| 1 |
MENG Dapeng, DAI Yao, XU Ying, et al. Energy, economic and environmental evaluations for the separation of ethyl acetate/ethanol/water mixture via distillation and pervaporation unit[J]. Process Safety and Environmental Protection, 2020, 140: 14-25.
|
| 2 |
ERNEST Flick W. Industrial solvents handbook[M]. 5th ed. New Jersey: Noyes Data Corporation, 1998: 818-819.
|
| 3 |
TOTH Andras Jozsef. Comprehensive evaluation and comparison of advanced separation methods on the separation of ethyl acetate-ethanol-water highly non-ideal mixture[J]. Separation and Purification Technology, 2019, 224: 490-508.
|
| 4 |
李群生, 王亚茹, 文放. 乙醇-水体系分离提纯过程新技术的研究[J]. 化工进展, 2015, 34(12): 4179-4184.
|
|
LI Qunsheng, WANG Yaru, WEN Fang. Research on the new technology of ethanol-water distillation[J]. Chemical Industry and Engineering Progress, 2015, 34(12): 4179-4184.
|
| 5 |
徐东芳, 胡佳静, 王丽丽, 等. 变压精馏分离乙醇-氯仿共沸物的动态特性[J]. 化工进展, 2016, 35(4): 1242-1249.
|
|
XU Dongfang, HU Jiajing, WANG Lili, et al. Dynamic characteristics of pressure-swing distillation for ethanol-chloroform separation[J]. Chemical Industry and Engineering Progress, 2016, 35(4): 1242-1249.
|
| 6 |
MA Yixin, CUI Peizhe, WANG Yongkun, et al. A review of extractive distillation from an azeotropic phenomenon for dynamic control[J]. Chinese Journal of Chemical Engineering, 2019, 27(7): 1510-1522.
|
| 7 |
张志刚, 张德彪, 张亲亲, 等. 基于COSMO-RS方法筛选离子液体分离乙酸乙酯-乙腈共沸物[J]. 化工学报, 2019, 70(1): 146-153.
|
|
ZHANG Zhigang, ZHANG Debiao, ZHANG Qinqin, et al. Screening of ionic liquids for separation of ethyl acetate-acetonitrile azeotrope based on COSMO-RS [J]. CIESC Journal, 2019, 70(1): 146-153.
|
| 8 |
GERBAUD Vincent, Ivonne RODRIGUEZ-DONIS, HEGELY Laszlo, et al. Review of extractive distillation. Process design, operation, optimization and control[J]. Chemical Engineering Research and Design, 2019, 141: 229-271.
|
| 9 |
DUAN Cong, LI Chunli. Novel energy-saving methods to improve the three-column extractive distillation process for separating ethyl acetate and ethanol using furfural[J]. Separation and Purification Technology, 2021, 272: 118887.
|
| 10 |
YANG Ao, ZOU Hechen, I-Lung CHIEN, et al. Optimal design and effective control of triple-column extractive distillation for separating ethyl acetate/ethanol/water with multiazeotrope[J]. Industrial & Engineering Chemistry Research, 2019, 58(17): 7265-7283.
|
| 11 |
AYUSO Miguel, Andrés CAÑADA-BARCALA, LARRIBA Marcos, et al. Enhanced separation of benzene and cyclohexane by homogeneous extractive distillation using ionic liquids as entrainers[J]. Separation and Purification Technology, 2020, 240: 116583.
|
| 12 |
李文秀, 张羽, 曹颖, 等. 离子液体用于四氢呋喃-乙醇-水三元共沸物系分离的研究[J]. 化工学报, 2020, 71(4): 1676-1682.
|
|
LI Wenxiu, ZHANG Yu, CAO Ying, et al. Study on separation of tetrahydrofuran-ethanol-water ternary azeotropesystem by ionic liquid[J]. CIESC Journal, 2020, 71(4): 1676-1682.
|
| 13 |
李文秀, 张琦, 张亲亲, 等. 含离子液体乙腈-正丙醇体系的等压汽液平衡[J]. 化工学报, 2015, 66(S1): 38-44.
|
|
LI Wenxiu, ZHANG Qi, ZHANG Qinqin, et al. Isobaric vapor-liquid equilibrium for system of acetonitrile-n-propanol system containing ionic liquids[J]. CIESC Journal, 2015, 66(S1): 38-44.
|
| 14 |
ZHANG Lianzheng, WANG Jie, YANG Lin, et al. Separation of isopropyl alcohol+isopropyl acetate azeotropic mixture: Selection of ionic liquids as entrainers and vapor-liquid equilibrium validation[J]. Chinese Journal of Chemical Engineering, 2022, 50: 326-334.
