Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (8): 4862-4870.DOI: 10.16085/j.issn.1000-6613.2025-0541
• Frontiers and trends in process modeling and simulation • Previous Articles
Construction of UNIFAC model for ionic liquid-carbon dioxide binary system
FU Yingxue1( ), LEI Yang1, CHEN Yuqiu2, LIU Xinyan1(
), LEI Yang1, CHEN Yuqiu2, LIU Xinyan1( )
)
- 1.School of Chemistry and Chemical Engineering, Wuhan University of Science and Technology, Wuhan 430000, Hubei, China
2.College of Chemical Engineering and Environment, China University of Petroleum, Beijing 102249, China
-
Received:2025-04-14Revised:2025-05-23Online:2025-09-08Published:2025-08-25 -
Contact:LIU Xinyan
离子液体-二氧化碳体系UNIFAC模型的构建
- 1.武汉科技大学化学与化工学院,湖北 武汉 430000
2.中国石油大学化学工程与环境学院,北京 102249
-
通讯作者:刘芯妍 -
作者简介:付映雪(1999—),女,博士研究生,研究方向为离子液体热力学模型。E-mail:Fuyingxue1999@163.com。 -
基金资助:国家自然科学基金青年项目(22208253)
CLC Number:
Cite this article
FU Yingxue, LEI Yang, CHEN Yuqiu, LIU Xinyan. Construction of UNIFAC model for ionic liquid-carbon dioxide binary system[J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4862-4870.
付映雪, 雷杨, 陈毓秋, 刘芯妍. 离子液体-二氧化碳体系UNIFAC模型的构建[J]. 化工进展, 2025, 44(8): 4862-4870.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2025-0541
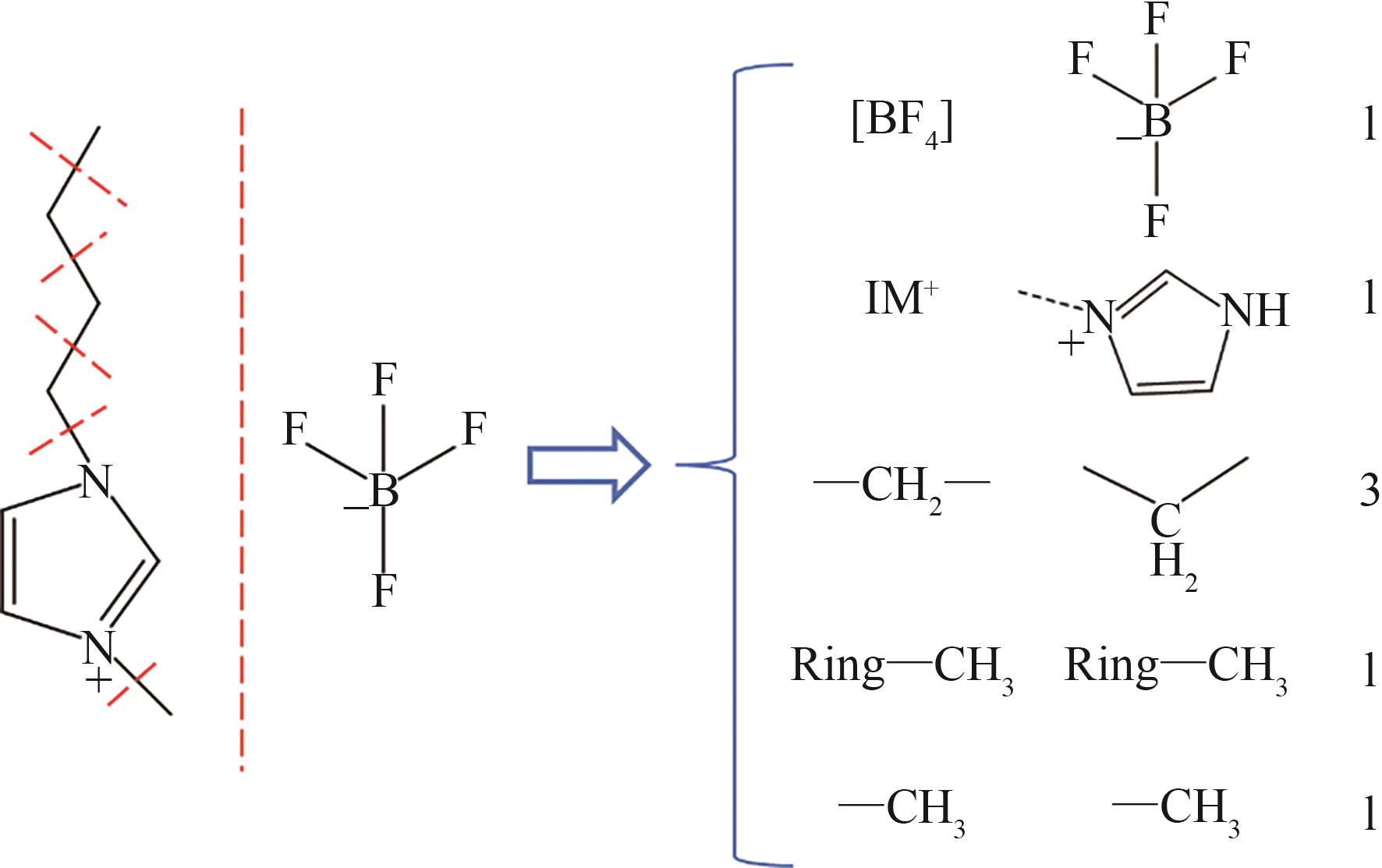
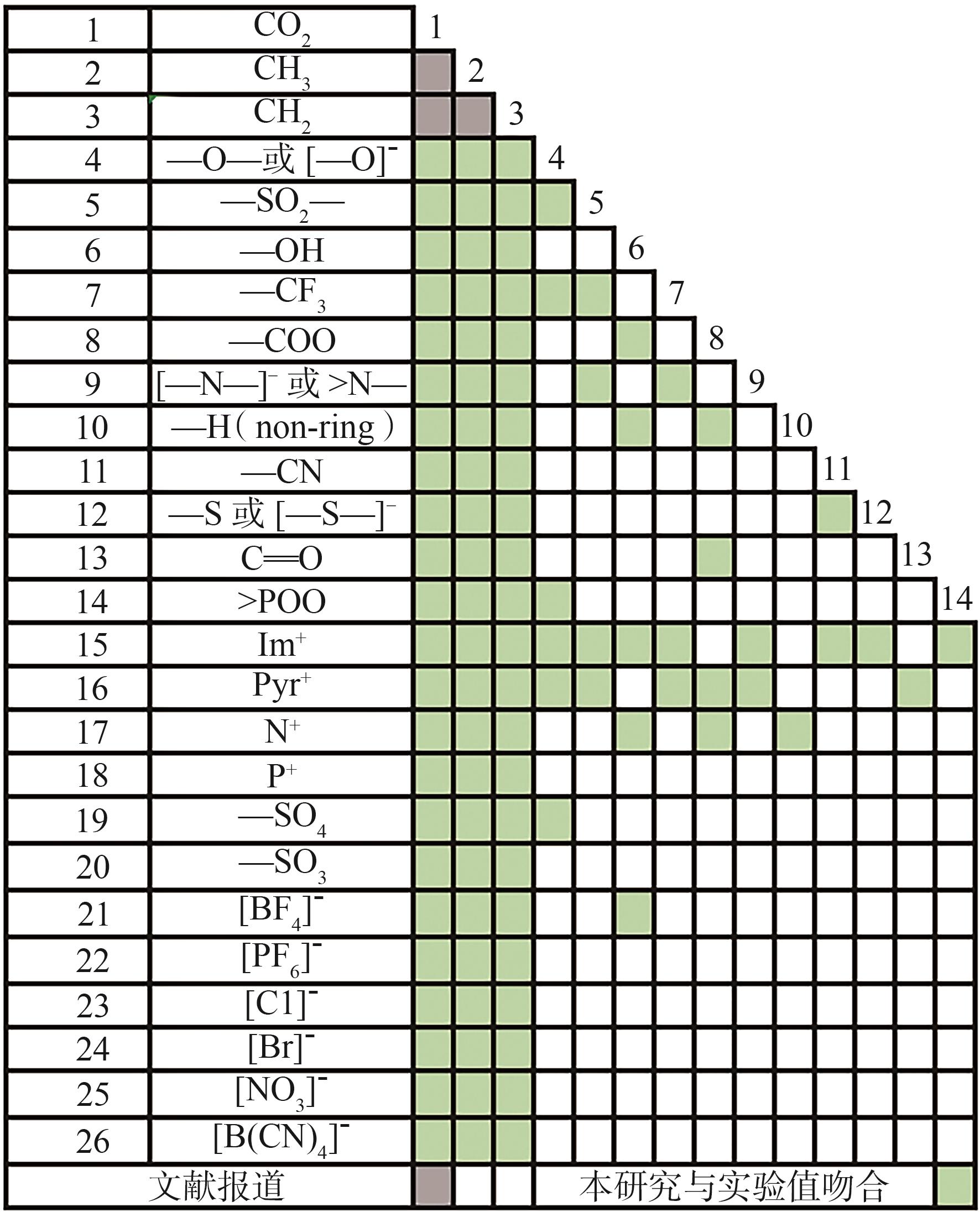
| 基团序号 | 基团化学式 | 基团结构式 |
|---|---|---|
| 1 | Im+ | 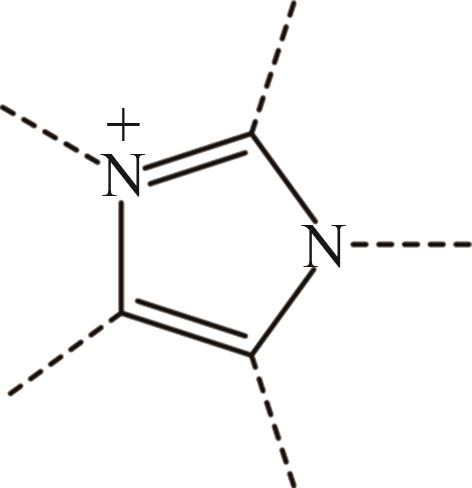 |
| 2 | Pyr+ | 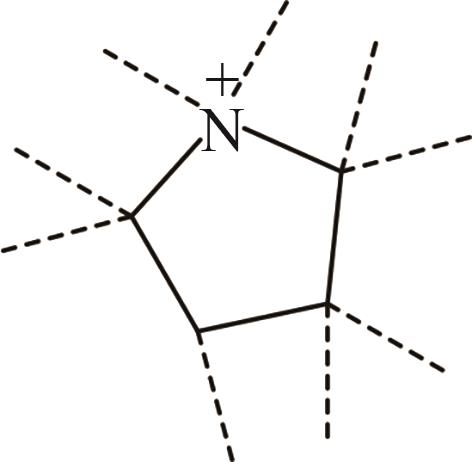 |
| 3 | N+ | 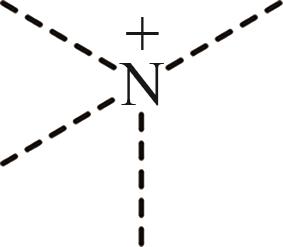 |
| 4 | P+ | 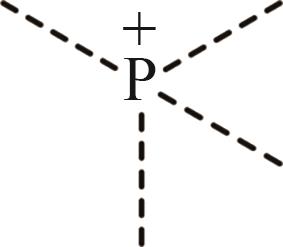 |
| 5 | [BF4]- | 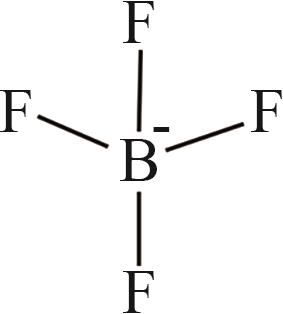 |
| 6 | [PF6]- | 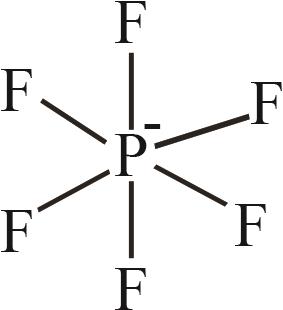 |
| 7 | [Cl]- | ---Cl |
| 8 | [Br]- | ---Br |
| 9 | [NO3]- | 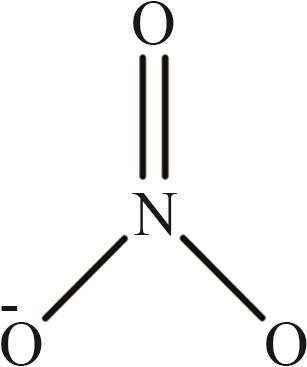 |
| 10 | —CN |  |
| 11 | —SO2— | 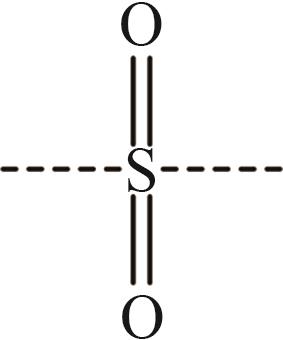 |
| 12 | —CH3 | 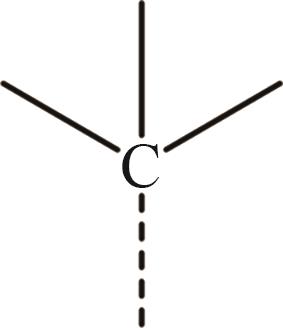 |
| 基团序号 | 基团化学式 | 基团结构式 |
|---|---|---|
| 1 | Im+ |  |
| 2 | Pyr+ |  |
| 3 | N+ |  |
| 4 | P+ |  |
| 5 | [BF4]- |  |
| 6 | [PF6]- |  |
| 7 | [Cl]- | ---Cl |
| 8 | [Br]- | ---Br |
| 9 | [NO3]- |  |
| 10 | —CN |  |
| 11 | —SO2— | 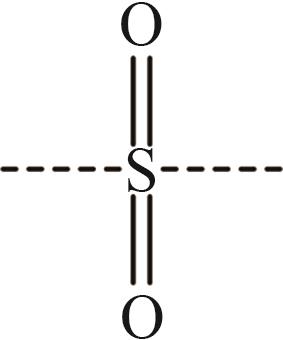 |
| 12 | —CH3 |  |
| 序号 | 基团片段 | 基团体积/Å3 | 基团表面积/Å2 | 序号 | 基团片段 | 基团体积/Å3 | 基团表面积/Å2 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| van der Waals | COSMO | van der Waals | COSMO | van der Waals | COSMO | van der Waals | COSMO | ||||
| a1 | Im+ | 76.5673 | 78.8425 | 67.0491 | 74.8054 | a31 | —H (non-ring) | 3.22×10-9 | 2.88×10-12 | 1.17×10-13 | 1.84×10-12 |
| a2 | Pyr+ | 93.0945 | 88.6008 | 68.7745 | 79.1905 | a32 | —S或[—S—]- | 41.4690 | 32.5233 | 26.0029 | 24.5613 |
| a3 | Py+ | 94.1034 | 95.2286 | 77.7260 | 86.4588 | a33 | BF3 | 56.1993 | 54.4049 | 46.7044 | 51.8305 |
| a4 | Pip+ | 112.8097 | 104.8166 | 82.1951 | 93.3278 | a34 | benzene ring | 95.5647 | 88.1758 | 75.9492 | 78.9774 |
| a5 | N+ | 18.7142 | 24.7863 | 7.1353 | 16.7011 | a35 | [—N—]-或>N— | 20.1460 | 15.9473 | 17.9156 | 16.7650 |
| a6 | P+ | 37.0570 | 40.4472 | 20.1218 | 35.8658 | a36 | C | 25.3835 | 24.9039 | 25.0013 | 25.6094 |
