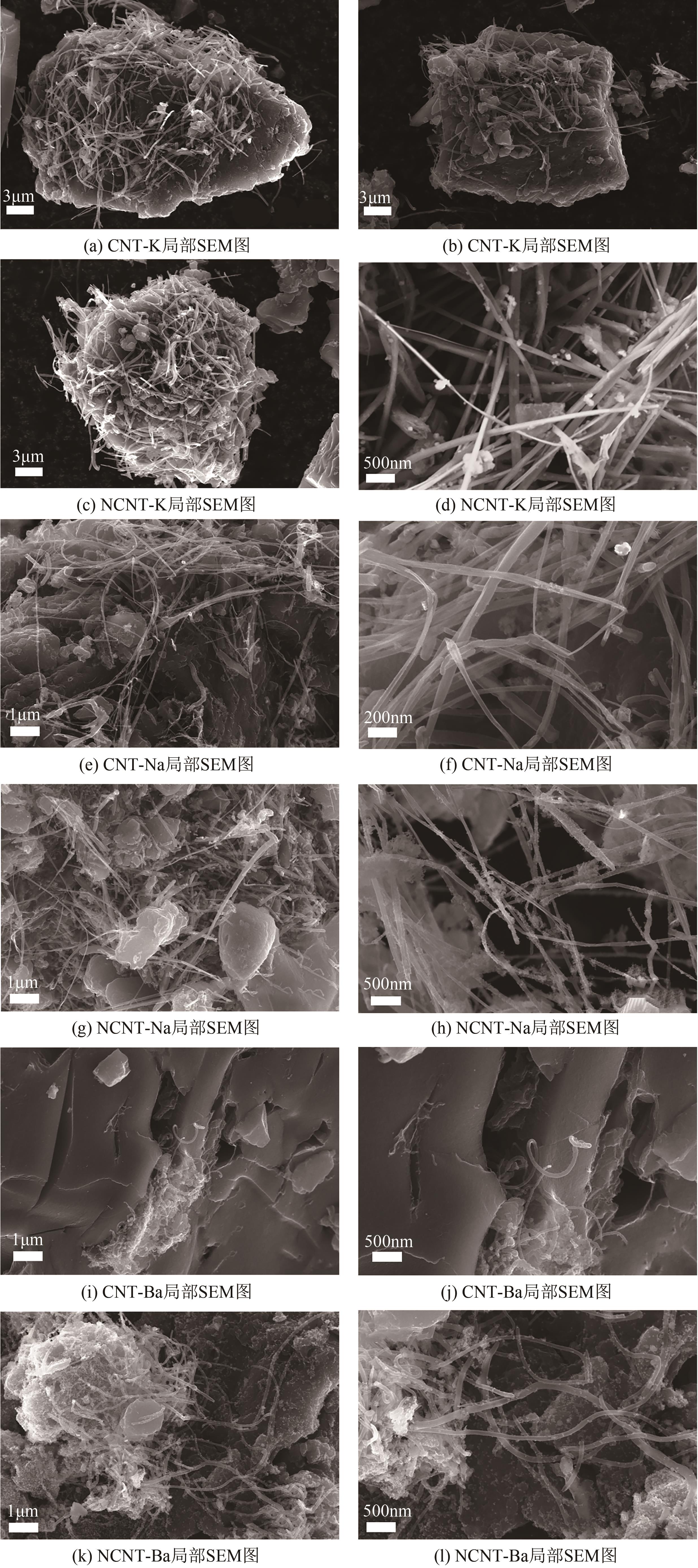
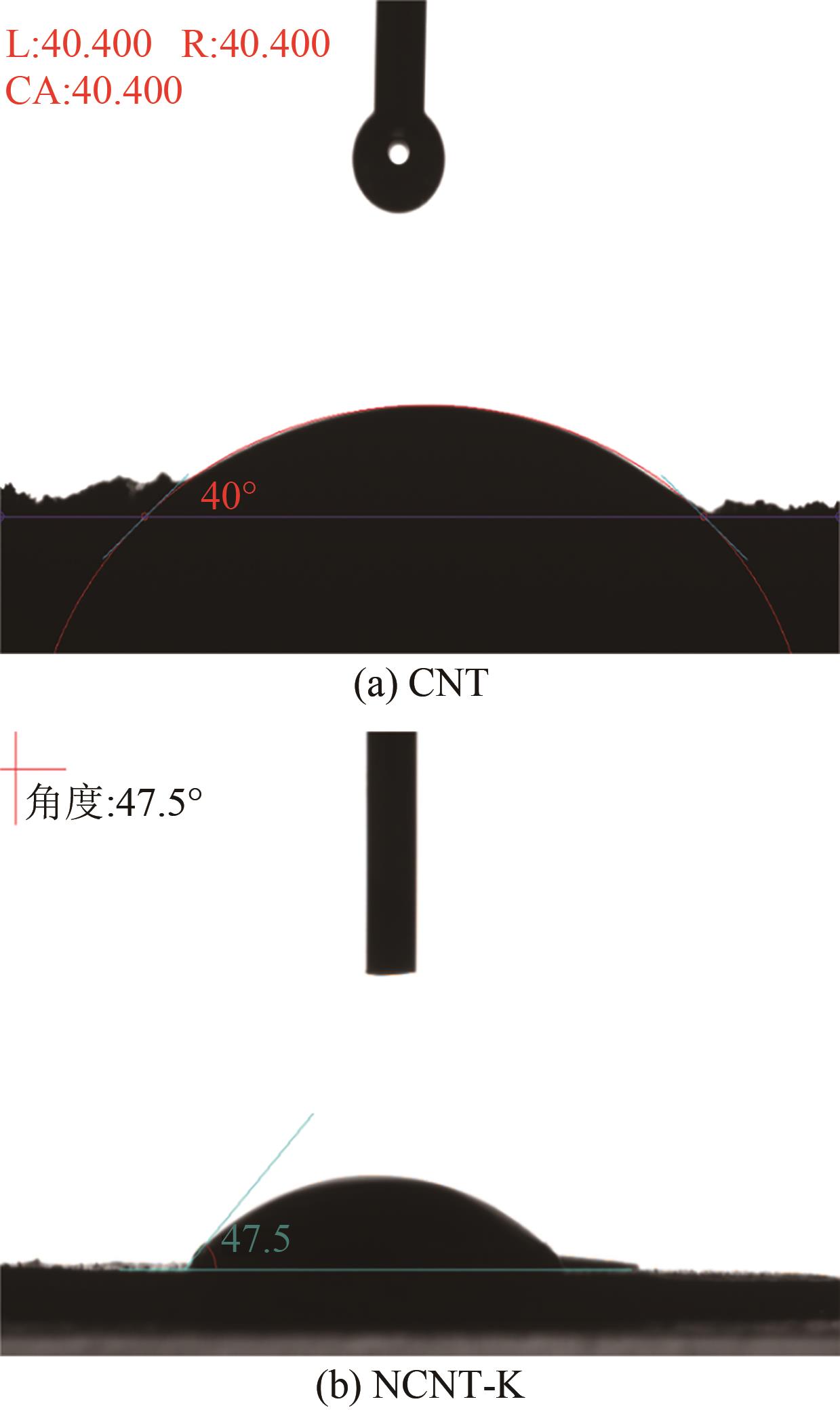
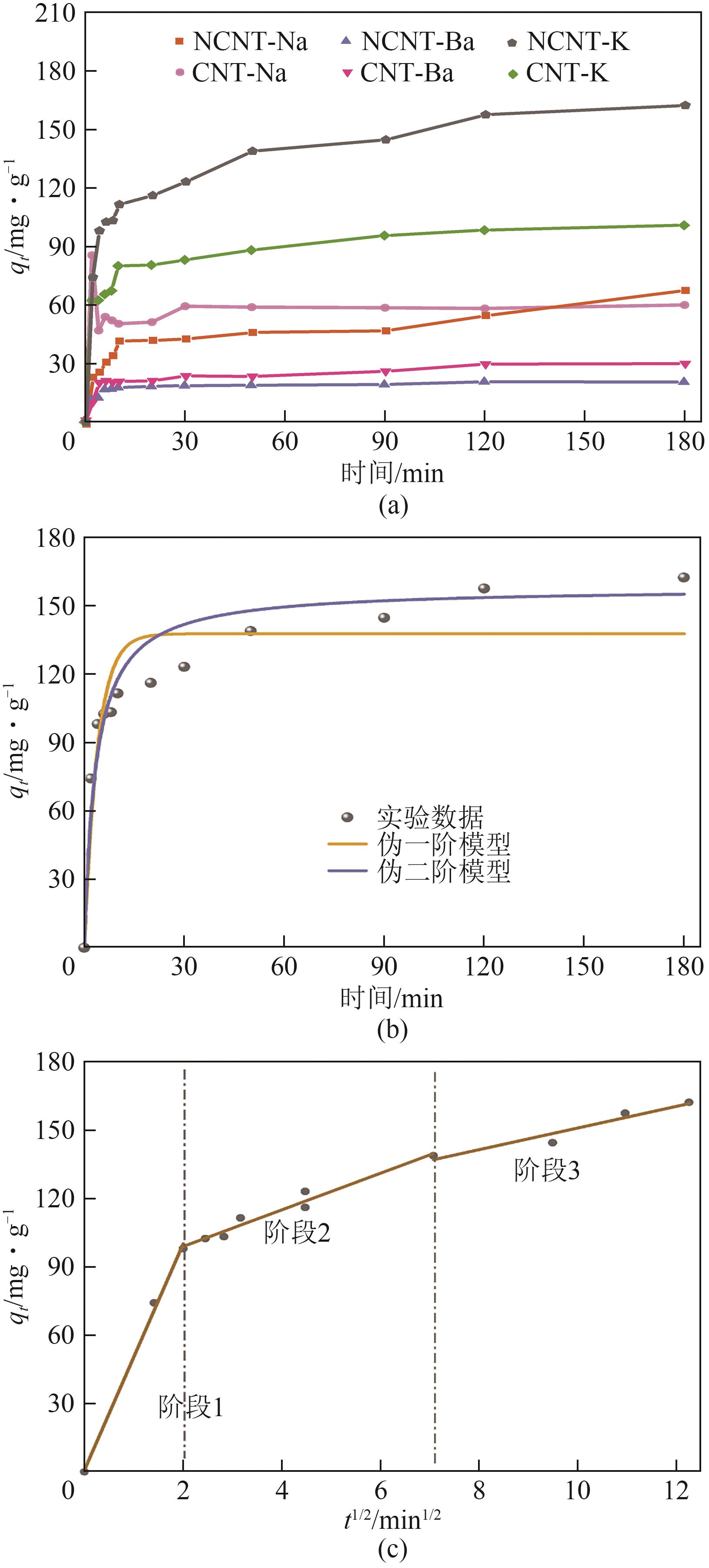
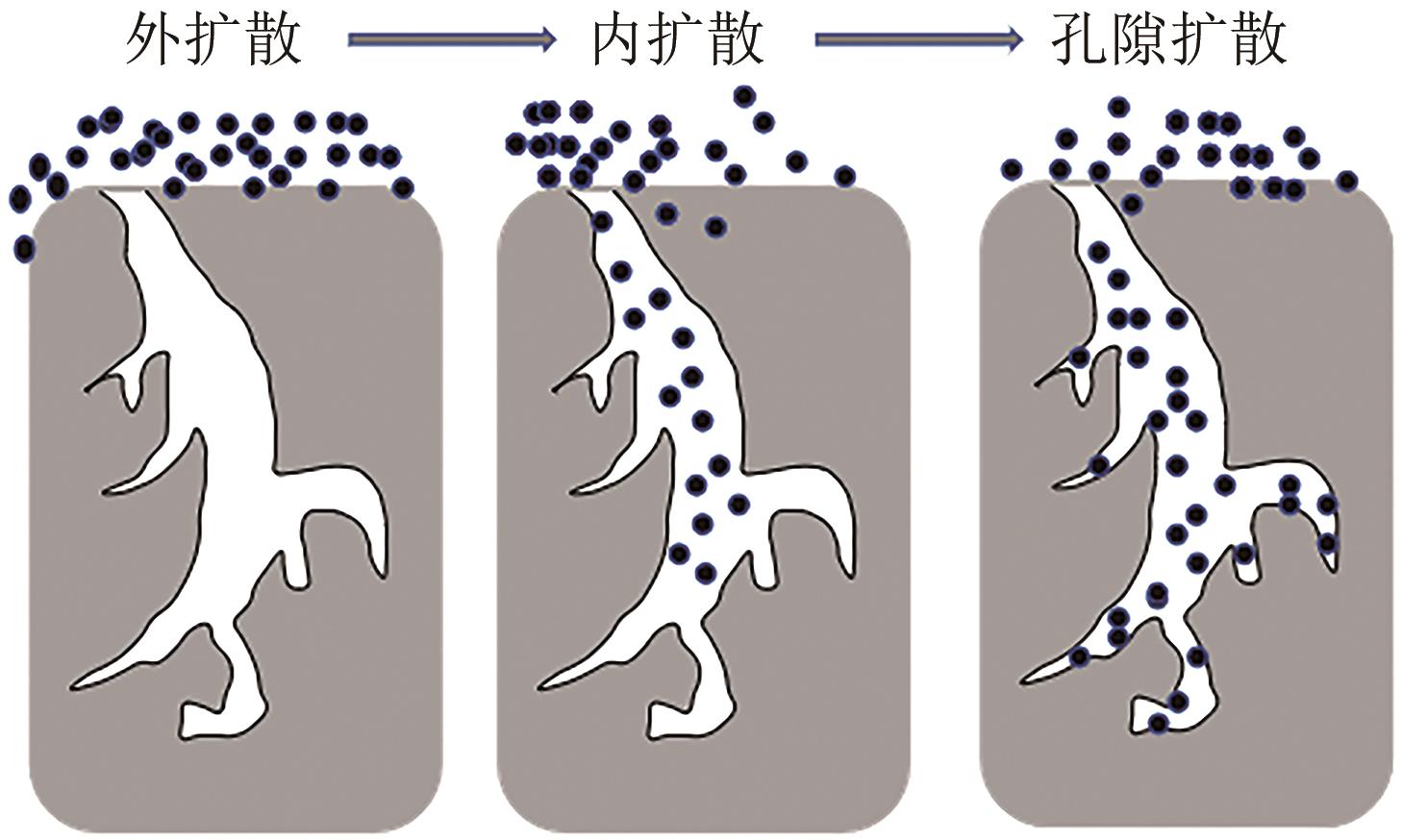
Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (7): 3985-3996.DOI: 10.16085/j.issn.1000-6613.2024-0906
• Materials science and technology • Previous Articles
Preparation of CNT composites from coal pyrolysis catalyzed by different alkali metals for adsorption of Rhodamine B
WANG Ying1( ), TANG Mengfei1, WANG Ying2, ZHANG Chuanfang1, ZHANG Guojie1, LIU Jun1,3, ZHAO Yuqiong1
), TANG Mengfei1, WANG Ying2, ZHANG Chuanfang1, ZHANG Guojie1, LIU Jun1,3, ZHAO Yuqiong1
- 1.State Key Laboratory of Clean and Efficient Coal Utilization, College of Chemical Engineering and Technology, Taiyuan University of Science and Technology, Taiyuan 030024, Shanxi, China
2.School of Electric Power, Civil Engineering and Architecture, Shanxi University, Taiyuan 030006, Shanxi, China
3.State Engineering Laboratory of Multiple Smoke and Gas Pollution Control Technologies and Equipments, School of Environment, Tsinghua University, Beijing 100084, China
-
Received:2024-06-03Revised:2024-08-19Online:2025-08-04Published:2025-07-25 -
Contact:WANG Ying
碱金属催化煤热解制备CNT复合材料用于吸附罗丹明B
王影1( ), 汤孟菲1, 王莹2, 张传芳1, 张国杰1, 刘俊1,3, 赵钰琼1
), 汤孟菲1, 王莹2, 张传芳1, 张国杰1, 刘俊1,3, 赵钰琼1
- 1.太原理工大学化学工程与技术学院,省部共建煤炭清洁高效利用国家重点实验室
1.山西 太原 030024,山西大学电力与建筑学院,山西 太原 030006
2.山西 太原 030024,清华大学环境学院
多重烟气污染控制技术与设备国家工程实验室,北京 100084
-
通讯作者:王影 -
作者简介:王影(1987—),男,博士研究生,讲师,研究方向为煤基碳材料,E-mail: wangying04@tyut.edu.cn。 -
基金资助:山西省基础研究计划(202203021222130);山西省基础研究计划(202303021211034);山西省基础研究计划(202303021211032);国家重点研发计划(2022YFC3701900)
CLC Number:
Cite this article
WANG Ying, TANG Mengfei, WANG Ying, ZHANG Chuanfang, ZHANG Guojie, LIU Jun, ZHAO Yuqiong. Preparation of CNT composites from coal pyrolysis catalyzed by different alkali metals for adsorption of Rhodamine B[J]. Chemical Industry and Engineering Progress, 2025, 44(7): 3985-3996.
王影, 汤孟菲, 王莹, 张传芳, 张国杰, 刘俊, 赵钰琼. 碱金属催化煤热解制备CNT复合材料用于吸附罗丹明B[J]. 化工进展, 2025, 44(7): 3985-3996.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-0906
| 工业分析Wad①/% | 元素分析Wd②/% | ||||||
|---|---|---|---|---|---|---|---|
| 灰分 | 水分 | 挥发分 | 固定碳③ | C | H | N | S |
| 5.53 | 3.25 | 30.91 | 60.33 | 73.75 | 4.42 | 1.45 | 0.22 |
| 工业分析Wad①/% | 元素分析Wd②/% | ||||||
