Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (11): 5831-5841.DOI: 10.16085/j.issn.1000-6613.2023-0010
• Materials science and technology • Previous Articles
CO2 capture and separation by ionic liquid-metal organic framework composite materials
CAO Mingmin( ), HAN Chengle, YANG Fang, CHEN Yuhuan(
), HAN Chengle, YANG Fang, CHEN Yuhuan( )
)
- School of Chemical Engineering and Technology, Hebei University of Technology, Tianjin 300130, China
-
Received:2023-01-04Revised:2023-02-24Online:2023-12-15Published:2023-11-20 -
Contact:CHEN Yuhuan
离子液体/金属有机框架复合材料捕集分离CO2
- 河北工业大学化工学院,天津 3001301
-
通讯作者:陈玉焕 -
作者简介:曹明敏(1997—),女,硕士研究生,研究方向为绿色功能材料。E-mail:986628549@qq.com。
CLC Number:
Cite this article
CAO Mingmin, HAN Chengle, YANG Fang, CHEN Yuhuan. CO2 capture and separation by ionic liquid-metal organic framework composite materials[J]. Chemical Industry and Engineering Progress, 2023, 42(11): 5831-5841.
曹明敏, 韩铖乐, 杨芳, 陈玉焕. 离子液体/金属有机框架复合材料捕集分离CO2[J]. 化工进展, 2023, 42(11): 5831-5841.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-0010
| 体系 | 吸附量 | 理想选择性α | 文献 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ILs | MOFs | CO2/N2 | CO2/CH4 | ||||||
| MOFs@ILs | 30%(质量分数)[BMIm][MeSO3] | ZIF-8 | 约12.5mL(STP)/g | 约1.5 | 约2 | [ | |||
| 30%(质量分数)[BMIm][CF3SO3] | ZIF-8 | 约11.8mL(STP)/g | 约0.7 | 约3 | [ | ||||
| 30%(质量分数)[BMIm][MeSO4] | ZIF-8 | 约12.7mL(STP)/g | 约3.3 | 约1.8 | [ | ||||
| 30%(质量分数)[BMIm][OCSO4] | ZIF-8 | 约5.5mL(STP)/g | 约0.6 | 约1.4 | [ | ||||
| 5%(质量分数)[BMIm][AC] | HKUST-1 | 4.4mmol/g | [ | ||||||
| 10%(质量分数)[BMIm][AC] | HKUST-1 | 2.9mmol/g | [ | ||||||
| 20%(质量分数)[BMIm][AC] | HKUST-1 | 1.9mmol/g | [ | ||||||
| 3.44%(质量分数)[BMIm][BF4] | UiO-66 | 约32cm3/g | 8.1 | [ | |||||
| 7.8%(质量分数)[BMIm][BF4] | ZIF-8 | 约12cm3/g | 7.5 | [ | |||||
| 2.38%(质量分数)[BMIm][BF4] | CuBTC | 约8cm3/g | 15.3 | [ | |||||
| 30%(质量分数)[EMIm][DEP] | CuBTC | 约44mL/g | 7.4 | [ | |||||
| 30%(质量分数)[BMIm][SCN] | ZIF-8 | 约6.5mL/g | 约11 | 约5 | [ | ||||
| 5%(质量分数)[BMIm][BF4] | ZIF-8 | 约250mL/g | 15.81 | 5.57 | [ | ||||
| 9.3%(摩尔分数)[BMIm]2[CoCl4] | ZIF-8 | 0.43mmol/g | 7.5 | 2.7 | [ | ||||
| 9.3%(摩尔分数)[BMIm]2[NiCl4] | ZIF-8 | 0.40mmol/g | 7.5 | 2.7 | [ | ||||
| 9.3%(摩尔分数)[BMIm]2[FeCl4] | ZIF-8 | 0.41mmol/g | 7.1 | 2.8 | [ | ||||
| 9.3%(摩尔分数)[BMIm]2[Co(NCS)4] | ZIF-8 | 0.30mmol/g | 6.4 | 2.4 | [ | ||||
| 9.3%(摩尔分数)[BMIm]2[MnCl4] | ZIF-8 | 0.32mmol/g | 7.9 | 3.3 | [ | ||||
| 50%(质量分数)[EMIm][DCN] | MIL-101(Al) | 约3.2mmol/g | [ | ||||||
| PIL | MIL-101(Cr) | 62cm3/g | [ | ||||||
| 20%(质量分数)[BMIm][BF4] | ZIF-8 | 10.5mL(STP)/g | 约8 | 约3 | [ | ||||
| IL@MOFs | [C2OHMIM][DCA] | ZIF-8 | 3mL(STP)/g | 11 | [ | ||||
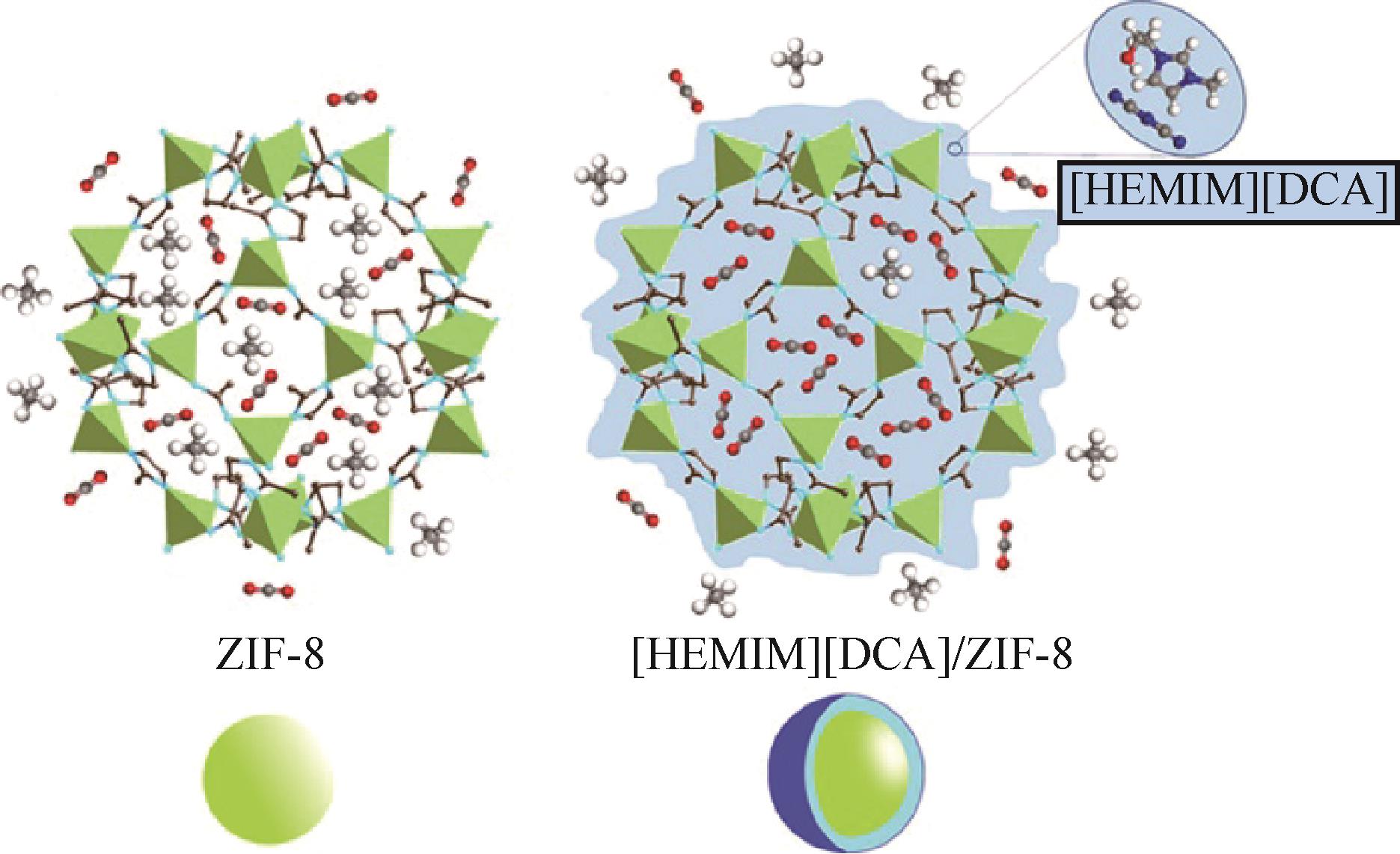
| [HemIm][DCA] | ZIF-8 | 8.6cm3/g | 30 | [ | |||||
| IL@MOFs@IL | [TETA]L | ZIF-8 | 1.53mmol/g | 1688① | 260② | [ | |||
| IL-MOFs | [PMIm][SO3]-NaCl | UiO-67 | 47.9cm3/g | 44.21② | [ | ||||
| [NAIm]Br | ZIF-67 | 24.8cm3/g | [ | ||||||
| PIL-NH2 | Cu3(BTC)2 | 19.5cm3/g | [ | ||||||
| 体系 | 吸附量 | 理想选择性α | 文献 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ILs | MOFs | CO2/N2 | CO2/CH4 | ||||||
| MOFs@ILs | 30%(质量分数)[BMIm][MeSO3] | ZIF-8 | 约12.5mL(STP)/g | 约1.5 | 约2 | [ | |||
| 30%(质量分数)[BMIm][CF3SO3] | ZIF-8 | 约11.8mL(STP)/g | 约0.7 | 约3 | [ | ||||
| 30%(质量分数)[BMIm][MeSO4] | ZIF-8 | 约12.7mL(STP)/g | 约3.3 | 约1.8 | [ | ||||
