Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (S1): 400-410.DOI: 10.16085/j.issn.1000-6613.2023-0424
• Fine chemicals • Previous Articles Next Articles
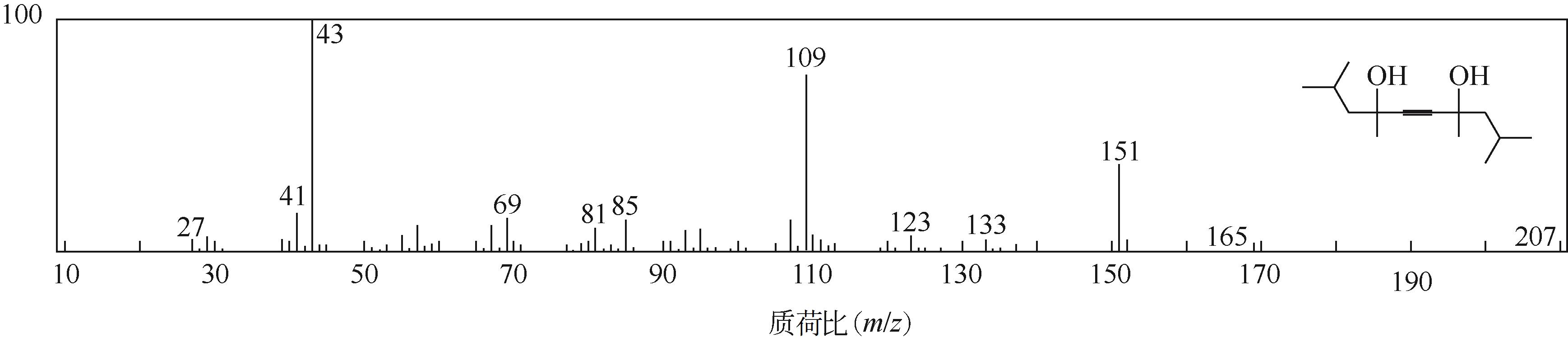
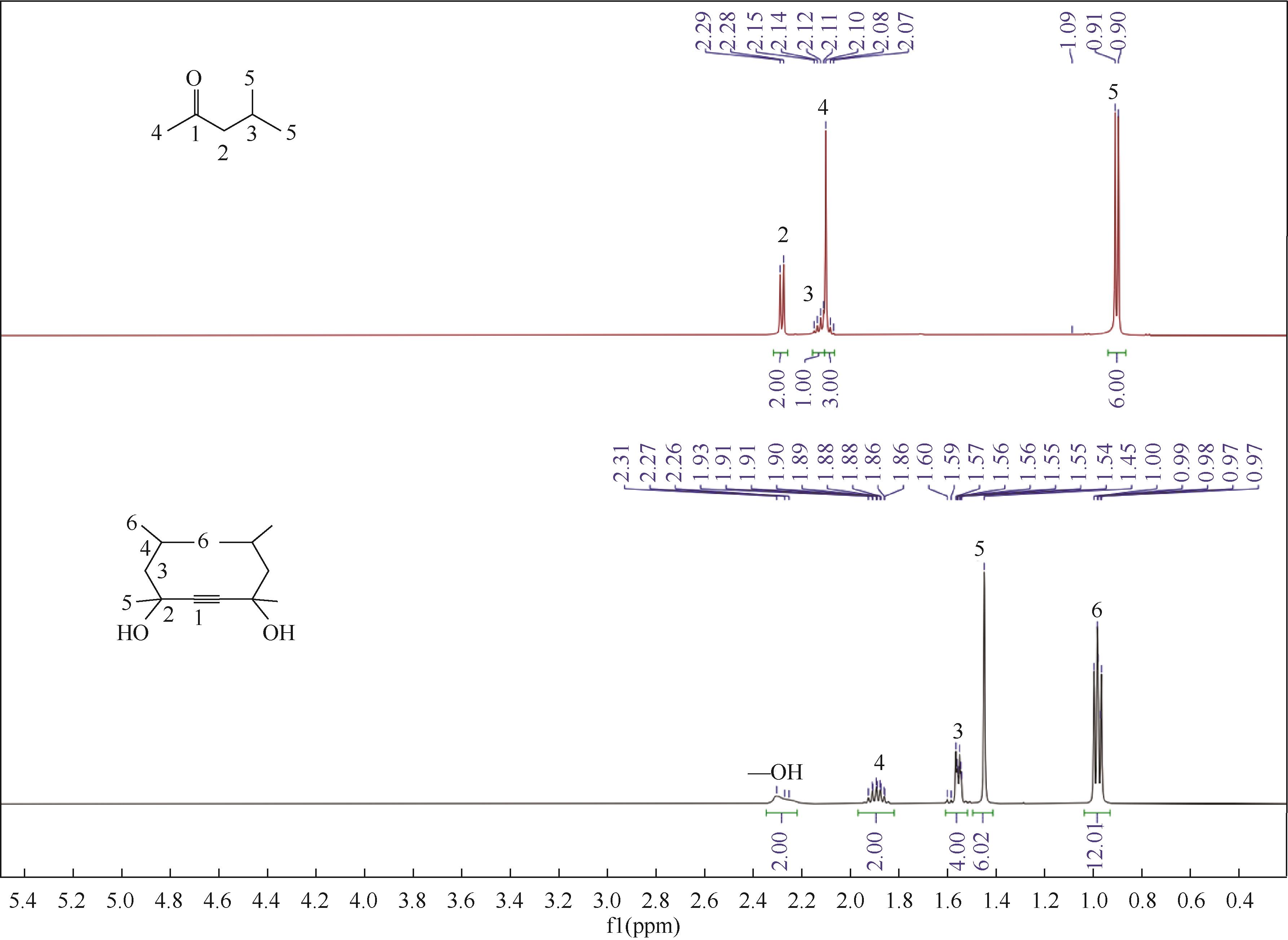
Green synthesis of gemini surfactant decyne diol
- College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, Fujian, China
-
Received:2023-03-21Revised:2023-07-09Online:2023-11-30Published:2023-10-25 -
Contact:LI Sifang
双子表面活性剂癸炔二醇的绿色合成
- 厦门大学化学化工学院,福建 厦门 361005
-
通讯作者:黎四芳 -
作者简介:王正坤(1993—),男,硕士研究生,研究方向为精细化工。E-mail:zkwang@stu.xmu.edu.cn。
CLC Number:
Cite this article
WANG Zhengkun, LI Sifang. Green synthesis of gemini surfactant decyne diol[J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 400-410.