|---|---|---|---|---|---|---|---|
| 灰分 | 水分 | 挥发分 | 固定碳③ | C | H | N | S |
| 5.53 | 3.25 | 30.91 | 60.33 | 73.75 | 4.42 | 1.45 | 0.22 |
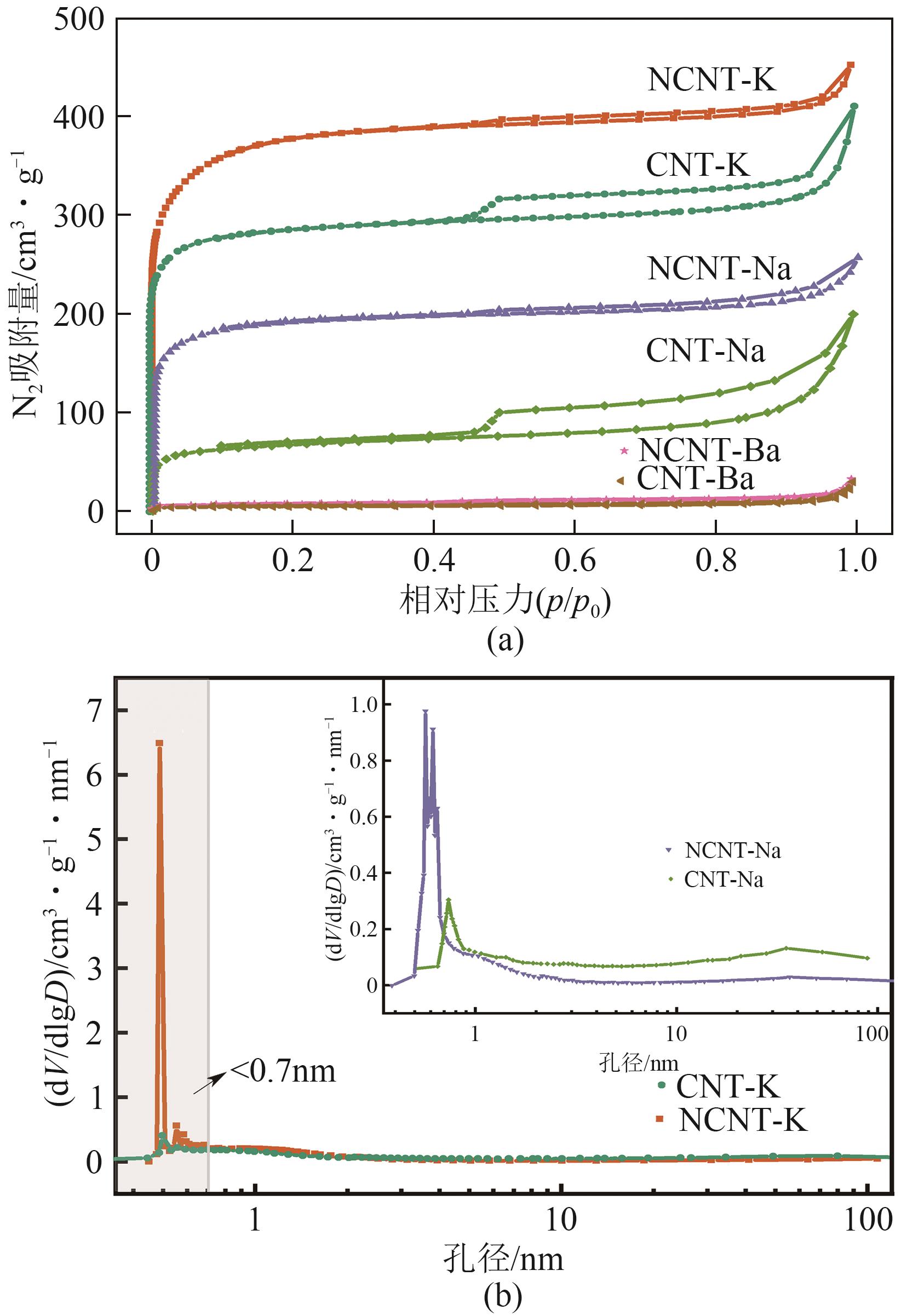
| 复合材料 | 比表面积/m2·g-1 | 微孔比表面积/m2·g-1 | 孔容/cm3·g-1 | 微孔体积/cm3·g-1 | 微孔含量/% | 平均粒径/nm |
|---|---|---|---|---|---|---|
| CNT-K | 1128 | 1090 | 0.580 | 0.443 | 76.38 | 2.082 |
| NCNT-K | 1439 | 1384 | 0.669 | 0.584 | 87.29 | 1.932 |
| CNT-Na | 252 | 198 | 0.259 | 0.104 | 40.15 | 4.745 |
| NCNT-Na | 726 | 700 | 0.370 | 0.292 | 78.92 | 2.077 |
| CNT-Ba | 18 | 11 | 0.037 | 0.008 | 21.62 | 9.294 |
| NCNT-Ba | 15 | 6 | 0.034 | 0.007 | 20.59 | 16.037 |
| 复合材料 | 比表面积/m2·g-1 | 微孔比表面积/m2·g-1 | 孔容/cm3·g-1 | 微孔体积/cm3·g-1 | 微孔含量/% | 平均粒径/nm |
|---|---|---|---|---|---|---|
| CNT-K | 1128 | 1090 | 0.580 | 0.443 | 76.38 | 2.082 |
| NCNT-K | 1439 | 1384 | 0.669 | 0.584 | 87.29 | 1.932 |
| CNT-Na | 252 | 198 | 0.259 | 0.104 | 40.15 | 4.745 |
| NCNT-Na | 726 | 700 | 0.370 | 0.292 | 78.92 | 2.077 |
| CNT-Ba | 18 | 11 | 0.037 | 0.008 | 21.62 | 9.294 |
| NCNT-Ba | 15 | 6 | 0.034 | 0.007 | 20.59 | 16.037 |
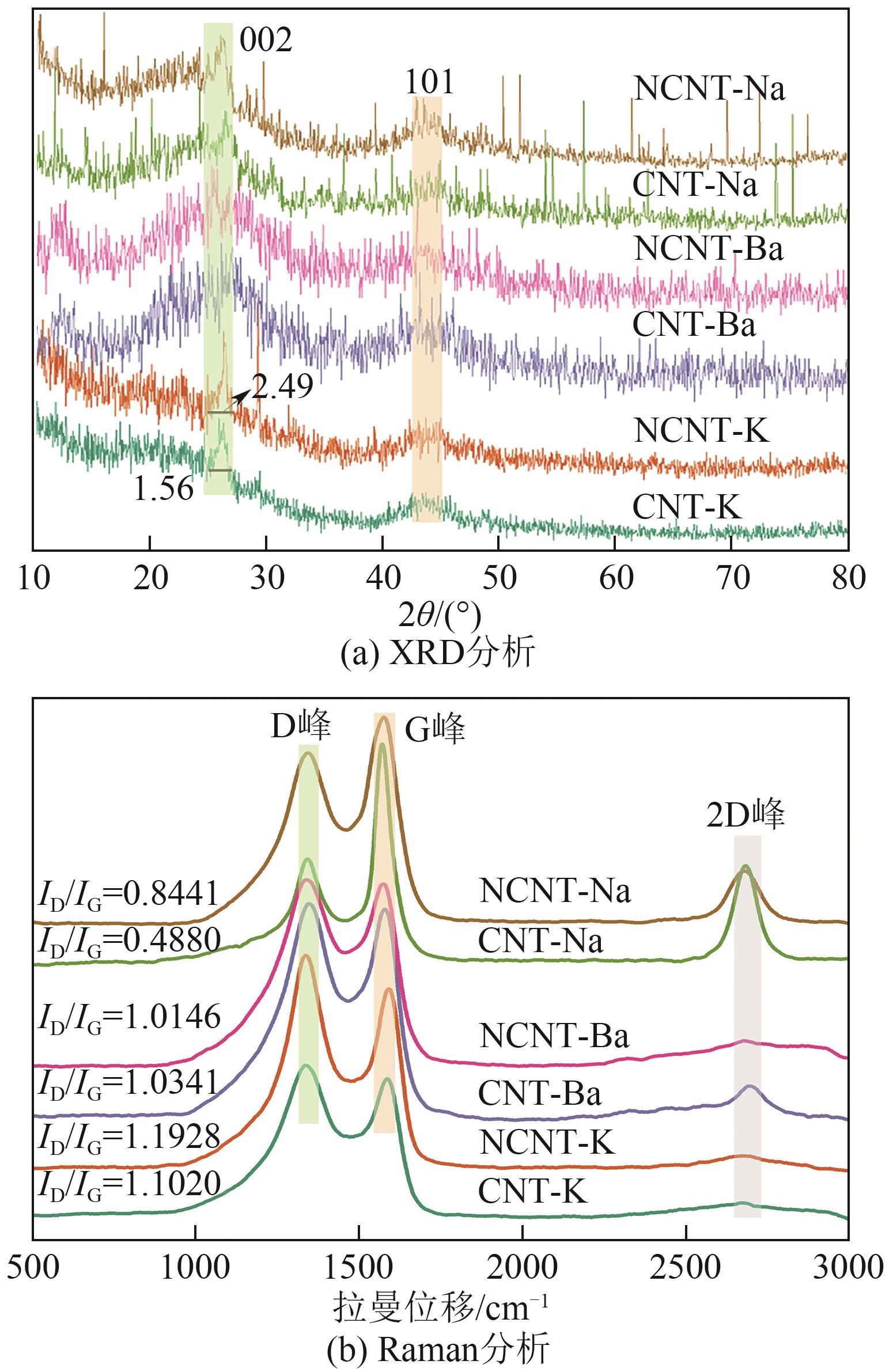
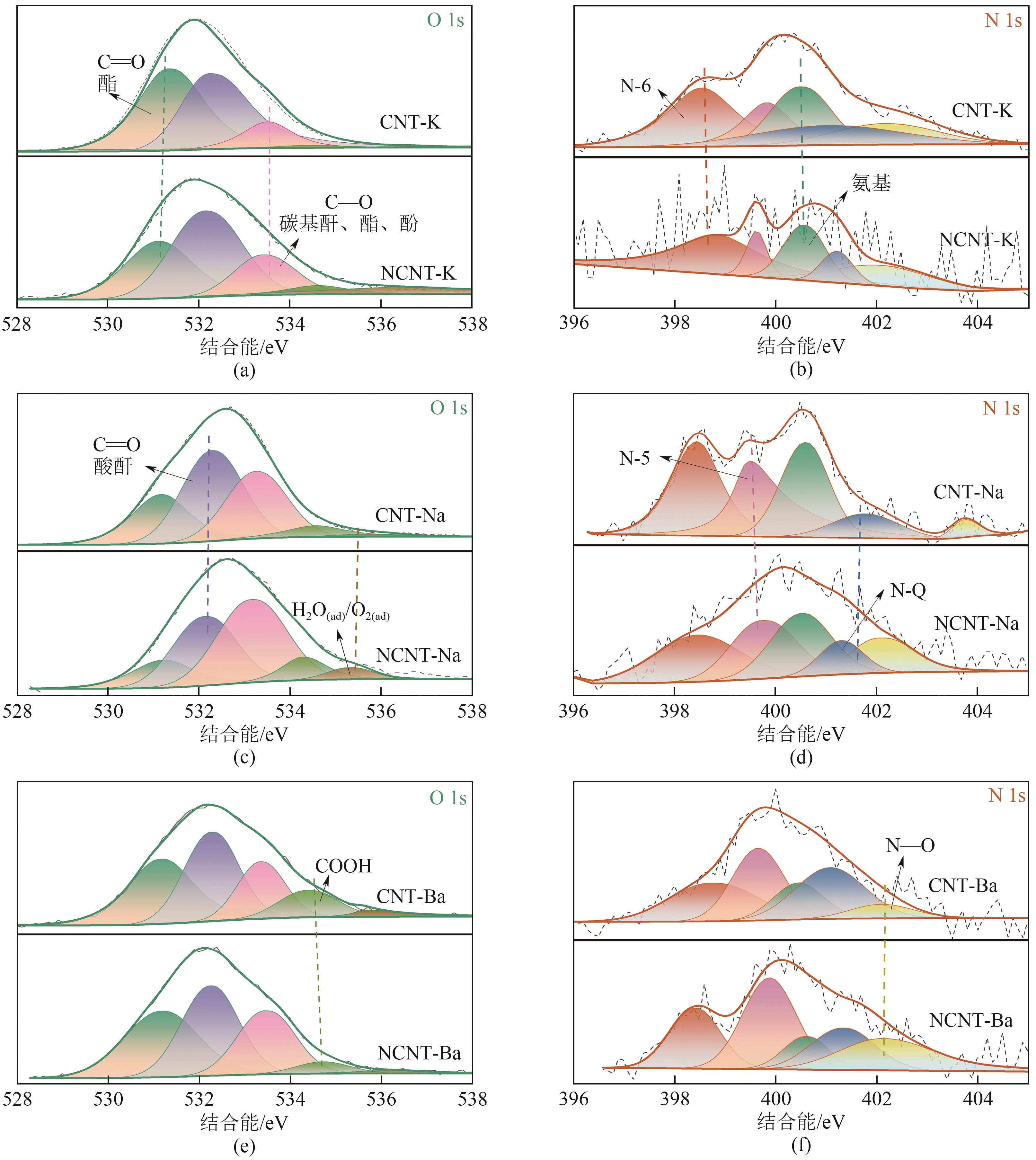
| 复合材料 | C原子分数/% | N原子分数/% | N物种比例/% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N-6 | N-5 | —NH2 | N-Q | N—O | |||||||
| CNT-K | 84.41 | 1.47 | 29.17 | 15.38 | 22.33 | 14.25 | 18.88 | ||||
| NCNT-K | 90.47 | 0.91 | 42.21 | 16.92 | 16.63 | 11.32 | 12.92 | ||||
| CNT-Na | 80.06 | 1.52 | 29.89 | 30.14 | 27.35 | 9.60 | 3.02 | ||||
| NCNT-Na | 87.37 | 1.47 | 14.84 | 26.19 | 24.70 | 24.78 | 9.49 | ||||
| CNT-Ba | 87.64 | 1.79 | 25.11 | 27.80 | 13.86 | 26.58 | 6.64 | ||||
| NCNT-Ba | 86.10 | 1.75 | 19.83 | 31.40 | 9.64 | 17.18 | 21.95 | ||||
| 复合材料 | O原子分数/% | O物种比例/% | |||||||||
| C | C | C—O | COOH | H2O(ad) | |||||||
| CNT-K | 14.12 | 44.25 | 43.16 | 10.82 | 1.32 | 0.46 | |||||
| NCNT-K | 8.62 | 55.82 | 19.72 | 15.69 | 7.57 | 1.20 | |||||
| CNT-Na | 18.41 | 22.47 | 40.43 | 30.04 | 5.76 | 1.30 | |||||
| NCNT-Na | 11.16 | 13.59 | 32.24 | 42.03 | 8.16 | 3.97 | |||||
| CNT-Ba | 10.57 | 32.22 | 34.26 | 19.16 | 11.29 | 3.07 | |||||
| NCNT-Ba | 12.15 | 33.93 | 35.01 | 24.95 | 4.33 | 1.78 | |||||
| 复合材料 | C原子分数/% | N原子分数/% | N物种比例/% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N-6 | N-5 | —NH2 | N-Q | N—O | |||||||
