nguyen, et al. Efficient mercury removal from wastewater by pistachio wood wastes-derived activated carbon prepared by chemical activation using a novel activating agent[J]. Journal of Environmental Management, 2018, 223: 1001-1009. |
| [8] | Rahim SHAHROKHI-SHAHRAKI, BENALLY Chelsea, EL-DIN Mohamed Gamal, et al. High efficiency removal of heavy metals using tire-derived activated carbon vs commercial activated carbon: Insights into the adsorption mechanisms[J]. Chemosphere, 2021, 264: 128455. |
| [9] | DU Boyu, WANG Yumeng, ZHENG Qian, et al. A novel modified lignin-based adsorbent for removal of malachite green and Pb2+ ions from wastewater[J]. Separation and Purification Technology, 2024, 330: 125495. |
| [10] | QI Xin, YIN Hua, ZHU Minghan, et al. MgO-loaded nitrogen and phosphorus self-doped biochar: High-efficient adsorption of aquatic Cu2+, Cd2+, and Pb2+ and its remediation efficiency on heavy metal contaminated soil[J]. Chemosphere, 2022, 294: 133733. |
| [11] | ABDELRAHMAN Ehab A, ABOU EL-REASH Y G, YOUSSEF Hany M, et al. Utilization of rice husk and waste aluminum cans for the synthesis of some nanosized zeolite, zeolite/zeolite, and geopolymer/zeolite products for the efficient removal of Co(Ⅱ), Cu(Ⅱ), and Zn(Ⅱ) ions from aqueous media[J]. Journal of Hazardous Materials, 2021, 401: 123813. |
| [12] | JENA Kishore K, SURESH KUMAR REDDY K, KARANIKOLOS Georgios N, et al. L-Cysteine and silver nitrate based metal sulfide and Zeolite-Y nano adsorbent for efficient removal of mercury (Ⅱ) ion from wastewater[J]. Applied Surface Science, 2023, 611: 155777. |
| [13] | JOSEPH Ifeoma V, TOSHEVA Lubomira, DOYLE Aidan M. Simultaneous removal of Cd(Ⅱ), Co(Ⅱ), Cu(Ⅱ), Pb(Ⅱ), and Zn(Ⅱ) ions from aqueous solutions via adsorption on FAU-type zeolites prepared from coal fly ash[J]. Journal of Environmental Chemical Engineering, 2020, 8(4): 103895. |
| [14] | HE Tong, LI Qian, LIN Tong, et al. Recent progress on highly efficient removal of heavy metals by layered double hydroxides[J]. Chemical Engineering Journal, 2023, 462: 142041. |
| [15] | MA Lijiao, WANG Qing, ISLAM Saiful M, et al. Highly selective and efficient removal of heavy metals by layered double hydroxide intercalated with the MoS4 2– ion[J]. Journal of the American Chemical Society, 2016, 138(8): 2858-2866. |
| [16] | LIU Junqin, WU Pingxiao, LI Shuaishuai, et al. Synergistic deep removal of As(Ⅲ) and Cd(Ⅱ) by a calcined multifunctional MgZnFe-CO3 layered double hydroxide: Photooxidation, precipitation and adsorption[J]. Chemosphere, 2019, 225: 115-125. |
| [17] | WANG Yubing, LI Shanshan, WU Xiaoxi, et al. Nitrogen-Based conjugated microporous polymers for efficient Hg(Ⅱ) removal from Water: Performance and mechanism[J]. Chemical Engineering Journal, 2023, 471: 144659. |
| [18] | ZHANG Jiarui, HE Bianyan, WANG Yubing, et al. Reticular poly(pyrrole methylene)s synthesized by synchronous-cross-linking process for the capture of Hg(Ⅱ) from water: Adsorption performance and mechanism[J]. Journal of Environmental Chemical Engineering, 2024, 12(3): 112625. |
| [19] | LIU Yunpeng, ZHANG Wenlong, ZHAO Chengcheng, et al. Study on the synthesis of poly(pyrrole methane)s with the hydroxyl in different substituent position and their selective adsorption for Pb2+ [J]. Chemical Engineering Journal, 2019, 361: 528-537. |
| [20] | WANG Zhenyu, ZHANG Aijing, ZHU Mengyuan, et al. Efficient removal of Cr(Ⅵ) through adsorption with reduced Cr(Ⅲ) sequestration by highly hydrophilic poly(pyrrole methane)[J]. Separation and Purification Technology, 2025, 354: 129122. |
| [21] | WANG Shengye, XIAO Ke, MO Yayuan, et al. Selenium(Ⅵ) and copper(Ⅱ) adsorption using polyethyleneimine-based resins: Effect of glutaraldehyde crosslinking and storage condition[J]. Journal of Hazardous Materials, 2020, 386: 121637. |
