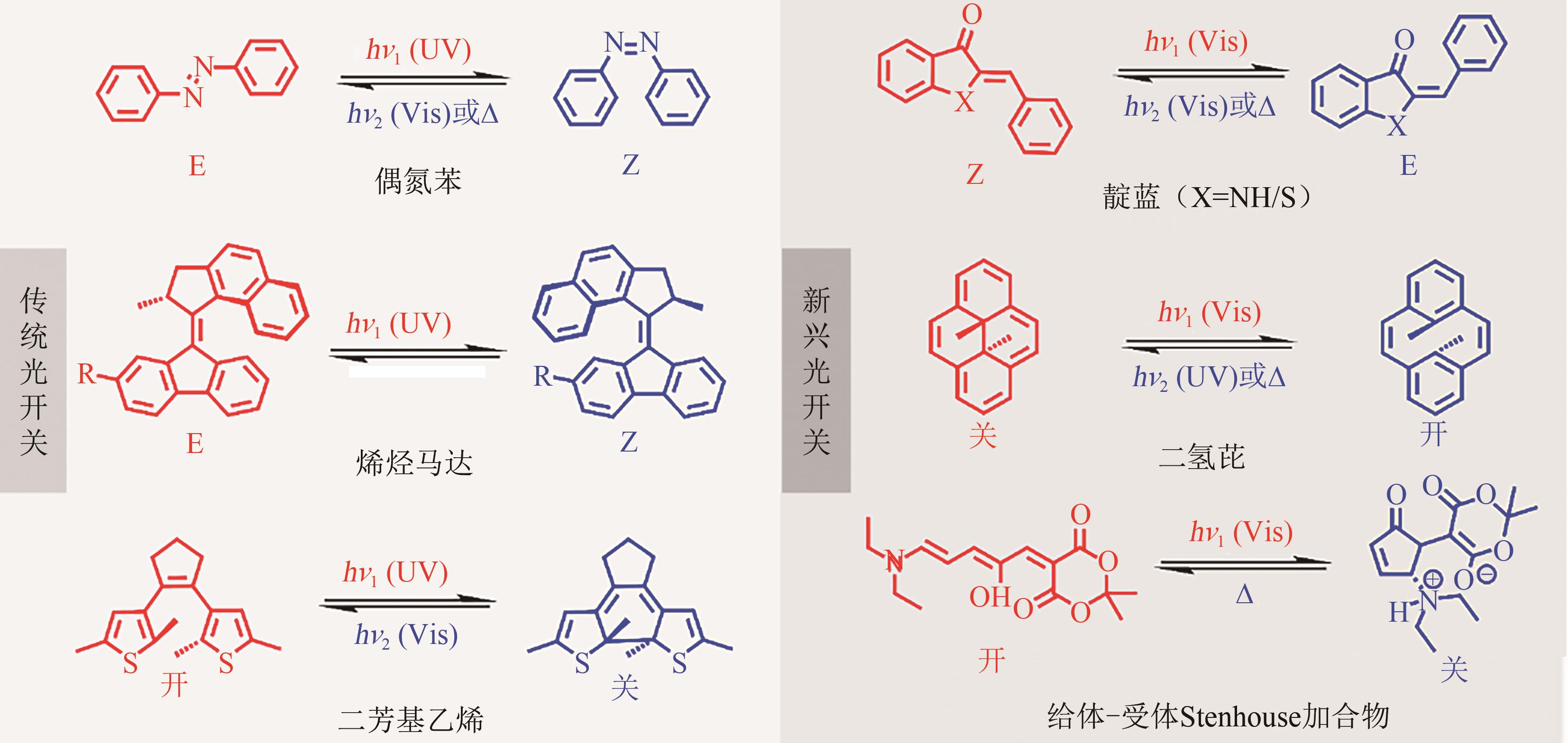
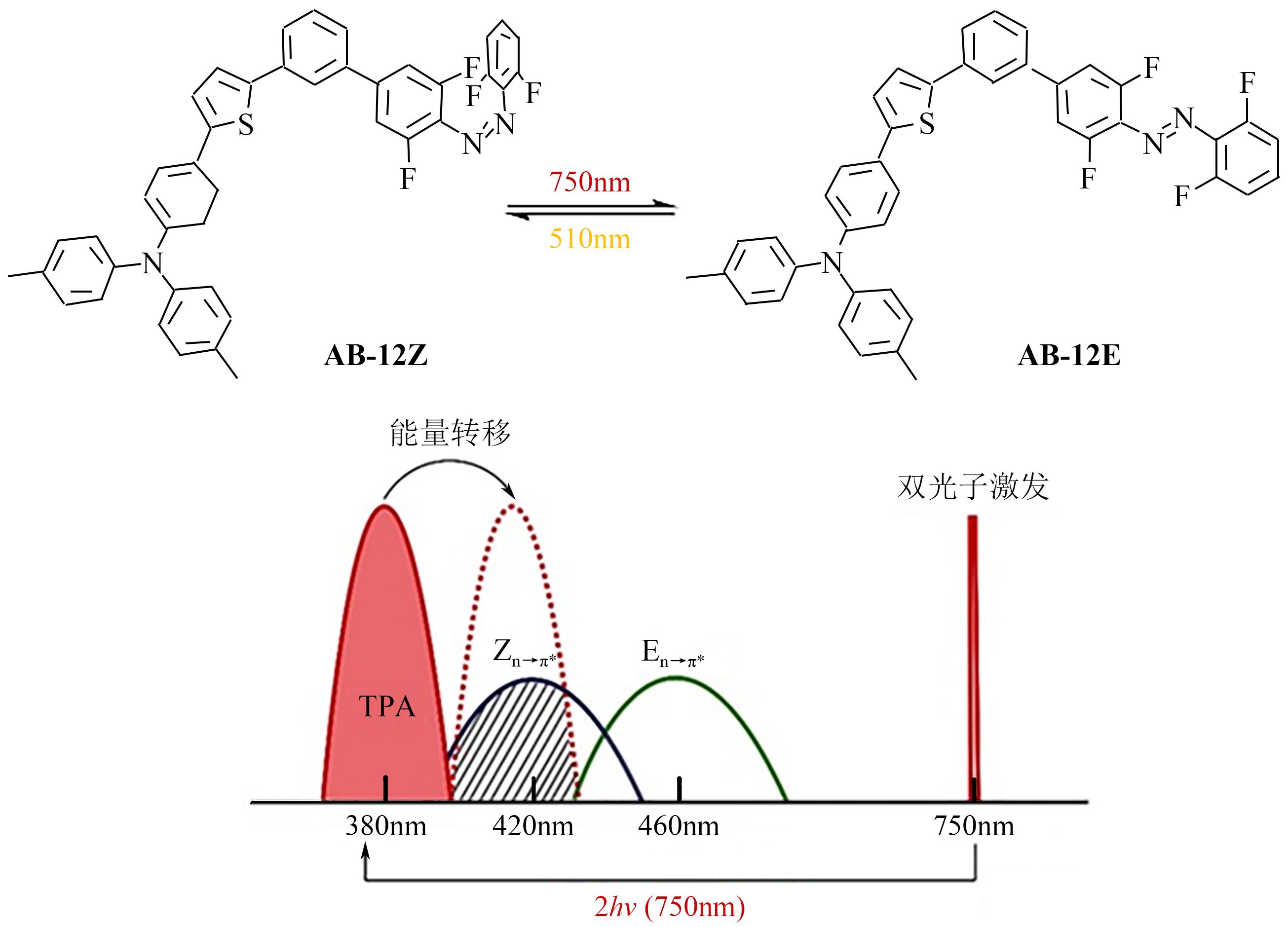
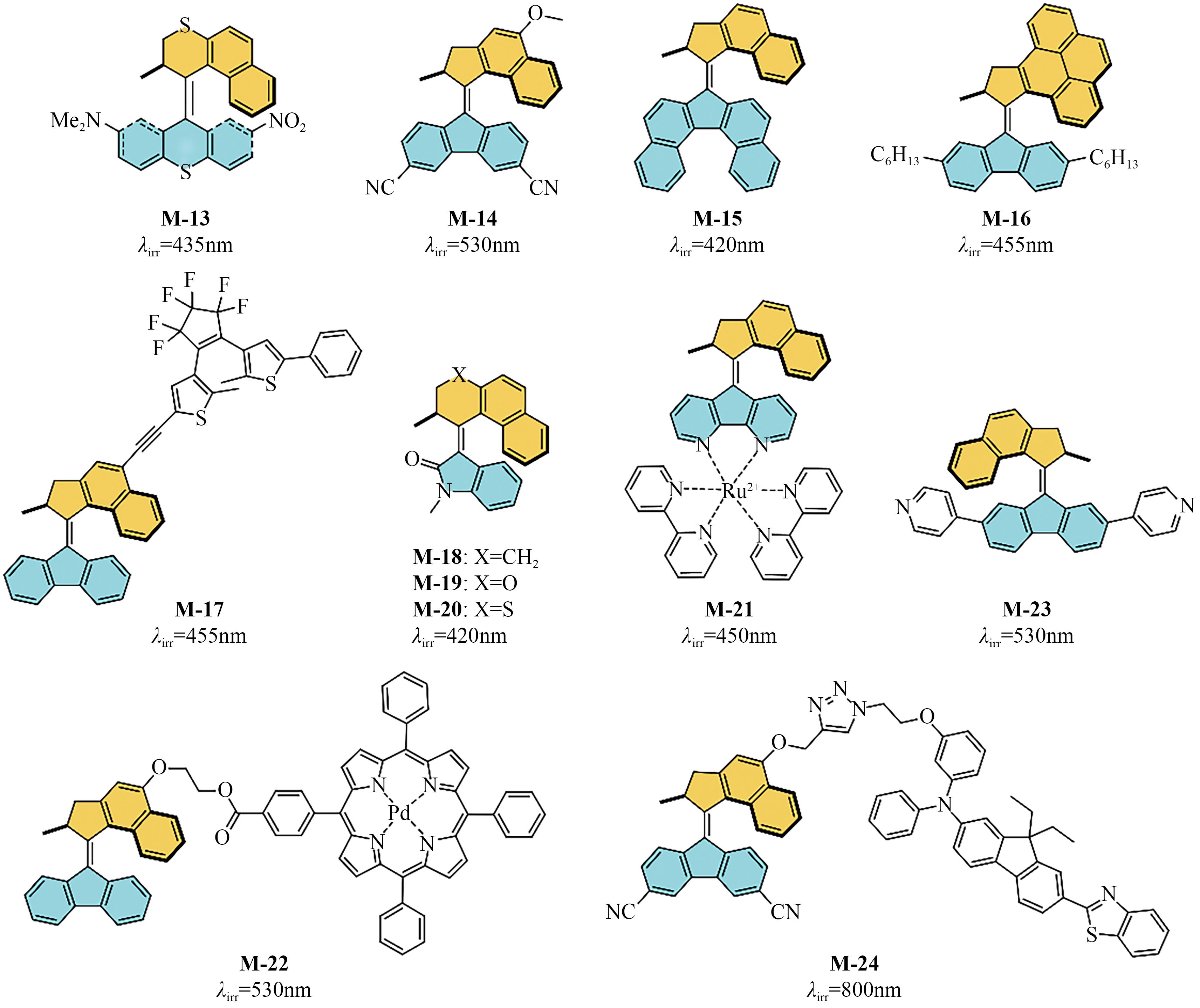
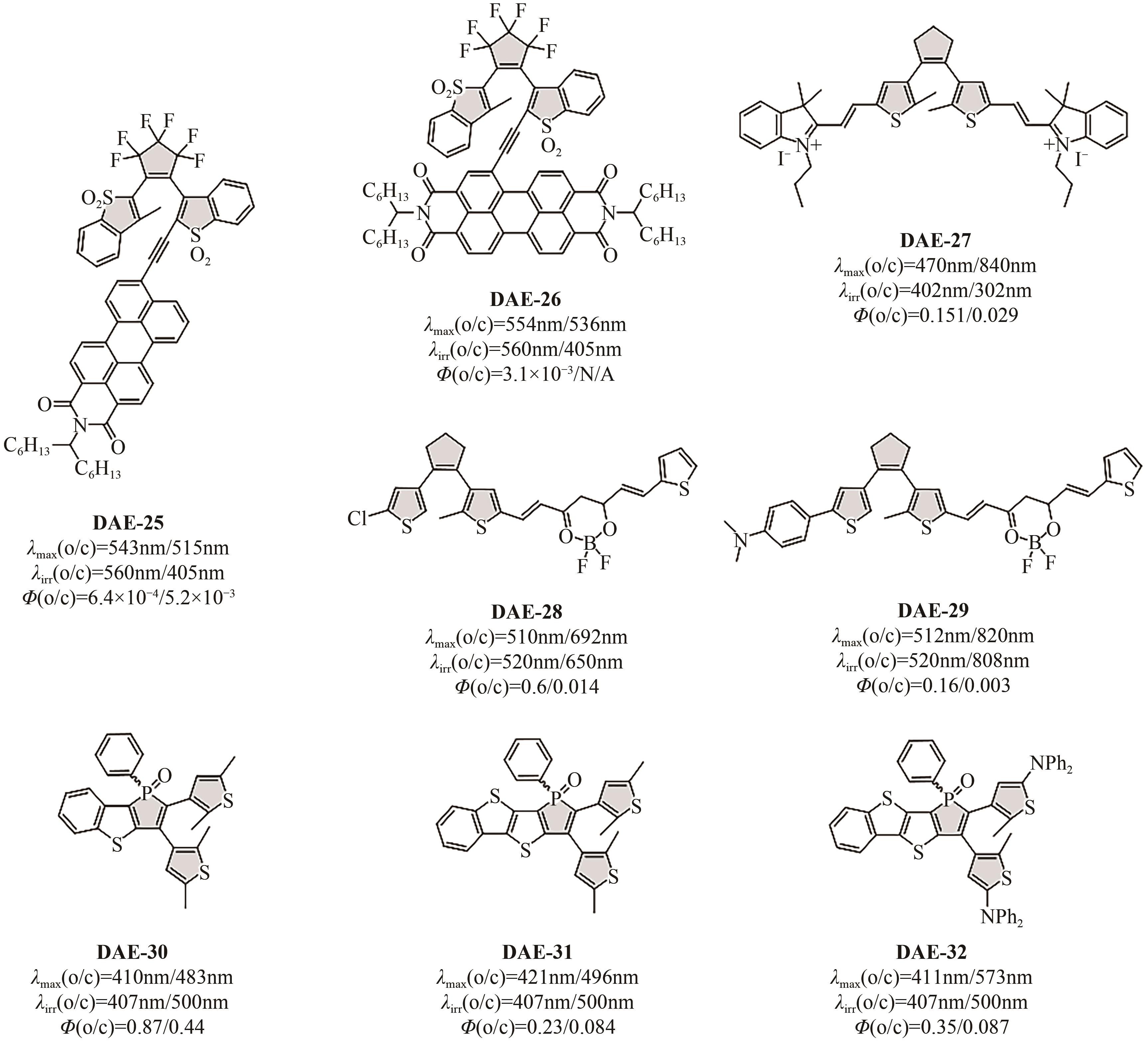
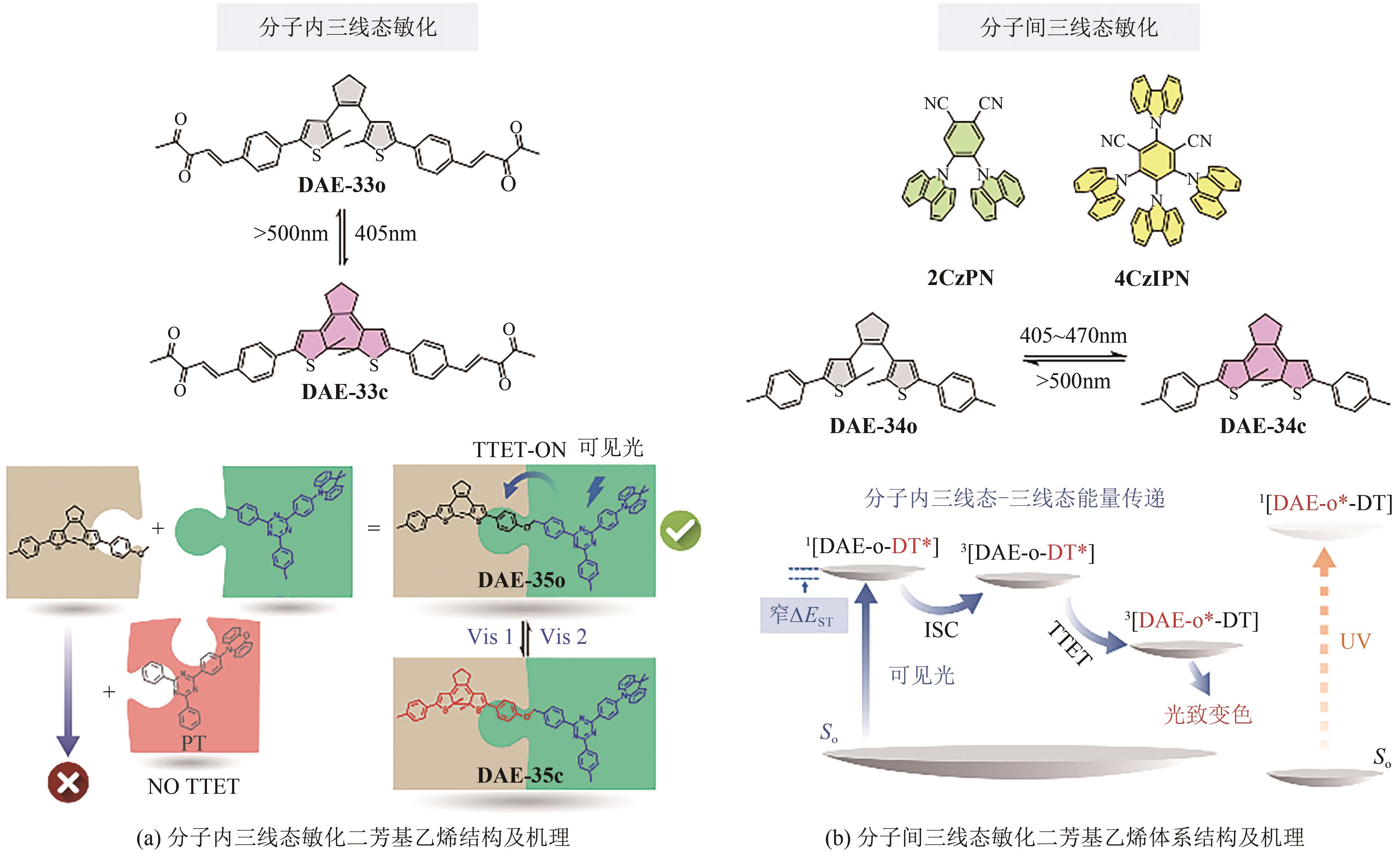
Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (8): 4058-4075.DOI: 10.16085/j.issn.1000-6613.2023-0894
Previous Articles Next Articles
Recent progress of long-wavelength-light-driven photoswitches
ZHANG Zhiwei( ), YANG Weixin, ZHANG Junji(
), YANG Weixin, ZHANG Junji( )
)
- Institute of Fine Chemicals, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai 200237, China
-
Received:2023-05-30Revised:2023-08-14Online:2023-09-19Published:2023-08-15 -
Contact:ZHANG Junji
长波长驱动光开关染料分子研究进展
- 华东理工大学化学与分子工程学院,精细化工研究所,上海 200237
-
通讯作者:张隽佶 -
作者简介:张志伟(1980—),博士,研究方向为光致变色染料。E-mail:zhiweizhang@ecust.edu.cn。 -
基金资助:国家自然科学基金(22122803)
CLC Number:
Cite this article
ZHANG Zhiwei, YANG Weixin, ZHANG Junji. Recent progress of long-wavelength-light-driven photoswitches[J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4058-4075.
