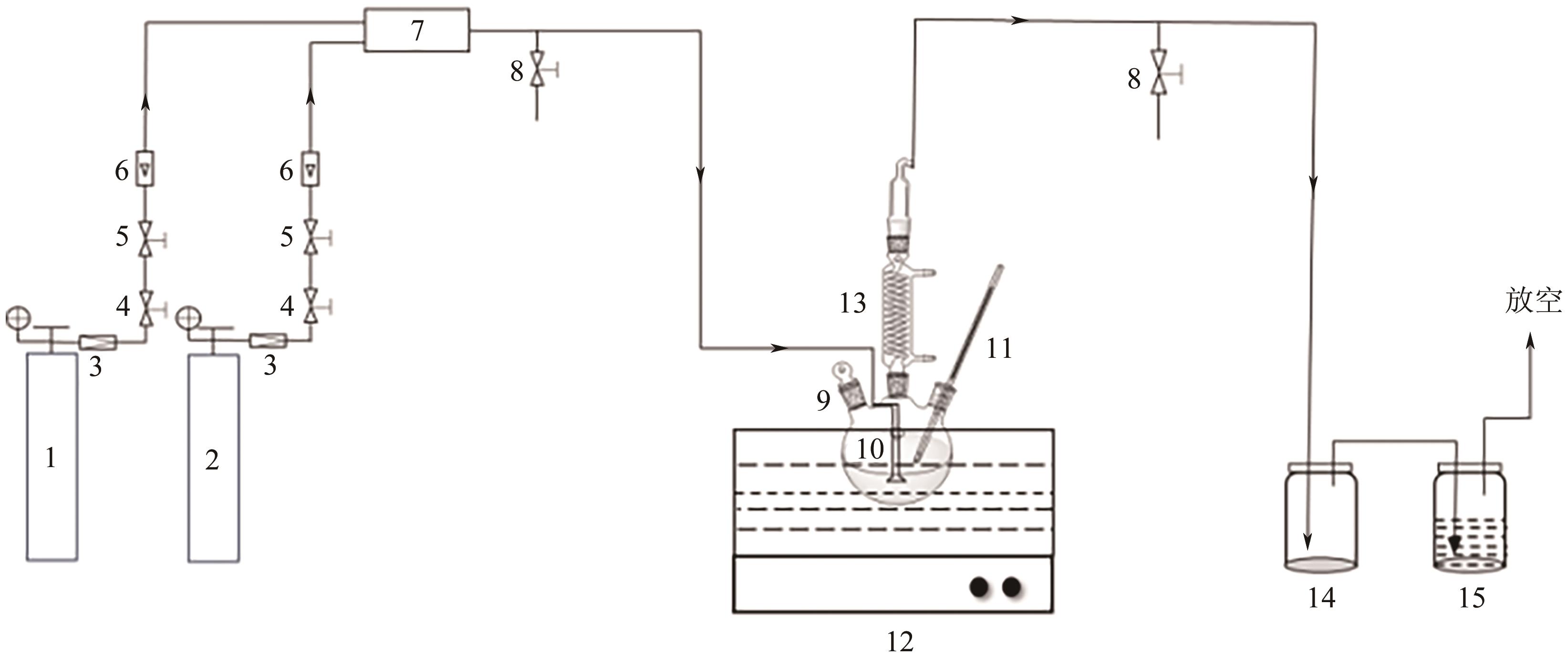
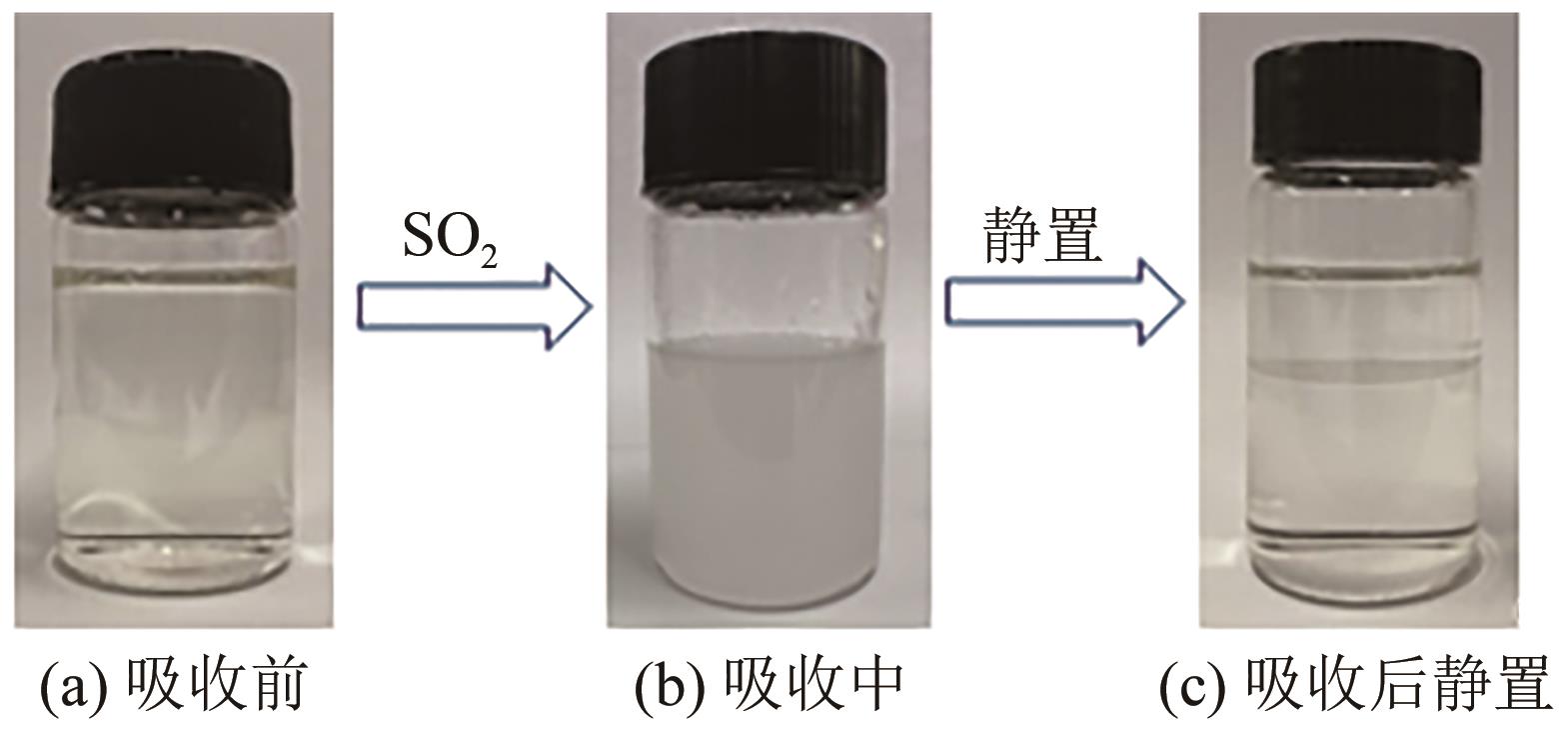
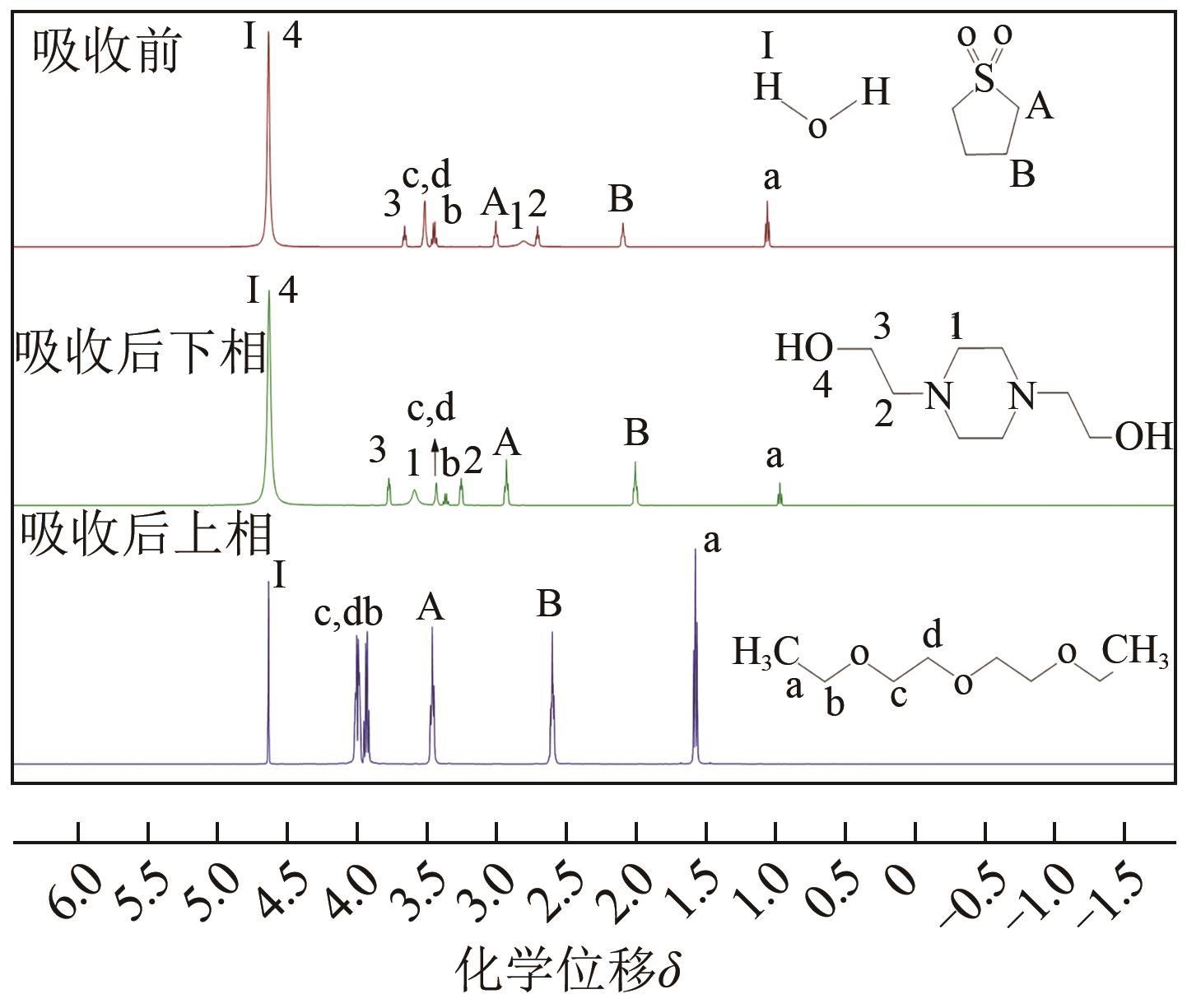
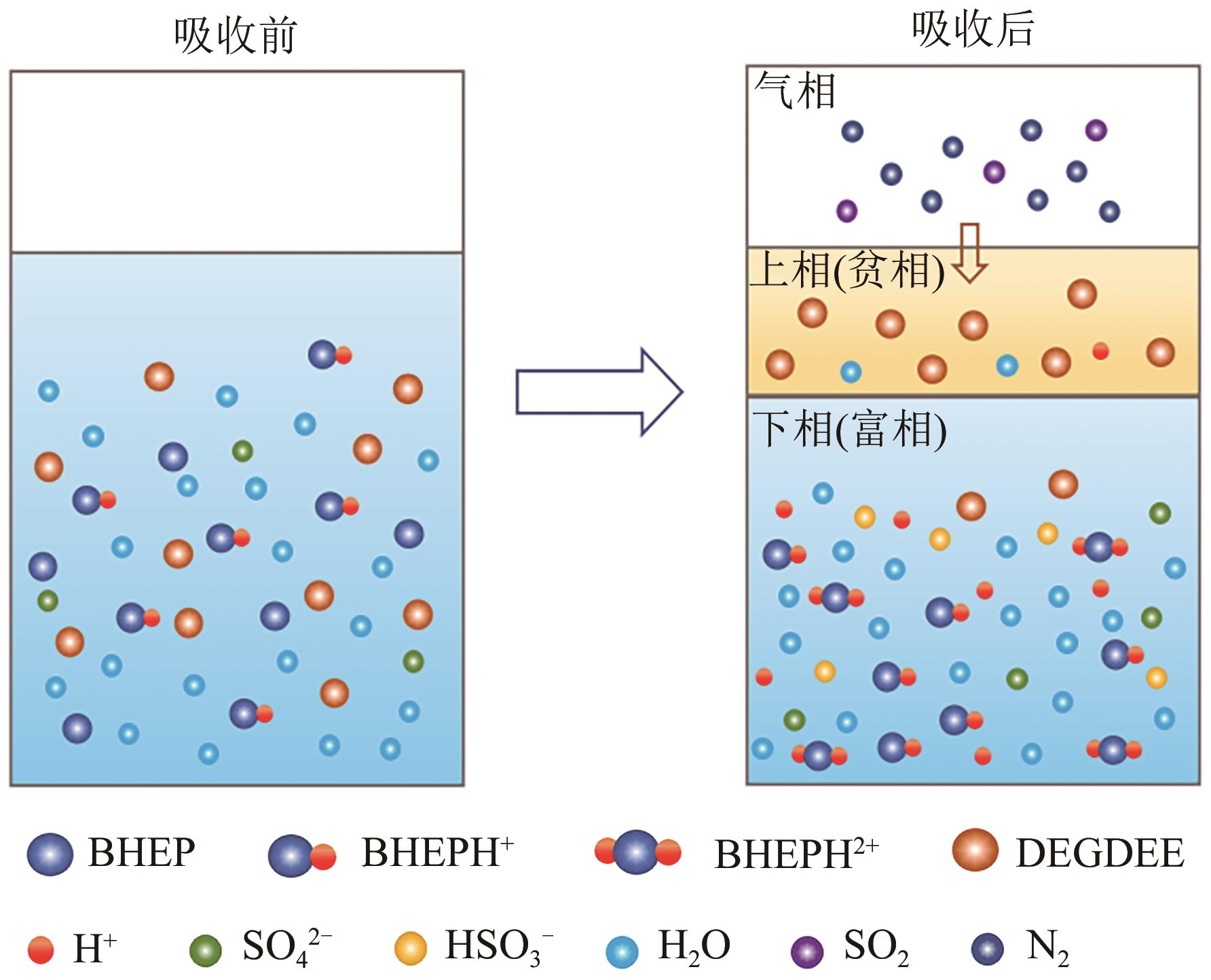
Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (10): 5890-5900.DOI: 10.16085/j.issn.1000-6613.2023-1603
• Resources and environmental engineering • Previous Articles
Phase-change absorption for SO2 capture by a novel aqueous BHEP/ether solution
DIAO Zhongxiu1( ), ZHENG Yu1, WEI Fengyu1(