| CNT-K | 84.41 | 1.47 | 29.17 | 15.38 | 22.33 | 14.25 | 18.88 | ||||
| NCNT-K | 90.47 | 0.91 | 42.21 | 16.92 | 16.63 | 11.32 | 12.92 | ||||
| CNT-Na | 80.06 | 1.52 | 29.89 | 30.14 | 27.35 | 9.60 | 3.02 | ||||
| NCNT-Na | 87.37 | 1.47 | 14.84 | 26.19 | 24.70 | 24.78 | 9.49 | ||||
| CNT-Ba | 87.64 | 1.79 | 25.11 | 27.80 | 13.86 | 26.58 | 6.64 | ||||
| NCNT-Ba | 86.10 | 1.75 | 19.83 | 31.40 | 9.64 | 17.18 | 21.95 | ||||
| 复合材料 | O原子分数/% | O物种比例/% | |||||||||
| C | C | C—O | COOH | H2O(ad) | |||||||
| CNT-K | 14.12 | 44.25 | 43.16 | 10.82 | 1.32 | 0.46 | |||||
| NCNT-K | 8.62 | 55.82 | 19.72 | 15.69 | 7.57 | 1.20 | |||||
| CNT-Na | 18.41 | 22.47 | 40.43 | 30.04 | 5.76 | 1.30 | |||||
| NCNT-Na | 11.16 | 13.59 | 32.24 | 42.03 | 8.16 | 3.97 | |||||
| CNT-Ba | 10.57 | 32.22 | 34.26 | 19.16 | 11.29 | 3.07 | |||||
| NCNT-Ba | 12.15 | 33.93 | 35.01 | 24.95 | 4.33 | 1.78 | |||||
| 参数 | 伪一阶 | 伪二阶 | 参数 | 第一步 | 第二步 | 第三步 |
|---|---|---|---|---|---|---|
| k/min-1 | 0.2531 | 0.0002 | K/mg·g-0.5∙t-0.5 | 49.63 | 8.04 | 4.76 |
| qe/mg·g-1 | 137.60 | 157.81 | C | 0.90 | 82.89 | 103.46 |
| R2 | 0.8708 | 0.9320 | R2 | 0.9942 | 0.9567 | 0.9041 |
| 参数 | 伪一阶 | 伪二阶 | 参数 | 第一步 | 第二步 | 第三步 |
|---|---|---|---|---|---|---|
| k/min-1 | 0.2531 | 0.0002 | K/mg·g-0.5∙t-0.5 | 49.63 | 8.04 | 4.76 |
| qe/mg·g-1 | 137.60 | 157.81 | C | 0.90 | 82.89 | 103.46 |
| R2 | 0.8708 | 0.9320 | R2 | 0.9942 | 0.9567 | 0.9041 |
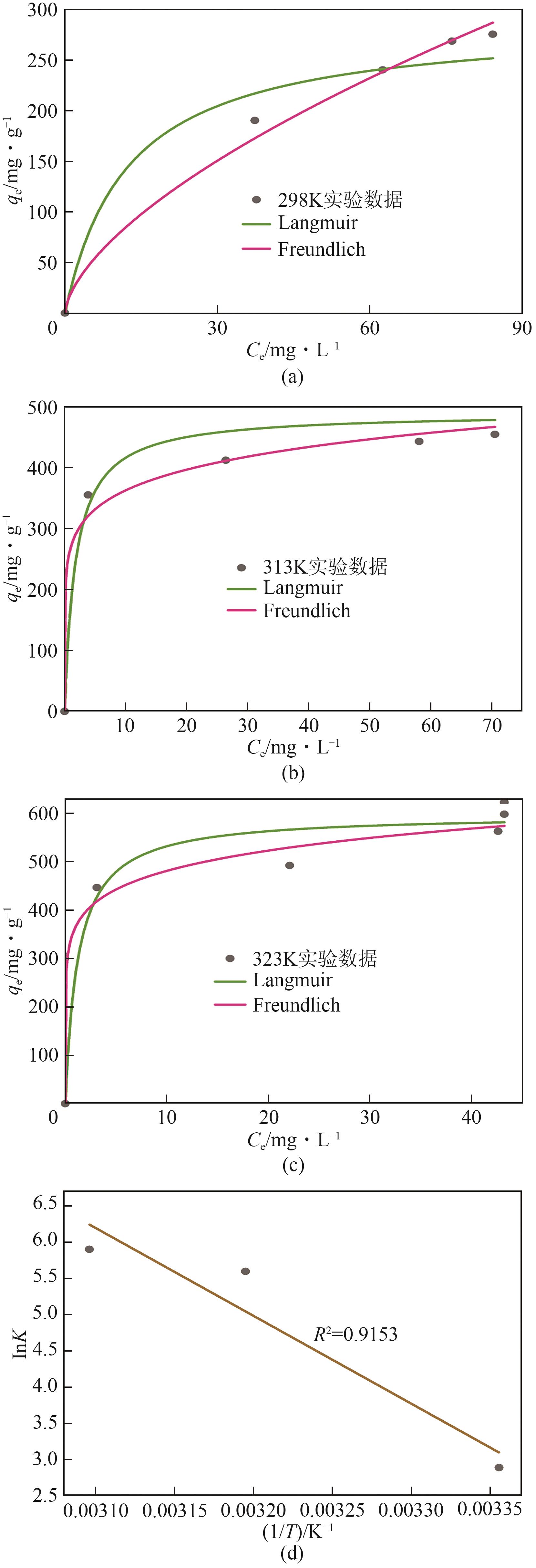
| 温度 | Langmuir模型 | Freundlich模型 | qe/mg·g-1 | ||||
|---|---|---|---|---|---|---|---|
| qmax/mg·g-1 | KL/L·mg-1 | R2 | n | KF/mg·g-1·L1/n ·mg-1/n | R2 | ||
| 298K | 288.15 | 1.61 | 0.9675 | 1.64 | 17.92 | 0.9914 | 275.13 |
| 313K | 490.17 | 0.56 | 0.9707 | 7.74 | 269.41 | 0.9897 | 454.43 |
| 323K | 598.26 | 0.80 | 0.9723 | 8.31 | 364.81 | 0.9877 | 598.09 |
| 温度 | Langmuir模型 | Freundlich模型 | qe/mg·g-1 | ||||
|---|---|---|---|---|---|---|---|
| qmax/mg·g-1 | KL/L·mg-1 | R2 | n | KF/mg·g-1·L1/n ·mg-1/n | R2 | ||
| 298K | 288.15 | 1.61 | 0.9675 | 1.64 | 17.92 | 0.9914 | 275.13 |
| 313K | 490.17 | 0.56 | 0.9707 | 7.74 | 269.41 | 0.9897 | 454.43 |
| 323K | 598.26 | 0.80 | 0.9723 | 8.31 | 364.81 | 0.9877 | 598.09 |
| 温度 | ∆G⊖/kJ·mol-1 | ∆H⊖/kJ·mol-1 | ∆S⊖/kJ·mol-1·K-1 |
|---|---|---|---|
| 298K | -7.15 | +0.11 | +4.65 |
| 313K | -14.56 | ||
| 323K | -15.84 |