王正坤, 黎四芳. 双子表面活性剂癸炔二醇的绿色合成[J]. 化工进展, 2023, 42(S1): 400-410.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-0424
| 实验因素 | 水平 | ||
|---|---|---|---|
| -1 | 0 | 1 | |
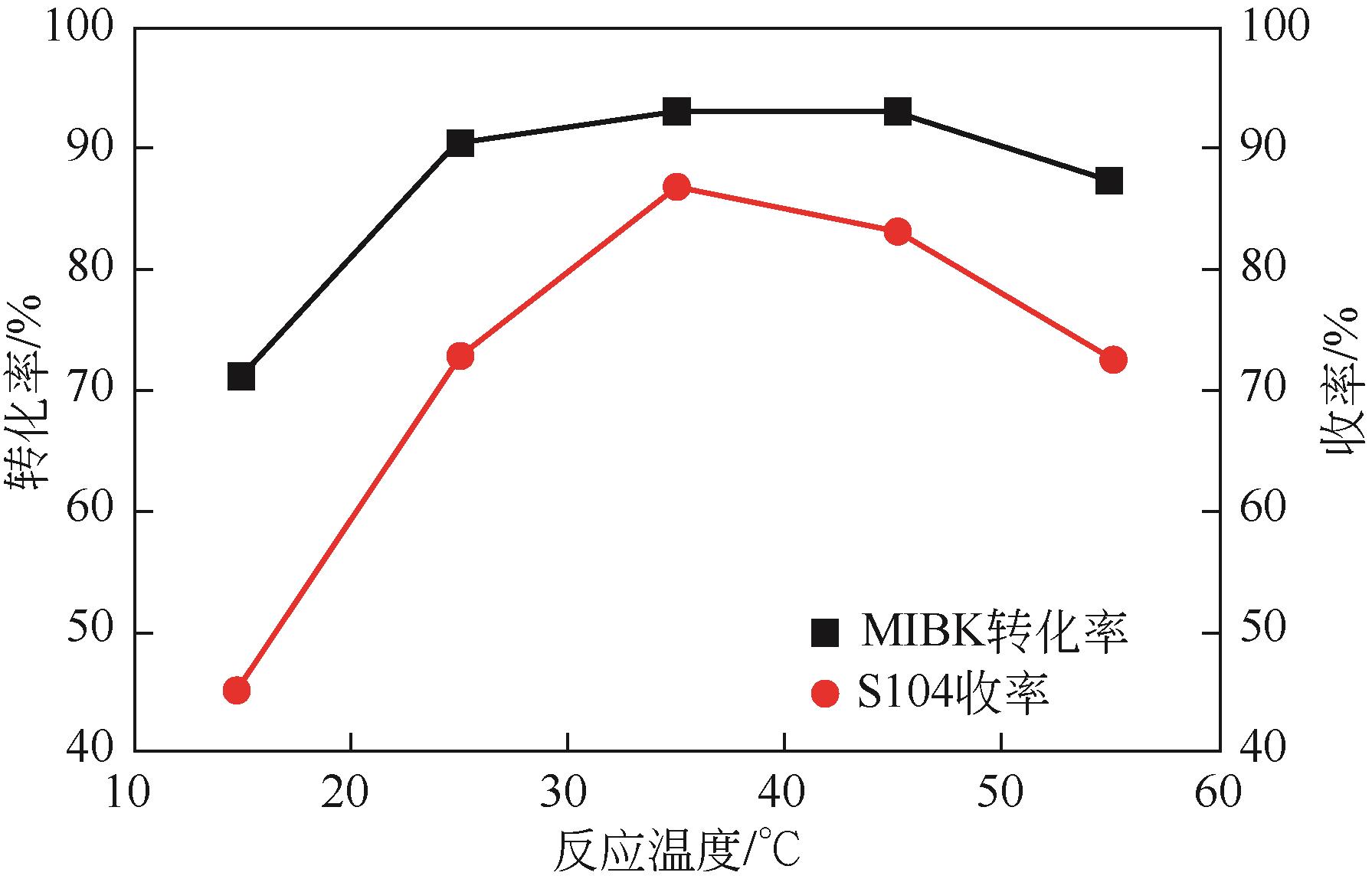
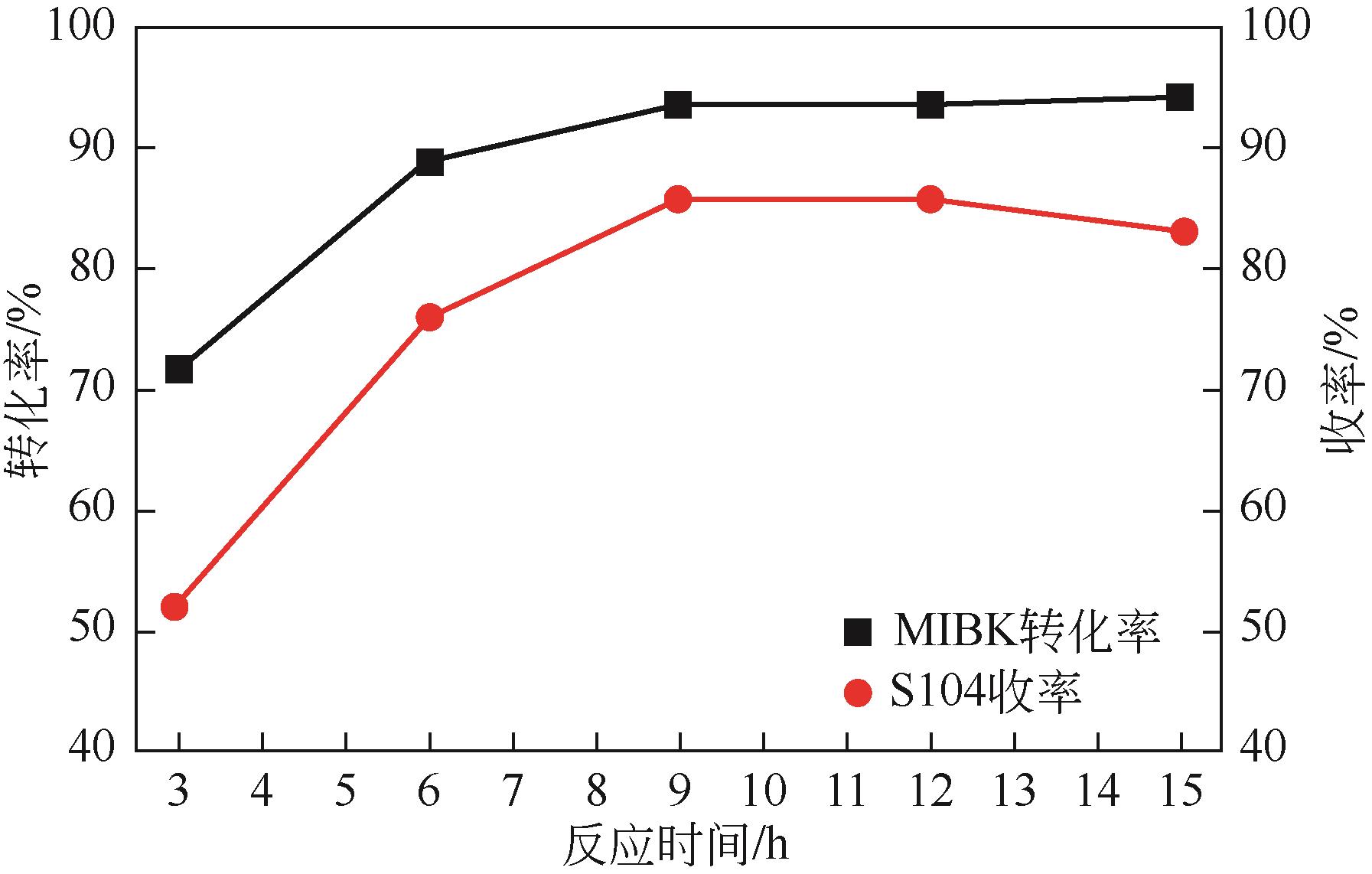
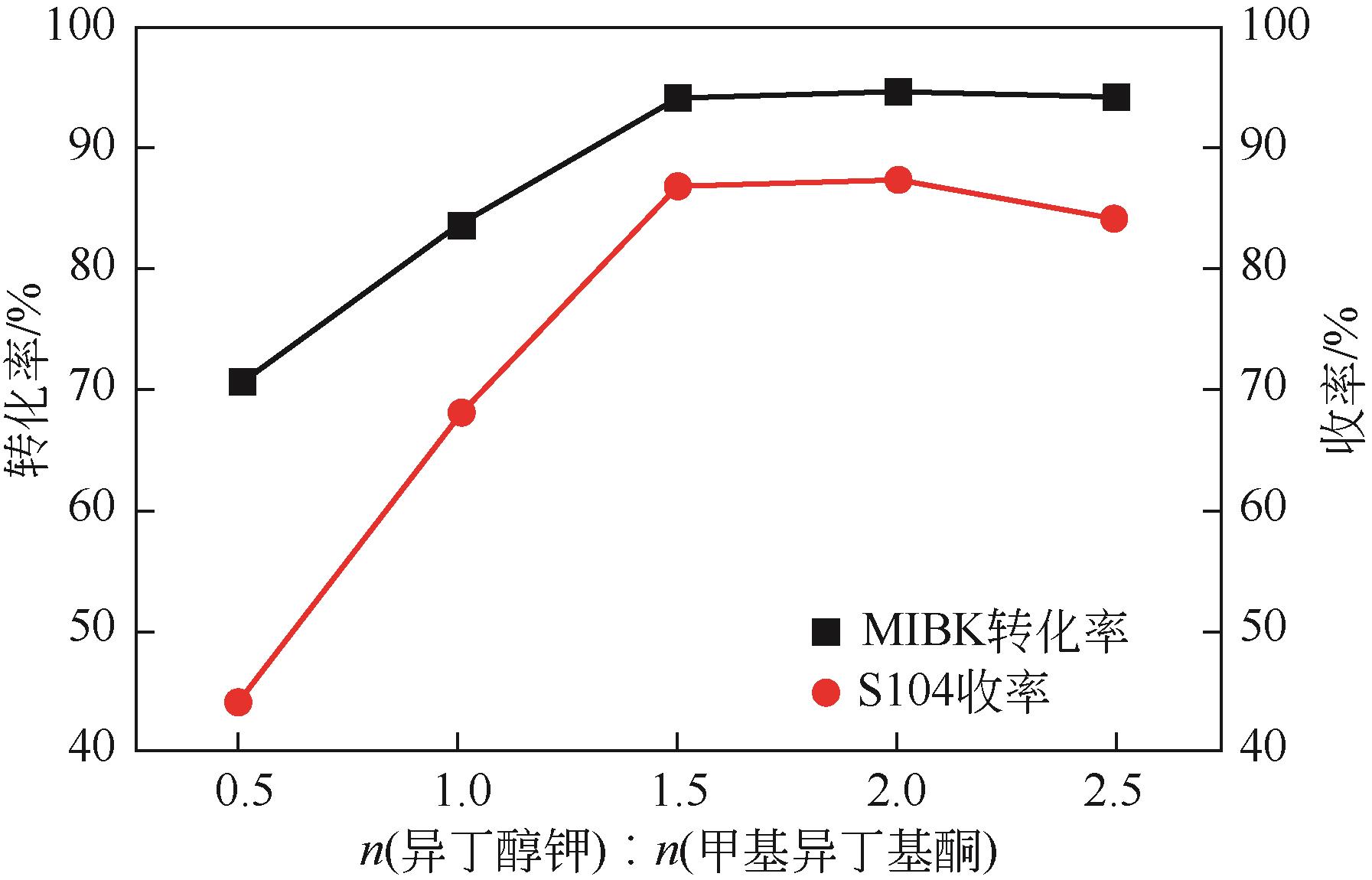
| 反应温度(A)/℃ | 25 | 35 | 45 |
| 反应时间(B)/h | 6 | 9 | 12 |
| 异丁醇钾与甲基异丁基酮摩尔比(C) | 1∶1 | 1.5∶1 | 2∶1 |
| 溶剂用量(D) | 3∶1 | 4.5∶1 | 6∶1 |
| 实验因素 | 水平 | ||
|---|---|---|---|
| -1 | 0 | 1 | |
| 反应温度(A)/℃ | 25 | 35 | 45 |
| 反应时间(B)/h | 6 | 9 | 12 |
| 异丁醇钾与甲基异丁基酮摩尔比(C) | 1∶1 | 1.5∶1 | 2∶1 |
| 溶剂用量(D) | 3∶1 | 4.5∶1 | 6∶1 |
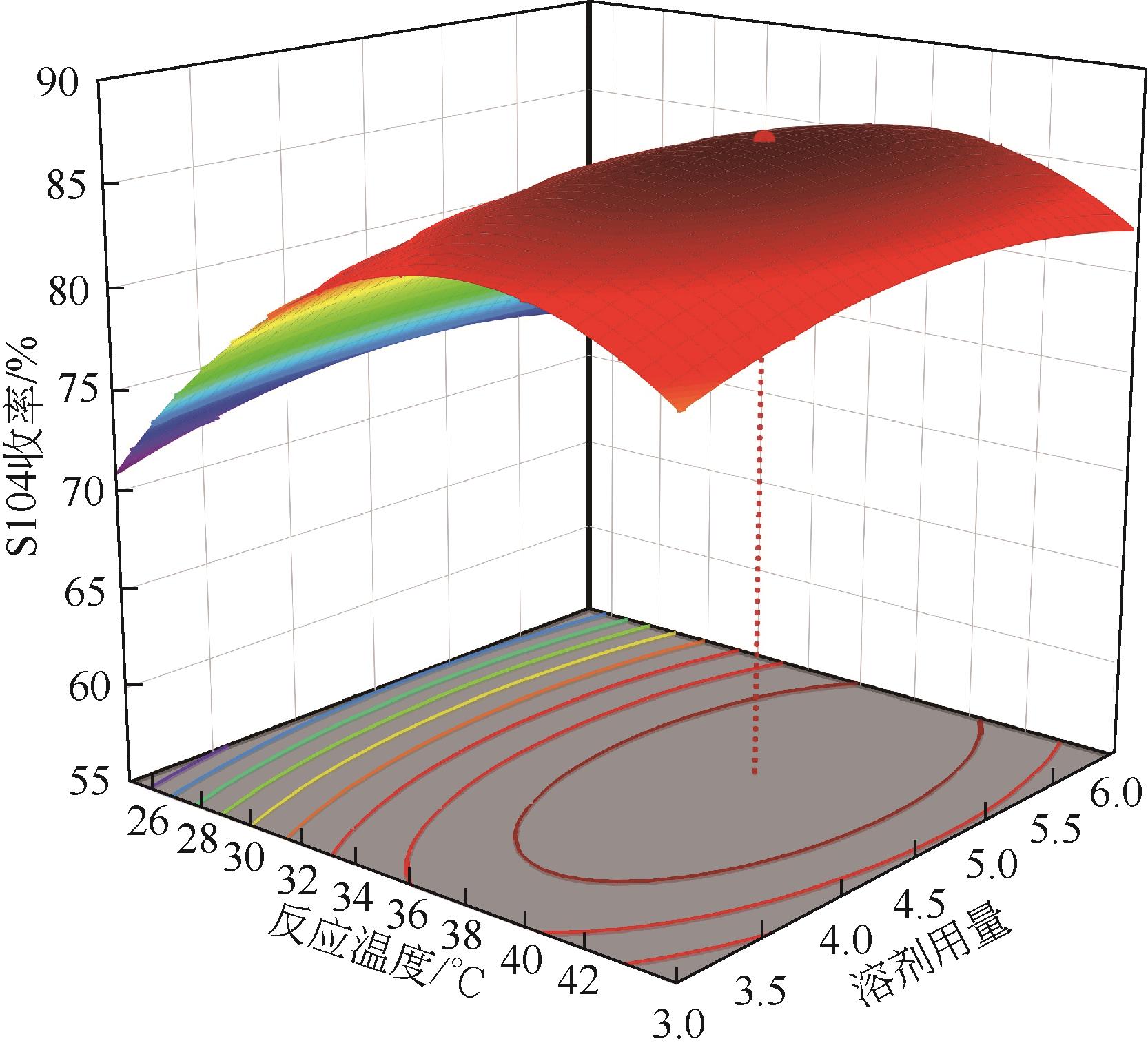
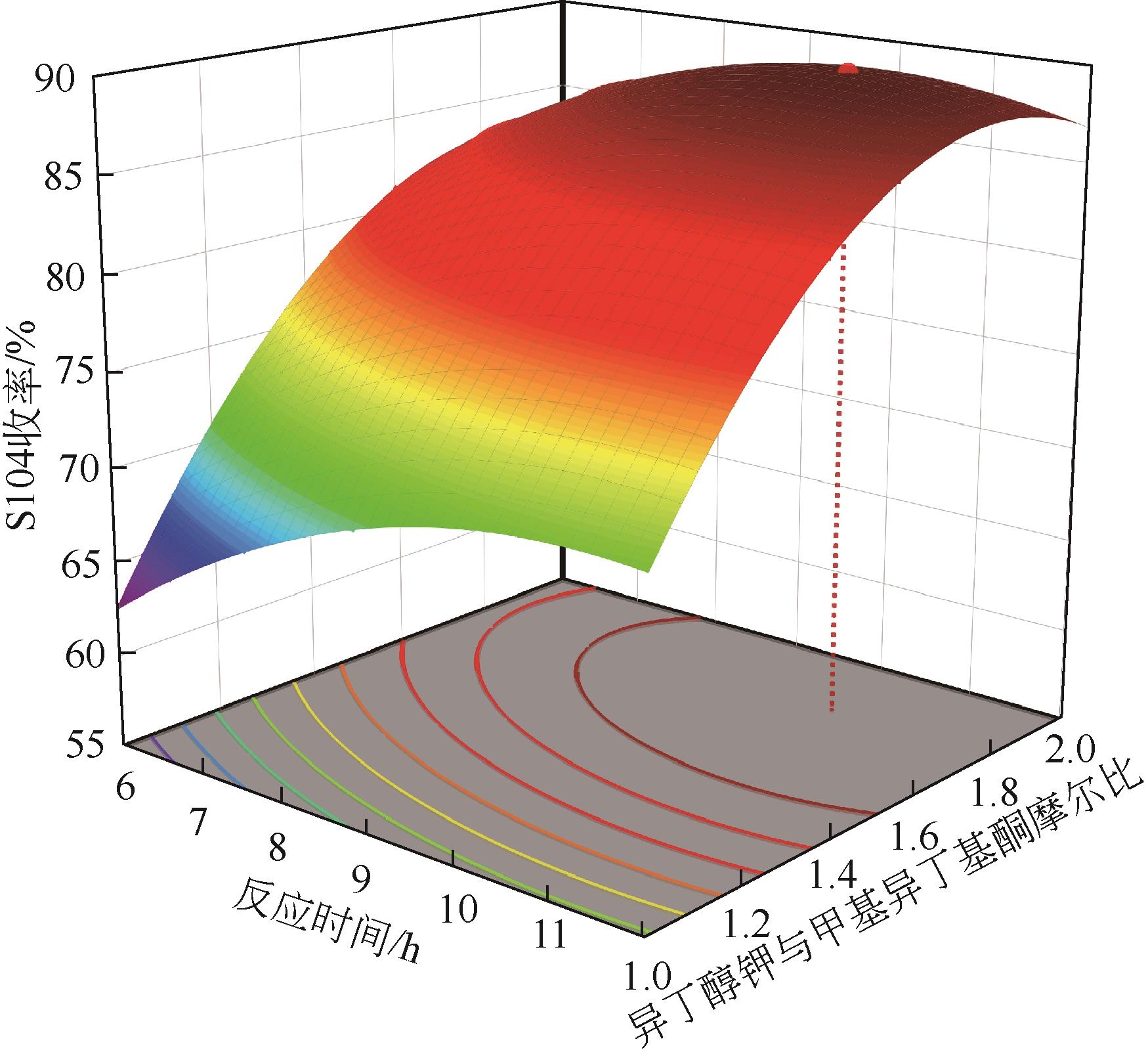
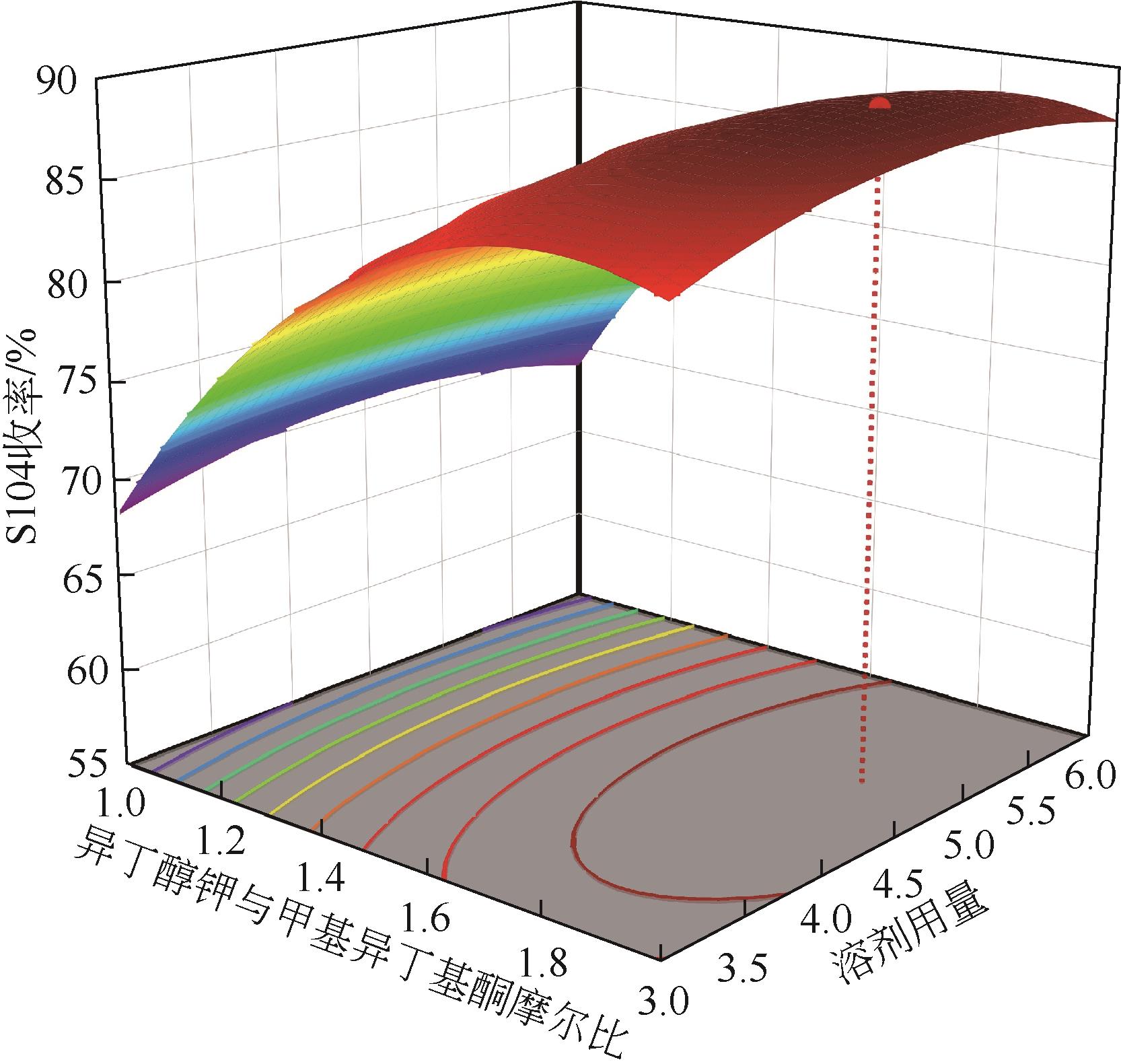
| 序号 | 实验因素 | S104收率/% | |||
|---|---|---|---|---|---|
| A/℃ | B/h | C | D | ||
| 1 | 25 | 9 | 1 | 4.5 | 56.78 |
| 2 | 35 | 12 | 1 | 4.5 | 70.95 |
| 3 | 35 | 12 | 2 | 4.5 | 86.01 |
| 4 | 35 | 6 | 1.5 | 3 | 75.86 |
| 5 | 35 | 9 | 1.5 | 4.5 | 87.48 |
| 6 | 45 | 9 | 2 | 4.5 | 80.87 |
| 7 | 45 | 6 | 1.5 | 4.5 | 76.77 |
| 8 | 35 | 9 | 1.5 | 4.5 | 84.61 |
| 9 | 35 | 12 | 1.5 | 6 | 86.42 |
| 10 | 35 | 6 | 1.5 | 6 | 75.09 |
| 11 | 35 | 9 | 2 | 3 | 85.88 |
| 12 | 25 | 9 | 1.5 | 6 | 71.58 |
| 13 | 25 | 9 | 2 | 4.5 | 76.97 |
| 14 | 35 | 6 | 2 | 4.5 | 83.86 |
| 15 | 35 | 9 | 1.5 | 4.5 | 86.41 |
| 16 | 35 | 9 | 1.5 | 4.5 | 84.89 |
| 17 | 35 | 9 | 1 | 3 | 68.77 |
| 18 | 45 | 9 | 1.5 | 6 | 83.54 |
| 19 | 25 | 9 | 1.5 | 3 | 69.03 |
| 20 | 35 | 9 | 1.5 | 4.5 | 86.59 |
| 21 | 35 | 9 | 2 | 6 | 88.23 |
| 22 | 25 | 6 | 1.5 | 4.5 | 69.02 |
| 23 | 35 | 9 | 1 | 6 | 69.23 |
| 24 | 35 | 12 | 1.5 | 3 | 83.4 |
| 25 | 45 | 9 | 1.5 | 3 | 80.98 |
| 26 | 45 | 9 | 1 | 4.5 | 68.96 |
| 27 | 35 | 6 | 1 | 4.5 | 62.98 |
| 28 | 45 | 12 | 1.5 | 4.5 | 83.87 |
| 29 | 25 | 12 | 1.5 | 4.5 | 74.32 |
| 序号 | 实验因素 | S104收率/% | |||
|---|---|---|---|---|---|
| A/℃ | B/h | C | D | ||
| 1 | 25 | 9 | 1 | 4.5 | 56.78 |
| 2 | 35 | 12 | 1 | 4.5 | 70.95 |
| 3 | 35 | 12 | 2 | 4.5 | 86.01 |
