Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (9): 5193-5206.DOI: 10.16085/j.issn.1000-6613.2023-1903
• Resources and environmental engineering • Previous Articles
Development trend of sulfide solid electrolytes for solid-state lithium batteries in the context of “dual carbon goals”
GUO Pei1( ), CUI Cancan2, KONG Dejie2, HUANG Sheng1(
), CUI Cancan2, KONG Dejie2, HUANG Sheng1( )
)
- 1.School of Public Administration, Yanshan University, Qinhuangdao 066004, Hebei, China
2.School of Environmental and Chemical Engineering, Yanshan University, Qinhuangdao 066004, Hebei, China
-
Received:2023-10-28Revised:2023-12-26Online:2024-09-30Published:2024-09-15 -
Contact:HUANG Sheng
“双碳”背景下固态锂电池用硫化物固态电解质的发展趋势
- 1.燕山大学公共管理学院,河北 秦皇岛 066004
2.燕山大学环境与化学工程学院,河北 秦皇岛 066004
-
通讯作者:黄晟 -
作者简介:郭沛(1982—),男,副研究员,博士研究生,研究方向为能源环境政策与治理。E-mail:guopei@ysu.edu.cn。
CLC Number:
Cite this article
GUO Pei, CUI Cancan, KONG Dejie, HUANG Sheng. Development trend of sulfide solid electrolytes for solid-state lithium batteries in the context of “dual carbon goals”[J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5193-5206.
郭沛, 崔灿灿, 孔德洁, 黄晟. “双碳”背景下固态锂电池用硫化物固态电解质的发展趋势[J]. 化工进展, 2024, 43(9): 5193-5206.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-1903
| 电解质 | 优点 | 缺点 | 离子电导率/S·cm-1 |
|---|---|---|---|
| 玻璃态电解质 | 在全固态电池中利用率很高,合成简单 | 离子电导率相对较低 | <10-3 |
| 玻璃-陶瓷态电解质 | 离子电导率较高 | 合成步骤复杂 | >10-3 |
| Thio-LISICON型电解质 | 合成简单,热稳定性好,电子电导率小 | 离子电导率相对较低 | <10-3 |
| Li11-x M2-x P1+x S12(M=Ge, Sn, Si)结构 | 离子电导率高 | 成本较高,空气稳定性差 | >10-3 |
| 硫银锗矿型电解质 | 电化学稳定性好 | 空气稳定性较差 | >10-3 |
| 电解质 | 优点 | 缺点 | 离子电导率/S·cm-1 |
|---|---|---|---|
| 玻璃态电解质 | 在全固态电池中利用率很高,合成简单 | 离子电导率相对较低 | <10-3 |
| 玻璃-陶瓷态电解质 | 离子电导率较高 | 合成步骤复杂 | >10-3 |
| Thio-LISICON型电解质 | 合成简单,热稳定性好,电子电导率小 | 离子电导率相对较低 | <10-3 |
| Li11-x M2-x P1+x S12(M=Ge, Sn, Si)结构 | 离子电导率高 | 成本较高,空气稳定性差 | >10-3 |
| 硫银锗矿型电解质 | 电化学稳定性好 | 空气稳定性较差 | >10-3 |
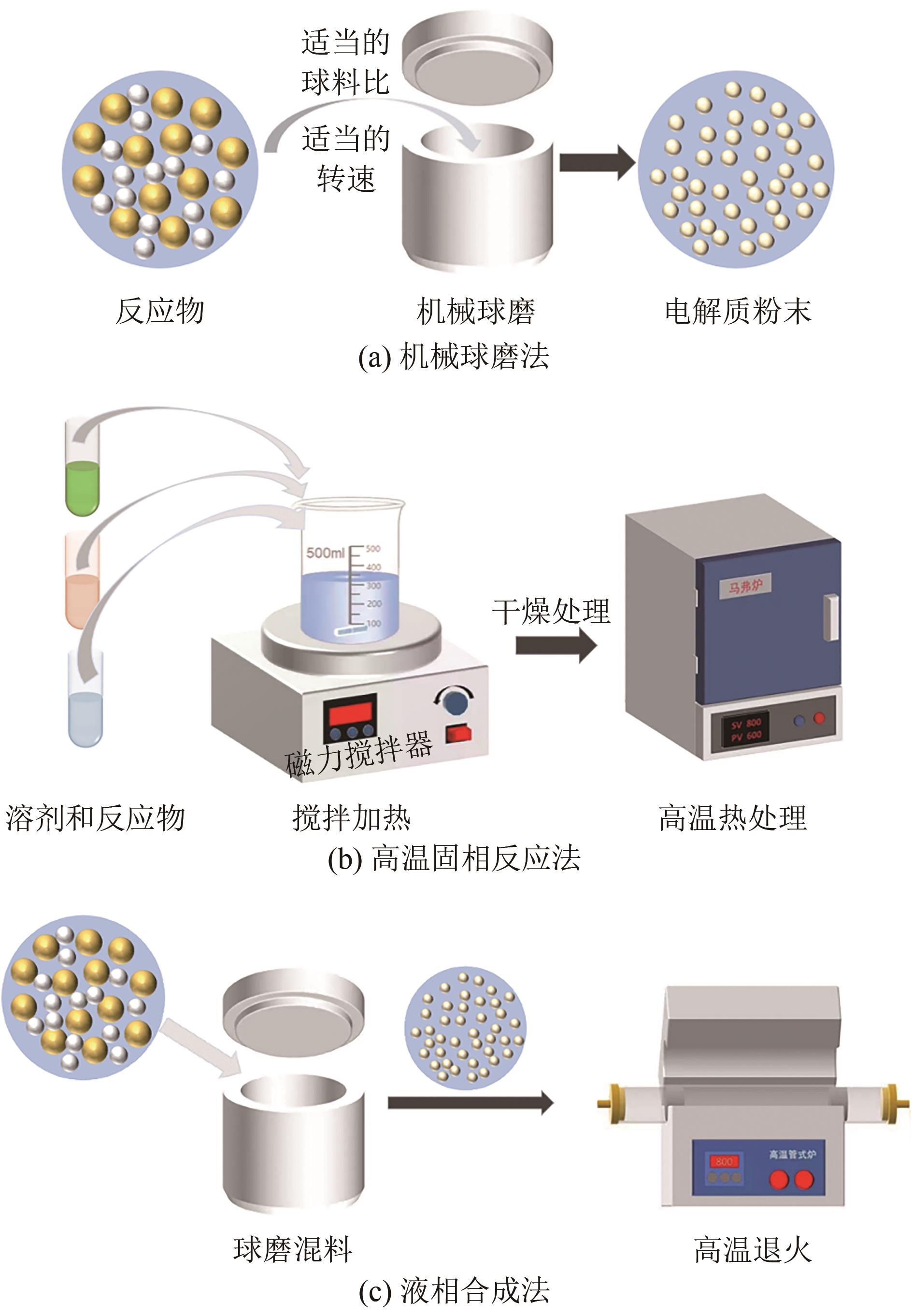
| 合成方法 | 操作步骤 | 离子电导率 /S·cm-1 | 操作环境 | 生产规模 |
|---|---|---|---|---|
| 机械球磨 | 复杂 | <10-3 | 友好 | 较小 |
| 机械球磨后退火 | 复杂 | >10-3 | 友好 | 较小 |
| 高温固相合成 | 简单 | >10-3 | 友好 | 较大 |
| 液相合成 | 复杂 | <10-4 | 苛刻 | 较大 |
| 合成方法 | 操作步骤 | 离子电导率 /S·cm-1 | 操作环境 | 生产规模 |
|---|---|---|---|---|
| 机械球磨 | 复杂 | <10-3 | 友好 | 较小 |
| 机械球磨后退火 | 复杂 | >10-3 | 友好 | 较小 |
| 高温固相合成 | 简单 | >10-3 | 友好 | 较大 |
| 液相合成 | 复杂 | <10-4 | 苛刻 | 较大 |
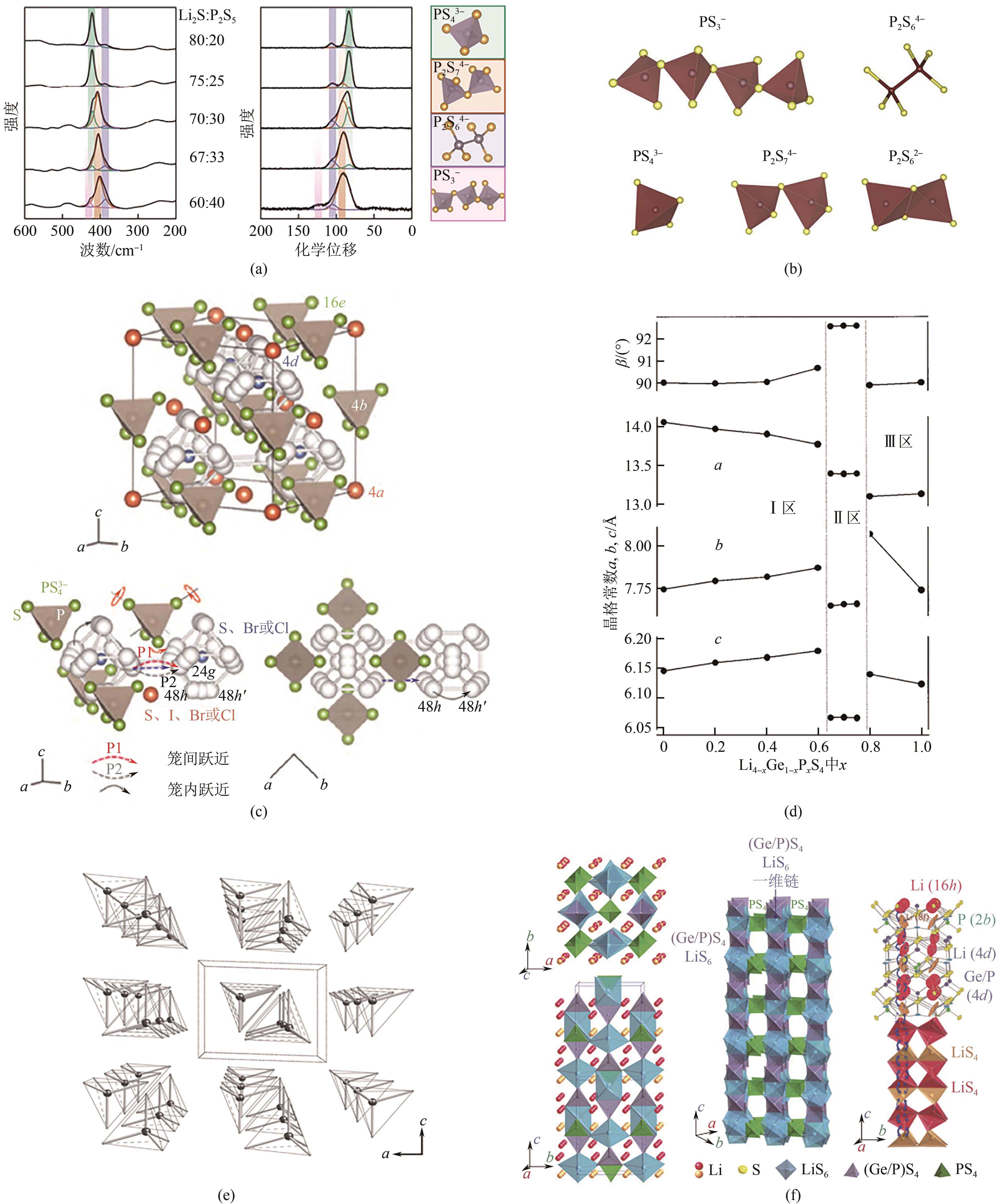
| 1 | KANNO Ryoji, MURAYAMA Masahiro. Lithium ionic conductor Thio-LISICON: The Li2S-GeS2-P2S5 system[J]. Journal of the Electrochemical Society, 2001, 148(7): A742. |
| 2 | MIZUNO F, HAYASHI A, TADANAGA K, et al. New, highly ion-conductive crystals precipitated from Li2S-P2S5 glasses[J]. Advanced Materials, 2005, 17(7): 918-921. |
