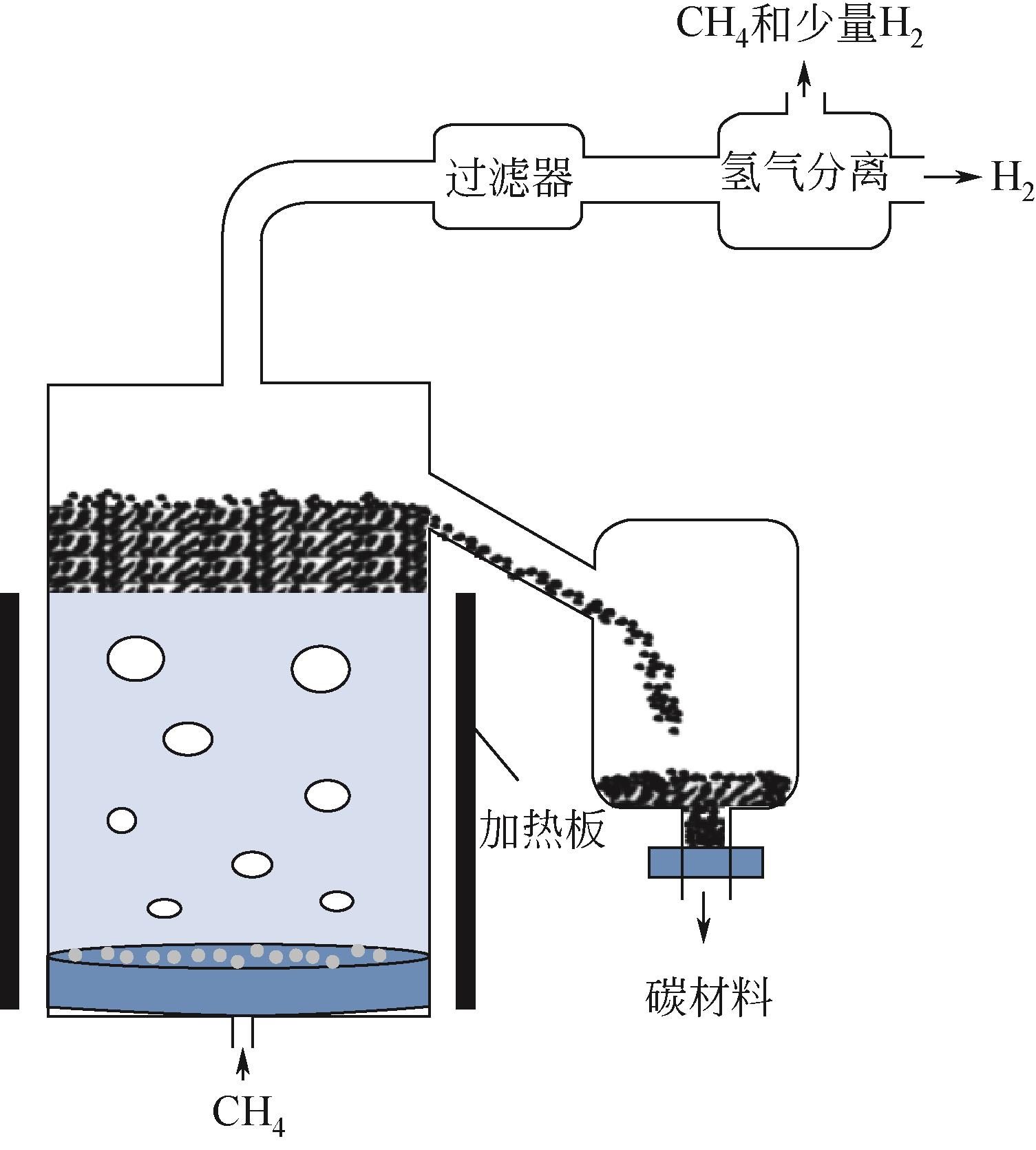
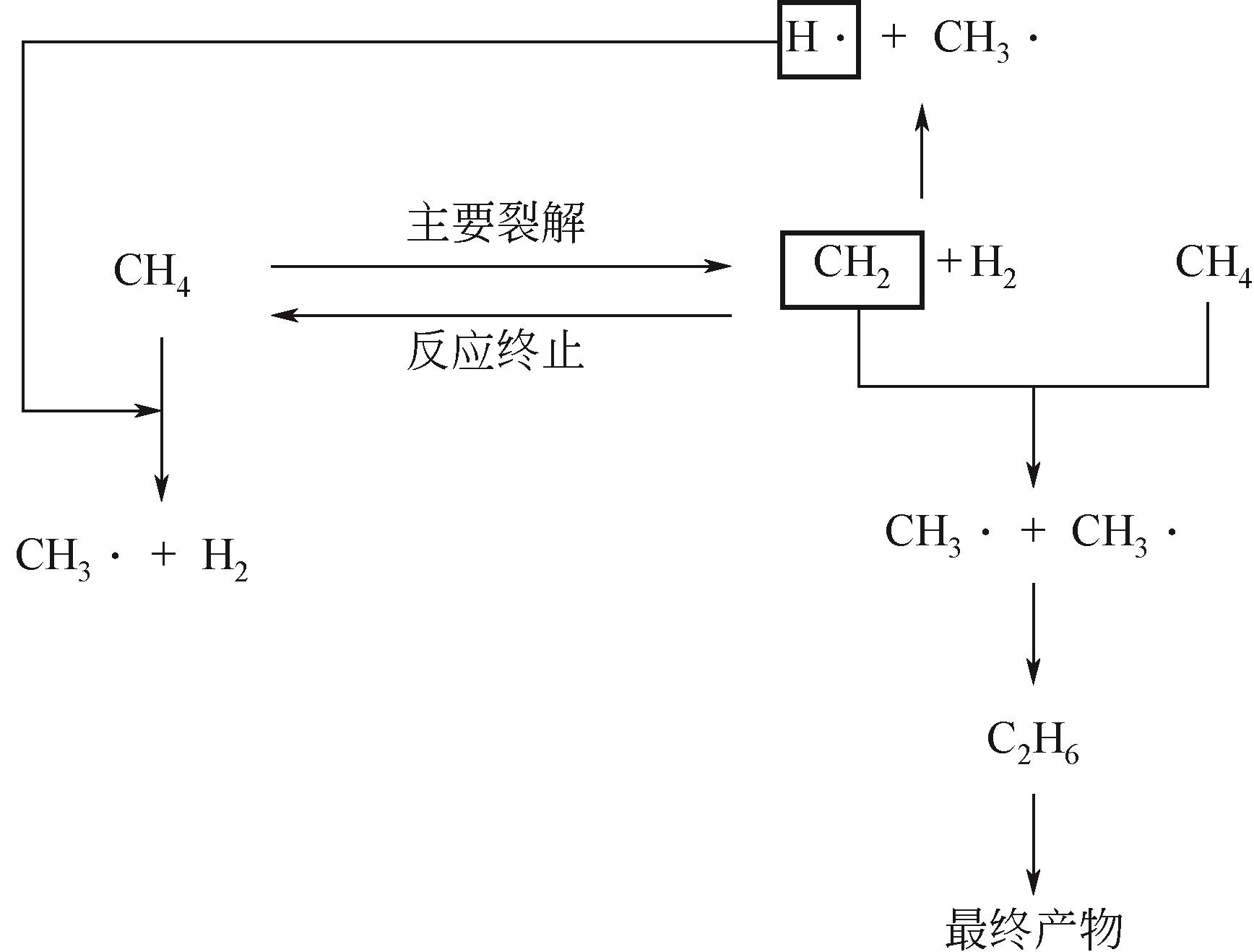
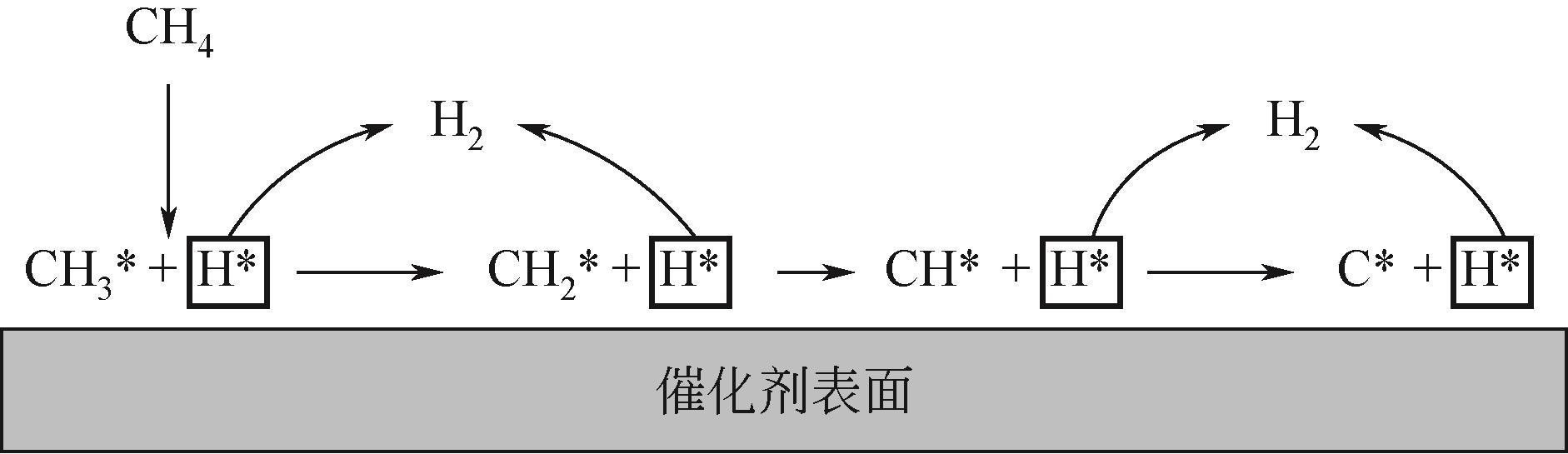
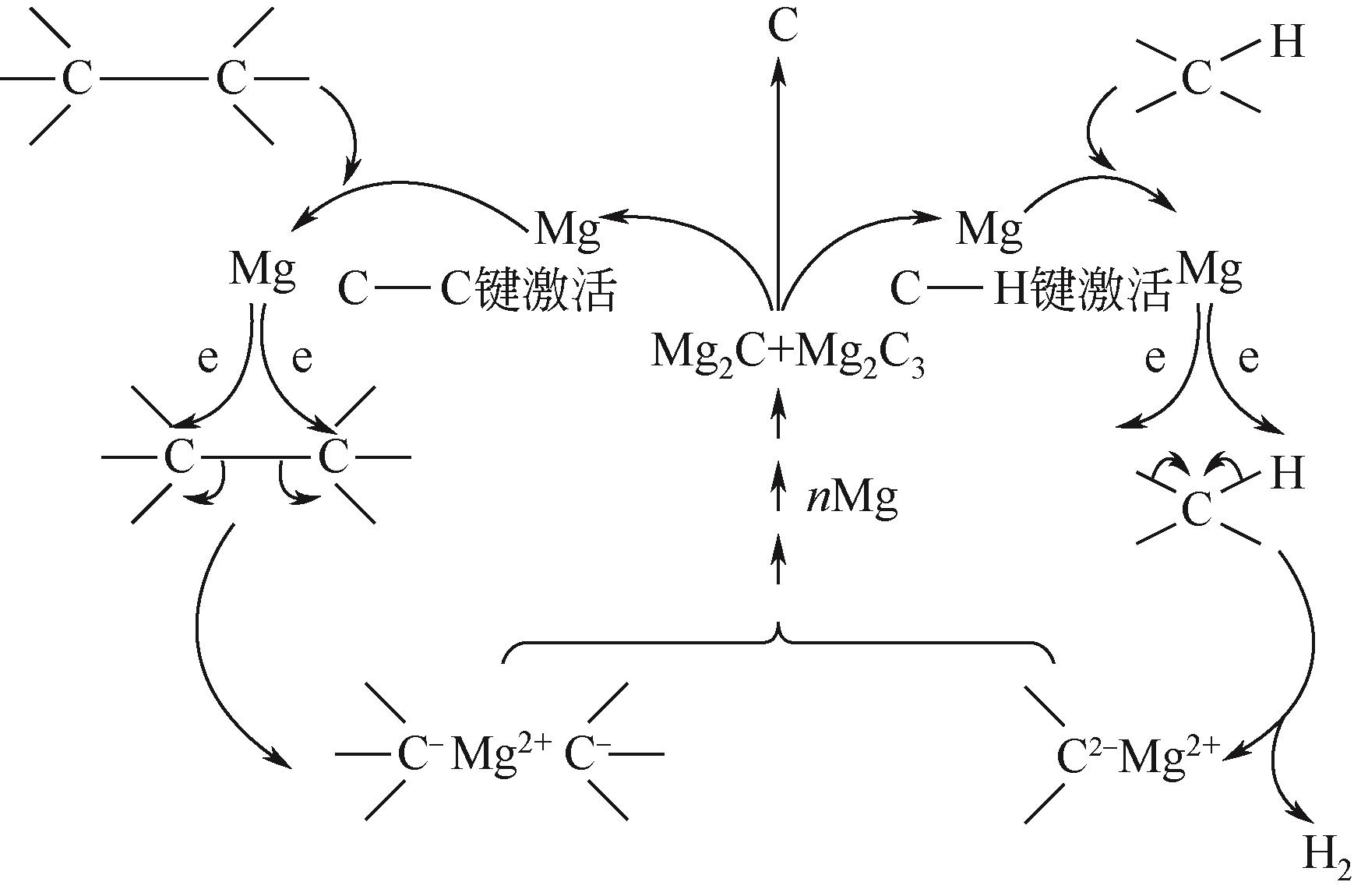
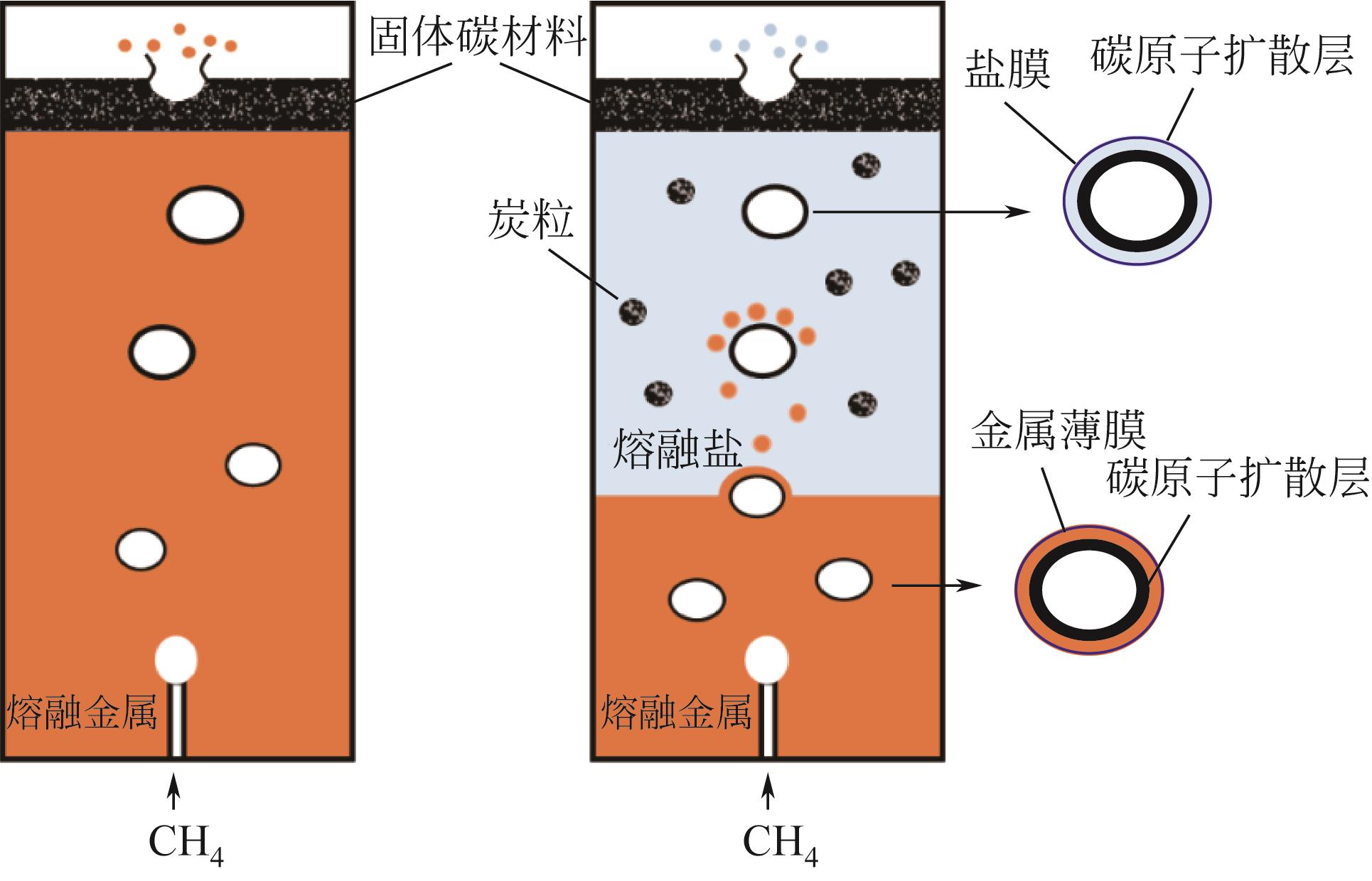
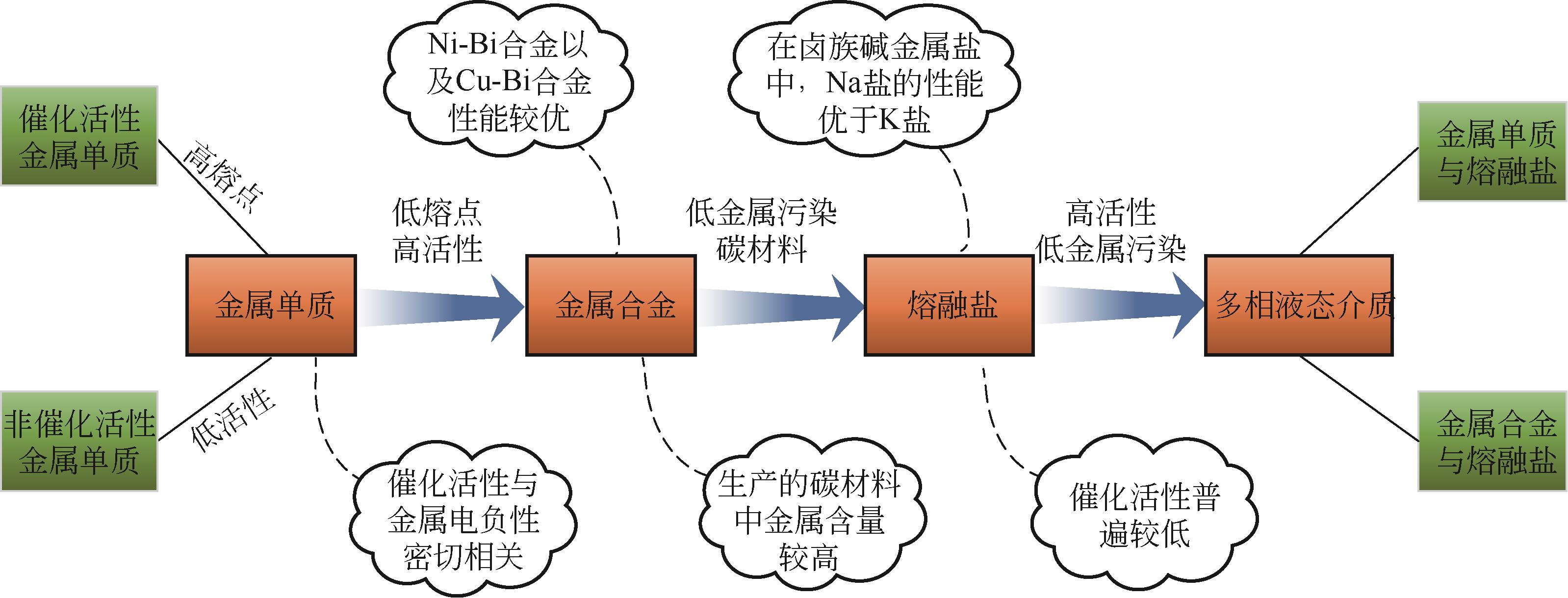
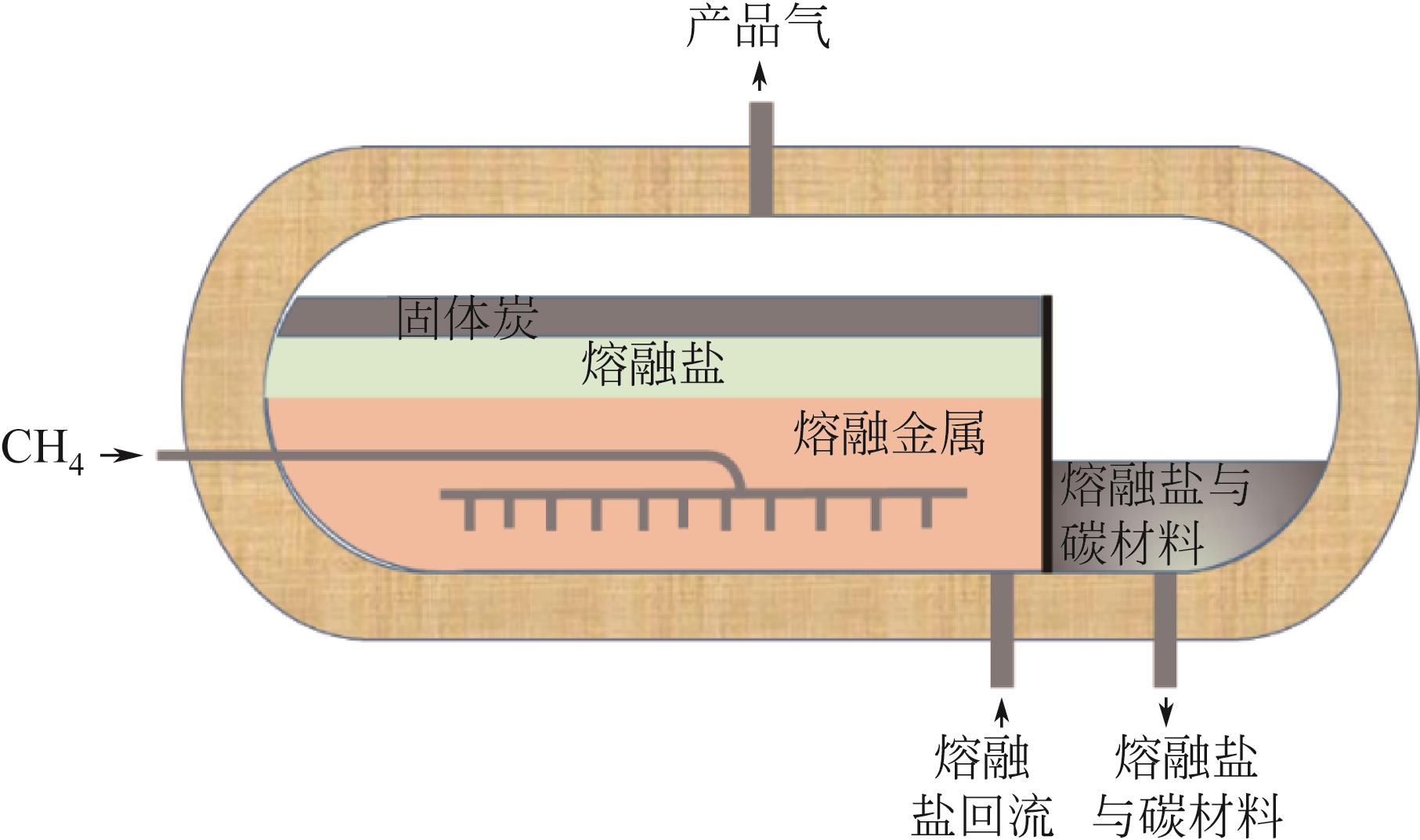
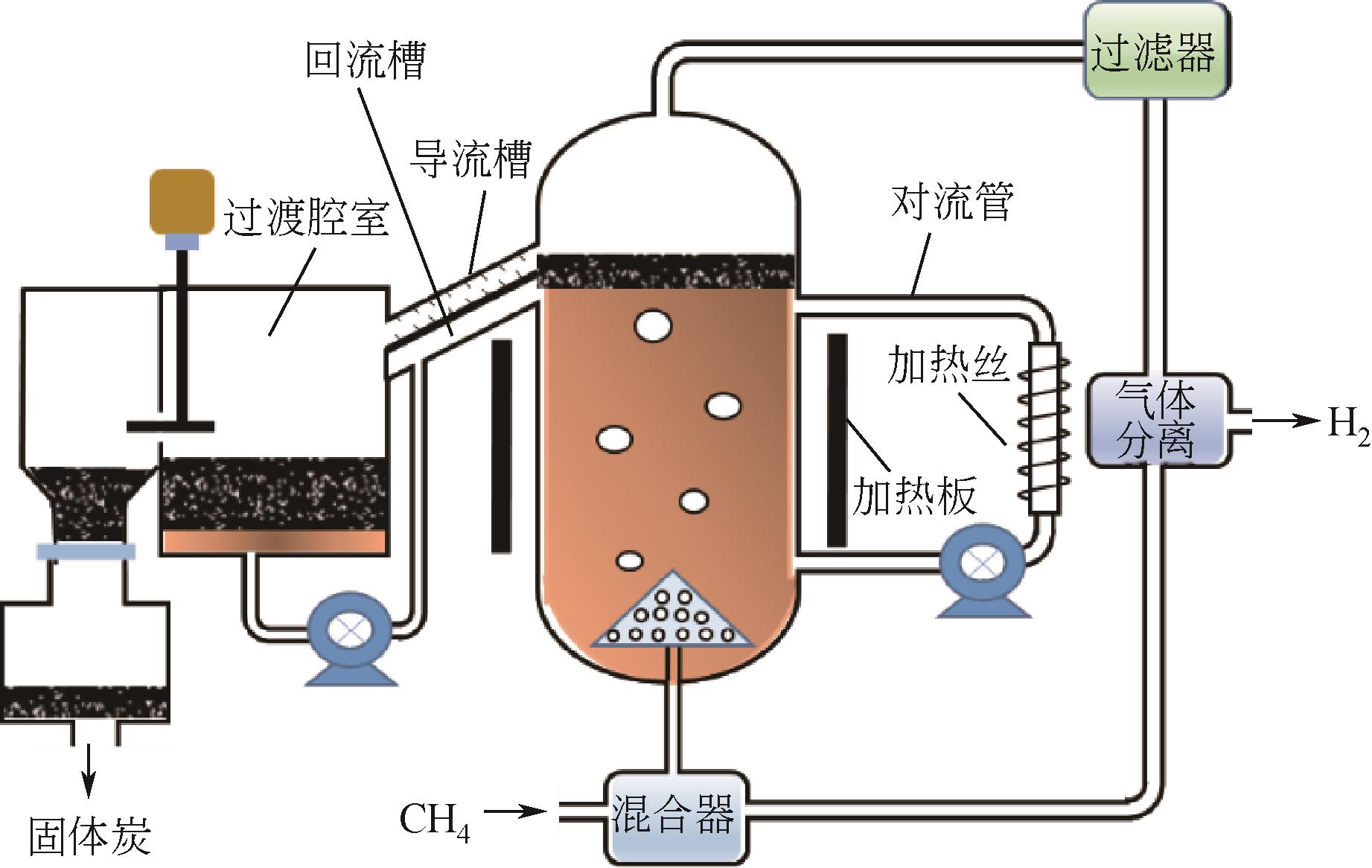
Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (3): 1270-1280.DOI: 10.16085/j.issn.1000-6613.2022-0902
• Energy processes and technology • Previous Articles Next Articles
Development of methane pyrolysis based on molten metal technology for coproduction of hydrogen and solid carbon products
HE Yangdong( ), CHANG Honggang, WANG Dan, CHEN Changjie, LI Yaxin
), CHANG Honggang, WANG Dan, CHEN Changjie, LI Yaxin
- Research Institute of Natural Gas Technology, PetroChina Southwest Oil & Gasfield Company, Chengdu 610213, Sichuan, China
-
Received:2022-05-16Revised:2022-08-29Online:2023-04-10Published:2023-03-15 -
Contact:HE Yangdong
熔融金属法甲烷裂解制氢和碳材料研究进展
- 中国石油西南油气田公司天然气研究院,四川 成都 610213
-
通讯作者:何阳东 -
作者简介:何阳东(1992—),男,博士后,研究方向为氢能及碳捕集。E-mail: heyd01@petrochina.com.cn。 -
基金资助:中国石油西南油气田公司博士后基金(20220306-11)
CLC Number:
Cite this article
HE Yangdong, CHANG Honggang, WANG Dan, CHEN Changjie, LI Yaxin. Development of methane pyrolysis based on molten metal technology for coproduction of hydrogen and solid carbon products[J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1270-1280.
何阳东, 常宏岗, 王丹, 陈昌介, 李雅欣. 熔融金属法甲烷裂解制氢和碳材料研究进展[J]. 化工进展, 2023, 42(3): 1270-1280.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-0902
| 熔融介质 | 装载高度/mm | 纯化方式① | C/% | Ni/% | Bi/% | Na/% | K/% | Br/% |
|---|---|---|---|---|---|---|---|---|
| NiBi | 350 | — | 17.36 | 12.98 | 69.66 | — | — | — |
| NiBi | 350 | 真空加热 | 51.82 | 1.84 | 46.34 | — | — | — |
| NiBi | 350 | 酸洗 | 58.81 | 4.09 | 37.10 | — | — | — |
| NiBi/KBr | 110/110 | 水洗+真空加热 | 85.63 | 0.82 | 1.10 | — | 3.73 | 8.72 |
| NiBi/KBr | 240/110 | 水洗 | 85.68 | 0.22 | 0.66 | — | 4.38 | 9.06 |
| NiBi/KBr | 240/110 | 水洗+真空加热 | 98.22 | 0.23 | 0.32 | — | 0.36 | 0.87 |
| NiBi/KBr | 110/240 | 水洗 | 74.19 | 0.06 | 0.42 | — | 8.07 | 17.26 |