| 30%(质量分数)[BMIm][OCSO4] | ZIF-8 | 约5.5mL(STP)/g | 约0.6 | 约1.4 | [ | ||||
| 5%(质量分数)[BMIm][AC] | HKUST-1 | 4.4mmol/g | [ | ||||||
| 10%(质量分数)[BMIm][AC] | HKUST-1 | 2.9mmol/g | [ | ||||||
| 20%(质量分数)[BMIm][AC] | HKUST-1 | 1.9mmol/g | [ | ||||||
| 3.44%(质量分数)[BMIm][BF4] | UiO-66 | 约32cm3/g | 8.1 | [ | |||||
| 7.8%(质量分数)[BMIm][BF4] | ZIF-8 | 约12cm3/g | 7.5 | [ | |||||
| 2.38%(质量分数)[BMIm][BF4] | CuBTC | 约8cm3/g | 15.3 | [ | |||||
| 30%(质量分数)[EMIm][DEP] | CuBTC | 约44mL/g | 7.4 | [ | |||||
| 30%(质量分数)[BMIm][SCN] | ZIF-8 | 约6.5mL/g | 约11 | 约5 | [ | ||||
| 5%(质量分数)[BMIm][BF4] | ZIF-8 | 约250mL/g | 15.81 | 5.57 | [ | ||||
| 9.3%(摩尔分数)[BMIm]2[CoCl4] | ZIF-8 | 0.43mmol/g | 7.5 | 2.7 | [ | ||||
| 9.3%(摩尔分数)[BMIm]2[NiCl4] | ZIF-8 | 0.40mmol/g | 7.5 | 2.7 | [ | ||||
| 9.3%(摩尔分数)[BMIm]2[FeCl4] | ZIF-8 | 0.41mmol/g | 7.1 | 2.8 | [ | ||||
| 9.3%(摩尔分数)[BMIm]2[Co(NCS)4] | ZIF-8 | 0.30mmol/g | 6.4 | 2.4 | [ | ||||
| 9.3%(摩尔分数)[BMIm]2[MnCl4] | ZIF-8 | 0.32mmol/g | 7.9 | 3.3 | [ | ||||
| 50%(质量分数)[EMIm][DCN] | MIL-101(Al) | 约3.2mmol/g | [ | ||||||
| PIL | MIL-101(Cr) | 62cm3/g | [ | ||||||
| 20%(质量分数)[BMIm][BF4] | ZIF-8 | 10.5mL(STP)/g | 约8 | 约3 | [ | ||||
| IL@MOFs | [C2OHMIM][DCA] | ZIF-8 | 3mL(STP)/g | 11 | [ | ||||
| [HemIm][DCA] | ZIF-8 | 8.6cm3/g | 30 | [ | |||||
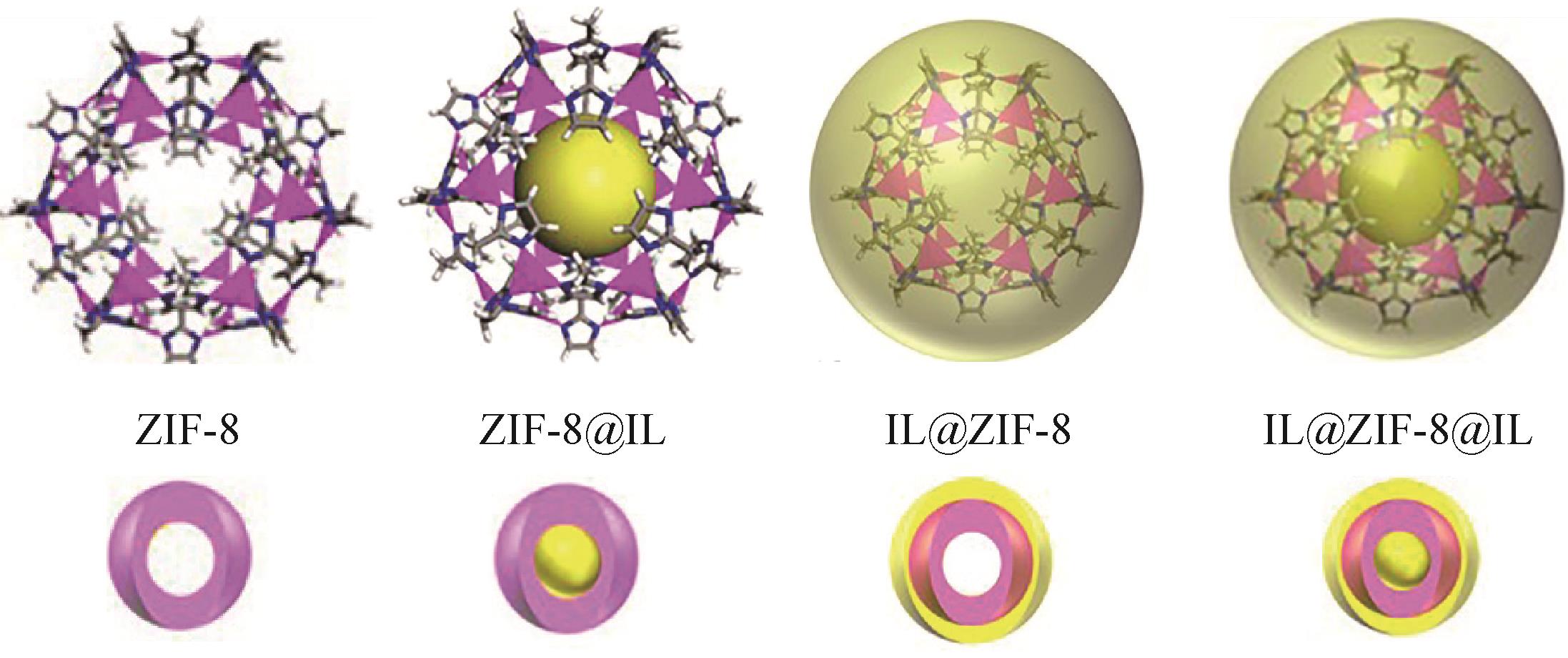
| IL@MOFs@IL | [TETA]L | ZIF-8 | 1.53mmol/g | 1688① | 260② | [ | |||
| IL-MOFs | [PMIm][SO3]-NaCl | UiO-67 | 47.9cm3/g | 44.21② | [ | ||||
| [NAIm]Br | ZIF-67 | 24.8cm3/g | [ | ||||||
| PIL-NH2 | Cu3(BTC)2 | 19.5cm3/g | [ | ||||||
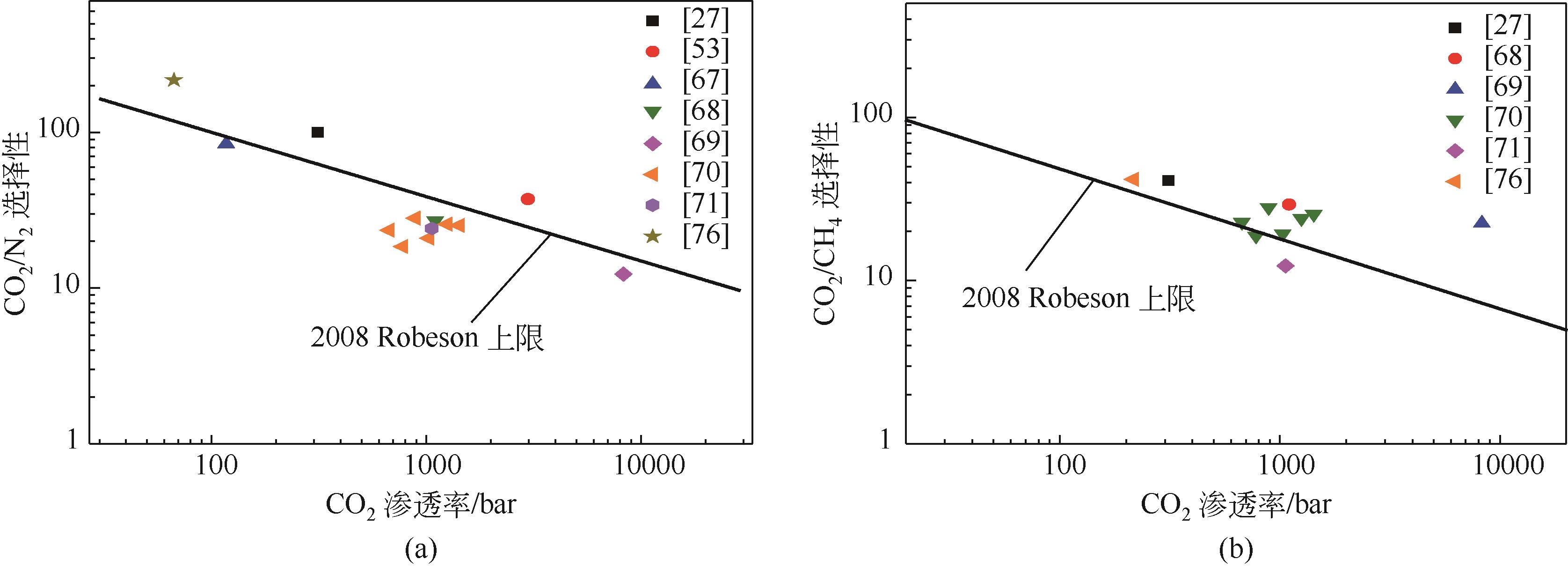
| 填料 | 聚合物基质 | 填料质量分数 /% | 操作条件 | 渗透率 /bar | 选择性 | 文献 | ||
|---|---|---|---|---|---|---|---|---|
| T/K | Δp/bar | CO2/N2 | CO2/CH4 | |||||
| 6%(体积分数)[BMIm][Tf2N]/ZIF-8 | PSF | 303 | 6 | 约312 | 100 | 41 | [ | |
| 2%(质量分数)[EMIm][C(CN)3]/Mg-MOF-74 | Matrimid®5218 | 2 | 303 | 0.7 | 36.13 | [ | ||
| 2%(质量分数)TMGA/MOF-5 | Matrimid®5218 | 2 | 303 | 0.7 | 35.48 | [ | ||
| 8%(质量分数)[BMIm][PF6]/ZIF-8 | Pebax | 25 | 298 | 2 | 117 | 84.5 | [ | |
| 50%(质量分数)[C3NH2BIm][Tf2N]/NH2-MIL-101(Cr) | PIM-1 | 5 | 298 | 3 | 2979 | 37.24 | [ | |
| 67%(质量分数)[EMIm][Tf2N]/HKUST-1 | 6FDA-Durene Polyimide | 30 | 298 | 1.985 | 1101.6 | 27.1 | 29.3 | [ |
| 83%(质量分数)[BMIm][Tf2N]/UiO-66-NH2 | PIM-1 | 10 | 293 | 1 | 8283.4 | 12.3 | 22.5 | [ |
| 5%(质量分数)[BMIm][BF4]/ZIF-67 | 6FDA-Durene Polyimide | 10 | 298 | 2 | 779.01 | 18.48 | 18.76 | [ |
| 5%(质量分数)[BMIm][BF4]/ZIF-67 | 6FDA-Durene Polyimide | 20 | 298 | 2 | 1254.64 | 25.70 | 24.00 | [ |
| 5%(质量分数)[EMIm][Tf2N]/ZIF-67 | 6FDA-Durene Polyimide | 10 | 298 | 2 | 1030.9 | 20.87 | 19.40 | [ |
| 5%(质量分数)[EMIm][Tf2N]/ZIF-67 | 6FDA-Durene Polyimide | 20 | 298 | 2 | 1426 | 25.28 | 25.49 | [ |
| 5%(质量分数)[BMIm][Tf2N]/ZIF-67 | 6FDA-Durene Polyimide | 10 | 298 | 2 | 672 | 23.54 | 22.77 | [ |
| 5%(质量分数)[BMIm][Tf2N]/ZIF-67 | 6FDA-Durene/(P) | 20 | 298 | 2 | 889 | 28.09 | 28.05 | [ |
| [EMIm][Tf2N]/ZIF-8 | P[VBIm][Tf2N] | 25.8 | 308 | 3.5 | 1062.4 | 24.2 | 12.34 | [ |
| [BMIm][BF4]/Zr-Fc MOF | Polycarbonate | 293 | 0.49 | 66.8 | 216.9 | 41.7 | [ | |
| 填料 | 聚合物基质 | 填料质量分数 /% | 操作条件 | 渗透率 /bar | 选择性 | 文献 | ||
|---|---|---|---|---|---|---|---|---|
| T/K | Δp/bar | CO2/N2 | CO2/CH4 | |||||
| 6%(体积分数)[BMIm][Tf2N]/ZIF-8 | PSF | 303 | 6 | 约312 | 100 | 41 | [ | |