| [22] | BALLAV Niladri, MAITY Arjun, MISHRA Shivani B. High efficient removal of chromium(Ⅵ) using glycine doped polypyrrole adsorbent from aqueous solution[J]. Chemical Engineering Journal, 2012, 198: 536-546. |
| [23] | LIN Guo, ZENG Biao, LI Jing, et al. A systematic review of metal organic frameworks materials for heavy metal removal: Synthesis, applications and mechanism[J]. Chemical Engineering Journal, 2023, 460: 141710. |
| [24] | FU Kaixing, LIU Xia, Chunyu LYU, et al. Superselective Hg(Ⅱ) removal from water using a thiol-laced MOF-based sponge monolith: Performance and mechanism[J]. Environmental Science & Technology, 2022, 56(4): 2677-2688. |
| [25] | LI Yuhang, WANG Chongchen, ZENG Xu, et al. Seignette salt induced defects in Zr-MOFs for boosted Pb(Ⅱ) adsorption: Universal strategy and mechanism insight[J]. Chemical Engineering Journal, 2022, 442: 136276. |
| [26] | AHMADIJOKANI Farhad, TAJAHMADI Shima, BAHI Addie, et al. Ethylenediamine-functionalized Zr-based MOF for efficient removal of heavy metal ions from water[J]. Chemosphere, 2021, 264: 128466. |
| [27] | REN Xueying, WANG Chongchen, LI Yang, et al. Ag(Ⅰ) removal and recovery from wastewater adopting NH2-MIL-125 as efficient adsorbent: A 3Rs (reduce, recycle and reuse) approach and practice[J]. Chemical Engineering Journal, 2022, 442: 136306. |
| [28] | WANG Chen, XIONG Chao, ZHANG Xusheng, et al. External optimization of Zr-MOF with mercaptosuccinic acid for efficient recovery of gold from solution: Adsorption performance and DFT calculation[J]. Separation and Purification Technology, 2022, 296: 121329. |
| [29] | GENDY Eman A, IFTHIKAR Jerosha, Jawad ALI, et al. Removal of heavy metals by covalent organic frameworks (COFs): A review on its mechanism and adsorption properties[J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105687. |
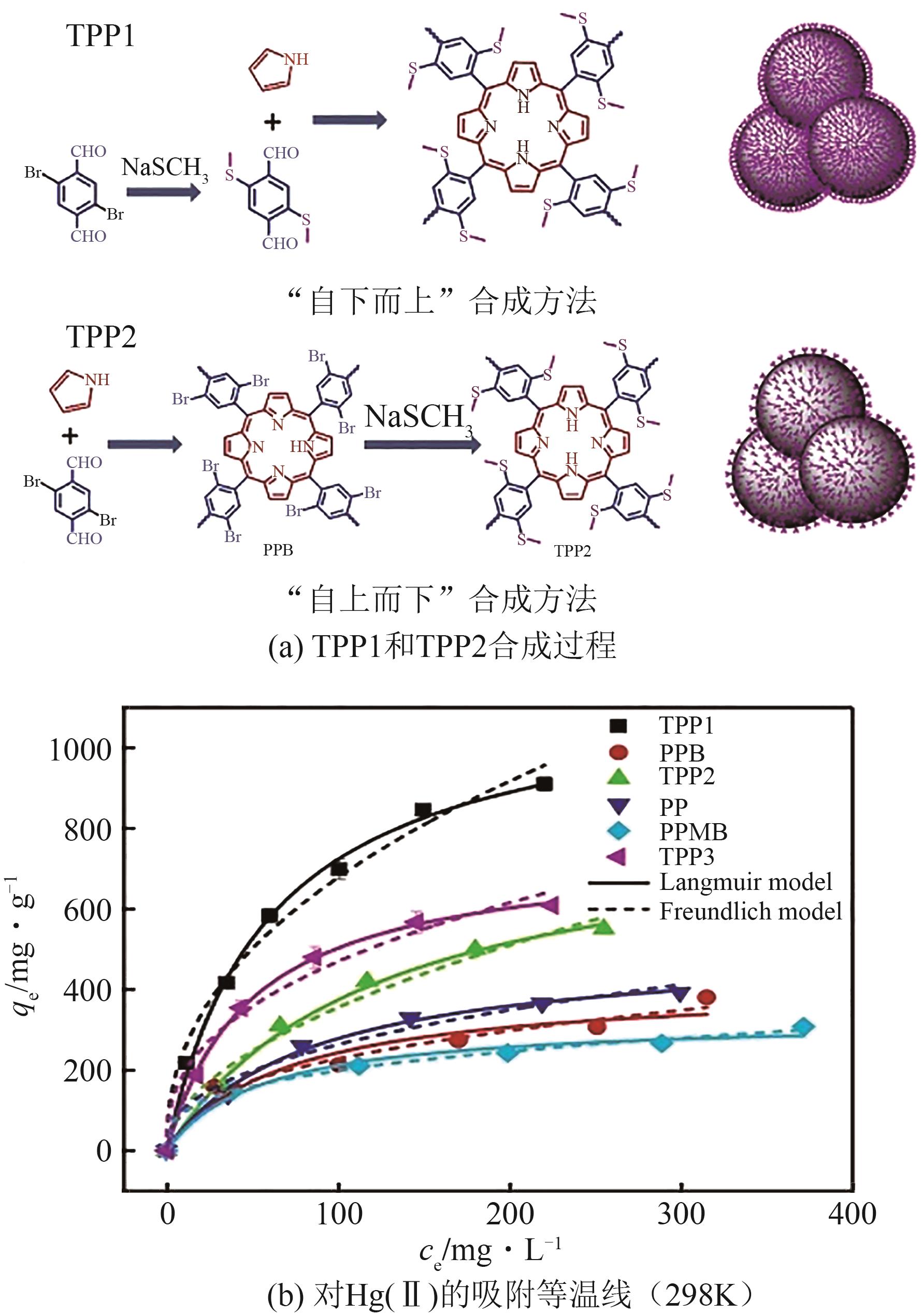
| [30] | MA Zhiyao, LIU Fuyang, LIU Nengsheng, et al. Facile synthesis of sulfhydryl modified covalent organic frameworks for high efficient Hg(Ⅱ) removal from water[J]. Journal of Hazardous Materials, 2021, 405: 124190. |
| [31] | WANG Shuai, WANG Hao, WANG Shixing, et al. Novel magnetic covalent organic framework for the selective and effective removal of hazardous metal Pb(Ⅱ) from solution: Synthesis and adsorption characteristics[J]. Separation and Purification Technology, 2023, 307: 122783. |
| [32] | ZHU Ruomeng, ZHANG Pengling, ZHANG Xinxin, et al. Fabrication of synergistic sites on an oxygen-rich covalent organic framework for efficient removal of Cd(Ⅱ) and Pb(Ⅱ) from water[J]. Journal of Hazardous Materials, 2022, 424: 127301. |
| [33] | KOTP Mohammed G, TORAD Nagy L, NARA Hiroki, et al. Tunable thiophene-based conjugated microporous polymers for the disposal of toxic hexavalent chromium[J]. Journal of Materials Chemistry A, 2023, 11(27): 15022-15032. |
| [34] | CÔTÉ Adrien P, BENIN Annabelle I, OCKWIG Nathan W, et al. Porous, crystalline, covalent organic frameworks[J]. Science, 2005, 310(5751): 1166-1170. |
| [35] | William TILFORD R, GEMMILL William R, LOYE Hans-Conrad ZUR, et al. Facile synthesis of a highly crystalline, covalently linked porous boronate network[J]. Chemistry of Materials, 2006, 18(22): 5296-5301. |
| [36] | BOJDYS Michael J, JEROMENOK Jekaterina, THOMAS Arne, et al. Rational extension of the family of layered, covalent, triazine-based frameworks with regular porosity[J]. Advanced Materials, 2010, 22(19): 2202-2205. |
| [37] | DONG Pengfei, XU Xinyu, LUO Rengan, et al. Postsynthetic annulation of three-dimensional covalent organic frameworks for boosting CO2 photoreduction[J]. Journal of the American Chemical Society, 2023, 145(28): 15473-15481. |