| 温度 | ∆G⊖/kJ·mol-1 | ∆H⊖/kJ·mol-1 | ∆S⊖/kJ·mol-1·K-1 |
|---|---|---|---|
| 298K | -7.15 | +0.11 | +4.65 |
| 313K | -14.56 | ||
| 323K | -15.84 |
| [1] | AMALINA Farah, RAZAK Abdul Syukor ABD, KRISHNAN Santhana, et al. A review of eco-sustainable techniques for the removal of Rhodamine B dye utilizing biomass residue adsorbents[J]. Physics and Chemistry of the Earth, Parts A/B/C, 2022, 128: 103267. |
| [2] | BENI Ali Aghababai, ESMAEILI Akbar. Biosorption, an efficient method for removing heavy metals from industrial effluents: A review[J]. Environmental Technology & Innovation, 2020, 17: 100503. |
| [3] | Byung-Moon JUN, LEE Hyun-Kyu, PARK Sungbin, et al. Purification of uranium-contaminated radioactive water by adsorption: A review on adsorbent materials[J]. Separation and Purification Technology, 2021, 278: 119675. |
| [4] | LI Shuangjun, YUAN Xiangzhou, DENG Shuai, et al. A review on biomass-derived CO2 adsorption capture: Adsorbent, adsorber, adsorption, and advice[J]. Renewable and Sustainable Energy Reviews, 2021, 152: 111708. |
| [5] | 林少华, 武海霞, 高莉苹, 等. 改性碳纳米管及其复合材料在废水处理中的应用现状及展望[J]. 化工进展, 2021, 40(6): 3466-3479. |
| LIN Shaohua, WU Haixia, GAO Liping, et al. Current status and future prospects of modified carbon nanotube and its composite materials application for wastewater treatment[J]. Chemical Industry and Engineering Progress, 2021, 40(6): 3466-3479. | |
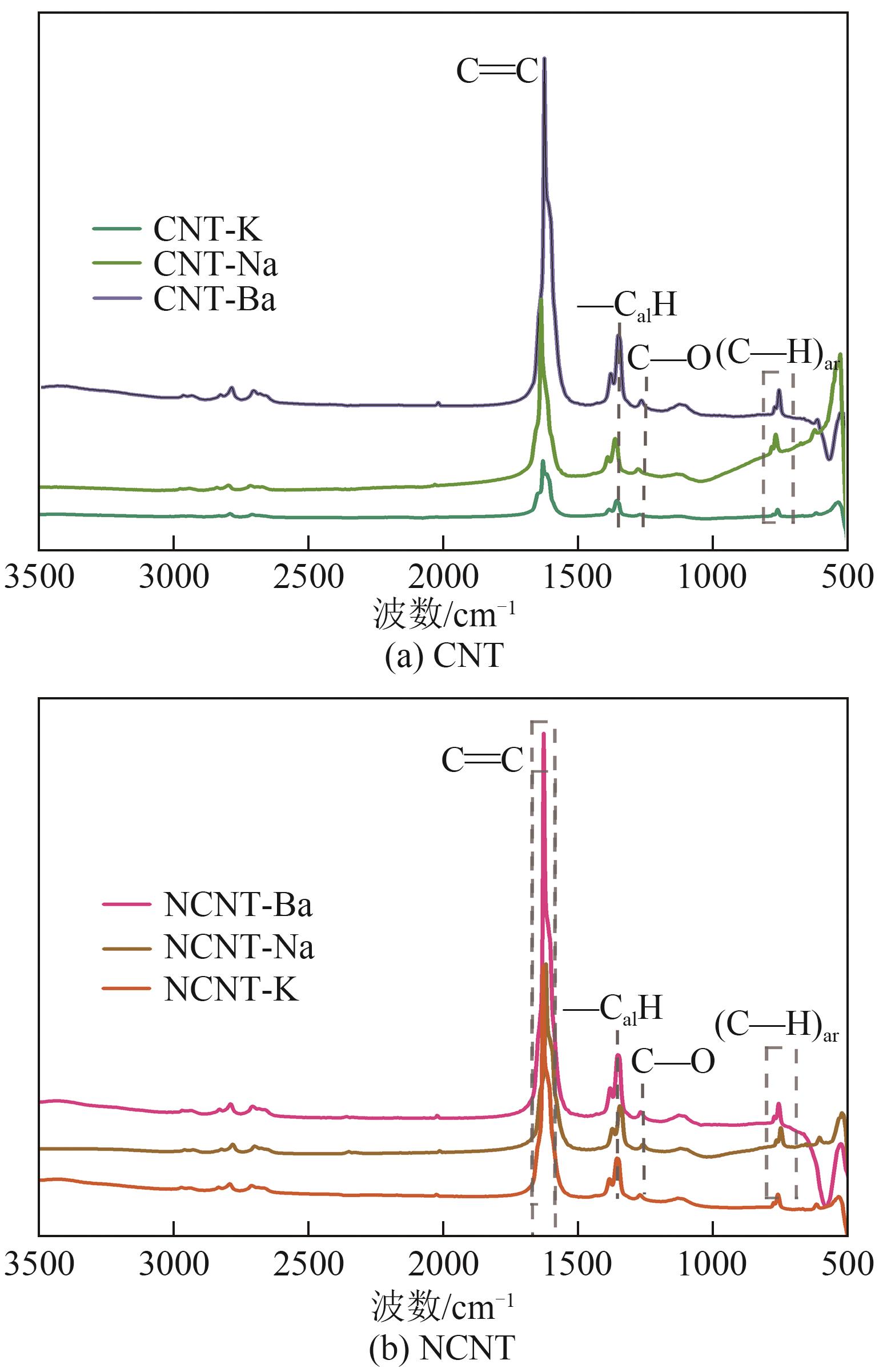
| [6] | 张德懿, 雷龙艳, 尚永花. 氮掺杂对碳材料性能的影响研究进展[J]. 化工进展, 2016, 35(3): 831-836. |
| ZHANG Deyi, LEI Longyan, SHANG Yonghua. Effect of the nitrogen doping on the performance of nano-structure carbon materials: A review[J]. Chemical Industry and Engineering Progress, 2016, 35(3): 831-836. | |
| [7] | KUMAR Sumeet, Jayanta DAS. Carbon nanotubes, nanochains and quantum dots synthesized through the chemical treatment of charcoal powder[J]. Journal of Molecular Structure, 2021, 1227: 129419. |
| [8] | OMAR Sarah, OMAR Mirna, ATTIA Nour F, et al. Rational engineering and fabrication of efficient nanophotocatalysts based on ZnO-SrO-CdS for pharmaceutical pollutants based wastewater degradation[J]. Surfaces and Interfaces, 2024, 45: 103817. |
| [9] | WANG Gen, GAO Ge, YANG Shengjiong, et al. Magnetic mesoporous carbon nanospheres from renewable plant phenol for efficient hexavalent chromium removal[J]. Microporous and Mesoporous Materials, 2021, 310: 110623. |