| 3 | DEISEROTH Hans-Jörg, KONG Shiao-Tong, ECKERT Hellmut, et al. Li6PS5X: A class of crystalline Li-rich solids with an unusually high Li+ mobility[J]. Angewandte Chemie International Edition, 2008, 47(4): 755-758. |
| 4 | KAMAYA Noriaki, HOMMA Kenji, YAMAKAWA Yuichiro, et al. A lithium superionic conductor[J]. Nature Materials, 2011, 10: 682-686. |
| 5 | KATO Yuki, HORI Satoshi, SAITO Toshiya, et al. High-power all-solid-state batteries using sulfide superionic conductors[J]. Nature Energy, 2016, 1(4): 16030. |
| 6 | IWASAKI Rui, HORI Satoshi, KANNO Ryoji, et al. Weak anisotropic lithium-ion conductivity in single crystals of Li10GeP2S12 [J]. Chemistry of Materials, 2019, 31(10): 3694-3699. |
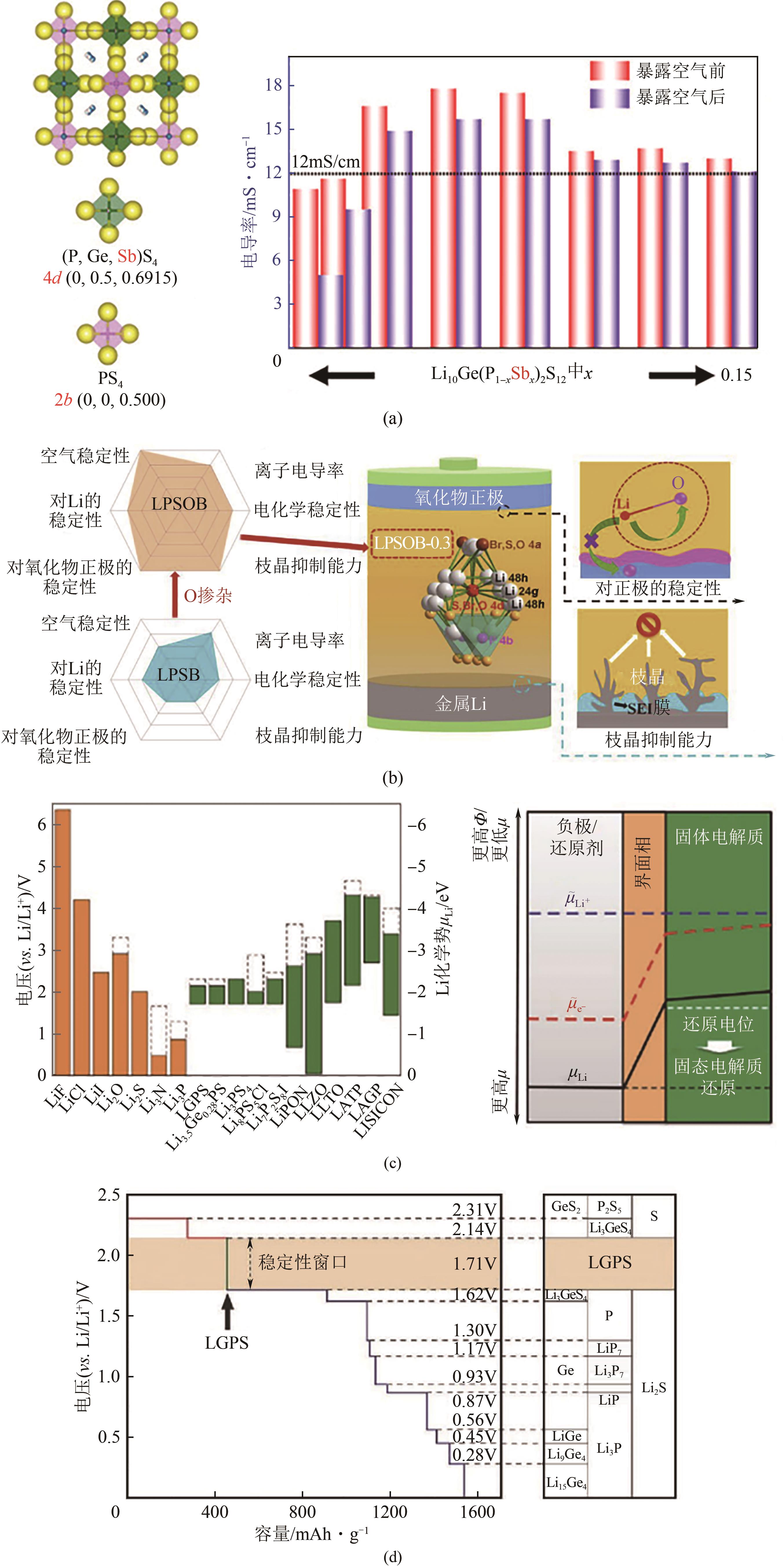
| 7 | LIANG Jianwen, CHEN Ning, LI Xiaona, et al. Li10Ge(P1- x Sb x )2S12 lithium-ion conductors with enhanced atmospheric stability[J]. Chemistry of Materials, 2020, 32(6): 2664-2672. |
| 8 | LU Pushun, LIU Lilu, WANG Shuo, et al. Superior all-solid-state batteries enabled by a gas-phase-synthesized sulfide electrolyte with ultrahigh moisture stability and ionic conductivity[J]. Advanced Materials, 2021, 33(32): e2100921. |
| 9 | LU Pushun, XIA Yu, SUN Guochen, et al. Realizing long-cycling all-solid-state Li-In||TiS2 batteries using Li6+ x M x As1- x S5I (M=Si, Sn) sulfide solid electrolytes[J]. Nature Communications, 2023, 14: 4077. |
| 10 | MANTHIRAM Arumugam, YU Xingwen, WANG Shaofei. Lithium battery chemistries enabled by solid-state electrolytes[J]. Nature Reviews Materials, 2017, 2(4): 16103. |
| 11 | UJIIE Satoshi, HAYASHI Akitoshi, TATSUMISAGO Masahiro. Structure, ionic conductivity and electrochemical stability of Li2S-P2S5-LiI glass and glass-ceramic electrolytes[J]. Solid State Ionics, 2012, 211: 42-45. |
| 12 | CHRISTIAN Dietrich, WEBER Dominik A, SEAN Culver, et al. Synthesis, structural characterization, and lithium ion conductivity of the lithium thiophosphate Li2P2S6 [J]. Inorganic Chemistry, 2017, 56(11): 6681-6687. |
| 13 | YAMANE Hisanori, SHIBATA Masatoshi, SHIMANE Yukio, et al. Crystal structure of a superionic conductor, Li7P3S11 [J]. Solid State Ionics, 2007, 178(15/16/17/18): 1163-1167. |
| 14 | MERCIER R, J-P MALUGANI, FAHYS B, et al. Structure du tetrathiophosphate de lithium[J]. Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry, 1982, 38(7): 1887-1890. |
| 15 | KONG Shiao Tong, Ozgül GÜN, KOCH Barbara, et al. Structural characterisation of the Li argyrodites Li7PS6 and Li7PSe6 and their solid solutions: Quantification of site preferences by MAS-NMR spectroscopy[J]. Chemistry, 2010, 16(17): 5138-5147. |
