Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (2): 1054-1062.DOI: 10.16085/j.issn.1000-6613.2023-1971
• Materials science and technology • Previous Articles
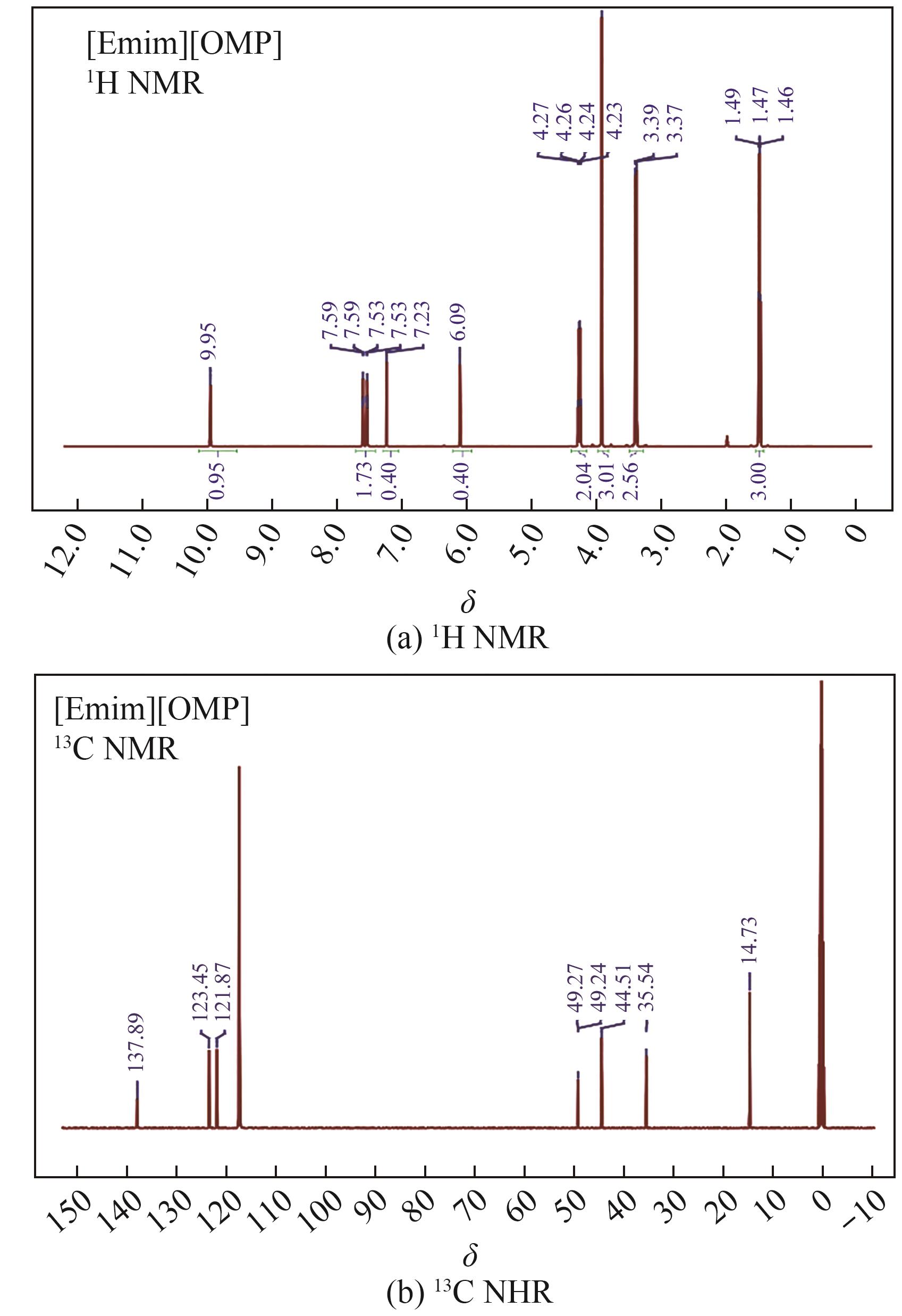
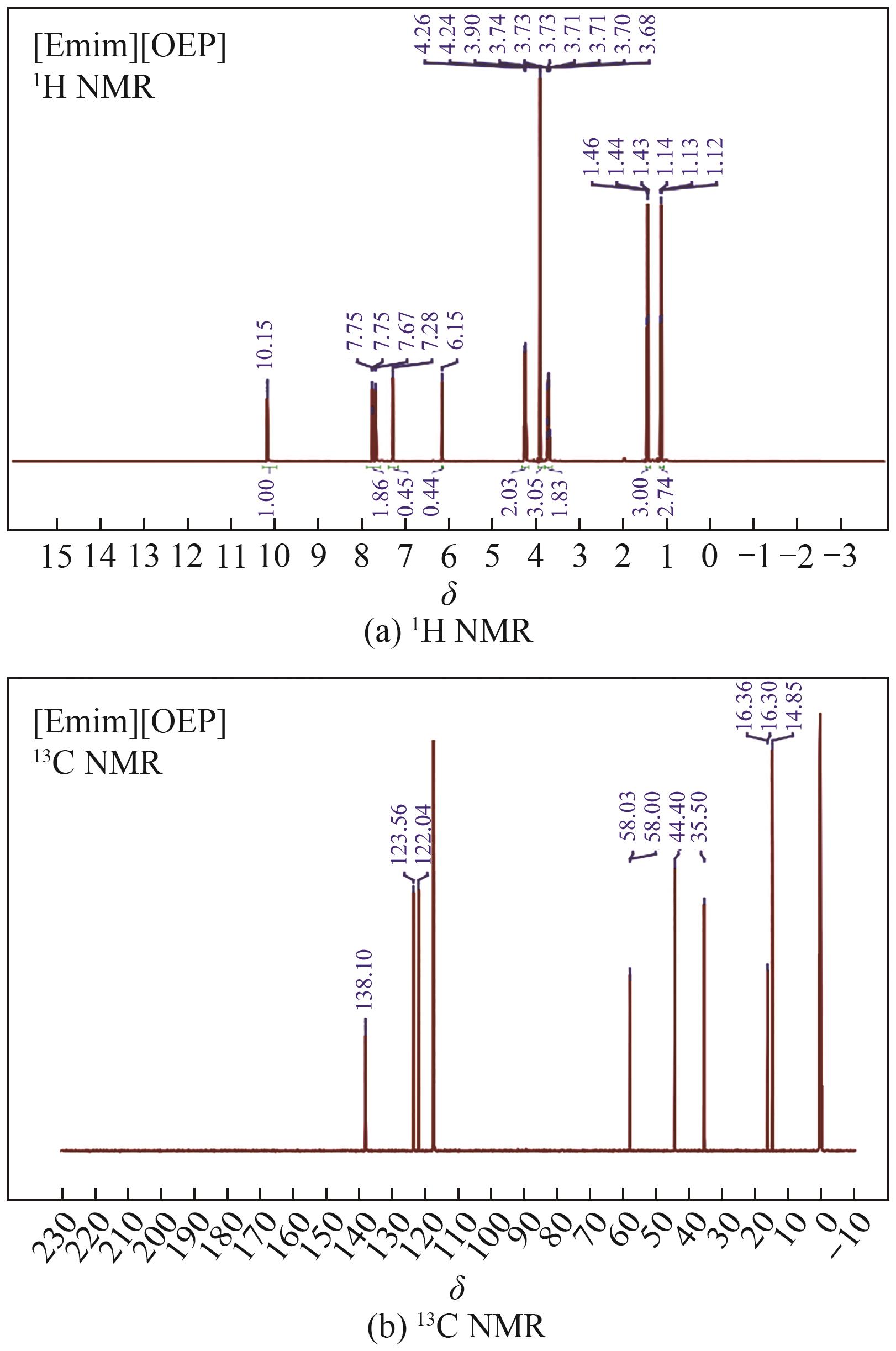
Thermodynamic properties of 1-ethyl-3-methylimidazolium methyl phosphonate and 1-ethyl-3-methylimidazolium ethyl phosphonate
LIU Zepeng( ), ZENG Jijun, LIAO Yuanhao, TANG Xiaobo, ZHAO Bo, HAN Sheng, ZHANG Wei(
), ZENG Jijun, LIAO Yuanhao, TANG Xiaobo, ZHAO Bo, HAN Sheng, ZHANG Wei( )
)
- State Key Laboratory of Fluorine & Nitrogen Chemicals, Xi'an Modern Chemistry Research Institute, Xi'an 710065, Shaanxi, China
-
