Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (3): 1426-1436.DOI: 10.16085/j.issn.1000-6613.2022-0970
• Materials science and technology • Previous Articles Next Articles
Research progress of porous aromatic frameworks based on uranium extraction from seawater
GUO Shuaishuai1( ), CHEN Jinlu2, JIN Liangchenglong1, TAO Zui1, CHEN Xiaoli2, PENG Guowen1,2,3(
), CHEN Jinlu2, JIN Liangchenglong1, TAO Zui1, CHEN Xiaoli2, PENG Guowen1,2,3( )
)
- 1.School of Resource & Environment and Safety Engineering, University of South China, Hengyang 421001, Hunan, China
2.School of Chemistry and Chemical Engineering, University of South China, Hengyang 421001, Hunan, China
3.Engineering Technology Research Center for Decommissioning Treatment of Uranium Tailings Pond in Hunan Province, University of South China, Hengyang 421001, Hunan, China
-
Received:2022-05-26Revised:2022-07-21Online:2023-04-10Published:2023-03-15 -
Contact:PENG Guowen
基于海水提铀的多孔芳香框架材料研究进展
郭帅帅1( ), 陈锦路2, 金梁程龙1, 陶醉1, 陈小丽2, 彭国文1,2,3(
), 陈锦路2, 金梁程龙1, 陶醉1, 陈小丽2, 彭国文1,2,3( )
)
- 1.南华大学资源环境与安全工程学院,湖南 衡阳 421001
2.南华大学化学化工学院,湖南 衡阳 421001
3.南华大学湖南省铀尾矿库退役治理工程技术研究中心,湖南 衡阳 421001
-
通讯作者:彭国文 -
作者简介:郭帅帅(1996—),男,硕士研究生,研究方向为新型功能材料。E-mail:guoss1996@163.com。 -
基金资助:中央军委装备发展部预研项目(32101070503)
CLC Number:
Cite this article
GUO Shuaishuai, CHEN Jinlu, JIN Liangchenglong, TAO Zui, CHEN Xiaoli, PENG Guowen. Research progress of porous aromatic frameworks based on uranium extraction from seawater[J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1426-1436.
郭帅帅, 陈锦路, 金梁程龙, 陶醉, 陈小丽, 彭国文. 基于海水提铀的多孔芳香框架材料研究进展[J]. 化工进展, 2023, 42(3): 1426-1436.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-0970
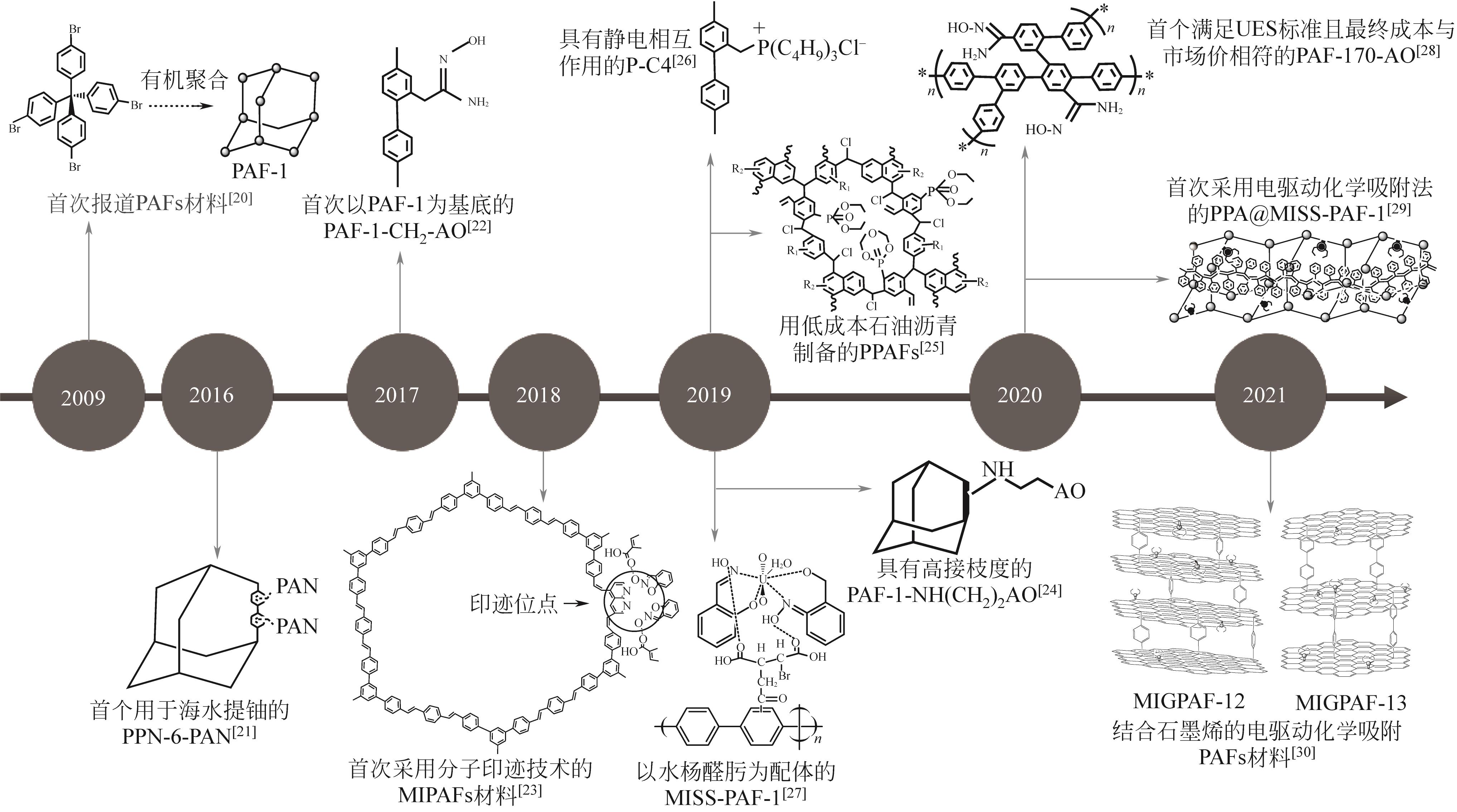
| 合成反应/合成技术 | 催化剂 | 特性 | 制备的PAFs材料 | 参考文献 |
|---|---|---|---|---|
| Yamamoto-type Ullmann偶联反应 | 铜、镍 | 反应条件温和、功能基团适应性强 | PAF-1、PPN-6-PAN | [ |
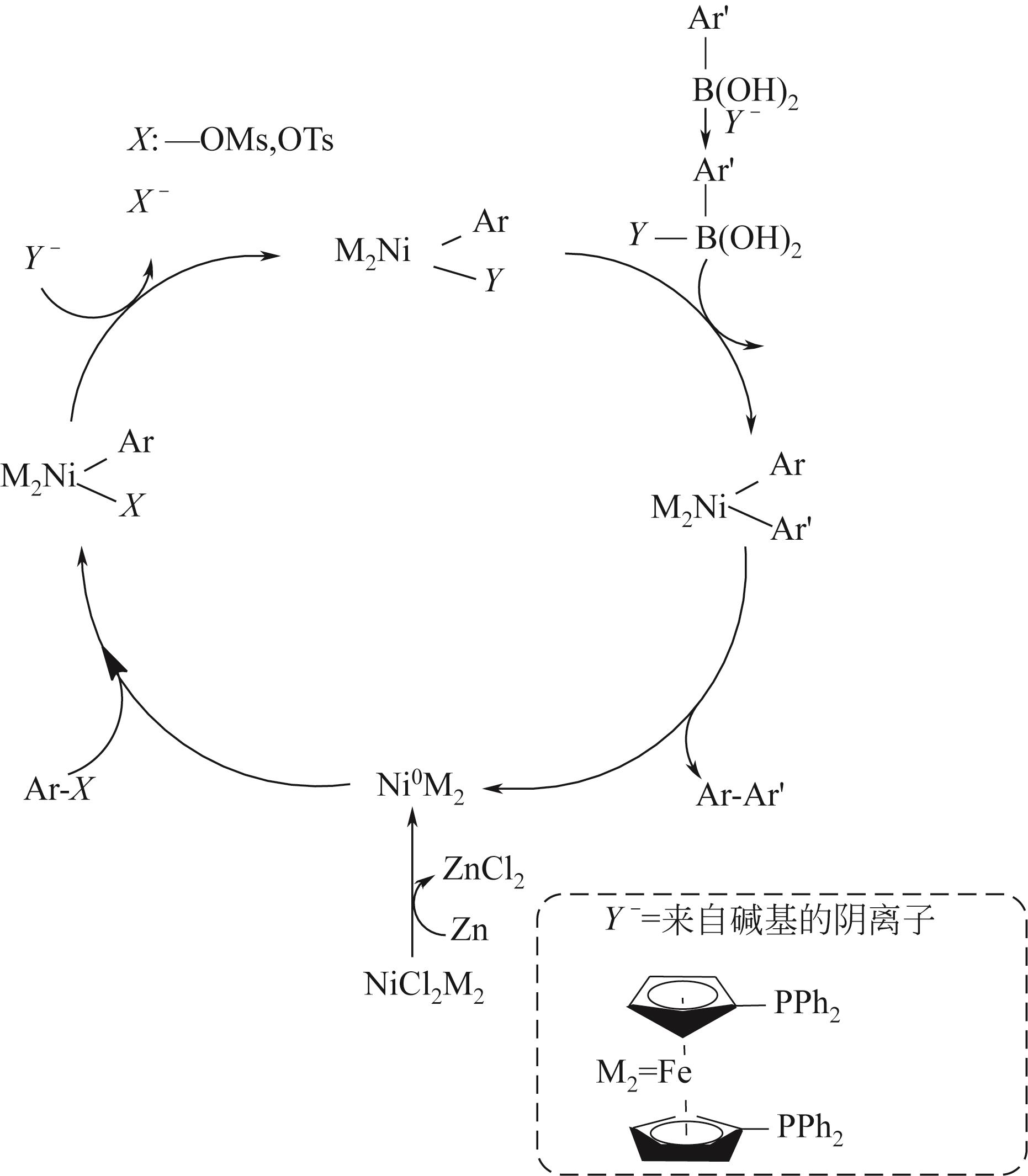
| Suzuki偶联反应 | 钯、镍 | 操作简便、亲水性较好、副产物无毒 | MIGPAF-12/13 | [ |
| Heck偶联反应 | 钯 | 催化活性高、官能团耐受性高 | MIPAFs | [ |
| Scholl反应 | AlCl3 | 可在碱性条件下反应,成本较低 | PAF-170/171/172-AO | [ |
| 傅-克烷基化反应 | AlCl3 | 可在强酸条件下反应,操作简单 | PPAF-3 | [ |
| 原子转移自由基聚合技术 | — | 增强引发反应的速率,控制反应的程度 | PPN-6-PAN | [ |
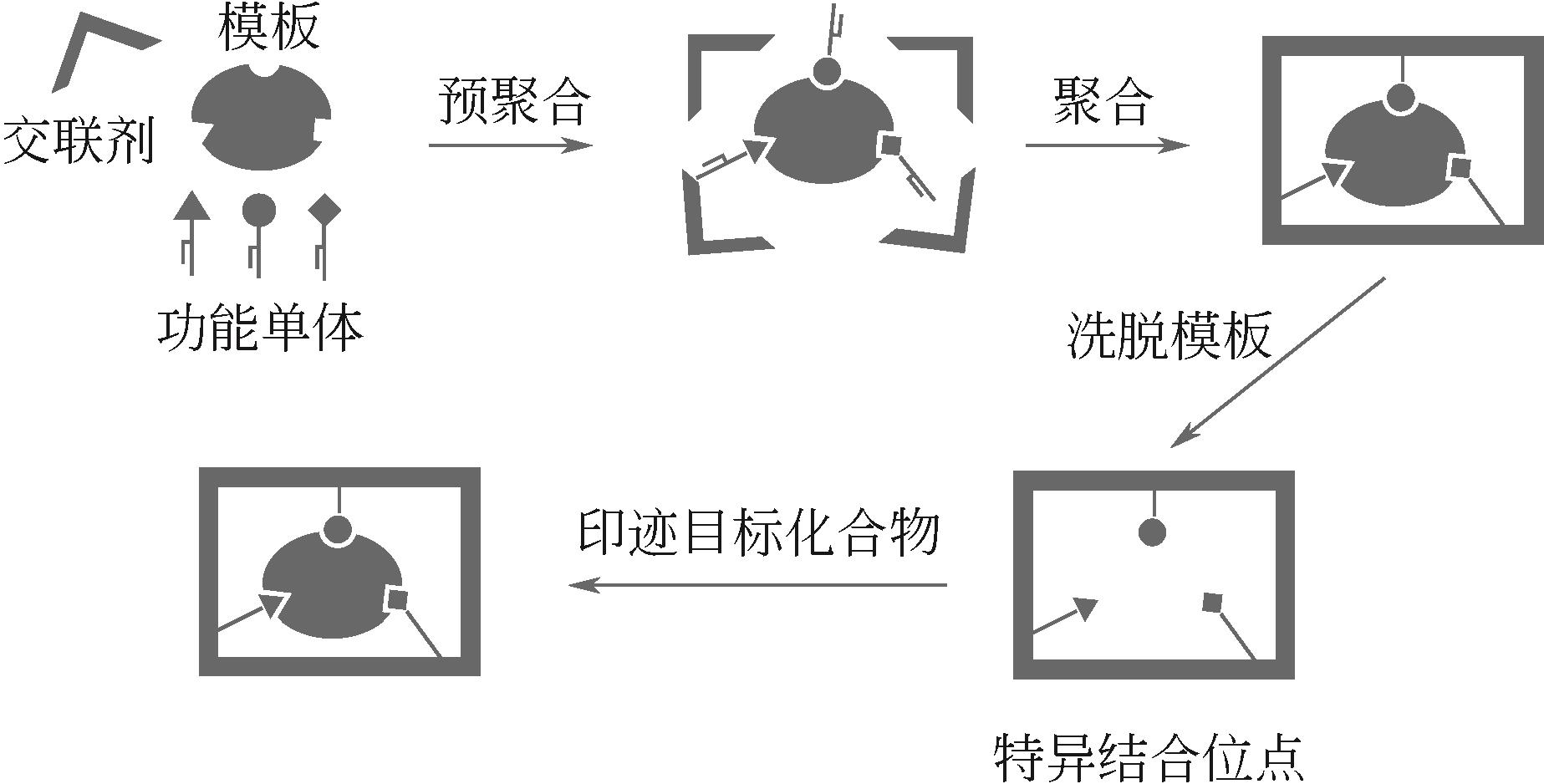
| 分子印迹技术 | — | 提高架构稳定性、增强铀选择性 | MISS-PAF-1、PPA@MISS-PAF-1、MIPAFs、MIGPAF-12/13 | [ |
| 合成反应/合成技术 | 催化剂 | 特性 | 制备的PAFs材料 | 参考文献 |
|---|---|---|---|---|
| Yamamoto-type Ullmann偶联反应 | 铜、镍 | 反应条件温和、功能基团适应性强 | PAF-1、PPN-6-PAN | [ |
| Suzuki偶联反应 | 钯、镍 | 操作简便、亲水性较好、副产物无毒 | MIGPAF-12/13 | [ |
| Heck偶联反应 | 钯 | 催化活性高、官能团耐受性高 | MIPAFs | [ |
| Scholl反应 | AlCl3 | 可在碱性条件下反应,成本较低 | PAF-170/171/172-AO | [ |
| 傅-克烷基化反应 | AlCl3 | 可在强酸条件下反应,操作简单 | PPAF-3 | [ |
| 原子转移自由基聚合技术 | — | 增强引发反应的速率,控制反应的程度 | PPN-6-PAN | [ |
| 分子印迹技术 | — | 提高架构稳定性、增强铀选择性 | MISS-PAF-1、PPA@MISS-PAF-1、MIPAFs、MIGPAF-12/13 | [ |
| 材料名称 | 配体官能团 | 孔径 /nm | 比表面积 /m2·g-1 | Kd/mL·g-1 | 吸附机理 | 参考文献 |
|---|---|---|---|---|---|---|
| PPN-6-PAN | 偕胺肟 | — | 19.5 | — | 通过密度泛函理论(DFT)研究表明吸附剂通过AO(供电子体)与铀酰(受电子体)进行结合的,结合基序为η2型 | [ |
| PAF-1-CH2-AO | 偕胺肟 | 0.7 | 855 | 1.05×106 | 通过X射线吸收精细结构谱(XAFS)分析铀与吸附剂的结合基序与η2型相似,且相邻偕胺肟基团之间存在协同结合 | [ |
| PAF-1-CH2NHAO | 偕胺肟 | — | — | — | 通过扩展X射线吸收精细结构谱(EXAFS)阐明了两种吸附剂的胺肟与铀酰是以2∶1方式结合的 | [ |
| PAF-1-NH(CH2)2AO | 465 | 1.15×107 | ||||
| PAF-170-AO | 偕胺肟 | 0.4~4 | 312 | 9.37×106 | 通过X射线能谱(EDS)和X射线光电子能谱(XPS)证明了铀酰离子与偕胺肟基团进行了螯合作用 | [ |
| PAF-171-AO | 425 | — | ||||
| PAF-172-AO | 541 | — | ||||
| PPAF-3 | 含磷配体 | — | 37 | — | 通过XPS和傅里叶变换红外光谱(FTIR)表明吸附剂是通过磷酸配体的P | [ |
| P-C4 | 含磷配体 | 7.67 | 110 | 2.2×107 | 通过与N-C2对比发现除阴离子与阳离子之间的静电相互作用外,还可能存在UO | [ |
| MIPAF-11a | 水杨醛肟 | — | 524 | — | 通过FTIR和XPS表明吸附剂通过水杨醛肟的N、O原子与铀进行配位的 | [ |
| MIPAF-11b | 297 | — | ||||
| MIPAF-11c | 182 | 6.98×105 | ||||
| MIPAF-11d | 95 | — | ||||
| MISS-PAF-1 | 双水杨醛肟 | — | 412 | 1.4×107 | 通过FTIR和XPS表明吸附剂通过—OH和—C | [ |
| PPA@MISS-PAF-1 | 双水杨醛肟 | — | 117 | 2.18×107 | 除与MISS-PAF-1相同的吸附机理,该吸附剂在非对称交流电化学(AACE)方法下通过PPA产生的扩大电场来提高对铀的吸附性能 | [ |