), ZHENG Yu1, WEI Fengyu1( ), SONG Xiaoliang2, SU Wenguo2, LI Shuquan2
), SONG Xiaoliang2, SU Wenguo2, LI Shuquan2
- 1.School of Chemistry and Chemical Engineering, Hefei University of Technology, Hefei 230009, Anhui, China
2.Shuangdun Environment Technology Company, Wuxi 214205, Jiangsu, China
-
Received:2023-09-11Revised:2024-01-20Online:2024-10-29Published:2024-10-15 -
Contact:WEI Fengyu
新型的BHEP-醚水溶液相变吸收二氧化硫
刁忠秀1( ), 郑宇1, 魏凤玉1(
), 郑宇1, 魏凤玉1( ), 宋小良2, 苏文国2, 李淑全2
), 宋小良2, 苏文国2, 李淑全2
- 1.合肥工业大学化学与化工学院,安徽 合肥 230009
2.双盾环境科技有限公司,江苏 无锡 214205
-
通讯作者:魏凤玉 -
作者简介:刁忠秀(1999—),女,硕士研究生,研究方向为化工传质与分离技术。E-mail:diaozhongxiu@gmail.com。
CLC Number:
Cite this article
DIAO Zhongxiu, ZHENG Yu, WEI Fengyu, SONG Xiaoliang, SU Wenguo, LI Shuquan. Phase-change absorption for SO2 capture by a novel aqueous BHEP/ether solution[J]. Chemical Industry and Engineering Progress, 2024, 43(10): 5890-5900.
