Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (1): 40-52.DOI: 10.16085/j.issn.1000-6613.2022-1545
Previous Articles Next Articles
Progress in metabolic engineering of microorganisms for CO2 fixation
TAO Yuxuan1( ), GUO Liang1, GAO Cong1, SONG Wei2, CHEN Xiulai1(
), GUO Liang1, GAO Cong1, SONG Wei2, CHEN Xiulai1( )
)
- 1.State Key Laboratory of Food Science and Technology, Jiangnan University, Wuxi 214122, Jiangsu, China
2.School of Life Sciences and Health Engineering, Jiangnan University, Wuxi 214122, Jiangsu, China
-
Received:2022-08-22Revised:2022-10-29Online:2023-02-20Published:2023-01-25 -
Contact:CHEN Xiulai
代谢工程改造微生物固定二氧化碳研究进展
- 1.江南大学食品科学与技术国家重点实验室,江苏 无锡 214122
2.江南大学生命科学与健康工程学院,江苏 无锡 214122
-
通讯作者:陈修来 -
作者简介:陶雨萱(1997—),女,博士研究生,研究方向为微生物代谢工程。E-mail:15106192105@163.com。 -
基金资助:国家重点研发计划(2021YFC2103500);国家自然科学基金(22122806);江苏省自然科学基金(BK20211529)
CLC Number:
Cite this article
TAO Yuxuan, GUO Liang, GAO Cong, SONG Wei, CHEN Xiulai. Progress in metabolic engineering of microorganisms for CO2 fixation[J]. Chemical Industry and Engineering Progress, 2023, 42(1): 40-52.
陶雨萱, 郭亮, 高聪, 宋伟, 陈修来. 代谢工程改造微生物固定二氧化碳研究进展[J]. 化工进展, 2023, 42(1): 40-52.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-1545
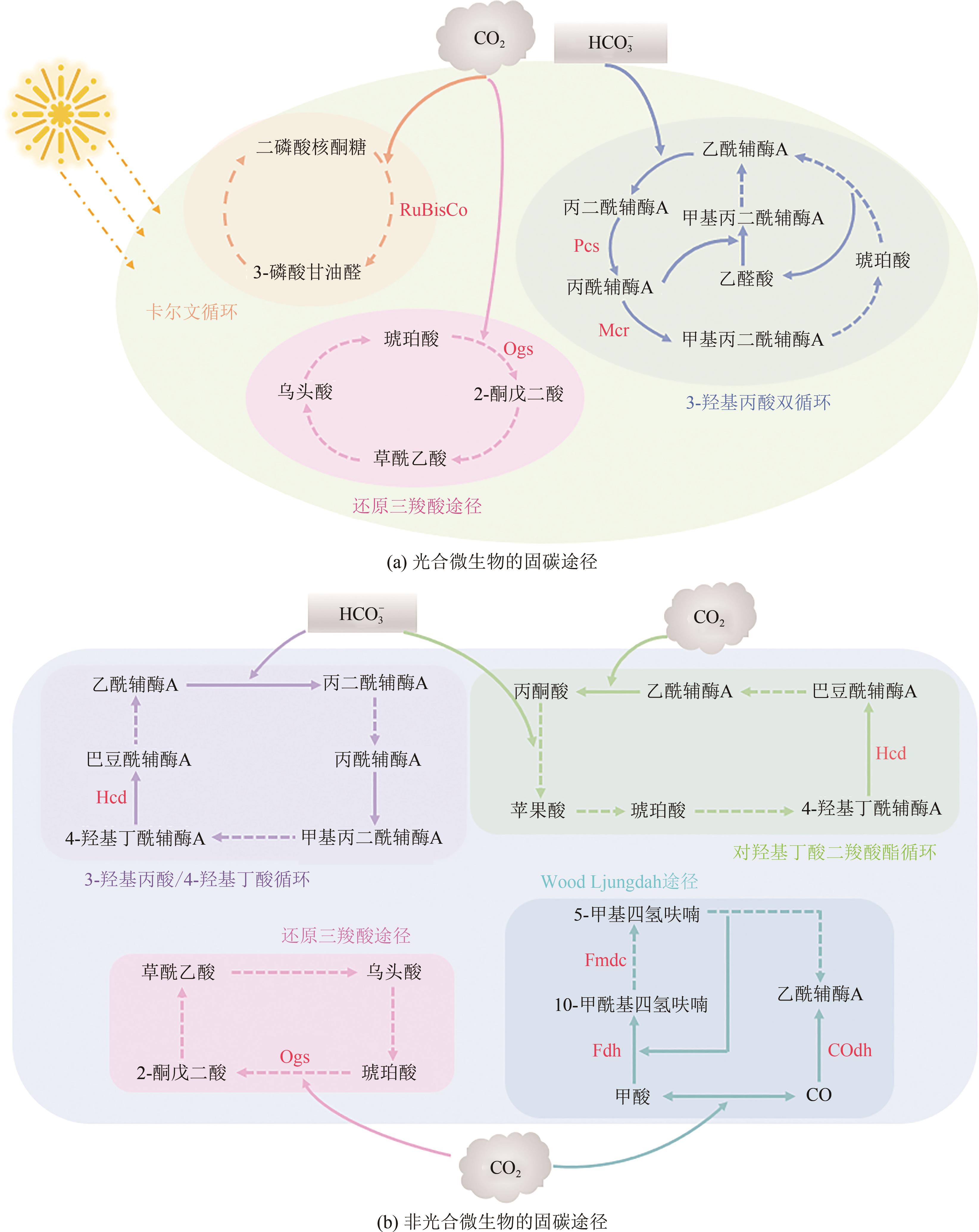
| CO2固定途径 | 固定CO2/HCO | 消耗能量 | 重要产物 | 氧气需求 | 参考文献 |
|---|---|---|---|---|---|
| CBB循环 | 3mol CO2 | 9mol ATP + 6mol NAD(P)H | 3-磷酸甘油醛 | 好氧 | [ |
| RTCA途径 | 2mol CO2 | 2mol ATP + 4mol NAD(P)H | 乙酰辅酶A | 厌氧/微氧 | [ |
| 3-HP双循环 | 3mol HCO | 5mol ATP + 5mol NAD(P)H | 丙酮酸 | 好氧 | [ |
| WL途径 | 2mol CO2 | 1mol ATP + 4mol NAD(P)H | 乙酰辅酶A | 厌氧 | [ |
| DC-4HB循环 | 1mol CO2 + 1mol HCO | 3mol ATP + 4mol NAD(P)H | 乙酰辅酶A | 厌氧/微氧 | [ |
| 3HP-4HB循环 | 2mol HCO | 4mol ATP + 4mol NAD(P)H | 乙酰辅酶A | 好氧 | [ |
| CO2固定途径 | 固定CO2/HCO | 消耗能量 | 重要产物 | 氧气需求 | 参考文献 |
|---|---|---|---|---|---|
| CBB循环 | 3mol CO2 | 9mol ATP + 6mol NAD(P)H | 3-磷酸甘油醛 | 好氧 | [ |
| RTCA途径 | 2mol CO2 | 2mol ATP + 4mol NAD(P)H | 乙酰辅酶A | 厌氧/微氧 | [ |
| 3-HP双循环 | 3mol HCO | 5mol ATP + 5mol NAD(P)H | 丙酮酸 | 好氧 | [ |
| WL途径 | 2mol CO2 | 1mol ATP + 4mol NAD(P)H | 乙酰辅酶A | 厌氧 | [ |
| DC-4HB循环 | 1mol CO2 + 1mol HCO | 3mol ATP + 4mol NAD(P)H | 乙酰辅酶A | 厌氧/微氧 | [ |
| 3HP-4HB循环 | 2mol HCO | 4mol ATP + 4mol NAD(P)H | 乙酰辅酶A | 好氧 | [ |
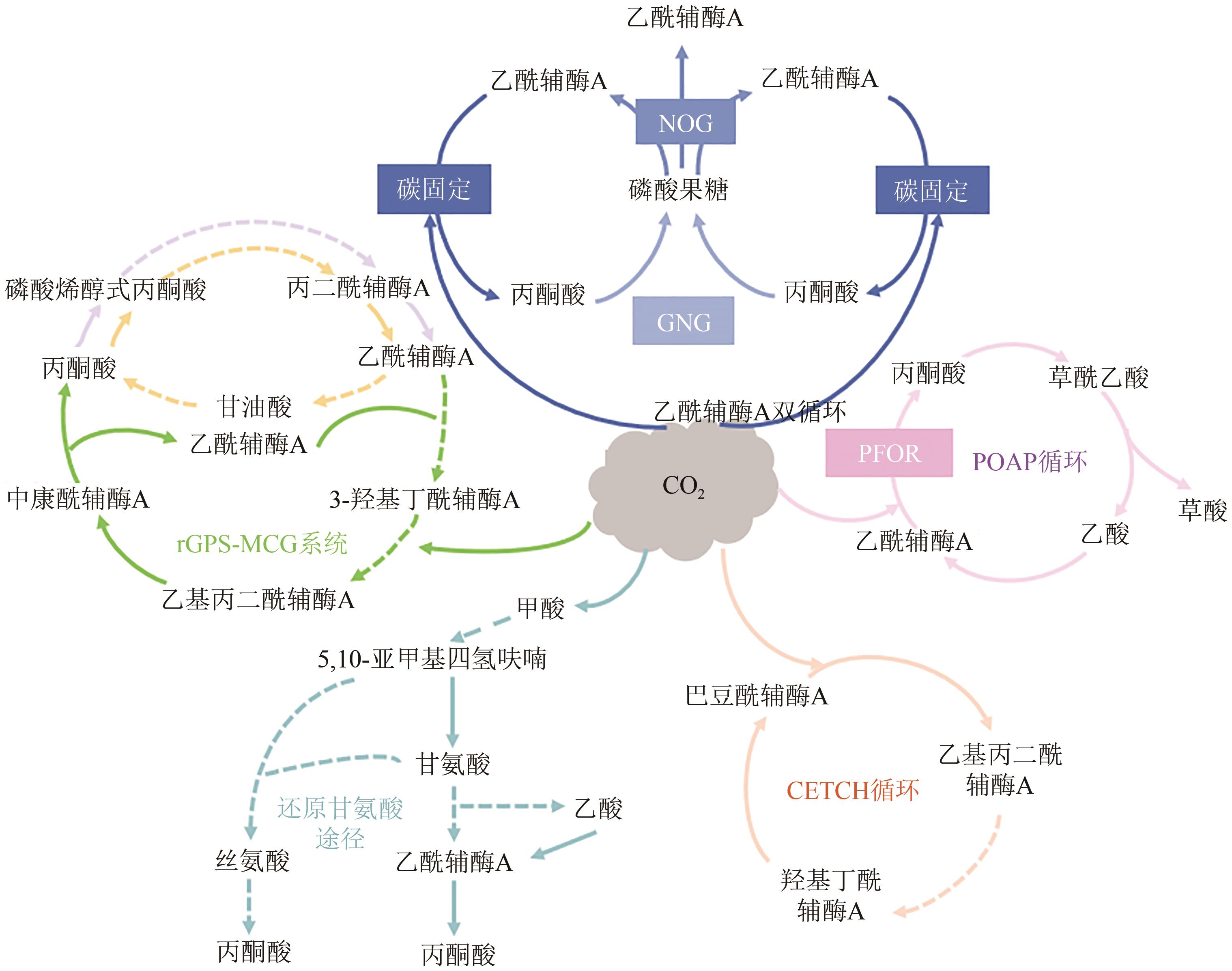
| CO2固定途径 | 固定CO2/HCO3- | 消耗能量 | 固碳效率 | 氧气需求 | 参考文献 |
|---|---|---|---|---|---|
| CETCH循环 | 1mol CO2 | 1mol ATP + 4mol NAD(P)H | 5nmol CO2/(mg 蛋白·min) | 好氧 | [ |
| rGly途径 | 1mol CO2 | 2mol ATP + 4mol NAD(P)H | — | 好氧 | [ |
| POAP循环 | 2mol CO2 | 2mol ATP和1mol NAD(P)H | 8.0nmol CO2/(mg CO2固定酶·min) | 厌氧 | [ |
| acetyl-CoA双循环 | 2mol CO2 | 消耗2mol乙酰辅酶A并生产3mol | — | 厌氧 | [ |