| [38] | LIN Song, DIERCKS Christian S, ZHANG Yue biao, et al. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water[J]. Science, 2015, 349(6253): 1208-1213. |
| [39] | LANNI Laura M, William TILFORD R, BHARATHY Muktha, et al. Enhanced hydrolytic stability of self-assembling alkylated two-dimensional covalent organic frameworks[J]. Journal of the American Chemical Society, 2011, 133(35): 13975-13983. |
| [40] | YANG Shaoxiong, LI Xia, QIN Yu, et al. Modulating the stacking model of covalent organic framework isomers with different generation efficiencies of reactive oxygen species[J]. ACS Applied Materials & Interfaces, 2021, 13(25): 29471-29481. |
| [41] | WANG Xiaohan, ENOMOTO Riku, MURAKAMI Yoichi. Ionic additive strategy to control nucleation and generate larger single crystals of 3D covalent organic frameworks[J]. Chemical Communications, 2021, 57(54): 6656-6659. |
| [42] | FENG Xiao, CHEN Long, DONG Yuping, et al. Porphyrin-based two-dimensional covalent organic frameworks: Synchronized synthetic control of macroscopic structures and pore parameters[J]. Chemical Communications, 2011, 47(7): 1979-1981. |
| [43] | KUHN Pierre, ANTONIETTI Markus, THOMAS Arne. Porous, covalent triazine-based frameworks prepared by ionothermal synthesis[J]. Angewandte Chemie International Edition, 2008, 47(18): 3450-3453. |
| [44] | POUREBRAHIMI Sina, PIROOZ Majid, KAZEMEINI Mohammad, et al. Synthesis, characterization, and gas (SO2, CO2, NO2, CH4, CO, NO, and N2) adsorption properties of the CTF-1 covalent triazine framework-based porous polymer: Experimental and DFT studies[J]. Journal of Porous Materials, 2024, 31(2): 643-657. |
| [45] | ZHU Xiang, TIAN Chengcheng, MAHURIN Shannon M, et al. A superacid-catalyzed synthesis of porous membranes based on triazine frameworks for CO2 separation[J]. Journal of the American Chemical Society, 2012, 134(25): 10478-10484. |
| [46] | ZHANG Siquan, CHENG Guang, GUO Liping, et al. Strong-base-assisted synthesis of a crystalline covalent triazine framework with high hydrophilicity via benzylamine monomer for photocatalytic water splitting[J]. Angewandte Chemie International Edition, 2020, 59(15): 6007-6014. |
| [47] | LAN Zhi'an, WU Meng, FANG Zhongpu, et al. Ionothermal synthesis of covalent triazine frameworks in a NaCl-KCl-ZnCl2 eutectic salt for the hydrogen evolution reaction[J]. Angewandte Chemie International Edition, 2022, 61(18): e202201482. |
| [48] | GUAN Xinyu, MA Yunchao, LI Hui, et al. Fast, ambient temperature and pressure ionothermal synthesis of three-dimensional covalent organic frameworks[J]. Journal of the American Chemical Society, 2018, 140(13): 4494-4498. |
| [49] | BISWAL Bishnu P, CHANDRA Suman, KANDAMBETH Sharath, et al. Mechanochemical synthesis of chemically stable isoreticular covalent organic frameworks[J]. Journal of the American Chemical Society, 2013, 135(14): 5328-5331. |
| [50] | Hongzhou LYU, ZHAO Xiaoli, NIU Hongyun, et al. Ball milling synthesis of covalent organic framework as a highly active photocatalyst for degradation of organic contaminants[J]. Journal of Hazardous Materials, 2019, 369: 494-502. |
| [51] | Gobinda DAS, BALAJI SHINDE Digambar, KANDAMBETH Sharath, et al. Mechanosynthesis of imine, β-ketoenamine, and hydrogen-bonded imine-linked covalent organic frameworks using liquid-assisted grinding[J]. Chemical Communications, 2014, 50(84): 12615-12618. |
| [52] | BROWN Normanda, ALSUDAIRY Ziad, BEHERA Ranjan, et al. Green mechanochemical synthesis of imine-linked covalent organic frameworks for high iodine capture[J]. Green Chemistry, 2023, 25(16): 6287-6296. |
| [53] | EMMERLING Sebastian T, GERMANN Luzia S, JULIEN Patrick A, et al. In situ monitoring of mechanochemical covalent organic framework formation reveals templating effect of liquid additive[J]. Chem, 2021, 7(6): 1639-1652. |