| 16 | MERCIER R, MALUGANI J P, FAHYS B, et al. Synthese, structure cristalline et analyse vibrationnelle de l’hexathiohypodiphosphate de lithium Li4P2S6 [J]. Journal of Solid State Chemistry, 1982, 43(2): 151-162. |
| 17 | TATSUMISAGO Masahiro. Glassy materials based on Li2S for all-solid-state lithium secondary batteries[J]. Solid State Ionics, 2004, 175(1/2/3/4): 13-18. |
| 18 | ITO Seitaro, NAKAKITA Moeka, AIHARA Yuichi, et al. A synthesis of crystalline Li7P3S11 solid electrolyte from 1,2-dimethoxyethane solvent[J]. Journal of Power Sources, 2014, 271: 342-345. |
| 19 | KANNO Ryoji, HATA Takayuki, KAWAMOTO Yoji, et al. Synthesis of a new lithium ionic conductor, Thio-LISICON-lithium germanium sulfide system[J]. Solid State Ionics, 2000, 130(1/2): 97-104. |
| 20 | MURAYAMA Masahiro, KANNO Ryoji, KAWAMOTO Yoji, et al. Structure of the Thio-LISICON, Li4GeS4 [J]. Solid State Ionics, 2002, 154/155: 789-794. |
| 21 | KAIB Thomas, HADDADPOUR Sima, KAPITEIN Manuel, et al. New lithium chalcogenidotetrelates, LiChT: Synthesis and characterization of the Li+-conducting tetralithium ortho-Sulfidostannate Li4SnS4 [J]. Chemistry of Materials, 2012, 24(11): 2211-2219. |
| 22 | SAHU Gayatri, LIN Zhan, LI Juchuan, et al. Air-stable, high-conduction solid electrolytes of arsenic-substituted Li4SnS4 [J]. Energy & Environmental Science, 2014, 7(3): 1053-1058. |
| 23 | HORI Satoshi, KATO Masahiko, SUZUKI Kota, et al. Phase diagram of the Li4GeS4-Li3PS4 quasi-binary system containing the superionic conductor Li10GeP2S12 [J]. Journal of the American Ceramic Society, 2015, 98(10): 3352-3360. |
| 24 | MURAYAMA Masahiro, KANNO Ryoji, IRIE Michihiko, et al. Synthesis of new lithium ionic conductor Thio-LISICON—Lithium silicon sulfides system[J]. Journal of Solid State Chemistry, 2002, 168(1): 140-148. |
| 25 | MINAFRA Nicolò, CULVER Sean P, LI Cheng, et al. Influence of the lithium substructure on the diffusion pathways and transport properties of the Thio-LISICON Li4Ge1- x Sn x S4 [J]. Chemistry of Materials, 2019, 31(10): 3794-3802. |
| 26 | KUHN Alexander, DUPPEL Viola, LOTSCH Bettina V. Tetragonal Li10GeP2S12 and Li7GePS8-exploring the Li ion dynamics in LGPS Li electrolytes[J]. Energy & Environmental Science, 2013, 6(12): 3548-3552. |
| 27 | BHANDARI Arihant, BHATTACHARYA Jishnu. Origin of fast ion conduction in Li10GeP2S12, a superionic conductor[J]. The Journal of Physical Chemistry C, 2016, 120(51): 29002-29010. |
| 28 | Shyue Ping ONG, MO Yifei, RICHARDS William Davidson, et al. Phase stability, electrochemical stability and ionic conductivity of the Li10±1MP2X12 (M=Ge, Si, Sn, Al or P, and X=O, S or Se) family of superionic conductors[J]. Energy & Environmental Science, 2013, 6(1): 148-156. |