Received:2023-11-13Revised:2024-01-08Online:2024-03-07Published:2024-02-25 -
Contact:ZHANG Wei
离子液体1-乙基-3-甲基咪唑亚磷酸甲酯盐与1-乙基-3-甲基咪唑亚磷酸乙酯盐的热物性
刘泽鹏( ), 曾纪珺, 廖袁淏, 唐晓博, 赵波, 韩升, 张伟(
), 曾纪珺, 廖袁淏, 唐晓博, 赵波, 韩升, 张伟( )
)
- 西安近代化学研究所,氟氮化工资源高效开发与利用国家重点实验室,陕西 西安 710065
-
通讯作者:张伟 -
作者简介:刘泽鹏(1999—),男,硕士研究生,研究方向为催化反应工程。E-mail:2456708892@qq.com。 -
基金资助:陕西省科技厅重点研发计划(2021ZDLGY13-07)
CLC Number:
Cite this article
LIU Zepeng, ZENG Jijun, LIAO Yuanhao, TANG Xiaobo, ZHAO Bo, HAN Sheng, ZHANG Wei. Thermodynamic properties of 1-ethyl-3-methylimidazolium methyl phosphonate and 1-ethyl-3-methylimidazolium ethyl phosphonate[J]. Chemical Industry and Engineering Progress, 2024, 43(2): 1054-1062.