| [54] | HAMZEHPOOR Ehsan, EFFATY Farshid, BORCHERS Tristan H, et al. Mechanochemical synthesis of boroxine-linked covalent organic frameworks[J]. Angewandte Chemie International Edition, 2024: e202404539. |
| [55] | CAMPBELL Neil L, CLOWES Rob, RITCHIE Lyndsey K, et al. Rapid microwave synthesis and purification of porous covalent organic frameworks[J]. Chemistry of Materials, 2009, 21(2): 204-206. |
| [56] | XU Lina, XU Jia, SHAN Baotian, et al. TpPa-2-incorporated mixed matrix membranes for efficient water purification[J]. Journal of Membrane Science, 2017, 526: 355-366. |
| [57] | ZHANG Wang, LI Cun, YUAN Yu peng, et al. Highly energy- and time-efficient synthesis of porous triazine-based framework: Microwave-enhanced ionothermal polymerization and hydrogen uptake[J]. Journal of Materials Chemistry, 2010, 20(31): 6413-6415. |
| [58] | ZHAO Wei, YAN Peiyao, YANG Haofan, et al. Using sound to synthesize covalent organic frameworks in water[J]. Nature Synthesis, 2022, 1: 87-95. |
| [59] | LIU Chunhua, PARK Eunsol, JIN Yinghua, et al. Separation of arylenevinylene macrocycles with a surface-confined two-dimensional covalent organic framework[J]. Angewandte Chemie International Edition, 2018, 57(29): 8984-8988. |
| [60] | ZHANG Penghui, WANG Zhifang, WANG Sa, et al. Fabricating industry-compatible olefin-linked COF resins for oxoanion pollutant scavenging[J]. Angewandte Chemie International Edition, 2022, 61(52): e202213247. |
| [61] | GU He, LIU Xiaolu, WANG Suhua, et al. COF-based composites: Extraordinary removal performance for heavy metals and radionuclides from aqueous solutions[J]. Reviews of Environmental Contamination and Toxicology, 2022, 260(1): 23. |
| [62] | GUO Weikang, LIU Jiale, TAO Haijuan, et al. Covalent organic framework nanoarchitectonics: Recent advances for precious metal recovery[J]. Advanced Materials, 2024, 36(33): 2405399. |
| [63] | SUN Qi, AGUILA Briana, PERMAN Jason, et al. Postsynthetically modified covalent organic frameworks for efficient and effective mercury removal[J]. Journal of the American Chemical Society, 2017, 139(7): 2786-2793. |
| [64] | GHAZI Zahid Ali, KHATTAK Abdul Muqsit, IQBAL Rashid, et al. Adsorptive removal of Cd2+ from aqueous solutions by a highly stable covalent triazine-based framework[J]. New Journal of Chemistry, 2018, 42(12): 10234-10242. |
| [65] | LU Xiaofan, JI Wenhua, YUAN Lin, et al. Preparation of carboxy-functionalized covalent organic framework for efficient removal of Hg2+ and Pb2+ from water[J]. Industrial & Engineering Chemistry Research, 2019, 58(38): 17660-17667. |
| [66] | 贾昊, 姜红新, 李明堂, 等. 羧基共价有机骨架材料对Cd(Ⅱ)的吸附性能及其机理研究[J]. 农业环境科学学报, 2023, 42(1): 177-187. |
| JIA Hao, JIANG Hongxin, LI Mingtang, et al Study on the adsorption performance and mechanism of carboxyl covalent organic framework materials for Cd ( Ⅱ)[J]. Journal of Agricultural Environmental Sciences, 2023, 42 (1): 177-187. | |
| [67] | XIANG Dawei, ZHU Rong, CHEN Yuefeng, et al. Preparation of amidoxime modified covalent organic framework for efficient adsorption of lead ions in aqueous solution[J]. Chemical Engineering Journal, 2024, 492: 152292. |
| [68] | LI Mingyan, CHEN Liangjun, DU Jiawei, et al. Thiol-ene click reaction modified triazinyl-based covalent organic framework for Pb (Ⅱ) ion effective removal[J]. ACS Applied Materials & Interfaces, 2024, 16(7): 8688-8696. |
| [69] | WANG Heping, HE Tengteng, QUAN Dandan, et al. Thiosemicarbazide-linked covalent organic framework: Preparation, properties and applications[J]. ChemistrySelect, 2021, 6(42): 11490-11495. |
