Chemical Industry and Engineering Progress ›› 2022, Vol. 41 ›› Issue (8): 4571-4579.DOI: 10.16085/j.issn.1000-6613.2021-1963
• Resources and environmental engineering • Previous Articles Next Articles
Synthesis of polymeric ionic liquid and its performance on adsorption desulfurization
SHAN Qingwen( ), ZHANG Juan(
), ZHANG Juan( ), WANG Yajuan, LIU Wenqiang
), WANG Yajuan, LIU Wenqiang
- College of Chemical and Pharmaceutical Engineering, Hebei University of Science and Technology, Shijiazhuang 050000, Hebei, China
-
Received:2021-09-13Revised:2022-01-20Online:2022-08-22Published:2022-08-25 -
Contact:ZHANG Juan
聚合离子液体的合成及其吸附脱硫性能
- 河北科技大学化学与制药工程学院,河北 石家庄 050000
-
通讯作者:张娟 -
作者简介:单清雯(1997—),女,硕士研究生,研究方向为吸附脱硫。E-mail:814251749@qq.com 。 -
基金资助:国家自然科学基金(21106032);河北省自然科学基金(B2021103012)
CLC Number:
Cite this article
SHAN Qingwen, ZHANG Juan, WANG Yajuan, LIU Wenqiang. Synthesis of polymeric ionic liquid and its performance on adsorption desulfurization[J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4571-4579.
单清雯, 张娟, 王亚娟, 刘文强. 聚合离子液体的合成及其吸附脱硫性能[J]. 化工进展, 2022, 41(8): 4571-4579.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2021-1963
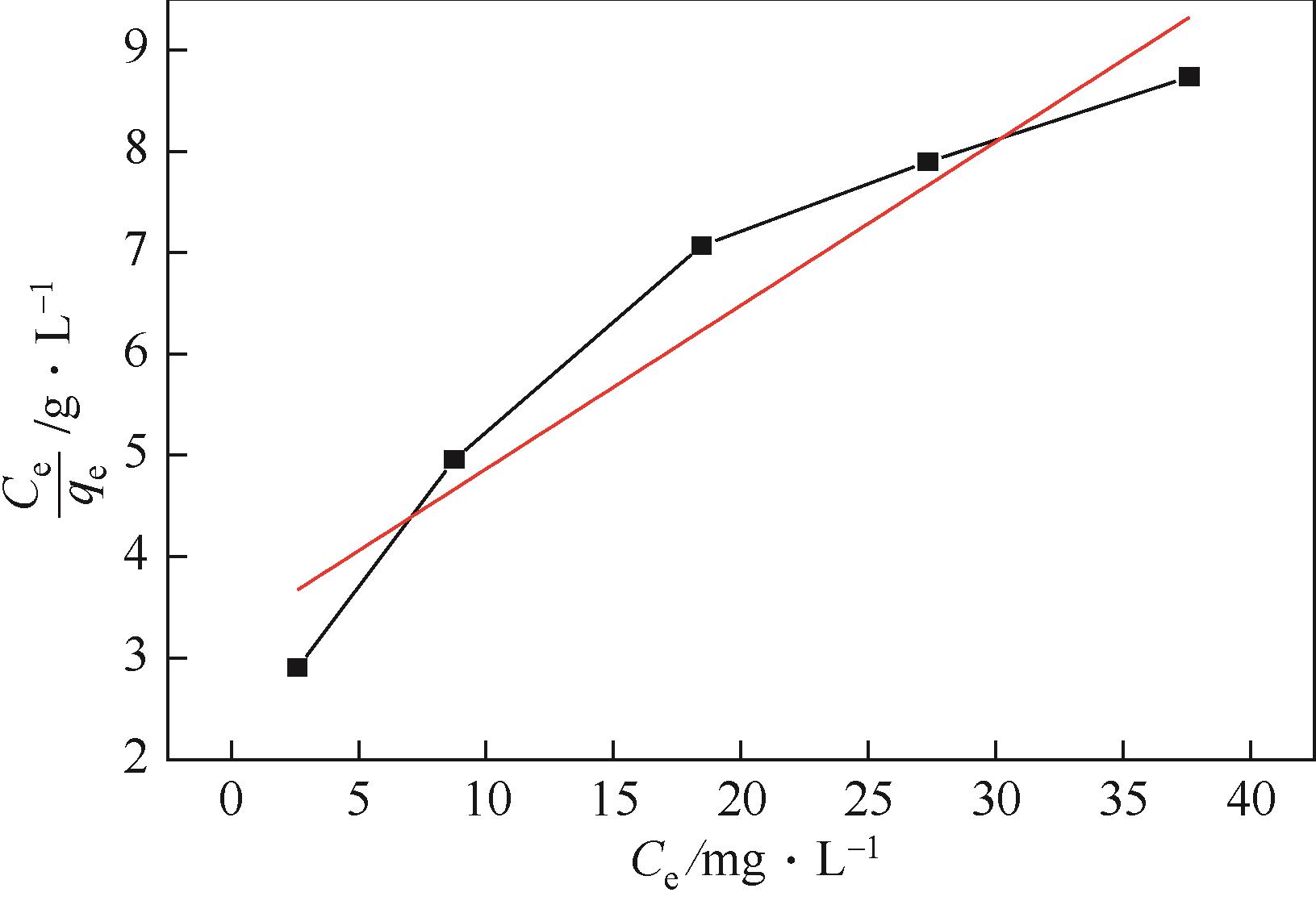
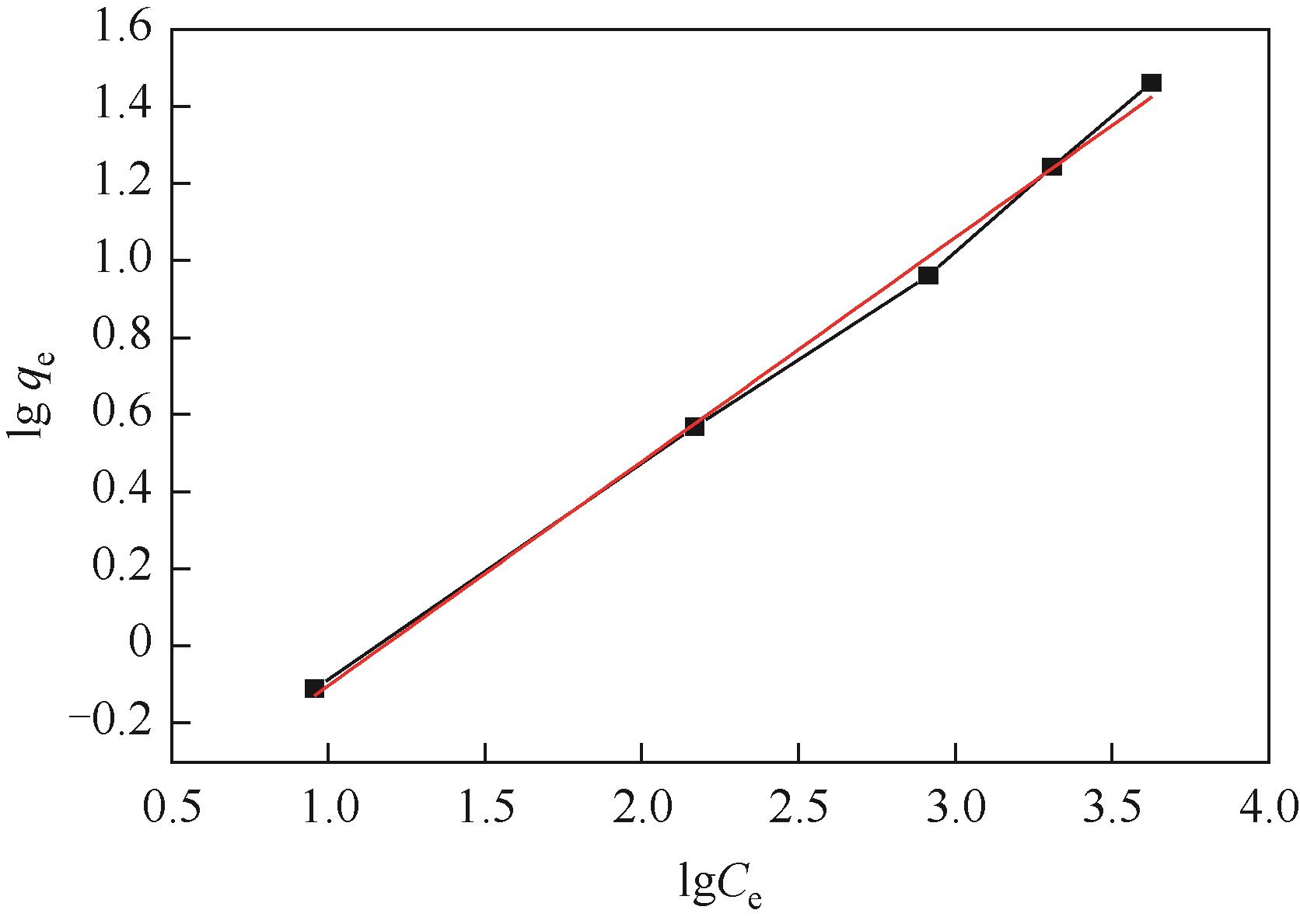
| 等温吸附模型 | 参数 | 数值 |
|---|---|---|
| Langmuir | qm/mg·g-1 | 6.198 |
| KL | 20.156 | |
| R2 | 0.89416 | |
| Freundlich | KF | 0.5048 |
| 1/n | 0.581 | |
| R2 | 0.99632 |
| 等温吸附模型 | 参数 | 数值 |
|---|---|---|
| Langmuir | qm/mg·g-1 | 6.198 |
| KL | 20.156 | |
| R2 | 0.89416 | |
| Freundlich | KF | 0.5048 |
| 1/n | 0.581 | |
| R2 | 0.99632 |
| 吸附剂 | 硫化物 种类 | 脱硫量 /mg·g-1 | 参考 文献 |
|---|---|---|---|
| Ni/ZSM-5 | DBT | 0.496 | [ |
| 过渡金属化合物负载的硅胶体系 | DBT | 0.5184 | [ |
| Ni-Ce/Al2O3-SiO2 | DBT | 3.97 | [ |
| Cu(Ⅰ)-Y 分子筛 | DBT | 4.925 | [ |
