Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (12): 6239-6250.DOI: 10.16085/j.issn.1000-6613.2023-0067
• Energy processes and technology • Previous Articles
Research progress on nickel-based oxygen evolution electrode prepared by electrodeposition for alkaline water electrolysis
ZHANG Jing1,2( ), HE Yeheng1(
), HE Yeheng1( ), WANG Jingjing1, XIA Bowen1, ZHAO Qinfeng1, WANG Yanfei1, YU Yinglong1, SHAO Chenyi1, LONG Chuan1
), WANG Jingjing1, XIA Bowen1, ZHAO Qinfeng1, WANG Yanfei1, YU Yinglong1, SHAO Chenyi1, LONG Chuan1
- 1.Petrochemical Research Institute of PetroChina, Beijing 102206, China
2.College of Chemical Engineering and Environment, China University of Petroleum (Beijing), Beijing 102249, China
-
Received:2023-01-13Revised:2023-04-25Online:2024-01-08Published:2023-12-25 -
Contact:HE Yeheng
电沉积法制备碱性电解水镍基析氧电极的研究进展
张静1,2( ), 贺业亨1(
), 贺业亨1( ), 王晶晶1, 夏博文1, 赵秦峰1, 王延飞1, 余颖龙1, 邵晨熠1, 龙川1
), 王晶晶1, 夏博文1, 赵秦峰1, 王延飞1, 余颖龙1, 邵晨熠1, 龙川1
- 1.中国石油石油化工研究院,北京 102206
2.中国石油大学(北京)化学工程与环境学院,北京 102249
-
通讯作者:贺业亨 -
作者简介:张静(1998—),女,硕士研究生,研究方向为电催化水分解。E-mail:zhangjing092543@163.com。 -
基金资助:石油化工研究院自主管理基金(2021-YK-05-12)
CLC Number:
Cite this article
ZHANG Jing, HE Yeheng, WANG Jingjing, XIA Bowen, ZHAO Qinfeng, WANG Yanfei, YU Yinglong, SHAO Chenyi, LONG Chuan. Research progress on nickel-based oxygen evolution electrode prepared by electrodeposition for alkaline water electrolysis[J]. Chemical Industry and Engineering Progress, 2023, 42(12): 6239-6250.
张静, 贺业亨, 王晶晶, 夏博文, 赵秦峰, 王延飞, 余颖龙, 邵晨熠, 龙川. 电沉积法制备碱性电解水镍基析氧电极的研究进展[J]. 化工进展, 2023, 42(12): 6239-6250.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-0067
| 催化剂名称 | 基底 | 电流密度 /mA·cm-2 | 过电位 /mV | 塔菲尔斜率 /mV·dec-1 | 电解液 | 沉积液 | 参考文献 |
|---|---|---|---|---|---|---|---|
| α-Ni(OH)2·0.75H2O/NF | NF | 100 | 357① | 128.7 | 1.0mol/L KOH | NiCl2 | [ |
| Ni(OH)2/NF/N-CNTs | NF/N-CNTs | 10 | 254 | 84 | 1.0mol/L KOH | Ni(NO3)2·6H2O | [ |
| α-Ni(OH)2/NF | NF | 10 | 192① | 108 | 1.0mol/L KOH | Ni(NO3)2·6H2O | [ |
| NiO/NiNDs@NF | NF | 50 | 360① | 90 | 1.0mol/L KOH | Ni(NO3)2乙腈溶液 | [ |
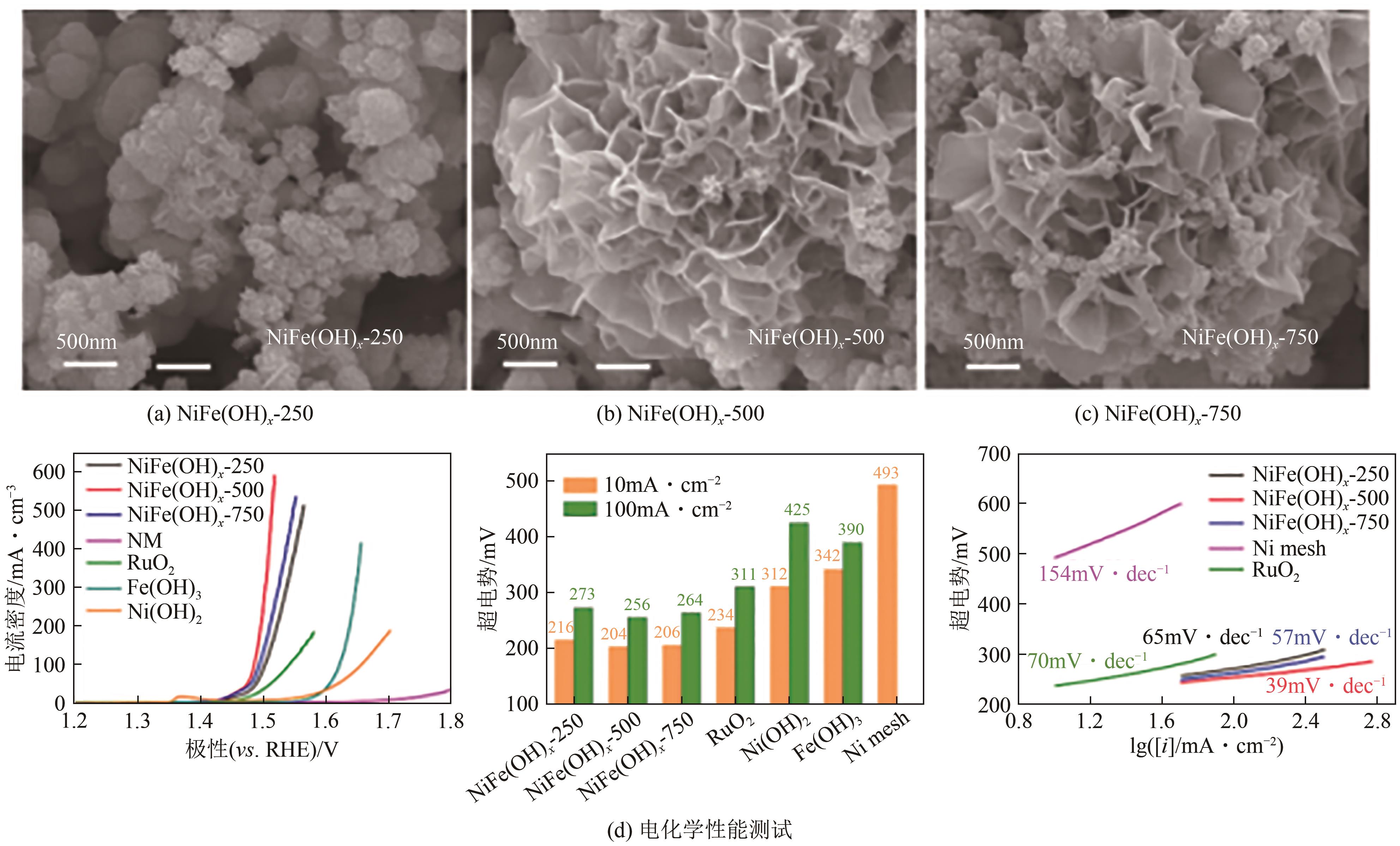
| NiFe(OH)x-500/Ni | 镍网 | 10 | 204① | 39 | 1.0mol/L KOH | Ni(NO3)2·6H2O+ FeSO4·7H2O | [ |
| NiFe/NF | NF | 10 | 191① | 44.1 | 1.0mol/L KOH | NiCl2·6H2O+FeCl2·4H2O | [ |
| NiFe-LDHs/NF | NF | 10 | 283① | 47.76 | 1.0mol/L KOH | Ni(NO3)2·6H2O+ Fe(NO3) 2·9H2O | [ |
| Ni(Co0.5Fe0.5)/NF | NF | 10 | 209① | 54 | 1.0mol/L KOH | Ni(NO3)2+Co(NO3)2+FeSO4 | [ |
| Ag@NiCo LDH/NF | NF | 100 | 304① | 64.6 | 1.0mol/L KOH | Ni(NO3)2·6H2O+Co(NO3)2·6H2O | [ |
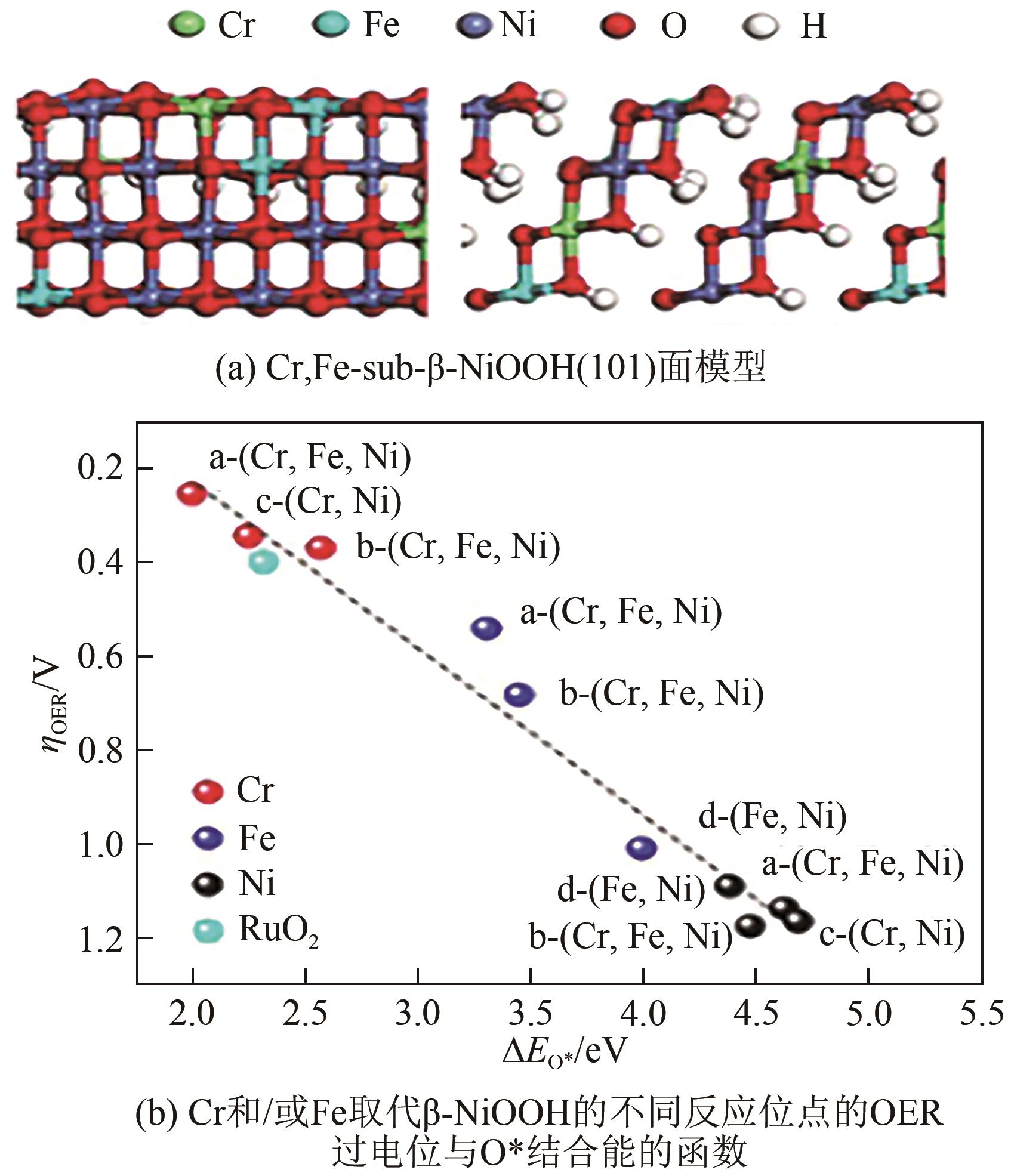
| NiFeCr/NF | NF | — | —① | 36 | 1.0mol/L KOH | Ni(NO3)2·6H2O+Fe(NO3)2·9H2O+ Cr(NO3)2·9H2O | [ |
| NiFeMo/NF | NF | 10 | 306① | 77.1 | 30% KOH | NiSO4·6H2O+NaCl+H3BO3+ FeSO4·6H2O+Na3C6H5O7·2H2O+ Na2MoO4·2H2O | [ |