| a7 | S+ | 33.2359 | 39.1128 | 27.9854 | 36.0758 | a37 | —CF2— | 31.4475 | 33.5980 | 25.3543 | 30.3875 |
| a8 | [BF4]- | 61.2174 | 62.2874 | 47.1405 | 55.6563 | a38 | —CF3 | 40.5394 | 44.4884 | 33.0376 | 41.7422 |
| a9 | [PF6]- | 87.1326 | 89.2392 | 67.7333 | 82.2673 | a39 | pyraz | 80.8593 | 81.3180 | 65.7994 | 74.4410 |
| a10 | [Cl]- | 40.4777 | 27.0595 | 22.8040 | 16.5167 | a40 | F (ring) | 0 | 5.4318 | 1.1015 | 5.0601 |
| a11 | [Br]- | 48.1779 | 33.5811 | 29.3039 | 22.7081 | a41 | quin | 152.0713 | 149.0973 | 121.1241 | 130.8098 |
| a12 | [NO3]- | 55.6096 | 46.2657 | 40.2931 | 40.6047 | a42 | thiaz | 85.7933 | 90.0874 | 70.8378 | 80.9204 |
| a13 | [Aces]- | 159.4382 | 142.2671 | 128.8563 | 130.6366 | a43 | [ASF6]- | 93.7932 | 95.5519 | 74.5103 | 89.9849 |
| a14 | [BMB]- | 203.5653 | 187.1462 | 168.1461 | 171.9940 | a44 | Guan | 73.5587 | 73.1337 | 59.2302 | 69.4544 |
| a15 | [BMLB]- | 250.6386 | 221.2287 | 203.8728 | 209.2854 | a45 | [BCl4]- | 133.6517 | 118.2315 | 103.1094 | 107.6575 |
| a16 | [BOB]- | 160.6194 | 150.2053 | 136.9377 | 139.2503 | a46 | [BClF3]- | 80.5704 | 76.6895 | 63.2229 | 69.5261 |
| a17 | —CN | 28.3720 | 27.8822 | 25.8283 | 26.2420 | a47 | —COO | 50.9436 | 41.4938 | 39.5836 | 37.1174 |
| a18 | —SO2— | 48.4627 | 44.2438 | 38.6278 | 41.2222 | a48 | >POO | 66.2032 | 53.0061 | 52.0701 | 51.9813 |
| a19 | [GaCl4]- | 151.8823 | 133.3315 | 121.8828 | 127.5031 | a49 | [BBPhB]- | 449.3889 | 410.0913 | 343.7077 | 358.4873 |
| a20 | [AlCl4]- | 150.1398 | 140.3866 | 120.2852 | 119.1695 | a50 | [ClO4]- | 75.6789 | 64.7725 | 57.7302 | 59.8847 |
| a21 | —CH3 | 22.5282 | 20.2353 | 17.1410 | 18.4048 | a51 | isoquin | 153.6530 | 149.9763 | 125.8740 | 134.9791 |
| a22 | —CH2— | 23.7306 | 20.3829 | 21.2030 | 21.1868 | a52 | morph | 102.4235 | 97.5111 | 74.6057 | 86.0866 |
| a23 | >CH—或[—CH—]- | 24.1542 | 20.2246 | 18.0718 | 19.6552 | a53 | [BSB]- | 302.9004 | 280.0698 | 245.5971 | 248.8058 |
| a24 | >C<或 [>C]- | 23.0386 | 18.4646 | 9.7866 | 12.9116 | a54 | —SO3 | 70.8050 | 59.4857 | 56.4512 | 55.8970 |
| a25 | . | 17.4088 | 17.8537 | 12.0279 | 15.5348 | a55 | —SO4 | 82.1872 | 70.4684 | 67.1649 | 66.5858 |
| a26 | . | 19.7661 | 18.1608 | 17.4641 | 18.0304 | a56 | [Imidphf]- | 130.9107 | 123.9470 | 112.3453 | 122.4850 |
| a27 | 20.3524 | 17.8412 | 20.6311 | 19.6341 | a57 | [salicylate]- | 155.9603 | 140.0384 | 122.5853 | 123.0451 | |
| a28 | —H (ring) | 0.3479 | 0.5889 | 0 | 0 | a58 | [B(CN)4]- | 144.2575 | 136.6109 | 126.7380 | 128.0408 |
| a29 | —O—或[—O]- | 11.8365 | 11.3277 | 12.1500 | 11.8530 | a59 | I- | 61.4400 | 37.3200 | 43.4100 | 36.1700 |
| a30 | —OH | 9.7960 | 9.8020 | 7.0168 | 8.3065 | b0 | 拟合常数 | 11.3393 | 8.8960 | 44.4916 | 36.1650 |
| 序号 | 基团片段 | 基团体积/Å3 | 基团表面积/Å2 | 序号 | 基团片段 | 基团体积/Å3 | 基团表面积/Å2 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| van der Waals | COSMO | van der Waals | COSMO | van der Waals | COSMO | van der Waals | COSMO | ||||
| a1 | Im+ | 76.5673 | 78.8425 | 67.0491 | 74.8054 | a31 | —H (non-ring) | 3.22×10-9 | 2.88×10-12 | 1.17×10-13 | 1.84×10-12 |
| a2 | Pyr+ | 93.0945 | 88.6008 | 68.7745 | 79.1905 | a32 | —S或[—S—]- | 41.4690 | 32.5233 | 26.0029 | 24.5613 |