| NiBi/KBr | 110/240 | 水洗+真空加热 | 88.47 | 0.10 | 0.00 | — | 3.60 | 7.83 |
| NiBi/KBr | 110/240 | 水洗+酸洗 | 84.63 | 0 | 0.00 | — | 4.98 | 10.39 |
| NiBi/NaBr | 110/240 | 水洗 | 95.06 | 0.18 | 0.56 | 1.06 | — | 3.14 |
| NiBi/NaBr | 110/240 | 水洗+真空加热 | 97.40 | 0.18 | 0.00 | 0.59 | — | 1.83 |
| NiBi/NaBr | 110/240 | 水洗+酸洗 | 97.34 | 0 | 0.00 | 1.15 | — | 1.51 |
| 熔融介质 | 装载高度/mm | 纯化方式① | C/% | Ni/% | Bi/% | Na/% | K/% | Br/% |
|---|---|---|---|---|---|---|---|---|
| NiBi | 350 | — | 17.36 | 12.98 | 69.66 | — | — | — |
| NiBi | 350 | 真空加热 | 51.82 | 1.84 | 46.34 | — | — | — |
| NiBi | 350 | 酸洗 | 58.81 | 4.09 | 37.10 | — | — | — |
| NiBi/KBr | 110/110 | 水洗+真空加热 | 85.63 | 0.82 | 1.10 | — | 3.73 | 8.72 |
| NiBi/KBr | 240/110 | 水洗 | 85.68 | 0.22 | 0.66 | — | 4.38 | 9.06 |
| NiBi/KBr | 240/110 | 水洗+真空加热 | 98.22 | 0.23 | 0.32 | — | 0.36 | 0.87 |
| NiBi/KBr | 110/240 | 水洗 | 74.19 | 0.06 | 0.42 | — | 8.07 | 17.26 |
| NiBi/KBr | 110/240 | 水洗+真空加热 | 88.47 | 0.10 | 0.00 | — | 3.60 | 7.83 |
| NiBi/KBr | 110/240 | 水洗+酸洗 | 84.63 | 0 | 0.00 | — | 4.98 | 10.39 |
| NiBi/NaBr | 110/240 | 水洗 | 95.06 | 0.18 | 0.56 | 1.06 | — | 3.14 |
| NiBi/NaBr | 110/240 | 水洗+真空加热 | 97.40 | 0.18 | 0.00 | 0.59 | — | 1.83 |
| NiBi/NaBr | 110/240 | 水洗+酸洗 | 97.34 | 0 | 0.00 | 1.15 | — | 1.51 |
| 成本 | SMR | 质子交换膜电解水 | 熔融金属法 |
|---|---|---|---|
| 总装置成本/106USD | 42 | 495.8 | 40.8 |
| 总投资成本/106USD | 252.1 | 829.1 | 349.7 |
| 运行成本/106USD·a-1 | 94.5 | 582.4 | 122 |
| 原料成本/106USD·a-1 | 63.2 | 543.8 | 92.8 |
| 净电输出/MWe | 12.6① | — | 5.4① |
| 1kg H2的碳排放/kg | 9.3 | 0 | 2.5 |
| 氢气售价(IRR=10%)/USD·kg-1 | 1.26 | 7.13② | 1.39③ |
| 与SMR工艺IRR收益为10%相等时,碳税价格/USD·t-1 | — | 585 | 18 |
| 成本 | SMR | 质子交换膜电解水 | 熔融金属法 |
|---|---|---|---|
| 总装置成本/106USD | 42 | 495.8 | 40.8 |
| 总投资成本/106USD | 252.1 | 829.1 | 349.7 |
| 运行成本/106USD·a-1 | 94.5 | 582.4 | 122 |
| 原料成本/106USD·a-1 | 63.2 | 543.8 | 92.8 |
| 净电输出/MWe | 12.6① | — | 5.4① |
| 1kg H2的碳排放/kg | 9.3 | 0 | 2.5 |
| 氢气售价(IRR=10%)/USD·kg-1 | 1.26 | 7.13② | 1.39③ |
| 与SMR工艺IRR收益为10%相等时,碳税价格/USD·t-1 | — | 585 | 18 |
| CO2捕集条件 | SMR | 熔融金属法 (碳供能) | 熔融金属法 (氢供能) | 熔融金属法 (天然气供能) | 熔融金属法 (电供能) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| — | MDEA脱碳 | — | MEA脱碳 | — | MEA脱碳 | ||||||
