Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (1): 417-434.DOI: 10.16085/j.issn.1000-6613.2022-0586
• Resources and environmental engineering • Previous Articles Next Articles
An overview of natural mineral catalytic oxidation of refractory organic contaminants in wastewater
WANG Qinghong( ), JIANG Chenxu, WANG Xin, YU Meiqi, ZHU Shuai, LI Yiming, CHEN Chunmao(
), JIANG Chenxu, WANG Xin, YU Meiqi, ZHU Shuai, LI Yiming, CHEN Chunmao( )
)
- State Key Laboratory of Heavy Oil Processing, College of Chemical Engineering and Environment, China University of Petroleum-Beijing, Beijing 102249, China
-
Received:2022-04-07Revised:2022-07-26Online:2023-02-20Published:2023-01-25 -
Contact:CHEN Chunmao
天然矿物催化氧化水中难降解有机污染物研究进展
王庆宏( ), 姜晨旭, 王鑫, 余美琪, 朱帅, 李一鸣, 陈春茂(
), 姜晨旭, 王鑫, 余美琪, 朱帅, 李一鸣, 陈春茂( )
)
- 中国石油大学(北京) 化学工程与环境学院,重质油国家重点实验室,北京 102249
-
通讯作者:陈春茂 -
作者简介:王庆宏(1984—),女,博士,副教授,研究方向为水处理技术及污泥资源化。E-mail:wangqhqh@163.com。 -
基金资助:中国石油科技创新基金(2020D-5007-0505);中国石油大学(北京)科研启动基金(2462021YJRC016)
CLC Number:
Cite this article
WANG Qinghong, JIANG Chenxu, WANG Xin, YU Meiqi, ZHU Shuai, LI Yiming, CHEN Chunmao. An overview of natural mineral catalytic oxidation of refractory organic contaminants in wastewater[J]. Chemical Industry and Engineering Progress, 2023, 42(1): 417-434.
王庆宏, 姜晨旭, 王鑫, 余美琪, 朱帅, 李一鸣, 陈春茂. 天然矿物催化氧化水中难降解有机污染物研究进展[J]. 化工进展, 2023, 42(1): 417-434.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-0586
| 催化剂 | 优点 | 缺点 |
|---|---|---|
| 金属氧化物 | 催化活性高 | 金属溶出问题 |
| 碳材料 | 比表面积较高;催化活性高;成本低 | 容易失活 |
| 新型纳米结构碳材料 | 比表面积高;催化活性高 | 制备条件苛刻;成本高 |
| 天然矿石 | 催化活性较高;储量大;成本低 | 活性组分溶出 |
| 催化剂 | 优点 | 缺点 |
|---|---|---|
| 金属氧化物 | 催化活性高 | 金属溶出问题 |
| 碳材料 | 比表面积较高;催化活性高;成本低 | 容易失活 |
| 新型纳米结构碳材料 | 比表面积高;催化活性高 | 制备条件苛刻;成本高 |
| 天然矿石 | 催化活性较高;储量大;成本低 | 活性组分溶出 |
| 催化剂 | 污染物 | 反应条件 | 处理效率 | 反应机理 | 文献 |
|---|---|---|---|---|---|
| 针铁矿 | 双酚A (BPA) | [BPA]:20μmol/L [催化剂]:0.25g/L [H2O2]:0.1mmol pH:6.2 [EDDS]:0.1mmol | 反应时间:550 min [BPA]:80% | 螯合剂EDDS在针铁矿表面形成Fe-EDDS配合物,中性环境下生成 | [ |
| 磁铁矿 | 双酚A (BPA) | [BPA]:20μmol/L [催化剂]:0.2g/L [H2O2]:0.5mmol/L pH:6.2 [EDDS]:0.1mmol/L | 反应时间:600min [BPA]:70% | 中性pH处形成 | [ |
| 黄铁矿 | 双氯芬酸 (DCF) | [DCF]:0.017mmol/L [催化剂]:0.5~4.0mmol/L [H2O2]:1.0mmol/L pH:3 [反应温度]:25℃ | 反应时间:120s [DCF]:100% | Fe2+溶出形成均相Fenton体系,与加入的H2O2发生均相Fenton反应 | [ |
| 针铁矿 | 扑热息痛 (PC) | [PC]:1×10-4mol/L [催化剂]:1 g/L [H2O2] :5×10-3mol/L pH:3 [草酸]:5×10-4mol/L | 反应时间:4h [PC]:78% | 草酸和Fe(Ⅲ)生成络合物,在催化剂表面通过光子生成Fe(Ⅱ)草酸络合物,与H2O2反应生成·OH | [ |
| 合成层状黏土锂皂石 | 苯酚 (Phenol) | [Phenol]:1mmol/L [催化剂]:1.0g/L [H2O2]:50mmol/L pH:3.0 [反应温度]:30℃ | 反应时间:5min [Phenol]:100% | 未探究 | [ |
| 纳米零价铁负载高岭土 | 偶氮染料直接黑(DBG) | [DBG]:100mg/L [催化剂]:0.6g/L [H2O2]:33mmol pH:7.06 [反应温度]:30℃ | [DBG]:87.22% TOC:54.06% | 具有高吸附性,催化剂表面裸露 金属离子催化H2O2分解产生·OH | [ |
| Fe负载硅藻土 | 碱性品红 | [碱性品红]:15mmol/L [催化剂]:0.7g [H2O2]:5mmol/L pH:3.0 [反应温度]:50℃ | 反应时间:60min 碱性品红: 98.3% | 催化剂表面金属离子非均相催化H2O2产生·OH | [ |
| La-Fe MMT | 亚甲基蓝(MB)、 罗丹明B(RhB) | [MB]:100mg/L [RhB]:100mg/L [催化剂]:1.0g/L [H2O2]:30mmol pH:7.07 | [MB]:97% [RhB]:96% | 镧氧化物在二元催化剂体系同时具有碱和酸两种性质,Fe3+/Fe2+和La2+/La3+循环促进·OH 自由基产生 | [ |
| Fe负载球黏土 | 蒽醌染料活性 蓝4(RB4) | [RB4]:50mg/L [催化剂]:5g/L [H2O2]:8mmol pH:3.0 [反应温度]:30℃ | 反应时间:140min [RB4]:99% | 未探究 | [ |
| 催化剂 | 污染物 | 反应条件 | 处理效率 | 反应机理 | 文献 |
|---|---|---|---|---|---|
| 针铁矿 | 双酚A (BPA) | [BPA]:20μmol/L [催化剂]:0.25g/L [H2O2]:0.1mmol pH:6.2 [EDDS]:0.1mmol | 反应时间:550 min [BPA]:80% | 螯合剂EDDS在针铁矿表面形成Fe-EDDS配合物,中性环境下生成 | [ |
| 磁铁矿 | 双酚A (BPA) | [BPA]:20μmol/L [催化剂]:0.2g/L [H2O2]:0.5mmol/L pH:6.2 [EDDS]:0.1mmol/L | 反应时间:600min [BPA]:70% | 中性pH处形成 | [ |
| 黄铁矿 | 双氯芬酸 (DCF) | [DCF]:0.017mmol/L [催化剂]:0.5~4.0mmol/L [H2O2]:1.0mmol/L pH:3 [反应温度]:25℃ | 反应时间:120s [DCF]:100% | Fe2+溶出形成均相Fenton体系,与加入的H2O2发生均相Fenton反应 | [ |
| 针铁矿 | 扑热息痛 (PC) | [PC]:1×10-4mol/L [催化剂]:1 g/L [H2O2] :5×10-3mol/L pH:3 [草酸]:5×10-4mol/L | 反应时间:4h [PC]:78% | 草酸和Fe(Ⅲ)生成络合物,在催化剂表面通过光子生成Fe(Ⅱ)草酸络合物,与H2O2反应生成·OH | [ |
| 合成层状黏土锂皂石 | 苯酚 (Phenol) | [Phenol]:1mmol/L [催化剂]:1.0g/L [H2O2]:50mmol/L pH:3.0 [反应温度]:30℃ | 反应时间:5min [Phenol]:100% | 未探究 | [ |
| 纳米零价铁负载高岭土 | 偶氮染料直接黑(DBG) | [DBG]:100mg/L [催化剂]:0.6g/L [H2O2]:33mmol pH:7.06 [反应温度]:30℃ | [DBG]:87.22% TOC:54.06% | 具有高吸附性,催化剂表面裸露 金属离子催化H2O2分解产生·OH | [ |
| Fe负载硅藻土 | 碱性品红 | [碱性品红]:15mmol/L [催化剂]:0.7g [H2O2]:5mmol/L pH:3.0 [反应温度]:50℃ | 反应时间:60min 碱性品红: 98.3% | 催化剂表面金属离子非均相催化H2O2产生·OH | [ |
| La-Fe MMT | 亚甲基蓝(MB)、 罗丹明B(RhB) | [MB]:100mg/L [RhB]:100mg/L [催化剂]:1.0g/L [H2O2]:30mmol pH:7.07 | [MB]:97% [RhB]:96% | 镧氧化物在二元催化剂体系同时具有碱和酸两种性质,Fe3+/Fe2+和La2+/La3+循环促进·OH 自由基产生 | [ |
| Fe负载球黏土 | 蒽醌染料活性 蓝4(RB4) | [RB4]:50mg/L [催化剂]:5g/L [H2O2]:8mmol pH:3.0 [反应温度]:30℃ | 反应时间:140min [RB4]:99% | 未探究 | [ |
| 催化剂 | 污染物 | 反应条件 | 处理效率 | 反应机理 | 文献 |
|---|---|---|---|---|---|
| Fe负载膨润土 | 偶氮染料橙Ⅱ | [橙Ⅱ] :1g/L [催化剂]:1.0g/L;[H2O2]:10mmol pH:3 [反应温度]:30℃ 1×8W UVC | 反应时间:120min [橙Ⅱ]:接近100% TOC:接近100% | 催化剂表面Fe(Ⅲ)与光子反应生成Fe(Ⅱ),激活H2O2反应生成·OH,污染物吸附在催化剂表面,发生表面反应 | [ |
| Fe负载锂皂石 | 偶氮染料橙Ⅱ | [橙Ⅱ]:0.1mmol [催化剂]:1.0g/L;[H2O2]:4.8mmol pH:3 1×8W UVC | 反应时间:120min [橙Ⅱ]:100% TOC:75% | 未探究 | [ |
| Fe负载锂皂石 | 活性红HE-3B | [HE-3B]:100mg/L [催化剂]:1.0g/L;[H2O2]:500mg/L pH:3.0 2×8W UVC | 反应时间:120min [活性红HE-3B]:100% TOC:70% | 催化剂表面Fe(Ⅲ)与光子反应生成Fe(Ⅱ),激活H2O2反应生成·OH,H2O2与催化剂产生·OH | [ |
| 催化剂 | 污染物 | 反应条件 | 处理效率 | 反应机理 | 文献 |
|---|---|---|---|---|---|
| Fe负载膨润土 | 偶氮染料橙Ⅱ | [橙Ⅱ] :1g/L [催化剂]:1.0g/L;[H2O2]:10mmol pH:3 [反应温度]:30℃ 1×8W UVC | 反应时间:120min [橙Ⅱ]:接近100% TOC:接近100% | 催化剂表面Fe(Ⅲ)与光子反应生成Fe(Ⅱ),激活H2O2反应生成·OH,污染物吸附在催化剂表面,发生表面反应 | [ |
| Fe负载锂皂石 | 偶氮染料橙Ⅱ | [橙Ⅱ]:0.1mmol [催化剂]:1.0g/L;[H2O2]:4.8mmol pH:3 1×8W UVC | 反应时间:120min [橙Ⅱ]:100% TOC:75% | 未探究 | [ |
| Fe负载锂皂石 | 活性红HE-3B | [HE-3B]:100mg/L [催化剂]:1.0g/L;[H2O2]:500mg/L pH:3.0 2×8W UVC | 反应时间:120min [活性红HE-3B]:100% TOC:70% | 催化剂表面Fe(Ⅲ)与光子反应生成Fe(Ⅱ),激活H2O2反应生成·OH,H2O2与催化剂产生·OH | [ |
| 催化剂 | 污染物 | 反应条件 | 处理效率 | 反应机理 | 文献 |
|---|---|---|---|---|---|
| 黄铁矿 | 磺胺甲𫫇唑(SMX) | [SMX]:1mmol/L [阳极]:25cm2 BDD板 [阴极]:碳毡阴极(15cm × 4cm × 0.5cm) [催化剂]:2.0g/L pH:3 [O2]:1L/min [反应温度]:25℃ | 反应时间:8h [SMX]:100% [TOC]:95% | 阴极原位生成H2O2,与溶液中的Fe2+发生均相Fenton反应 | [ |