| a3 | Py+ | 94.1034 | 95.2286 | 77.7260 | 86.4588 | a33 | BF3 | 56.1993 | 54.4049 | 46.7044 | 51.8305 |
| a4 | Pip+ | 112.8097 | 104.8166 | 82.1951 | 93.3278 | a34 | benzene ring | 95.5647 | 88.1758 | 75.9492 | 78.9774 |
| a5 | N+ | 18.7142 | 24.7863 | 7.1353 | 16.7011 | a35 | [—N—]-或>N— | 20.1460 | 15.9473 | 17.9156 | 16.7650 |
| a6 | P+ | 37.0570 | 40.4472 | 20.1218 | 35.8658 | a36 | C | 25.3835 | 24.9039 | 25.0013 | 25.6094 |
| a7 | S+ | 33.2359 | 39.1128 | 27.9854 | 36.0758 | a37 | —CF2— | 31.4475 | 33.5980 | 25.3543 | 30.3875 |
| a8 | [BF4]- | 61.2174 | 62.2874 | 47.1405 | 55.6563 | a38 | —CF3 | 40.5394 | 44.4884 | 33.0376 | 41.7422 |
| a9 | [PF6]- | 87.1326 | 89.2392 | 67.7333 | 82.2673 | a39 | pyraz | 80.8593 | 81.3180 | 65.7994 | 74.4410 |
| a10 | [Cl]- | 40.4777 | 27.0595 | 22.8040 | 16.5167 | a40 | F (ring) | 0 | 5.4318 | 1.1015 | 5.0601 |
| a11 | [Br]- | 48.1779 | 33.5811 | 29.3039 | 22.7081 | a41 | quin | 152.0713 | 149.0973 | 121.1241 | 130.8098 |
| a12 | [NO3]- | 55.6096 | 46.2657 | 40.2931 | 40.6047 | a42 | thiaz | 85.7933 | 90.0874 | 70.8378 | 80.9204 |
| a13 | [Aces]- | 159.4382 | 142.2671 | 128.8563 | 130.6366 | a43 | [ASF6]- | 93.7932 | 95.5519 | 74.5103 | 89.9849 |
| a14 | [BMB]- | 203.5653 | 187.1462 | 168.1461 | 171.9940 | a44 | Guan | 73.5587 | 73.1337 | 59.2302 | 69.4544 |
| a15 | [BMLB]- | 250.6386 | 221.2287 | 203.8728 | 209.2854 | a45 | [BCl4]- | 133.6517 | 118.2315 | 103.1094 | 107.6575 |
| a16 | [BOB]- | 160.6194 | 150.2053 | 136.9377 | 139.2503 | a46 | [BClF3]- | 80.5704 | 76.6895 | 63.2229 | 69.5261 |
| a17 | —CN | 28.3720 | 27.8822 | 25.8283 | 26.2420 | a47 | —COO | 50.9436 | 41.4938 | 39.5836 | 37.1174 |
| a18 | —SO2— | 48.4627 | 44.2438 | 38.6278 | 41.2222 | a48 | >POO | 66.2032 | 53.0061 | 52.0701 | 51.9813 |
| a19 | [GaCl4]- | 151.8823 | 133.3315 | 121.8828 | 127.5031 | a49 | [BBPhB]- | 449.3889 | 410.0913 | 343.7077 | 358.4873 |
| a20 | [AlCl4]- | 150.1398 | 140.3866 | 120.2852 | 119.1695 | a50 | [ClO4]- | 75.6789 | 64.7725 | 57.7302 | 59.8847 |
| a21 | —CH3 | 22.5282 | 20.2353 | 17.1410 | 18.4048 | a51 | isoquin | 153.6530 | 149.9763 | 125.8740 | 134.9791 |
| a22 | —CH2— | 23.7306 | 20.3829 | 21.2030 | 21.1868 | a52 | morph | 102.4235 | 97.5111 | 74.6057 | 86.0866 |
| a23 | >CH—或[—CH—]- | 24.1542 | 20.2246 | 18.0718 | 19.6552 | a53 | [BSB]- | 302.9004 | 280.0698 | 245.5971 | 248.8058 |
| a24 | >C<或 [>C]- | 23.0386 | 18.4646 | 9.7866 | 12.9116 | a54 | —SO3 | 70.8050 | 59.4857 | 56.4512 | 55.8970 |
| a25 | . | 17.4088 | 17.8537 | 12.0279 | 15.5348 | a55 | —SO4 | 82.1872 | 70.4684 | 67.1649 | 66.5858 |
| a26 | . | 19.7661 | 18.1608 | 17.4641 | 18.0304 | a56 | [Imidphf]- | 130.9107 | 123.9470 | 112.3453 | 122.4850 |
| a27 | 20.3524 | 17.8412 | 20.6311 | 19.6341 | a57 | [salicylate]- | 155.9603 | 140.0384 | 122.5853 | 123.0451 | |
| a28 | —H (ring) | 0.3479 | 0.5889 | 0 | 0 | a58 | [B(CN)4]- | 144.2575 | 136.6109 | 126.7380 | 128.0408 |
| a29 | —O—或[—O]- | 11.8365 | 11.3277 | 12.1500 | 11.8530 | a59 | I- | 61.4400 | 37.3200 | 43.4100 | 36.1700 |
| a30 | —OH | 9.7960 | 9.8020 | 7.0168 | 8.3065 | b0 | 拟合常数 | 11.3393 | 8.8960 | 44.4916 | 36.1650 |
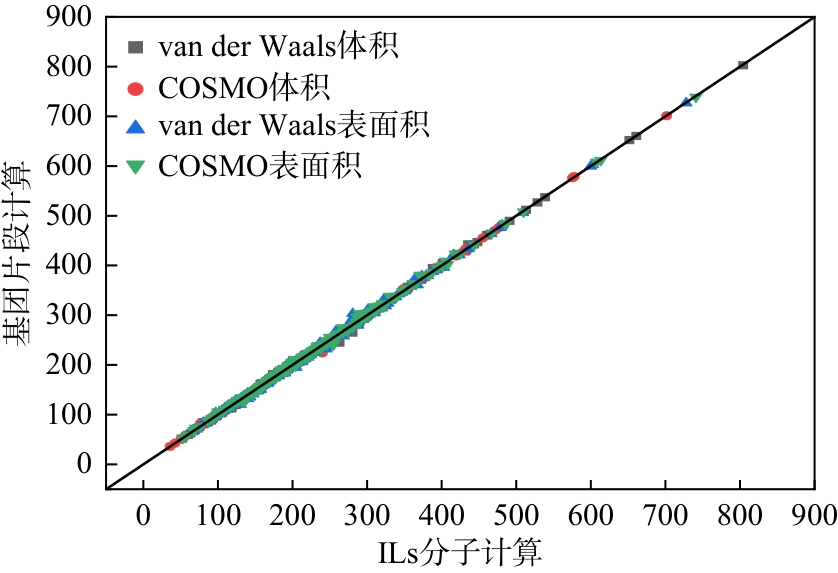