| [10] | ZHANG Xialan, WANG Xin, CHENG Ting, et al. A novel mesoporous carbon nanospheres-based adsorbent material with desirable performances for dyes removal[J]. Journal of Molecular Liquids, 2023, 390: 123091. |
| [11] | AI Lunhong, ZHANG Chunying, LIAO Fang, et al. Removal of methylene blue from aqueous solution with magnetite loaded multi-wall carbon nanotube: Kinetic, isotherm and mechanism analysis[J]. Journal of Hazardous Materials, 2011, 198: 282-290. |
| [12] | VARGUES F, BRION M A, ROSA DA COSTA A M, et al. Development of a magnetic activated carbon adsorbent for the removal of common pharmaceuticals in wastewater treatment[J]. International Journal of Environmental Science and Technology, 2021, 18(9): 2805-2818. |
| [13] | AUNG Myat Thandar, SHIMABUKU Kyle K, Natalia SOARES-QUINETE, et al. Leveraging DOM UV absorbance and fluorescence to accurately predict and monitor short-chain PFAS removal by fixed-bed carbon adsorbers[J]. Water Research, 2022, 213: 118146. |
| [14] | SHI Wen, WANG Hui, YAN Jinlong, et al. Wheat straw derived biochar with hierarchically porous structure for bisphenol A removal: Preparation, characterization, and adsorption properties[J]. Separation and Purification Technology, 2022, 289: 120796. |
| [15] | XIAO Ye, AZAIEZ Jalel, HILL Josephine M. Erroneous application of pseudo-second-order adsorption kinetics model: Ignored assumptions and spurious correlations[J]. Industrial & Engineering Chemistry Research, 2018, 57(7): 2705-2709. |
| [16] | MATOS Beatriz, BATISTA Mary, PIRES João. Efficient adsorption of carbon dioxide and methane on activated carbon prepared from glycerol with potassium acetate[J]. Environmental Chemistry Letters, 2023, 21(3): 1265-1270. |
| [17] | ELWADOOD Samar N ABD, K Suresh Kumar REDDY, WAHEDI Yasser AL, et al. Hybrid salt-enriched micro-sorbents for atmospheric water sorption[J]. Journal of Water Process Engineering, 2023, 52: 103560. |
| [18] | HE Zhiqin, LI Yun, QI Benkun. A new and low-cost surface-functionalized corn straw adsorbent for adsorptive removal of sodium dodecylbenzene sulfonate: Adsorbent preparation and adsorption performance[J]. Separation and Purification Technology, 2023, 309: 122999. |