| rGPS-MCG系统 | 2mol HCO3– | 5mol ATP + 5mol NAD(P)H | 28.5nmol CO2/(mg核心蛋白·min) | 好氧/厌氧 | [ |
| CO2固定途径 | 固定CO2/HCO3- | 消耗能量 | 固碳效率 | 氧气需求 | 参考文献 |
|---|---|---|---|---|---|
| CETCH循环 | 1mol CO2 | 1mol ATP + 4mol NAD(P)H | 5nmol CO2/(mg 蛋白·min) | 好氧 | [ |
| rGly途径 | 1mol CO2 | 2mol ATP + 4mol NAD(P)H | — | 好氧 | [ |
| POAP循环 | 2mol CO2 | 2mol ATP和1mol NAD(P)H | 8.0nmol CO2/(mg CO2固定酶·min) | 厌氧 | [ |
| acetyl-CoA双循环 | 2mol CO2 | 消耗2mol乙酰辅酶A并生产3mol | — | 厌氧 | [ |
| rGPS-MCG系统 | 2mol HCO3– | 5mol ATP + 5mol NAD(P)H | 28.5nmol CO2/(mg核心蛋白·min) | 好氧/厌氧 | [ |
| 1 | LIU Z, WANG K, CHEN Y, Third-generation biorefineries as the means to produce fuels and chemicals from CO2 [J]. Nature Catalysis, 2020, 3: 274-288. |
| 2 | HUGHES T P, BAIRD A H, BELLWOOD D R, et al. Climate change, human impacts, and the resilience of coral reefs[J]. Science, 2003, 301(5635): 929-933. |
| 3 | XU X Y, KENTISH S E and MARTIN G J O. Direct air capture of CO2 by microalgae with buoyant beads encapsulating carbonic anhydrase[J]. ACS Sustainable Chemistry and Engineering, 2021, 9(29): 9698-9706. |
| 4 | DE VISSER E, HENDRIKS C, BARRIO M, et al. Dynamis CO2 quality recommendations[J]. International Journal of Greenhouse Gas Control, 2008, 2(4): 478-484. |
| 5 | WALL T F. Combustion processes for carbon capture[J]. Proceedings of the Combustion Institute, 2007, 31(1): 31-47. |
| 6 | BRUNETTI A, SCURA F, BARBIERI G, et al. Membrane technologies for CO2 separation[J]. Journal of Membrane Science, 2010, 359: 115-125. |
| 7 | LEUNG D Y C, CARAMANNA G and MAROTO-VALER M M. An overview of current status of carbon dioxide capture and storage technologies[J]. Renewable and Sustainable Energy Reviews, 2014, 39: 426-443. |
| 8 | LIANG F, ENGLUND E, LINDBERG P, et al. Engineered cyanobacteria with enhanced growth show increased ethanol production and higher biofuel to biomass ratio[J]. Metabolic Engineering, 2018, 46: 51-59. |
| 9 | MOON S Y, HONG S H, KIM T Y, et al. Metabolic engineering of Escherichia coli for the production of malic acid[J]. Biochemical Engineering Journal, 2008, 40: 312-320. |
| 10 | GASSLER T, SAUER M, GASSER B, et al. The industrial yeast Pichia pastoris is converted from a heterotroph into an autotroph capable of growth on CO2 [J]. Nature Biotechnology, 2020, 38: 210-216. |
| 11 | GONG F, ZHU H, ZHANG Y, et al. Biological carbon fixation: From natural to synthetic[J]. Journal of CO2 Utilization, 2018, 28: 221-227. |
| 12 | GONG F, CAI Z, LI Y. Synthetic biology for CO2 fixation[J]. Science China Life Sciences, 2016, 59(11): 1106-1114. |
| 13 | CALVIN M, BENSO A A. The path of carbon in photosynthesis[J]. Science, 1948, 107: 476-480. |