| (NMDA-Pc/Ni2+)/SiO2 | DBT | 6.196 | 本实验 |
| 吸附剂 | 硫化物 种类 | 脱硫量 /mg·g-1 | 参考 文献 |
|---|---|---|---|
| Ni/ZSM-5 | DBT | 0.496 | [ |
| 过渡金属化合物负载的硅胶体系 | DBT | 0.5184 | [ |
| Ni-Ce/Al2O3-SiO2 | DBT | 3.97 | [ |
| Cu(Ⅰ)-Y 分子筛 | DBT | 4.925 | [ |
| (NMDA-Pc/Ni2+)/SiO2 | DBT | 6.196 | 本实验 |
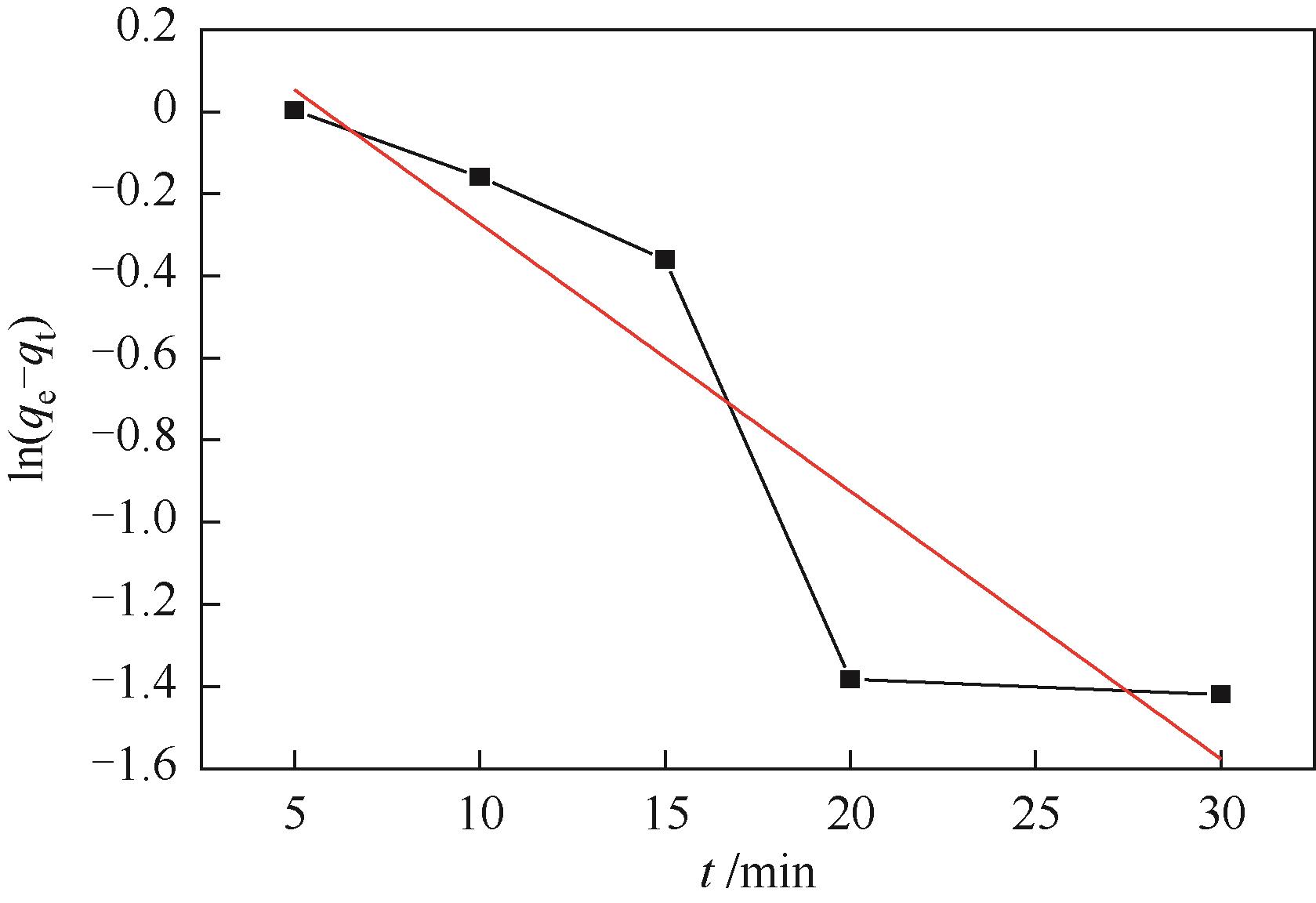
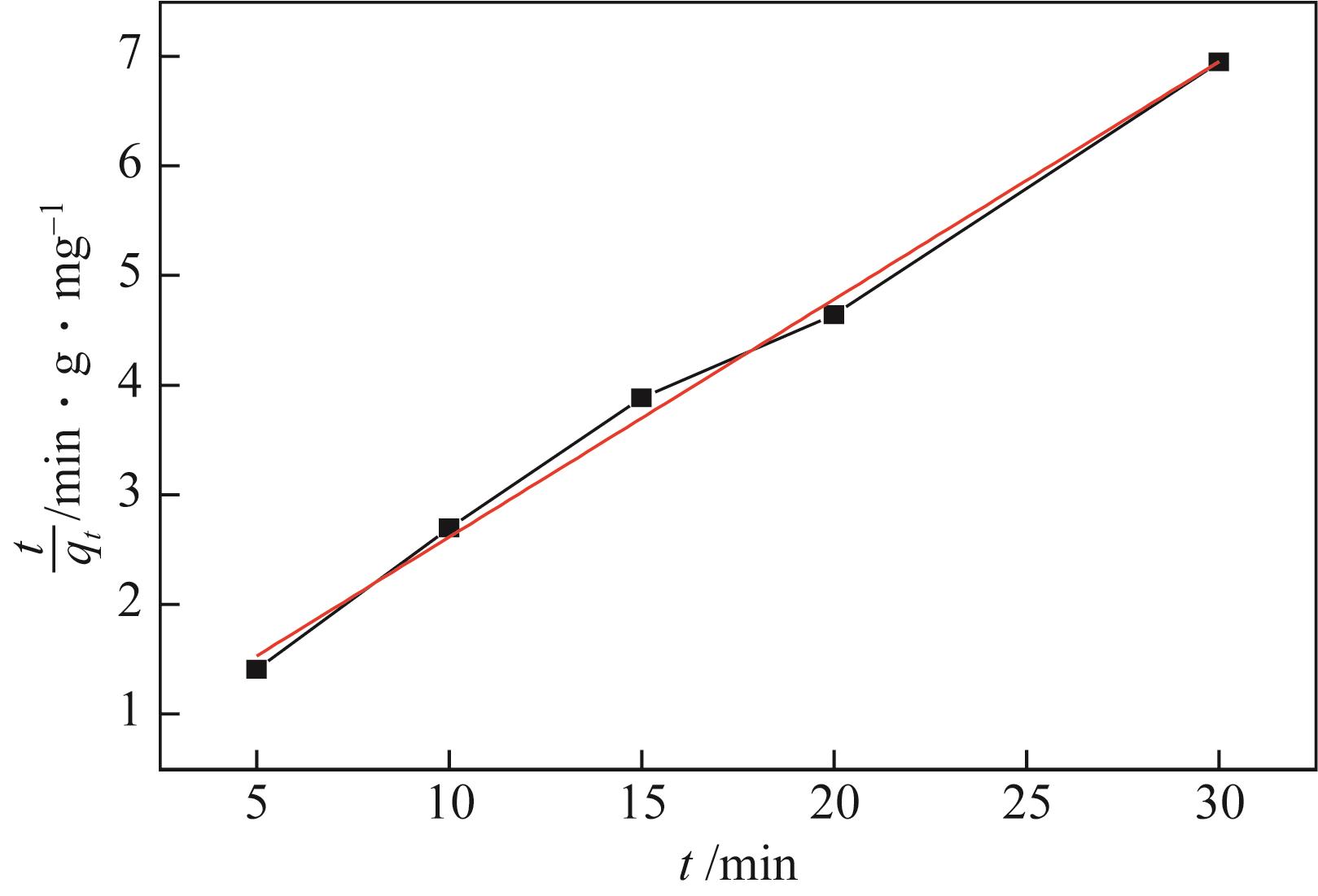
| 模型 | 参数 | 数值 |
|---|---|---|
| 拟一级动力学 | k1/min-1 | 0.06523 |
| qe,cal/mg·g-1 | 1.4619 | |
| R2 | 0.78241 | |
| 拟二级动力学 | k2/min-1 | 0.1063 |
| qe,cal/mg·g-1 | 4.6081 | |
| R2 | 0.99416 |
| 模型 | 参数 | 数值 |
|---|---|---|
| 拟一级动力学 | k1/min-1 | 0.06523 |
| qe,cal/mg·g-1 | 1.4619 | |
| R2 | 0.78241 | |
| 拟二级动力学 | k2/min-1 | 0.1063 |
| qe,cal/mg·g-1 | 4.6081 | |
| R2 | 0.99416 |
| 1 | WU Jianxiang, GAO Yilong, ZHANG Wei, et al. Deep desulfurization by oxidation using an active ionic liquid-supported Zr metal-organic framework as catalyst[J]. Applied Organometallic Chemistry, 2015, 29(2): 96-100. |
| 2 | SURYAWANSHI N B, BHANDARI V M, SOROKHAIBAM L G, et al. Investigating adsorptive deep desulfurization of fuels using metal-modified adsorbents and process intensification by acoustic cavitation[J]. Industrial & Engineering Chemistry Research, 2019, 58(18): 7593-7606. |
| 3 | 张健, 韩磊, 刘树伟, 等. 大孔Ni-Mo/Al2O3催化剂上重馏分油加氢脱硫生产低硫船用燃料油[J]. 石油化工, 2021, 50(2): 117-122. |
| ZHANG Jian, HAN Lei, LIU Shuwei, et al. Hydrodesulfurization of heavy distillate oil over macroporous Ni-Mo/Al2O3 catalyst with the aim of producing low-sulfur marine fuel[J]. Petrochemical Technology, 2021, 50(2): 117-122. | |
| 4 | FOX E B, LIU Zhongwen, LIU Zhaotie. Ultraclean fuels production and utilization for the twenty-first century: advances toward sustainable transportation fuels[J]. Energy & Fuels, 2013, 27(11): 6335-6338. |