| MIGPAF-12 | 双水杨醛肟 | 0.8 | 178 | — | 通过XPS、FTIR、XAFS表明吸附剂在-1.3V下产生放大的铀酰局域浓度与双水杨醛肟配体结合产生协同电极,提高了铀吸附效率 | [ |
| MIGPAF-13 | 1.1 | 290 | 2.0×106 |
| 材料名称 | 配体官能团 | 孔径 /nm | 比表面积 /m2·g-1 | Kd/mL·g-1 | 吸附机理 | 参考文献 |
|---|---|---|---|---|---|---|
| PPN-6-PAN | 偕胺肟 | — | 19.5 | — | 通过密度泛函理论(DFT)研究表明吸附剂通过AO(供电子体)与铀酰(受电子体)进行结合的,结合基序为η2型 | [ |
| PAF-1-CH2-AO | 偕胺肟 | 0.7 | 855 | 1.05×106 | 通过X射线吸收精细结构谱(XAFS)分析铀与吸附剂的结合基序与η2型相似,且相邻偕胺肟基团之间存在协同结合 | [ |
| PAF-1-CH2NHAO | 偕胺肟 | — | — | — | 通过扩展X射线吸收精细结构谱(EXAFS)阐明了两种吸附剂的胺肟与铀酰是以2∶1方式结合的 | [ |
| PAF-1-NH(CH2)2AO | 465 | 1.15×107 | ||||
| PAF-170-AO | 偕胺肟 | 0.4~4 | 312 | 9.37×106 | 通过X射线能谱(EDS)和X射线光电子能谱(XPS)证明了铀酰离子与偕胺肟基团进行了螯合作用 | [ |
| PAF-171-AO | 425 | — | ||||
| PAF-172-AO | 541 | — | ||||
| PPAF-3 | 含磷配体 | — | 37 | — | 通过XPS和傅里叶变换红外光谱(FTIR)表明吸附剂是通过磷酸配体的P | [ |
| P-C4 | 含磷配体 | 7.67 | 110 | 2.2×107 | 通过与N-C2对比发现除阴离子与阳离子之间的静电相互作用外,还可能存在UO | [ |
| MIPAF-11a | 水杨醛肟 | — | 524 | — | 通过FTIR和XPS表明吸附剂通过水杨醛肟的N、O原子与铀进行配位的 | [ |
| MIPAF-11b | 297 | — | ||||
| MIPAF-11c | 182 | 6.98×105 | ||||
| MIPAF-11d | 95 | — | ||||
| MISS-PAF-1 | 双水杨醛肟 | — | 412 | 1.4×107 | 通过FTIR和XPS表明吸附剂通过—OH和—C | [ |
| PPA@MISS-PAF-1 | 双水杨醛肟 | — | 117 | 2.18×107 | 除与MISS-PAF-1相同的吸附机理,该吸附剂在非对称交流电化学(AACE)方法下通过PPA产生的扩大电场来提高对铀的吸附性能 | [ |
| MIGPAF-12 | 双水杨醛肟 | 0.8 | 178 | — | 通过XPS、FTIR、XAFS表明吸附剂在-1.3V下产生放大的铀酰局域浓度与双水杨醛肟配体结合产生协同电极,提高了铀吸附效率 | [ |
| MIGPAF-13 | 1.1 | 290 | 2.0×106 |
| 材料名称 | 循环性能 /次 | 铀水溶液 | 模拟海水 | 实际海水 | 参考 文献 | |||
|---|---|---|---|---|---|---|---|---|
| 铀吸附量/mg·g-1 | 吸附条件 | 铀吸附量/mg·g-1 | 吸附条件 | 铀吸附量/mg·g-1 | 吸附条件 | |||
| PPN-6-PAN | — | — | — | 65.2 | 24h, 60mg/L | 4.81 | 42d, 80mg/L | [ |
| PAF-1-CH2-AO | 2 | 304 | 12h, 7.36mg/L | 40 | 7.05mg/L | — | — | [ |
| PAF-1-CH2NHAO | — | 102 | 24h, 1~400mg/L | — | — | — | — | [ |
| PAF-1-NH(CH2)2AO | 3 | 385 | 24h, 1~400mg/L | — | — | 36.5 | 7d, 8mg/L | |
| PAF-170-AO | 10 | — | — | 702 | 24h, 7mg/L | 6 | 21d, 3.3μg/L | [ |
| 8.92 | 60d, 3.3μg/L | |||||||
| PAF-171-AO | — | — | — | 608 | 24h, 7mg/L | — | — | |
| PAF-172-AO | — | — | — | 569 | 24h, 7mg/L | — | — | |
| 材料名称 | 循环性能 /次 | 铀水溶液 | 模拟海水 | 实际海水 | 参考 文献 | |||
|---|---|---|---|---|---|---|---|---|
| 铀吸附量/mg·g-1 | 吸附条件 | 铀吸附量/mg·g-1 | 吸附条件 | 铀吸附量/mg·g-1 | 吸附条件 | |||
| PPN-6-PAN | — | — | — | 65.2 | 24h, 60mg/L | 4.81 | 42d, 80mg/L | [ |
| PAF-1-CH2-AO | 2 | 304 | 12h, 7.36mg/L | 40 | 7.05mg/L | — | — | [ |
| PAF-1-CH2NHAO | — | 102 | 24h, 1~400mg/L | — | — | — | — | [ |
| PAF-1-NH(CH2)2AO | 3 | 385 | 24h, 1~400mg/L | — | — | 36.5 | 7d, 8mg/L | |
| PAF-170-AO | 10 | — | — | 702 | 24h, 7mg/L | 6 | 21d, 3.3μg/L | [ |
| 8.92 | 60d, 3.3μg/L | |||||||
| PAF-171-AO | — | — | — | 608 | 24h, 7mg/L | — | — | |
| PAF-172-AO | — | — | — | 569 | 24h, 7mg/L | — | — | |
吸附剂 名称 | 循环性能 /次 | 铀水溶液 | 参考 文献 | |
|---|---|---|---|---|
| 铀吸附量/mg·g-1 | 吸附条件 | |||
| PPAF-3 | 5 | 147.6 | 40min, 10~250mg/L | [ |
| P-C4 | — | 670 | 4h, 20~175mg/L | [ |
吸附剂 名称 | 循环性能 /次 | 铀水溶液 | 参考 文献 | |
|---|---|---|---|---|
| 铀吸附量/mg·g-1 | 吸附条件 | |||
| PPAF-3 | 5 | 147.6 | 40min, 10~250mg/L | [ |
| P-C4 | — | 670 | 4h, 20~175mg/L | [ |
| 吸附剂名称 | 循环性能 /次 | 铀水溶液 | 模拟海水 | 实际海水 | 参考 文献 | |||
|---|---|---|---|---|---|---|---|---|
| 铀吸附量/mg·g-1 | 吸附条件 | 铀吸附量/mg·g-1 | 吸附条件 | 铀吸附量/mg·g-1 | 吸附条件 | |||
| MIPAF-11a | — | 0.02 | 40min, 20mg/L | — | — | — | — | [ |
| MIPAF-11b | — | 21.4 | 40min, 20mg/L | — | — | — | — | |
| MIPAF-11c | 10 | 37.28 | 40min, 20mg/L | 35.44 | 1h, 7.05mg/L | — | — | |
| MIPAF-11d | 35.48 | 40min, 20mg/L | — | — | — | — | ||
| MISS-PAF-1 | 4 | 253 | 50~200mg/L | 73.26 | 7.05mg/L | 5.79 | 56d, 4.4μg/L | [ |
| PPA@MISS-PAF-1 | 10 | — | — | — | — | 307.3 | 60min, 8mg/L | [ |
| 16.5 | 90d, 3.3μg/L | |||||||
| MIGPAF-12 | — | — | — | — | — | 367.4 | 60min, 8mg/L | [ |
| MIGPAF-13 | 6 | — | — | — | — | 419.2 | 60min, 8mg/L | |
| 吸附剂名称 | 循环性能 /次 | 铀水溶液 | 模拟海水 | 实际海水 | 参考 文献 | |||
|---|---|---|---|---|---|---|---|---|
