Chemical Industry and Engineering Progress ›› 2022, Vol. 41 ›› Issue (S1): 260-268.DOI: 10.16085/j.issn.1000-6613.2022-1150
• Materials science and technology • Previous Articles Next Articles
Proton transport in metal-organic frameworks and their applications in proton exchange membranes
GAO Weitao( ), YIN Qinan, TU Ziqiang, GONG Fan, LI Yang, XU Hong, WANG Cheng(
), YIN Qinan, TU Ziqiang, GONG Fan, LI Yang, XU Hong, WANG Cheng( ), MAO Zongqiang
), MAO Zongqiang
- Institute of Nuclear and New Energy Technology, Tsinghua University, Beijing 100084, China
-
Received:2022-06-20Revised:2022-08-03Online:2022-11-10Published:2022-10-20 -
Contact:WANG Cheng
金属有机框架材料中的质子传导及其在质子交换膜中的应用
高帷韬( ), 殷屺男, 涂自强, 龚繁, 李阳, 徐宏, 王诚(
), 殷屺男, 涂自强, 龚繁, 李阳, 徐宏, 王诚( ), 毛宗强
), 毛宗强
- 清华大学核能与新能源技术研究院,北京 100084
-
通讯作者:王诚 -
作者简介:高帷韬(1996—),男,博士研究生,研究方向为燃料电池。E-mail:gwt19@mails.tsinghua.edu.cn。 -
基金资助:国家重点研发计划(2018YFE0202000)
CLC Number:
Cite this article
GAO Weitao, YIN Qinan, TU Ziqiang, GONG Fan, LI Yang, XU Hong, WANG Cheng, MAO Zongqiang. Proton transport in metal-organic frameworks and their applications in proton exchange membranes[J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 260-268.
高帷韬, 殷屺男, 涂自强, 龚繁, 李阳, 徐宏, 王诚, 毛宗强. 金属有机框架材料中的质子传导及其在质子交换膜中的应用[J]. 化工进展, 2022, 41(S1): 260-268.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-1150
| 传导方式 | 优势 | 举例 | |
|---|---|---|---|
| 借助功能客体分子进行质子传导 | 主体MOF和客体分子之间能够形成协同质子传导;可通过选择不同的客体分子构建额外的氢键网络 | 将功能化铜基金属-有机多面体引入介孔PCN-777[ | (80℃/90% RH) |
| 酸化Ni-MOF-74[ | (80℃/95% RH) | ||
| 借助反离子进行质子传导 | 带电的反离子可促进连续氢键网络的形成 | Fe(THO)·Fe(SO4)(DMA)3[ | (25℃/98% RH) |
| {[(Me2NH2)3(SO4)]2[Zn2(ox)3]} n[ | (25℃/98% RH) | ||
| 借助有机配体中的非配位官能团进行质子传导 | 能够通过控制配体中的官能团,来实现对质子电导率的良好调节和精准控制 | MIL-53-(Fe)-(COOH)2[ | (80℃/98% RH) |
| BUT-8(Cr)A[ | (80℃/100% RH) | ||
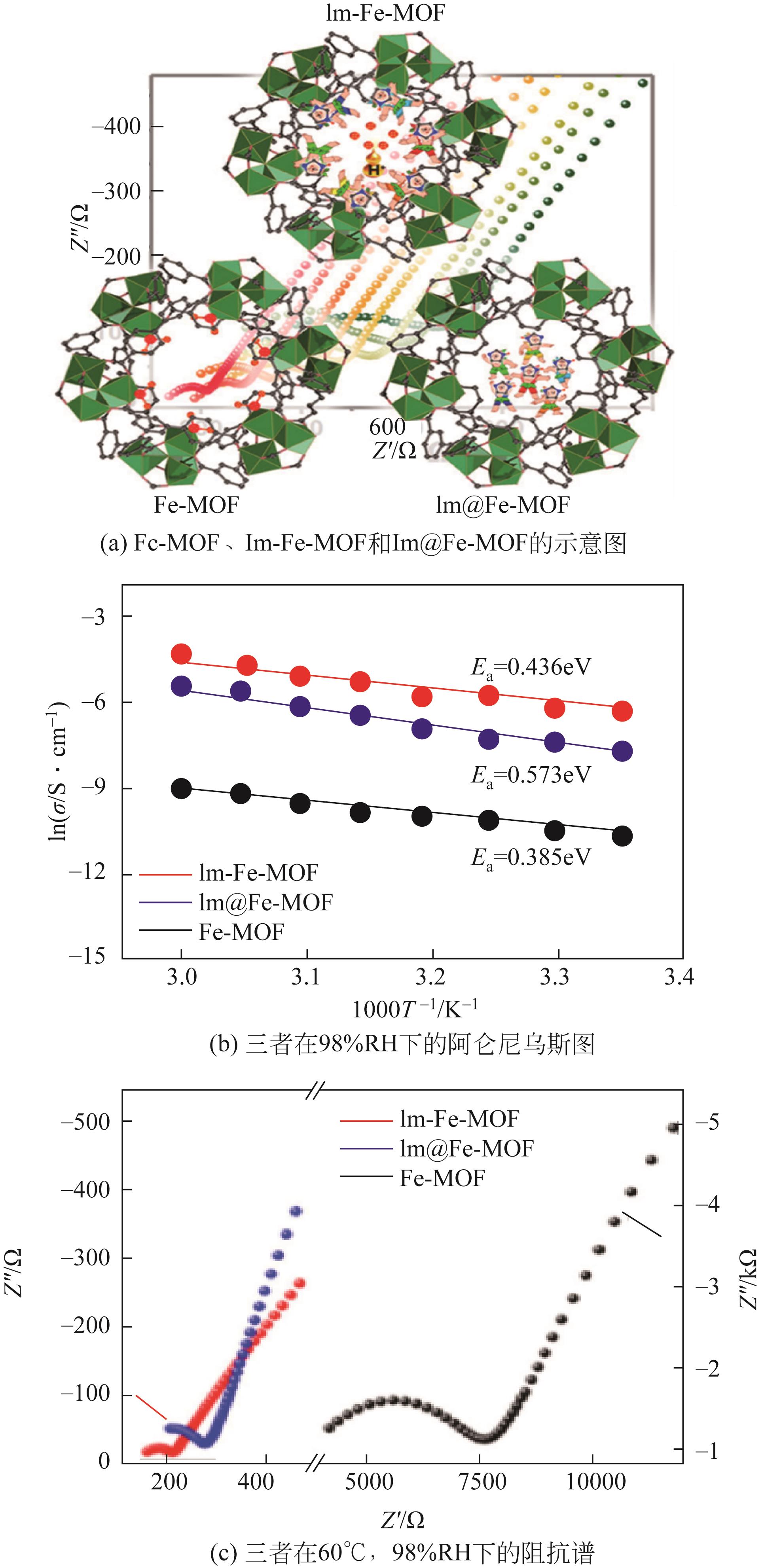
| 借助与金属中心配位的功能分子进行质子传导 | 功能分子能够被固定于金属中心的空配位,从而形成规则排列的质子传导路径 | Im-Fe-MOF (咪唑配位的Fe-MOF)[ | (60℃/98% RH) |
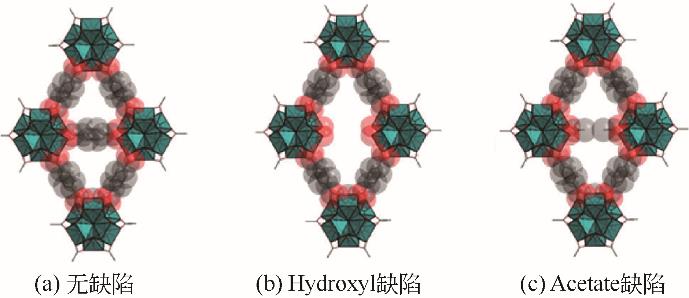
| 借助MOFs中的缺陷来促进质子传导 | 缺陷能够形成促进质子传导的特殊位点(路易斯酸位点、质子捕获位点等);同时缺陷能够增加孔道体积,从而增加质子的迁移率 | 磺化UIO-66[ | (65℃/95% RH) |
| Zr6O4(OH)6(O2C-C6H4-CO2)5[ | (65℃/95% RH) | ||
| 传导方式 | 优势 | 举例 | |
|---|---|---|---|
| 借助功能客体分子进行质子传导 | 主体MOF和客体分子之间能够形成协同质子传导;可通过选择不同的客体分子构建额外的氢键网络 | 将功能化铜基金属-有机多面体引入介孔PCN-777[ | (80℃/90% RH) |
| 酸化Ni-MOF-74[ | (80℃/95% RH) | ||
| 借助反离子进行质子传导 | 带电的反离子可促进连续氢键网络的形成 | Fe(THO)·Fe(SO4)(DMA)3[ | (25℃/98% RH) |