| 2%(质量分数)[EMIm][C(CN)3]/Mg-MOF-74 | Matrimid®5218 | 2 | 303 | 0.7 | 36.13 | [ | ||
| 2%(质量分数)TMGA/MOF-5 | Matrimid®5218 | 2 | 303 | 0.7 | 35.48 | [ | ||
| 8%(质量分数)[BMIm][PF6]/ZIF-8 | Pebax | 25 | 298 | 2 | 117 | 84.5 | [ | |
| 50%(质量分数)[C3NH2BIm][Tf2N]/NH2-MIL-101(Cr) | PIM-1 | 5 | 298 | 3 | 2979 | 37.24 | [ | |
| 67%(质量分数)[EMIm][Tf2N]/HKUST-1 | 6FDA-Durene Polyimide | 30 | 298 | 1.985 | 1101.6 | 27.1 | 29.3 | [ |
| 83%(质量分数)[BMIm][Tf2N]/UiO-66-NH2 | PIM-1 | 10 | 293 | 1 | 8283.4 | 12.3 | 22.5 | [ |
| 5%(质量分数)[BMIm][BF4]/ZIF-67 | 6FDA-Durene Polyimide | 10 | 298 | 2 | 779.01 | 18.48 | 18.76 | [ |
| 5%(质量分数)[BMIm][BF4]/ZIF-67 | 6FDA-Durene Polyimide | 20 | 298 | 2 | 1254.64 | 25.70 | 24.00 | [ |
| 5%(质量分数)[EMIm][Tf2N]/ZIF-67 | 6FDA-Durene Polyimide | 10 | 298 | 2 | 1030.9 | 20.87 | 19.40 | [ |
| 5%(质量分数)[EMIm][Tf2N]/ZIF-67 | 6FDA-Durene Polyimide | 20 | 298 | 2 | 1426 | 25.28 | 25.49 | [ |
| 5%(质量分数)[BMIm][Tf2N]/ZIF-67 | 6FDA-Durene Polyimide | 10 | 298 | 2 | 672 | 23.54 | 22.77 | [ |
| 5%(质量分数)[BMIm][Tf2N]/ZIF-67 | 6FDA-Durene/(P) | 20 | 298 | 2 | 889 | 28.09 | 28.05 | [ |
| [EMIm][Tf2N]/ZIF-8 | P[VBIm][Tf2N] | 25.8 | 308 | 3.5 | 1062.4 | 24.2 | 12.34 | [ |
| [BMIm][BF4]/Zr-Fc MOF | Polycarbonate | 293 | 0.49 | 66.8 | 216.9 | 41.7 | [ | |
| 1 | NEJAT P, JOMEHZADEH F, TAHERI M, et al. A global review of energy consumption, CO2 emissions and policy in the residential sector (with an overview of the top ten CO2 emitting countries)[J]. Renewable and Sustainable Energy Reviews, 2015, 43: 843-862. |
| 2 | BAUER N, MOURATIADOU I, LUDERER G, et al. Global fossil energy markets and climate change mitigation—an analysis with REMIND[J]. Climatic Change, 2016, 136(1): 69-82. |
| 3 | RAO A, RUBIN E. A technical, economic, and environmental assessment of amine-based CO2 capture technology for power plant greenhouse gas control[J]. Environmental Science & Technology, 2002, 36(20): 4467-4475. |
| 4 | RAMDIN M, LOOS T, VLUGT T. State-of-the-art of CO2 capture with ionic liquids[J]. Industrial & Engineering Chemistry Research, 2012, 51(24): 8149-8177. |
| 5 | HASIB-UR-RAHMAN M, SIAJ M, LARACHI F. Ionic liquids for CO2 capture—Development and progress[J]. Chemical Engineering and Processing: Process Intensification, 2010, 49(4): 313-322. |
| 6 | COTA I, MARTINEZ F. Recent advances in the synthesis and applications of metal organic frameworks doped with ionic liquids for CO2 adsorption[J]. Coordination Chemistry Reviews, 2017, 351: 189-204. |
| 7 | KINIK F, UZUN A, KESKIN S. Ionic liquid/metal-organic framework composites: From synthesis to applications[J]. ChemSusChem, 2017, 10(14): 2842-2863. |
| 8 | ZEESHAN M, KULAK H, KAVAK S, et al. Influence of anion size and electronic structure on the gas separation performance of ionic liquid/ZIF-8 composites[J]. Microporous and Mesoporous Materials, 2020, 306: 110446. |
| 9 | XIA X, HU G, LI W, et al. Understanding reduced CO2 uptake of ionic liquid/metal-organic framework (IL/MOF) composites[J]. ACS Applied Nano Materials, 2019, 2(9): 6022-6029. |
| 10 | MOHAMEDALI M, HENNI A, IBRAHIM H. Markedly improved CO2 uptake using imidazolium-based ionic liquids confined into HKUST-1 frameworks[J]. Microporous and Mesoporous Materials, 2019, 284: 98-110. |