| 铀吸附量/mg·g-1 | 吸附条件 | 铀吸附量/mg·g-1 | 吸附条件 | 铀吸附量/mg·g-1 | 吸附条件 | |||
| MIPAF-11a | — | 0.02 | 40min, 20mg/L | — | — | — | — | [ |
| MIPAF-11b | — | 21.4 | 40min, 20mg/L | — | — | — | — | |
| MIPAF-11c | 10 | 37.28 | 40min, 20mg/L | 35.44 | 1h, 7.05mg/L | — | — | |
| MIPAF-11d | 35.48 | 40min, 20mg/L | — | — | — | — | ||
| MISS-PAF-1 | 4 | 253 | 50~200mg/L | 73.26 | 7.05mg/L | 5.79 | 56d, 4.4μg/L | [ |
| PPA@MISS-PAF-1 | 10 | — | — | — | — | 307.3 | 60min, 8mg/L | [ |
| 16.5 | 90d, 3.3μg/L | |||||||
| MIGPAF-12 | — | — | — | — | — | 367.4 | 60min, 8mg/L | [ |
| MIGPAF-13 | 6 | — | — | — | — | 419.2 | 60min, 8mg/L | |
| 1 | FUKAYA Y, GOTO M. Sustainable and safe energy supply with seawater uranium fueled HTGR and its economy[J]. Annals of Nuclear Energy, 2017, 99: 19-27. |
| 2 | ABNEY C W, MAYES R T, SAITO T, et al. Materials for the recovery of uranium from seawater[J]. Chemical Reviews, 2017, 117(23): 13935-14013. |
| 3 | TANG Xingrui, LIU Yan, LIU Min, et al. Sulfur edge in molybdenum disulfide nanosheets achieves efficient uranium binding and electrocatalytic extraction in seawater[J]. Nanoscale, 2022, 14(17): 6285-6290. |
| 4 | SINGHAL P, JHA S K, PANDEY S P, et al. Rapid extraction of uranium from sea water using Fe3O4 and humic acid coated Fe3O4 nanoparticles[J]. Journal of Hazardous Materials, 2017, 335: 152-161. |
| 5 | XU Xiao, ZHANG Hongjun, AO Junxuan, et al. 3D hierarchical porous amidoxime fibers speed up uranium extraction from seawater[J]. Energy & Environmental Science, 2019, 12(6): 1979-1988. |
| 6 | WANG Hui, YAO Huiqin, CHEN Lihong, et al. Highly efficient capture of uranium from seawater by layered double hydroxide composite with benzamidoxime[J]. The Science of the Total Environment, 2021, 759: 143483. |
| 7 | SUN Ye, LIU Rongrong, WEN Shunxi, et al. Antibiofouling ultrathin poly(amidoxime) membrane for enhanced U(VI) recovery from wastewater and seawater[J]. ACS Applied Materials & Interfaces, 2021, 13(18): 21272-21285. |
| 8 | KUSHWAHA S, PATEL K. Catalyst: uranium extraction from seawater, a paradigm shift in resource recovery[J]. Chem, 2021, 7(2): 271-274. |
| 9 | 沈江南, 林龙, 陈卫军, 等. 吸附法海水提铀材料研究进展[J]. 化工进展, 2011, 30(12): 2586-2592. |
| SHEN Jiangnan, LIN Long, CHEN Weijun, et al. Recent development of uranium extraction materials from sea-water[J]. Chemical Industry and Engineering Progress, 2011, 30(12): 2586-2592. | |
| 10 | CHEN Peng, HE Xihong, PANG Maobin, et al. Iodine capture using Zr-based metal-organic frameworks (Zr-MOFs): adsorption performance and mechanism[J]. ACS Applied Materials & Interfaces, 2020, 12(18): 20429-20439. |
| 11 | ZHANG Wen, YE Gang, CHEN Jing. New insights into the uranium adsorption behavior of mesoporous SBA-15 silicas decorated with alkylphosphine oxide ligands[J]. RSC Advances, 2016, 6(2): 1210-1217. |
| 12 | 曹炎森, 王洪江, 喻清. 槟榔渣活性炭对废水中铀(Ⅵ)的吸附实验[J]. 矿业研究与开发, 2019, 39(10): 95-98. |
| CAO Yansen, WANG Hongjiang, YU Qing. Adsorption experiments of areca residue activated carbon on uranium(Ⅵ) in wastewater[J]. Mining Research and Development, 2019, 39(10): 95-98. | |
| 13 | 熊正为, 熊巍滔, 王劲松, 等. 偕胺肟乙烷基硅材料对铀(Ⅵ)的吸附机理研究[J]. 环境科学与技术, 2016, 39(10): 47-51. |
| XIONG Zhengwei, XIONG Weitao, WANG Jinsong, et al. Adsorption mechanism of U( Ⅵ ) by amidoxime ethane-bridged periodic mesoporous silicas[J]. Environmental Science & Technology, 2016, 39(10): 47-51. | |
| 14 | 杨晓丹, 王玉如, 李敏睿. 纳米零价铁的制备、改性及对废水中重金属和有机污染物的去除[J]. 化工进展, 2019, 38(7): 3412-3424. |
| YANG Xiaodan, WANG Yuru, LI Minrui. Preparation, modification of nanoscale zero valent iron and its application for the removal of heavy metals and organic pollutants from wastewater[J]. Chemical Industry and Engineering Progress, 2019, 38(7): 3412-3424. | |
| 15 | ZHANG Wen, DONG Xiuting, MU Yingxin, et al. Constructing adjacent phosphine oxide ligands confined in mesoporous Zr-MOFs for uranium capture from acidic medium[J]. Journal of Materials Chemistry A, 2021, 9(31): 16685-16691. |