| 5 | CHANDRA SRIVASTAVA V. An evaluation of desulfurization technologies for sulfur removal from liquid fuels[J]. RSC Adv., 2012, 2(3): 759-783. |
| 6 | 刘璇, 崔颖娜, 尹静梅, 等. 金属有机骨架材料在吸附脱硫领域的应用[J]. 化工进展, 2020, 39(8): 3163-3176. |
| LIU Xuan, CUI Yingna, YIN Jingmei, et al. Application of meta-organic frameworks materials in adsorptive desulfurization[J]. Chemical Industry and Engineering Progress, 2020, 39(8): 3163-3176. | |
| 7 | 张娟, 任腾杰, 胡颜荟, 等. MCM-41分子筛负载金属酞菁在氧化脱硫反应中的催化性能[J]. 化工学报, 2014, 65(8): 3012-3018. |
| ZHANG Juan, REN Tengjie, HU Yanhui, et al. Catalytic performance of metal phthalocyanine loaded on MCM-41 molecular sieve in oxidation desulfurization[J]. CIESC Journal, 2014, 65(8): 3012-3018. | |
| 8 | 易成高, 于寒颖, 赵欢, 等. 石油和天然气生物脱硫技术分析和展望[J]. 石油化工, 2010, 39(6): 681-687. |
| YI Chenggao, YU Hanying, ZHAO Huan, et al. Technique analysis and prospect of biologic desulphurization process for crude oil and natural gas[J]. Petrochemical Technology, 2010, 39(6): 681-687. | |
| 9 | 张娟, 李俊盼, 任腾杰, 等. [C n mim]Br/FeCl3型离子液体萃取脱除二苯并噻吩[J]. 化工学报, 2013, 64(10): 3647-3651. |
| ZHANG Juan, LI Junpan, REN Tengjie, et al. Extraction desulfurization of dibenzothiophene with [C3~8mim]Br/FeCl3 ionic liquids[J]. CIESC Journal, 2013, 64(10): 3647-3651. | |
| 10 | 刘卉, 高金森, 赵亮. 吸附脱除噻吩类硫化物机理的研究进展[J]. 石油化工, 2010, 39(9): 1059-1065. |
| LIU Hui, GAO Jinsen, ZHAO Liang. Advances in adsorptive desulfurization mechanism for thiophene-type sulfide[J]. Petrochemical Technology, 2010, 39(9): 1059-1065. | |
| 11 | WANG Sihua, ZU Yun, QIN Yucai, et al. Fabrication of effective desulfurization species active sites in the CeY zeolites and the adsorption desulfurization mechanisms[J]. Journal of Fuel Chemistry and Technology, 2020, 48( 1): 52-62. |