| AARD | 体积 | 比表面积 |
|---|---|---|
| COSMO | 0.0041 | 0.0119 |
| van der Waals | 0.0062 | 0.0090 |
| AARD | 体积 | 比表面积 |
|---|---|---|
| COSMO | 0.0041 | 0.0119 |
| van der Waals | 0.0062 | 0.0090 |
| 序号 | 基团 | 计算方法 | |||
|---|---|---|---|---|---|
| COSMO | van der Waals | ||||
| Qk | Rk | Qk | Rk | ||
| 1 | Im+ | 1.8023 | 3.1302 | 1.6154 | 3.0401 |
| 2 | Pyr+ | 1.9078 | 3.5177 | 1.6568 | 3.6960 |
| 3 | N+ | 0.4023 | 0.9842 | 0.1720 | 0.7428 |
| 4 | P+ | 0.8642 | 1.6060 | 0.4847 | 1.4714 |
| 5 | [BF4]- | 1.3410 | 2.4731 | 1.1357 | 2.4306 |
| 6 | [PF6]- | 1.9820 | 3.5431 | 1.6318 | 3.4594 |
| 7 | [Cl]- | 0.3980 | 1.0744 | 0.5493 | 1.6072 |
| 8 | [Br]- | 0.5471 | 1.3332 | 0.7059 | 1.9129 |
| 9 | [NO3]- | 0.9781 | 1.8371 | 0.9707 | 2.2079 |
| 10 | —CN | 0.6322 | 1.1069 | 0.6223 | 1.1264 |
| 11 | —SO2— | 0.9931 | 1.7565 | 0.9307 | 1.9240 |
| 12 | —CH3 | 0.4433 | 0.8036 | 0.4129 | 0.8945 |
| 13 | —CH2— | 0.5105 | 0.8092 | 0.5108 | 0.9422 |
| 14 | —O— | 0.2855 | 0.4498 | 0.2927 | 0.4701 |
| 15 | —OH | 0.2002 | 0.3891 | 0.1691 | 0.3891 |
| 16 | —H (non-ring) | 0 | 0 | 0 | 0 |
| 17 | —S | 0.5917 | 1.2912 | 0.6264 | 1.6465 |
| 18 | >N— | 0.4040 | 0.6333 | 0.4317 | 0.8000 |
| 19 | C | 0.6170 | 0.9886 | 0.6023 | 1.0077 |
| 20 | —CF3 | 1.0056 | 1.7664 | 0.7960 | 1.6096 |
| 21 | —COO | 0.8943 | 1.6473 | 0.9536 | 2.0225 |
| 22 | >POO | 1.2523 | 2.1047 | 1.2545 | 2.6284 |
| 23 | —SO3 | 1.3467 | 2.3620 | 1.3600 | 2.8114 |
| 24 | —SO4 | 1.6043 | 2.7979 | 1.6180 | 3.2632 |
| 25 | [B(CN)4]- | 3.0847 | 5.4239 | 3.0534 | 5.7276 |
| 序号 | 基团 | 计算方法 | |||
|---|---|---|---|---|---|
| COSMO | van der Waals | ||||
| Qk | Rk | Qk | Rk | ||
| 1 | Im+ | 1.8023 | 3.1302 | 1.6154 | 3.0401 |
| 2 | Pyr+ | 1.9078 | 3.5177 | 1.6568 | 3.6960 |
| 3 | N+ | 0.4023 | 0.9842 | 0.1720 | 0.7428 |
| 4 | P+ | 0.8642 | 1.6060 | 0.4847 | 1.4714 |
| 5 | [BF4]- | 1.3410 | 2.4731 | 1.1357 | 2.4306 |
| 6 | [PF6]- | 1.9820 | 3.5431 | 1.6318 | 3.4594 |
| 7 | [Cl]- | 0.3980 | 1.0744 | 0.5493 | 1.6072 |
| 8 | [Br]- | 0.5471 | 1.3332 | 0.7059 | 1.9129 |
| 9 | [NO3]- | 0.9781 | 1.8371 | 0.9707 | 2.2079 |
| 10 | —CN | 0.6322 | 1.1069 | 0.6223 | 1.1264 |
| 11 | —SO2— | 0.9931 | 1.7565 | 0.9307 | 1.9240 |
| 12 | —CH3 | 0.4433 | 0.8036 | 0.4129 | 0.8945 |
| 13 | —CH2— | 0.5105 | 0.8092 | 0.5108 | 0.9422 |
| 14 | —O— | 0.2855 | 0.4498 | 0.2927 | 0.4701 |
| 15 | —OH | 0.2002 | 0.3891 | 0.1691 | 0.3891 |
| 16 | —H (non-ring) | 0 | 0 | 0 | 0 |
| 17 | —S | 0.5917 | 1.2912 | 0.6264 | 1.6465 |
| 18 | >N— | 0.4040 | 0.6333 | 0.4317 | 0.8000 |
| 19 | C | 0.6170 | 0.9886 | 0.6023 | 1.0077 |
| 20 | —CF3 | 1.0056 | 1.7664 | 0.7960 | 1.6096 |
| 21 | —COO | 0.8943 | 1.6473 | 0.9536 | 2.0225 |
| 22 | >POO | 1.2523 | 2.1047 | 1.2545 | 2.6284 |
| 23 | —SO3 | 1.3467 | 2.3620 | 1.3600 | 2.8114 |
| 24 | —SO4 | 1.6043 | 2.7979 | 1.6180 | 3.2632 |
| 25 | [B(CN)4]- | 3.0847 | 5.4239 | 3.0534 | 5.7276 |
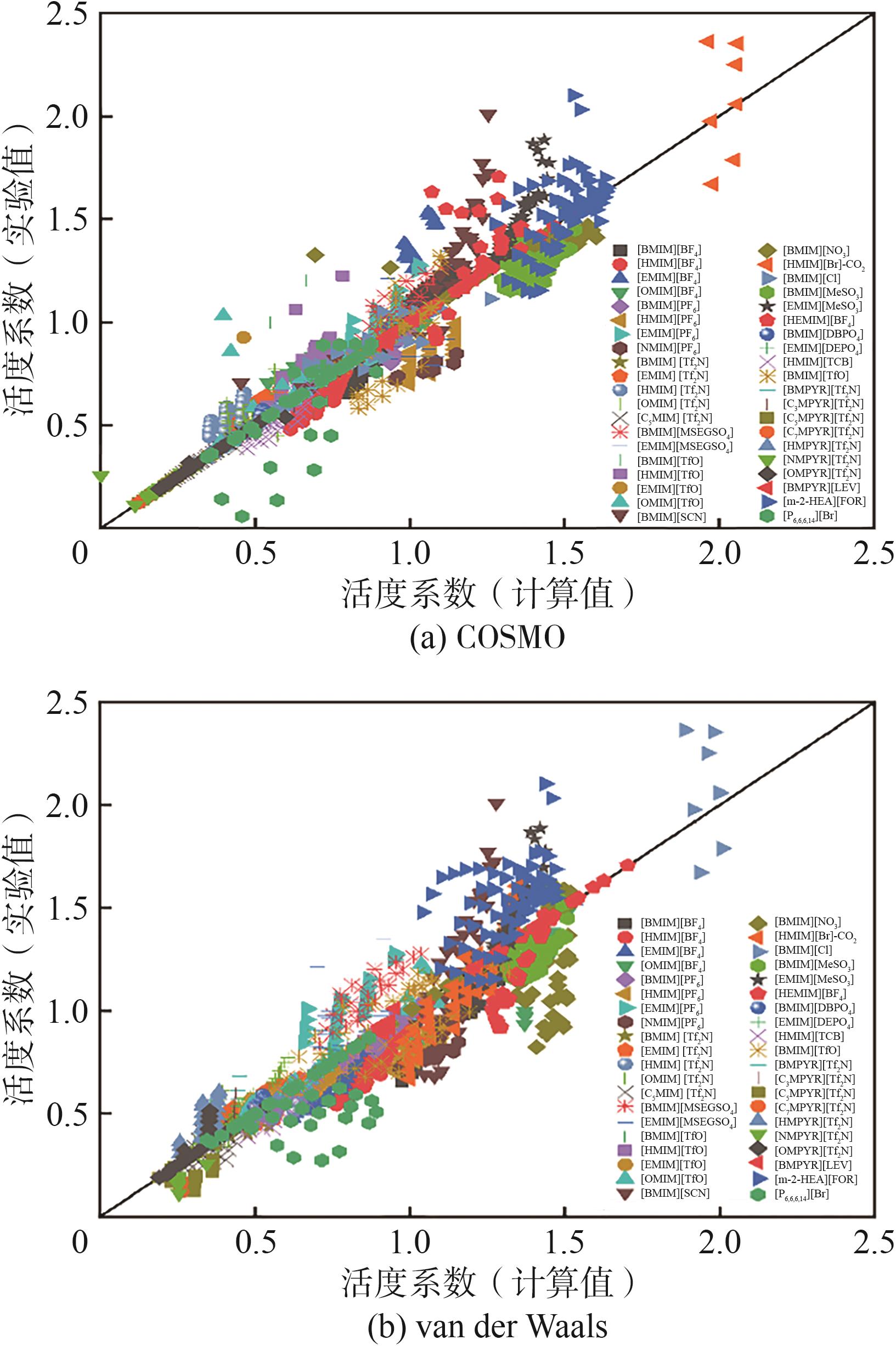
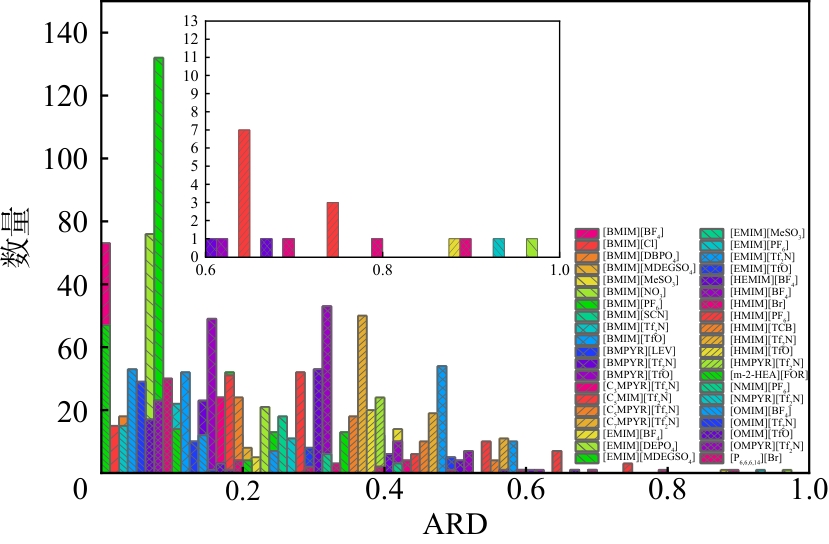
| 序号 | 体系 | 样本数量 | AARD/% | |
|---|---|---|---|---|
| COSMO | van der Walls | |||
| 总计 | 1524 | 7.86 | 12.57 | |
| 1 | [BMIM][BF4]-CO2 [HMIM][BF4]-CO2 [EMIM][BF4]-CO2 [OMIM][BF4]-CO2 | 229 | 8.30 | 15.08 |