| 11 | HUSSAIN S, DONG H, ZHANG Y, et al. Impregnation of 1-n-butyl-3-methylimidazolium dicyanide [BMIM][DCA] into ZIF-8 as a versatile sorbent for efficient and selective separation of CO2 [J]. Industrial & Engineering Chemistry Research, 2022, 61(1): 706-715. |
| 12 | YU T, CAI Q, LIAN G, et al. Mechanisms behind high CO2/CH4 selectivity using ZIF-8 metal organic frameworks with encapsulated ionic liquids: A computational study[J]. Chemical Engineering Journal, 2021, 419: 1385-8947. |
| 13 | FERREIRA T, ESTEVES L, ESPERANCA J, et al. Unveiling the temperature influence on the sorptive behaviour of ZIF-8 composite materials impregnated with [C n MIM][B(CN)4] ionic liquids[J]. Processes, 2022, 10(2): 247. |
| 14 | ZEESHAN M, GULBALKAN H, DURAK O, et al. An integrated computational-experimental hierarchical approach for the rational design of an IL/UiO-66 composite offering infinite CO2 selectivity[J]. Advanced Functional Materials, 2022, 32(35): 2204149. |
| 15 | GAO W, ZHENG W, SUN W, et al. Understanding the effective capture of H2S/CO2 from natural gas using ionic liquid@MOF composites[J]. The Journal of Physical Chemistry C, 2022, 126(46): 19872-19882. |
| 16 | ZEESHAN M, NOZARI V, YAGCI M B, et al. Core-shell type ionic liquid/metal organic framework composite: An exceptionally high CO2/CH4 selectivity[J]. Journal of the American Chemical Society, 2018, 140(32): 10113-10116. |
| 17 | HAN G, YU N, LIU D, et al. Stepped enhancement of CO2 adsorption and separation in IL-ZIF-IL composites with shell-interlayer-core structure[J]. AIChE Journal, 2021, 67(2): e17112. |
| 18 | BAN Y, LI Z, LI Y, et al. Confinement of ionic liquids in nanocages: Tailoring the molecular sieving properties of ZIF-8 for membrane-Based CO2 capture [J]. Angewandte Chemie International Edition, 2015, 54(51): 15483 –15487. |
| 19 | WANG J, XIE D, ZHANG Z, et al. Efficient adsorption separation of acetylene and ethylene via supported ionic liquid on metal-organic framework[J]. AIChE Journal, 2017, 63(6): 2165-2175. |
| 20 | SILVA F, MAGALHÃES G, JARDIM E, et al. CO2 adsorption on ionic liquid-modified Cu-BTC: Experimental and simulation study[J]. Adsorption Science & Technology, 2015, 33(2): 223-242. |
| 21 | POLAT H, KAVAK S, KULAK H, et al. CO2 separation from flue gas mixture using [BMIM][BF4]/MOF composites: Linking high-throughput computational screening with experiments[J]. Chemical Engineering Journal, 2020, 394: 124916. |
| 22 | ZEESHAN M, GULBALKAN H, HASLAK Z, et al. Doubling CO2/N2 separation performance of CuBTC by incorporation of 1-n-ethyl-3-methylimidazolium diethyl phosphate[J]. Microporous and Mesoporous Materials, 2021, 316: 110947. |
| 23 | ZEESHAN M, KESKIN S, UZUN A. Enhancing CO2/CH4 and CO2/N2 separation performances of ZIF-8 by post-synthesis modification with [BMIM][SCN][J]. Polyhedron, 2018, 155: 485-492. |
| 24 | SEZGINEL K, KESKIN S, UZUN A. Tuning the gas separation performance of CuBTC by ionic liquid incorporation[J]. Langmuir: The ACS Journal of Surfaces and Colloids, 2016, 32(4): 1139-1147. |
| 25 | KAVAK S, POLAT H, KULAK H, et al. MIL-53 (Al) as a versatile platform for ionic-liquid/MOF composites to enhance CO2 selectivity over CH4 and N2 [J]. Chemistry, an Asian Journal, 2019, 14(20): 3655-3667. |