| 16 | ZHANG Wen, BU An, JI Qingyuan, et al. pKa-Directed incorporation of phosphonates into MOF-808 via ligand exchange: stability and adsorption properties for uranium[J]. ACS Applied Materials & Interfaces, 2019, 11(37): 33931-33940. |
| 17 | 彭莹, 张晓文, 李密, 等. 金属有机框架材料吸附分离水中铀的应用[J]. 化工进展, 2019, 38(7): 3227-3242. |
| PENG Ying, ZHANG Xiaowen, LI Mi, et al. Application of metal-organic frameworks in adsorption and separation of uranium from water[J]. Chemical Industry and Engineering Progress, 2019, 38(7): 3227-3242. | |
| 18 | LI Zhongyue, ZHU Ruomeng, ZHANG Pengling, et al. Functionalized polyarylether-based COFs for rapid and selective extraction of uranium from aqueous solution[J]. Chemical Engineering Journal, 2022, 434: 134623. |
| 19 | 陈琦, 王文涛, 张志鹏, 等. 共价有机框架材料对放射性核素吸附的研究进展[J]. 化工进展, 2021, 40(S2): 241-255. |
| CHEN Qi, WANG Wentao, ZHANG Zhipeng, et al. Progress of covalent framework for radionuclides absorption[J]. Chemical Industry and Engineering Progress, 2021, 40(S2): 241-255. | |
| 20 | Teng BEN, REN Hao, MA Shengqian, et al. Targeted synthesis of a porous aromatic framework with high stability and exceptionally high surface area[J]. Angewandte Chemie International Edition, 2009, 48(50): 9457-9460. |
| 21 | YUE Yanfeng, ZHANG Chenxi, TANG Qing, et al. A poly(acrylonitrile)-functionalized porous aromatic framework synthesized by atom-transfer radical polymerization for the extraction of uranium from seawater[J]. Industrial & Engineering Chemistry Research, 2016, 55(15): 4125-4129. |
| 22 | LI Baiyan, SUN Qi, ZHANG Yiming, et al. Functionalized porous aromatic framework for efficient uranium adsorption from aqueous solutions[J]. ACS Applied Materials & Interfaces, 2017, 9(14): 12511-12517. |
| 23 | YUAN Ye, YANG Yajie, MA Xujiao, et al. Molecularly imprinted porous aromatic frameworks and their composite components for selective extraction of uranium ions[J]. Advanced Materials, 2018, 30(12): 1706507. |
| 24 | AGUILA B, SUN Q, CASSADY H, et al. Design strategies to enhance amidoxime chelators for uranium recovery[J]. ACS Applied Materials & Interfaces, 2019, 11(34): 30919-30926. |
| 25 | WANG Tao, XU Meiyun, HAN Xiaoli, et al. Petroleum pitch-based porous aromatic frameworks with phosphonate ligand for efficient separation of uranium from radioactive effluents[J]. Journal of Hazardous Materials, 2019, 368: 214-220. |
| 26 | SHEN Yinglin, CHU Nini, YANG Suliang, et al. Quaternary phosphonium-grafted porous aromatic framework for preferential uranium adsorption in alkaline solution[J]. Industrial & Engineering Chemistry Research, 2019, 58(39): 18329-18335. |
| 27 | YUAN Y, MENG QH, FAHEEM M, et al. A molecular coordination template strategy for designing selective porous aromatic framework materials for uranyl capture[J]. ACS Central Science, 2019, 5(8): 1432-1439. |
| 28 | LI Zhangnan, MENG Qinghao, YANG Yajie, et al. Constructing amidoxime-modified porous adsorbents with open architecture for cost-effective and efficient uranium extraction[J]. Chemical Science, 2020, 11(18): 4747-4752. |
| 29 | WANG Zeyu, MENG Qinghao, MA Rongchen, et al. Constructing an ion pathway for uranium extraction from seawater[J]. Chem, 2020, 6(7): 1683-1691. |
| 30 | WANG Zeyu, MA Rongchen, MENG Qinghao, et al. Constructing uranyl-specific nanofluidic channels for unipolar ionic transport to realize ultrafast uranium extraction[J]. Journal of the American Chemical Society, 2021, 143(36): 14523-14529. |
| 31 | PEI Cuiying, Teng BEN, QIU Shilun. Great prospects for PAF-1 and its derivatives[J]. Materials Horizons, 2015, 2(1): 11-21. |
| 32 | YANG Yuting, WANG Tienan, JING Xiaofei, et al. Phosphine-based porous aromatic frameworks for gold nanoparticle immobilization with superior catalytic activities[J]. Journal of Materials Chemistry A, 2019, 7(16): 10004-10009. |