| 12 | BAGHERI M, MASOOMI M Y, MORSALI A. High organic sulfur removal performance of a cobalt based metal-organic framework[J]. Journal of Hazardous Materials, 2017, 331: 142-149. |
| 13 | BLANCO-BRIEVA G, CAMPOS-MARTIN J M, AL-ZAHRANI S M, et al. Effectiveness of metal-organic frameworks for removal of refractory organo-sulfur compound present in liquid fuels[J]. Fuel, 2011, 90(1): 190-197. |
| 14 | 肖永厚, 朱科润, 董晓莹, 等. 燃油选择性吸附脱硫的多孔材料研究进展[J]. 化工进展, 2020, 39(6): 2241-2250. |
| XIAO Yonghou, ZHU Kerun, DONG Xiaoying, et al. Research progress on porous materials for desulfurization of fuel by selective adsorption[J]. Chemical Industry and Engineering Progress, 2020, 39(6): 2241-2250. | |
| 15 | 杨诗, 蔡阳, 李长平, 等. 磷钨酸负载锆基金属有机骨架PTA@MOF-808的制备及其吸附脱硫性能[J]. 化工学报, 2021, 72(3): 1722-1731. |
| YANG Shi, CAI Yang, LI Changping, et al. Preparation of phosphotungstic acid loaded Zr-based metal-organic framework PTA@MOF-808 and its adsorption desulfurization performance[J]. CIESC Journal, 2021, 72(3): 1722-1731. | |
| 16 | ZHANG Junheng, CHEN Shiyuan, HE Qianjun, et al. Toughening benzoxazines with hyperbranched polymeric ionic liquids: effect of cations and anions[J]. Reactive and Functional Polymers, 2018, 133: 37-44. |
| 17 | 李春喜, 熊佳丽, 孟洪, 等. 从ILs到PILs: 聚合离子液体介孔材料的制备性质及结构调控方法[J]. 化工进展, 2014, 33(8): 1941-1950. |
| LI Chunxi, XIONG Jiali, MENG Hong, et al. From ILs to PILs: synthesis and structure tuning of poly ionic liquids mesoporous materials[J]. Chemical Industry and Engineering Progress, 2014, 33(8): 1941-1950. | |
| 18 | 冯建朋, 张香平, 尚大伟, 等. 离子液体中电化学还原CO2研究评述与展望[J]. 化工学报, 2018, 69(1): 69-75. |
| FENG Jianpeng, ZHANG Xiangping, SHANG Dawei, et al. Review and prospect of CO2 electro-reduction in ionic liquids[J]. CIESC Journal, 2018, 69(1): 69-75. | |