| 黄铁矿 | 四环素 (TC) | [TC]:0.2mmol/L [阳极]:24cm2 BDD板 [阴极]:碳毡阴极(15cm×4cm×0.5cm) [催化剂]:2.0mg/L pH:3 [O2]:1L/min [反应温度]:25℃ | 反应时间:480min TOC:96% | 阴极原位生成H2O2,与溶液中的Fe2+发生均相Fenton反应 | [ |
| 黄铁矿 | 左氧氟沙星 | [左氧氟沙星]:0.23mmol/L [阳极]:6cm2 BDD薄膜电极 [阴极]:60cm2碳毡阴极 [催化剂]:2.0g/L pH:3 [O2]:1L/min [反应温度]:25℃ [电流]:300mA | 反应时间:8h TOC:95% | 未探究 | [ |
| 黄铁矿 | 酪醇 (TY) | [TY]:0.3mmol [阳极]:6cm2 BDD薄膜沉积导电硅片 [阴极]:60cm2碳毡电极 [催化剂]:1g/L pH:3.0 [反应温度]:25℃ [电流]:300mA | 反应时间:6h [TY]:100% [TOC]: 89% | 1.0g/L黄铁矿提供0.20mmol Fe2+,发生均相Fenton反应 | [ |
| 黄铁矿 | 罗丹明B(RhB) | [RhB]:10mg/L [阳极]:25cm2 石墨电极 [阴极]:25cm2 石墨电极 [催化剂]:10mg/L [极板间距]:4cm pH:3 [电压]:8V | 反应时间:180min [RhB]:99.2% | Fe2+溶出,与H2O2形成均相Fenton体系 | [ |
| 黄铜矿 | 四环素(TC) | [TC]:0.20mmol/L [阳极]:高4.5cm、内径3cm的圆柱形Pt网或25cm2薄膜BDD置于Nb衬底上 [阴极]:三维碳毡(15cm×4cm×0.5cm) [催化剂]:1.0g/L pH:3 [反应温度]:25℃ [电流]:500mA | 反应时间:360min TOC:98% | 黄铜矿溶出Fe2+和Cu2+协同均相反应;·OH攻击Cu(II)-羧酸配合物 | [ |
| Fe2O3负载高岭土 | 依诺沙星(ENXN) | [ENXN]:0.25mmol/L [阳极]:Pt网或BDD电极 (5cm×4cm) [阴极]:70cm2三维碳毡 [催化剂]:0.3g pH:2.0 [反应温度]:25℃ [电流]:300mA | 反应时间:7h TOC:98% | 催化剂表面形成活性铁物种,与电化学协同生成·OH的非均相反应 | [ |
| 催化剂 | 污染物 | 反应条件 | 处理效率 | 反应机理 | 文献 |
|---|---|---|---|---|---|
| 黄铁矿 | 磺胺甲𫫇唑(SMX) | [SMX]:1mmol/L [阳极]:25cm2 BDD板 [阴极]:碳毡阴极(15cm × 4cm × 0.5cm) [催化剂]:2.0g/L pH:3 [O2]:1L/min [反应温度]:25℃ | 反应时间:8h [SMX]:100% [TOC]:95% | 阴极原位生成H2O2,与溶液中的Fe2+发生均相Fenton反应 | [ |
| 黄铁矿 | 四环素 (TC) | [TC]:0.2mmol/L [阳极]:24cm2 BDD板 [阴极]:碳毡阴极(15cm×4cm×0.5cm) [催化剂]:2.0mg/L pH:3 [O2]:1L/min [反应温度]:25℃ | 反应时间:480min TOC:96% | 阴极原位生成H2O2,与溶液中的Fe2+发生均相Fenton反应 | [ |
| 黄铁矿 | 左氧氟沙星 | [左氧氟沙星]:0.23mmol/L [阳极]:6cm2 BDD薄膜电极 [阴极]:60cm2碳毡阴极 [催化剂]:2.0g/L pH:3 [O2]:1L/min [反应温度]:25℃ [电流]:300mA | 反应时间:8h TOC:95% | 未探究 | [ |
| 黄铁矿 | 酪醇 (TY) | [TY]:0.3mmol [阳极]:6cm2 BDD薄膜沉积导电硅片 [阴极]:60cm2碳毡电极 [催化剂]:1g/L pH:3.0 [反应温度]:25℃ [电流]:300mA | 反应时间:6h [TY]:100% [TOC]: 89% | 1.0g/L黄铁矿提供0.20mmol Fe2+,发生均相Fenton反应 | [ |
| 黄铁矿 | 罗丹明B(RhB) | [RhB]:10mg/L [阳极]:25cm2 石墨电极 [阴极]:25cm2 石墨电极 [催化剂]:10mg/L [极板间距]:4cm pH:3 [电压]:8V | 反应时间:180min [RhB]:99.2% | Fe2+溶出,与H2O2形成均相Fenton体系 | [ |
| 黄铜矿 | 四环素(TC) | [TC]:0.20mmol/L [阳极]:高4.5cm、内径3cm的圆柱形Pt网或25cm2薄膜BDD置于Nb衬底上 [阴极]:三维碳毡(15cm×4cm×0.5cm) [催化剂]:1.0g/L pH:3 [反应温度]:25℃ [电流]:500mA | 反应时间:360min TOC:98% | 黄铜矿溶出Fe2+和Cu2+协同均相反应;·OH攻击Cu(II)-羧酸配合物 | [ |
| Fe2O3负载高岭土 | 依诺沙星(ENXN) | [ENXN]:0.25mmol/L [阳极]:Pt网或BDD电极 (5cm×4cm) [阴极]:70cm2三维碳毡 [催化剂]:0.3g pH:2.0 [反应温度]:25℃ [电流]:300mA | 反应时间:7h TOC:98% | 催化剂表面形成活性铁物种,与电化学协同生成·OH的非均相反应 | [ |
| 催化剂 | 污染物 | 反应条件 | 处理效率 | 反应机理 | 文献 |
|---|---|---|---|---|---|
| 水镁石 | 活性艳红X-3B | [活性艳红X-3B]:50mg/L [催化剂]:0.5g [O3]:0.3mg/min pH:6.4 [反应温度]:25℃ | 反应时间:15min [活性艳红X-3B]: 89% COD:32.5% | Mg(OH)2溶解,产生大量OH-,均相臭氧直接氧化催化机理 | [ |
| 苯酚 | [苯酚]:100mg/L [催化剂]:0.5g [O3]:72mg/L pH:10.8 | 反应时间:15min [苯酚]:50.3% COD:57.8% | Mg(OH)2溶解,产生大量OH-,均相臭氧直接氧化催化机理 | [ | |
斜发沸石、 丝光沸石 | 亚甲基蓝 (MB) | [MB]:94μmol/L [催化剂]:15g/L [O3]:125μmol/L pH:8.0 [反应温度]:20℃ | 反应时间:50min [MB]:接近100% | 表面羟基 (S—OH2+,S—OH,S—O—) 催化臭氧分解生成·OH | [ |
| 铝土矿 | 2,4,6-三氯苯甲醚(TCA) | [TCA]:100ng/L [催化剂]:200mg/L [O3]:0.5mg/L pH:6.0 [反应温度]:20℃ | 反应时间:10min [TCA]:95.2% | 铝土矿Al—O、Si—O表面活性位点存在表面羟基,催化臭氧分解生成·OH | [ |
| 四方硫铁矿 | N, N-二甲基乙酰胺(DMAC) | [DMAC]:10mg/L [催化剂]:3.5g/L [O3]:300mL/min pH:6.8 [反应温度]:30℃ | 反应时间:20min [DMAC]:96.6% | Fe金属和表面羟基活性组分促进·OH生成,S2-作为电子供体促进体系中Fe(Ⅱ)和Fe(Ⅲ)循环 | [ |
| 焙烧磁铁矿 | 活性红-120 (RR-120) | [RR-120]:100mg/L [催化剂]:0.2g [O3]:(1±0.08)mg/min pH:11.0 [反应温度]:20℃ | 反应时间:10min [RR-120]:100% TOC:96.1% | 焙烧后主要成分为Fe2O3,比表面积增大,表面羟基促进·OH生成 | [ |
焙烧铝矾土 (Al2O3、SiO2、 Fe2O3、TiO2) | 对硝基苯酚 | [对硝基苯酚]:300mg/L [催化剂]:5g/L [O3]:(2.46 ± 0.1)mg/min pH:5.0 [反应温度]:25℃ | 反应时间:10min TOC: 73.5% | 比表面积增大,表面羟基促进·OH生成 | [ |
| 焙烧锰砂矿(MnO、Fe2O3、SiO2、Al2O3) | 苯胺 | [苯胺]:200mg/L [催化剂]:3g/L [O3]:(1.76 ± 0.1)mg/min pH:5.0 [反应温度]:25℃ | 反应时间:10min COD: 69.2% | 比表面积增大,催化剂表面和溶液中的·OH氧化起主导作用,同时存在臭氧直接氧化 | [ |
| Co改性赤泥 | 苯扎贝特 (BZF) | [BZF]:2.76μmol/L [催化剂]:50mg/L [O3]:0.5mg/L pH:6.68 | 反应时间:30min [BZF]:80% | 表面负载Co形成介孔,比表面积增大,生成Co—O—活性反应位点,增加表面羟基,增加臭氧分解生成·OH | [ |
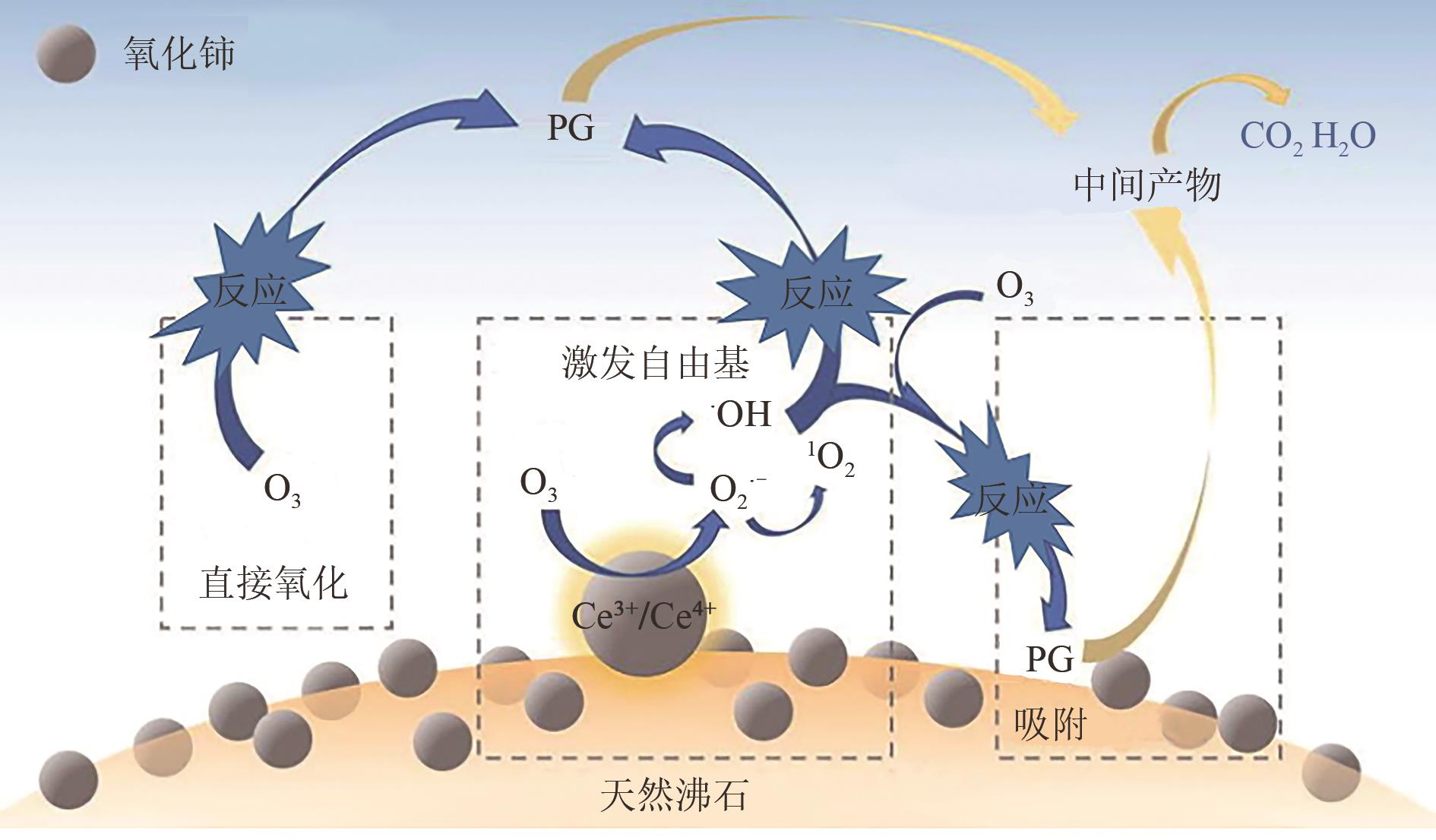
| Ce负载天然沸石 | 青霉素G (PG) | [PG]:50mg/L [催化剂]:2.0g/L [O3]:6mg/min pH:4.5 | 反应时间:20min [PG]:99.5% | Ce3+/Ce4+电子转移促进O3产生 | [ |
| 改性铝土矿 | 2,4,6-三氯苯甲醚 (TCA) | [TCA]:28.2μg/L [催化剂]:0.5g/L [O3]:0.62mg/L pH:6.5 | 反应时间:40min [TCA]:90.0% | 催化剂微孔吸附,表面羟基催化臭氧 分解生成·OH | [ |
改性针铁矿 纳米粒子 | 磺胺嘧啶 (SSZ) | [SSZ]:10mg/L [催化剂]:1.5g/L [O3]:5mg/min pH:7.0 [反应温度]:25℃ | 反应时间:40min COD: 69.2% | 比表面积增大,增加表面羟基的密度,促进表面吸附和·OH生成 | [ |
| 催化剂 | 污染物 | 反应条件 | 处理效率 | 反应机理 | 文献 |
|---|---|---|---|---|---|
| 水镁石 | 活性艳红X-3B | [活性艳红X-3B]:50mg/L [催化剂]:0.5g [O3]:0.3mg/min pH:6.4 [反应温度]:25℃ | 反应时间:15min [活性艳红X-3B]: 89% COD:32.5% | Mg(OH)2溶解,产生大量OH-,均相臭氧直接氧化催化机理 | [ |
| 苯酚 | [苯酚]:100mg/L [催化剂]:0.5g [O3]:72mg/L pH:10.8 | 反应时间:15min [苯酚]:50.3% COD:57.8% | Mg(OH)2溶解,产生大量OH-,均相臭氧直接氧化催化机理 | [ | |
斜发沸石、 丝光沸石 | 亚甲基蓝 (MB) | [MB]:94μmol/L [催化剂]:15g/L [O3]:125μmol/L pH:8.0 [反应温度]:20℃ | 反应时间:50min [MB]:接近100% | 表面羟基 (S—OH2+,S—OH,S—O—) 催化臭氧分解生成·OH | [ |
| 铝土矿 | 2,4,6-三氯苯甲醚(TCA) | [TCA]:100ng/L [催化剂]:200mg/L [O3]:0.5mg/L pH:6.0 [反应温度]:20℃ | 反应时间:10min [TCA]:95.2% | 铝土矿Al—O、Si—O表面活性位点存在表面羟基,催化臭氧分解生成·OH | [ |
| 四方硫铁矿 | N, N-二甲基乙酰胺(DMAC) | [DMAC]:10mg/L [催化剂]:3.5g/L [O3]:300mL/min pH:6.8 [反应温度]:30℃ | 反应时间:20min [DMAC]:96.6% | Fe金属和表面羟基活性组分促进·OH生成,S2-作为电子供体促进体系中Fe(Ⅱ)和Fe(Ⅲ)循环 | [ |
| 焙烧磁铁矿 | 活性红-120 (RR-120) | [RR-120]:100mg/L [催化剂]:0.2g [O3]:(1±0.08)mg/min pH:11.0 [反应温度]:20℃ | 反应时间:10min [RR-120]:100% TOC:96.1% | 焙烧后主要成分为Fe2O3,比表面积增大,表面羟基促进·OH生成 | [ |
焙烧铝矾土 (Al2O3、SiO2、 Fe2O3、TiO2) | 对硝基苯酚 | [对硝基苯酚]:300mg/L [催化剂]:5g/L [O3]:(2.46 ± 0.1)mg/min pH:5.0 [反应温度]:25℃ | 反应时间:10min TOC: 73.5% | 比表面积增大,表面羟基促进·OH生成 | [ |
| 焙烧锰砂矿(MnO、Fe2O3、SiO2、Al2O3) | 苯胺 | [苯胺]:200mg/L [催化剂]:3g/L [O3]:(1.76 ± 0.1)mg/min pH:5.0 [反应温度]:25℃ | 反应时间:10min COD: 69.2% | 比表面积增大,催化剂表面和溶液中的·OH氧化起主导作用,同时存在臭氧直接氧化 | [ |
| Co改性赤泥 | 苯扎贝特 (BZF) | [BZF]:2.76μmol/L [催化剂]:50mg/L [O3]:0.5mg/L pH:6.68 | 反应时间:30min [BZF]:80% | 表面负载Co形成介孔,比表面积增大,生成Co—O—活性反应位点,增加表面羟基,增加臭氧分解生成·OH | [ |