| 14 | ALTAŞ N, ASLAN A S, KARATAŞ E, et al. Heterologous production of extreme alkaline thermostable NAD+- dependent formate dehydrogenase with wide-range pH activity from Myceliophthora thermophila [J]. Process Biochemistry, 2017, 61: 110-118. |
| 15 | HUGLER M, MENENDEZ C, SCHAGGER H, et al. Malonyl-coenzyme A reductase from Chloroflexus aurantiacus, a key enzyme of the 3-hydroxypropionate cycle for autotrophic CO2 fixation[J]. Journal of Bacteriology, 2002, 184(9): 2404-2410. |
| 16 | KUMAR M, SUNDARAM S, GNANSOUNOU E, et al. Carbon dioxide capture, storage and production of biofuel and biomaterials by bacteria: A review[J]. Bioresource Technology, 2018, 247: 1059-1068. |
| 17 | HUBER H, GALLENBERGER M, LAHN U, et al. A dicarboxylate/4-hydroxybutyrate autotrophic carbon assimilation cycle in the hyperthermophilic Archaeum Ignicoccus hospitalis [J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(22): 7851-7856. |
| 18 | BERG I A, KOCKELKORN D, BUCKEL W, et al. A 3-hydroxypropionate/4-hydroxybutyrate autotrophic carbon dioxide assimilation pathway in Archaea [J]. Science, 2007, 318: 1782-1786. |
| 19 | MOHAN S V, MODESTRA J A, AMULYA K, et al. A circular bioeconomy with biobased products from CO2 sequestration[J]. Trends in Biotechnology, 2016, 34(6): 506-519. |
| 20 | SHI L X, THEG S M. The chloroplast protein import system: from algae to trees[J]. BBA Molecular Cell Research, 2013, 1833(2): 314-331. |
| 21 | FUCHS G. Alternative pathways of carbon dioxide fixation: Insights into the early evolution of life?[J]. Annual Review of Microbiology, 2011, 65: 631-658. |
| 22 | JAJESNIAK P, ALI H E M O and WONG T S. Carbon dioxide capture and utilization using biological systems: opportunities and challenges[J]. Journal of Bioprocessing and Biotechniques, 2014, 4(3): 1000155. |
| 23 | ZHAO T, LI Y, ZHANG Y. Biological carbon fixation: A thermodynamic perspective[J]. Green Chemistry, 2021, 23(20): 7852-7864. |
| 24 | BARENHOLZ U, DAVIDI D, REZNIK E, et al. Design principles of autocatalytic cycles constrain enzyme kinetics and force low substrate saturation at flux branch points[J]. Elife, 2017, 6: e20667. |
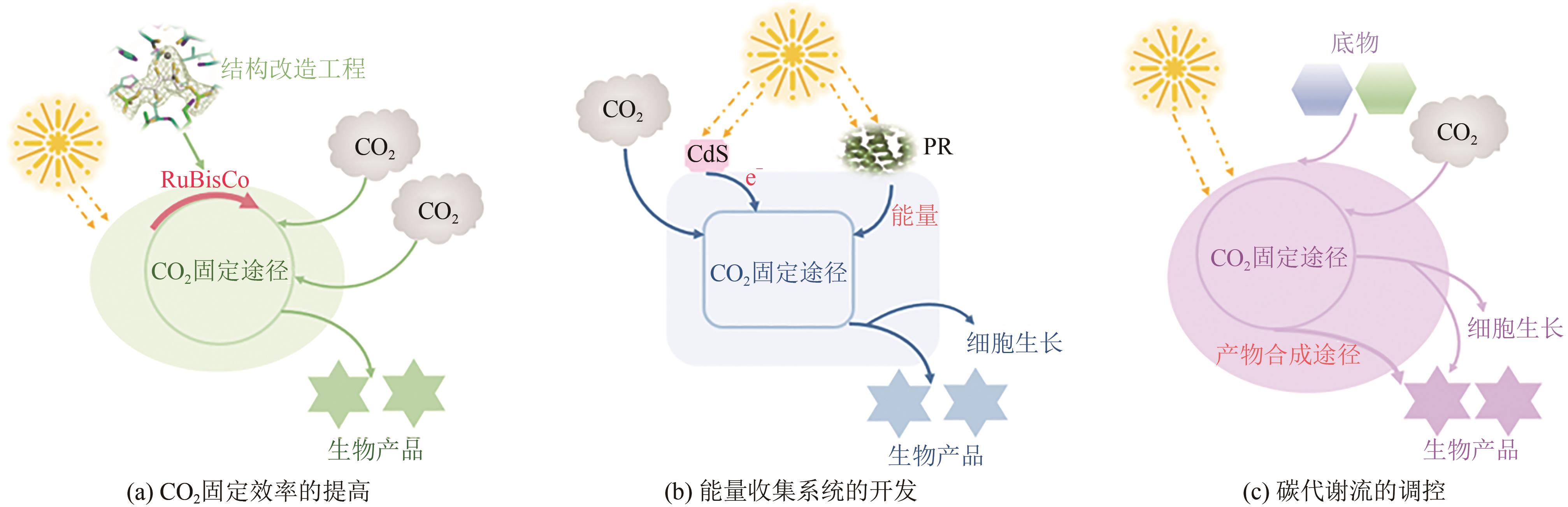
| 25 | HU G, LI Y, YE C, et al. Engineering microorganisms for enhanced CO2 sequestration[J]. Trends in Biotechnology, 2019, 37(5): 532-547. |
| 26 | LIANG F, LINDBLAD P. Synechocystis PCC 6803 overexpressing RuBisCo grow faster with increased photosynthesis[J]. Metabolic Engineering Communications, 2017, 4: 29-36. |
| 27 | ANDREWS T J, LORIMER G H. Rubisco structure, mechanisms, and prospects for improvement[J]. The Biochemistry of Plants, 1987, 10: 131-218. |
| 28 | NISHITANI Y, YOSHIDA S, FUJIHASHI M, et al. Structure-based catalytic optimization of a type Ⅲ RuBisCo from a hyperthermophile[J]. The Journal of Biological Chemistry, 2010, 285(50): 39339-39347. |