| 19 | CHEN Yuanzhe, ZHANG Fengwei, FANG Yiyun, et al. Phosphotungstic acid containing ionic liquid immobilized on magnetic mesoporous silica rod catalyst for the oxidation of dibenzothiophene with H2O2 [J]. Catalysis Communications, 2013, 38: 54-58. |
| 20 | 孙爽, 李未康, 张娟, 等. 聚合离子液体的吸附分离应用研究进展[J]. 现代化工, 2017, 37(6): 38-42. |
| SUN Shuang, LI Weikang, ZHANG Juan, et al. Research progress on application of polymeric ionic liquids in adsorption separation[J]. Modern Chemical Industry, 2017, 37(6): 38-42. | |
| 21 | QIAN Wenjing, TEXTER J, YAN Feng. Frontiers in poly(ionic liquid)s: syntheses and applications[J]. Chemical Society Reviews, 2017, 46(4): 1124-1159. |
| 22 | 李春喜, 许慧慧, 朱学习, 等. 吡啶基聚合离子液体的制备及其对油中噻吩硫的吸附性能[J]. 化工学报, 2016, 67(7): 2880-2886. |
| LI Chunxi, XU Huihui, ZHU Xuexi, et al. Synthesis of pyridinium based polymerized ionic liquid and its adsorptive desulfurization performance for thiophenic sulfurs from oil[J]. CIESC Journal, 2016, 67(7): 2880-2886. | |
| 23 | ZHANG Juan, SUN Shuang, BIAN Yuhang, et al. Adsorptive desulfurization of metal phthalocyanine functionalized poly-ionic liquids grafted to silica gel[J]. Fuel, 2018, 220: 513-520. |
| 24 | BI Wentao, ZHU Tao, PARK D W, et al. Sorption of carbon dioxide by ionic liquid-based sorbents[J]. Asia-Pacific Journal of Chemical Engineering, 2012, 7(1): 86-92. |
| 25 | HAN Peng, ZHANG Hongming, QIU Xuepeng, et al. Palladium within ionic liquid functionalized mesoporous silica SBA-15 and its catalytic application in room-temperature Suzuki coupling reaction[J]. Journal of Molecular Catalysis A: Chemical, 2008, 295(1/2): 57-67. |
| 26 | SAHOO S, KUMAR P, LEFEBVRE F, et al. Oxidative kinetic resolution of alcohols using chiral Mn-salen complex immobilized onto ionic liquid modified silica[J]. Applied Catalysis A: General, 2009, 354(1/2): 17-25. |