| 33 | LU Weigang, SCULLEY Julian P, YUAN Daqiang, et al. Polyamine-tethered porous polymer networks for carbon dioxide capture from flue gas[J]. Angewandte Chemie International Edition, 2012, 51(30): 7480-7484. |
| 34 | YAN Zhuojun, YUAN Ye, TIAN Yuyang, et al. Highly efficient enrichment of volatile iodine by charged porous aromatic frameworks with three sorption sites[J]. Angewandte Chemie International Edition, 2015, 54(43): 12733-12737. |
| 35 | LIAO Xinrong, YU Guoqiang, LUO Ruiqing, et al. Thiol/methylthio-functionalized porous aromatic frameworks for simultaneous capture of aromatic pollutants and Hg(II) from water[J]. Journal of Hazardous Materials, 2021, 418: 126244. |
| 36 | ZHANG Lei, SUN Jinshi, SUN Fuxing, et al. Facile synthesis of ultrastable porous aromatic frameworks by Suzuki-Miyaura coupling reaction for adsorption removal of organic dyes[J]. Chemistry, 2019, 25(15): 3903-3908. |
| 37 | ZHANG Panpan, ZOU Xiaoqin, SONG Jian, et al. Anion substitution in porous aromatic frameworks: boosting molecular permeability and selectivity for membrane acetylene separation[J]. Advanced Materials, 2020, 32(30): e1907449. |
| 38 | MENG Qinghao, HUANG Yihan, DENG Dan, et al. Porous aromatic framework nanosheets anchored with lewis pairs for efficient and recyclable heterogeneous catalysis[J]. Advanced Science, 2020, 7(22): 2000067. |
| 39 | JING Liping, SUN Jinshi, SUN Fuxing, et al. Porous aromatic framework with mesopores as a platform for a super-efficient heterogeneous Pd-based organometallic catalysis[J]. Chemical Science, 2018, 9(14): 3523-3530. |
| 40 | TIAN Yuyang, ZHU Guangshan. Porous aromatic frameworks (PAFs)[J]. Chemical Reviews, 2020, 120(16): 8934-8986. |
| 41 | PHUKAN S, MAHANTA A, KAKATI D, et al. Green chemical synthesis of Pd nanoparticles for use as efficient catalyst in Suzuki-Miyaura cross-coupling reaction[J]. Applied Organometallic Chemistry, 2019, 33(3): e4758. |
| 42 | SUZUKI A. Recent advances in the cross-coupling reactions of organoboron derivatives with organic electrophiles, 1995-1998[J]. Journal of Organometallic Chemistry, 1999, 576(1/2): 147-168. |
| 43 | YAO Shuwen, YANG Xiao, YU Miao, et al. High surface area hypercrosslinked microporous organic polymer networks based on tetraphenylethylene for CO2 capture[J]. Journal of Materials Chemistry A, 2014, 2(21): 8054-8059. |
| 44 | SHAFIEI N, NASROLLAHZADEH M, BARAN T, et al. Pd nanoparticles loaded on modified chitosan-Unye bentonite microcapsules: a reusable nanocatalyst for Sonogashira coupling reaction[J]. Carbohydrate Polymers, 2021, 262: 117920. |
| 45 | YUE Y F, MAYES R T, KIM J S, et al. Seawater uranium sorbents: preparation from a mesoporous copolymer initiator by atom-transfer radical polymerization[J]. Angewandte Chemie International Edition, 2013, 52(50): 13458-13462. |
| 46 | 唐燕春, 艾长军, 马敬红, 等. 原子转移自由基聚合机理及其应用[J]. 合成技术及应用, 2007, 22(4): 38-40. |
| TANG Yanchun, AI Changjun, MA Jinghong, et al. The mechanism and application of atom transfer radical polymerization[J]. Synthetic Technology & Application, 2007, 22(4): 38-40. | |
| 47 | YUAN Ye, YANG Yajie, ZHU Guangshan. Molecularly imprinted porous aromatic frameworks for molecular recognition[J]. ACS Central Science, 2020, 6(7): 1082-1094. |
| 48 | JIA Jiangtao, CHEN Zhijie, JIANG Hao, et al. Extremely hydrophobic POPs to access highly porous storage media and capturing agent for organic vapors[J]. Chem, 2019, 5(1): 180-191. |
| 49 | YUAN Daqiang, LU Weigang, ZHAO Dan, et al. Highly stable porous polymer networks with exceptionally high gas-uptake capacities[J]. Advanced Materials, 2011, 23(32): 3723-3725. |
| 50 | 张亦琳, 延永, 葛颖, 等. Ni催化的Suzuki偶联反应[J]. 化学通报, 2019, 82(5): 404-414. |
| ZHANG Yilin, YAN Yong, GE Ying, et al. Ni-catalyzed Suzuki coupling reaction[J]. Chemistry, 2019, 82(5): 404-414. | |
| 51 | CHEN Lingxin, XU Shoufang, LI Jinhua. Recent advances in molecular imprinting technology: current status, challenges and highlighted applications[J]. Chemical Society Reviews, 2011, 40(5): 2922-2942. |