| Ce负载天然沸石 | 青霉素G (PG) | [PG]:50mg/L [催化剂]:2.0g/L [O3]:6mg/min pH:4.5 | 反应时间:20min [PG]:99.5% | Ce3+/Ce4+电子转移促进O3产生 | [ |
| 改性铝土矿 | 2,4,6-三氯苯甲醚 (TCA) | [TCA]:28.2μg/L [催化剂]:0.5g/L [O3]:0.62mg/L pH:6.5 | 反应时间:40min [TCA]:90.0% | 催化剂微孔吸附,表面羟基催化臭氧 分解生成·OH | [ |
改性针铁矿 纳米粒子 | 磺胺嘧啶 (SSZ) | [SSZ]:10mg/L [催化剂]:1.5g/L [O3]:5mg/min pH:7.0 [反应温度]:25℃ | 反应时间:40min COD: 69.2% | 比表面积增大,增加表面羟基的密度,促进表面吸附和·OH生成 | [ |
| 催化剂 | 污染物 | 反应条件 | 处理效率 | 反应机理 | 文献 |
|---|---|---|---|---|---|
| 磁铁矿纳米颗粒 | 2,4,4'-三氯联苯(PCB28) | [PCB28]:2.5μmol [催化剂]:1.0g/L [PS]:2.0mmol pH:7.0 反应温度:25℃ | 反应时间:240min [PCB28]:71% | 溶解和表面Fe(Ⅱ)活化PS生成 | [ |
| 磁铁矿纳米颗粒 | 磺胺甲氧嘧啶(SMM) | [SMM]:0.06mmol/L [催化剂]:2.4mmol [PS]:1.2 mmol pH:6.4 反应温度:25℃ | 反应时间:15min [SMM]:100% | 溶解和表面Fe(Ⅱ)活化PS生成 | [ |
| 黄铁矿 | 对氯苯胺(PCA) | [PCA]:0.1mmol/L [催化剂]:0.5g/L [PS]:0.5mmol pH:7.0 反应温度:20℃ | 反应时间:240min [PCA]:91.02% | 溶解和表面Fe(Ⅱ)活化PS生成 | [ |
| 磁黄铁矿 | 乙硫氨酯(IPETC) | [IPETC]:0.28mmol [催化剂]:0.8g/L [PS]:1.4mmol pH:6.0 反应温度:20℃ | TOC:62.84% | 溶解和表面Fe(Ⅱ)活化PS生成 | [ |
| 赤泥 | 对羟基苯甲酸酯 | [对羟基苯甲酸酯]:0.8mg/L [催化剂]:2g/L [PS]:2g/L pH:3.0 反应温度:25℃ | 反应时间:120min [对羟基苯甲酸酯]:接近100% | 赤泥中Fe2+溶解后非均相催化反应 | [ |
| 赤泥粉 | 磺胺嘧啶(SDZ) | [SDZ]:4μmol [催化剂]:2g/L [PS]:1.75mmol pH:8.0 反应温度:(20 ± 2)℃ | 反应时间:180min [SDZ]:94.0% | 溶解和表面Fe(Ⅱ)活化过硫酸盐产生 | [ |
| 磁铁矿 | 偶氮染料(AO7) | [AO7]:25mg/L [催化剂:0.8g/L [PDS]:10mmol pH:6.0 [阳极]:5cm ×11.9cm Ti/RuO2-IrO2平板 [阴极]:5cm ×11.9cm不锈钢平板 电流密度:8.4mA/cm2 | 反应时间:60min [AO7]:100% | 溶解和表面Fe(Ⅱ)活化过硫酸盐产生 | [ |
| 针铁矿 | 偶氮染料橙Ⅱ | [橙Ⅱ]:50mg/L [催化剂]:0.5g/L [PDS]:2g/L pH:7.0 Na2SO4:50mmol [阳极]:5cm ×11.9cm Ti/RuO2-IrO2平板 [阴极]:5cm ×11.9cm 不锈钢平板 电流密度:8.4mA/cm2 | 反应时间:120min; [橙Ⅱ]:92.1% TOC:12.8% | 溶解和表面Fe(Ⅱ)活化过硫酸盐产生 | [ |
| 催化剂 | 污染物 | 反应条件 | 处理效率 | 反应机理 | 文献 |
|---|---|---|---|---|---|
| 磁铁矿纳米颗粒 | 2,4,4'-三氯联苯(PCB28) | [PCB28]:2.5μmol [催化剂]:1.0g/L [PS]:2.0mmol pH:7.0 反应温度:25℃ | 反应时间:240min [PCB28]:71% | 溶解和表面Fe(Ⅱ)活化PS生成 | [ |
| 磁铁矿纳米颗粒 | 磺胺甲氧嘧啶(SMM) | [SMM]:0.06mmol/L [催化剂]:2.4mmol [PS]:1.2 mmol pH:6.4 反应温度:25℃ | 反应时间:15min [SMM]:100% | 溶解和表面Fe(Ⅱ)活化PS生成 | [ |
| 黄铁矿 | 对氯苯胺(PCA) | [PCA]:0.1mmol/L [催化剂]:0.5g/L [PS]:0.5mmol pH:7.0 反应温度:20℃ | 反应时间:240min [PCA]:91.02% | 溶解和表面Fe(Ⅱ)活化PS生成 | [ |
| 磁黄铁矿 | 乙硫氨酯(IPETC) | [IPETC]:0.28mmol [催化剂]:0.8g/L [PS]:1.4mmol pH:6.0 反应温度:20℃ | TOC:62.84% | 溶解和表面Fe(Ⅱ)活化PS生成 | [ |
| 赤泥 | 对羟基苯甲酸酯 | [对羟基苯甲酸酯]:0.8mg/L [催化剂]:2g/L [PS]:2g/L pH:3.0 反应温度:25℃ | 反应时间:120min [对羟基苯甲酸酯]:接近100% | 赤泥中Fe2+溶解后非均相催化反应 | [ |
| 赤泥粉 | 磺胺嘧啶(SDZ) | [SDZ]:4μmol [催化剂]:2g/L [PS]:1.75mmol pH:8.0 反应温度:(20 ± 2)℃ | 反应时间:180min [SDZ]:94.0% | 溶解和表面Fe(Ⅱ)活化过硫酸盐产生 | [ |
| 磁铁矿 | 偶氮染料(AO7) | [AO7]:25mg/L [催化剂:0.8g/L [PDS]:10mmol pH:6.0 [阳极]:5cm ×11.9cm Ti/RuO2-IrO2平板 [阴极]:5cm ×11.9cm不锈钢平板 电流密度:8.4mA/cm2 | 反应时间:60min [AO7]:100% | 溶解和表面Fe(Ⅱ)活化过硫酸盐产生 | [ |
| 针铁矿 | 偶氮染料橙Ⅱ | [橙Ⅱ]:50mg/L [催化剂]:0.5g/L [PDS]:2g/L pH:7.0 Na2SO4:50mmol [阳极]:5cm ×11.9cm Ti/RuO2-IrO2平板 [阴极]:5cm ×11.9cm 不锈钢平板 电流密度:8.4mA/cm2 | 反应时间:120min; [橙Ⅱ]:92.1% TOC:12.8% | 溶解和表面Fe(Ⅱ)活化过硫酸盐产生 | [ |
| 1 | 冀滨弘, 章非娟. 难降解有机污染物的处理技术[J]. 重庆环境科学, 1998(5): 36-40. |
| JI Binhong, ZHANG Feijuan. Treatment technology of nondegradable organics[J]. Chongqing Environmental Science, 1998(5): 36-40. | |
| 2 | 陈春茂, 曹越, 胡景泽, 等. 难降解石油化工废水臭氧氧化处理催化剂研究进展[J]. 工业水处理, 2020, 40(4): 1-5, 88. |
| CHEN Chunmao, CAO Yue, HU Jingze, et al. An overview of catalysts in ozonation treatment of refractory petrochemical wastewaters [J]. Industrial Water Treatment, 2020, 40(4): 1-5, 88. | |
| 3 | CHEN Chunmao, YOZA B A, CHEN Hongshuo, et al. Manganese sand ore is an economical and effective catalyst for ozonation of organic contaminants in petrochemical wastewater [J]. Water, Air, & Soil Pollution, 2015, 226(6): 1-11. |
| 4 | 董振, 刘亮, 郝艳, 等. 偶氮染料废水处理技术的研究进展[J]. 水处理技术, 2017, 43(4): 6-10. |
| DONG Zhen, LIU Liang, HAO Yan, et al. Research progress on the treatment of azo dye containing wastewater[J]. Technology of Water Treatment, 2017, 43(4): 6-10. | |
| 5 | 张呈程. 煤化工废水难降解有机物的处理研究[J]. 山西化工, 2021, 41(4): 205-207. |
| ZHANG Chengcheng. Study on treatment of refractory organic matter in coal chemical wastewater[J]. Shanxi Chemical Industry, 2021, 41(4): 205-207. | |
| 6 | CRALL L W, HAMILL R L. Antibiotics: polyethers[M]//CONSIDINE G D. Van Nostrand's Encyclopedia of Chemistry.5th ed. New York: Wiley, 2005: 23-35. |
| 7 | 王嘉玮, 魏红, 杨小雨, 等. 渭河西安段磺胺类抗生素的分布特征及生态风险评价[J]. 环境化学, 2017, 36(12): 2574-2583. |
| WANG Jiawei, WEI Hong, YANG Xiaoyu, et al. Occurrence and ecological risk of sulfonamide antibiotics in the surface water of the Weihe Xi’an section[J]. Environmental Chemistry, 2017, 36(12): 2574-2583. | |
| 8 | 任峰. 水处理工艺对消毒副产物生成及其前体物控制[J]. 净水技术, 2020, 39(12): 87-93. |
| REN Feng. DBPs formation and precursors control by water treatment processes[J]. Water Purification Technology, 2020, 39(12): 87-93. | |
| 9 | WANG Jianlong, ZHUAN Run. Degradation of antibiotics by advanced oxidation processes: an overview[J]. Science of the Total Environment, 2020, 701: 135023. |
| 10 | MAZIVILA S J, RICARDO I A, LEITÃO J M M, et al. A review on advanced oxidation processes: from classical to new perspectives coupled to two- and multi-way calibration strategies to monitor degradation of contaminants in environmental samples[J]. Trends in Environmental Analytical Chemistry, 2019, 24: e00072. |
| 11 | LIU Yong, ZHAO Yang, WANG Jianlong. Fenton/Fenton-like processes with in-situ production of hydrogen peroxide/hydroxyl radical for degradation of emerging contaminants: advances and prospects[J]. Journal of Hazardous Materials, 2021, 404(Pt B): 124191. |
| 12 | SHANG Yanan, XU Xing, GAO Baoyu, et al. Single-atom catalysis in advanced oxidation processes for environmental remediation[J]. Chemical Society Reviews, 2021, 50(8): 5281-5322. |
| 13 | LIU Ya, CHEN Chunmao, DUAN Xiaoguang, et al. Carbocatalytic ozonation toward advanced water purification[J]. Journal of Materials Chemistry A, 2021, 9(35): 18994-19024. |