刘泽鹏, 曾纪珺, 廖袁淏, 唐晓博, 赵波, 韩升, 张伟. 离子液体1-乙基-3-甲基咪唑亚磷酸甲酯盐与1-乙基-3-甲基咪唑亚磷酸乙酯盐的热物性[J]. 化工进展, 2024, 43(2): 1054-1062.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-1971
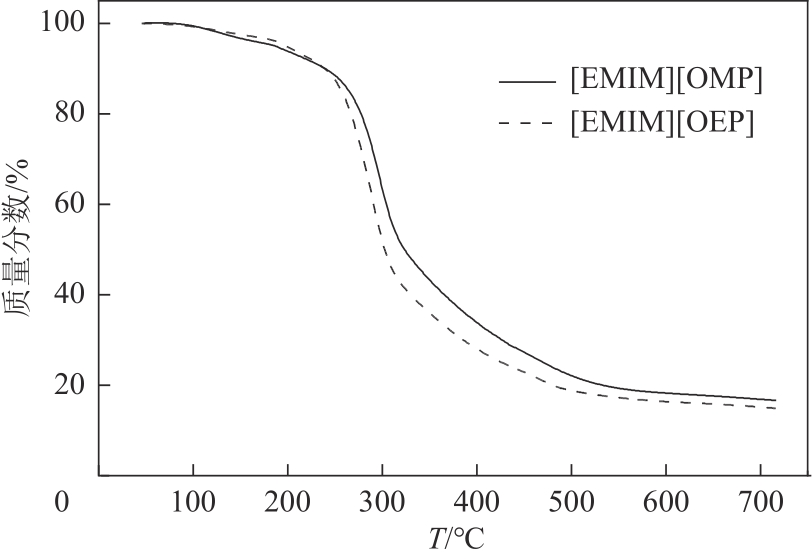
| 离子液体 | Tstart /℃ | Tonset /℃ |
|---|---|---|
| [Emim][OMP] | 172.5 | 271.0 |
| [Emim][OEP] | 167.8 | 259.2 |
| 离子液体 | Tstart /℃ | Tonset /℃ |
|---|---|---|
| [Emim][OMP] | 172.5 | 271.0 |
| [Emim][OEP] | 167.8 | 259.2 |
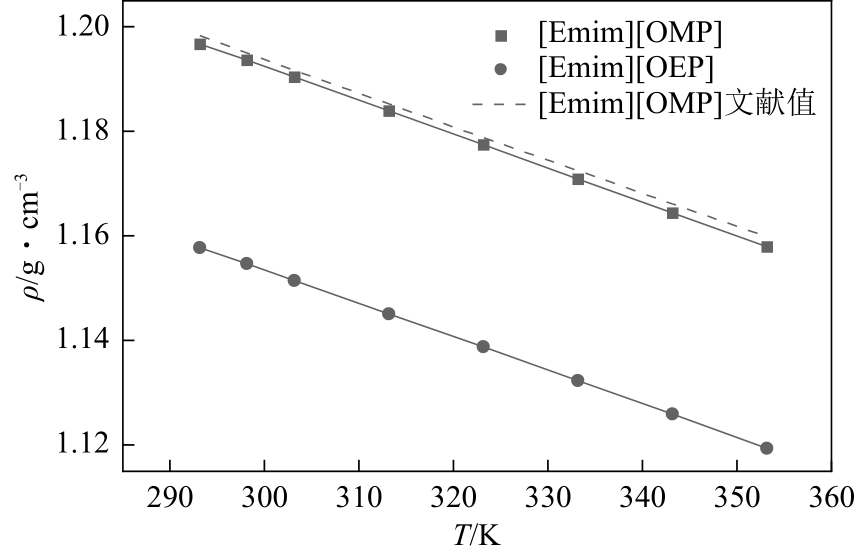
| T/K | ρ /g | ||
|---|---|---|---|
| [Emim][OMP] | [Emim][OMP](ref) [ | [Emim][OEP] | |
| 293.15 | 1.1967 | 1.1983 | 1.1578 |
| 298.15 | 1.1936 | — | 1.1547 |
| 303.15 | 1.1904 | 1.1917 | 1.1515 |
| 313.15 | 1.1840 | 1.1853 | 1.1451 |
| 323.15 | 1.1775 | 1.1788 | 1.1388 |
| 333.15 | 1.1709 | 1.1725 | 1.1323 |
| 343.15 | 1.1644 | 1.1661 | 1.1259 |
| 353.15 | 1.1580 | 1.1599 | 1.1194 |
| T/K | ρ /g | ||
|---|---|---|---|
| [Emim][OMP] | [Emim][OMP](ref) [ | [Emim][OEP] | |
| 293.15 | 1.1967 | 1.1983 | 1.1578 |
| 298.15 | 1.1936 | — | 1.1547 |
| 303.15 | 1.1904 | 1.1917 | 1.1515 |
| 313.15 | 1.1840 | 1.1853 | 1.1451 |
| 323.15 | 1.1775 | 1.1788 | 1.1388 |
| 333.15 | 1.1709 | 1.1725 | 1.1323 |
| 343.15 | 1.1644 | 1.1661 | 1.1259 |
| 353.15 | 1.1580 | 1.1599 | 1.1194 |
| 离子液体 | α/10-4K-1 | ρ0/g·cm-3 | R2 | Δ |
|---|---|---|---|---|
| [Emim][OMP] | 5.49543 | 0.34085 | 0.99991 | 0.0068% |
| [Emim][OEP] | 5.61784 | 0.31135 | 0.99992 | 0.0070% |
| 离子液体 | α/10-4K-1 | ρ0/g·cm-3 | R2 | Δ |
|---|---|---|---|---|
| [Emim][OMP] | 5.49543 | 0.34085 | 0.99991 | 0.0068% |
| [Emim][OEP] | 5.61784 | 0.31135 | 0.99992 | 0.0070% |
| 离子液体 | M/g∙mol-3 | Vm/nm3 | S⊖/J∙mol-1∙K-1 | UPOT/kJ∙mol-1 | |
|---|---|---|---|---|---|
| [Emim][OMP] | 206.179 | 0.2868 | 387.00 | 459.54 | |
| [Emim][OEP] | 220.212 | 0.3167 | 424.27 | 447.97 | |
| 离子液体 | M/g∙mol-3 | Vm/nm3 | S⊖/J∙mol-1∙K-1 | UPOT/kJ∙mol-1 | |
|---|---|---|---|---|---|
| [Emim][OMP] | 206.179 | 0.2868 | 387.00 | 459.54 | |
| [Emim][OEP] | 220.212 | 0.3167 | 424.27 | 447.97 | |
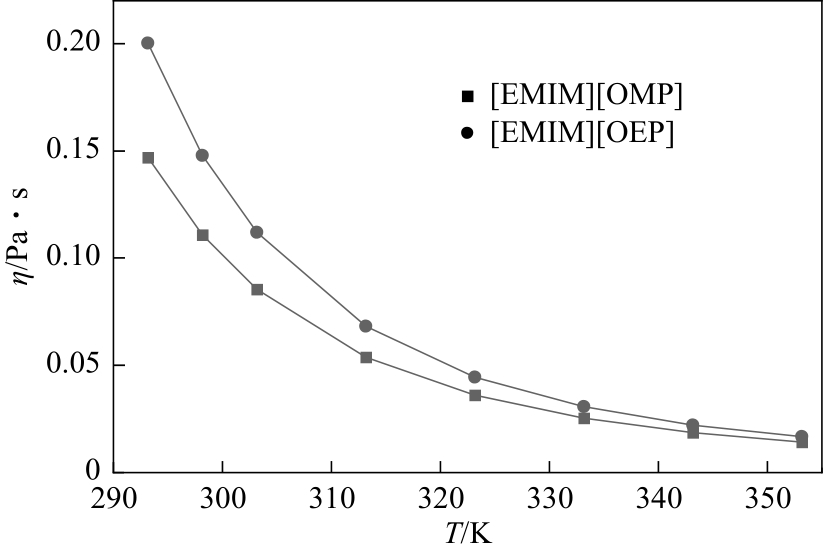
| T/K | η/Pa·s | |
|---|---|---|
| [Emim][OMP] | [Emim][OEP] | |
| 293.15 | 0.147010 | 0.200193 |
| 298.15 | 0.110750 | 0.147847 |
| 303.15 | 0.085334 | 0.111877 |
| 313.15 | 0.053695 | 0.068143 |
| 323.15 | 0.035999 | 0.044478 |
| 333.15 | 0.025411 | 0.030706 |
| 343.15 | 0.018716 | 0.022174 |
| 353.15 | 0.014278 | 0.016649 |
| T/K | η/Pa·s | |
|---|---|---|
| [Emim][OMP] | [Emim][OEP] | |
| 293.15 | 0.147010 | 0.200193 |
| 298.15 | 0.110750 | 0.147847 |
| 303.15 | 0.085334 | 0.111877 |
| 313.15 | 0.053695 | 0.068143 |
| 323.15 | 0.035999 | 0.044478 |
| 333.15 | 0.025411 | 0.030706 |
| 343.15 | 0.018716 | 0.022174 |
| 353.15 | 0.014278 | 0.016649 |
| 离子液体 | R2 | Δ | |||