| 反应温度/℃ | 890 | 890 | 1200 | 1200 | 1200 | 1200 | 1200 | 1200 | |||
| 反应压力/MPa | 3.2 | 3.2 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| NG流率/kg·s-1 | 2.62 | 2.81 | 3.86 | 3.86 | 7.31 | 5.33 | 5.33 | 3.86 | |||
| H2流率/kg·s-1 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | |||
| 1mol CH4的H2产率/mol | 2.49 | 2.48 | 1.65 | 1.63 | 0.93 | 1.25 | 1.23 | 1.71 | |||
| 1kg H2的CO2排放量/kg | 9.18 | 1.57 | 5.26 | 0.45 | 1.46 | 6.16 | 0.56 | 1.01 | |||
| CO2减排率/% | — | 83 | 43 | 95 | 84 | 33 | 94 | 89 | |||
| CO2捕集条件 | SMR | 熔融金属法 (碳供能) | 熔融金属法 (氢供能) | 熔融金属法 (天然气供能) | 熔融金属法 (电供能) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| — | MDEA脱碳 | — | MEA脱碳 | — | MEA脱碳 | ||||||
| 反应温度/℃ | 890 | 890 | 1200 | 1200 | 1200 | 1200 | 1200 | 1200 | |||
| 反应压力/MPa | 3.2 | 3.2 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| NG流率/kg·s-1 | 2.62 | 2.81 | 3.86 | 3.86 | 7.31 | 5.33 | 5.33 | 3.86 | |||
| H2流率/kg·s-1 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | |||
| 1mol CH4的H2产率/mol | 2.49 | 2.48 | 1.65 | 1.63 | 0.93 | 1.25 | 1.23 | 1.71 | |||
| 1kg H2的CO2排放量/kg | 9.18 | 1.57 | 5.26 | 0.45 | 1.46 | 6.16 | 0.56 | 1.01 | |||
| CO2减排率/% | — | 83 | 43 | 95 | 84 | 33 | 94 | 89 | |||
| 1 | ABÁNADES A, RUBBIA C, SALMIERI D. Thermal cracking of methane into Hydrogen for a CO2-free utilization of natural gas[J]. International Journal of Hydrogen Energy, 2013, 38(20): 8491-8496. |
| 2 | HE Yangdong, ZHU Lin, FAN Junming, et al. Life cycle assessment of CO2 emission reduction potential of carbon capture and utilization for liquid fuel and power cogeneration[J]. Fuel Processing Technology, 2021, 221: 106924. |
| 3 | HE Yangdong, ZHU Lin, LI Luling, et al. Hydrogen and power cogeneration based on chemical looping combustion: is it capable of reducing carbon emissions and the cost of production?[J]. Energy & Fuels, 2020, 34(3): 3501-3512. |
| 4 | NOH Y G, LEE Y J, KIM J, et al. Enhanced efficiency in CO2-free hydrogen production from methane in a molten liquid alloy bubble column reactor with zirconia beads[J]. Chemical Engineering Journal, 2022, 428: 131095. |