| 14 | 孔涛, 任诺, 陈春茂, 等. 多金属氧化物催化臭氧氧化有机污染物的研究进展[J]. 工业水处理, 2021, 41(1): 1-18. |
| KONG Tao, REN Nuo, CHEN Chunmao, et al. Research progress of catalytic ozonation of organic contaminants by poly-metallic oxides[J]. Industrial Water Treatment, 2021, 41(1): 1-18. | |
| 15 | 陈春茂, 曹越, 郝康宏, 等. 利用炼化废弃物协同臭氧处理炼化废水的技术策略展望[J].辽宁石油化工大学学报, 2020, 40(4): 21-27. |
| CHEN Chunmao, CAO Yue, HAO Kanghong, et al. Prospects for the technical strategy of using refining wastes and ozone to treat refining wastewater[J]. Journal of Liaoning Shihua University, 2020, 40(4): 21-27. | |
| 16 | 游洋洋, 卢学强, 许丹宇, 等. 多相催化臭氧化水处理技术研究进展[J]. 环境工程, 2014, 32(1): 37-41, 54. |
| YOU Yangyang, LU Xueqiang, XU Danyu, et al. Review on heterogeneous catalytic ozonation in water treatment[J]. Environmental Engineering, 2014, 32(1): 37-41, 54. | |
| 17 | KOPPENOL W H. The centennial of the Fenton reaction[J]. Free Radical Biology and Medicine, 1993, 15(6): 645-651. |
| 18 | ERTUGAY N, ACAR F N. Removal of COD and color from Direct Blue 71 azo dye wastewater by Fenton's oxidation: kinetic study[J]. Arabian Journal of Chemistry, 2017, 10: S1158-S1163. |
| 19 | XAVIER S, GANDHIMATHI R, NIDHEESH P V, et al. Comparison of homogeneous and heterogeneous Fenton processes for the removal of reactive dye Magenta MB from aqueous solution[J]. Desalination and Water Treatment, 2015, 53(1): 109-118. |
| 20 | 杨振兴, 李烨莹, 叶芳芳, 等. 抗生素废水处理技术研究进展[J]. 现代化工, 2021, 41(1): 57-61. |
| YANG Zhenxing, LI Yeying, YE Fangfang, et al. Summary of progress in antibiotics wastewater treatment technology[J]. Modern Chemical Industry, 2021, 41(1): 57-61. | |
| 21 | HUANG Anqi, ZHI Dan, TANG Hongmei, et al. Effect of Fe2+, Mn2+ catalysts on the performance of electro-Fenton degradation of antibiotic ciprofloxacin, and expanding the utilizing of acid mine drainage[J]. Science of the Total Environment, 2020, 720: 137560. |
| 22 | 李蓉, 吴小宁, 王倩, 等. 非均相类Fenton体系中降解水中染料的固体催化剂研究进展[J]. 净水技术, 2019, 38(4): 70-73, 100. |
| LI Rong, WU Xiaoning, WANG Qian, et al. Review on solid catalysts for degradation of dyes in water in heterogeneous Fenton-like systems[J]. Water Purification Technology, 2019, 38(4): 70-73, 100. | |
| 23 | KASIRI M B, ALEBOYEH H, ALEBOYEH A. Degradation of Acid Blue 74 using Fe-ZSM5 zeolite as a heterogeneous photo-Fenton catalyst [J]. Applied Catalysis B: Environmental, 2008, 84(1/2): 9-15. |
| 24 | XU Lejin, WANG Jianlong. Fenton-like degradation of 2,4-dichlorophenol using Fe3O4 magnetic nanoparticles[J]. Applied Catalysis B: Environmental, 2012, 123/124: 117-126. |
| 25 | XUE Xiaofei, HANNA K, ABDELMOULA M, et al. Adsorption and oxidation of PCP on the surface of magnetite: kinetic experiments and spectroscopic investigations[J]. Applied Catalysis B: Environmental, 2009, 89(3-4): 432-440. |
| 26 | FONTECHA-CÁMARA M A, MORENO-CASTILLA C, LÓPEZ-RAMÓN M V, et al. Mixed iron oxides as Fenton catalysts for gallic acid removal from aqueous solutions[J]. Applied Catalysis B: Environmental, 2016, 196: 207-215. |
| 27 | LU Mingchun, CHEN J N, HUANG H H. Role of goethite dissolution in the oxidation of 2-chlorophenol with hydrogen peroxide[J]. Chemosphere, 2002, 46(1): 131-136. |
| 28 | WANG Xiaobing, CHEN Na, LIU Xiufan, et al. Ascorbate guided conversion of hydrogen peroxide to hydroxyl radical on goethite[J]. Applied Catalysis B: Environmental, 2021, 282: 119558. |
| 29 | 李联, 何平, 李洪, 等. Ti5O9-Ti4O7电极电化学处理2,4,6-三硝基苯酚废水[J]. 化工环保, 2015, 35(4): 420-425. |
| LI Lian, HE Pi, LI Hong, et al. Electrochemical treatment of 2,4,6-trinitrophenol wastewater on Ti5O9-Ti4O7 electrode[J]. Environmental Protection of Chemical Industry, 2015, 35(4): 420-425. | |
| 30 | DULOVA N, TRAPIDO M, DULOV A. Catalytic degradation of picric acid by heterogeneous Fenton-based processes[J]. Environmental Technology, 2011, 32(4): 439-446. |
| 31 | 张帅, 陈泉源, 卢钧, 等. 基于铜渣的类Fenton体系处理H酸废水的研究[J]. 工业水处理, 2020, 40(3): 39-42. |
| ZHANG Shuai, CHEN Quanyuan, LU Jun, et al. Study on treatment of H-acid wastewater by Fenton-like system based on copper slag[J]. Industrial Water Treatment, 2020, 40(3): 39-42. | |
| 32 | HUANG H H, LU Mingchun, CHEN J N. Catalytic decomposition of hydrogen peroxide and 2-chlorophenol with iron oxides[J]. Water Research, 2001, 35(9): 2291-2299. |
| 33 | TYRE B W, WATTS R J, MILLER G C. Treatment of four biorefractory contaminants in soils using catalyzed hydrogen peroxide[J]. Journal of Environmental Quality, 1991, 20(4): 832-838. |
| 34 | LU Mingchun. Oxidation of chlorophenols with hydrogen peroxide in the presence of goethite[J]. Chemosphere, 2000, 40(2): 125-130. |
| 35 | KWAN W P, VOELKER B M. Rates of hydroxyl radical generation and organic compound oxidation in mineral-catalyzed Fenton-like systems[J]. Environmental Science & Technology, 2003, 37(6): 1150-1158. |
| 36 | COSTA R C C, LELIS M F F, OLIVEIRA L C A, et al. Novel active heterogeneous Fenton system based on Fe3- x M x O4 (Fe, Co, Mn, Ni): the role of M2+ species on the reactivity towards H2O2 reactions[J]. Journal of Hazardous Materials, 2006, 129(1/2/3): 171-178. |
| 37 | COSTA R C C, DE FÁTIMA FONTES LELIS M, OLIVEIRA L C A, et al. Remarkable effect of Co and Mn on the activity of Fe3- x M x O4 promoted oxidation of organic contaminants in aqueous medium with H2O2 [J]. Catalysis Communications, 2003, 4(10): 525-529. |
| 38 | BALDRIAN P, MERHAUTOVÁ V, GABRIEL J, et al. Decolorization of synthetic dyes by hydrogen peroxide with heterogeneous catalysis by mixed iron oxides[J]. Applied Catalysis B: Environmental, 2006, 66(3/4): 258-264. |
| 39 | LORENZO D, DOMINGUEZ C M, ROMERO A, et al. Wet peroxide oxidation of chlorobenzenes catalyzed by goethite and promoted by Hydroxylamine[J]. Catalysts, 2019, 9(6): 553. |
| 40 | LIU Xinwen, WANG Feifeng, CHEN Zuliang, et al. Heterogeneous Fenton oxidation of Direct Black G in dye effluent using functional Kaolin-supported nanoscale zero iron[J]. Environmental Science and Pollution Research International, 2014, 21(3): 1936-1943. |