| 4 | 35 | 6 | 1.5 | 3 | 75.86 |
| 5 | 35 | 9 | 1.5 | 4.5 | 87.48 |
| 6 | 45 | 9 | 2 | 4.5 | 80.87 |
| 7 | 45 | 6 | 1.5 | 4.5 | 76.77 |
| 8 | 35 | 9 | 1.5 | 4.5 | 84.61 |
| 9 | 35 | 12 | 1.5 | 6 | 86.42 |
| 10 | 35 | 6 | 1.5 | 6 | 75.09 |
| 11 | 35 | 9 | 2 | 3 | 85.88 |
| 12 | 25 | 9 | 1.5 | 6 | 71.58 |
| 13 | 25 | 9 | 2 | 4.5 | 76.97 |
| 14 | 35 | 6 | 2 | 4.5 | 83.86 |
| 15 | 35 | 9 | 1.5 | 4.5 | 86.41 |
| 16 | 35 | 9 | 1.5 | 4.5 | 84.89 |
| 17 | 35 | 9 | 1 | 3 | 68.77 |
| 18 | 45 | 9 | 1.5 | 6 | 83.54 |
| 19 | 25 | 9 | 1.5 | 3 | 69.03 |
| 20 | 35 | 9 | 1.5 | 4.5 | 86.59 |
| 21 | 35 | 9 | 2 | 6 | 88.23 |
| 22 | 25 | 6 | 1.5 | 4.5 | 69.02 |
| 23 | 35 | 9 | 1 | 6 | 69.23 |
| 24 | 35 | 12 | 1.5 | 3 | 83.4 |
| 25 | 45 | 9 | 1.5 | 3 | 80.98 |
| 26 | 45 | 9 | 1 | 4.5 | 68.96 |
| 27 | 35 | 6 | 1 | 4.5 | 62.98 |
| 28 | 45 | 12 | 1.5 | 4.5 | 83.87 |
| 29 | 25 | 12 | 1.5 | 4.5 | 74.32 |
| 方差来源 | 平方和 | 自由度 | 均方 | F | P | 显著性 |
|---|---|---|---|---|---|---|
| 回归方程 | 1936.259 | 14 | 138.3042 | 51.60691 | < 0.0001 | + + |
| 反应温度A | 273.512 | 1 | 273.512 | 102.0584 | < 0.0001 | + + |
| 反应时间B | 142.761 | 1 | 142.761 | 53.26993 | < 0.0001 | + + |
| 异丁醇钾与甲基异丁基酮摩尔比C | 903.9352 | 1 | 903.9352 | 337.2949 | < 0.0001 | + + |
| 溶剂用量D | 8.619075 | 1 | 8.619075 | 3.216127 | 0.0945 | |
| AB | 0.81 | 1 | 0.81 | 0.302244 | 0.5911 | |
| AC | 17.1396 | 1 | 17.1396 | 6.395481 | 0.0241 | + |
| AD | 2.5×10-5 | 1 | 2.5×10-5 | 9.33×10-6 | 0.9976 | |
| BC | 8.4681 | 1 | 8.4681 | 3.159792 | 0.0972 | |
| BD | 3.591025 | 1 | 3.591025 | 1.339957 | 0.2664 | |
| CD | 0.893025 | 1 | 0.893025 | 0.333224 | 0.5729 | |
| A2 | 378.1677 | 1 | 378.1677 | 141.1097 | < 0.0001 | + + |
| B2 | 64.48482 | 1 | 64.48482 | 24.0619 | 0.0002 | + + |
| C2 | 298.6574 | 1 | 298.6574 | 111.4412 | < 0.0001 | + + |
| D2 | 25.18619 | 1 | 25.18619 | 9.397988 | 0.0084 | + + |
| 残差 | 37.51937 | 14 | 2.679955 | |||
| 失拟项 | 31.64865 | 10 | 3.164865 | 2.156373 | 0.2388 | |
| 纯误差 | 5.87072 | 4 | 1.46768 | |||
| 总和 | 1973.778 | 28 |