| {[(Me2NH2)3(SO4)]2[Zn2(ox)3]} n[ | (25℃/98% RH) | ||
| 借助有机配体中的非配位官能团进行质子传导 | 能够通过控制配体中的官能团,来实现对质子电导率的良好调节和精准控制 | MIL-53-(Fe)-(COOH)2[ | (80℃/98% RH) |
| BUT-8(Cr)A[ | (80℃/100% RH) | ||
| 借助与金属中心配位的功能分子进行质子传导 | 功能分子能够被固定于金属中心的空配位,从而形成规则排列的质子传导路径 | Im-Fe-MOF (咪唑配位的Fe-MOF)[ | (60℃/98% RH) |
| 借助MOFs中的缺陷来促进质子传导 | 缺陷能够形成促进质子传导的特殊位点(路易斯酸位点、质子捕获位点等);同时缺陷能够增加孔道体积,从而增加质子的迁移率 | 磺化UIO-66[ | (65℃/95% RH) |
| Zr6O4(OH)6(O2C-C6H4-CO2)5[ | (65℃/95% RH) | ||
| 常见应用方法 | 优势 | 劣势 | 举例 | |
|---|---|---|---|---|
| MOFs掺入高分子质子交换膜 | 方法简单,制备快速,能够大规模生产 | MOFs的添加量受限,且容易聚集,导致膜的透氢电流升高,机械性能下降 | 质量分数为1%的硫酸化的Zr-MOF-808掺入 Nafion中制得Nafion-SZM复合膜[ | (80℃/35% RH) |
| 质量分数为2%的200nm粒径的UIO-66与 Nafion制成复合质子交换膜[ | (110℃/95% RH) | |||
| 质量分数为2.5%的磷酸掺杂的HKUST-1与 Nafion制成复合质子交换膜[ | (25℃/100% RH) | |||
| 质量分数为10%的UIO-66与PBI制成复合质子交换膜[ | (160℃) | |||
| MOFs作为质子传导的主要介质组成MOFs&高分子复合膜 | 成本低廉,MOFs添加量较大 | 与现有高分子质子交换膜相比,其在耐久性和质子电导率等方面存在差距 | {[Ca(D-Hpmpc)(H2O)2]·2HO0.5} n 和50% 的PVP制备成复合质子交换膜[ | (25℃/97% RH) |
| 质量分数为55%的MOF-808与PVDF制成的复合质子交换膜[ | (42℃/99% RH) | |||
| (NH4)3Zr(H2/3PO4)3与10%质量分数的PVDF混合制备的复合质子交换膜[ | (180℃/0% RH) | |||
| 基于新型膜制备技术的MOFs质子交换膜 | 可具有一些特殊优势,例如克服高分子膜长程无序的缺陷 | 制备周期长,难以满足实际生产要求 | 吸附1-(3-氨基丙基)-咪唑的Fe-MIL-101-NH2与磺化聚苯醚键连制备出的高温质子交换膜[ | (160℃/0.15% RH) |
| 质量分数为10%的氨基磺酸掺杂的MIL-101与PVDF和PVP共纺丝制成复合膜[ | (160℃/0% RH) | |||
| 纤维素与UiO-66-NH2的共混纳米纤维复合磺化聚砜基体制备的复合膜[ | (80℃/100% RH) | |||
| 常见应用方法 | 优势 | 劣势 | 举例 | |
|---|---|---|---|---|
| MOFs掺入高分子质子交换膜 | 方法简单,制备快速,能够大规模生产 | MOFs的添加量受限,且容易聚集,导致膜的透氢电流升高,机械性能下降 | 质量分数为1%的硫酸化的Zr-MOF-808掺入 Nafion中制得Nafion-SZM复合膜[ | (80℃/35% RH) |
| 质量分数为2%的200nm粒径的UIO-66与 Nafion制成复合质子交换膜[ | (110℃/95% RH) | |||
| 质量分数为2.5%的磷酸掺杂的HKUST-1与 Nafion制成复合质子交换膜[ | (25℃/100% RH) | |||
| 质量分数为10%的UIO-66与PBI制成复合质子交换膜[ | (160℃) | |||
| MOFs作为质子传导的主要介质组成MOFs&高分子复合膜 | 成本低廉,MOFs添加量较大 | 与现有高分子质子交换膜相比,其在耐久性和质子电导率等方面存在差距 | {[Ca(D-Hpmpc)(H2O)2]·2HO0.5} n 和50% 的PVP制备成复合质子交换膜[ | (25℃/97% RH) |
| 质量分数为55%的MOF-808与PVDF制成的复合质子交换膜[ | (42℃/99% RH) | |||
| (NH4)3Zr(H2/3PO4)3与10%质量分数的PVDF混合制备的复合质子交换膜[ | (180℃/0% RH) | |||
| 基于新型膜制备技术的MOFs质子交换膜 | 可具有一些特殊优势,例如克服高分子膜长程无序的缺陷 | 制备周期长,难以满足实际生产要求 | 吸附1-(3-氨基丙基)-咪唑的Fe-MIL-101-NH2与磺化聚苯醚键连制备出的高温质子交换膜[ | (160℃/0.15% RH) |
| 质量分数为10%的氨基磺酸掺杂的MIL-101与PVDF和PVP共纺丝制成复合膜[ | (160℃/0% RH) | |||
| 纤维素与UiO-66-NH2的共混纳米纤维复合磺化聚砜基体制备的复合膜[ | (80℃/100% RH) | |||