| 41 | 侯少文, 王欢, 王舒英, 等. 硅藻土负载铁类芬顿体系催化降解碱性品红[J]. 化学工程师, 2020, 34(10): 9-12. |
| HOU Shaowen, WANG Huan, WANG Shuying, et al. Catalytic degradation of basic fuchsin by iron-supported diatomite Fenton-like system[J]. Chemical Engineer, 2020, 34(10): 9-12. | |
| 42 | FIDA H, GAO Ke, GUO Sheng, et al. Heterogeneous Fenton degradation of organic dyes in batch and fixed bed using La-Fe montmorillonite as catalyst[J]. Journal of Colloid and Interface Science, 2017, 490: 859-868. |
| 43 | IURASCU B, SIMINICEANU I, VIONE D, et al. Phenol degradation in water through a heterogeneous photo-Fenton process catalyzed by Fe-treated laponite[J]. Water Research, 2009, 43(5): 1313-1322. |
| 44 | HUANG Wenyu, LUO Mengqi, WEI Chaoshuai, et al. Enhanced heterogeneous photo-Fenton process modified by magnetite and EDDS: BPA degradation[J]. Environmental Science and Pollution Research International, 2017, 24(11): 10421-10429. |
| 45 | BAE S, KIM D, LEE W. Degradation of diclofenac by pyrite catalyzed Fenton oxidation[J]. Applied Catalysis B: Environmental, 2013, 134/135: 93-102. |
| 46 | MAMERI Y, DEBBACHE N, BENACHERINE M E M, et al. Heterogeneous photodegradation of paracetamol using goethite/H2O2 and goethite/oxalic acid systems under artificial and natural light[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2016, 315: 129-137. |
| 47 | HUANG Wenyu, BRIGANTE M, WU Fenget al. Effect of ethylenediamine-N,N'-disuccinic acid on Fenton and photo-Fenton processes using goethite as an iron source: optimization of parameters for bisphenol A degradation[J]. Environmental Science and Pollution Research International, 2013, 20(1): 39-50. |
| 48 | HASSAN H, HAMEED B H. Fe-clay as effective heterogeneous Fenton catalyst for the decolorization of Reactive Blue 4[J]. Chemical Engineering Journal, 2011, 171(3): 912-918. |
| 49 | 马冬梅, 陈泉源, 何晋保. 异相类Fenton氧化处理T酸废母液的催化剂[J]. 环境工程学报, 2015, 9(6): 2601-2606. |
| MA Dongmei, CHEN Quanyuan, HE Jinbao. Catalysts of heterogeneous Fenton-like oxidation reaction for treatment of spent liquor of T-acid crystallization[J]. Chinese Journal of Environmental Engineering, 2015, 9(6): 2601-2606. | |
| 50 | YANG Ying, DENG Qinzu, YAN Wei, et al. Comparative study of glyphosate removal on goethite and magnetite: adsorption and photo-degradation[J]. Chemical Engineering Journal, 2018, 352: 581-589. |
| 51 | 张钰, 何燕, 邹彩琼, 等. 铁锰矿类Fenton异相光催化降解有毒有机染料[J]. 环境化学, 2010, 29(6): 1032-1037. |
| ZHANG Yu, HE Yan, ZOU Caiqiong, et al. Photo-degradation of organic dye by iron-manganese ore as heterogeneous Fenton-like catalyst[J]. Environmental Chemistry, 2010, 29(6): 1032-1037. | |
| 52 | MOLINA R, SEGURA Y, MARTÍNEZ F, et al. Immobilization of active and stable goethite coated-films by a dip-coating process and its application for photo-Fenton systems[J]. Chemical Engineering Journal, 2012, 203: 212-222. |
| 53 | CHENG Mingming, SONG Wenjing, MA Wanhong, et al. Catalytic activity of iron species in layered clays for photodegradation of organic dyes under visible irradiation[J]. Applied Catalysis B: Environmental, 2008, 77(3/4): 355-363. |
| 54 | FENG Jiyun, HU Xijun, YUE P L, et al. Discoloration and mineralization of Reactive Red HE-3B by heterogeneous photo-Fenton reaction[J]. Water Research, 2003, 37(15): 3776-3784. |
| 55 | SHARMA V K, FENG Mingbao. Water depollution using metal-organic frameworks-catalyzed advanced oxidation processes: a review[J]. Journal of Hazardous Materials, 2019, 372: 3-16. |
| 56 | HU Jinshan, ZHANG Pengfei, AN Weijia, et al. In-situ Fe-doped g-C3N4 heterogeneous catalyst via photocatalysis-Fenton reaction with enriched photocatalytic performance for removal of complex wastewater[J]. Applied Catalysis B: Environmental, 2019, 245: 130-142. |
| 57 | 李君敬, 刘惠玲, 程修文. 氯酚废水处理方法研究进展[J]. 工业水处理, 2013, 33(9): 1-5. |
| LI Junjing, LIU Huiling, CHENG Xiuwe. Progress in the treatment methods for wastewater containing chlorophenols[J]. Industrial Water Treatment, 2013, 33(9): 1-5. | |
| 58 | LI Linghui, LI Yan, LI Yanzhang, et al. Natural wolframite as a novel visible-light photocatalyst towards organics degradation and bacterial inactivation[J]. Catalysis Today, 2020, 358: 177-183. |
| 59 | 李灵慧, 李艳, 黎晏彰, 等. 天然黑钨矿可见光催化活性的实验研究[J]. 地学前缘, 2019, 26(4): 287-294. |
| LI Linghui, LI Yan, LI Yanzhang, et al. Experimental study on the photocatalytic activity of natural wolframite under natural light[J]. Earth Science Frontiers, 2019, 26(4): 287-294. | |
| 60 | FENG Jiyun, HU Xijun, YUE P L. Novel bentonite clay-based Fe-nanocomposite as a heterogeneous catalyst for photo-Fenton discoloration and mineralization of Orange II[J]. Environmental Science & Technology, 2004, 38(1): 269-275. |
| 61 | FENG Jiyun, HU Xijun, YUE P L, et al. Degradation of azo-dye Orange II by a photoassisted Fenton reaction using a novel composite of iron oxide and silicate nanoparticles as a catalyst[J]. Industrial & Engineering Chemistry Research, 2003, 42(10): 2058-2066. |
| 62 | BRILLAS E, SIRÉS I, OTURAN M A. Electro-Fenton process and related electrochemical technologies based on Fenton's reaction chemistry[J]. Chemical Reviews, 2009, 109(12): 6570-6631. |
| 63 | BARHOUMI N, OTURAN N, OLVERA-VARGAS H, et al. Pyrite as a sustainable catalyst in electro-Fenton process for improving oxidation of sulfamethazine. Kinetics, mechanism and toxicity assessment[J]. Water Research, 2016, 94: 52-61. |
| 64 | CHOI K, BAE S, LEE W. Degradation of pyrene in cetylpyridinium chloride-aided soil washing wastewater by pyrite Fenton reaction[J]. Chemical Engineering Journal, 2014, 249: 34-41. |
| 65 | CHOI K, BAE S, LEE W. Degradation of off-gas toluene in continuous pyrite Fenton system[J]. Journal of Hazardous Materials, 2014, 280: 31-37. |
| 66 | CHE H, BAE S, LEE W. Degradation of trichloroethylene by Fenton reaction in pyrite suspension[J]. Journal of Hazardous Materials, 2011, 185(2): 1355-1361. |