| 29 | BRON Philipp, JOHANSSON Sebastian, ZICK Klaus, et al. Li10SnP2S12: An affordable lithium superionic conductor[J]. Journal of the American Chemical Society, 2013, 135(42): 15694-15697. |
| 30 | BRON Philipp, DEHNEN Stefanie, ROLING Bernhard. Li10Si0.3Sn0.7P2S12—A low-cost and low-grain-boundary-resistance lithium superionic conductor[J]. Journal of Power Sources, 2016, 329: 530-535. |
| 31 | KUHN Alexander, Jürgen KÖHLER, LOTSCH Bettina V. Single-crystal X-ray structure analysis of the superionic conductor Li10GeP2S12 [J]. Physical Chemistry Chemical Physics, 2013, 15(28): 11620-11622. |
| 32 | SUN Yulong, SUZUKI Kota, HARA Kosuke, et al. Oxygen substitution effects in Li10GeP2S12 solid electrolyte[J]. Journal of Power Sources, 2016, 324: 798-803. |
| 33 | HORI Satoshi, SUZUKI Kota, HIRAYAMA Masaaki, et al. Lithium superionic conductor Li9.42Si1.02P2.1S9.96O2.04 with Li10GeP2S12-type structure in the Li2S-P2S5-SiO2 pseudoternary system: Synthesis, electrochemical properties, and structure-composition relationships[J]. Frontiers in Energy Research, 2016, 4: 38. |
| 34 | BAI Yang, ZHAO Yanbiao, LI Weidong, et al. New insight for solid sulfide electrolytes LSiPSI by using Si/P/S as the raw materials and I doping[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(15): 12930-12937. |
| 35 | HAYASHI Akitoshi, NISHIO Yusuke, KITAURA Hirokazu, et al. Novel technique to form electrode-electrolyte nanointerface in all-solid-state rechargeable lithium batteries[J]. Electrochemistry Communications, 2008, 10(12): 1860-1863. |
| 36 | HANGHOFER I, BRINEK M, EISBACHER S L, et al. Substitutional disorder: Structure and ion dynamics of the argyrodites Li6PS5Cl, Li6PS5Br and Li6PS5I[J]. Physical Chemistry Chemical Physics: PCCP, 2019, 21(16): 8489-8507. |
| 37 | RAYAVARAPU Prasada Rao, SHARMA Neeraj, PETERSON Vanessa K, et al. Variation in structure and Li+-ion migration in argyrodite-type Li6PS5X (X=Cl, Br, I) solid electrolytes[J]. Journal of Solid State Electrochemistry, 2012, 16(5): 1807-1813. |
| 38 | ZHANG Zhuoran, ZHANG Jianxing, JIA Huanhuan, et al. Enhancing ionic conductivity of solid electrolyte by lithium substitution in halogenated Li-argyrodite[J]. Journal of Power Sources, 2020, 450: 227601. |
| 39 | PECHER Oliver, KONG Shiao-Tong, GOEBEL Thorsten, et al. Atomistic characterisation of Li+ mobility and conductivity in Li7- x PS6- x I x argyrodites from molecular dynamics simulations, solid-state NMR, and impedance spectroscopy[J]. Chemistry: A European Journal, 2010, 16(28): 8347-8354. |
| 40 | ZHANG Zhuoran, SUN Yulong, DUAN Xianbao, et al. Design and synthesis of room temperature stable Li-argyrodite superionic conductors via cation doping[J]. Journal of Materials Chemistry A, 2019, 7(6): 2717-2722. |