| 2 | [BMIM][PF6]-CO2 [HMIM][PF6]-CO2 [EMIM][PF6]-CO2 [NMIM][PF6]-CO2 | 224 | 9.70 | 9.02 |
| 3 | [BMIM] [Tf2N]-CO2 [EMIM] [Tf2N]-CO2 [HMIM] [Tf2N]-CO2 [OMIM] [Tf2N]-CO2 [C5MIM] [Tf2N]-CO2 | 248 | 10.70 | 11.11 |
| 4 | [BMIM][MSEGSO4] -CO2 [EMIM][MSEGSO4] -CO2 | 55 | 13.24 | 16.51 |
| 5 | [BMIM][TfO]-CO2 [HMIM][TfO] -CO2 [EMIM][TfO] -CO2 [OMIM] [TfO]-CO2 | 107 | 7.56 | 4.60 |
| 6 | [BMIM][SCN]-CO2 | 47 | 10.06 | 18.76 |
| 7 | [BMIM][NO3]-CO2 | 80 | 3.51 | 13.98 |
| 8 | [BMIM][Cl]-CO2 | 21 | 7.44 | 13.98 |
| 9 | [HMIM][Br]-CO2 | 7 | 10.18 | 11.91 |
| 10 | [BMIM][MeSO3]-CO2 [EMIM][MeSO3]-CO2 | 79 | 9.82 | 10.26 |
| 11 | [HEMIM][OH]-CO2 | 44 | 7.48 | 9.38 |
| 12 | [BMIM][DBPO4]-CO2 [EMIM][DEPO4]-CO2 | 40 | 3.68 | 3.35 |
| 13 | [HMIM][TCB]-CO2 | 32 | 10.01 | 11.11 |
| 14 | [BMPYR][Tf2N]-CO2 [HMPYR][Tf2N]-CO2 [NMPYR][Tf2N]-CO2 [OMPYR][Tf2N]-CO2 [C3MPYR][Tf2N]-CO2 [C5MPYR][Tf2N]-CO2 [C7MPYR][Tf2N]-CO2 | 144 | 1.03 | 21.99 |
| 15 | [BMPYR][TfO]-CO2 | 49 | 0.05 | 13.86 |
| 16 | [BMPYR][LEV]-CO2 | 10 | 0.78 | 2.51 |
| 17 | [m-2-HEA][FOR]-CO2 | 65 | 7.27 | 14.21 |
| 18 | [P6,6,6,14][Br]-CO2 | 43 | 12.31 | 15.08 |
| 序号 | 体系 | 样本数量 | AARD/% | |
|---|---|---|---|---|
| COSMO | van der Walls | |||
| 总计 | 1524 | 7.86 | 12.57 | |
| 1 | [BMIM][BF4]-CO2 [HMIM][BF4]-CO2 [EMIM][BF4]-CO2 [OMIM][BF4]-CO2 | 229 | 8.30 | 15.08 |
| 2 | [BMIM][PF6]-CO2 [HMIM][PF6]-CO2 [EMIM][PF6]-CO2 [NMIM][PF6]-CO2 | 224 | 9.70 | 9.02 |
| 3 | [BMIM] [Tf2N]-CO2 [EMIM] [Tf2N]-CO2 [HMIM] [Tf2N]-CO2 [OMIM] [Tf2N]-CO2 [C5MIM] [Tf2N]-CO2 | 248 | 10.70 | 11.11 |
| 4 | [BMIM][MSEGSO4] -CO2 [EMIM][MSEGSO4] -CO2 | 55 | 13.24 | 16.51 |
| 5 | [BMIM][TfO]-CO2 [HMIM][TfO] -CO2 [EMIM][TfO] -CO2 [OMIM] [TfO]-CO2 | 107 | 7.56 | 4.60 |
| 6 | [BMIM][SCN]-CO2 | 47 | 10.06 | 18.76 |
| 7 | [BMIM][NO3]-CO2 | 80 | 3.51 | 13.98 |
| 8 | [BMIM][Cl]-CO2 | 21 | 7.44 | 13.98 |
| 9 | [HMIM][Br]-CO2 | 7 | 10.18 | 11.91 |
| 10 | [BMIM][MeSO3]-CO2 [EMIM][MeSO3]-CO2 | 79 | 9.82 | 10.26 |
| 11 | [HEMIM][OH]-CO2 | 44 | 7.48 | 9.38 |
| 12 | [BMIM][DBPO4]-CO2 [EMIM][DEPO4]-CO2 | 40 | 3.68 | 3.35 |
| 13 | [HMIM][TCB]-CO2 | 32 | 10.01 | 11.11 |
| 14 | [BMPYR][Tf2N]-CO2 [HMPYR][Tf2N]-CO2 [NMPYR][Tf2N]-CO2 [OMPYR][Tf2N]-CO2 [C3MPYR][Tf2N]-CO2 [C5MPYR][Tf2N]-CO2 [C7MPYR][Tf2N]-CO2 | 144 | 1.03 | 21.99 |
| 15 | [BMPYR][TfO]-CO2 | 49 | 0.05 | 13.86 |
| 16 | [BMPYR][LEV]-CO2 | 10 | 0.78 | 2.51 |
| 17 | [m-2-HEA][FOR]-CO2 | 65 | 7.27 | 14.21 |
| 18 | [P6,6,6,14][Br]-CO2 | 43 | 12.31 | 15.08 |
| [1] | LI Yi, SHANG Jiahui, ZHANG Chi, et al. The role of freshwater eutrophication in greenhouse gas emissions: A review[J]. Science of The Total Environment, 2021, 768: 144582. |
| [2] | KROON M C, ZUBEIR L F. Deep eutectic solvents for sustainable CO2 capture[C]//Abu Dhabi International Petroleum Exhibition & Conference. Abu Dhabi, UAE: Society of Petroleum Engineers, 2016. |
| [3] | YANG Sheng, QIAN Yu, YANG Siyu. Development of a full CO2 capture process based on the rectisol wash technology[J]. Industrial & Engineering Chemistry Research, 2016, 55(21): 6186-6193. |