| Ni0.65Ga0.30Fe0.05/NF | NF | 10 | 200 | 42 | 1.0mol/L KOH | Ga(NO3)3∙xH2O+Ni(NO3)2∙6H2O+ Fe(NO3)2∙9H2O | [ |
| NiFeSn/NF | NF | 10 | 253① | 61.5 | 30% KOH | NiSO4·6H2O+NaCl+H3BO3+ FeSO4·6H2O+C6H5Na3O7·2H2O+ SnCl2·6H2O | [ |
| Ni-Fe-W-Mo/Ni | 镍网 | 10 | 152① | — | 5.35mol/L KOH | NiSO4·6H2O+FeSO4·7H2O+ Na2WO4·2H2O+C6H8O7·7H2O+ Na2MoO4·2H2O+抗坏血酸 | [ |
| Ni(OH)2/Ni3S2/NF | NF | 10 | 210① | 72 | 1.0mol/L KOH | NiCl2·6H2O+CS(NH2)2+NaCl+H2SO4 | [ |
| NiFeS/NF | NF | 10 | 287① | 64.74 | 1.0mol/L KOH | NiSO4·6H2O+CoCl2·6H2O+CH4N2S+H3BO3+ C6H5Na3O7·2H2O+NaCl | [ |
| NiFe-Pi/P-12/NF | NF | 20 | 255① | 50 | 1.0mol/L KOH | NiCl2·6H2O+FeCl3·6H2O+NaOH+柠檬酸溶液 | [ |
| NiFeCuP@Ni3S2/NF | NF | 10 | 230 | 42 | 1.0mol/L KOH | NaH2PO4+NH4Cl+Ni(NO3)2·6H2O+ FeSO4·7H2O+CuSO4·5H2O | [ |
| Ni-S-P/NF | NF | 10 | 219① | 82 | 1.0mol/L KOH | Ni(NO3)2·6H2O+CH4N2S Ni(NO3)2·6H2O+NaH2PO2+CH3COONa | [ |
| NiSP/NF | NF | 10 | 281① | 68.51 | 1.0mol/L KOH | NiSO4·6H2O+CH4N2S+Na2HPO4·2H2O+ C6H5Na3O7·2H2O+NaCl | [ |
| 催化剂名称 | 基底 | 电流密度 /mA·cm-2 | 过电位 /mV | 塔菲尔斜率 /mV·dec-1 | 电解液 | 沉积液 | 参考文献 |
|---|---|---|---|---|---|---|---|
| α-Ni(OH)2·0.75H2O/NF | NF | 100 | 357① | 128.7 | 1.0mol/L KOH | NiCl2 | [ |
| Ni(OH)2/NF/N-CNTs | NF/N-CNTs | 10 | 254 | 84 | 1.0mol/L KOH | Ni(NO3)2·6H2O | [ |
| α-Ni(OH)2/NF | NF | 10 | 192① | 108 | 1.0mol/L KOH | Ni(NO3)2·6H2O | [ |
| NiO/NiNDs@NF | NF | 50 | 360① | 90 | 1.0mol/L KOH | Ni(NO3)2乙腈溶液 | [ |
| NiFe(OH)x-500/Ni | 镍网 | 10 | 204① | 39 | 1.0mol/L KOH | Ni(NO3)2·6H2O+ FeSO4·7H2O | [ |
| NiFe/NF | NF | 10 | 191① | 44.1 | 1.0mol/L KOH | NiCl2·6H2O+FeCl2·4H2O | [ |
| NiFe-LDHs/NF | NF | 10 | 283① | 47.76 | 1.0mol/L KOH | Ni(NO3)2·6H2O+ Fe(NO3) 2·9H2O | [ |
| Ni(Co0.5Fe0.5)/NF | NF | 10 | 209① | 54 | 1.0mol/L KOH | Ni(NO3)2+Co(NO3)2+FeSO4 | [ |
| Ag@NiCo LDH/NF | NF | 100 | 304① | 64.6 | 1.0mol/L KOH | Ni(NO3)2·6H2O+Co(NO3)2·6H2O | [ |
| NiFeCr/NF | NF | — | —① | 36 | 1.0mol/L KOH | Ni(NO3)2·6H2O+Fe(NO3)2·9H2O+ Cr(NO3)2·9H2O | [ |
| NiFeMo/NF | NF | 10 | 306① | 77.1 | 30% KOH | NiSO4·6H2O+NaCl+H3BO3+ FeSO4·6H2O+Na3C6H5O7·2H2O+ Na2MoO4·2H2O | [ |
| Ni0.65Ga0.30Fe0.05/NF | NF | 10 | 200 | 42 | 1.0mol/L KOH | Ga(NO3)3∙xH2O+Ni(NO3)2∙6H2O+ Fe(NO3)2∙9H2O | [ |
| NiFeSn/NF | NF | 10 | 253① | 61.5 | 30% KOH | NiSO4·6H2O+NaCl+H3BO3+ FeSO4·6H2O+C6H5Na3O7·2H2O+ SnCl2·6H2O | [ |
| Ni-Fe-W-Mo/Ni | 镍网 | 10 | 152① | — | 5.35mol/L KOH | NiSO4·6H2O+FeSO4·7H2O+ Na2WO4·2H2O+C6H8O7·7H2O+ Na2MoO4·2H2O+抗坏血酸 | [ |
| Ni(OH)2/Ni3S2/NF | NF | 10 | 210① | 72 | 1.0mol/L KOH | NiCl2·6H2O+CS(NH2)2+NaCl+H2SO4 | [ |
| NiFeS/NF | NF | 10 | 287① | 64.74 | 1.0mol/L KOH | NiSO4·6H2O+CoCl2·6H2O+CH4N2S+H3BO3+ C6H5Na3O7·2H2O+NaCl | [ |
| NiFe-Pi/P-12/NF | NF | 20 | 255① | 50 | 1.0mol/L KOH | NiCl2·6H2O+FeCl3·6H2O+NaOH+柠檬酸溶液 | [ |