张志伟, 杨伟鑫, 张隽佶. 长波长驱动光开关染料分子研究进展[J]. 化工进展, 2023, 42(8): 4058-4075.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-0894
| 48 | LI Ziyong, DAI Yijie, LU Zhiqiang, et al. Efficient green light-excited switches based on dithienylethenes with BF2-doped π-conjugated systems[J]. Chemical Communications, 2019, 55(89): 13430-13433. |
| 49 | WU Nathan Man-Wai, Maggie NG, Wai Han LAM, et al. Photochromic heterocycle-fused thieno[3,2-b]phosphole oxides as visible light switches without sacrificing photoswitching efficiency[J]. Journal of the American Chemical Society, 2017, 139(42): 15142-15150. |
| 50 | FREDRICH Sebastian, Robert GÖSTL, HERDER Martin, et al. Switching diarylethenes reliably in both directions with visible light[J]. Angewandte Chemie International Edition, 2016, 55(3): 1208-1212. |
| 51 | ZHANG Zhiwei, ZHANG Junji, WU Bin, et al. Diarylethenes with a narrow singlet-triplet energy gap sensitizer: A simple strategy for efficient visible-light photochromism[J]. Advanced Optical Materials, 2018, 6(6): 1700847. |
| 52 | ZHANG Zhiwei, WANG Wenhui, JIN Peipei, et al. A building-block design for enhanced visible-light switching of diarylethenes[J]. Nature Communications, 2019, 10: 4232. |
| 53 | XI Hancheng, ZHANG Zhipeng, ZHANG Weiwei, et al. All-visible-light-activated dithienylethenes induced by intramolecular proton transfer[J]. Journal of the American Chemical Society, 2019, 141(46): 18467-18474. |
| 54 | BOYER John-Christopher, CARLING Carl-Johan, GATES Byron D, et al. Two-way photoswitching using one type of near-infrared light, upconverting nanoparticles, and changing only the light intensity[J]. Journal of the American Chemical Society, 2010, 132(44): 15766-15772. |
| 55 | MORI Kazuya, ISHIBASHI Yukihide, MATSUDA Hirohisa, et al. One-color reversible control of photochromic reactions in a diarylethene derivative: Three-photon cyclization and two-photon cycloreversion by a near-infrared femtosecond laser pulse at 1.28μm[J]. Journal of the American Chemical Society, 2011, 133(8): 2621-2625. |
| 56 | DITTMANN Marc, GRAUPNER Franziska F, MAERZ Benjamin, et al. Photostability of 4, 4’-dihydroxythioindigo, a mimetic of indigo[J]. Angewandte Chemie International Edition, 2014, 53(2): 591-594. |
| 57 | HUANG Chung-Yang, BONASERA Aurelio, HRISTOV Lachezar, et al. N,N’-disubstituted indigos as readily available red-light photoswitches with tunable thermal half-lives[J]. Journal of the American Chemical Society, 2017, 139(42): 15205-15211. |
| 58 | PETERMAYER Christian, THUMSER Stefan, KINK Florian, et al. Hemiindigo: Highly bistable photoswitching at the biooptical window[J]. Journal of the American Chemical Society, 2017, 139(42): 15060-15067. |
| 59 | MAERZ Benjamin, WIEDBRAUK Sandra, OESTERLING Sven, et al. Making fast photoswitches faster—Using Hammett analysis to understand the limit of donor-acceptor approaches for faster hemithioindigo photoswitches[J]. Chemistry-A European Journal, 2014, 20(43): 13984-13992. |
| 60 | KINK Florian, COLLADO Marina Polo, WIEDBRAUK Sandra, et al. Frontispiece: Bistable photoswitching of hemithioindigo with green and red light: Entry point to advanced molecular digital information processing[J]. Chemistry-A European Journal, 2017, 23(26): 6237-6243. |
| 61 | ZWEIG Joshua E, NEWHOUSE Timothy R. Isomer-specific hydrogen bonding as a design principle for bidirectionally quantitative and redshifted hemithioindigo photoswitches[J]. Journal of the American Chemical Society, 2017, 139(32): 10956-10959. |
| 62 | MITCHELL Reginald H, BRKIC Zinka, SAURO Vittorio A, et al. A photochromic, electrochromic, thermochromic Ru complexed benzannulene: an organometallic example of the dimethyldihydropyrene-metacyclophanediene valence isomerization[J]. Journal of the American Chemical Society, 2003, 125(25): 7581-7585. |