| 26 | FERREIRA T, VERA A, DE MOURA B, et al. Paramagnetic ionic liquid/metal organic framework composites for CO2/CH4 and CO2/N2 separations[J]. Frontiers in Chemistry, 2020, 8: 590191. |
| 27 | AIJAZ A, AKITA T, YANG H, et al. From ionic-liquid@metal-organic framework composites to heteroatom-decorated large-surface area carbons: Superior CO2 and H2 uptake[J]. Chemical Communications, 2014, 50(49): 6498-6501. |
| 28 | MOHAMED A, KROKIDAS P, ECONOMOU I. CO2 selective metal organic framework ZIF-8 modified through ionic liquid encapsulation: A computational study[J]. Journal of Computational Science, 2018, 27: 183-191. |
| 29 | YU T, CAI Q, LIAN G, et al. Mechanisms behind high CO2/CH4 selectivity using ZIF-8 metal organic frameworks with encapsulated ionic liquids: a computational study[J]. Chemical Engineering Journal, 2021, 419: 129638. |
| 30 | MOHAMED A, KROKIDAS P, ECONOMOU I. Encapsulation of [bmim+][Tf2N-] in different ZIF-8 metal analogues and evaluation of their CO2 selectivity over CH4 and N2 using molecular simulation[J]. Molecular Systems Design & Engineering, 2020, 5(7): 1230-1238. |
| 31 | MOHAMED A, MONCHO S, KROKIDAS P, et al. Computational investigation of the performance of ZIF-8 with encapsulated ionic liquids towards CO2 capture[J]. Molecular Physics, 2019, 117(23/24): 3791-3805. |
| 32 | LAN Y, YAN T, TONG M, et al. Large-scale computational assembly of ionic liquid/MOF composites: Synergistic effect in the wire-tube conformation for efficient CO2/CH4 separation[J]. Journal of Materials Chemistry A, 2019, 7(20): 12556-12564. |
| 33 | OLIVEIRA L, GONÇALVES R, GONÇALVES D, et al. Superior performance of mesoporous MOF MIL-100 (Fe) impregnated with ionic liquids for CO2 adsorption[J]. Journal of Chemical and Engineering Data, 2019, 64(5): 2221-2228. |
| 34 | MAURYA M, SINGH J. Effect of ionic liquid impregnation in highly water-stable metal-organic frameworks, covalent organic frameworks, and carbon-based adsorbents for post-combustion flue gas treatment[J]. Energy & Fuels, 2019, 33(4): 3421-3428. |
| 35 | KINIK F P, ALTINTAS C, BALCI V, et al. [BMIM][PF6] incorporation doubles CO2 selectivity of ZIF-8: Elucidation of interactions and their consequences on performance[J]. ACS Applied Materials & Interfaces, 2016, 8(45): 30992-31005. |
| 36 | XUE W, LI Z, HUANG H, et al. Effects of ionic liquid dispersion in metal-organic frameworks and covalent organic frameworks on CO2 capture: A computational study[J]. Chemical Engineering Science, 2016, 140: 1-9. |
| 37 | BARA J, CARLISLE T, GABRIEL C, et al. Guide to CO2 separations in imidazolium-based room-temperature ionic liquids[J]. Industrial & Engineering Chemistry Research, 2009, 48(6): 2739-2751. |
| 38 | ALMANTARIOTIS D, GEFFLAUT T, PÁDUA A, et al. Effect of fluorination and size of the alkyl side-chain on the solubility of carbon dioxide in 1-alkyl-3-methylimidazolium bis(trifluoromethylsulfonyl) amide ionic liquids[J]. The Journal of Physical Chemistry B, 2010, 114(10): 3608-3617. |
| 39 | DING M, JIANG H. Incorporation of imidazolium-based poly(ionic liquid) s into a metal-organic framework for CO2 capture and conversion[J]. ACS Catalysis, 2018, 8(4): 3194-3201. |
| 40 | KOYUTURK B, ALTINTAS C, KINIK F P, et al. Improving gas separation performance of ZIF-8 by [BMIM][BF4] incorporation: Interactions and their consequences on performance[J]. The Journal of Physical Chemistry C, 2017, 121(19): 10370-10381. |