|
| 15 |
张清珍, 代成娜, 韩敬莉, 等. 萃取蒸馏脱除油品中硫的过程模拟与优化[J]. 化工进展, 2016, 35(8): 2553-2560.
|
|
ZHANG Qingzhen, DAI Chengna, HAN Jingli, et al. Desulfurization of oil products by extractive distillation: Simulation and optimization[J]. Chemical Industry and Engineering Progress. 2016, 35(8): 2553-2560.
|
| 16 |
高腾飞, 李国选, 雷志刚. 从催化裂化柴油中分离联苯的溶剂筛选: 实验和计算热力学[J]. 化工学报, 2022, 73(12): 5314-5323.
|
|
GAO Tengfei, LI Guoxuan, LEI Zhigang. Solvents selection for separation of biphenyl from FCC diesel: Experimental and computational thermodynamics[J]. CIESC Journal, 2022, 73(12): 5314-5323.
|
| 17 |
SALLEH M. Zulhaziman M, HADJ-KALI Mohamed K, HASHIM Mohd A,et al. Ionic liquids for the separation of benzene and cyclohexane - COSMO-RS screening and experimental validation[J]. Journal of Molecular Liquids, 2018, 266: 51-61.
|
| 18 |
MALIK Huzaifa, KHAN Huma Warsi, HASSAN SHAH Mansoor Ul, et al. Screening of ionic liquids as green entrainers for ethanol water separation by extractive distillation: COSMO-RS prediction and aspen plus simulation[J]. Chemosphere, 2022, 311(Pt 2): 136901.
|
| 19 |
VERMA Vijay Kumar, BANERJEE Tamal. Ionic liquids as entrainers for water+ethanol, water+2-propanol, and water+THF systems: A quantum chemical approach[J]. The Journal of Chemical Thermodynamics, 2010, 42(7): 909-919.
|
| 20 |
Vicent ORCHILLÉS A, MIGUEL Pablo J, LLOPIS Francisco J, et al. Isobaric vapor-liquid equilibria for the extractive distillation of ethanol+water mixtures using 1-ethyl-3-methylimidazolium dicyanamide[J]. Journal of Chemical & Engineering Data, 2011, 56(12): 4875-4880.
|
| 21 |
TSANAS Christos, TZANI Andromachi, PAPADOPOULOS Achilleas, et al. Ionic liquids as entrainers for the separation of the ethanol/water system[J]. Fluid Phase Equilibria, 2014, 379: 148-156.
|
| 22 |
GE Yun, ZHANG Lianzhong, YUAN Xingcai, et al. Selection of ionic liquids as entrainers for separation of (water+ethanol)[J]. The Journal of Chemical Thermodynamics, 2008, 40(8): 1248-1252.
|
| 23 |
ANDREATTA Alfonsina E, CHARNLEY Matthew P, BRENNECKE Joan F. Using ionic liquids to break the ethanol-ethyl acetate azeotrope[J]. ACS Sustainable Chemistry & Engineering, 2015, 3(12): 3435-3444.
|
| 24 |
LI Rui, CUI Xianbao, ZHANG Ying, et al. Vapor-liquid equilibrium and liquid-liquid equilibrium of ethyl acetate+ethanol+1-ethyl-3-methylimidazolium acetate[J]. Journal of Chemical & Engineering Data, 2012, 57(3): 911-917.
|
| 25 |
LI Qunsheng, ZHANG Jiguo, LEI Zhigang, et al. Isobaric vapor-liquid equilibrium for ethyl acetate plus ethanol+1-ethyl-3-methylimidazolium tetrafluoroborate[J]. Journal of Chemical and Engineering Data, 2009, 54(2): 193-197.
|
| 26 |
Vicent ORCHILLÉS A, MIGUEL Pablo J, VERCHER Ernesto, et al. Isobaric vapor-liquid equilibria for ethyl acetate+ethanol+1-ethyl-3-methylimidazolium trifluoromethanesulfonate at 100 kPa[J]. Journal of Chemical and Engineering Data, 2007, 52(6): 2325-2330.
|
| 27 |
ZHANG Lianzhong, YUAN Xingcai, QIAO Bingbang, et al. Isobaric vapor-liquid equilibria for water+ethanol+ethyl acetate+1-butyl-3-methylimidazolium acetate at low water mole fractions[J]. Journal of Chemical and Engineering Data, 2008, 53(7): 1595-1601.
|
| 28 |
MA Shoutao, SHANG Xianyong, LI Lumin, et al. Energy-saving thermally coupled ternary extractive distillation process using ionic liquids as entrainer for separating ethyl acetate-ethanol-water ternary mixture[J]. Separation and Purification Technology, 2019, 226: 337-349.