| 方差来源 | 平方和 | 自由度 | 均方 | F | P | 显著性 |
|---|---|---|---|---|---|---|
| 回归方程 | 1936.259 | 14 | 138.3042 | 51.60691 | < 0.0001 | + + |
| 反应温度A | 273.512 | 1 | 273.512 | 102.0584 | < 0.0001 | + + |
| 反应时间B | 142.761 | 1 | 142.761 | 53.26993 | < 0.0001 | + + |
| 异丁醇钾与甲基异丁基酮摩尔比C | 903.9352 | 1 | 903.9352 | 337.2949 | < 0.0001 | + + |
| 溶剂用量D | 8.619075 | 1 | 8.619075 | 3.216127 | 0.0945 | |
| AB | 0.81 | 1 | 0.81 | 0.302244 | 0.5911 | |
| AC | 17.1396 | 1 | 17.1396 | 6.395481 | 0.0241 | + |
| AD | 2.5×10-5 | 1 | 2.5×10-5 | 9.33×10-6 | 0.9976 | |
| BC | 8.4681 | 1 | 8.4681 | 3.159792 | 0.0972 | |
| BD | 3.591025 | 1 | 3.591025 | 1.339957 | 0.2664 | |
| CD | 0.893025 | 1 | 0.893025 | 0.333224 | 0.5729 | |
| A2 | 378.1677 | 1 | 378.1677 | 141.1097 | < 0.0001 | + + |
| B2 | 64.48482 | 1 | 64.48482 | 24.0619 | 0.0002 | + + |
| C2 | 298.6574 | 1 | 298.6574 | 111.4412 | < 0.0001 | + + |
| D2 | 25.18619 | 1 | 25.18619 | 9.397988 | 0.0084 | + + |
| 残差 | 37.51937 | 14 | 2.679955 | |||
| 失拟项 | 31.64865 | 10 | 3.164865 | 2.156373 | 0.2388 | |
| 纯误差 | 5.87072 | 4 | 1.46768 | |||
| 总和 | 1973.778 | 28 |
| 循环次数 | 甲基异丁基酮转化率/% | S104收率/% |
|---|---|---|
| 新配氢氧化钾溶液 | 92.46 | 85.91 |
| 1 | 89.14 | 83.19 |
| 2 | 90.29 | 83.77 |
| 3 | 93.82 | 87.16 |
| 4 | 93.11 | 86.87 |
| 5 | 91.22 | 84.52 |
| 循环次数 | 甲基异丁基酮转化率/% | S104收率/% |
|---|---|---|
| 新配氢氧化钾溶液 | 92.46 | 85.91 |
| 1 | 89.14 | 83.19 |
| 2 | 90.29 | 83.77 |
| 3 | 93.82 | 87.16 |
| 4 | 93.11 | 86.87 |
| 5 | 91.22 | 84.52 |
| 1 | GALGOCI E C, CHAN S Y, KHALIL Y. Innovative, gemini-type molecular defoamer technology for improved coating aesthetics[J]. JCT Research, 2006, 3(1): 77-85. |
| 2 | LEE F J. Acetylenic glycol-based surfactants for use in fountain solutions[J]. American Ink Maker, 1998,76(9): 30-36. |
| 3 | ZHANG P, KING D M, KARWACKI E J. Evaluating the impact of an acetylenic diol-type surfactant on DUV lithography performance[J]. Micro, 2002,20(6): 51-55. |