| 27 | ZHAO Dishun, ZHANG Juan, DUAN Erhong, et al. Adsorption equilibrium and kinetics of dibenzothiophene from n-octane on bamboo charcoal[J]. Applied Surface Science, 2008, 254(10): 3242-3247. |
| 28 | SRIVASTAV A, SRIVASTAVA V C. Adsorptive desulfurization by activated alumina[J]. Journal of Hazardous Materials, 2009, 170(2/3): 1133-1140. |
| 29 | MITCHELL L A, LEVAN M D. Development of adsorption equilibrium relations for mixtures from pure component isotherms and Henry’s law behavior with components in excess[J]. Industrial & Engineering Chemistry Research, 2014, 53(40): 15531-15537. |
| 30 | KUNDU S, GUPTA A K. Arsenic adsorption onto iron oxide-coated cement (IOCC): regression analysis of equilibrium data with several isotherm models and their optimization[J]. Chemical Engineering Journal, 2006, 122(1/2): 93-106. |
| 31 | SARDA K K, BHANDARI A, PANT K K, et al. Deep desulfurization of diesel fuel by selective adsorption over Ni/Al2O3 and Ni/ZSM-5 extrudates[J]. Fuel, 2012, 93: 86-91. |
| 32 | MA Xiaoliang, SUN Lu, SONG Chunshan. A new approach to deep desulfurization of gasoline, diesel fuel and jet fuel by selective adsorption for ultra-clean fuels and for fuel cell applications[J]. Catalysis Today, 2002, 77(1/2): 107-116. |
| 33 | XU Xinhai, ZHANG Shuyang, LI Peiwen, et al. Equilibrium and kinetics of Jet-A fuel desulfurization by selective adsorption at room temperatures[J]. Fuel, 2013, 111: 172-179. |
| 34 | HERNÁNDEZ-MALDONADO A J, YANG R T. Desulfurization of commercial liquid fuels by selective adsorption via π-complexation with Cu( Ⅰ )-Y zeolite[J]. Industrial & Engineering Chemistry Research, 2003, 42(13): 3103-3110. |