| 63 | KLAUE Kristin, GARMSHAUSEN Yves, HECHT Stefan. Taking photochromism beyond visible: Direct one-photon NIR photoswitches operating in the biological window[J]. Angewandte Chemie International Edition, 2018, 57(5): 1414-1417. |
| 64 | KLAUE Kristin, HAN Wenjie, LIESFELD Pauline, et al. Donor-acceptor dihydropyrenes switchable with near-infrared light[J]. Journal of the American Chemical Society, 2020, 142(27): 11857-11864. |
| 65 | LERCH Michael M, SZYMANSKI Wiktor, FERINGA Ben L. The (photo)chemistry of Stenhouse photoswitches: Guiding principles and system design[J]. Chemical Society Reviews, 2018, 47(6): 1910-1937. |
| 66 | HELMY Sameh, LEIBFARTH Frank A, Saemi OH, et al. Photoswitching using visible light: A new class of organic photochromic molecules[J]. Journal of the American Chemical Society, 2014, 136(23): 8169-8172. |
| 67 | HEMMER James R, POELMA Saemi O, TREAT Nicolas, et al. Tunable visible and near infrared photoswitches[J]. Journal of the American Chemical Society, 2016, 138(42): 13960-13966. |
| 68 | HEMMER James R, PAGE Zachariah A, CLARK Kyle D, et al. Controlling dark equilibria and enhancing donor-acceptor stenhouse adduct photoswitching properties through carbon acid design[J]. Journal of the American Chemical Society, 2018, 140(33): 10425-10429. |
| 69 | LERCH Michael M, DI DONATO Mariangela, LAURENT Adèle D, et al. Solvent effects on the actinic step of donor-acceptor Stenhouse adduct photoswitching[J]. Angewandte Chemie International Edition, 2018, 57(27): 8063-8068. |
| 70 | KUMAR Kamlesh, KNIE Christopher, David BLÉGER, et al. A chaotic self-oscillating sunlight-driven polymer actuator[J]. Nature Communications, 2016, 7: 11975. |
| 71 | WANG Yingying, LI Mengwei, YAN Chunmei, et al. Diazocine as a versatile building block enables excellent photoswitching and chromic properties in self-assembled organogels[J]. CCS Chemistry, 2022, 4(2): 704-712. |
| 1 | SINESHCHEKOV Oleg A, SPUDICH John L. Sensory rhodopsin signaling in green flagellate algae[M]//Handbook of Photosensory Receptors. Weinheim, FRG: Wiley-VCH Verlag GmbH & Co. KGaA, 2005: 25-42. |
| 2 | CARBONELL Carlos, VALLES Daniel, WONG Alexa M, et al. Polymer brush hypersurface photolithography[J]. Nature Communications, 2020, 11: 1244. |
| 3 | PHAM Thanh Chung, van Nghia NGUYEN, CHOI Yeonghwan, et al. Recent strategies to develop innovative photosensitizers for enhanced photodynamic therapy[J]. Chemical Reviews, 2021, 121(21): 13454-13619. |
| 4 | CRESPI Stefano, SIMETH Nadja A, Burkhard KÖNIG. Heteroaryl azo dyes as molecular photoswitches[J]. Nature Reviews Chemistry, 2019, 3(3): 133-146. |
| 5 | ZHANG Junji, TIAN He. The endeavor of diarylethenes: New structures, high performance, and bright future[J]. Advanced Optical Materials, 2018, 6(6): 1701278. |
| 6 | TIAN He, ZHANG Junji. Photochromic materials: preparation, properties and applications[M]. New York, John Wiley & Sons Inc., 2016. |
| 7 | WU Yue, XIE Yongshu, ZHANG Qiong, et al. Quantitative photoswitching in bis(dithiazole)ethene enables modulation of light for encoding optical signals[J]. Angewandte Chemie International Edition, 2014, 53(8): 2090-2094. |
| 8 | Jana VOLARIĆ, SZYMANSKI Wiktor, SIMETH Nadja A, et al. Molecular photoswitches in aqueous environments[J]. Chemical Society Reviews, 2021, 50(22): 12377-12449. |
| 9 | ZHAO Hui, Soumyo SEN, UDAYABHASKARARAO T, et al. Reversible trapping and reaction acceleration within dynamically self-assembling nanoflasks[J]. Nature Nanotechnology, 2016, 11(1): 82-88. |
| 10 | HOU Lili, ZHANG Xiaoyan, COTELLA Giovanni F, et al. Optically switchable organic light-emitting transistors[J]. Nature Nanotechnology, 2019, 14(4): 347-353. |
| 11 | JIA Chuancheng, Migliore Agostino, XIN Na, et al. Covalently bonded single-molecule junctions with stable and reversible photoswitched conductivity[J]. Science, 2016, 352(6292): 1443-1445. |