| 1 | JIAO Kui, XUAN Jin, DU Qing, et al. Designing the next generation of proton-exchange membrane fuel cells[J]. Nature, 2021, 595(7867): 361-369. |
| 2 | GAO Weitao, HU Zunyan, HUANG Haiyan, et al. All-condition economy evaluation method for fuel cell systems: System efficiency contour map[J]. eTransportation, 2021, 9: 100127. |
| 3 | 高帷韬, 雷一杰, 张勋, 等. 质子交换膜燃料电池研究进展[J]. 化工进展, 2022, 41(3): 1539-1555. |
| GAO Weitao, LEI Yijie, ZHANG Xun, et al. An overview of proton exchange membrane fuel cell[J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1539-1555. | |
| 4 | 梁茜, 王诚, 雷一杰, 等. 金属有机框架材料在质子交换膜燃料电池中的潜在应用[J]. 化学进展, 2018, 30(11): 1770-1783. |
| LIANG Xi, WANG Cheng, LEI Yijie, et al. Potential applications of metal organic framework-based materials for proton exchange membrane fuel cells[J]. Progress in Chemistry, 2018, 30(11): 1770-1783. | |
| 5 | HICKNER M A, GHASSEMI H, KIM Y S, et al. Alternative polymer systems for proton exchange membranes (PEMs)[J]. Chemical Reviews, 2004, 104(10): 4587-4611. |
| 6 | KARIMI M B, MOHAMMADI F, HOOSHYARI K. Recent approaches to improve Nafion performance for fuel cell applications: A review[J]. International Journal of Hydrogen Energy, 2019, 44(54): 28919-28938. |
| 7 | 吴魁, 解东来. 高温质子交换膜研究进展[J]. 化工进展, 2012, 31(10): 2202-2206, 2220. |
| WU Kui, XIE Donglai. Research progress in high temperature proton exchange membranes[J]. Chemical Industry and Engineering Progress, 2012, 31(10): 2202-2206, 2220. | |
| 8 | 伍斌. MOFs基质子交换膜的制备及性能研究[D]. 合肥: 中国科学技术大学, 2015. |
| WU Bin. The preparation and charactrizations of MOFs based proton exchange membranes[D]. Hefei: University of Science and Technology of China, 2015. | |
| 33 | GUI Daxiang, DAI Xing, TAO Zetian, et al. Unique proton transportation pathway in a robust inorganic coordination polymer leading to intrinsically high and sustainable anhydrous proton conductivity[J]. Journal of the American Chemical Society, 2018, 140(19): 6146-6155. |
| 34 | WU Bin, LIN Xiaocheng, GE Liang, et al. A novel route for preparing highly proton conductive membrane materials with metal-organic frameworks[J]. Chemical Communications, 2013, 49(2): 143-145. |
| 35 | WU Bin, LIANG Ge, LIN Xiaocheng, et al. Immobilization of N-(3-aminopropyl)-imidazole through MOFs in proton conductive membrane for elevated temperature anhydrous applications[J]. Journal of Membrane Science, 2014, 458: 86-95. |