| 29 | DURAO P, AIGNER H, NAGY P, et al. Opposing effects of folding and assembly chaperones on evolvability of Rubisco[J]. Nature Chemical Biology, 2015, 11(2): 148-155. |
| 30 | YANG F, ZHANG J, CAI Z, et al. Exploring the oxygenase function of Form II Rubisco for production of glycolate from CO2 [J]. AMB Express, 2021, 11(65). |
| 31 | ZHOU J, ZHANG F, MENG H, et al. Introducing extra NADPH consumption ability significantly increases the photosynthetic efficiency and biomass production of cyanobacteria[J]. Metabolic Engineering, 2016, 38: 217-227. |
| 32 | BAR-EVEN A. Daring metabolic designs for enhanced plant carbon fixation[J]. Plant Science, 2018, 273: 71-83. |
| 33 | BAR-EVEN A, NOOR E, FLAMHOLZ A, et al. Design and analysis of metabolic pathways supporting formatotrophic growth for electricity-dependent cultivation of microbes[J]. Biochimica et Biophysica Acta, 2013, 1827(8-9): 1039-1047. |
| 34 | BAR-EVEN A, NOOR E, LEWIS N E, et al. Design and analysis of synthetic carbon fixation pathways[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(19): 8889-8894. |
| 35 | DEVI M P, MOHAN S V. CO2 supplementation to domestic wastewater enhances microalgae lipid accumulation under mixotrophic microenvironment: Effect of sparging period and interval[J]. Bioresource Technology, 2012, 112: 116-123. |
| 36 | PALOVAARA J, AKRAM N, BALTAR F, et al. Stimulation of growth by proteorhodopsin phototrophy involves regulation of central metabolic pathways in marine planktonic bacteria[J]. Proceedings of the Combustion Institute, 2014, 111(35): E3650-3658. |
| 37 | CHEN Q, VAN DER STEEN J B, DEKKER H L, et al. Expression of holo-proteorhodopsin in Synechocystis sp. PCC 6803[J]. Metabolic Engineering, 2016, 35: 83-94. |
| 38 | KIRST H, FORMIGHIERI C, MELIS A. Maximizing photosynthetic efficiency and culture productivity in cyanobacteria upon minimizing the phycobilisome light-harvesting antenna size[J]. Biochimica et Biophysica Acta, 2014, 1837(10): 1653-1664. |
| 39 | GARTZIA-RIVERO L, BANUELOS J, LOPEZ-ARBELOA I. Photoactive nanomaterials inspired by nature: ltl zeolite doped with laser dyes as artificial light harvesting systems[J]. Materials, 2017, 10(5): ma10050495. |
| 40 | CHOWDHURY F A, TRUDEAU M L, GUO H, et al. A photochemical diode artificial photosynthesis system for unassisted high efficiency overall pure water splitting[J]. Nature Communications, 2018, 9: 1707. |
| 41 | ZHANG X, WU Z, ZHANG X, et al. Highly selective and active CO2 reduction electrocatalysts based on cobalt phthalocyanine/carbon nanotube hybrid structures[J]. Nature Communications, 2017, 8: 14675. |