| 41 | MINAFRA Nicolò, CULVER Sean P, KRAUSKOPF Thorben, et al. Effect of Si substitution on the structural and transport properties of superionic Li-argyrodites[J]. Journal of Materials Chemistry A, 2018, 6(2): 645-651. |
| 42 | TATSUMISAGO M, MINAMI T. Lithium ion conducting glasses prepared by rapid quenching[J]. Materials Chemistry and Physics, 1987, 18(1/2): 1-17. |
| 43 | OHTOMO Takamasa, HAYASHI Akitoshi, TATSUMISAGO Masahiro, et al. Characteristics of the Li2O-Li2S-P2S5 glasses synthesized by the two-step mechanical milling[J]. Journal of Non-Crystalline Solids, 2013, 364: 57-61. |
| 44 | TATSUMISAGO M, HAMA S, HAYASHI A, et al. New lithium ion conducting glass-ceramics prepared from mechanochemical Li2S-P2S5 glasses[J]. Solid State Ionics, 2002, 154/155: 635-640. |
| 45 | PHUC Nguyen Huu Huy, MORIKAWA Kei, TOTANI Mitsuhiro, et al. Chemical synthesis of Li3PS4 precursor suspension by liquid-phase shaking[J]. Solid State Ionics, 2016, 285: 2-5. |
| 46 | BOULINEAU Sylvain, COURTY Matthieu, TARASCON Jean-Marie, et al. Mechanochemical synthesis of Li-argyrodite Li6PS5X (X=Cl, Br, I) as sulfur-based solid electrolytes for all solid state batteries application[J]. Solid State Ionics, 2012, 221: 1-5. |
| 47 | SEINO Yoshikatsu, Tsuyoshi OTA, TAKADA Kazunori, et al. A sulphide lithium super ion conductor is superior to liquid ion conductors for use in rechargeable batteries[J]. Energy & Environmental Science, 2014, 7(2): 627-631. |
| 48 | HOMMA Kenji, YONEMURA Masao, KOBAYASHI Takeshi, et al. Crystal structure and phase transitions of the lithium ionic conductor Li3PS4 [J]. Solid State Ionics, 2011, 182(1): 53-58. |
| 49 | KUHN Alexander, GERBIG Oliver, ZHU Changbao, et al. A new ultrafast superionic Li-conductor: Ion dynamics in Li11Si2PS12 and comparison with other tetragonal LGPS-type electrolytes[J]. Physical Chemistry Chemical Physics: PCCP, 2014, 16(28): 14669-14674. |
| 50 | YU Chuang, GANAPATHY Swapna, DE KLERK Niek J J, et al. Unravelling Li-ion transport from picoseconds to seconds: Bulk versus interfaces in an argyrodite Li6PS5Cl-Li2S all-solid-state Li-ion battery[J]. Journal of the American Chemical Society, 2016, 138(35): 11192-11201. |
| 51 | LIU Zengcai, FU Wujun, PAYZANT Andrew, et al. Anomalous high ionic conductivity of nanoporous β‑Li3PS4 [J]. J. Am. Chem. Soc, 2013, 135: 975-978. |
| 52 | CALPA Marcela, ROSERO-NAVARRO Nataly Carolina, MIURA Akira, et al. Instantaneous preparation of high lithium-ion conducting sulfide solid electrolyte Li7P3S11 by a liquid phase process[J]. RSC Advances, 2017, 7(73): 46499-46504. |