| 41 | THOMAS A, AHAMED R, PRAKASH M. Selection of a suitable ZIF-8/ionic liquid (IL) based composite for selective CO2 capture: The role of anions at the interface[J]. RSC Advances, 2020, 10(64): 39160-39170. |
| 42 | THOMAS A, MAIYELVAGANAN K, KAMALAKANNAN S, et al. Density functional theory studies on zeolitic imidazolate framework-8 and ionic liquid-based composite materials[J]. ACS Omega, 2019, 4(27): 22655-22666. |
| 43 | MA J, YING Y, GUO X, et al. Fabrication of mixed-matrix membrane containing selective and facilitated CO2 transport metal-organic framework composite with task-specific ionic liquid for efficient CO2 separation[J]. Journal of Materials Chemistry A, 2016, 4(19): 7281-7288. |
| 44 | MCEWEN J, HAYMAN J, YAZAYDIN A. A comparative study of CO2, CH4 and N2 adsorption in ZIF-8, Zeolite-13X and BPL activated carbon[J]. Chemical Physics, 2013, 412: 72-76. |
| 45 | KURISINGAL J, RACHURI Y, PILLAI R, et al. Ionic-liquid-functionalized UiO-66 framework: An experimental and theoretical study on the cycloaddition of CO2 and epoxides[J]. ChemSusChem, 2019, 12(5): 1033-1042. |
| 46 | DING L, YAO B, JIANG W, et al. Bifunctional imidazolium-based ionic liquid decorated UiO-67 type MOF for selective CO2 adsorption and catalytic property for CO2 cycloaddition with epoxides[J]. Inorganic Chemistry, 2017, 56(4): 2337-2344. |
| 47 | XUE W, WANG L, LI Y, et al. Reticular chemistry for ionic liquid-functionalized metal-organic frameworks with high selectivity for CO2 [J]. ACS Sustainable Chemistry & Engineering, 2020, 8(50): 18558-18567. |
| 48 | 李庆朝. 金属-有机骨架材料ZIF-8的改性及其在CO2捕获-转化中的应用[D]. 太原: 太原理工大学, 2017. |
| LI Qingchao. Modification of metal-organic frameworks ZIF-8 and their application in CO2 capture and conversion[D]. Taiyuan: Taiyuan University of Technology, 2017. | |
| 49 | 许少勃. 面向CO2高效捕获与转化的功能化咪唑离子液体基有机无机复合材料[D]. 太原: 太原理工大学, 2018. |
| XU Shaobo. Functionalized imidazolium ionic liquids based organicinorganic hybrid materials for efficient CO2 capture and conversion[D]. Taiyuan: Taiyuan University of Technology, 2018. | |
| 50 | SONG X, YU J, WEI M, et al. Ionic liquids-functionalized zeolitic imidazolate framework for carbon dioxide adsorption[J]. Materials, 2019, 12(15): 2361. |
| 51 | CHEN C, FENG N, GUO Q, et al. Surface engineering of a chromium metal-organic framework with bifunctional ionic liquids for selective CO2 adsorption: Synergistic effect between multiple active sites[J]. Journal of Colloid and Interface Science, 2018, 521: 91-101. |
| 52 | YANG G, YU J, PENG S, et al. Poly(ionic liquid)-modified metal organic framework for carbon dioxide adsorption[J]. Polymers, 2020, 12(2): 370. |
| 53 | YAO B, DING L, LI F, et al. Chemically cross-linked MOF membrane generated from imidazolium-based ionic liquid-decorated UiO-66 type NMOF and its application toward CO2 separation and conversion[J]. ACS Applied Materials & Interfaces, 2017, 9(44): 38919-38930. |
| 54 | AVILA J, LEPRE L, SANTINI C, et al. High-performance porous ionic liquids for low-pressure CO2 capture[J]. Angewandte Chemie International Edition, 2021, 60(23): 12876-12882. |