|
| 29 |
PAN Qi, SHANG Xianyong, MA Shoutao, et al. Control comparison of extractive distillation configurations for separating ethyl acetate-ethanol-water ternary mixture using ionic liquids as entrainer[J]. Separation and Purification Technology, 2020, 236: 116290.
|
| 30 |
ZHANG Yu, PAN Yanqiu, ZHANG Tao, et al. A comprehensive method of ionic liquid screening and experimental verification for simultaneous separation of multiple sulfides from oil[J]. Separation and Purification Technology, 2023, 315: 123714.
|
| 31 |
FREIRE Mara G, TELES Ana Rita R, ROCHA Marisa A A, et al. Thermophysical characterization of ionic liquids able to dissolve biomass[J]. Journal of Chemical and Engineering Data, 2011, 56(12): 4813-4822.
|
| 32 |
雷志刚, 王洪有, 许峥, 等. 萃取精馏的研究进展[J]. 化工进展, 2001, 20(9): 6-9.
|
|
LEI Zhigang, WANG Hongyou, XU Zheng, et al. A review of extractive distillation[J]. Chemical Industry and Engineering Progress, 2001, 20(9): 6-9.
|
| 33 |
EIDEN Philipp, BULUT Safak, Tobias KÖCHNER, et al. In silico predictions of the temperature-dependent viscosities and electrical conductivities of functionalized and nonfunctionalized ionic liquids[J]. Journal of Physical Chemistry B, 2011, 115(2): 300-309.
|
| 34 |
WANG Ping, XU Dongmei, YAN Peisong, et al. Separation of azeotrope (ethanol and ethyl methyl carbonate) by different imidazolium-based ionic liquids: Ionic liquids interaction analysis and phase equilibrium measurements[J]. Journal of Molecular Liquids, 2018, 261: 89-95.
|
| 35 |
Marek ŁUSZCZYK, MALANOWSKI Stanislaw K. Vapor-liquid equilibrium in α-methylbenzenemethanol + water[J]. Journal of Chemical and Engineering Data, 2006, 51(5): 1735-1739.
|
| 36 |
HONG Guibing, LEE Ming-Jer, LIN Ho-Mu. Multiphase coexistence for mixtures containing water, 2-propanol, and ethyl acetate[J]. Fluid Phase Equilibria, 2002, 203(1/2): 227-245.
|
| 37 |
YANG Tzu-Huai, JESSIE LUE Shingjiang. UNIQUAC and UNIQUAC-HB models for the sorption behavior of ethanol/water mixtures in a cross-linked polydimethylsiloxane membrane[J]. Journal of Membrane Science, 2012, 415: 534-545.
|
| 38 |
SUN Guangming, HUANG Weijia, ZHENG Danxing, et al. Vapor-liquid equilibrium prediction of ammonia-ionic liquid working pairs of absorption cycle using UNIFAC model[J]. Chinese Journal of Chemical Engineering, 2014, 22(1): 72-78.