| 53 | XU R C, XIA X H, YAO Z J, et al. Preparation of Li7P3S11 glass-ceramic electrolyte by dissolution-evaporation method for all-solid-state lithium ion batteries[J]. Electrochimica Acta, 2016, 219: 235-240. |
| 54 | YAO Xiayin, LIU Deng, WANG Chunsheng, et al. High-energy all-solid-state lithium batteries with ultralong cycle life[J]. Nano Letters, 2016, 16(11): 7148-7154. |
| 55 | WANG Yuxing, LU Dongping, BOWDEN Mark, et al. Mechanism of formation of Li7P3S11 solid electrolytes through liquid phase synthesis[J]. Chemistry of Materials, 2018, 30(3): 990-997. |
| 56 | TERAGAWA Shingo, Keigo ASO, TADANAGA Kiyoharu, et al. Liquid-phase synthesis of a Li3PS4 solid electrolyte using N-methylformamide for all-solid-state lithium batteries[J]. Journal of Materials Chemistry A, 2014, 2(14): 5095-5099. |
| 57 | CHOI Sunho, Jiu ANN, Jiyae DO, et al. Application of rod-like Li6PS5Cl directly synthesized by a liquid phase process to sheet-type electrodes for all-solid-state lithium batteries[J]. Journal of the Electrochemical Society, 2018, 166(3): A5193-A5200. |
| 58 | ZHOU Laidong, PARK Kern-Ho, SUN Xiaoqi, et al. Solvent-engineered design of argyrodite Li6PS5X (X=Cl, Br, I) solid electrolytes with high ionic conductivity[J]. ACS Energy Letters, 2019, 4(1): 265-270. |
| 59 | YUBUCHI So, HAYASHI Akitoshi, TATSUMISAGO Masahiro. Liquid-phase synthesis of argyrodite-type Li6PS5Br solid electrolyte with high lithium-ion conductivity[J]. Materials Science, 2016, MA2016-02: 3982. |
| 60 | PEARSON Ralph G. Hard and soft acids and bases[J]. Journal of the American Chemical Society, 1963, 85(22): 3533-3539. |
| 61 | LU Pushun, WU Dengxu, CHEN Liquan, et al. Air stability of solid-state sulfide batteries and electrolytes[J]. Electrochemical Energy Reviews, 2022, 5(3): 3. |
| 62 | HAYASHI Akitoshi, MURAMATSU Hiromasa, OHTOMO Takamasa, et al. Improvement of chemical stability of Li3PS4 glass electrolytes by adding M x O y (M=Fe, Zn, and Bi) nanoparticles[J]. Journal of Materials Chemistry A, 2013, 1(21): 6320-6326. |
| 63 | OHTOMO Takamasa, HAYASHI Akitoshi, TAYSUMISAGO Masahiro,et al. All-solid-state batteries with Li2O-Li2S-P2S5 glass electrolytes synthesized by two-step mechanical milling [J]. Journal of Solid State Electrochemistry, 2013, 17: 2551-2557. |
| 64 | ZHANG Zhixia, ZHANG Long, YAN Xinlin, et al. All-in-one improvement toward Li6PS5Br-Based solid electrolytes triggered by compositional tune[J]. Journal of Power Sources, 2019, 410/411: 162-170. |
| 65 | LIU Gaozhan, XIE Dongjiu, WANG Xuelong, et al. High air-stability and superior lithium ion conduction of Li3+3 x P1- x Zn x S4- x O x by aliovalent substitution of ZnO for all-solid-state lithium batteries[J]. Energy Storage Materials, 2019, 17: 266-274. |