|---|---|---|---|---|---|
| [Emim][OMP] | 1.6190 | 784.91 | 177.91 | >0.99999 | 0.01% |
| [Emim][OEP] | 1.4700 | 822.49 | 179.18 | >0.99999 | 0.04% |
| 离子液体 | R2 | Δ | |||
|---|---|---|---|---|---|
| [Emim][OMP] | 1.6190 | 784.91 | 177.91 | >0.99999 | 0.01% |
| [Emim][OEP] | 1.4700 | 822.49 | 179.18 | >0.99999 | 0.04% |
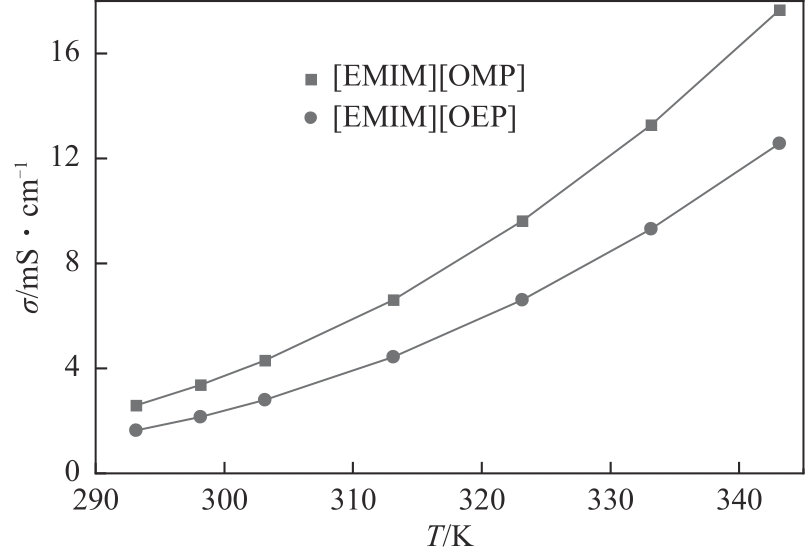
| T/K | σ/mS·cm-1 | |
|---|---|---|
| [Emim][OMP] | [Emim][OEP] | |
| 293.15 | 2.582 | 1.639 |
| 298.15 | 3.376 | 2.156 |
| 303.15 | 4.305 | 2.791 |
| 313.15 | 6.619 | 4.445 |
| 323.15 | 9.616 | 6.604 |
| 333.15 | 13.270 | 9.321 |
| 343.15 | 17.660 | 12.570 |
| T/K | σ/mS·cm-1 | |
|---|---|---|
| [Emim][OMP] | [Emim][OEP] | |
| 293.15 | 2.582 | 1.639 |
| 298.15 | 3.376 | 2.156 |
| 303.15 | 4.305 | 2.791 |
| 313.15 | 6.619 | 4.445 |
| 323.15 | 9.616 | 6.604 |
| 333.15 | 13.270 | 9.321 |
| 343.15 | 17.660 | 12.570 |
| 离子液体 | B | R2 | Δ | ||
|---|---|---|---|---|---|
| [Emim][OMP] | 1439.9 | 724.7 | 178.50 | >0.99999 | 0.35% |
| [Emim][OEP] | 1158.2 | 726.0 | 182.63 | >0.99999 | 0.73% |
| 离子液体 | B | R2 | Δ | ||
|---|---|---|---|---|---|
| [Emim][OMP] | 1439.9 | 724.7 | 178.50 | >0.99999 | 0.35% |
| [Emim][OEP] | 1158.2 | 726.0 | 182.63 | >0.99999 | 0.73% |
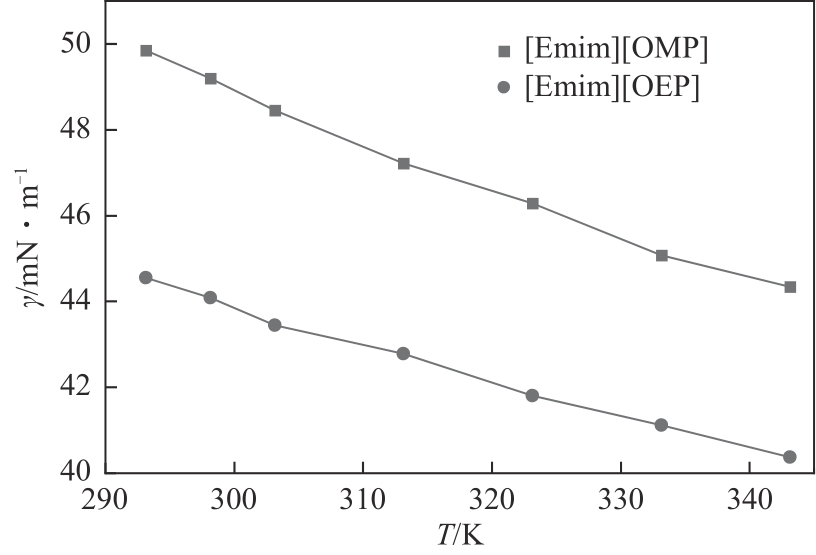
| T/K | γ/mN·m-1 | |
|---|---|---|
| [Emim][OMP] | [Emim][OEP] | |
| 293.15 | 49.85 | 44.55 |
| 298.15 | 49.19 | 44.08 |
| 303.15 | 48.45 | 43.45 |
| 313.15 | 47.22 | 42.78 |
| 323.15 | 46.28 | 41.81 |
| 333.15 | 45.07 | 41.12 |
| 343.15 | 44.34 | 40.37 |
| 298.15(ref) [ | 49.0 | — |
| 343.15(ref) [ | 46.3 | — |
| T/K | γ/mN·m-1 | |
|---|---|---|
| [Emim][OMP] | [Emim][OEP] | |
| 293.15 | 49.85 | 44.55 |
| 298.15 | 49.19 | 44.08 |
| 303.15 | 48.45 | 43.45 |
| 313.15 | 47.22 | 42.78 |
| 323.15 | 46.28 | 41.81 |
| 333.15 | 45.07 | 41.12 |
| 343.15 | 44.34 | 40.37 |
| 298.15(ref) [ | 49.0 | — |
| 343.15(ref) [ | 46.3 | — |
| 离子液体 | Hγ /mJ·m-2 | Sγ / mJ·m-2·K-1 | R2 | Δ |
|---|---|---|---|---|
| [Emim][OMP] | 172.5 | 271.0 | 0.99175 | 0.31% |
| [Emim][OEP] | 167.8 | 259.2 | 0.99453 | 0.19% |
| 离子液体 | Hγ /mJ·m-2 | Sγ / mJ·m-2·K-1 | R2 | Δ |
|---|---|---|---|---|
| [Emim][OMP] | 172.5 | 271.0 | 0.99175 | 0.31% |
| [Emim][OEP] | 167.8 | 259.2 | 0.99453 | 0.19% |
| 1 | WILKES John S. A short history of ionic liquids—From molten salts to neoteric solvents[J]. Green Chemistry, 2002, 4(2): 73-80. |
| 2 | OHNO, HIROYUKI. Importance and possibility of ionic liquids [M]. New York: John Wiley & Sons, Inc. 2005. |
| 3 | MACFARLANE Douglas R, PRINGLE Jennifer M, JOHANSSON Katarina M, et al. Lewis base ionic liquids[J]. Chemical Communications, 2006(18): 1905-1917. |