| 4 | JIAO Qingbin, ZHU Chunlin, TAN Xin, et al. The effect of ultrasonic vibration and surfactant additive on fabrication of 53.5gr/mm silicon echelle grating with low surface roughness in alkaline KOH solution[J]. Ultrasonics Sonochemistry, 2018, 40: 937-943. |
| 5 | TEDESCHI R J. Acetylene-based chemicals from coal and other natural resources[M]. New York: M. Dekker, 1982. |
| 6 | KELLAND M A, DIRDAL E G. Powerful synergy of acetylenic diol surfactants with kinetic hydrate inhibitor polymers—Choosing the correct synergist aqueous solubility[J]. Energy & Fuels, 2021, 35(19): 15721-15727. |
| 7 | CHRISTINE Louis. New additives for waterbased coatings: A new class of defoamers is born! [J]. Pitture e Vernici, European Coatings, 2003,79(20): 7-19. |
| 8 | DOUGLAS Bohn. The global waterborne coatings market keeps growing[J]. European Coatings Journal, 2021, 10: 16-17. |
| 9 | TROFIMOV B A, SCHMIDT E Y E. Reactions of acetylenes in superbasic media. recent advances[J]. Russian Chemical Reviews, 2014, 83(7): 600-619. |
| 10 | TROFIMOV B A, NOSYREVA V V, MAL'KINA A G. The role of potassium cation in the favorskii ethynylation of acetone: Effect of dibenzo-18-crown-6[J]. Russian Journal of Organic Chemistry, 2005, 41(9): 1254-1259. |
| 11 | DANILKINA N, VASIL'EVA A A, BALOVA I. A.E.Favorskii’s scientific legacy in modern organic chemistry: Prototropic acetylene-allene isomerization and the acetylene zipper reaction[J]. Russian Chemical Reviews, 2020, 89: 125-171. |
| 12 | TEDESCHI R J, WILSON M F, SCANLON J, et al. The reaction of alkali metal hydroxides with tertiary acetylenic carbinols and glycols[J]. The Journal of Organic Chemistry, 1963, 28(9): 2480-2483. |
| 13 | TEDESCHI R J. The mechanism of base-catalyzed ethynylation in donor solvents[J]. The Journal of Organic Chemistry, 1965, 30(9): 3045-3049. |
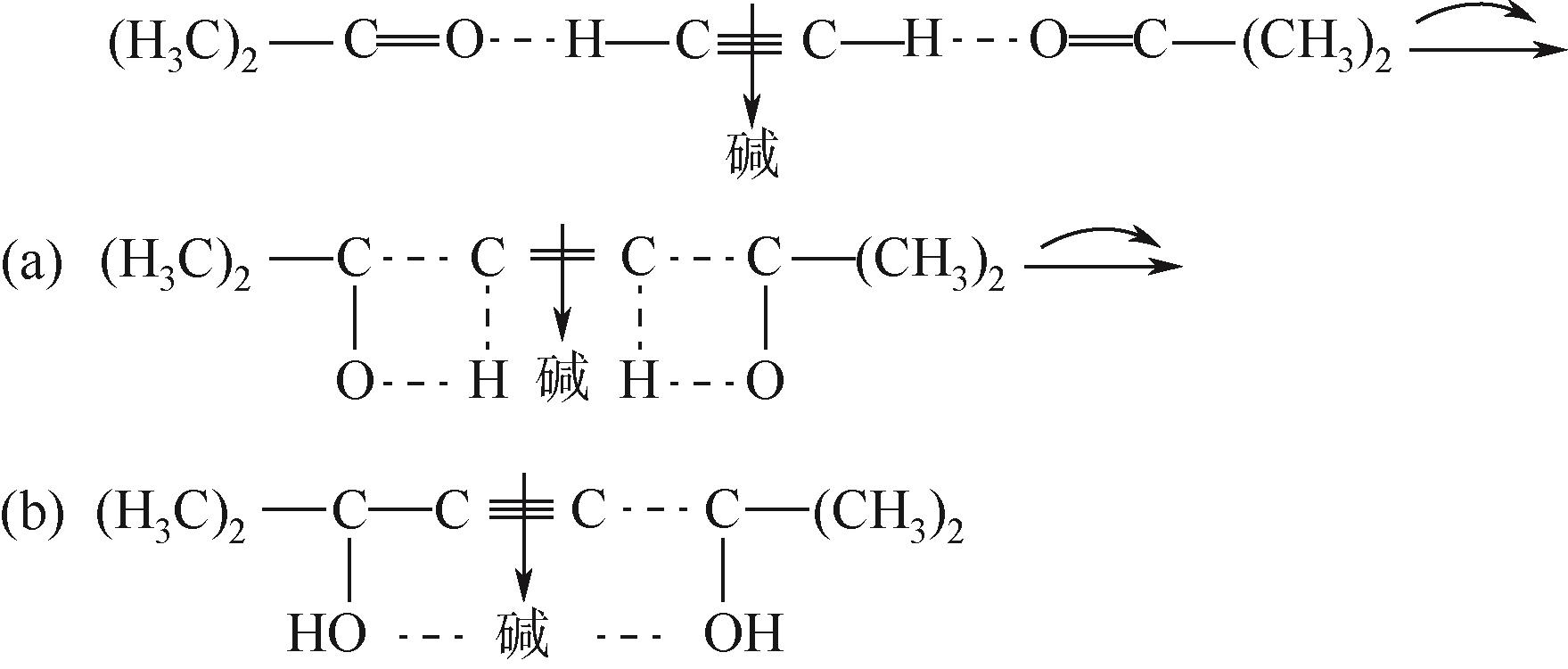
| 14 | 周飞, 陶川东, 李杰灵, 等. 游离碱对合成2,5-二甲基己炔二醇的影响[J]. 华东科技: 综合, 2018, 1(12): 399-401. |