| 5 | ABÁNADES A, RUIZ E, FERRUELO E M, et al. Experimental analysis of direct thermal methane cracking[J]. International Journal of Hydrogen Energy, 2011, 36(20): 12877-12886. |
| 6 | SERBAN M, LEWIS M A, MARSHALL C L, et al. Hydrogen production by direct contact pyrolysis of natural gas[J]. Energy & Fuels, 2003, 17(3): 705-713. |
| 7 | AO Dongyi, TANG Yongliang, XU Xiaofeng, et al. Highly conductive PDMS composite mechanically enhanced with 3D-graphene network for high-performance EMI shielding application[J]. Nanomaterials (Basel, Switzerland), 2020, 10(4): 768. |
| 8 | GEIßLER T, ABÁNADES A, HEINZEL A,et al. Hydrogen production via methane pyrolysis in a liquid metal bubble column reactor with a packed bed[J]. Chemical Engineering Journal, 2016, 299: 192-200. |
| 9 | CHEN C J, BACK M H, BACK R A. The thermal decomposition of methane. Ⅱ. Secondary reactions, autocatalysis and carbon formation; non-Arrhenius behaviour in the reaction of CH3 with ethane[J]. Canadian Journal of Chemistry, 1976, 54(20): 3175-3184. |
| 10 | CHEN C J, BACK M H, BACK R A. Mechanism of the thermal decomposition of methane[J]. ACS Symposium Series, 1976(32): 1-16. |
| 11 | ROSCOE J M, THOMPSON M J. Thermal decomposition of methane: Autocatalysis[J]. International Journal of Chemical Kinetics, 1985, 17(9): 967-990. |
| 12 | KHAN M S, CRYNES B L. Survey of recent methane pyrolysis literature[J]. Industrial & Engineering Chemistry, 1970, 62(10): 54-59. |
| 13 | KEVORKIAN V, HEATH C E, BOUDART M. The decomposition of methane in shock waves[J]. The Journal of Physical Chemistry, 1960, 64(8): 964-968. |
| 14 | KOZLOV G I, KNORRE V G. Single-pulse shock tube studies on the kinetics of the thermal decomposition of methane[J]. Combustion and Flame, 1962, 6: 253-263. |
| 15 | CHEN Q Q, LUA A C. Kinetic reaction and deactivation studies on thermocatalytic decomposition of methane by electroless nickel plating catalyst[J]. Chemical Engineering Journal, 2020, 389: 124366. |
| 16 | WANG Jiaofei, LI Xiaoming, ZHOU Yang, et al. Mechanism of methane decomposition with hydrogen addition over activated carbon via in-situ pyrolysis-electron impact ionization time-of-flight mass spectrometry[J]. Fuel, 2020, 263: 116734. |