| [70] | LI Ya, WANG Chang, MA Shujuan, et al. Fabrication of hydrazone-linked covalent organic frameworks using alkyl amine as building block for high adsorption capacity of metal ions[J]. ACS Applied Materials & Interfaces, 2019, 11(12): 11706-11714. |
| [71] | YANG Cheng xiong, LIU Chang, CAO Yi meng, et al. Facile room-temperature solution-phase synthesis of a spherical covalent organic framework for high-resolution chromatographic separation[J]. Chemical Communications, 2015, 51(61): 12254-12257. |
| [72] | LI Guiliang, YE Jianrong, FANG Qile, et al. Amide-based covalent organic frameworks materials for efficient and recyclable removal of heavy metal lead ( Ⅱ)[J]. Chemical Engineering Journal, 2019, 370: 822-830. |
| [73] | QIAN Hai long, ZHU Meng si, DU Mei lan, et al. Engineering linkage as functional moiety into irreversible thiourea-linked covalent organic framework for ultrafast adsorption of Hg(Ⅱ)[J]. Journal of Hazardous Materials, 2022, 427: 128156. |
| [74] | YUSRAN Yusran, GUAN Xinyu, LI Hui, et al. Postsynthetic functionalization of covalent organic frameworks[J]. National Science Review, 2020, 7(1): 170-190. |
| [75] | Laura MERÍ-BOFÍ, ROYUELA Sergio, ZAMORA Félix, et al. Thiol grafted imine-based covalent organic frameworks for water remediation through selective removal of Hg(Ⅱ)[J]. Journal of Materials Chemistry A, 2017, 5(34): 17973-17981. |
| [76] | WANG Lizhi, WANG Jiajia, WANG You, et al. Thioether-functionalized porphyrin-based polymers for Hg2+ efficient removal in aqueous solution[J]. Journal of Hazardous Materials, 2022, 429: 128303. |
| [77] | FU Quanbin, ZHANG Tingting, SUN Xin, et al. Pyridine-based covalent organic framework for efficient and selective removal of Hg(Ⅱ) from water: Adsorption behavior and adsorption mechanism investigations[J]. Chemical Engineering Journal, 2023, 454: 140154. |
| [78] | SHAN Houchao, LI Shufeng, YANG Zhen, et al. Triazine-based N-rich porous covalent organic polymer for the effective detection and removal of Hg(Ⅱ) from an aqueous solution[J]. Chemical Engineering Journal, 2021, 426: 130757. |
| [79] | ZHU Bin, ZHU Longyi, DENG Shengyuan, et al. A fully π-conjugated covalent organic framework with dual binding sites for ultrasensitive detection and removal of divalent heavy metal ions[J]. Journal of Hazardous Materials, 2023, 459: 132081. |
| [80] | DINARI Mohammad, HATAMI Mohammad. Novel N-riched crystalline covalent organic framework as a highly porous adsorbent for effective cadmium removal[J]. Journal of Environmental Chemical Engineering, 2019, 7(1): 102907. |
| [81] | GUAN Xinyu, LI Hui, MA Yunchao, et al. Chemically stable polyarylether-based covalent organic frameworks[J]. Nature Chemistry, 2019, 11(6): 587-594. |
| [82] | CUI Fuzhi, LIANG Rongrran, QI Qiaoyan, et al. Efficient removal of Cr(Ⅵ) from aqueous solutions by a dual-pore covalent organic framework[J]. Advanced Sustainable Systems, 2019, 3(4): 1800150. |
| [83] | TIAN Yuan, XU Shunqi, LIANG Rongran, et al. Construction of two heteropore covalent organic frameworks with Kagome lattices[J]. CrystEngComm, 2017, 19(33): 4877-4881. |
| [84] | BABUJOHN Nisar Ahamed, ELURI Amoluck, NABEELA V P. One pot synthesis of crystalline covalent organic polymers with tunable pores for the removal of gold and toxic organic pollutants[J]. Chemical Engineering Journal, 2023, 464: 142459. |
| [85] | HUANG Ning, ZHAI Lipeng, XU Hong, et al. Stable covalent organic frameworks for exceptional mercury removal from aqueous solutions[J]. Journal of the American Chemical Society, 2017, 139(6): 2428-2434. |
| [86] | DING San yuan, DONG Ming, WANG Ya wen, et al. Thioether-based fluorescent covalent organic framework for selective detection and facile removal of mercury(Ⅱ)[J]. Journal of the American Chemical Society, 2016, 138(9): 3031-3037. |