| ZHOU Fei, TAO Chuandong, LI Jieling, et al. Effect of free base on the synthesis of 2,5 -dimethylhexynediol[J]. East China Technology: Comprehensive, 2018, 1(12): 399-401. | |
| 15 | 方善伦, 王丽蓉, 张征林. 叔炔醇的合成及用途[J]. 天然气化工(Cl化学与化工), 2003, 28(6): 43-47. |
| FANG Shanlun, WANG Lirong, ZHANG Zhenglin. Synthesis and application of tertiary alkynols[J]. Natural Gas Chemical Industry (C1 Chemistry and Technology), 2003, 28(6): 43-47. | |
| 16 | SHACHAT N, BAGNELL J J. Catalytic ethynylation of Ketones[J]. The Journal of Organic Chemistry, 1962, 27(5): 1498-1504. |
| 17 | GERHARD Thelen. Process for the manufacture of alkynediols by reaction of ketones with acetylene: US4960959[P]. 1990-10-02. |
| 18 | 齐建国, 周全凯, 金凤龙. 一种合成癸炔二醇的工艺: CN102964216A[P]. 2013-03-13. |
| QI Jianguo, ZHOU Quankai, JIN Fenglong. Decyne glycol synthesizing technology: CN102964216A[P]. 2013-03-13. | |
| 19 | 王立峰, 杨智, 孙健. 一种炔二醇系列产品的清洁生产工艺: CN103304376A[P]. 2013-09-18. |
| WANG Lifeng, YANG Zhi, SUN Jian. Clean production process of alkyne diol serial products: CN103304376A[P]. 2013-09-18. | |
| 20 | 程霜莉, 张一甫, 黄波. 一种炔二醇系列产品的生产方法: CN103242140A[P]. 2013-08-14. |
| CHENG Shuangli, ZHANG Yifu, HUANG Bo. Production method of acetylenic diol series products: CN103242140A[P]. 2013-08-14. | |
| 21 | 钟传蓉, 卢艾. 炔醇的应用与生产[J]. 油田化学, 2000, 17(3): 285-288. |
| ZHONG Chuanrong, LU Ai. The uses and production of alkynols: A review[J]. Oilfield Chemistry, 2000, 17(3): 285-288. | |
| 22 | LI Sifang. Manufacture of fine chemicals from acetylene[M]. Berlin: De Gruyter, 2021. |
| 23 | 芦艾, 饶增科. 醇钾催化合成甲基戊炔醇[J]. 化学工程师, 1998, 12(5): 5-7. |
| LU Ai, RAO Zengke. Synthesis of methyl pentynol with potassium alcoholate used as catalyst[J]. Chemical Engineer, 1998, 12(5): 5-7. | |
| 24 | KINDLER Alois, BRUNNER Melanie, TRAGUT Christian, et al. Method for producing alkyne diols: US6297407[P]. 2001-10-02. |
| 25 | 刘星玉. 2,5-二甲基己炔二醇的反应工程开发[J]. 天然气化工(C1化学与化工), 1996, 21(6): 36-40. |
| LIU Xingyu. Reaction engineering development of 2,5 dimethyl hexynediol[J]. Natural Gas Chemical Industry(C1 Chemistry and Technology), 1996, 21(6): 36-40. | |