| 42 | SAKIMOTO K K, WONG A B, YANG P D. Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production[J]. Science, 2016, 351(6268): 74-77. |
| 43 | DEMPO Y, OHTA E, NAKAYAMA Y, et al. Molar-based targeted metabolic profiling of cyanobacterial strains with potential for biological production[J]. Metabolites, 2014, 4(2): 499-516. |
| 44 | ANGERMAYR S A, GORCHS ROVIRA A, HELLINGWERF K J. Metabolic engineering of cyanobacteria for the synthesis of commodity products[J]. Trends in Biotechnology, 2015, 33(6): 352-361. |
| 45 | KUDOH K, KAWANO Y, HOTTA S, et al. Prerequisite for highly efficient isoprenoid production by cyanobacteria discovered through the over-expression of 1-deoxy-d-xylulose 5-phosphate synthase and carbon allocation analysis[J]. Journal of Bioscience and Bioengineering, 2014, 118(1): 20-28. |
| 46 | NGAN C Y, WONG C H, CHOI C, et al. Lineage-specific chromatin signatures reveal a regulator of lipid metabolism in microalgae[J]. Nature Plants, 2015, 1: 15107. |
| 47 | MATSON M M, ATSUMI S. Photomixotrophic chemical production in cyanobacteria[J]. Current Opinion in Biotechnology, 2018, 50: 65-71. |
| 48 | CLAASSENS N J, SOUSA D Z, DOS SANTOS V A, et al. Harnessing the power of microbial autotrophy[J]. Nature Reviews Microbiology, 2016, 14(11): 692-706. |
| 49 | HENARD C A, SMITH H, DOWE N, et al. Bioconversion of methane to lactate by an obligate methanotrophic bacterium[J]. Scientific Reports, 2016, 6: 21585. |
| 50 | SCHWANDER T, BORZYSKOWSKI L S, BURGENER S, et al. A synthetic pathway for the fixation of carbon dioxide in vitro[J]. Science, 2016, 354(6314): 900-904. |
| 51 | BROWN S H, BASHKIROVA L, BERKA R, et al. Metabolic engineering of Aspergillus oryzae NRRL 3488 for increased production of L-malic acid[J]. Applied Microbiology and Biotechnology, 2013, 97(20): 8903-8912. |
| 52 | KUPRIYANOVA E, VILLAREJO A, MARKELOVA A, et al. Extracellular carbonic anhydrases of the stromatolite-forming cyanobacterium Microcoleus chthonoplastes [J]. Microbiology, 2007, 153(4): 1149-1156. |
| 53 | BONACCI W, TENG P K, AFONSO B, et al. Modularity of a carbon-fixing protein organelle[J]. Proceedings of the Combustion Institute, 2012, 109(2): 478-483. |
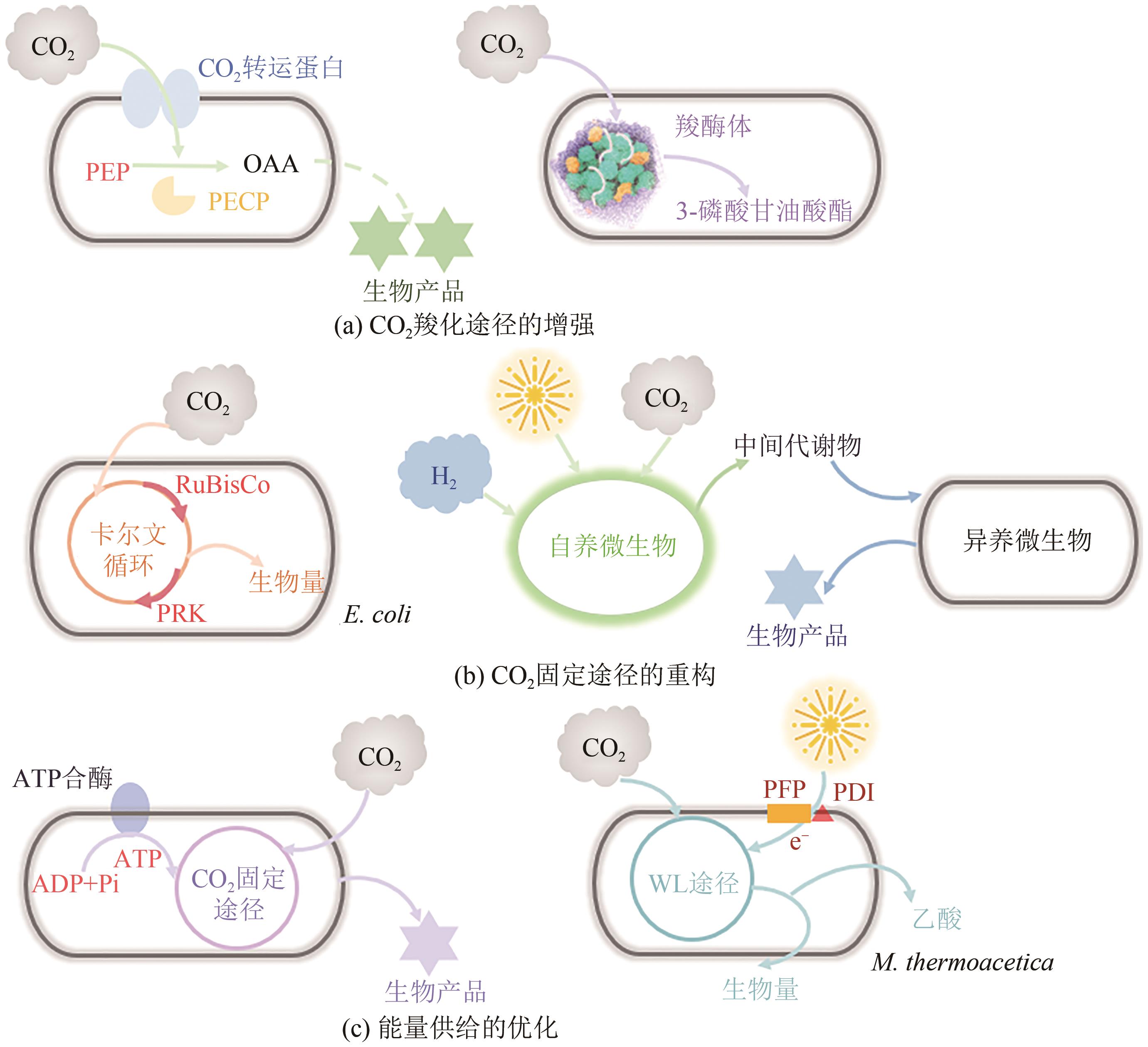
| 54 | ZHANG Y, ZHOU J, ZHANG Y, et al. Auxiliary module promotes the synthesis of carboxysomes in E. coli to achieve high-efficiency CO2 assimilation[J]. ACS Synthetic Biology, 2021, 10(4): 707-715. |
| 55 | MATTOZZI M, ZIESACK M, VOGES M J, et al. Expression of the sub-pathways of the Chloroflexus aurantiacus 3-hydroxypropionate carbon fixation bicycle in E. coli: Toward horizontal transfer of autotrophic growth[J]. Metabolic Engineering, 2013, 16: 130-139. |