| 52 | DEMIR S, BRUNE N K, VAN HUMBECK J F, et al. Extraction of lanthanide and actinide ions from aqueous mixtures using a carboxylic acid-functionalized porous aromatic framework[J]. ACS Central Science, 2016, 2(4): 253-265. |
| 53 | 王聪聪. 基于肖尔反应的多孔芳香骨架及其复合材料处理挥发性有机物的性能研究[D]. 长春: 吉林大学, 2021. |
| WANG Congcong. Performance of porous aromatic framework based on Scholl reaction and its composites in the treatment of volatile organic compounds[D]. Changchun: Jilin University, 2021. | |
| 54 | 龙威, 陈秋灵, 杨诗霖. 新型磁性复合生物吸附剂吸附铀的实验研究[J]. 中国无机分析化学, 2022, 12(2): 7-15. |
| LONG Wei, CHEN Qiuling, YANG Shilin. Experimental research on uranium adsorption by new magnetic composite biological adsorbent[J]. Chinese Journal of Inorganic Analytical Chemistry, 2022, 12(2): 7-15. | |
| 55 | 廖云. 多孔纳米复合吸附剂的制备及其对铀(Ⅵ)离子吸附性能研究[D]. 上海: 东华大学, 2019. |
| LIAO Yun. Preparation of porous nanocomposite adsorbents and their adsorption properties for uranium(Ⅵ) ions[D]. Shanghai: Donghua University, 2019. | |
| 56 | 杨春雁, 杨卫亚, 凌凤香, 等. 负载型金属催化剂表面金属分散度的测定[J]. 化工进展, 2010, 29(8): 1468-1473, 1501. |
| YANG Chunyan, YANG Weiya, LING Fengxiang, et al. Determination of metal dispersion on supported metal catalyst surface[J]. Chemical Industry and Engineering Progress, 2010, 29(8): 1468-1473, 1501. | |
| 57 | ABNEY C W, MAYES R T, PIECHOWICZ M, et al. XAFS investigation of polyamidoxime-bound uranyl contests the paradigm from small molecule studies[J]. Energy & Environmental Science, 2016, 9(2): 448-453. |
| 58 | KELLEY S P, BARBER P S, MULLINS P H, et al. Structural clues to UO₂²⁺/VO₂⁺ competition in seawater extraction using amidoxime-based extractants[J]. Chemical Communications, 2014, 50(83): 12504-12507. |
| 59 | SUN Q, AGUILA B, EARL L D, et al. Covalent organic frameworks as a decorating platform for utilization and affinity enhancement of chelating sites for radionuclide sequestration[J]. Advanced Materials, 2018, 30(20): e1705479. |
| 60 | AGUILA B, SUN Q, PERMAN J A, et al. Efficient mercury capture using functionalized porous organic polymer[J]. Advanced Materials, 2017, 29(31): 1700665. |
| 61 | SUN Q, ZHU L, AGUILA B, et al. Optimizing radionuclide sequestration in anion nanotraps with record pertechnetate sorption[J]. Nature Communications, 2019, 10: 1646. |
| 62 | SUN Q, AGUILA B, PERMAN J, et al. Bio-inspired nano-traps for uranium extraction from seawater and recovery from nuclear waste[J]. Nature Communications, 2018, 9: 1644. |
| 63 | MAZARÍO E, STEMPER J, HELAL A S, et al. New iron oxide nanoparticles catechol-grafted with bis(amidoxime)s for uranium(VI) depletion of aqueous solution[J]. Journal of Nanoscience and Nanotechnology, 2019, 19(8): 4911-4919. |
| 64 | LI Buyi, GUAN Zhenhong, YANG Xinjia, et al. Multifunctional microporous organic polymers[J]. Journal of Materials Chemistry A, 2014, 2(30): 11930-11939. |
| 65 | YAN G Y, VIRARAGHAVAN T. Heavy metal removal in a biosorption column by immobilized M. rouxii biomass[J]. Bioresource Technology, 2001, 78(3): 243-249. |
| 66 | LIU Mingxue, DONG Faqin, YAN Xiuying, et al. Biosorption of uranium by Saccharomyces cerevisiae and surface interactions under culture conditions[J]. Bioresource Technology, 2010, 101(22): 8573-8580. |
| 67 | GIVAJA G, BLAKE A J, WILSON C, et al. Macrocyclic diiminodipyrromethane complexes: structural analogues of Pac-Man porphyrins[J]. Chemical Communications, 2003(19): 2508-2509. |
| 68 | WU Qunyan, WANG Congzhi, LAN Jianhui, et al. Theoretical insight into the binding affinity enhancement of serine with the uranyl ion through phosphorylation[J]. RSC Advances, 2016, 6(74): 69773-69781. |
| 69 | CAI Xiang, TAN Shaozao, LIN Minsong, et al. Synergistic antibacterial brilliant blue/reduced graphene oxide/quaternary phosphonium salt composite with excellent water solubility and specific targeting capability[J]. Langmuir, 2011, 27(12): 7828-7835. |