| 26 | BADISCHE Anilin, SODA Fabric, AKTIENGESELL Schaft. Production of tertiary acetylenic glycols by reaction of acetylene with ketones: GB1329815A[P]. 1973-09-12. |
| 27 | BADISCHE Anilin, SODA Fabric, AKTIENGESELL Schaft. Production of acetylene glycols: GB1354011A[P]. 1974-5-22. |
| 28 | 刘栋昌, 李世模. 酮的乙炔化反应的化学平衡[J]. 成都科技大学学报, 1993, 25(6): 31-39. |
| LIU Dongchang, LI Shimo. Chemical equilibrium of the ethynation of ketones[J]. Journal of Chengdu University of Science and Technology, 1993, 25(6): 31-39. | |
| 29 | 方聪, 刘怡雪, 黎四芳. 爱德万甜中间体3-羟基-4-甲氧基肉桂醛的合成[J]. 化工进展, 2022, 41(11): 6053-6060. |
| FANG Cong, LIU Yixue, LI Sifang. Synthesis of 3-hydroxy-4-methoxycinnamaldehyde, an intermediate of advantame[J]. Chemical Industry and Engineering Progress, 2022, 41(11): 6053-6060. | |
| 30 | HORT E V, TAYLOR P. Kirk-Othmer encyclopedia of chemical technology, Vol. 1[M]. 5th ed. New Jersey: John Wiley and Sons, 2003. |
| [1] | LI Mengyuan, GUO Fan, LI Qunsheng. Simulation and optimization of the third and fourth distillation columns in the recovery section of polyvinyl alcohol production [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 113-123. |
| [2] | ZHANG Ruijie, LIU Zhilin, WANG Junwen, ZHANG Wei, HAN Deqiu, LI Ting, ZOU Xiong. On-line dynamic simulation and optimization of water-cooled cascade refrigeration system [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 124-132. |
| [3] | WANG Fu'an. Consumption and emission reduction of the reactor of 300kt/a propylene oxide process [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 213-218. |
| [4] | SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298. |
| [5] | ZHENG Qian, GUAN Xiushuai, JIN Shanbiao, ZHANG Changming, ZHANG Xiaochao. Photothermal catalysis synthesis of DMC from CO2 and methanol over Ce0.25Zr0.75O2 solid solution [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 319-327. |