| 36 | BAI Zhongxiong, LIU Shucheng, CHEN Ping, et al. Enhanced proton conduction of imidazole localized in one-dimensional Ni-metal-organic framework nanofibers[J]. Nanotechnology, 2020, 31(12): 125702. |
| 37 | DOU Yibo, ZHANG Wenjing, KAISER Andreas. Electrospinning of metal-organic frameworks for energy and environmental applications[J]. Advanced Science, 2020, 7(3): 1902590. |
| 38 | SUN Lian, GU Quanchao, WANG Honglei, et al. Anhydrous proton conductivity of electrospun phosphoric acid-doped PVP-PVDF nanofibers and composite membranes containing MOF fillers[J]. RSC Advances, 2021, 11(47): 29527-29536. |
| 39 | WANG Shubo, LIN Yuan, YANG Jian, et al. UiO-66-NH2 functionalized cellulose nanofibers embedded in sulfonated polysulfone as proton exchange membrane[J]. International Journal of Hydrogen Energy, 2021, 46(36): 19106-19115. |
| 40 | DAI Xiu, LI Xu, WANG Xinlong. Morphology controlled porous poly(lactic acid)/zeolitic imidazolate framework-8 fibrous membranes with superior PM2.5 capture capacity[J]. Chemical Engineering Journal, 2018, 338: 82-91. |
| 9 | AGMON Noam. The grotthuss mechanism[J]. Chemical Physics Letters, 1995, 244(5/6): 456-462. |
| 10 | KREUER K D, RABENAU A, WEPPNER W. Vehicle mechanism, a new model for the interpretation of the conductivity of fast proton conductors[J]. Angewandte Chemie International Edition in English, 1982, 21(3): 208-209. |
| 11 | LIM D W, KITAGAWA H. Proton transport in metal-organic frameworks[J]. Chemical Reviews, 2020, 120(16): 8416-8467. |
| 12 | LEE J, LIM D W, DEKURA S, et al. MOP × MOF: Collaborative combination of metal-organic polyhedra and metal-organic framework for proton conductivity[J]. ACS Applied Materials & Interfaces, 2019, 11(13): 12639-12646. |
| 13 | PHANG W J, LEE W R, YOO K, et al. pH-dependent proton conducting behavior in a metal-organic framework material[J]. Angewandte Chemie, 2014, 126(32): 8523-8527. |
| 14 | WEI Yongsheng, HU Xiaopeng, HAN Zhen, et al. Unique proton dynamics in an efficient MOF-based proton conductor[J]. Journal of the American Chemical Society, 2017, 139(9): 3505-3512. |
| 15 | NGUYEN N T T, FURUKAWA H, GÁNDARA F, et al. Three-dimensional metal-catecholate frameworks and their ultrahigh proton conductivity[J]. Journal of the American Chemical Society, 2015, 137(49): 15394-15397. |