刁忠秀, 郑宇, 魏凤玉, 宋小良, 苏文国, 李淑全. 新型的BHEP-醚水溶液相变吸收二氧化硫[J]. 化工进展, 2024, 43(10): 5890-5900.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-1603
| 溶剂种类 | 分子式 | 沸点/℃ | 闪点/℃ | 水溶解性 | 吸收前 | 吸收后 |
|---|---|---|---|---|---|---|
| 三乙二醇单甲醚(TriEGME) | C7H16O4 | 233.9 | 95.30 | 互溶 | 液相 | 液相 |
| 三乙二醇二甲醚(TriEGDME) | C8H18O4 | 249.0 | 118.30 | 互溶 | 液相 | 液相① |
| 二乙二醇二乙醚(DEGDEE) | C8H18O3 | 180.0 | 71.10 | 微溶 | 液相 | 液-液 |
| 二丙二醇单丙醚(DPGPE) | C9H20O3 | 242.9 | 100.70 | 不溶 | 液-液 | 液-液 |
| 溶剂种类 | 分子式 | 沸点/℃ | 闪点/℃ | 水溶解性 | 吸收前 | 吸收后 |
|---|---|---|---|---|---|---|
| 三乙二醇单甲醚(TriEGME) | C7H16O4 | 233.9 | 95.30 | 互溶 | 液相 | 液相 |
| 三乙二醇二甲醚(TriEGDME) | C8H18O4 | 249.0 | 118.30 | 互溶 | 液相 | 液相① |
| 二乙二醇二乙醚(DEGDEE) | C8H18O3 | 180.0 | 71.10 | 微溶 | 液相 | 液-液 |
| 二丙二醇单丙醚(DPGPE) | C9H20O3 | 242.9 | 100.70 | 不溶 | 液-液 | 液-液 |
| DEGDEE用量/% | 吸收前 | 吸收后 | 分相时间/min | 黏度/mPa·s | 下相体积占比/% | 分配系数 | |
|---|---|---|---|---|---|---|---|
| 吸收前 | 吸收后下相 | ||||||
| 0 | 液相 | 液相 | 不分相 | 5.62 | 8.25 | 100 | — |
| 5 | 液相 | 液相 | 不分相 | 7.46 | 9.13 | 100 | — |
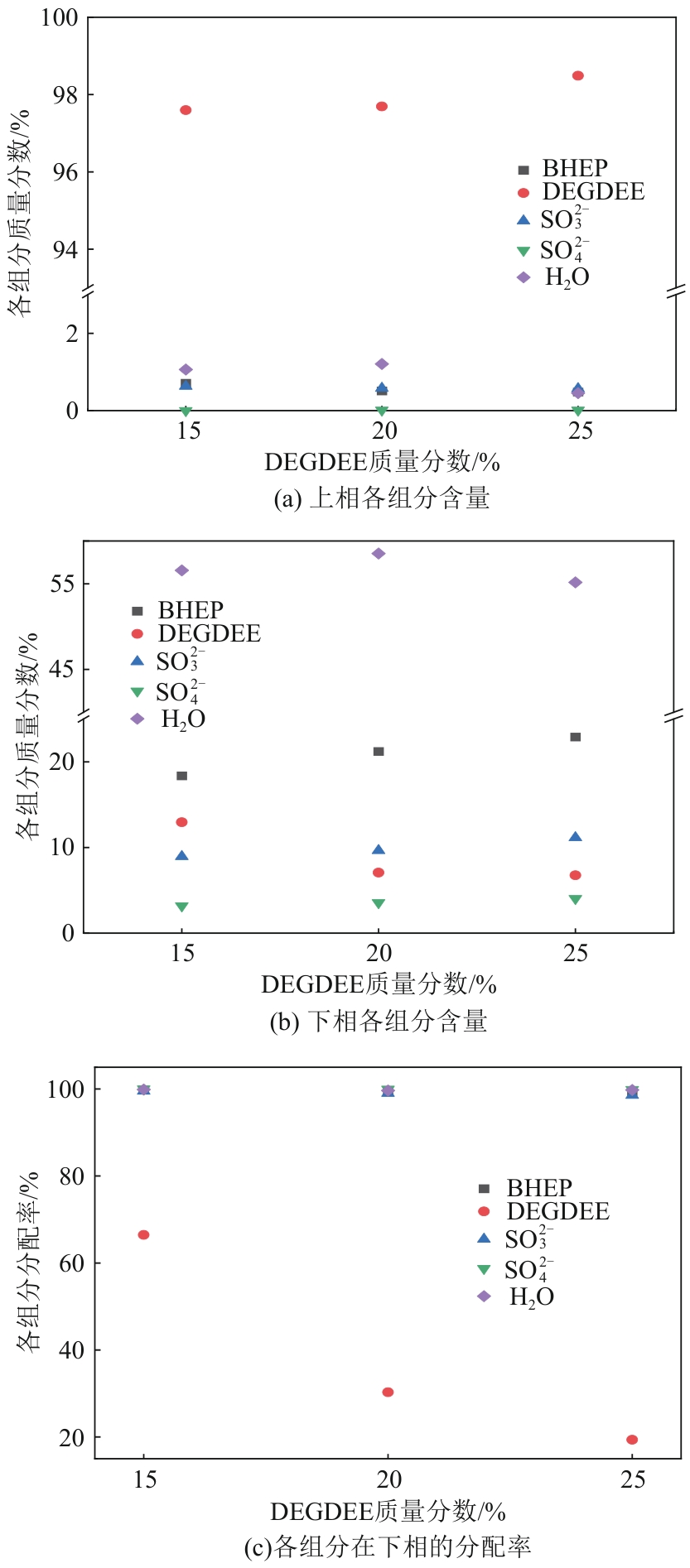
| 10 | 液相 | 液-液 | 30 | 8.03 | 10.59 | 98.70 | 14.30 |
| 15 | 液相 | 液-液 | 10 | 9.5 | 11.28 | 91.86 | 17.26 |
| 20 | 液相 | 液-液 | 3 | 10.12 | 13.03 | 83.33 | 19.40 |
| 25 | 液-液 | 液-液 | 0 | 11.33 | 16.75 | 73.68 | 21.37 |
| DEGDEE用量/% | 吸收前 | 吸收后 | 分相时间/min | 黏度/mPa·s | 下相体积占比/% | 分配系数 | |
|---|---|---|---|---|---|---|---|
| 吸收前 | 吸收后下相 | ||||||
| 0 | 液相 | 液相 | 不分相 | 5.62 | 8.25 | 100 | — |
| 5 | 液相 | 液相 | 不分相 | 7.46 | 9.13 | 100 | — |
| 10 | 液相 | 液-液 | 30 | 8.03 | 10.59 | 98.70 | 14.30 |
| 15 | 液相 | 液-液 | 10 | 9.5 | 11.28 | 91.86 | 17.26 |
| 20 | 液相 | 液-液 | 3 | 10.12 | 13.03 | 83.33 | 19.40 |
| 25 | 液-液 | 液-液 | 0 | 11.33 | 16.75 | 73.68 | 21.37 |
| BHEP用量/% | 吸收前 | 吸收后 | 分相时间/min | 黏度/mPa·s | 下相体积占比/% | |
|---|---|---|---|---|---|---|
| 吸收前 | 吸收后下相 | |||||
| 10 | 液相 | 液-液 | 35 | 3.47 | 7.50 | 91.86 |
| 15 | 液相 | 液-液 | 10 | 5.72 | 10.41 | 84.62 |
| 20 | 液相 | 液-液 | 5 | 9.56 | 11.79 | 79.22 |
| 25 | 液-液 | 液-液 | 0 | 13.28 | 18.47 | 77.92 |
| BHEP用量/% | 吸收前 | 吸收后 | 分相时间/min | 黏度/mPa·s | 下相体积占比/% | |
|---|---|---|---|---|---|---|
| 吸收前 | 吸收后下相 | |||||
| 10 | 液相 | 液-液 | 35 | 3.47 | 7.50 | 91.86 |
| 15 | 液相 | 液-液 | 10 | 5.72 | 10.41 | 84.62 |
| 20 | 液相 | 液-液 | 5 | 9.56 | 11.79 | 79.22 |
| 25 | 液-液 | 液-液 | 0 | 13.28 | 18.47 | 77.92 |
| 1 | RAYNAL Ludovic, ALIX Pascal, BOUILLON Pierre-Antoine, et al. The DMX™ process: An original solution for lowering the cost of post-combustion carbon capture[J]. Energy Procedia, 2011, 4: 779-786. |
| 2 | ZHANG Shihan, SHEN Yao, WANG Lidong, et al. Phase change solvents for post-combustion CO2 capture: Principle, advances, and challenges[J]. Applied Energy, 2019, 239: 876-897. |
| 3 | 张卫风, 周武, 王秋华. 相变吸收捕集烟气中CO2技术的发展现状[J]. 化工进展, 2022, 41(4): 2090-2101. |
| ZHANG Weifeng, ZHOU Wu, WANG Qiuhua. Recent developments of phase-change absorption technology for CO2 capture from flue gas[J]. Chemical Industry and Engineering Progress, 2022, 41(4): 2090-2101. | |
| 4 | 刘飞. 胺基两相吸收剂捕集二氧化碳机理研究[D]. 杭州: 浙江大学, 2020. |