| [4] | KOSTYANAYA Margarita I, NOVITSKII Eduard G, BAZHENOV Stepan D. CO2 absorption/desorption on gas-liquid membrane contactors using monoethanolamine solvent: Comparison of porous and composite hollow fibers[J]. Key Engineering Materials, 2020, 869: 321-335. |
| [5] | AHMAD Naveed, LIN Xingyu, WANG Xiaoxiao, et al. Understanding the CO2 capture performance by MDEA-based deep eutectics solvents with excellent cyclic capacity[J]. Fuel, 2021, 293: 120466. |
| [6] | SHANG Dawei, LIU Xinyan, BAI Lu, et al. Ionic liquids in gas separation processing[J]. Current Opinion in Green and Sustainable Chemistry, 2017, 5: 74-81. |
| [7] | SCHOLES Colin A, ANDERSON Clare J, STEVENS Geoff W, et al. Membrane gas separation-physical solvent absorption combined plant simulations for pre-combustion capture[J]. Energy Procedia, 2013, 37: 1039-1049. |
| [8] | SHARMA Pooja, SHARMA Shubham, KUMAR Harsh. Introduction to ionic liquids, applications and micellization behaviour in presence of different additives[J]. Journal of Molecular Liquids, 2024, 393: 123447. |
| [9] | EBRAHIMI Mohammad, FATYEYEVA Kateryna, KUJAWSKI Wojciech. Different approaches for the preparation of composite ionic liquid-based membranes for proton exchange membrane fuel cell applications-recent advancements[J]. Membranes, 2023, 13(6): 593. |
| [10] | FRIESS Karel, Pavel IZÁK, Magda KÁRÁSZOVÁ, et al. A review on ionic liquid gas separation membranes[J]. Membranes, 2021, 11(2): 97. |
| [11] | WATKINS Tylan, KUMAR Ashok, BUTTRY Daniel A. Designer ionic liquids for reversible electrochemical deposition/dissolution of magnesium[J]. Journal of the American Chemical Society, 2016, 138(2): 641-650. |
| [12] | TAHERI Mohsen, ZHU Ruisong, YU Gangqiang, et al. Ionic liquid screening for CO2 capture and H2S removal from gases: The syngas purification case[J]. Chemical Engineering Science, 2021, 230: 116199. |
| [13] | RASHID Taslim Ur. Ionic liquids: Innovative fluids for sustainable gas separation from industrial waste stream[J]. Journal of Molecular Liquids, 2021, 321: 114916. |
| [14] | GREER Adam J, JACQUEMIN Johan, HARDACRE Christopher. Industrial applications of ionic liquids[J]. Molecules, 2020, 25(21): 5207. |
| [15] | CHEN Yuqiu, LIU Xinyan, WOODLEY John M, et al. Gas solubility in ionic liquids: UNIFAC-IL model extension[J]. Industrial & Engineering Chemistry Research, 2020, 59(38): 16805-16821. |
| [16] | KLAMT Andreas, ECKERT Frank. COSMO-RS: A novel and efficient method for the a priori prediction of thermophysical data of liquids[J]. Fluid Phase Equilibria, 2000, 172(1): 43-72. |
| [17] | FANG Jing, ZHAO Rui, SU Weiyi, et al. A molecular design method based on the COSMO-SAC model for solvent selection in ionic liquid extractive distillation[J]. AIChE Journal, 2016, 62(8): 2853-2869. |
| [18] | COTO Baudilio, Inmaculada SUÁREZ, TENORIO Maria José, et al. Oil acidity reduction by extraction with imidazolium ionic liquids: Experimental, COSMO description and reutilization study[J]. Separation and Purification Technology, 2021, 254: 117529. |
| [19] | Urszula DOMAŃSKA, Michał WLAZŁO, Kamil PADUSZYŃSKI. Extraction of butan-1-ol from aqueous solution using ionic liquids: An effect of cation revealed by experiments and thermodynamic models[J]. Separation and Purification Technology, 2018, 196: 71-81. |