| 70 | 楚妮妮. 多孔芳香骨架阴离子交换树脂的制备及对铀的吸附研究[D]. 兰州: 兰州大学, 2020. |
| CHU Nini. Preparation and adsorption for uranium of pore aromatic framework anion exchange resin[D]. Lanzhou: Lanzhou University, 2020. | |
| 71 | GAO Hui, DING Lei, BAI Hua, et al. Pitch-based hyper-cross-linked polymers with high performance for gas adsorption[J]. Journal of Materials Chemistry A, 2016, 4(42): 16490-16498. |
| 72 | MONIER M, ELSAYED N H. Selective extraction of uranyl ions using ion-imprinted chelating microspheres[J]. Journal of Colloid and Interface Science, 2014, 423: 113-122. |
| 73 | METILDA P, GLADIS J M, VENKATESWARAN G, et al. Investigation of the role of chelating ligand in the synthesis of ion-imprinted polymeric resins on the selective enrichment of uranium(VI)[J]. Analytica Chimica Acta, 2007, 587(2): 263-271. |
| 74 | SHOLL D S, LIVELY R P. Seven chemical separations to change the world[J]. Nature, 2016, 532(7600): 435-437. |
| 75 | LIU Chong, HSU Po Chun, XIE Jin, et al. A half-wave rectified alternating current electrochemical method for uranium extraction from seawater[J]. Nature Energy, 2017, 2: 17007. |
| 76 | KEENER M, HUNT C, CARROLL T G, et al. Redox-switchable carboranes for uranium capture and release[J]. Nature, 2020, 577(7792): 652-655. |
| 77 | 王万兵, 高晓辉, 李怀阳, 等. 石墨烯/导电聚合物复合防腐蚀材料制备及应用研究进展[J]. 化工进展, 2020, 39(3): 1080-1089. |
| WANG Wanbing, GAO Xiaohui, LI Huaiyang, et al. Progress of preparation and application of graphene/conductive polymer composite anticorrosion materials[J]. Chemical Industry and Engineering Progress, 2020, 39(3): 1080-1089. | |
| 78 | ZHANG Kun, LIU Wei, GAO Yuliang, et al. A high-performance lithium metal battery with ion-selective nanofluidic transport in a conjugated microporous polymer protective layer[J]. Advanced Materials, 2021, 33(5): 2006323. |
| 79 | RABAJCZYK A, ZIELECKA M, CYGAŃCZUK K, et al. The use of polymer membranes to counteract the risk of environmental of soil and water contamination[J]. Membranes, 2021, 11(6): 426. |
| 80 | DAHANAYAKA M, LIU B, SRIKANTH N, et al. Ionised graphene oxide membranes for seawater desalination[J]. Desalination, 2020, 496: 114637. |
| 81 | WANG Yiran, ZHANG Wen, ZENG Xianjie, et al. Membranes for separation of alkali/alkaline earth metal ions: a review[J]. Separation and Purification Technology, 2021, 278: 119640. |
| 82 | GLADIS J M, RAO T P. Synthesis and analytical applications of uranyl ion imprinted polymer particles[J]. Analytical Letters, 2003, 36(10): 2107-2121. |
| 83 | WANG Zhimei, ZHANG Di, XIAO Xilin, et al. A highly sensitive and selective sensor for trace uranyl(VI) ion based on a graphene-coated carbon paste electrode modified with ion imprinted polymer[J]. Microchemical Journal, 2020, 155: 104767. |
| 84 | 王泽宇. 导电多孔芳香骨架的设计合成及其在海水提铀领域的应用研究[D]. 长春: 东北师范大学, 2021. |
| WANG Zeyu. Target preparation of conductive porous aromatic frameworks for uranium extraction from seawater[D]. Changchun: Northeast Normal University, 2021. | |
| 85 | 关荣锋, 王杏, 田大垒, 等. 有机酸掺杂聚苯胺的制备及其结构分析[J]. 化工进展, 2009, 28(11): 1978-1981. |
| GUAN Rongfeng, WANG Xing, TIAN Dalei, et al. Preparation and structural analysis of polyaniline doped with organic acid[J]. Chemical Industry and Engineering Progress, 2009, 28(11): 1978-1981. | |
| 86 | KASIAN O, GEIGER S, MAYRHOFER K J J, et al. Electrochemical on-line ICP-MS in electrocatalysis research[J]. Chemical Record, 2019, 19(10): 2130-2142. |
| 87 | BAER D R, ARTYUSHKOVA K, RICHARD BRUNDLE C, et al. Practical guides for X-ray photoelectron spectroscopy: first steps in planning, conducting, and reporting XPS measurements[J]. Journal of Vacuum Science & Technology A, 2019, 37(3): 031401. |
| 88 | MARSUSI F, DRUMMOND N D, VERSTRAETE M J. The physics of single-side fluorination of graphene: DFT and DFT + U studies[J]. Carbon, 2019, 144: 615-627. |