| LIU Fei. Study on carbon dioxide capture mechanism of amine-based two-phase absorbent[D]. Hangzhou: Zhejiang University, 2020. | |
| 5 | 涂巍巍, 方佳伟, 李竹石, 等. 基于MEA的CO2相变化吸收剂的开发[J]. 中国科学: 化学, 2018, 48(6): 641-647. |
| TU Weiwei, FANG Jiawei, LI Zhushi, et al. Development of MEA phase change absorbent[J]. Scientia Sinica (Chimica), 2018, 48(6): 641-647. | |
| 6 | YE Qing, WANG Xinlei, LU Yongqi. Screening and evaluation of novel biphasic solvents for energy-efficient post-combustion CO2 capture[J]. International Journal of Greenhouse Gas Control, 2015, 39: 205-214. |
| 7 | 沈丽, 刘凡, 沈遥, 等. 新型AEP-相变吸收剂捕集CO2研究[J]. 高校化学工程学报, 2021, 35(6): 1067-1072. |
| SHEN Li, LIU Fan, SHEN Yao, et al. Study on novel AEP-based biphasic solvents for CO2 capture[J]. Journal of Chemical Engineering of Chinese Universities, 2021, 35(6): 1067-1072. | |
| 8 | SHEN Yao, JIANG Chenkai, ZHANG Shihan, et al. Biphasic solvent for CO2 capture: Amine property-performance and heat duty relationship[J]. Applied Energy, 2018, 230: 726-733. |
| 9 | 桑伟, 唐建峰, 花亦怀, 等. 物理溶剂及有机胺的性质对相变吸收性能的影响[J]. 化工进展, 2023, 42(4): 2151-2159. |
| SANG Wei, TANG Jianfeng, HUA Yihuai, et al. Effects of physical solvent and amine properties on the performance of biphasic solvent[J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2151-2159. | |
| 10 | 赵文波, 李广振, 许胜超, 等. 相变吸收酸性气体的发展现状[J]. 化工进展, 2021, 40(1): 401-414. |
| ZHAO Wenbo, LI Guangzhen, XU Shengchao, et al. Recent developments of acid gas absorption by phase-change[J]. Chemical Industry and Engineering Progress, 2021, 40(1): 401-414. | |
| 11 | XU Shengchao, ZHAO Wenbo, XIE Xuhao, et al. Dual effects of long-chain alcohols in phase change capture of SO2: Increasing mutual solubility and decreasing product viscosity[J]. Journal of Molecular Liquids, 2021, 328: 115461. |
| 12 | 李雪霏, 陈玲, 许胜超, 等. N,N-二甲基正辛胺/十六烷混合体系液液相变吸收SO2 [J]. 化学学报, 2019, 77(12): 1287-1293. |
| LI Xuefei, CHEN Ling, XU Shengchao, et al. Liquid-liquid phase-change absorption of SO2 using N,N-dimethyl-n-octylamine mixed with hexadecane[J]. Acta Chimica Sinica, 2019, 77(12): 1287-1293. | |
| 13 | CHAI Muyuan, ZHAO Wenbo, LI Genming, et al. Novel SO2 phase-change absorbent: Mixture of N,N-dimethylaniline and liquid paraffin[J]. Industrial & Engineering Chemistry Research, 2018, 57(37): 12502-12510. |
| 14 | LI Genming, ZHAO Wenbo, CHAI Muyuan, et al. Liquid-liquid phase-change absorption of SO2 using N,N-dimethylcyclohexylamine as absorbent and liquid paraffin as solvent[J]. Journal of Hazardous Materials, 2018, 360: 89-96. |
| 15 | HELDEBRANT David J, KOECH Phillip K, YONKER Clement R. A reversible zwitterionic SO2-binding organic liquid[J]. Energy & Environmental Science, 2010, 3(1): 111-113. |
| 16 | 沈紫薇, 常栋渊, 郭本帅, 等. 水对无水相变吸收剂捕集CO2性能的影响[J]. 低碳化学与化工, 2023(4): 107-113. |
| SHEN Ziwei, CHANG Dongyuan, GUO Benshuai, et al. Effect of water on CO2 capture performance of anhydrous phase change absorbent[J]. Low-Carbon Chemistry and Chemical Engineering, 2023(4): 107-113. | |
| 17 | DUAN Erhong, GUO Bin, ZHANG Miaomiao, et al. Efficient capture of SO2 by a binary mixture of caprolactam tetrabutyl ammonium bromide ionic liquid and water[J]. Journal of Hazardous Materials, 2011, 194: 48-52. |