| [19] | ZHI Fangke, ZHOU Wenjing, CHEN Jingru, et al. Adsorption properties of active biochar: Overlooked role of the structure of biomass[J]. Bioresource Technology, 2023, 387: 129695. |
| [20] | ZHANG Tiankai, ZHANG Yongfa, WANG Qi, et al. Mechanism of K-catalyzed transformation of solid carbon structure into carbon nanotubes in coal[J]. Fuel Processing Technology, 2020, 204: 106409. |
| [21] | 张天开. Fe/K催化煤热解直接制备碳纳米管机理研究[D]. 太原: 太原理工大学, 2020. |
| ZHANG Tiankai. Study on the mechanism of direct preparation of carbon nanotubes from coal pyrolysis catalyzed by Fe/K[D]. Taiyuan: Taiyuan University of Technology, 2020. | |
| [22] | EL-SEESY Ahmed I, WALY Mahmoud S, EL-BATSH Hesham M, et al. Enhancement of the diesel fuel characteristics by using nitrogen-doped multi-walled carbon nanotube additives[J]. Process Safety and Environmental Protection, 2023, 171: 561-577. |
| [23] | GUO Dawei, WU Jiabo, FENG Dongdong, et al. Mechanism of efficient magnetic biochar for typical aqueous organic contaminant combined-adsorption removal[J]. Fuel Processing Technology, 2023, 247: 107795. |
| [24] | DAULAY Amru, ASTUTI Widi, SUMARDI Slamet, et al. Synthesis and characteristics of Na-A zeolite from coal fly ash and application for adsorption of cerium(Ⅲ)[J]. Journal of Rare Earths, 2025, 43(1): 171-179. |
| [25] | CHEN Hsi-Chao, CHIU Hsuan-Yi, HUANG Kuoting. Raman spectroscopy on 3-D acid-functional single-walled carbon nanotubes for flexible transparent-conducting films deposited with vacuum-filtration and dip-coating[J]. Diamond and Related Materials, 2019, 92: 1-8. |
| [26] | ELLISON Candice, ABDELSAYED Victor, SMITH Mark W. Analysis of char structure and composition from microwave and conventional pyrolysis/gasification of low and middle rank coals[J]. Fuel, 2023, 354: 129301. |
| [27] | DENIS Pablo A. Heteroatom codoped graphene: The importance of nitrogen[J]. ACS Omega, 2022, 7(50): 45935-45961. |
| [28] | XU Xiangju, YANG Chen, YANG Zhi, et al. Carbon nanotube growth from alkali metal salt nanoparticles[J]. Carbon, 2014, 80: 490-495. |
| [29] | JIANG Qinyuan, WANG Fei, LI Run, et al. Synthesis of ultralong carbon nanotubes with ultrahigh yields[J]. Nano Letters, 2023, 23(2): 523-532. |
| [30] | KANG Jian, DUAN Xiaoguang, WANG Chen, et al. Nitrogen-doped bamboo-like carbon nanotubes with Ni encapsulation for persulfate activation to remove emerging contaminants with excellent catalytic stability[J]. Chemical Engineering Journal, 2018, 332: 398-408. |