| 17 | YADAV M D, DASGUPTA K, PATWARDHAN A W, et al. Kinetic study of single-walled carbon nanotube synthesis by thermocatalytic decomposition of methane using floating catalyst chemical vapour deposition[J]. Chemical Engineering Science, 2019, 196: 91-103. |
| 18 | WANG K, LI W S, ZHOU X P. Hydrogen generation by direct decomposition of hydrocarbons over molten magnesium[J]. Journal of Molecular Catalysis A: Chemical, 2008, 283(1/2): 153-157. |
| 19 | ZHOU Lu, ENAKONDA L R, HARB M, et al. Fe catalysts for methane decomposition to produce hydrogen and carbon nano materials[J]. Applied Catalysis B: Environmental, 2017, 208: 44-59. |
| 20 | PLEVAN M, GEIßLER T, ABÁNADES A, et al. Thermal cracking of methane in a liquid metal bubble column reactor: Experiments and kinetic analysis[J]. International Journal of Hydrogen Energy, 2015, 40(25): 8020-8033. |
| 21 | TANG Yongliang, PENG Peng, WANG Shuangyue, et al. Continuous production of graphite nanosheets by bubbling chemical vapor deposition using molten copper[J]. Chemistry of Materials, 2017, 29(19): 8404-8411. |
| 22 | ZENG J R, TARAZKAR M, PENNEBAKER T, et al. Catalytic methane pyrolysis with liquid and vapor phase tellurium[J]. ACS Catalysis, 2020, 10(15): 8223-8230. |
| 23 | PÉREZ B J L, MEDRANO JIMÉNEZ J A, BHARDWAJ R, et al. Methane pyrolysis in a molten gallium bubble column reactor for sustainable hydrogen production: Proof of concept & techno-economic assessment[J]. International Journal of Hydrogen Energy, 2020, 46(7): 4917-4935. |
| 24 | UPHAM D C, AGARWAL V, KHECHFE A, et al. Catalytic molten metals for the direct conversion of methane to hydrogen and separable carbon[J]. Science, 2017, 358(6365): 917-921. |
| 25 | PALMER C, BUNYAN E, GELINAS J, et al. CO2-Free hydrogen production by catalytic pyrolysis of hydrocarbon feedstocks in molten Ni-Bi[J]. Energy & Fuels, 2020, 34(12): 16073-16080. |
| 26 | 敖东羿. 多层高品质石墨烯的大量制备及其应用研究[D]. 成都: 电子科技大学, 2020. |
| AO Dongyi. Study on massive production of multilayer high-quality graphene and its applications[D]. Chengdu: University of Electronic Science and Technology of China, 2020. | |