| 18 | 杨福绅. 燃煤烟气碳捕集相变化吸收剂开发及杂质脱除技术研究[D]. 北京: 北京化工大学, 2019. |
| YANG Fushen. Development of phase change absorbent for carbon capture in coal-fired flue gas and study on impurity removal technology[D]. Beijing: Beijing University of Chemical Technology, 2019. | |
| 19 | WEI Fengyu, HE Yuan, XUE Pan, et al. Mass transfer performance for low SO2 absorption into aqueous N,N′-bis(2-hydroxypropyl) piperazine solution in a θ-ring packed column[J]. Industrial & Engineering Chemistry Research, 2014, 53(11): 4462-4468. |
| 20 | 刘建芳. 无机酸和Fe2+/Fe3+对有机胺吸收/解吸SO2气体的影响[D]. 合肥: 合肥工业大学, 2012. |
| LIU Jianfang. Effects of inorganic acids and Fe2+/Fe3+ on absorption/desorption of SO2 gas by organic amines[D]. Hefei: Hefei University of Technology, 2012. | |
| 21 | 魏凤玉. 一种吸收捕集SO2的水系液-液相变吸收剂及其制备方法及其应用: CN116196728A[P]. 2023-06-02. |
| WEI Fengyu. The invention relates to an aqueous liquid-liquid variable absorber for absorbing and trapping SO, a preparation method and application thereof: CN116196728A[P]. 2023-06-02. | |
| 22 | 魏凤玉, 童晨. 哌嗪类二胺PA-A-硫酸动态吸收模拟烟气中的二氧化硫[J]. 化工进展, 2010, 29(S2): 330-333. |
| WEI Fengyu, TONG Chen. Dynamic absorption of SO2 in simulated flue gas with piperazines diamine(PA-A)-sulfuric acid solution[J]. Chemical Industry and Engineering Progress, 2010, 29(S2): 330-333. | |
| 23 | 孙志豪, 郭子东, 陈俊, 等. 哌嗪类有机胺脱除二氧化硫性能及机理探讨[J]. 化工进展, 2019, 38(S1): 46-51. |
| SUN Zhihao, GUO Zidong, CHEN Jun, et al. Performances and mechanism of piperazine-based organic amines removal of SO2 [J]. Chemical Industry and Engineering Progress, 2019, 38(S1): 46-51. | |
| 24 | 华东理工大学, 四川大学. 分析化学[M]. 7版. 北京: 高等教育出版社, 2018: 43-192. |
| East China University of Science and Technology, Sichuan University. Analytical chemistry[M]. 7th ed. Beijing: Higher Education Press, 2018: 43-192. | |
| 25 | 袁存光, 祝优珍, 田晶, 等. 现代仪器分析[M]. 北京: 化学工业出版社, 2012. |
| YUAN Cunguang, ZHU Youzhen, TIAN Jing, et al. Modern instrumental analysis[M]. Beijing: Chemical Industry Press, 2012. | |
| 26 | ZHANG J, MISCH R, TAN Y, et al. Novel thermomorphic biphasic amine solvents for CO2 absorption and low-temperature extractive regeneration[J]. Chemical Engineering & Technology, 2011, 34(9): 1481-1489. |
| 27 | 刘彪. 离去基团法制备端氨基聚醚及其吸收解吸二氧化碳的研究[D]. 昆明: 昆明理工大学, 2015. |
| LIU Biao. Study on preparation of amino-terminated polyether by leaving group method and its absorption and desorption of carbon dioxide[D]. Kunming: Kunming University of Science and Technology, 2015. | |
| 28 | MACHIDA Hiroshi, Kazuki OBA, TOMIKAWA Takashi, et al. Development of phase separation solvent for CO2 capture by aqueous (amine + ether) solution[J]. The Journal of Chemical Thermodynamics, 2017, 113: 64-70. |
| 29 | 唐思扬, 李星宇, 鲁厚芳, 等. 低能耗化学吸收碳捕集技术展望[J]. 化工进展, 2022, 41(3): 1102-1106. |
| TANG Siyang, LI Xingyu, LU Houfang, et al. Perspective on low-energy chemical absorption for CO2 capture[J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1102-1106. | |
| 30 | 翁诗甫, 徐怡庄. 傅里叶变换红外光谱分析[M]. 3版. 北京: 化学工业出版社, 2016: 490-507. |