| 16 | NAGARKAR S S, UNNI S M, SHARMA A, et al. Two-in-one: inherent anhydrous and water-assisted high proton conduction in a 3D metal-organic framework[J]. Angewandte Chemie International Edition, 2014, 53(10): 2638-2642. |
| 17 | SHIGEMATSU Akihito, YAMADA Teppei, KITAGAWA Hiroshi. Wide control of proton conductivity in porous coordination polymers[J]. Journal of the American Chemical Society, 2011, 133(7): 2034-2036. |
| 18 | ROUGHT Peter, MARSH Christopher, PILI Simona, et al. Modulating proton diffusion and conductivity in metal-organic frameworks by incorporation of accessible free carboxylic acid groups[J]. Chemical Science, 2019, 10(5): 1492-1499. |
| 19 | YANG Fan, XU Gang, DOU Yibo, et al. A flexible metal-organic framework with a high density of sulfonic acid sites for proton conduction[J]. Nature Energy, 2017, 2(11): 877-883. |
| 20 | JEONG N C, SAMANTA B, LEE C Y, et al. Coordination-chemistry control of proton conductivity in the iconic metal-organic framework material HKUST-1[J]. Journal of the American Chemical Society, 2012, 134(1): 51-54. |
| 21 | ZHANG Fengming, DONG Longzhang, QIN Junsheng, et al. Effect of imidazole arrangements on proton-conductivity in metal-organic frameworks[J]. Journal of the American Chemical Society, 2017, 139(17): 6183-6189. |
| 22 | BUREEKAEW Sareeya, HORIKE Satoshi, HIGUCHI Masakazu, et al. One-dimensional imidazole aggregate in aluminium porous coordination polymers with high proton conductivity[J]. Nature Materials, 2009, 8(10): 831-836. |
| 23 | TAYLOR J M, KOMATSU T, DEKURA S, et al. The role of a three dimensionally ordered defect sublattice on the acidity of a sulfonated metal-organic framework[J]. Journal of the American Chemical Society, 2015, 137(35): 11498-11506. |
| 24 | TAYLOR J M, DEKURA S, IKEDA R, et al. Defect control to enhance proton conductivity in a metal-organic framework[J]. Chemistry of Materials, 2015, 27(7): 2286-2289. |
| 25 | LI Xiaomin, DONG Longzhang, LI Shunli, et al. Synergistic conductivity effect in a proton sources-coupled metal-organic framework[J]. ACS Energy Letters, 2017, 2(10): 2313-2318. |
| 26 | PATEL H A, MANSOR N, GADIPELLI S, et al. Superacidity in nafion/MOF hybrid membranes retains water at low humidity to enhance proton conduction for fuel cells[J]. ACS Applied Materials & Interfaces, 2016, 8(45): 30687-30691. |