| 12 | EISENREICH Fabian, KATHAN Michael, DALLMANN Andre, et al. A photoswitchable catalyst system for remote-controlled (co)polymerization in situ [J]. Nature Catalysis, 2018, 1(7): 516-522. |
| 13 | XU Wencong, SUN Shaodong, WU Si. Photoinduced reversible solid-to-liquid transitions for photoswitchable materials[J]. Angewandte Chemie International Edition, 2019, 58(29): 9712-9740. |
| 14 | Wiktor SZYMAŃSKI, BEIERLE John M, KISTEMAKER Hans A V, et al. Reversible photocontrol of biological systems by the incorporation of molecular photoswitches[J]. Chemical Reviews, 2013, 113(8): 6114-6178. |
| 15 | LUBBE Anouk S, SZYMANSKI Wiktor, FERINGA Ben L. Recent developments in reversible photoregulation of oligonucleotide structure and function[J]. Chemical Society Reviews, 2017, 46(4): 1052-1079. |
| 16 | LI Ziyuan, LIU Yingya, LI Yuanjie, et al. High-preservation single-cell operation through a photo-responsive hydrogel-nanopipette system[J]. Angewandte Chemie International Edition, 2021, 60(10): 5157-5161. |
| 17 | Katharina HÜLL, MORSTEIN Johannes, TRAUNER Dirk. In vivo photopharmacology[J]. Chemical Reviews, 2018, 118(21): 10710-10747. |
| 18 | FUCHTER Matthew J. On the promise of photopharmacology using photoswitches: A medicinal chemist’s perspective[J]. Journal of Medicinal Chemistry, 2020, 63(20): 11436-11447. |
| 19 | CHAI Xianzhi, HAN Haihao, SEDGWICK Adam C, et al. Photochromic fluorescent probe strategy for the super-resolution imaging of biologically important biomarkers[J]. Journal of the American Chemical Society, 2020, 142(42): 18005-18013. |
| 20 | KALKA Katrin, MERK Hans, MUKHTAR Hasan. Photodynamic therapy in dermatology[J]. Journal of the American Academy of Dermatology, 2000, 42(3): 389-413. |
| 21 | FRAZIER Claude C. Photodynamic therapy in dermatology[J]. International Journal of Dermatology, 1996, 35(5): 312-316. |
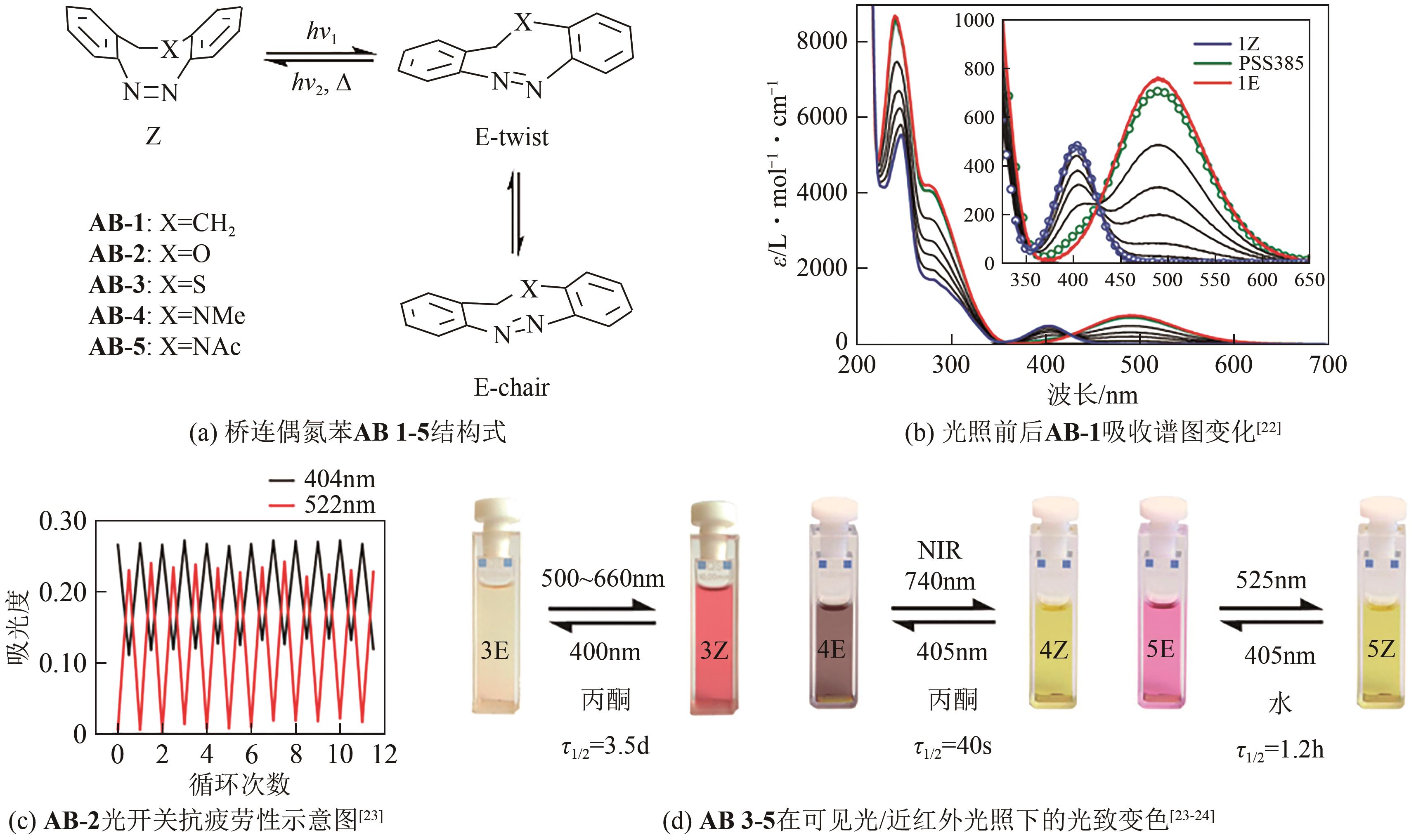
| 22 | SIEWERTSEN R, NEUMANN H, BUCHHEIM-STEHN B, et al. Highly efficient reversible Z-E photoisomerization of a bridged azobenzene with visible light through resolved S(1)(n pi*) absorption bands[J]. Journal of the American Chemical Society, 2009, 131(43): 15594-15595. |
| 23 | HAMMERICH Melanie, Christian SCHÜTT, Cosima STÄHLER, et al. Heterodiazocines: Synthesis and photochromic properties, Trans to Cis switching within the bio-optical window[J]. Journal of the American Chemical Society, 2016, 138(40): 13111-13114. |
| 24 | LENTES Pascal, STADLER Eduard, Fynn RÖHRICHT, et al. Nitrogen bridged diazocines: Photochromes switching within the near-infrared region with high quantum yields in organic solvents and in water[J]. Journal of the American Chemical Society, 2019, 141(34): 13592-13600. |
| 72 | ZHAO Yunhan, MA Liangwei, HUANG Zizhao, et al. Visible light activated organic room-temperature phosphorescence based on triplet-to-singlet Förster-resonance energy transfer[J]. Advanced Optical Materials, 2022, 10(8): 2102701. |
| 73 | LI Ziyuan, WANG Chen, LI Jiang, et al. Functional DNA structures and their biomedical applications[J]. CCS Chemistry, 2020, 2(5): 707-728. |
| 74 | ZHENG Zhigang, HU Honglong, ZHANG Zhipeng, et al. Digital photoprogramming of liquid-crystal superstructures featuring intrinsic chiral photoswitches[J]. Nature Photonics, 2022, 16(3): 226-234. |
| 75 | VELEMA Willem. A, SZYMAŃSKI Wiktor, FERINGA Ben L, Photopharmacology: Beyond proof of principle[J]. Journal of the American Chemical Society, 2014, 136(6): 2178-2191. |
| 25 | BEHARRY Andrew A, SADOVSKI Oleg, Andrew WOOLLEY G. Azobenzene photoswitching without ultraviolet light[J]. Journal of the American Chemical Society, 2011, 133(49): 19684-19687. |
| 26 | SAMANTA Subhas, BEHARRY Andrew A, SADOVSKI Oleg, et al. Photoswitching azo compounds in vivo with red light[J]. Journal of the American Chemical Society, 2013, 135(26): 9777-9784. |