| NiFeCuP@Ni3S2/NF | NF | 10 | 230 | 42 | 1.0mol/L KOH | NaH2PO4+NH4Cl+Ni(NO3)2·6H2O+ FeSO4·7H2O+CuSO4·5H2O | [ |
| Ni-S-P/NF | NF | 10 | 219① | 82 | 1.0mol/L KOH | Ni(NO3)2·6H2O+CH4N2S Ni(NO3)2·6H2O+NaH2PO2+CH3COONa | [ |
| NiSP/NF | NF | 10 | 281① | 68.51 | 1.0mol/L KOH | NiSO4·6H2O+CH4N2S+Na2HPO4·2H2O+ C6H5Na3O7·2H2O+NaCl | [ |
| 1 | DORNING Monica A, DIFFENDORFER Jay E, LOSS Scott R, et al. Review of indicators for comparing environmental effects across energy sources[J]. Environmental Research Letters, 2019, 14(10): 103002. |
| 2 | SOLARIN Sakiru Adebola. An environmental impact assessment of fossil fuel subsidies in emerging and developing economies[J]. Environmental Impact Assessment Review, 2020, 85: 106443. |
| 3 | MARTINS Florinda, FELGUEIRAS Carlos, SMITKOVA Miroslava, et al. Analysis of fossil fuel energy consumption and environmental impacts in European countries[J]. Energies, 2019, 12(6): 964. |
| 4 | VAKULCHUK Roman, OVERLAND Indra, SCHOLTEN Daniel. Renewable energy and geopolitics: A review[J]. Renewable and Sustainable Energy Reviews, 2020, 122: 109547. |
| 5 | DOU Yuhai, HE Chunting, ZHANG Lei, et al. Approaching the activity limit of CoSe2 for oxygen evolution via Fe doping and Co vacancy[J]. Nature Communications, 2020, 11(1): 1664. |
| 6 | WAN Jiawei, ZHAO Zhenghang, SHANG Huishan, et al. In situ phosphatizing of triphenylphosphine encapsulated within metal-organic frameworks to design atomic Co1-P1N3 interfacial structure for promoting catalytic performance[J]. Journal of the American Chemical Society, 2020, 142(18): 8431-8439. |
| 7 | YUN Qinbai, LU Qipeng, ZHANG Xiao, et al. Three-dimensional architectures constructed from transition-metal dichalcogenide nanomaterials for electrochemical energy storage and conversion[J]. Angewandte Chemie International Edition, 2018, 57(3): 626-646. |
| 8 | 中国氢能源及燃料电池产业创新战略联盟. 中国氢能源及燃料电池产业白皮书2020[M]. 北京: 人民日报出版社, 2021. |
| China Hydrogen Energy and Fuel Cell Industry Innovation Strategic Alliance. White Paper on hydrogen energy and fuel cell industry in China 2020[M]. Beijing: People Daily Press, 2021. | |
| 9 | 程明睿, 高宏. 绿氢已成为未来维护能源安全的重要方向[J]. 科技中国, 2022(10): 60-65. |
| CHENG Mingrui, GAO Hong. Green hydrogen has become an important direction to maintain energy security in the future[J]. Scitech in China, 2022(10): 60-65. | |
| 10 | LEE Hae In, MEHDI Muhammad, KIM Sang Kyung, et al. Advanced Zirfon-type porous separator for a high-rate alkaline electrolyser operating in a dynamic mode[J]. Journal of Membrane Science, 2020, 616: 118541. |
| 11 | HASAN Md Mahedi, ISLAM Tamanna, SHAH Syed Shaheen, et al. Supporting electrolyte interaction with the AACVD synthesized Rh thin film influences the OER activity[J]. International Journal of Hydrogen Energy, 2022, 47(67): 28740-28751. |