| 66 | WU Jinghua, SHEN Lin, ZHANG Zhihua, et al. All-solid-state lithium batteries with sulfide electrolytes and oxide cathodes[J]. Electrochemical Energy Reviews, 2021, 4(1): 101-135. |
| 67 | WU Jinghua, LIU Sufu, HAN Fudong, et al. Lithium/sulfide all-solid-state batteries using sulfide electrolytes[J]. Advanced Materials, 2021, 33(6): e2000751. |
| 68 | TREVEY James, JANG Jum Suk, JUNG Yoon Seok, et al. Glass-ceramic Li2S-P2S5 electrolytes prepared by a single step ball billing process and their application for all-solid-state lithium-ion batteries[J]. Electrochemistry Communications, 2009, 11:1830-1833. |
| 69 | KANNO R, MURAYAMA M, New Lithium ionic conductor, Thio-LISICON, and its application to all solid-state ceramic battery[J]. Journal of the Japanese Association for Crystal Growth, 2004, 46(1):9-15. |
| 70 | YAMAMOTO Hidekazu, MACHIDA Nobuya, SHIGEMATSU Toshihiko. A mixed-former effect on lithium-ion conductivities of the Li2S-GeS2-P2S5 amorphous materials prepared by a high-energy ball-milling process[J]. Solid State Ionics, 2004, 175(1/2/3/4): 707-711. |
| 71 | ZHU Yizhou, HE Xingfeng, MO Yifei. Origin of outstanding stability in the lithium solid electrolyte materials: Insights from thermodynamic analyses based on first-principles calculations[J]. ACS Applied Materials & Interfaces, 2015, 7(42): 23685-23693. |
| 72 | HAN Fudong, ZHU Yizhou, HE Xingfeng, et al. Electrochemical stability of Li10GeP2S12 and Li7La3Zr2O12 solid electrolytes[J]. Advanced Energy Materials, 2016, 6(8): 1501590. |
| 73 | YU Chuang, VAN EIJCK Lambert, GANAPATHY Swapna, et al. Synthesis, structure and electrochemical performance of the argyrodite Li6PS5Cl solid electrolyte for Li-ion solid state batteries[J]. Electrochimica Acta 2016, 215: 93-99. |
| 74 | ZHENG Bizhu, LIU Xiangsi, ZHU Jianping, et al. Unraveling (electro)-chemical stability and interfacial reactions of Li10SnP2S12 in all-solid-state Li batteries[J]. Nano Energy 2020, 67:104252. |
| 75 | LI Guoyao, WU Shaoping, ZHENG Hongpeng, et al. Sn-O dual-substituted chlorine-rich argyrodite electrolyte with enhanced moisture and electrochemical stability[J]. Advanced Functional Materials, 2023, 33:2211805. |
| [1] | ZHANG Changsheng, WEN Song, ZHAO Jinchong, LU Fangxu, JIANG Jie. Advances in chemical deoxidation of oxygen-containing organic gases in chemical processes [J]. Chemical Industry and Engineering Progress, 2024, 43(2): 903-912. |
| [2] | MAO Shanjun, WANG Zhe, WANG Yong. Group recognition hydrogenation: From concept to application [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3917-3922. |