| 27 | PALMER C, TARAZKAR M, KRISTOFFERSEN H H, et al. Methane pyrolysis with a molten Cu-Bi alloy catalyst[J]. ACS Catalysis, 2019, 9(9): 8337-8345. |
| 28 | RIEDEWALD F, SOUSA-GALLAGHER M. Novel waste printed circuit board recycling process with molten salt[J]. Methods X, 2015, 2: 100-106. |
| 29 | RAHIMI N, KANG D, GELINAS J, et al. Solid carbon production and recovery from high temperature methane pyrolysis in bubble columns containing molten metals and molten salts[J]. Carbon, 2019, 151: 181-191. |
| 30 | WICHTERLE K. Breakup of gas bubbles rising in molten metals[J]. Steel Research International, 2010, 81(5): 356-361. |
| 31 | KANG D, RAHIMI N, GORDON M J, et al. Catalytic methane pyrolysis in molten MnCl2-KCl[J]. Applied Catalysis B: Environmental, 2019, 254: 659-666. |
| 32 | KANG D, PALMER C, MANNINI D, et al. Catalytic methane pyrolysis in molten alkali chloride salts containing iron[J]. ACS Catalysis, 2020, 10(13): 7032-7042. |
| 33 | PARKINSON B, PATZSCHKE C F, NIKOLIS D, et al. Methane pyrolysis in monovalent alkali halide salts: Kinetics and pyrolytic carbon properties[J]. International Journal of Hydrogen Energy, 2021, 46(9): 6225-6238. |
| 34 | PARKINSON B, TABATABAEI M, UPHAM D C, et al. Hydrogen production using methane: Techno-economics of decarbonizing fuels and chemicals[J]. International Journal of Hydrogen Energy, 2018, 43(5): 2540-2555. |
| 35 | 芶富均, 陈建军, 叶宗标, 等. 一种催化辅助甲烷裂解制氢的设备: CN113213423A[P]. 2021-08-06. |
| GOU Fujun, CHEN Jianjun, YE Zongbiao,et al. Catalysis-assisted methane cracking hydrogen production equipment: CN113213423A[P]. 2021-08-06. | |
| 36 | KUDINOV I V, PIMENOV A A, KRYUKOV Y A, et al. A theoretical and experimental study on hydrodynamics, heat exchange and diffusion during methane pyrolysis in a layer of molten tin[J]. International Journal of Hydrogen Energy, 2021, 46(17): 10183-10190. |
| 37 | PARKINSON B, MATTHEWS J W, MCCONNAUGHY T B, et al. Techno-economic analysis of methane pyrolysis in molten metals: Decarbonizing natural gas[J]. Chemical Engineering & Technology, 2017, 40(6): 1022-1030. |