| [31] | WEI Kexin, Tao LYU, WANG Ting, et al. High adsorption of methyl orange by nitrogen-doped activated carbon derived from kraft lignin via self-activation[J]. Surfaces and Interfaces, 2023, 42: 103484. |
| [32] | HE Haijie, CHAI Kuan, WU Tao, et al. Adsorption of rhodamine B from simulated waste water onto kaolin-bentonite composites[J]. Materials, 2022, 15(12): 4058. |
| [33] | FODIL M, MAANE S, AVALOS RAMIREZ A, et al. Adsorption of lead from wastewater using olive leaf powder as biosorbent[J]. International Journal of Environmental Science and Technology, 2024, 21(3): 2615-2626. |
| [34] | GHASEMZADEH Hasan, BABAEI Saeed, TESSON Stéphane, et al. From excess to absolute adsorption isotherm: The effect of the adsorbed density[J]. Chemical Engineering Journal, 2021, 425: 131495. |
| [1] | GAO Jiaojiao, YAN Shiyu, YANG Taishun, XIE Shangzhi, YANG Yanjuan, XU Jing. Effect of alumina support crystal structure of Ru-based catalysts on polyethylene hydrogenolysis performance [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 3917-3927. |
| [2] | LIANG Shuwei, YU Jie, XIE Zhongyin, PEI Jianlu, LIN Zhongxin, CHEN Zexiang. Covalent organic frameworks for radioactive gaseous iodine adsorption [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 3965-3975. |
| [3] | HAN Pei, LI Jinjian, KE Tian, ZHANG Zhiguo, BAO Zongbi, REN Qilong, YANG Qiwei. Advances in adsorption separation of sulfur hexafluoride/nitrogen by novel porous materials [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3592-3617. |
| [4] | REN Pengkun, ZHONG Zhaoping, ZHANG Xiaoni, YANG Yuxuan, RAN Zhenzhen. Preparation of sludge-sawdust-based activated carbon and its adsorption performance for benzene series VOCs [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3031-3040. |
| [5] | ZHOU Meimei, HE Jiahui, XIANG Wanting, SHANG Jiaxin, WEI Xinyu, SUN Mimi, ZOU Wei, LUO Pingping. Electrospun PVA/SiO2 nanofibers loaded with A-TiO2/BiOBr for enhanced visible light photocatalytic activity [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3084-3092. |