| [87] | CAO Ying, HU Xue, ZHU Changqian, et al. Sulfhydryl functionalized covalent organic framework as an efficient adsorbent for selective Pb(Ⅱ) removal[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2020, 600: 125004. |
| [88] | ZHOU Zhiming, ZHONG Wanfu, CUI Kaixun, et al. A covalent organic framework bearing thioether pendant arms for selective detection and recovery of Au from ultra-low concentration aqueous solution[J]. Chemical Communications, 2018, 54(71): 9977-9980. |
| [89] | JIANG Yunzhe, LIU Chuanyao, HUANG Aisheng. EDTA-functionalized covalent organic framework for the removal of heavy-metal ions[J]. ACS Applied Materials & Interfaces, 2019, 11(35): 32186-32191. |
| [90] | ZHANG Du, CHEN Yiping, WANG Jiajia, et al. Melamine-functionalization of the carbonyl-rich polymers for iodine vapor and Hg2+ capture[J]. Chemical Engineering Journal, 2023, 460: 141669. |
| [91] | ZHAO Jie, QIAO Zelong, HE Yuncheng, et al. Anion-regulated ionic covalent organic frameworks for highly selective recovery of gold from E-waste[J]. Angewandte Chemie International Edition, 2025,64(2): e202414366. |
| [92] | YANG Zhenlian, GU Yangyi, YUAN Baoling, et al. Thio-groups decorated covalent triazine frameworks for selective mercury removal[J]. Journal of Hazardous Materials, 2021, 403: 123702. |
| [93] | WANG Wei, GONG Minjuan, ZHU Donghai, et al. Post-synthetic thiol modification of covalent organic frameworks for mercury(Ⅱ) removal from water[J]. Environmental Science and Ecotechnology, 2023, 14: 100236. |
| [94] | WANG Huaizhen, CHAN Michael Ho-Yeung, Vivian Wing -Wah YAM. Heavy-metal ions removal and iodine capture by terpyridine covalent organic frameworks[J]. Small Methods, 2024,8(11): 2400465. |
| [95] | MA Qi, LIU Xiaoyun, QIAN Jun, et al. Preparation of covalent organic framework with carboxy and triazine for efficient removal of Pb2+ ions[J]. Separation and Purification Technology, 2023, 323: 124368. |
| [96] | LIU Na, SHI Liangfeng, HAN Xianghao, et al. A heteropore covalent organic framework for adsorptive removal of Cd (Ⅱ) from aqueous solutions with high efficiency[J]. Chinese Chemical Letters, 2020, 31(2): 386-390. |
| [97] | ZHU Donghai, ZHOU Shuangxi, ZHOU Ziming, et al. Highly efficient and selective removal of Cr(Ⅵ) by covalent organic frameworks: Structure, performance and mechanism[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2020, 600: 124910. |
| [98] | ABNEY Carter W, MAYES Richard T, SAITO Tomonori, et al. Materials for the recovery of uranium from seawater[J]. Chemical Reviews, 2017, 117(23): 13935-14013. |
| [99] | CAO Doudou, CUI Fengchao, ZHANG Cheng, et al. In situ synthesis of polyamidoxime chains inside the negative-charged confining fields for efficient adsorption of uranyl ions[J]. Advanced Functional Materials, 2025, 35(3): 2413729. |
| [100] | KUMAR Ajay, ARYA Kushal, MEHRA Sanjay, et al. Luminescent Zn-MOF@COF hybrid for selective decontamination of Cu(Ⅱ) ions and methylene blue dye in aqueous media[J]. Separation and Purification Technology, 2024, 340: 126756. |
| [101] | OLIVEIRA Ana Rita, CORREIA António Alberto, RASTEIRO Maria Graça. Heavy metals removal from aqueous solutions by multiwall carbon nanotubes: Effect of MWCNTs dispersion[J]. Nanomaterials 2021, 11(8): 2082. |
| [102] | LIU Xin, WANG Xun, JIANG Wei, et al. Covalent organic framework modified carbon nanotubes for removal of uranium (Ⅵ) from mining wastewater[J]. Chemical Engineering Journal, 2022, 450: 138062. |
| [103] | MARTINSON Carol A, REDDY K J. Adsorption of arsenic(Ⅲ) and arsenic(Ⅴ) by cupric oxide nanoparticles[J]. Journal of Colloid and Interface Science, 2009, 336(2): 406-411. |
| [104] | WANG Longlong, XU Haomiao, QIU Yixiang, et al. Utilization of Ag nanoparticles anchored in covalent organic frameworks for mercury removal from acidic waste water[J]. Journal of Hazardous Materials, 2020, 389: 121824. |