| [6] | ZHAO Wei, ZHAO Deyin, LI Shihan, LIU Hongda, SUN Jin, GUO Yanqiu. Synthesis and application of triazine drag reducing agent for nature gas pipeline [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 391-399. |
| [7] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [8] | LI Chunli, HAN Xiaoguang, LIU Jiapeng, WANG Yatao, WANG Chenxi, WANG Honghai, PENG Sheng. Research progress of liquid distributors in packed columns [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4479-4495. |
| [9] | GENG Yuanze, ZHOU Junhu, ZHANG Tianyou, ZHU Xiaoyu, YANG Weijuan. Homogeneous/heterogeneous coupled combustion of heptane in a partially packed bed burner [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4514-4521. |
| [10] | GAO Yanjing. Analysis of international research trend of single-atom catalysis technology [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4667-4676. |
| [11] | LI Dongze, ZHANG Xiang, TIAN Jian, HU Pan, YAO Jie, ZHU Lin, BU Changsheng, WANG Xinye. Research progress of NO x reduction by carbonaceous substances for denitration in cement kiln [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4882-4893. |
| [12] | WANG Chen, BAI Haoliang, KANG Xue. Performance study of high power UV-LED heat dissipation and nano-TiO2 photocatalytic acid red 26 coupling system [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4905-4916. |
| [13] | XIANG Yang, HUANG Xun, WEI Zidong. Recent progresses in the activity and selectivity improvement of electrocatalytic organic synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4005-4014. |
| [14] | HUANG Yufei, LI Ziyi, HUANG Yangqiang, JIN Bo, LUO Xiao, LIANG Zhiwu. Research progress on catalysts for photocatalytic CO2 and CH4 reforming [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4247-4263. |
| [15] | WU Haibo, WANG Xilun, FANG Yanxiong, JI Hongbing. Progress of the development and application of 3D printing catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3956-3964. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||