| 4 | KAZUHIDE Ueno, HIROYUKI Tokuda, MASAYOSHI Watanabe. Ionicity in ionic liquids: Correlation with ionic structure and physicochemical properties[J]. Physical Chemistry Chemical Physics: PCCP, 2010, 12(8): 1649-1658. |
| 5 | ZHOU Zhibin, HAJIME Matsumoto, KUNIAKI Tatsumi. Structure and properties of new ionic liquids based on alkyl- and alkenyltrifluoroborates[J]. Chemphyschem: a European Journal of Chemical Physics and Physical Chemistry, 2005, 6(7): 1324-1332. |
| 6 | ANDERSON Jared L, DING Rongfang, ELLERN Arkady, et al. Structure and properties of high stability geminal dicationic ionic liquids[J]. Journal of the American Chemical Society, 2005, 127(2): 593-604. |
| 7 | APPETECCHI Giovanni B, MONTANINO Maria, ZANE Daniela, et al. Effect of the alkyl group on the synthesis and the electrochemical properties of N-alkyl-N-methyl-pyrrolidinium bis(trifluoromethanesulfonyl)imide ionic liquids[J]. Electrochimica Acta, 2009, 54(4): 1325-1332. |
| 8 | DZYUBA Sergei V, BARTSCH Richard A. Influence of structural variations in 1-alkyl(aralkyl)-3-methylimidazolium hexafluorophosphates and bis(trifluoromethylsulfonyl)imides on physical properties of the ionic liquids[J]. Chemphyschem: a European Journal of Chemical Physics and Physical Chemistry, 2002, 3(2): 161-166. |
| 9 | KUNZE Miriam, JEONG Sangsik, PAILLARD Elie, et al. Melting behavior of pyrrolidinium-based ionic liquids and their binary mixtures[J]. The Journal of Physical Chemistry C, 2010, 114(28): 12364-12369. |
| 10 | SEKI Shiro, KOBAYASHI Takeshi, KOBAYASHI Yo, et al. Effects of cation and anion on physical properties of room-temperature ionic liquids[J]. Journal of Molecular Liquids, 2010, 152(1/2/3): 9-13. |
| 11 | HIROYUKI Tokuda, KUNIKAZU Ishii, Abu Bin Hasan Susan Md, et al. Physicochemical properties and structures of room-temperature ionic liquids. 3. Variation of cationic structures[J]. The Journal of Physical Chemistry B, 2006, 110(6): 2833-2839. |
| 12 | RAMAJO B, BLANCO D, RIVERA N, et al. Long-term thermal stability of fatty acid anion-based ionic liquids[J]. Journal of Molecular Liquids, 2021, 328: 115492. |
| 13 | LIU Kexin, WANG Zhuyi, SHI Liyi, et al. Ionic liquids for high performance lithium metal batteries[J]. Journal of Energy Chemistry, 2021, 59: 320-333. |
| 14 | REN Tianlin, MA Xiwen, WU Xiaoqiong, et al. Degradation of imidazolium ionic liquids in a thermally activated persulfate system[J]. Chemical Engineering Journal, 2021, 412: 128624. |
| 15 | HIROSAWA Kazu, FUJII Kenta, HASHIMOTO Kei, et al. Solvated structure of cellulose in a phosphonate-based ionic liquid[J]. Macromolecules, 2017, 50(17): 6509-6517. |
| 16 | HAN Yunyan, QIAO Dan, GUO Yuexia, et al. Influence of competitive adsorption on lubricating property of phosphonate ionic liquid additives in PEG[J]. Tribology Letters, 2016, 64(2): 22. |