| [105] | LEUS Karen, FOLENS Karel, NICOMEL Nina Ricci, et al. Removal of arsenic and mercury species from water by covalent triazine framework encapsulated γ-Fe2O3 nanoparticles[J]. Journal of Hazardous Materials, 2018, 353: 312-319. |
| [106] | LIANG Pei, LIU Sijia, LI Mei, et al. Effective adsorption and removal of Cr(Ⅵ) from wastewater using magnetic composites prepared by synergistic effect of polypyrrole and covalent organic frameworks[J]. Separation and Purification Technology, 2024, 336: 126222. |
| [107] | YI Tan, ZHAO Hanyu, MO Qi, et al. From cellulose to cellulose nanofibrils—A comprehensive review of the preparation and modification of cellulose nanofibrils[J]. Materials,2020,13(22): 5062. |
| [108] | WANG Yixiang, CHEN Lingyun. Impacts of nanowhisker on formation kinetics and properties of all-cellulose composite gels[J]. Carbohydrate Polymers, 2011, 83(4): 1937-1946. |
| [109] | ZHAO Bingbing, FU Xu, DI Yaoyue, et al. Covalent organic framework@cellulose nanofibrils@carboxymethyl cellulose composite hydrogel beads for the removal of nickel ions from aqueous solutions[J]. Journal of Molecular Structure, 2024, 1312: 138619. |
| [110] | ZHANG Luwei, MA Shujuan, CHEN Yao, et al. Facile fabrication of biomimetic chitosan membrane with honeycomb-like structure for enrichment of glycosylated peptides[J]. Analytical Chemistry, 2019, 91(4): 2985-2993. |
| [111] | ZHANG Luwei, LI Ya, WANG Yan, et al. Integration of covalent organic frameworks into hydrophilic membrane with hierarchical porous structure for fast adsorption of metal ions[J]. Journal of Hazardous Materials, 2021, 407: 124390. |
| [112] | ZHONG Juan, CAO Yiwen, ZHU Jianhui, et al. Facile construction of phenolic hydroxyl anchored covalent organic frameworks/chitosan composite aerogels for efficient adsorption of Pb(Ⅱ) from water[J]. Separation and Purification Technology, 2025, 354: 129087. |
| [113] | TRAN Hai Nguyen, YOU Shengjie, Ahmad HOSSEINI-BANDEGHARAEI, et al. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review[J]. Water Research, 2017, 120: 88-116. |
| [114] | XU Yang, ZHAO Xiaodong, HUA Weiwei, et al. Spatially confined coordination platform in covalent organic framework for selective uranium adsorption from aqueous solutions[J]. Separation and Purification Technology, 2024, 345: 127307. |
| [115] | BI Changlong, ZHANG Chunhong, XU Wenda, et al. Highly efficient antibacterial adsorbent for recovering uranium from seawater based on molecular structure design of PCN-222 post-engineering[J]. Desalination, 2023, 545: 116169. |
| [116] | XIONG Xiaohong, YU Zhiwu, GONG Lele, et al. Ammoniating covalent organic framework (COF) for high-performance and selective extraction of toxic and radioactive uranium ions[J]. Advanced Science, 2019, 6(16): 1900547. |
| [117] | GUPTA Krishna M, ZHANG Kang, JIANG Jianwen. Efficient removal of Pb2+ from aqueous solution by an ionic covalent-organic framework: Molecular simulation study[J]. Industrial & Engineering Chemistry Research, 2018, 57(18): 6477-6482. |
| [118] | ZHOU Shuangxi, ZHOU Ziming, ZHU Donghai, et al. Preparation of covalent triazine-based framework for efficient Cr(Ⅵ) removal from water[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2022, 632: 127757. |
| [119] | SHENG Xin, SHI Hui, YOU Deng, et al. Specific π δ +-π δ - interaction enables conjugated microporous polymers for highly selective capture of Pd(Ⅱ)[J]. Chemical Engineering Journal, 2022, 437: 135367. |
| [120] | PEARSON Ralph G. Hard and soft acids and bases[J]. Journal of the American Chemical Society, 1963, 85(22): 3533-3539. |