| 27 | DONNADIO Anna, NARDUCCI Riccardo, CASCIOLA Mario, et al. Mixed membrane matrices based on nafion/UiO-66/SO3H-UiO-66 nano-MOFs: revealing the effect of crystal size, sulfonation, and filler loading on the mechanical and conductivity properties[J]. ACS Applied Materials & Interfaces, 2017, 9(48): 42239-42246. |
| 28 | KIM H J, TALUKDAR K, CHOI S J. Tuning of nafion® by HKUST-1 as coordination network to enhance proton conductivity for fuel cell applications[J]. Journal of Nanoparticle Research, 2016, 18(2): 1-6. |
| 29 | EREN E O, ÖZKAN N, DEVRIM Y. Preparation of polybenzimidazole/ZIF-8 and polybenzimidazole/UiO-66 composite membranes with enhanced proton conductivity[J]. International Journal of Hydrogen Energy, 2022, 47(45): 19690-19701. |
| 30 | ZHANG Jin, BAI Huijuan, REN Qiu, et al. Extra water- and acid-stable MOF-801 with high proton conductivity and its composite membrane for proton-exchange membrane[J]. ACS Applied Materials & Interfaces, 2018, 10(34): 28656-28663. |
| 31 | LIANG Xiaoqiang, ZHANG Feng, FENG Wei, et al. From metal-organic framework (MOF) to MOF-polymer composite membrane: Enhancement of low-humidity proton conductivity[J]. Chemical Science, 2013, 4(3): 983-992. |
| 32 | LUO Hongbin, WANG Mei, LIU Shaoxian, et al. Proton conductance of a superior water-stable metal-organic framework and its composite membrane with poly(vinylidene fluoride)[J]. Inorganic Chemistry, 2017, 56(7): 4169-4175. |
| [1] | CHEN Kuangyin, LI Ruilan, TONG Yang, SHEN Jianhua. Structure design of gas diffusion layer in proton exchange membrane fuel cell [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 246-259. |
| [2] | XU Jiaheng, LI Yongsheng, LUO Chunhuan, SU Qingquan. Optimization of methanol steam reforming process [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 41-46. |
| [3] | YANG Xiazhen, PENG Yifan, LIU Huazhang, HUO Chao. Regulation of active phase of fused iron catalyst and its catalytic performance of Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 310-318. |
| [4] | ZHANG Qi, ZHAO Hong, RONG Junfeng. Research progress of anti-toxicity electrocatalysts for oxygen reduction reaction in PEMFC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4677-4691. |
| [5] | PAN Yichang, ZHOU Rongfei, XING Weihong. Advanced microporous membranes for efficient separation of same-carbon-number hydrocarbon mixtures: State-of-the-art and challenges [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3926-3942. |