| [20] | BERALDO Cleiton S, LIANG Xiaodong, FOLLEGATTI-ROMERO Luis A. Predicting the solubility of gases in imidazolium-based ionic liquids with SAFT-VR Mie EoS by a novel approach based on COSMO[J]. Chemical Engineering Science, 2024, 285: 119610. |
| [21] | HAN Jingli, DAI Chengna, LEI Zhigang, et al. Gas drying with ionic liquids[J]. AIChE Journal, 2018, 64(2): 606-619. |
| [22] | DAI Chengna, LEI Zhigang, CHEN Biaohua. Gas solubility in long-chain imidazolium-based ionic liquids[J]. AIChE Journal, 2017, 63(6): 1792-1798. |
| [23] | LIU Xinyan, ZHOU Teng, ZHANG Xiangping, et al. Application of COSMO-RS and UNIFAC for ionic liquids based gas separation[J]. Chemical Engineering Science, 2018, 192: 816-828. |
| [24] | VENKATRAMAN Vishwesh, ALSBERG Bjørn Kåre. Predicting CO2 capture of ionic liquids using machine learning[J]. Journal of CO2 Utilization, 2017, 21: 162-168. |
| [25] | SONG Zhen, SHI Huaiwei, ZHANG Xiang, et al. Prediction of CO2 solubility in ionic liquids using machine learning methods[J]. Chemical Engineering Science, 2020, 223: 115752. |
| [33] | LU Tian, CHEN Feiwu. Multiwfn: A multifunctional wavefunction analyzer[J]. Journal of Computational Chemistry, 2012, 33(5): 580-592. |
| [26] | LEI Zhigang, DAI Chengna, LIU Xing, et al. Extension of the UNIFAC model for ionic liquids[J]. Industrial and Engineering Chemistry Research, 2012, 51(37): 12135-12144. |
| [27] | LEI Zhigang, DAI Chengna, YANG Qian, et al. UNIFAC model for ionic liquid-CO(H2) systems: An experimental and modeling study on gas solubility[J]. AIChE Journal, 2014, 60(12): 4222-4231. |
| [28] | DONG Yichun, GUO Yanyan, ZHU Ruisong, et al. UNIFAC model for ionic liquids. 2. Revision and extension[J]. Industrial & Engineering Chemistry Research, 2020, 59(21): 10172-10184. |
| [29] | CHEN Guzhong, SONG Zhen, QI Zhiwen, et al. Neural recommender system for the activity coefficient prediction and UNIFAC model extension of ionic liquid-solute systems[J]. AIChE Journal, 2021, 67(4): e17171. |
| [30] | ZHU Peng, KANG Xuejing, LATIF Ullah, et al. A reliable database for ionic volume and surface: Its application to predict molar volume and density of ionic liquid[J]. Industrial & Engineering Chemistry Research, 2019, 58(23): 10073-10083. |
| [31] | HUANG Ying, DONG Haifeng, ZHANG Xiangping, et al. A new fragment contribution-corresponding states method for physicochemical properties prediction of ionic liquids[J]. AIChE Journal, 2013, 59(4): 1348-1359. |
| [32] | DITCHFIELD R, HEHRE W J, POPLE J A. Self‐consistent molecular‐orbital methods. IX. An extended Gaussian‐type basis for molecular‐orbital studies of organic molecules[J]. The Journal of Chemical Physics, 1971, 54(2): 724-728. |
| [1] | HAN Yingna, LI Li, ZHANG Linzi, AN Jinze, LI Wenxiu, ZHANG Tao. Separation of methanol-acetonitrile azeotrope by ionic liquid extractive distillation [J]. Chemical Industry and Engineering Progress, 2025, 44(2): 660-668. |
| [2] | LI Xin, WANG Wei, ZHANG Yu, XIE Qiuyu, YUAN Hao. Separation of ethyl acetate+ethanol+water system: Ionic liquids screening, vapor liquid equilibrium and process simulation [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 75-85. |