| WENG Shifu, XU Yizhuang. Fourier transform infrared spectrum analysis[M]. 3rd ed. Beijing: Chemical Industry Press, 2016: 490-507. | |
| 31 | FINNEY J L, BOWRON D T. Anion bridges and salting out[J]. Current Opinion in Colloid & Interface Science, 2004, 9(1/2): 59-63. |
| 32 | 金显杭. 面向CO2捕集的相变吸收剂开发及应用研究[D]. 北京: 北京化工大学, 2017. |
| JIN Xianhang. The development and application of phase change absorbents for CO2 capture[D]. Beijing: Beijing University of Chemical Technology, 2017. | |
| 33 | 马婷. 几种类型有机溶剂吸收SO2的研究[D]. 太原: 太原理工大学, 2019. |
| MA Ting. Research on absorbing SO2 by several different type organic solvents[D]. Taiyuan: Taiyuan University of Technology, 2019. | |
| 34 | 王蓉. 物理溶剂对N-甲基二乙醇胺体系相变化吸收剂液液相平衡及吸收性能的影响[D]. 北京: 北京化工大学, 2021. |
| WANG Rong. Effect of physical solvents on liquid-liquid equilibrium and absorption performance of phase change absorbent in N-methyldiethanolamine system[D]. Beijing: Beijing University of Chemical Technology, 2021. | |
| 35 | SHAMIRI A, SHAFEEYAN M S, TEE H C, et al. Absorption of CO2 into aqueous mixtures of glycerol and monoethanolamine[J]. Journal of Natural Gas Science and Engineering, 2016, 35: 605-613. |
| 36 | 何园. 有机二胺吸收SO2的传质及其动力学研究[D]. 合肥: 合肥工业大学, 2013. |
| HE Yuan. Absorption of SO2 into aqueous organic diamine: Mass transfer and reaction kinetics[D]. Hefei: Hefei University of Technology, 2013. | |
| 37 | CHEN Kaihong, LIN Wenjun, YU Xini, et al. Designing of anion-functionalized ionic liquids for efficient capture of SO2 from flue gas[J]. AIChE Journal, 2015, 61(6): 2028-2034. |
| 38 | 张建斌, 李强, 刘占英, 等. 聚乙二醇及其水溶液吸收SO2机理研究[J]. 化学工程, 2010, 38(12): 76-79. |
| ZHANG Jianbin, LI Qiang, LIU Zhanying, et al. Absorption mechanism of SO2 in polyethylene glycol and its aqueous solution[J]. Chemical Engineering (China), 2010, 38(12): 76-79. | |
| 39 | UYANGA Itoro J, IDEM Raphael O. Studies of SO2- and O2-induced degradation of aqueous MEA during CO2 capture from power plant flue gas streams[J]. Industrial & Engineering Chemistry Research, 2007, 46(8): 2558-2566. |
| 40 | 谢江鹏. 二氧化硫捕集过程胺基吸收剂筛选与工艺流程匹配研究[D]. 兰州: 兰州理工大学, 2022. |
| XIE Jiangpeng. Study on screening of amino absorbent and matching of process flow in sulfur dioxide capture process[D]. Lanzhou: Lanzhou University of Technology, 2022. | |
| 41 | 张宇, 薛攀, 魏凤玉. 哌嗪类有机胺对二氧化硫的吸收及解吸性能研究[J]. 应用化工, 2014, 43(7): 1237-1239, 1242. |
| ZHANG Yu, XUE Pan, WEI Fengyu. Study on the properties of absorption and desorption of SO2 by piperazines organic amines in aqueous solution[J]. Applied Chemical Industry, 2014, 43(7): 1237-1239, 1242. |
| [1] | GENG Xiumei, ZHANG Feng, ZHANG Xiang, SHAN Meixia, ZHANG Yatao. Research progress on the stability of Pebax-based mixed matrix membranes for CO2 separation [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 4996-5012. |
| [2] | CAO Shuyang, SHI Jingbo, DONG Youming, LYU Jianxiong. Water adsorption and desorption isotherms and thermodynamic properties of Eucalyptus obliqua woods at different temperatures [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5095-5105. |