| [6] | JIANG Bolong, CUI Yanyan, SHI Shunjie, CHANG Jiacheng, JIANG Nan, TAN Weiqiang. Synthesis of transition metal Co3O4/ZnO-ZIF oxygen reduction catalyst by Co/Zn-ZIF template method and its electricity generation performance [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3066-3076. |
| [7] | ZHU Yajing, XU Yan, JIAN Meipeng, LI Haiyan, WANG Chongchen. Progress of metal-organic frameworks for uranium extraction from seawater [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3029-3048. |
| [8] | MA Zhejie, ZHANG Wenli, ZHAO Xuankai, LI Ping. Progress on the influence of oxygen mass transfer resistance in PEMFC cathode catalyst layer [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2860-2873. |
| [9] | ZHANG Jinhui, ZHANG Huan, ZHU Xinfeng, SONG Zhongxian, KANG Haiyan, LIU Hongpan, DENG Wei, HOU Guangchao, LI Guiting, HUANG Zhenzhen. Research progress of UiO-66 materials for adsorption and photocatalytic oxidation of typical organic compounds [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 445-456. |
| [10] | ZHU Xiao, ZHU Junyong, ZHANG Yatao. Research progress of metal organic framework/polyamide thin film nanocomposite membrane [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4314-4326. |
| [11] | GAO Weitao, LEI Yijie, ZHANG Xun, HU Xiaobo, SONG Pingping, ZHAO Qing, WANG Cheng, MAO Zongqiang. An overview of proton exchange membrane fuel cell [J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1539-1555. |
| [12] | ZHANG Dong, ZHANG Rui, ZHANG Bin, AN Zhoujian, LEI Che. Research progress of combined cooling-heat-and-power systems based on PEMFC [J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1608-1621. |
| [13] | CHEN Yuntao, DONG Xiaoxu, WANG Yang, WANG Jiannan, CUI Mei, HUANG Renliang. Preparation and application of mercapto-functionalized Zr-MOFs/polyester fabric composite [J]. Chemical Industry and Engineering Progress, 2022, 41(2): 854-861. |
| [14] | MENG Zihao, LI Qingyun, LIU Youyan, LIN Dongliang, TANG Aixing. MOF-immobilized lipase-catalyzed epoxidation of limonene in a single-phase system [J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6540-6548. |
| [15] | GAO Yifei, YI Qun, QI Kai, GAO Lili, LI Xuelian. Research status and application in H2/CH4 separation of MOFs-based membrane [J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6395-6407. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||