| 17 | HAN Yunyan, QIAO Dan, ZHANG Lin, et al. Study of tribological performance and mechanism of phosphonate ionic liquids for steel/aluminum contact[J]. Tribology International, 2015, 84: 71-80. |
| 18 | HIRAGA Yuya, KATO Aya, SATO Yoshiyuki, et al. Densities at pressures up to 200 MPa and atmospheric pressure viscosities of ionic liquids 1-ethyl-3-methylimidazolium methylphosphate, 1-ethyl-3-methylimidazolium diethylphosphate, 1-butyl-3-methylimidazolium acetate, and 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide[J]. Journal of Chemical & Engineering Data, 2015, 60(3): 876-885. |
| 19 | HASSE Benjamin, LEHMANN Julia, ASSENBAUM Daniel, et al. Viscosity, interfacial tension, density, and refractive index of ionic liquids[EMIM][MeSO3], [EMIM][MeOHPO2], [EMIM][OcSO4], and[BBIM][NTf2]in dependence on temperature at atmospheric pressure[J]. Journal of Chemical & Engineering Data, 2009, 54(9): 2576-2583. |
| 20 | ALMEIDA Hugo F D, PASSOS Helena, LOPES-DA-SILVA José A, et al. Thermophysical properties of five acetate-based ionic liquids[J]. Journal of Chemical & Engineering Data, 2012, 57(11): 3005-3013. |
| 21 | CAO Yuanyuan, MU Tiancheng. Comprehensive investigation on the thermal stability of 66 ionic liquids by thermogravimetric analysis[J]. Industrial & Engineering Chemistry Research, 2014, 53(20): 8651-8664. |
| 22 | Pierre BONHÔTE, DIAS Ana Paula, PAPAGEORGIOU Nicholas, et al. Hydrophobic, highly conductive ambient-temperature molten salts[J]. Inorganic Chemistry, 1996, 35(5): 1168-1178. |
| 23 | ALMEIDA Hugo F D, TELES Ana Rita R, LOPES-DA-SILVA José A, et al. Influence of the anion on the surface tension of 1-ethyl-3-methylimidazolium-based ionic liquids[J]. The Journal of Chemical Thermodynamics, 2012, 54: 49-54. |
| 24 | 王义闹, 吴利丰. 基于平均相对误差绝对值最小的GM(1, 1)建模[J]. 华中科技大学学报(自然科学版), 2009, 37(10): 29-31. |
| WANG Yinao, WU Lifeng. Modeling GM(1, 1) based on the minimum of mean absolute percentage error[J]. Journal of Huazhong University of Science and Technology (Nature Science Edition), 2009, 37(10): 29-31. | |
| 25 | NEVES Catarina M S S, KIKI Adi Kurnia, COUTINHO João A P, et al. Systematic study of the thermophysical properties of imidazolium-based ionic liquids with cyano-functionalized anions[J]. The Journal of Physical Chemistry B, 2013, 117(35): 10271-10283. |
| 26 | 王晓玲, 王建英, 李小云, 等. 离子液体[C2mim]NO3与[C2mim][MetSO4]的热力学性能研究[J]. 河北科技大学学报, 2011, 32(2): 103-106. |
| WANG Xiaoling, WANG Jianying, LI Xiaoyun, et al. Study on thermophysical properties of ionic liquids of 1-ethyl-3-methylimid azolium nitrate and 1-ethyl-3-methylimidazolium methylsulfate[J]. Journal of Hebei University of Science and Technology, 2011, 32(2): 103-106. | |