|
| 39 |
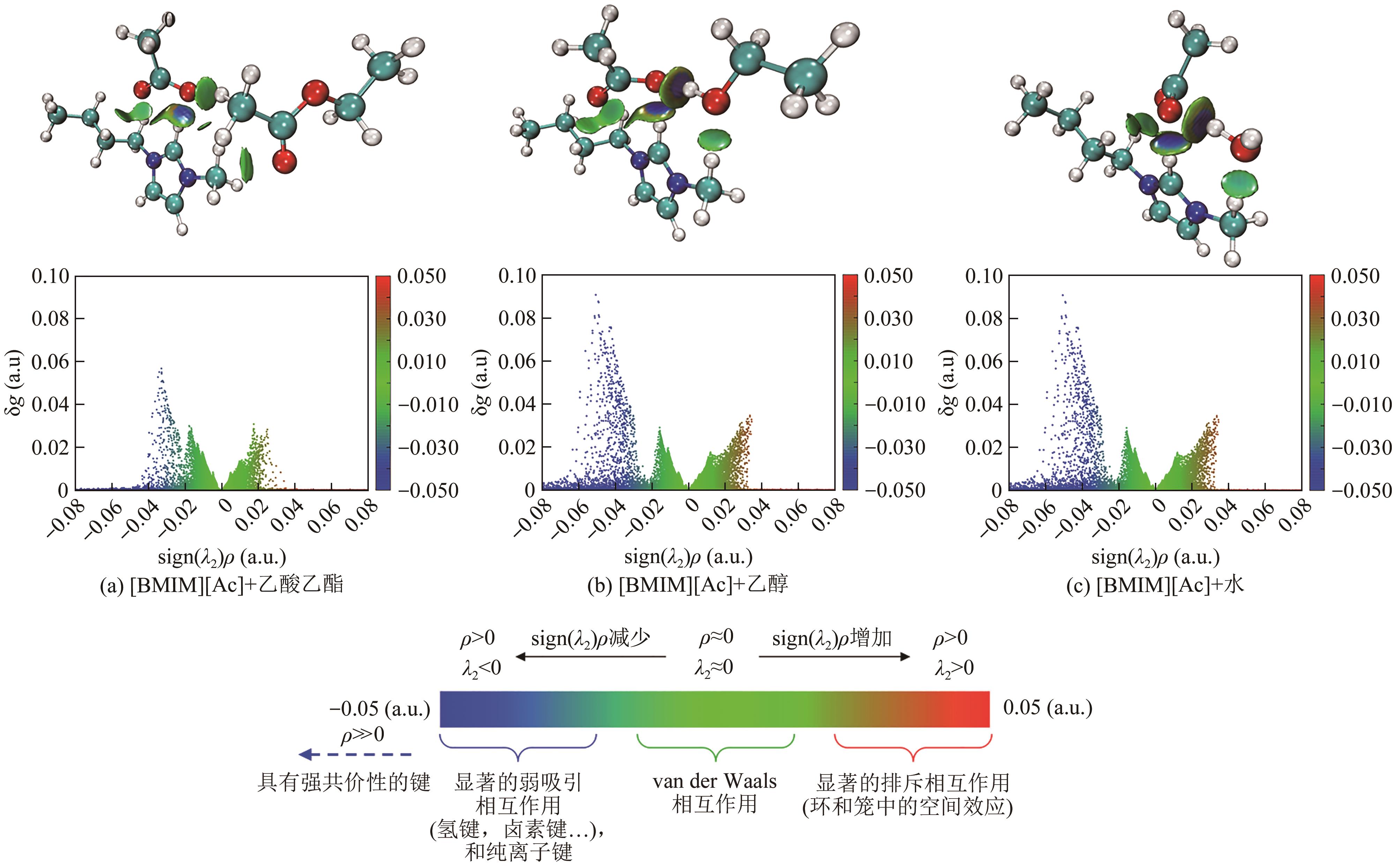
LU Tian, CHEN Qinxue. Independent gradient model based on Hirshfeld partition: A new method for visual study of interactions in chemical systems[J]. Journal of Computational Chemistry, 2022, 43(8): 539-555.
|
| 40 |
LU Tian, CHEN Feiwu. Multiwfn: A multifunctional wavefunction analyzer[J]. Journal of Computational Chemistry, 2012, 33(5): 580-592.
|
| 41 |
HUMPHREY William, DALKE Andrew, SCHULTEN Klaus. VMD: Visual molecular dynamics[J]. Journal of Molecular Graphics, 1996, 14(1): 33-38.
|
| 42 |
VALDERRAMA José O, SANGA Wilson W, LAZZÚS Juan A. Critical properties, normal boiling temperature, and acentric factor of another 200 ionic liquids[J]. Industrial & Engineering Chemistry Research, 2008, 47(4): 1318-1330.
|
| 43 |
LUYBEN William L. Distillation design and control using AspenTM simulation[M]. New Jersey: John Wiley & Sons Inc, 2013: 87-89.
|
| 44 |
LUYBEN William L, YU Cheng-Ching. Reactive distillation design and control[M]. New Jersey: John Wiley & Sons Inc, 2008: 42-43.
|
| 45 |
WISNIAK Jaime, ORTEGA Juan, Luis FERNÁNDEZ. A fresh look at the thermodynamic consistency of vapour-liquid equilibria data[J]. The Journal of Chemical Thermodynamics, 2017, 105: 385-395.
|
| 46 |
YUE Kun, ZHOU Guowei. Isobaric vapor-liquid equilibrium for ethyl acetate+ethanol with ionic liquids [MMIM][DMP] and [OMIM][PF6] as entrainers[J]. Journal of Molecular Liquids, 2022, 348: 118404.
|
| 47 |
CHEN Zhengrun, DAI Yasen, CHI Shuxiu, et al. Analysis and intensification of energy saving process for separation of azeotrope by ionic liquid extractive distillation based on molecular dynamics simulation[J]. Separation and Purification Technology, 2021, 276: 119254.
|
| 48 |
ZEESHAN Muhammad, NOZARI Vahid, KESKIN Seda, et al. Structural factors determining thermal stability limits of ionic liquid/MOF composites: Imidazolium ionic liquids combined with CuBTC and ZIF-8[J]. Industrial & Engineering Chemistry Research, 2019, 58(31): 14124-14138.
|
 ), WANG Wei(
), WANG Wei( ), ZHANG Yu, XIE Qiuyu, YUAN Hao
), ZHANG Yu, XIE Qiuyu, YUAN Hao