| 12 | SUNTIVICH Jin, Kevin J MAY, GASTEIGER Hubert A, et al. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles[J]. Science, 2011, 334(6061): 1383-1385. |
| 13 | Boon Siang YEO. Oxygen evolution by stabilized single Ru atoms[J]. Nature Catalysis, 2019, 2(4): 284-285. |
| 14 | 刘太楷, 邓春明, 张亚鹏. 电解水制氢发展概况之一: 碱式电解水[J].材料研究与应用, 2019, 13(4): 339-346. |
| LIU Taikai, DENG Chunming, ZHANG Yapeng. Development of hydrogen production by electrolysis of water I: Alkaline electrolysis of water[J]. Materials Research and Application, 2019, 13(4): 339-346. | |
| 15 | YAO Mengqi, WANG Ni, HU Wencheng, et al. Novel hydrothermal electrodeposition to fabricate mesoporous film of Ni0.8Fe0.2 nanosheets for high performance oxygen evolution reaction[J]. Applied Catalysis B: Environmental, 2018, 233: 226-233. |
| 16 | THIRUMAL V, YUVAKKUMAR R, SENTHIL KUMAR P, et al. Morphology investigation on direct growth ultra-long CNTs by chemical vapour deposition method for high performance HER applications[J]. Fuel, 2022, 330: 125532. |
| 17 | GONG Feilong, YE Sheng, LIU Mengmeng, et al. Boosting electrochemical oxygen evolution over yolk-shell structured O-MoS2 nanoreactors with sulfur vacancy and decorated Pt nanoparticles[J]. Nano Energy, 2020, 78: 105284. |
| 18 | GUO Daying, ZENG Zhihao, WAN Zhixin, et al. A CoN-based OER electrocatalyst capable in neutral medium: Atomic layer deposition as rational strategy for fabrication[J]. Advanced Functional Materials, 2021, 31(24): 2101324. |
| 19 | ZAI Shifeng, DONG Anqi, LI Jian, et al. Low-crystallinity mesoporous NiGaFe hydroxide nanosheets on macroporous Ni foam for high-efficiency oxygen evolution electrocatalysis[J]. Journal of Materials Chemistry A, 2021, 9(10): 6223-6231. |
| 20 | BO Xin, HOCKING Rosalie K, ZHOU Si, et al. Capturing the active sites of multimetallic (oxy) hydroxides for the oxygen evolution reaction[J]. Energy & Environmental Science, 2020, 13(11): 4225-4237. |
| 21 | TONG Wenming, FORSTER Mark, DIONIGI Fabio, et al. Electrolysis of low-grade and saline surface water[J]. Nature Energy, 2020, 5(5): 367-377. |
| 22 | SONG Jiajia, WEI Chao, HUANG Zhenfeng, et al. A review on fundamentals for designing oxygen evolution electrocatalysts[J]. Chemical Society Reviews, 2020, 49(7): 2196-2214. |
| 23 | MEDFORD Andrew J, VOJVODIC Aleksandra, HUMMELSHØJ Jens S, et al. From the Sabatier principle to a predictive theory of transition-metal heterogeneous catalysis[J]. Journal of Catalysis, 2015, 328: 36-42. |
| 24 | JIANG Wenjie, TANG Tang, ZHANG Yun, et al. Synergistic modulation of non-precious-metal electrocatalysts for advanced water splitting[J]. Accounts of Chemical Research, 2020, 53(6): 1111-1123. |
| 25 | 李荻. 电化学原理[M]. 3版. 北京: 北京航空航天大学出版社, 2008. |