| 27 | MACFARLANE Douglas R, KAR Mega, PRINGLE Jennifer M. Fundamentals of ionic liquids: From chemistry to applications [M/OB]. New York:John Wiley & Sons, 2017. DOI: 10.1002/9783527340033 . |
| 28 | ZHOU Zhibin, HAJIME Matsumoto, KUNIAKI Tatsumi. Cyclic quaternary ammonium ionic liquids with perfluoroalkyltrifluoroborates: Synthesis, characterization, and properties[J]. Chemistry, 2006, 12(8): 2196-2212. |
| 29 | TAO Duanjian, HU Wenjing, CHEN Fengfeng, et al. Low-viscosity tetramethylguanidinum-based ionic liquids with different phenolate anions: Synthesis, characterization, and physical properties[J]. Journal of Chemical & Engineering Data, 2014, 59(12): 4031-4038. |
| 30 | BITTNER Bożena, WROBEL Rafal J, MILCHERT Eugeniusz. Physical properties of pyridinium ionic liquids[J]. The Journal of Chemical Thermodynamics, 2012, 55: 159-165. |
| 31 | BRAUER Ulises G, DE LA HOZ Andreah T, MILLER Kevin M. The effect of counteranion on the physicochemical and thermal properties of 4-methyl-1-propyl-1,2,4-triazolium ionic liquids[J]. Journal of Molecular Liquids, 2015, 210: 286-292. |
| 32 | PLECHKOVA Natalia V, SEDDON Kenneth R. Applications of ionic liquids in the chemical industry[J]. Chemical Society Reviews, 2008, 37(1): 123-150. |
| 33 | Anouti MÉRIÈM, MAGALY Caillon-Caravanier, YOSRA Dridi, et al. Synthesis and characterization of new pyrrolidinium based protic ionic liquids. Good and superionic liquids[J]. The Journal of Physical Chemistry B, 2008, 112(42): 13335-13343. |
| 34 | MANN Sarah K, BROWN Steven P, MACFARLANE Douglas R. Structure effects on the ionicity of protic ionic liquids[J]. Chemphyschem: A European Journal of Chemical Physics and Physical Chemistry, 2020, 21(13): 1444-1454. |
| 35 | MOHAMMAD Tariq, FREIRE Mara G, Saramago Benilde, et al. Surface tension of ionic liquids and ionic liquid solutions[J]. Chemical Society Reviews, 2012, 41(2): 829-868. |
| 36 | KOLBECK C, LEHMANN J, LOVELOCK K R J, et al. Density and surface tension of ionic liquids[J]. The Journal of Physical Chemistry B, 2010, 114(51): 17025-17036. |
| [1] | YE Zhendong, LIU Han, LYU Jing, ZHANG Yaning, LIU Hongzhi. Optimization of thermochemical energy storage reactor based on calcium and magnesium binary salt hydrates [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4307-4314. |
| [2] | CHEN Weiyang, SONG Xin, YIN Yaran, ZHANG Xianming, ZHU Chunying, FU Taotao, MA Youguang. Effect of liquid viscosity on bubble interface in the rectangular microchannel [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3468-3477. |
| [3] | XIE Zhiwei, WU Zhangyong, ZHU Qichen, JIANG Jiajun, LIANG Tianxiang, LIU Zhenyang. Viscosity properties and magnetoviscous effects of Ni0.5Zn0.5Fe2O4 vegetable oil-based magnetic fluid [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3623-3633. |