| 35 | CHENG Bei, LE Yao, CAI Weiquan, et al. Synthesis of hierarchical Ni(OH)2 and NiO nanosheets and their adsorption kinetics and isotherms to Congo red in water[J]. Journal of Hazardous Materials, 2011, 185(2/3): 889-897. |
| [1] | SHENG Weiwu, CHENG Yongpan, CHEN Qiang, LI Xiaoting, WEI Jia, LI Linge, CHEN Xianfeng. Operating condition analysis of the microbubble and microdroplet dual-enhanced desulfurization reactor [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 142-147. |
| [2] | WANG Shengyan, DENG Shuai, ZHAO Ruikai. Research progress on carbon dioxide capture technology based on electric swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 233-245. |
| [3] | CUI Shoucheng, XU Hongbo, PENG Nan. Simulation analysis of two MOFs materials for O2/He adsorption separation [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 382-390. |
| [4] | CHEN Chongming, CHEN Qiu, GONG Yunqian, CHE Kai, YU Jinxing, SUN Nannan. Research progresses on zeolite-based CO2 adsorbents [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 411-419. |
| [5] | XU Chunshu, YAO Qingda, LIANG Yongxian, ZHOU Hualong. Research progress on functionalization strategies of covalent organic frame materials and its adsorption properties for Hg(Ⅱ) and Cr(Ⅵ) [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 461-478. |
| [6] | GU Yongzheng, ZHANG Yongsheng. Dynamic behavior and kinetic model of Hg0 adsorption by HBr-modified fly ash [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 498-509. |
| [7] | GUO Qiang, ZHAO Wenkai, XIAO Yonghou. Numerical simulation of enhancing fluid perturbation to improve separation of dimethyl sulfide/nitrogen via pressure swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 64-72. |