| [3] | LIAO Xu, ZHOU Jun, LUO Jie, ZENG Ruilin, WANG Zeyu, LI Zunhua, LIN Jinqing. Research progress on CO2 cycloaddition reaction catalyzed by porous ionic polymers [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 4925-4940. |
| [4] | XIANG Rui, AI Bo, WU Gaosheng, LI Yuzhe, ZONG Rui, XU Baoyun, DU Lijun. Measurement and regression of solid-liquid binary equilibrium data for lithium battery additive FEC-VC system [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4246-4252. |
| [5] | LI Siwen, LEI Min, LIU Yushuang, DONG Zhaoqi, XUE Lili, ZHAO Jianshe. Research progress of ionic liquid-based heteropolyacids in fuel oxidation desulfurization [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3322-3335. |
| [6] | LIU Zepeng, ZENG Jijun, TANG Xiaobo, ZHAO Bo, HAN Sheng, LIAO Yuanhao, ZHANG Wei. Thermodynamic properties of four alkyl imidazolium phosphate ionic liquids [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1484-1491. |
| [7] | LIU Zepeng, ZENG Jijun, LIAO Yuanhao, TANG Xiaobo, ZHAO Bo, HAN Sheng, ZHANG Wei. Thermodynamic properties of 1-ethyl-3-methylimidazolium methyl phosphonate and 1-ethyl-3-methylimidazolium ethyl phosphonate [J]. Chemical Industry and Engineering Progress, 2024, 43(2): 1054-1062. |
| [8] | QI Yabing, LIU Ziyan. Research advances of extraction separation of phenolic compounds by ionic liquids [J]. Chemical Industry and Engineering Progress, 2024, 43(10): 5765-5777. |
| [9] | RONG Fanding, DING Zexiang, CAO Yifeng, CHEN Lihang, YANG Liu, SHEN Fuxing, YANG Qiwei, BAO Zongbi. Progress in enhanced separation of compounds differing in unsaturated bonds by ionic liquids [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 198-214. |
| [10] | CHEN Yaoji, REN Chengyu, HU Daqing, LU Hanfeng, GE Chunliang, CUI Guokai. Carbon monoxide conversion via ionic liquids intensification [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 124-134. |
| [11] | MI Zehao, HUA Er. Theoretical analysis of CO2 absorption by polyamines-TFSA type protic ionic liquids [J]. Chemical Industry and Engineering Progress, 2023, 42(11): 6015-6030. |
| [12] | CAO Mingmin, HAN Chengle, YANG Fang, CHEN Yuhuan. CO2 capture and separation by ionic liquid-metal organic framework composite materials [J]. Chemical Industry and Engineering Progress, 2023, 42(11): 5831-5841. |
| [13] | CHEN Yu, LIU Chong, QIU Yuhui, BI Zixin, MU Tiancheng. Ionic liquids and deep eutectic solvents for green recycle of spent lithium-ion batteries [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 485-496. |
| [14] | SHAN Qingwen, ZHANG Juan, WANG Yajuan, LIU Wenqiang. Synthesis of polymeric ionic liquid and its performance on adsorption desulfurization [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4571-4579. |
| [15] | RUAN Jiawei, YE Xiangzhu, CHEN Lifang, QI Zhiwen. Recent progress in synthesis of organic carbonates from carbon dioxide catalyzed by ionic liquids and deep eutectic solvents [J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1176-1186. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||