| 55 | ZHAO X, YUAN Y, LI P, et al. A polyether amine modified metal organic framework enhanced the CO2 adsorption capacity of room temperature porous liquids[J]. Chemical Communications, 2019, 55(87): 13179-13182. |
| 56 | 郭自兴. 离子液体调控MOF分离通道及其混合基质膜CO2分离性能探究[D]. 大连: 大连理工大学, 2020. |
| GUO Zixing. Ionic liquid modulation of MOF cage size and its mixed matrix membrane for CO2 separatioin[D]. Dalian: Dalian University of Technology, 2020. | |
| 57 | MONTEIRO B, NABAIS A, ALMEIDA|PAZ F, et al. Front cover: Membranes with a low loading of metal-organic framework‐supported ionic liquids for CO2/N2 separation in CO2 capture[J]. Energy Technology, 2017, 5(12): 2158-2162. |
| 58 | GUO Z, ZHENG W, YAN X, et al. Ionic liquid tuning nanocage size of MOFs through a two-step adsorption/infiltration strategy for enhanced gas screening of mixed-matrix membranes[J]. Journal of Membrane Science, 2020, 605: 118101. |
| 59 | LIN R, GE L, DIAO H, et al. Ionic liquids as the MOFs/polymer interfacial binder for efficient membrane separation[J]. ACS Applied Materials & Interfaces, 2016, 8(46): 32041-32049. |
| 60 | LU J, ZHANG X, XU L, et al. Preparation of amino-functional UiO-66/PIMs mixed matrix membranes with [bmim][Tf2N] as regulator for enhanced gas separation[J]. Membranes, 2021, 11(1): 35. |
| 61 | VU M, LIN R, DIAO H, et al. Effect of ionic liquids (ILs) on MOFs/polymer interfacial enhancement in mixed matrix membranes[J]. Journal of Membrane Science, 2019, 587: 117157. |
| 62 | HAO L, LI P, YANG T, CHUNG T. Room temperature ionic liquid/ZIF-8 mixed-matrix membranes for natural gas sweetening and post-combustion CO2 capture[J]. Journal of Membrane Science, 2013, 436: 221-231. |
| 63 | NEVES L. Impact of ionic liquid structure and loading on gas sorption and permeation for ZIF-8-based composites and mixed matrix membranes[J]. Membranes, 2021, 12(1): 13. |
| 64 | CHEN W, ZHANG Z, YANG C, et al. PIM-based mixed-matrix membranes containing MOF-801/ionic liquid nanocomposites for enhanced CO2 separation performance[J]. Journal of Membrane Science, 2021, 636: 119581. |
| 65 | FERREIRA I, FERREIRA T, BARBOSA A, et al. Cr-based MOF/IL composites as fillers in mixed matrix membranes for CO2 separation[J]. Separation and Purification Technology, 2021, 276: 119303. |
| 66 | MARTĺNEZ-IZQUIERDO L, TÉLLEZ C, CORONAS J. Highly stable Pebax® Renew® thin-film nanocomposite membranes with metal organic framework ZIF-94 and ionic liquid [Bmim][BF 4] for CO2 capture[J]. Journal of Materials Chemistry A, 2022, 10: 18822-18833. |
| 67 | DENG Z, WAN T, CHEN D, et al. Photothermal-responsive microporous nanosheets confined ionic liquid for efficient CO2 separation[J]. Small, 2020, 16(34): e2002699. |
| 68 | GUPTA K, CHEN Y, JIANG J. Ionic liquid membranes supported by hydrophobic and hydrophilic metal-organic frameworks for CO2 capture[J]. The Journal of Physical Chemistry C, 2013, 117(11): 5792-5799. |
| 69 | ALI S, SHAH S, SHAH M, et al. Synthesis and performance evaluation of copper and magnesium-based metal organic framework supported ionic liquid membrane for CO2/N2 separation [J]. Chemosphere, 2023, 311: 136913. |
| [1] | YANG Xiazhen, PENG Yifan, LIU Huazhang, HUO Chao. Regulation of active phase of fused iron catalyst and its catalytic performance of Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 310-318. |