| [4] | ZHAO Yi, YANG Zhen, ZHANG Xinwei, WANG Gang, YANG Xuan. Molecular simulation of self-healing behavior of asphalt under different crack damage and healing temperature [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3147-3156. |
| [5] | SUN Zhengnan, LI Hongjing, JING Guolin, ZHANG Funing, YAN Biao, LIU Xiaoyan. Application of EVA and its modified polymer in crude oil pour point depressant field [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2987-2998. |
| [6] | WANG Yuzhuo, LI Gang. S,N co-doped three-dimensional graphene for all-solid-state supercapacitors [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 1974-1982. |
| [7] | LI Guangwen, HUA Qucheng, HUANG Zuoxin, DA Zhijian. Progress on polymethacrylate as viscosity index improvers for lube oil [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1562-1571. |
| [8] | YANG Juanjuan, HE Lin, HE Changqing, LI Xingang, SUI Hong. Treatment of oily sludge through multiphase compound conditioning and demulsification separation process [J]. Chemical Industry and Engineering Progress, 2023, 42(2): 614-623. |
| [9] | CHEN Wenzhe, WANG Shuang, ZHAI Yuling, LI Zhouhang. Effect of aggregation state on the thermal conductivity of nanofluids [J]. Chemical Industry and Engineering Progress, 2023, 42(11): 5700-5706. |
| [10] | CUI Tengda, WEN Hua, ZHAO Ying. Comparative analysis of deposition characteristics of modified droplets impacting lotus leaf surface [J]. Chemical Industry and Engineering Progress, 2023, 42(11): 5882-5890. |
| [11] | MI Zehao, HUA Er. Theoretical analysis of CO2 absorption by polyamines-TFSA type protic ionic liquids [J]. Chemical Industry and Engineering Progress, 2023, 42(11): 6015-6030. |
| [12] | ZHU Qichen, WU Zhangyong, WANG Zhiqiang, JIANG Jiajun, LI Xiang. Sedimentation stability and viscosity properties of silicone oil-based magnetic nanofluid at low temperature [J]. Chemical Industry and Engineering Progress, 2023, 42(10): 5101-5110. |
| [13] | ZHANG Hongming, LU Jiongyuan, WANG Sanfan. Research progress on molecular structure of anion exchange membrane for fuel cells [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 318-330. |
| [14] | LI Lu, BAO Sui, ZHANG Liming, WANG Ran, TAO Zhenghong, YANG Xingxiang. Preparation and characterization of carrageenan-konjac gum hybrid gel for encapsulating fragrance [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 376-381. |
| [15] | GUO Rui, LI Ping’an, ZHAO Yunfei. Synthesis and performance of silicon modified BPA-PA phenolic epoxy resin conductive adhesive [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4473-4480. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||