| LI Di. Electrochemical principle[M]. 3rd ed. Beijing: Beijing University of Aeronautics & Astronautics Press, 2008. | |
| 26 | WU Zhipeng, LU Xuefeng, ZANG Shuangquan, et al. Non-noble-metal-based electrocatalysts toward the oxygen evolution reaction[J]. Advanced Functional Materials, 2020, 30(15): 1910274. |
| 27 | WU Jian, SUBRAMANIAM Jayabal, LIU Yongqiang, et al. Facile assembly of Ni(OH)2 nanosheets on nitrogen-doped carbon nanotubes network as high-performance electrocatalyst for oxygen evolution reaction[J]. Journal of Alloys and Compounds, 2018, 731: 766-773. |
| 28 | WANG Jiaxin, SUN Xiaoliang, HU Hanbin, et al. Electrodeposition of defect-rich ternary NiCoFe layered double hydroxides: fine modulation of Co3+ for highly efficient oxygen evolution reaction[J]. Chemistry-A European Journal, 2022, 28(6): e202103601. |
| 29 | ALLEMAND Morgan, MARTIN Manuel H, REYTER David, et al. Synthesis of Cu-Pd alloy thin films by co-electrodeposition[J]. Electrochimica Acta, 2011, 56(21): 7397-7403. |
| 30 | ABBASPOUR Abdolkarim, Fatemeh NOROUZ-SARVESTANI. High electrocatalytic effect of Au-Pd alloy nanoparticles electrodeposited on microwave assisted sol-gel-derived carbon ceramic electrode for hydrogen evolution reaction[J]. International Journal of Hydrogen Energy, 2013, 38(4): 1883-1891. |
| 31 | HE Chuanglong, JIN Xiaobing, MA Peter X. Calcium phosphate deposition rate, structure and osteoconductivity on electrospun poly (L-lactic acid) matrix using electrodeposition or simulated body fluid incubation[J]. Acta Biomaterialia, 2014, 10(1): 419-427. |
| 32 | LEE Sol A, YANG Jin Wook, CHOI Sungkyun, et al. Nanoscale electrodeposition: dimension control and 3D conformality[J]. Exploration. 2021, 1(3): 20210012. |
| 33 | LI Mian, XIONG Yueping, LIU Xiaotian, et al. Facile synthesis of electrospun MFe2O4(M=Co, Ni, Cu, Mn) spinel nanofibers with excellent electrocatalytic properties for oxygen evolution and hydrogen peroxide reduction[J]. Nanoscale, 2015, 7(19): 8920-8930. |
| 34 | CHEN Junsheng, LI Hao, YU Zixun, et al. Octahedral coordinated trivalent cobalt enriched multimetal oxygen-evolution catalysts[J]. Advanced Energy Materials, 2020, 10(43): 2002593. |
| 35 | ZHENG Jingxu, ZHAO Qing, TANG Tian, et al. Reversible epitaxial electrodeposition of metals in battery anodes[J]. Science, 2019, 366(6465): 645-648. |
| 36 | ZHANG Huigang, NING Hailong, BUSBEE John, et al. Electroplating lithium transition metal oxides[J]. Science Advances, 2017, 3(5):e1602427. |
| 37 | YU Hongtao, QUAN Ting, MEI Shilin, et al. Prompt electrodeposition of Ni nanodots on Ni foam to construct a high-performance water-splitting electrode: efficient, scalable, and recyclable[J]. Nano-Micro Letters, 2019, 11(1): 41. |