| 27 | DONG Mingxin, BABALHAVAEJI Amirhossein, COLLINS Catherine V, et al. Near-infrared photoswitching of azobenzenes under physiological conditions[J]. Journal of the American Chemical Society, 2017, 139(38): 13483-13486. |
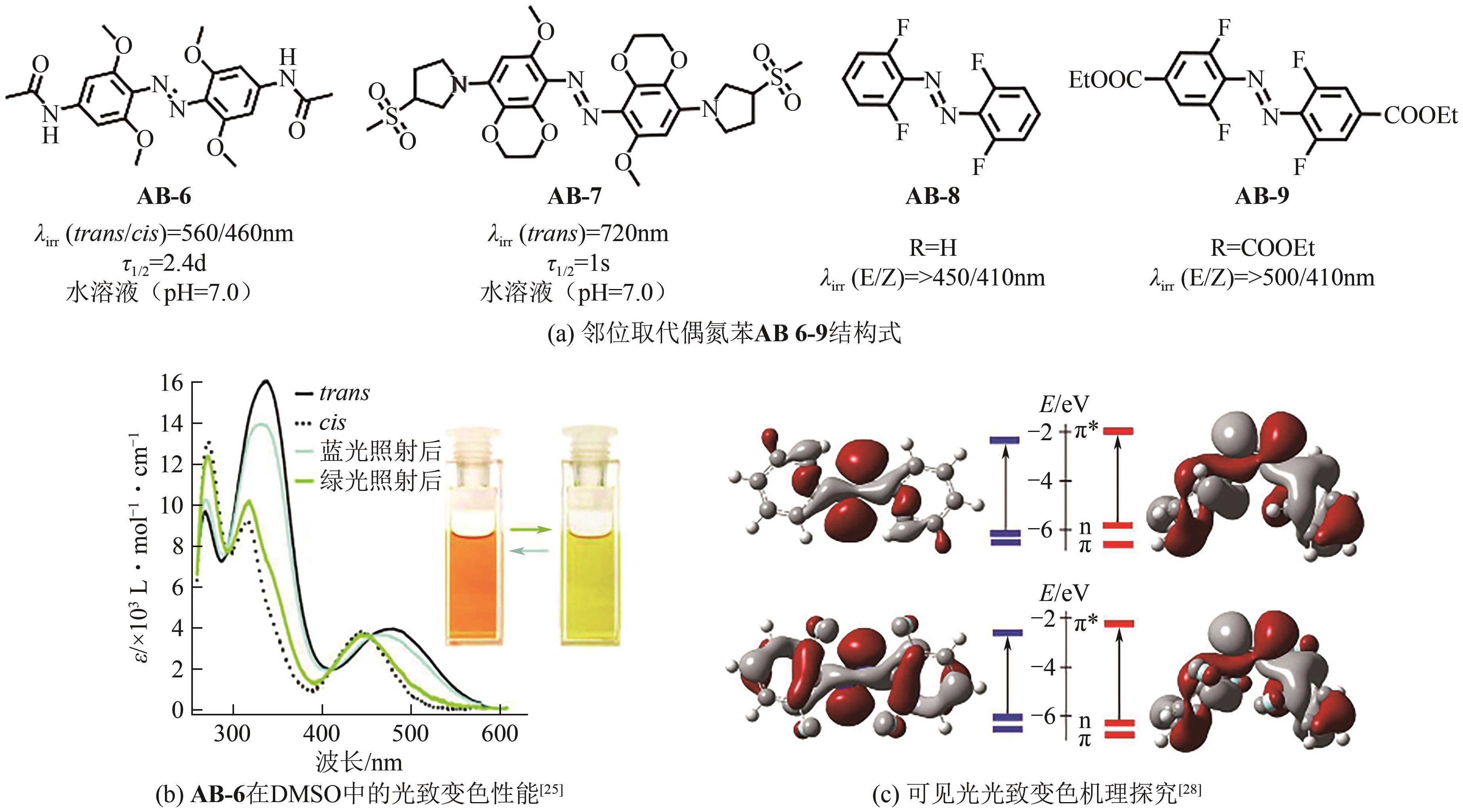
| 28 | David BLÉGER, SCHWARZ Jutta, BROUWER Albert M, et al. O-fluoroazobenzenes as readily synthesized photoswitches offering nearly quantitative two-way isomerization with visible light[J]. Journal of the American Chemical Society, 2012, 134(51): 20597-20600. |
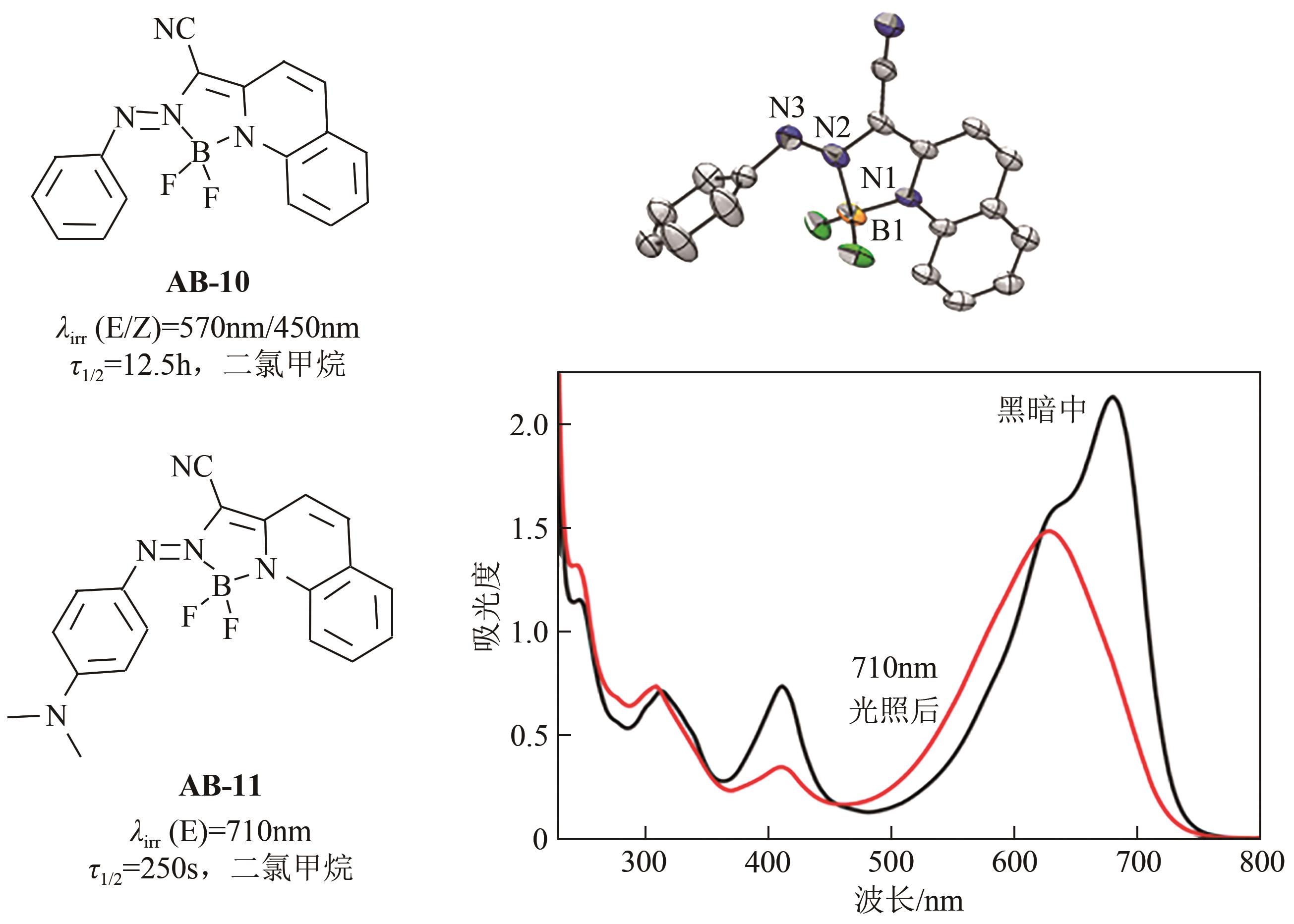
| 29 | YANG Yin, HUGHES Russell P, APRAHAMIAN Ivan. Visible light switching of a BF2-coordinated azo compound[J]. Journal of the American Chemical Society, 2012, 134(37): 15221-15224. |
| 30 | YANG Yin, HUGHES Russell P, APRAHAMIAN Ivan. Near-infrared light activated azo-BF2 switches[J]. Journal of the American Chemical Society, 2014, 136(38): 13190-13193. |
| 31 | WALKER Edwin, RENTZEPIS Peter M. A new dimension[J]. Nature Photonics, 2008, 2(7): 406-408. |
| 32 | MORENO Javier, GERECKE Mario, GRUBERT Lutz, et al. Sensitized two-NIR-photon Z→E isomerization of a visible-light-addressable bistable azobenzene derivative[J]. Angewandte Chemie International Edition, 2016, 55(4): 1544-1547. |
| 33 | KOUMURA N, ZIJLSTRA R W, VAN DELDEN R A, et al. Light-driven monodirectional molecular rotor[J]. Nature, 1999, 401(6749): 152-155. |
| 34 | WANG Jiaobing, FERINGA Ben L. Dynamic control of chiral space in a catalytic asymmetric reaction using a molecular motor[J]. Science, 2011, 331(6023): 1429-1432. |
| 35 | VAN DELDEN Richard A, KOUMURA Nagatoshi, SCHOEVAARS Annemarie, et al. A donor-acceptor substituted molecular motor: Unidirectional rotation driven by visible light[J]. Organic & Biomolecular Chemistry, 2003, 1(1): 33-35. |
| 36 | PFEIFER Lukas, Maximilian SCHERÜBL, FELLERT Maximilian, et al. Photoefficient 2nd generation molecular motors responsive to visible light[J]. Chemical Science, 2019, 10(38): 8768-8773. |
| 37 | SHI Zhaotao, HU Yixiong, HU Zhubin, et al. Visible-light-driven rotation of molecular motors in discrete supramolecular metallacycles[J]. Journal of the American Chemical Society, 2021, 143(1): 442-452. |
| 38 | van LEEUWEN Thomas, Jasper POL, ROKE Diederik, et al. Visible-light excitation of a molecular motor with an extended aromatic core[J]. Organic Letters, 2017, 19(6): 1402-1405. |
| 39 | ROKE Diederik, FERINGA Ben L, WEZENBERG Sander J. A visible-light-driven molecular motor based on pyrene[J]. Helvetica Chimica Acta, 2019, 102(2): e1800221. |
| 40 | ROKE Diederik, STUCKHARDT Constantin, DANOWSKI Wojciech, et al. Light-gated rotation in a molecular motor functionalized with a dithienylethene switch[J]. Angewandte Chemie International Edition, 2018, 57(33): 10515-10519. |
| 41 | ROKE Diederik, Metin SEN, DANOWSKI Wojciech, et al. Visible-light-driven tunable molecular motors based on oxindole[J]. Journal of the American Chemical Society, 2019, 141(18): 7622-7627. |
| 42 | WEZENBERG Sander J, CHEN Kuang-Yen, FERINGA Ben L. Visible-light-driven photoisomerization and increased rotation speed of a molecular motor acting as a ligand in a ruthenium(Ⅱ) complex[J]. Angewandte Chemie International Edition, 2015, 54(39): 11457-11461. |
| 43 | CNOSSEN Arjen, HOU Lili, POLLARD Michael M, et al. Driving unidirectional molecular rotary motors with visible light by intra- and intermolecular energy transfer from palladium porphyrin[J]. Journal of the American Chemical Society, 2012, 134(42): 17613-17619. |
| 44 | DANOWSKI Wojciech, CASTIGLIONI Fabio, SARDJAN Andy S, et al. Visible-light-driven rotation of molecular motors in a dual-function metal-organic framework enabled by energy transfer[J]. Journal of the American Chemical Society, 2020, 142(19): 9048-9056. |
| 45 | PFEIFER Lukas, HOANG Nong V, Maximilian SCHERÜBL, et al. Powering rotary molecular motors with low-intensity near-infrared light[J]. Science Advances, 2020, 6(44): eabb6165. |