| [8] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [9] | QIAN Sitian, PENG Wenjun, ZHANG Xianming. Comparative analysis of forming cyclic oligomers via PET melt polycondensation and cyclodepolymerization [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4808-4816. |
| [10] | YANG Ying, HOU Haojie, HUANG Rui, CUI Yu, WANG Bing, LIU Jian, BAO Weiren, CHANG Liping, WANG Jiancheng, HAN Lina. Coal tar phenol-based carbon nanosphere prepared by Stöber method for adsorption of CO2 [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 5011-5018. |
| [11] | ZHANG Zhen, LI Dan, CHEN Chen, WU Jinglan, YING Hanjie, QIAO Hao. Separation and purification of salivary acids with adsorption resin [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4153-4158. |
| [12] | CHEN Junjun, FEI Chang’en, DUAN Jintang, GU Xueping, FENG Lianfang, ZHANG Cailiang. Research progress on chemical modification of polyether ether ketone for the high bioactivity [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4015-4028. |
| [13] | WANG Yungang, JIAO Jian, DENG Shifeng, ZHAO Qinxin, SHAO Huaishuang. Experimental analysis of condensation heat transfer and synergistic desulfurization [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4230-4237. |
| [14] | JIANG Jing, CHEN Xiaoyu, ZHANG Ruiyan, SHENG Guangyao. Research progress of manganese-loaded biochar preparation and its application in environmental remediation [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4385-4397. |
| [15] | YU Jingwen, SONG Luna, LIU Yanchao, LYU Ruidong, WU Mengmeng, FENG Yu, LI Zhong, MI Jie. An indole-bearing hypercrosslinked polymer In-HCP for iodine adsorption from water [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3674-3683. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||