| 67 | NIDHEESH P V, OLVERA-VARGAS H, OTURAN N, et al. Heterogeneous electro-Fenton process: principles and applications[J]. Electro-Fenton Process, 2018: 85-110. |
| 68 | LABIADH L, OTURAN M A, PANIZZA M, et al. Complete removal of AHPS synthetic dye from water using new electro-Fenton oxidation catalyzed by natural pyrite as heterogeneous catalyst[J]. Journal of Hazardous Materials, 2015, 297: 34-41. |
| 69 | BARHOUMI N, OTURAN N, AMMAR S, et al. Enhanced degradation of the antibiotic tetracycline by heterogeneous electro-Fenton with pyrite catalysis[J]. Environmental Chemistry Letters, 2017, 15(4): 689-693. |
| 70 | BARHOUMI N, LABIADH L, OTURAN M A, et al. Electrochemical mineralization of the antibiotic levofloxacin by electro-Fenton-pyrite process[J]. Chemosphere, 2015, 141: 250-257. |
| 71 | LI Y, KAWASHIMA N, LI J, et al. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite[J]. Advances in Colloid and Interface Science, 2013, 197/198: 1-32. |
| 72 | BARHOUMI N, OLVERA-VARGAS H, OTURAN N, et al. Kinetics of oxidative degradation/mineralization pathways of the antibiotic tetracycline by the novel heterogeneous electro-Fenton process with solid catalyst chalcopyrite[J]. Applied Catalysis B: Environmental, 2017, 209: 637-647. |
| 73 | ÖZCAN A, ATILIR ÖZCAN A, DEMIRCI Y, et al. Preparation of Fe2O3 modified kaolin and application in heterogeneous electro-catalytic oxidation of enoxaci [J]. Applied Catalysis B: Environmental, 2017, 200: 361-371. |
| 74 | THIAM A, SALAZAR R, BRILLAS E, et al. In-situ dosage of Fe2+ catalyst using natural pyrite for thiamphenicol mineralization by photoelectro-Fenton process[J]. Journal of Environmental Management, 2020, 270: 110835. |
| 75 | AMMAR S, OTURAN M A, LABIADH L, et al. Degradation of tyrosol by a novel electro-Fenton process using pyrite as heterogeneous source of iron catalyst[J]. Water Research, 2015, 74: 77-87. |
| 76 | NIDHEESH P V, GANDHIMATHI R, VELMATHI S, et al. Magnetite as a heterogeneous electro Fenton catalyst for the removal of Rhodamine B from aqueous solution[J]. RSC Advances, 2014, 4(11): 5698-5708. |
| 77 | ANDREOZZI R, INSOLA A, CAPRIO V, et al. The use of manganese dioxide as a heterogeneous catalyst for oxalic acid ozonation in aqueous solution[J]. Applied Catalysis A: General, 1996, 138(1): 75-81. |
| 78 | LUO Kai, ZHAO Shixi, WANG Yifeng, et al. Synthesis of petal-like δ-MnO2 and its catalytic ozonation performance[J]. New Journal of Chemistry, 2018, 42(9): 6770-6777. |
| 79 | NAWAZ F, GAO Hongbin, XIE Yongbing, et al. Selection of active phase of MnO2 for catalytic ozonation of 4-nitrophenol[J]. Chemosphere, 2017, 168: 1457-1466. |
| 80 | SÁENZ-ROBLERO B, DURÁN J E, MASÍS-MORA M, et al. Removal of cimetidine, ketoprofen and naproxen by heterogeneous catalytic ozonation over volcanic sand at low pH[J]. Journal of Water Process Engineering, 2020, 37: 101461. |
| 81 | NEMATI SANI O, NAVAEI FEZABADY A A, YAZDANI M, et al. Catalytic ozonation of ciprofloxacin using γ-Al2O3 nanoparticles in synthetic and real wastewaters[J]. Journal of Water Process Engineering, 2019, 32: 100894. |
| 82 | QI Fei, XU Bingbing, CHEN Zhonglin, et al. Catalytic ozonation of 2-isopropyl-3-methoxypyrazine in water by γ-AlOOH and γ-Al2O3: comparison of removal efficiency and mechanism[J]. Chemical Engineering Journal, 2013, 219: 527-536. |
| 83 | YUAN Xiangjuan, YAN Xuan, XU Haiming, et al. Enhanced ozonation degradation of atrazine in the presence of nano-ZnO: performance, kinetics and effects[J]. Journal of Environmental Sciences-China, 2017, 61: 3-13. |
| 84 | DONG Yuming, WANG Guangli, JIANG Pingping, et al. Simple preparation and catalytic properties of ZnO for ozonation degradation of phenol in water[J]. Chinese Chemical Letters, 2011, 22(2): 209-212. |
| 85 | ZHU Hao, MA Wencheng, HAN Hongjun, et al. Catalytic ozonation of quinoline using nano-MgO: efficacy, pathways, mechanisms and its application to real biologically pretreated coal gasification wastewater[J]. Chemical Engineering Journal, 2017, 327: 91-99. |
| 86 | CHEN Jun, TIAN Shuanghong, LU Jiang, et al. Catalytic performance of MgO with different exposed crystal facets towards the ozonation of 4-chlorophenol[J]. Applied Catalysis A: General, 2015, 506: 118-125. |
| 87 | DONG Yuming, HE Kun, ZHAO Bo, et al. Catalytic ozonation of azo dye active brilliant red X-3B in water with natural mineral brucite[J]. Catalysis Communications, 2007, 8(11): 1599-1603. |
| 88 | 何坤, 董玉明, 李真, 等. 天然水镁石及其煅烧产物对臭氧化降解水中苯酚的效果研究[J]. 矿物岩石地球化学通报, 2007, 26(2): 155-159. |
| HE Kun, DONG Yuming, LI Zhen, et al. The decomposition of phenol in water under catalytic ozonation with natural brucite and magnesia[J]. Bulletin of Mineralogy, Petrology and Geochemistry, 2007, 26(2): 155-159. | |
| 89 | VALDÉS H, TARDÓN R F, ZAROR C A. Methylene blue removal from contaminated waters using heterogeneous catalytic ozonation promoted by natural zeolite: mechanism and kinetic approach[J]. Environmental Technology, 2012, 33(16/17/18): 1895-1903. |
| 90 | VALDÉS H, TARDÓN R F, ZAROR C A. Role of surface hydroxyl groups of acid-treated natural zeolite on the heterogeneous catalytic ozonation of methylene blue contaminated waters[J]. Chemical Engineering Journal, 2012, 211/212: 388-395. |
| 91 | VALDÉS H, FARFÁN V J, MANOLI J A, et al. Catalytic ozone aqueous decomposition promoted by natural zeolite and volcanic sand[J]. Journal of Hazardous Materials, 2009, 165(1/2/3): 915-922. |
| 92 | ALVER E, METIN A Ü. Anionic dye removal from aqueous solutions using modified zeolite: adsorption kinetics and isotherm studies[J]. Chemical Engineering Journal, 2012, 200/201/202: 59-67. |
| 93 | QI Fei, XU Bingbing, CHEN Zhonglin, et al. Ozonation catalyzed by the raw bauxite for the degradation of 2,4,6-trichloroanisole in drinking water[J]. Journal of Hazardous Materials, 2009, 168(1): 246-252. |