| [3] | LU Shijian, ZHANG Juanjuan, YANG Fei, LIU Ling, CHEN Siming, KANG Guojun, FANG Qinqin. Research progress of amine escape control technology by chemical absorption method [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4562-4570. |
| [4] | GU Songqi, SUN Fanfei, WEI Yao, SONG Xingfei, NAN Bing, LI Lina, HUANG Yuying. Time-resolved thermochemical in-situ XAFS methodology [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3747-3755. |
| [5] | LIU Kefeng, LIU Taoran, CAI Yong, HU Xuesheng, DONG Weigang, ZHOU Huaqun, GAO Fei. Progress in research and engineering demonstration of CO2 capture technology [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 2901-2914. |
| [6] | LIAO Changjian, ZHANG Kewei, WANG Jing, ZENG Xiangyu, JIN Ping, LIU Zhiyu. Progress on direct air capture of carbon dioxide [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 2031-2048. |
| [7] | MIAO Feng, XU Chuanlong, LI Jian, ZHANG Biao, HAN Shaopeng, TANG Guanghua. Online calibration of the wavelength of spectrometer based on SO2 absorption spectrum [J]. Chemical Industry and Engineering Progress, 2024, 43(2): 818-822. |
| [8] | SU Huihui, WANG Enlu, XU Yifei. Advances in research on capture of post-combustion carbon dioxide by liquid adsorbents [J]. Chemical Industry and Engineering Progress, 2024, 43(10): 5734-5747. |
| [9] | LI Jitong, WANG Gang, XIONG Yaxuan, XU Qian. Energy and exergy analysis of single-effect absorption refrigeration system with different refrigerants [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 104-112. |
| [10] | YANG Hanyue, KONG Lingzhen, CHEN Jiaqing, SUN Huan, SONG Jiakai, WANG Sicheng, KONG Biao. Decarbonization performance of downflow tubular gas-liquid contactor of microbubble-type [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 197-204. |
| [11] | WANG Shengyan, DENG Shuai, ZHAO Ruikai. Research progress on carbon dioxide capture technology based on electric swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 233-245. |
| [12] | ZHANG Fengqi, CUI Chengdong, BAO Xuewei, ZHU Weixuan, DONG Hongguang. Design and evaluation of sweetening process with amine solution absorption and multiple desorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 518-528. |
| [13] | SHI Keke, LIU Muzi, ZHAO Qiang, LI Jinping, LIU Guang. Properties and research progress of magnesium based hydrogen storage materials [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4731-4745. |
| [14] | TAN Jihuai, YU Min, ZHANG Tongtong, HUANG Nengkun, WANG Ziwen, ZHU Xinbao. Manufacturing of tannin polypropoxy ether carboxylates as efficient and improved migration resistance plasticizers for PVC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4847-4855. |
| [15] | SHAO Zhiguo, REN Wen, XU Shipei, NIE Fan, XU Yu, LIU Longjie, XIE Shuixiang, LI Xingchun, WANG Qingji, XIE Jiacai. Influence of final temperature on the distribution and characteristics of oil-based drilling cuttings pyrolysis products [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4929-4938. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||