| 56 | GONG F, LIU G, ZHAI X, et al. Quantitative analysis of an engineered CO2-fixing Escherichia coli reveals great potential of heterotrophic CO2 fixation[J]. Biotechnology for Biofuels, 2015, 8: 86. |
| 57 | ANTONOVSKY N, GLEIZER S, NOOR E, et al. Sugar synthesis from CO2 in Escherichia coli [J]. Cell, 2016, 166(1): 115-125. |
| 58 | FRADINHO J C, DOMINGOS J M, CARVALHO G, et al. Polyhydroxyalkanoates production by a mixed photosynthetic consortium of bacteria and algae[J]. Bioresource Technology, 2013, 132: 146-153. |
| 59 | SAID S BEN, TECON R, BORER B, et al. The engineering of spatially linked microbial consortia-potential and perspectives[J]. Current Opinion in Biotechnology, 2020, 62: 137-145. |
| 60 | HU P, CHAKRABORTY S, KUMAR A, et al. Integrated bioprocess for conversion of gaseous substrates to liquids[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(14): 3773-3778. |
| 61 | LOWE H, HOBMEIER K, MOOS M, et al. Photoautotrophic production of polyhydroxyalkanoates in a synthetic mixed culture of Synechococcus elongatus cscB and Pseudomonas putida cscAB [J]. Biotechnology for Biofuels, 2017, 10(190): 1-11. |
| 62 | TONG T, CHEN X, HU G, et al. Engineering microbial metabolic energy homeostasis for improved bioproduction[J]. Biotechnology Advances, 2021, 53: 107841. |
| 63 | BRAAKMAN R, SMITH E. Metabolic evolution of a deep-branching hyperthermophilic chemoautotrophic bacterium[J]. PLoS One, 2014, 9(2): e87950. |
| 64 | GUADALUPE-MEDINA V, WISSELINK H W, LUTTIK M A, et al. Carbon dioxide fixation by Calvin-Cycle enzymes improves ethanol yield in yeast[J]. Biotechnology for Biofuels, 2013, 6: 125. |
| 65 | HU G, ZHOU J, CHEN X, et al. Engineering synergetic CO2-fixing pathways for malate production[J]. Metabolic Engineering, 2018, 47: 496-504. |
| 66 | LIEW F, HENSTRA A M, KPKE M, et al. Metabolic engineering of Clostridium autoethanogenum for selective alcohol production[J]. Metabolic Engineering, 2017, 40: 104-114. |
| 67 | WU Z, WANG J, LIU J, et al. Engineering an electroactive Escherichia coli for the microbial electrosynthesis of succinate from glucose and CO2 [J]. Microbial Cell Factories, 2019, 18: 15. |
| 68 | HU G, LI Z, MA D, et al. Light-driven CO2 sequestration in Escherichia coli to achieve theoretical yield of chemicals[J]. Nature Catalysis, 2021, 4(5): 395-406. |
| 69 | GAI P, YU W, ZHAO H, et al. Solar-powered organic semiconductor-bacteria biohybrids for CO2 reduction into acetic acid[J]. Angewandte Chemie International Edition, 2020, 59(18): 7224-7229. |
| 70 | JONES S W, FAST A G, CARLSON E D, et al. CO2 fixation by anaerobic non-photosynthetic mixotrophy for improved carbon conversion[J]. Nature Communications, 2016, 7: 12800. |