| [2] | PAN Yichang, ZHOU Rongfei, XING Weihong. Advanced microporous membranes for efficient separation of same-carbon-number hydrocarbon mixtures: State-of-the-art and challenges [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3926-3942. |
| [3] | ZHU Yajing, XU Yan, JIAN Meipeng, LI Haiyan, WANG Chongchen. Progress of metal-organic frameworks for uranium extraction from seawater [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3029-3048. |
| [4] | XU Chunshu, YAO Qingda, LIANG Yongxian, ZHOU Hualong. Effects of graphene oxide/carbon nanotubes on the properties of several typical polymer materials [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3012-3028. |
| [5] | ZHANG Jianzhong, XU Sheng, FAN Jiashu, FEI Zhenyu, WANG Kun, HUANG Jian, CUI Fengbo, RAN Wenhua. Progress in characterization and analysis of glass fiber sizing [J]. Chemical Industry and Engineering Progress, 2023, 42(2): 821-838. |
| [6] | HUA Guoyan, XU Xiaoming, CHEN Yuxuan, ZHANG Yanhong, LIU Fuqiang. Progress and prospects of MOFs-based membranes for Mg-Li separation [J]. Chemical Industry and Engineering Progress, 2023, 42(11): 5776-5785. |
| [7] | YE Hao, HU Ping, WANG Ce, LIU Yong. Advances in research on magnetic fibrous electromagnetic wave absorbers [J]. Chemical Industry and Engineering Progress, 2023, 42(10): 5310-5321. |
| [8] | YANG Zhuangzhuang, LIU Yongjun, LIU Xingshe, LIU Zhe, YANG Lu, ZHANG Aining. Coalescence separation of oily sludge and removal effect of organic substances from coal chemical wastewater [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 538-545. |
| [9] | ZHANG Jinhui, ZHANG Huan, ZHU Xinfeng, SONG Zhongxian, KANG Haiyan, LIU Hongpan, DENG Wei, HOU Guangchao, LI Guiting, HUANG Zhenzhen. Research progress of UiO-66 materials for adsorption and photocatalytic oxidation of typical organic compounds [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 445-456. |
| [10] | GAO Weitao, YIN Qinan, TU Ziqiang, GONG Fan, LI Yang, XU Hong, WANG Cheng, MAO Zongqiang. Proton transport in metal-organic frameworks and their applications in proton exchange membranes [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 260-268. |
| [11] | CHEN Yu, LIU Chong, QIU Yuhui, BI Zixin, MU Tiancheng. Ionic liquids and deep eutectic solvents for green recycle of spent lithium-ion batteries [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 485-496. |
| [12] | QIN Jian, LIU Tianxia, WANG Jian, LU Xing. Preparation and tribological properties of oleic acid modified graphene/molybdenum disulfide composite lubricating additives [J]. Chemical Industry and Engineering Progress, 2022, 41(9): 4973-4985. |
| [13] | ZHU Xiao, ZHU Junyong, ZHANG Yatao. Research progress of metal organic framework/polyamide thin film nanocomposite membrane [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4314-4326. |
| [14] | SHAN Qingwen, ZHANG Juan, WANG Yajuan, LIU Wenqiang. Synthesis of polymeric ionic liquid and its performance on adsorption desulfurization [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4571-4579. |
| [15] | ZHENG Jinbao, LI Chen. Research progress in improving hydrophobicity of starch-based packaging materials [J]. Chemical Industry and Engineering Progress, 2022, 41(6): 3089-3102. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||