| [1] | FU Hongmei, LIU Dinghua, LIU Xiaoqin. Research progress on the separation of aromatic isomers using MOF materials [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5006-5017. |
| [2] | ZHANG Wenjing, HUANG Zhixin, LI Shiteng, DENG Shuai, LI Shuangjun. Biomass carbon aerogels for CO2 adsorbents [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5018-5032. |
| [3] | LI Xuejiao, JIANG Ning, LIU Xinhao, LI Di, XU Jiachuan. Research progress of thermal-oxidative aging mechanism and life prediction of polyolefin [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5075-5091. |
| [4] | WANG Rui, WANG Hailan, DAI Ruobin, WANG Zhiwei. Silicon fouling of reverse osmosis membrane for advanced treatment of industrial wastewater: Mechanisms, influencing factors and control strategies [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5315-5326. |
| [5] | CAO Jiangfei, LEI Xiaotong, HUANG Zhiyi, HUANG Jiankai, CHEN Fan, YANG Pianpian, XIE Chunsheng. Preparation of iron-nitrogen doped carbon microspheres and their activation for PS degradation of rhodamine B [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5406-5415. |
| [6] | ZENG Jin, GAO Yan, WANG Zhaopeng, XIE Yuyun, LIU Jun, LIANG Qi, WANG Chunying. Degradation mechanism of 2,4-dichlorophenoxyacetic acid by NaYF4:Yb,Tm composite TiO2/Bi2WO6 photocatalyst and evaluation of products toxicity [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5416-5431. |
| [7] | TANG Jian, CUI Wangwang, CHEN Jiakun, WANG Tianzheng, QIAO Junfei. Full lifecycle prediction model construction for dioxins in municipal solid waste incineration process: Method of coupling numerical simulation and fuzzy forest regression [J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4628-4647. |
| [8] | YANG Yong, ZHANG Zhao, WANG Dongliang, ZHOU Huairong, ZHAO Zihao, LI Yukun. Technical-economic evaluation for different separation strategies of xylene isomers [J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4732-4740. |
| [9] | MI Yifang, WANG Baoguo, WANG Wenqiang, SUN Guojin, CAO Zhihai. Preparation of nitrogen self-doped cyanobacterial biomass-based activated carbon for CO2 adsorption [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 4223-4232. |
| [10] | GAO Jiaojiao, YAN Shiyu, YANG Taishun, XIE Shangzhi, YANG Yanjuan, XU Jing. Effect of alumina support crystal structure of Ru-based catalysts on polyethylene hydrogenolysis performance [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 3917-3927. |
| [11] | RONG Liping, LI Zhiguo, WANG Gang, ZHANG Dayong, ZHOU Hongxia, MI Changhong, LI Xin, ZHAO Ying, ZHU Jinhua, LIU Xiaohui, LIU Ye. Advances in adhesives for wet bonding based on the catechol structure [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 3950-3964. |
| [12] | LIANG Shuwei, YU Jie, XIE Zhongyin, PEI Jianlu, LIN Zhongxin, CHEN Zexiang. Covalent organic frameworks for radioactive gaseous iodine adsorption [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 3965-3975. |
| [13] | WANG Ying, TANG Mengfei, WANG Ying, ZHANG Chuanfang, ZHANG Guojie, LIU Jun, ZHAO Yuqiong. Preparation of CNT composites from coal pyrolysis catalyzed by different alkali metals for adsorption of Rhodamine B [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 3985-3996. |
| [14] | ZHAO Baohua, LIU Xiaona, HU Yanyun, JIA Tiancong, XIE Qiang, HE Yan, MA Xiangshuai, MA Shuangchen. Comparison and development trend of traditional electroadsorption and flow electrode capacitive deion technology [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 4101-4116. |
| [15] | YE Xiaosheng, YUAN Ting, JIA Xin, REN Qingxia. Research progress on the removal of microcystin-LR by multicomponent composite nanomaterials [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 4144-4157. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||