| 38 | TIMMERBERG S, KALTSCHMITT M, FINKBEINER M. Hydrogen and hydrogen-derived fuels through methane decomposition of natural gas-GHG emissions and costs[J]. Energy Conversion and Management: X, 2020, 7: 100043. |
| 39 | ABÁNADES A, RATHNAM R K, GEIßLER T, et al. Development of methane decarbonisation based on liquid metal technology for CO2-free production of hydrogen[J]. International Journal of Hydrogen Energy, 2016, 41(19): 8159-8167. |
| 40 | STEINBERG M. Fossil fuel decarbonization technology for mitigating global warming[J]. International Journal of Hydrogen Energy, 1999, 24(8): 771-777. |
| 41 | RODAT S, ABÁNADS S, FLAMANT G. Co-production of hydrogen and carbon black from solar thermal methane splitting in a tubular reactor prototype[J]. Solar Energy, 2011, 85(4): 645-652. |
| 42 | DUFOUR J, GÁLVEZ J L, SERRANO D P, et al. Life cycle assessment of hydrogen production by methane decomposition using carbonaceous catalysts[J]. International Journal of Hydrogen Energy, 2010, 35(3): 1205-1212. |
| 43 | POSTELS S, ABÁNADES A, VON DER ASSEN N, et al. Life cycle assessment of hydrogen production by thermal cracking of methane based on liquid-metal technology[J]. International Journal of Hydrogen Energy, 2016, 41(48): 23204-23212. |
| [1] | SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298. |
| [2] | XIE Luyao, CHEN Songzhe, WANG Laijun, ZHANG Ping. Platinum-based catalysts for SO2 depolarized electrolysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 299-309. |
| [3] | YANG Jianping. PSE for feedstock consumption reduction in reaction system of HPPO plant [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 21-32. |
| [4] | LAI Shini, JIANG Lixia, LI Jun, HUANG Hongyu, KOBAYASHI Noriyuki. Research progress of ammonia blended fossil fuel [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4603-4615. |
| [5] | GE Quanqian, XU Mai, LIANG Xian, WANG Fengwu. Research progress on the application of MOFs in photoelectrocatalysis [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4692-4705. |
| [6] | SHI Keke, LIU Muzi, ZHAO Qiang, LI Jinping, LIU Guang. Properties and research progress of magnesium based hydrogen storage materials [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4731-4745. |