| 94 | XU Bingbing, QI Fei, ZHANG Jizhou, et al. Cobalt modified red mud catalytic ozonation for the degradation of bezafibrate in water: catalyst surface properties characterization and reaction mechanism[J]. Chemical Engineering Journal, 2016, 284: 942-952. |
| 95 | YUAN Lei, SHEN Jimin, CHEN Zhonglin, et al. Pumice-catalyzed ozonation degradation of p-chloronitrobenzene in aqueous solution[J]. Applied Catalysis B: Environmental, 2012, 117/118: 414-419. |
| 96 | PENG Jiali, YAN Jianfei, CHEN Qixuan, et al. Natural mackinawite catalytic ozonation for N, N-dimethylacetamide (DMAC) degradation in aqueous solution: kinetic, performance, biotoxicity and mechanism[J]. Chemosphere, 2018, 210: 831-842. |
| 97 | KHATAEE A, RAD T S, FATHINIA M. The role of clinoptilolite nanosheets in catalytic ozonation process: insights into the degradation mechanism, kinetics and the toxicity[J]. Journal of the Taiwan Institute of Chemical Engineers, 2017, 77: 205-215. |
| 98 | ALGBURI H R, AZIZ H A, ZWAIN H M, et al. Treatment of landfill leachate by heterogeneous catalytic ozonation with granular faujasite zeolite[J]. Environmental Engineering Science, 2021, 38(7): 635-644. |
| 99 | MOUSSAVI G, KHOSRAVI R, OMRAN N R. Development of an efficient catalyst from magnetite ore: characterization and catalytic potential in the ozonation of water toxic contaminants[J]. Applied Catalysis A: General, 2012, 445/446: 42-49. |
| 100 | CHEN Chunmao, CHEN Hongshuo, YU Ji, et al. p-Nitrophenol removal by bauxite ore assisted ozonation and its catalytic potential[J]. Clean-Soil, Air, Water, 2015, 43(7): 1010-1017. |
| 101 | WU Deli, LIU Ying, HE Hongping, et al. Magnetic pyrite cinder as an efficient heterogeneous ozonation catalyst and synergetic effect of deposited Ce[J]. Chemosphere, 2016, 155: 127-134. |
| 102 | ZHANG Jiali, XIONG Zhaokun, WEI Jian, et al. Catalytic ozonation of penicillin G using cerium-loaded natural zeolite (CZ): efficacy, mechanisms, pathways and toxicity assessment[J]. Chemical Engineering Journal, 2020, 383: 123144. |
| 103 | CHEN Yu, CHEN Chunmao, YOZA B A, et al. Efficient ozonation of reverse osmosis concentrates from petroleum refinery wastewater using composite metal oxide-loaded alumina[J]. Petroleum Science, 2017, 14(3): 605-615. |
| 104 | 梁涛, 马军, 王胜军, 等. O3/TiO2催化氧化工艺对饮用水中AOC的影响[J]. 环境科学, 2007, 28(9): 2004-2008. |
| LIANG Tao, MA Jun, WANG Shengjun, et al. Impacts of AOC by O3/TiO2 catalytic oxidation in drinking water[J]. Chinese Journal of Environmental Science, 2007, 28(9): 2004-2008. | |
| 105 | QI Fei, XU Bingbing, ZHAO Lun, et al. Comparison of the efficiency and mechanism of catalytic ozonation of 2,4,6-trichloroanisole by iron and manganese modified bauxite[J]. Applied Catalysis B: Environmental, 2012, 121/122: 171-181. |
| 106 | 张馨月, 田振邦, 段文杰, 等. γ-Al2O3球和蜂窝陶瓷负载Fe2O3臭氧氧化降解丙烯酸废水的研究[J]. 河南科学, 2017, 35(11): 1749-1754. |
| ZHANG Xinyue, TIAN Zhenbang, DUAN Wenjie, et al. Oxidative degradation of acrylic wastewater by ozone oxidation of γ-Al2O3 and honeycomb ceramics loaded Fe2O3 [J]. Henan Science, 2017, 35(11): 1749-1754. | |
| 107 | 邹剑锋. 在K-Co-Al2O3上催化臭氧化水中痕量N-二甲基亚硝胺(NDMA)[D]. 北京: 清华大学, 2010. |
| ZOU Jianfeng. Catalytic ozonation of trace NDMA in aqueous solution on K-Co-Al2O3 [D]. Beijing: Tsinghua University, 2010. | |
| 108 | 王香平, 李发祥, 耿文钊, 等. 陶粒/改性陶粒催化臭氧降解水杨酸废水[J]. 非金属矿, 2020, 43(3): 96-99. |
| WANG Xiangping, LI Faxiang, GENG Wenzhao, et al. Degradation of salicylic acid wastewater by catalytic ozonation with ceramsite/modified ceramsite[J]. Non-Metallic Mines, 2020, 43(3): 96-99. | |
| 109 | 张静, 马军, 杨忆新, 等. 陶粒负载纳米TiO2催化臭氧化降解水中微量硝基苯[J]. 环境科学, 2007, 28(10): 2208-2212. |
| ZHANG Jing, MA Jun, YANG Yixin, et al. Degradation of trace nitrobenzene in aqueous solution by ozone with catalysis of nanosized TiO2 supported on haydite[J]. Chinese Journal of Environmental Science, 2007, 28(10): 2208-2212. | |
| 110 | 周永强, 李青山, 韩长菊, 白玲. 海泡石的组成、结构、性质及其应用[J]. 化工时刊, 1999, 12: 7-10. |
| ZHOU Yongqiang, LI Qingshan, HAN Changju, et al. The composition structure properties and application of sepiolite[J]. Chemical Industry Times, 1999, 12: 7-10. | |
| 111 | 王洪华, 赵雅晴, 牛建瑞. Cu-Mn-Ce/海泡石的制备及其催化臭氧氧化布洛芬废水性能研究[J]. 现代化工, 2020, 40(11): 136-138, 143. |
| WANG Honghua, ZHAO Yaqing, NIU Jianrui. Preparation of Cu-Mn-Ce/sepiolite and its performance in catalyzing ozonation degradation of ibuprofen wastewater[J]. Modern Chemical Industry, 2020, 40(11): 136-138, 143. | |
| 112 | PELALAK R, ALIZADEH R, GHARESHABANI E. Enhanced heterogeneous catalytic ozonation of pharmaceutical pollutants using a novel nanostructure of iron-based mineral prepared via plasma technology: a comparative study[J]. Journal of Hazardous Materials, 2020, 392: 122269. |
| 113 | HAO Feifei, GUO Weilin, WANG Anqi, et al. Intensification of sonochemical degradation of ammonium perfluorooctanoate by persulfate oxidant[J]. Ultrasonics Sonochemistry, 2014, 21(2): 554-558. |
| 114 | ZHAO Y S, SUN Chao, SUN J Q, et al. Kinetic modeling and efficiency of sulfate radical-based oxidation to remove p-nitroaniline from wastewater by persulfate/Fe3O4 nanoparticles process[J]. Separation and Purification Technology, 2015, 142: 182-188. |
| 115 | FANG Guodong, DIONYSIOU D D, AL-ABED S R, et al. Superoxide radical driving the activation of persulfate by magnetite nanoparticles: implications for the degradation of PCBs[J]. Applied Catalysis B: Environmental, 2013, 129: 325-332. |
| 116 | RODRIGUEZ S, VASQUEZ L, COSTA D, et al. Oxidation of Orange G by persulfate activated by Fe(II), Fe(III) and zero valent iron (ZVI)[J]. Chemosphere, 2014, 101: 86-92. |
| 117 | MATZEK L W, CARTER K E. Activated persulfate for organic chemical degradation: a review[J]. Chemosphere, 2016, 151: 178-188. |