| [3] | JIN Yong, CHENG Yi, BAI Dingrong, ZHANG Chenxi, WEI Fei. Fluidization research and development in China [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2761-2780. |
| [4] | SHAO Bin, SUN Zheyi, ZHANG Yun, PAN Fenghongkang, ZHAO Kaiqing, HU Jun, LIU Honglai. Recent progresses in CO2 to syngas and high value-added products [J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1136-1151. |
| [5] | FAN Tao. Industrial application progress of lignite pyrolysis technology in eastern area of Inner Mongolia, China [J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1362-1370. |
| [6] | Yitao LIU, Minghui ZHU, Zixu YANG, Bo MENG, Weifeng TU, Yifan HAN. Advances of catalysts for direct synthesis of lower olefins from syngas [J]. Chemical Industry and Engineering Progress, 2021, 40(2): 594-604. |
| [7] | Zhongli JI, Xin LUAN, Linfeng MIAO. Overview of hot-gas filtration technology and equipment development [J]. Chemical Industry and Engineering Progress, 2020, 39(6): 2304-2311. |
| [8] | Rui WANG,Xue YANG,Ping MIAO. Progress in preparation research and industrial application of SO42-/ZrO2catalysts for light paraffin isomerization [J]. Chemical Industry and Engineering Progress, 2019, 38(11): 4930-4940. |
| [9] | Qijiang LIAO,Hongyun QIN,Mingliang ZHOU,Minqing ZHANG,Jinli ZHANG. Progress of researches and applications for high shear mixers [J]. Chemical Industry and Engineering Progress, 2019, 38(03): 1160-1175. |
| [10] | GUO Zhengdong, SU Mengjun, LIU Hanxiao, LI Yajun, QING Yizhao, LUO Yong, CHU Guangwen, CHEN Jianfeng. States-of-the-arts progress on fundamental research and industrial applications of rotating packed bed [J]. Chemical Industry and Engineering Progress, 2018, 37(04): 1335-1346. |
| [11] | ZHUANG Xiuzheng, YIN Xiuli, HUANG Yanqin, WU Chuangzhi. Research on hydrothermal treatment of sewage sludge and its industrial applications [J]. Chemical Industry and Engineering Progress, 2017, 36(11): 4224-4231. |
| [12] | WANG Shaofeng,XIANG Shuguang . Application and research progress of valve trays [J]. Chemical Industry and Engineering Progree, 2014, 33(07): 1677-1683. |
| [13] | AN Dongxu1,LI Xia1,XU Guangdi1,YU Tengxiang1,ZHU Dongsheng2. Application of variable cross-section twisted oval tube on freezing dehydrator [J]. Chemical Industry and Engineering Progree, 2013, 32(12): 2832-2836. |
| [14] | SHAO Pingjun. Industrial application of catalyst SLC-S [J]. Chemical Industry and Engineering Progree, 2013, 32(07): 1717-1720. |
| [15] | GAO Liya,TAN Xuejun,ZHANG Dongsheng,WANG Shufang,ZHAO Xinqiang,WANG Yanji. Progress of synthesis and application of hydroxylamine (salts) [J]. Chemical Industry and Engineering Progree, 2012, 31(09): 2043-2048. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||