| [7] | LIU Muzi, SHI Keke, ZHAO Qiang, LI Jinping, LIU Guang. Research progress of solid hydrogen storage materials [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4746-4769. |
| [8] | LI Zhiyuan, HUANG Yaji, ZHAO Jiaqi, YU Mengzhu, ZHU Zhicheng, CHENG Haoqiang, SHI Hao, WANG Sheng. Characterization of heavy metals during co-pyrolysis of sludge with PVC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4947-4956. |
| [9] | LI You, WU Yue, ZHONG Yu, LIN Qixuan, REN Junli. Pretreatment of wheat straw with acidic molten salt hydrate for xylose production and its effect on enzymatic hydrolysis efficiency [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4974-4983. |
| [10] | MAO Shanjun, WANG Zhe, WANG Yong. Group recognition hydrogenation: From concept to application [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3917-3922. |
| [11] | WANG Lanjiang, LIANG Yu, TANG Qiong, TANG Mingxing, LI Xuekuan, LIU Lei, DONG Jinxiang. Synthesis of highly dispersed Pt/HY catalyst by rapid pyrolysis of platinum precursors and its performance for deep naphthalene hydrogenation [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4159-4166. |
| [12] | GUO Jin, ZHANG Geng, CHEN Guohua, ZHU Ming, TAN Yue, LI Wei, XIA Li, HU Kun. Research progress on vehicle liquid hydrogen cylinder design [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4221-4229. |
| [13] | HUANG Yufei, LI Ziyi, HUANG Yangqiang, JIN Bo, LUO Xiao, LIANG Zhiwu. Research progress on catalysts for photocatalytic CO2 and CH4 reforming [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4247-4263. |
| [14] | ZHANG Yajuan, XU Hui, HU Bei, SHI Xingwei. Preparation of NiCoP/rGO/NF electrocatalyst by eletroless plating for efficient hydrogen evolution reaction [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4275-4282. |
| [15] | WANG Xiaohan, ZHOU Yasong, YU Zhiqing, WEI Qiang, SUN Jinxiao, JIANG Peng. Synthesis and hydrocracking performance of Y molecular sieves with different crystal sizes [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4283-4295. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||