| 71 | HU L, GUO S, WANG B, et al. Bio-valorization of C1 gaseous substrates into bioalcohols: Potentials and challenges in reducing carbon emissions[J]. Biotechnology Advances, 2022, 59: 107954. |
| 72 | XIAO L, LIU G, GONG F, et al. A Minimized synthetic carbon fixation cycle[J]. ACS Catalysis, 2021, 12(1): 799-808. |
| 73 | WU C, LO J, URBAN C, et al. Acetyl-CoA synthesis through a bicyclic carbon-fixing pathway in gas-fermenting bacteria[J]. Nature Synthesis, 2022, 1(8): 615-625. |
| 74 | LUO S, LIN P P, NIEH L Y, et al. A cell-free self-replenishing CO2-fixing system[J]. Nature Catalysis, 2022, 5(2): 154-162. |
| 75 | YISHAI O, BOUZON M, DORING V, et al. In vivo assimilation of one-carbon via a synthetic reductive glycine pathway in Escherichia coli [J]. ACS Synthetic Biology, 2018, 7(9): 2023-2028. |
| 76 | PAVAN M, REINMETS K, GARG S, et al. Advances in systems metabolic engineering of autotrophic carbon oxide-fixing biocatalysts towards a circular economy[J]. Metabolic Engineering, 2022, 71: 117-141. |
| 77 | RODRIGUES R M, GUAN X, IÑIGUEZ J A, et al. Perfluorocarbon nanoemulsion promotes the delivery of reducing equivalents for electricity-driven microbial CO2 reduction[J]. Nature Catalysis, 2019, 2(5): 407-414. |
| 78 | TREMBLAY P L, XU M, CHEN Y, et al. Nonmetallic abiotic-biological hybrid photocatalyst for visible water splitting and carbon dioxide reduction[J]. iScience, 2020, 23(1): 100784. |
| 79 | LI H, OPGENORTH P H, WERNICK D G, et al. Integrated electromicrobial conversion of CO2 to higher slcohols[J]. Science, 2012, 335(6076): 1596. |
| 80 | MILLER T E, BENEYTON T, SCHWANDER T, et al. Light-powered CO2 fixation in a chloroplast mimic with natural and synthetic parts[J]. Science, 2020, 368(6491): 649-654. |
| 81 | GLEIZER S, BEN-NISSAN R, BAR-ON Y M, et al. Conversion of Escherichia coli to generate all biomass carbon from CO2 [J]. Cell, 2019, 179(6): 1255-1263. |
| 82 | PENA D A, GASSER B, ZANGHELLINI J, et al. Metabolic engineering of Pichia pastoris [J]. Metabolic Engineering, 2018, 50: 2-15. |
| [1] | GUO Feng, ZHANG Shangjie, JIANG Yujia, JIANG Wankui, XIN Fengxue, ZHANG Wenming, JIANG Min. Biotransformation of one-carbon resources by yeast [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 30-39. |
| [2] | LIU Yali, ZHANG Hongwei, KANG Xiaorong. Effect and mechanisms of microplastics on anaerobic digestion of sludge [J]. Chemical Industry and Engineering Progress, 2022, 41(9): 5037-5046. |
| [3] | LI Ling, YU Yong, HU Yonghong. Research progress in production of lipstatinfermentation [J]. Chemical Industry and Engineering Progress, 2021, 40(4): 2251-2257. |