| 46 | FUKAMINATO Tuyoshi, HIROSE Takashi, Takao DOI, et al. Molecular design strategy toward diarylethenes that photoswitch with visible light[J]. Journal of the American Chemical Society, 2014, 136(49): 17145-17154. |
| 47 | HU Fang, CAO Meijiao, MA Xiang, et al. Visible-light-dependent photocyclization: Design, synthesis, and properties of a cyanine-based dithienylethene[J]. The Journal of Organic Chemistry, 2015, 80(15): 7830-7835. |
| [1] | ZHANG Huixia, ZHOU Lishan, ZHANG Chenglei, QIAN Guanglei, XIE Chenxin, ZHU Lingzhi. Preparation of Bi2S3/TiO2 nanocone photoanode and their photoelectrocatalysis degradation of hygromycin [J]. Chemical Industry and Engineering Progress, 2023, 42(10): 5548-5557. |
| [2] | LIU Haicheng, MENG Wushuang, HUANG Zhe, YOU Yu, HUA Ruiqi, CAO Mengru. Preparation of WO3/BiOCl0.7I0.3 photocatalyst and its photocatalytic degradation mechanism [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 255-264. |
| [3] | LUO Juxiang, CHENG Deshu, LI Mingchun, XIN Meihua. Preparation of P2VP-b-PSt nano-objects via visible light-mediated polymerization-induced self-assembly at room temperature [J]. Chemical Industry and Engineering Progress, 2021, 40(5): 2676-2684. |
| [4] | ZHU Enquan, MA Yuhua, AINIWA·Munire. Research progress of red phosphorus photocatalytic materials [J]. Chemical Industry and Engineering Progress, 2019, 38(s1): 139-143. |
| [5] | GONG Jiuyan, SONG Wendong, CHEN Jialin, LI Shijie, CAI Lu, JI Lili. Ag/AgBr-diatomaceous earth composite photocatalysts with superior photocatalytic performance under visible-light irridiation [J]. Chemical Industry and Engineering Progress, 2017, 36(09): 3309-3315. |
| [6] | WANG Ao, SUN Kang, JIANG Jianchun. Immobilized phthalocyanines for visible light photodegradation of organic pollutants in water and air [J]. Chemical Industry and Engineering Progress, 2017, 36(09): 3475-3484. |
| [7] | FANG Junhua, ZHANG Kai, ZHANG Wei, WANG Zhongyuan. Preparation of novel Sb2O3/BiOBr composite and decontamination of RhB [J]. Chemical Industry and Engineering Progress, 2017, 36(03): 1140-1146. |
| [8] | SHAO Xiankun, HAO Yonggan, LIU Tongxuan, HU Luyang, WANG Yuanyuan, LI Benxia. Research progress of Ag(Au)/semiconductor nanohybrid photocatalysts based on surface plasmon resonance [J]. Chemical Industry and Engineering Progree, 2016, 35(01): 131-137. |
| [9] | LI Jiade, FANG Wen, ZHOU Wanqin, YU Changlin. New development of silver-based semiconductor photo-catalyts [J]. Chemical Industry and Engineering Progree, 2015, 34(1): 113-118. |
| [10] | WANG Xiaowen,ZHANG Xiaochao,FAN Caimei. Research and development of BiOCl-based photocatalytic materials [J]. Chemical Industry and Engineering Progree, 2014, 33(01): 124-132. |
| [11] | YU Shui,LIU Yulu,LIU Xiaoqing,WANG Xiaojing. Preparation and light absorption mechanism of iron doped NaTaO3 nanoparticles [J]. Chemical Industry and Engineering Progree, 2012, 31(06): 1293-1297. |
| [12] | CHEN Qiuqiang1,XIE Hongqin1,ZHOU Wen1,CHEN Jifan2,HUANG Hao1,. Study on the performance of photocatalytic degradation of phenol using cerium doped nano-TiO2 under visible irradiation [J]. Chemical Industry and Engineering Progree, 2012, 31(05): 1043-1046. |
| [13] | SONG Weiwei,XUE Yongqiang,SU Lining,JU Hongbin. Preparation and photocatalytic activity of nano-nitrogen-doped TiO2 [J]. Chemical Industry and Engineering Progree, 2012, 31(05): 1057-1060. |
| [14] | XIAO Yifan,LIU Song,ZHANG Lianjun,CHEN Anqi . Visible light photocatalytic activity of carbon doped titania photocatalyst prepared from TiCl4 [J]. Chemical Industry and Engineering Progree, 2011, 30(3): 562-. |
| [15] | XIAO Yutang,LI Zhihua,XU Shuangshuang. Research advances of nonmetal doped titania nanotubes [J]. Chemical Industry and Engineering Progree, 2010, 29(7): 1235-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||