| 38 | LEI Yaqi, XU Tingting, YE Shenghua, et al. Engineering defect-rich Fe-doped NiO coupled Ni cluster nanotube arrays with excellent oxygen evolution activity[J]. Applied Catalysis B: Environmental, 2021, 285: 119809. |
| 39 | CAO Liming, CAO Qingcai, ZHANG Jia, et al. Electrochemically controlled synthesis of ultrathin nickel hydroxide nanosheets for electrocatalytic oxygen evolution[J]. Inorganic Chemistry, 2021, 60(5): 3365-3374. |
| 40 | YAO Kaili, ZHAI Muheng, NI Yonghong. α-Ni(OH)2·0.75H2O nanofilms on Ni foam from simple NiCl2 solution: Fast electrodeposition, formation mechanism and application as an efficient bifunctional electrocatalyst for overall water splitting in alkaline solution[J]. Electrochimica Acta, 2019, 301: 87-96. |
| 41 | ZHANG Ping, TAN Wenyu, HE Hanwei, et al. Binder-free quaternary Ni-Fe-W-Mo alloy as a highly efficient electrocatalyst for oxygen evolution reaction[J]. Journal of Alloys and Compounds, 2021, 853: 157265. |
| 42 | HAI Y, LIU L, GONG Y. Iron coordination polymer, Fe(oxalate)(H2O)2 nanorods grown on nickel foam via one-step electrodeposition as an efficient electrocatalyst for oxygen evolution reaction[J]. Inorganic Chemistry, 2021, 60(7): 5140-5152. |
| 43 | JIN Jun, XIA Jiangbing, QIAN Xin, et al. Exceptional electrocatalytic oxygen evolution efficiency and stability from electrodeposited NiFe alloy on Ni foam[J]. Electrochimica Acta, 2019, 299: 567-574. |
| 44 | WU Yihui, GAO Ying, HE Hanwei, et al. Electrodeposition of self-supported Ni-Fe-Sn film on Ni foam: An efficient electrocatalyst for oxygen evolution reaction[J]. Electrochimica Acta, 2019, 301: 39-46. |
| 45 | MLYNAREK G, PASZKIEWICZ M, RADNIECKA A. The effect of ferric ions on the behaviour of a nickelous hydroxide electrode[J]. Journal of Applied Electrochemistry, 1984, 14(2): 145-149. |
| 46 | TICHENOR Robert L. Nickel oxides-relation between electrochemical and foreign ion content[J]. Industrial & Engineering Chemistry, 1952, 44(5): 973-977. |
| 47 | LI Huixiu, ZHANG Lingling, WANG Shengping, et al. Accelerated oxygen evolution kinetics on NiFeAl-layered double hydroxide electrocatalysts with defect sites prepared by electrodeposition[J]. International Journal of Hydrogen Energy, 2019, 44(54): 28556-28565. |
| 48 | ZHOU Huan, ZHANG Hua, LAI Changgan, et al. Rapidly electrodeposited NiFe(OH) x as the catalyst for oxygen evolution reaction[J]. Inorganic Chemistry Communications, 2022, 139: 109350. |
| 49 | ETESAMI Mohammad, MOHAMAD Ahmad Azmin, NGUYEN Mai Thanh, et al. Benchmarking superfast electrodeposited bimetallic (Ni, Fe, Co, and Cu) hydroxides for oxygen evolution reaction[J]. Journal of Alloys and Compounds, 2021, 889: 161738. |