| [4] | GUO Liang, GAO Cong, ZHANG Li, CHEN Xiulai, LIU Liming. Advances in the suitability of artificial metabolic pathways [J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1252-1261. |
| [5] | ZHOU Zikang, XU Ping. Application and progress of global transcription regulation in microbial cell factory construction [J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1248-1251. |
| [6] | GAO Cong, GUO Liang, HU Guipeng, CHEN Xiulai, LIU Liming. Advances of metabolic flux regulation in microbial cell factories [J]. Chemical Industry and Engineering Progress, 2021, 40(12): 6807-6817. |
| [7] | Hong CHEN, Jun WU, Chen CHEN, Zhi TU, Li’e YU, Enzhe YANG, Min YANG, Benyi XIAO. Advances in biohydrogen production from anaerobic co-fermentation of organic wastes [J]. Chemical Industry and Engineering Progress, 2021, 40(1): 440-450. |
| [8] | Liju BAI, Bo HOU, Bo JIANG, Siming CHEN. Research progress of CO2 fixation by chemical absorbents enhanced microalgae [J]. Chemical Industry and Engineering Progress, 2020, 39(S2): 106-114. |
| [9] | Yan JIANG,Heping ZHOU,Zhe ZHANG,Hongbing LIU,Shunxiang SHEN. Bioremediation of contaminated sites by petroleum hydrocarbon under low temperature environment [J]. Chemical Industry and Engineering Progress, 2020, 39(2): 419-428. |
| [10] | Taotao TANG,Jiang LI,Zhao YANG,Fuliang XIANG,Yuehu WANG,Yancheng LI. Research progress in on microbial community structure of anaerobic digestion of sludge [J]. Chemical Industry and Engineering Progress, 2020, 39(1): 320-328. |
| [11] | Lu CHEN,Dingyu LIU,Baowei WANG,Yu jiao ZHAO,Guangtao JIA,Tao CHEN,Zhiwen WANG. Advances in acetyl coenzyme A metabolic engineering with Escherichia coli [J]. Chemical Industry and Engineering Progress, 2019, 38(9): 4218-4226. |
| [12] | Pengcheng CHANG, Yang YU, Ying WANG, Chun LI. Combinatorial regulation strategies for efficient synthesis of terpenoids in Saccharomyces cerevisiae [J]. Chemical Industry and Engineering Progress, 2019, 38(01): 598-605. |
| [13] | CHENG Shen, ZHANG Songhong, YUN Junxian. Recent advances in microbial synthesis of α-ketoisocaproate [J]. Chemical Industry and Engineering Progress, 2018, 37(12): 4821-4829. |
| [14] | YU Xinlei, MAO Yufeng, ZHANG Xiaoxia, LU Lingxue, WANG Zhiwen, CHEN Tao. Recent progress in microbial production of 3-hydroxypropionic acid [J]. Chemical Industry and Engineering Progress, 2018, 37(11): 4427-4436. |
| [15] | TANG Ruiqi, XIONG Liang, CHENG Cheng, ZHAO Xinqing, BAI Fengwu. Progress of research on construction and optimization of recombinant Saccharomyces cerevisiae strains for cellulosic ethanol production [J]. Chemical Industry and Engineering Progress, 2018, 37(08): 3119-3128. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||