| [6] | CHEN Chongming, LI Dong, YU Jinxing, CHE Kai, HE Wei, CHEN Chuanmin. Adsorption performance of titanium based MXene aerogel for Hg(Ⅱ) in desulfurization wastewater [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3112-3120. |
| [7] | LUO Yiwen, ZHAO Liang, ZHANG Yuhao, LIU Dongyang, GAO Jinsen, XU Chunming. Progress on separation materials and mechanisms of light hydrocarbons [J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2938-2954. |
| [8] | REN Shipeng, AN Yuan, LOU Chun, MEI Shengdong, LIU Kai, CHEN Xinjian. Online reconstruction of combustion temperature field distribution in furnace by integrating deep learning algorithm [J]. Chemical Industry and Engineering Progress, 2025, 44(4): 1923-1933. |
| [9] | WANG Guangya, DONG Meirong, ZHOU Jieheng, CHEN Xiang, LU Jidong. Boiler load monitoring method based on time series alignment of flame images [J]. Chemical Industry and Engineering Progress, 2025, 44(4): 1934-1944. |
| [10] | LI Ziliang, ZHANG Wei, HU Heng, WANG Yingjin, XU Na. mGAN-NN method for low-cost chemical process modeling based on generative adversarial networks [J]. Chemical Industry and Engineering Progress, 2025, 44(4): 1978-1986. |
| [11] | YUAN Mengli, SONG Yuncai, LI Wenying, FENG Jie. Heat and mass transfer law of photothermal-driven lignite fixed-bed gasification process [J]. Chemical Industry and Engineering Progress, 2025, 44(4): 2008-2019. |
| [12] | WANG Peigan, LI Leli, XIE Songzhuan, SONG Bingbing, KONG Qiaoping, LIU Gaige, MA Weiwei, SHI Xueqing. Phosphate adsorption mechanism of sludge-based FeCa-ALE composite material [J]. Chemical Industry and Engineering Progress, 2025, 44(4): 2365-2373. |
| [13] | CHEN Yuhang, LI Qiaoyan, LIANG Meisheng, SONG Tianyuan, WANG Yue, LI Simeng, ZHOU Yuxuan. Role of the Sn dopant on Cu/CeZrO2/γ-Al2O3 three-way catalyst: Enhancement of low-temperature activity and sulfur resistance [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1368-1377. |
| [14] | ZHU Shiyu, HE Yongjin, WANG Mingzi, CHEN Bilian. Research progress on microalgae to fix CO2 in flue gas from coal-fired power plants [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1666-1682. |
| [15] | ZHANG Yi, YAO Qiuxiang, SUN Ming. Adsorption performance of natural clinoptilolite based analcime and its modifications on Pb2+ [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1726-1738. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||