| 118 | TEEL A L, AHMAD M, WATTS R J. Persulfate activation by naturally occurring trace minerals[J]. Journal of Hazardous Materials, 2011, 196: 153-159. |
| 119 | 娄保锋, 朱利中, 杨坤. 苯及其取代物与对硝基苯胺在沉积物上的竞争吸附[J]. 中国环境科学, 2004, 24(3): 327-331. |
| LOU Baofeng, ZHU Lizhong, YANG Kun. Competitive sorption behavior between p-nitroaniline and benzene and its monosubstituent in sediment-water system[J]. China Environmental Science, 2004, 24(3): 327-331. | |
| 120 | FAN Jinhong, GU Lin, WU Deli, et al. Mackinawite (FeS) activation of persulfate for the degradation of p-chloroaniline: surface reaction mechanism and sulfur-mediated cycling of iron species[J]. Chemical Engineering Journal, 2018, 333: 657-664. |
| 121 | ZHANG Yongqing, TRAN H P, DU Xiaodong, et al. Efficient pyrite activating persulfate process for degradation of p-chloroaniline in aqueous systems: a mechanistic study[J]. Chemical Engineering Journal, 2017, 308: 1112-1119. |
| 122 | YUAN Yanmei, TAO Hong, FAN Jinhong, et al. Degradation of p-chloroaniline by persulfate activated with ferrous sulfide ore particles[J]. Chemical Engineering Journal, 2015, 268: 38-46. |
| 123 | OH S Y, KANG S G, KIM D W, et al. Degradation of 2,4-dinitrotoluene by persulfate activated with iron sulfides[J]. Chemical Engineering Journal, 2011, 172(2/3): 641-646. |
| 124 | SILVEIRA J E, CLARO E M T, PAZ W S, et al. Optimization of disperse Blue 3 mineralization by UV-LED/FeTiO3 activated persulfate using response surface methodology[J]. Journal of the Taiwan Institute of Chemical Engineers, 2018, 85: 66-73. |
| 125 | YAN Jingchun, LEI Min, ZHU Lihua, et al. Degradation of sulfamonomethoxine with Fe3O4 magnetic nanoparticles as heterogeneous activator of persulfate[J]. Journal of Hazardous Materials, 2011, 186(2): 1398-1404. |
| 126 | WU Bichao, GU Guohua, DENG Sha, et al. Efficient natural pyrrhotite activating persulfate for the degradation of O-isopropyl-N-ethyl thionocarbamate: iron recycle mechanism and degradation pathway[J]. Chemosphere, 2019, 224: 120-127. |
| 127 | MATTHAIOU V, FRONTISTIS Z, PETALA A, et al. Utilization of raw red mud as a source of iron activating the persulfate oxidation of paraben[J]. Process Safety and Environmental Protection, 2018, 119: 311-319. |
| 128 | FENG Yong, WU Deli, LIAO Changzhong, et al. Red mud powders as low-cost and efficient catalysts for persulfate activation: pathways and reusability of mineralizing sulfadiazine[J]. Separation and Purification Technology, 2016, 167: 136-145. |
| 129 | LIN Heng, ZHANG Hui, HOU Liwei. Degradation of C. I. Acid Orange 7 in aqueous solution by a novel electro/Fe3O4/PDS process[J]. Journal of Hazardous Materials, 2014, 276: 182-191. |
| 130 | LIN Heng, LI Yating, MAO Xiaoyu, et al. Electro-enhanced goethite activation of peroxydisulfate for the decolorization of Orange Ⅱ at neutral pH: efficiency, stability and mechanism[J]. Journal of the Taiwan Institute of Chemical Engineers, 2016, 65: 390-398. |
| [1] | ZHANG Mingyan, LIU Yan, ZHANG Xueting, LIU Yake, LI Congju, ZHANG Xiuling. Research progress of non-noble metal bifunctional catalysts in zinc-air batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 276-286. |
| [2] | SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298. |
| [3] | XIE Luyao, CHEN Songzhe, WANG Laijun, ZHANG Ping. Platinum-based catalysts for SO2 depolarized electrolysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 299-309. |
| [4] | YANG Xiazhen, PENG Yifan, LIU Huazhang, HUO Chao. Regulation of active phase of fused iron catalyst and its catalytic performance of Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 310-318. |
| [5] | ZHENG Qian, GUAN Xiushuai, JIN Shanbiao, ZHANG Changming, ZHANG Xiaochao. Photothermal catalysis synthesis of DMC from CO2 and methanol over Ce0.25Zr0.75O2 solid solution [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 319-327. |
| [6] | WANG Zhengkun, LI Sifang. Green synthesis of gemini surfactant decyne diol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 400-410. |
| [7] | WANG Lele, YANG Wanrong, YAO Yan, LIU Tao, HE Chuan, LIU Xiao, SU Sheng, KONG Fanhai, ZHU Canghai, XIANG Jun. Influence of spent SCR catalyst blending on the characteristics and deNO x performance for new SCR catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 489-497. |
| [8] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [9] | ZHANG Tingting, ZUO Xuqian, TIAN Lingdi, WANG Shimeng. Construction method of volatile organic compounds emission inventory and factor database in chemical industry park [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 549-557. |
| [10] | GENG Yuanze, ZHOU Junhu, ZHANG Tianyou, ZHU Xiaoyu, YANG Weijuan. Homogeneous/heterogeneous coupled combustion of heptane in a partially packed bed burner [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4514-4521. |
| [11] | CHENG Tao, CUI Ruili, SONG Junnan, ZHANG Tianqi, ZHANG Yunhe, LIANG Shijie, PU Shi. Analysis of impurity deposition and pressure drop increase mechanisms in residue hydrotreating unit [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4616-4627. |
| [12] | WANG Peng, SHI Huibing, ZHAO Deming, FENG Baolin, CHEN Qian, YANG Da. Recent advances on transition metal catalyzed carbonylation of chlorinated compounds [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4649-4666. |
| [13] | GAO Yanjing. Analysis of international research trend of single-atom catalysis technology [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4667-4676. |
| [14] | ZHANG Qi, ZHAO Hong, RONG Junfeng. Research progress of anti-toxicity electrocatalysts for oxygen reduction reaction in PEMFC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4677-4691. |
| [15] | GE Quanqian, XU Mai, LIANG Xian, WANG Fengwu. Research progress on the application of MOFs in photoelectrocatalysis [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4692-4705. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||