| 50 | HUANG Yu, WU Yihui, ZHANG Zejie, et al. Rapid electrodeposited of self-supporting Ni-Fe-Mo film on Ni foam as affordable electrocatalysts for oxygen evolution reaction[J]. Electrochimica Acta, 2021, 390: 138754. |
| 51 | LIU Shanshan, XU Xiufeng, LI Jisen. Silver decorated nickel-cobalt (oxy) hydroxides fabricated via surface reconstruction engineering for boosted electrocatalytic oxygen evolution and urea oxidation[J]. Dalton Transactions, 2022, 51(31): 11814-11822. |
| 52 | XU Qiucheng, JIANG Hao, ZHANG Haoxuan, et al. Heterogeneous interface engineered atomic configuration on ultrathin Ni(OH)2/Ni3S2 nanoforests for efficient water splitting[J]. Applied Catalysis B: Environmental, 2019, 242: 60-66. |
| 53 | CARTAGENA Santiago, BEDOYA-LORA Franky E, CALDERÓN Jorge A. Enhancement of anodically treated stainless steel by NiFeP-catalyst electrodeposition as bifunctional electrodes for water electrolysis[J]. Journal of the Electrochemical Society, 2022, 169(4): 044501. |
| 54 | LI Wenhui, CHEN Mingyue, LU Yu, et al. One-pot electrodeposition synthesis of NiFe-phosphate/phosphide hybrid nanosheet arrays for efficient water splitting[J]. Applied Surface Science, 2022, 598: 153717. |
| 55 | WAN Kai, LUO Jiangshui, ZHOU Chen, et al. Hierarchical porous Ni3S4 with enriched high-valence Ni sites as a robust electrocatalyst for efficient oxygen evolution reaction[J]. Advanced Functional Materials, 2019, 29(18): 1900315. |
| 56 | LV Jingjing, ZHAO Jun, FANG Hua, et al. Incorporating nitrogen-doped graphene quantum dots and Ni3S2 nanosheets: A synergistic electrocatalyst with highly enhanced activity for overall water splitting[J]. Small, 2017, 13(24): 1700264. |
| 57 | ZHANG Yanfang, LIN Li, LIU Juntong, et al. A hierarchical and branch-like NiCoS/NF material prepared by gradient electrodeposition method for oxygen evolution reaction[J]. International Journal of Hydrogen Energy, 2021, 46(74): 36629-36639. |
| 58 | KHODABAKHSHI Meysam, CHEN Shumin, YE Tian, et al. Hierarchical highly wrinkled trimetallic NiFeCu phosphide nanosheets on nanodendrite Ni3S2/Ni foam as an efficient electrocatalyst for the oxygen evolution reaction[J]. ACS Applied Materials & Interfaces, 2020, 12(32): 36268-36276. |
| 59 | XU Qingli, GAO Wenluan, WANG Miao, et al. Electrodeposition of NiS/Ni2P nanoparticles embedded in amorphous Ni(OH)2 nanosheets as an efficient and durable dual-functional electrocatalyst for overall water splitting[J]. International Journal of Hydrogen Energy, 2020, 45(4): 2546-2556. |
| 60 | ZHANG Yanfang, LIN Li, LIU Juntong, et al. Hierarchical sulphide-phosphide NiSP/NF catalyst prepared by gradient electrodeposition for oxygen evolution reaction[J]. Journal of Alloys and Compounds, 2022, 895:162675. |
| [1] | ZHANG Mingyan, LIU Yan, ZHANG Xueting, LIU Yake, LI Congju, ZHANG Xiuling. Research progress of non-noble metal bifunctional catalysts in zinc-air batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 276-286. |
| [2] | SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298. |
| [3] | XIE Luyao, CHEN Songzhe, WANG Laijun, ZHANG Ping. Platinum-based catalysts for SO2 depolarized electrolysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 299-309. |
| [4] | YANG Xiazhen, PENG Yifan, LIU Huazhang, HUO Chao. Regulation of active phase of fused iron catalyst and its catalytic performance of Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 310-318. |
| [5] | ZHANG Jie, BAI Zhongbo, FENG Baoxin, PENG Xiaolin, REN Weiwei, ZHANG Jingli, LIU Eryong. Effect of PEG and its compound additives on post-treatment of electrolytic copper foils [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 374-381. |
| [6] | WANG Lele, YANG Wanrong, YAO Yan, LIU Tao, HE Chuan, LIU Xiao, SU Sheng, KONG Fanhai, ZHU Canghai, XIANG Jun. Influence of spent SCR catalyst blending on the characteristics and deNO x performance for new SCR catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 489-497. |
| [7] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [8] | CHENG Tao, CUI Ruili, SONG Junnan, ZHANG Tianqi, ZHANG Yunhe, LIANG Shijie, PU Shi. Analysis of impurity deposition and pressure drop increase mechanisms in residue hydrotreating unit [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4616-4627. |
| [9] | WANG Peng, SHI Huibing, ZHAO Deming, FENG Baolin, CHEN Qian, YANG Da. Recent advances on transition metal catalyzed carbonylation of chlorinated compounds [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4649-4666. |
| [10] | ZHANG Qi, ZHAO Hong, RONG Junfeng. Research progress of anti-toxicity electrocatalysts for oxygen reduction reaction in PEMFC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4677-4691. |
| [11] | GE Quanqian, XU Mai, LIANG Xian, WANG Fengwu. Research progress on the application of MOFs in photoelectrocatalysis [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4692-4705. |
| [12] | WANG Weitao, BAO Tingyu, JIANG Xulu, HE Zhenhong, WANG Kuan, YANG Yang, LIU Zhaotie. Oxidation of benzene to phenol over aldehyde-ketone resin based metal-free catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4706-4715. |
| [13] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [14] | WU Haibo, WANG Xilun, FANG Yanxiong, JI Hongbing. Progress of the development and application of 3D printing catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3956-3964. |
| [15] | XIANG Yang, HUANG Xun, WEI Zidong. Recent progresses in the activity and selectivity improvement of electrocatalytic organic synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4005-4014. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||