| 89 | CAPUTO P, LOISE V, CRISPINI A, et al. The efficiency of bitumen rejuvenator investigated through powder X-ray diffraction (PXRD) analysis and T2-NMR spectroscopy[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2019, 571: 50-54. |
| 90 | IZUMI A, SHUDO Y, KAKARA T. XAFS and HAXPES analyses of the oxidation state of a copper surface buried under a phenolic resin nanofilm[J]. Applied Surface Science, 2022, 589: 152967. |
| 91 | LINDNER H, SCHNEIDER E. Review of cost estimates for uranium recovery from seawater[J]. Energy Economics, 2015, 49: 9-22. |
| 92 | KUO L J, PAN H B, WAI C M, et al. Investigations into the reusability of amidoxime-based polymeric adsorbents for seawater uranium extraction[J]. Industrial & Engineering Chemistry Research, 2017, 56(40): 11603-11611. |
| 93 | KIM J, TSOURIS C, OYOLA Y, et al. Uptake of uranium from seawater by amidoxime-based polymeric adsorbent: field experiments, modeling, and updated economic assessment[J]. Industrial & Engineering Chemistry Research, 2014, 53(14): 6076-6083. |
| [1] | SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298. |
| [2] | ZHAO Wei, ZHAO Deyin, LI Shihan, LIU Hongda, SUN Jin, GUO Yanqiu. Synthesis and application of triazine drag reducing agent for nature gas pipeline [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 391-399. |
| [3] | WANG Zhengkun, LI Sifang. Green synthesis of gemini surfactant decyne diol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 400-410. |
| [4] | WANG Shengyan, DENG Shuai, ZHAO Ruikai. Research progress on carbon dioxide capture technology based on electric swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 233-245. |
| [5] | YANG Ying, HOU Haojie, HUANG Rui, CUI Yu, WANG Bing, LIU Jian, BAO Weiren, CHANG Liping, WANG Jiancheng, HAN Lina. Coal tar phenol-based carbon nanosphere prepared by Stöber method for adsorption of CO2 [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 5011-5018. |
| [6] | YIN Xinyu, PI Pihui, WEN Xiufang, QIAN Yu. Application of special wettability materials for anti-hydrate-nucleation and anti-hydrate-adhesion in oil and gas pipelines [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4076-4092. |
| [7] | XIANG Yang, HUANG Xun, WEI Zidong. Recent progresses in the activity and selectivity improvement of electrocatalytic organic synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4005-4014. |
| [8] | XU Peiyao, CHEN Biaoqi, KANKALA Ranjith Kumar, WANG Shibin, CHEN Aizheng. Research progress of nanomaterials for synergistic ferroptosis anticancer therapy [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3684-3694. |
| [9] | LU Yang, ZHOU Jinsong, ZHOU Qixin, WANG Tang, LIU Zhuang, LI Bohao, ZHOU Lingtao. Leaching mechanism of Hg-absorption products on CeO2/TiO2 sorbentsin syngas [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3875-3883. |
| [10] | WANG Shuaiqi, WANG Congxin, WANG Xuelin, TIAN Zhijian. Solvent-free rapid synthesis of ZSM-12 zeolite [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3561-3571. |
| [11] | YU Xixi, ZHANG Jinshuai, LEI Wen, LIU Chengguo. Research progress of self-healing photocuring polymeric materials based on dynamic covalent bonds [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3589-3599. |
| [12] | WANG Zhicai, LIU Weiwei, ZHOU Cong, PAN Chunxiu, YAN Honglei, LI Zhanku, YAN Jingchong, REN Shibiao, LEI Zhiping, SHUI Hengfu. Synthesis and performance of a superplasticizer based on coal-based humic acid [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3634-3642. |
| [13] | CHEN Sen, YIN Pengyuan, YANG Zhenglu, MO Yiming, CUI Xili, SUO Xian, XING Huabin. Advances in the intelligent synthesis of functional solid materials [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3340-3348. |
| [14] | XU Chunshu, YAO Qingda, LIANG Yongxian, ZHOU Hualong. Effects of graphene oxide/carbon nanotubes on the properties of several typical polymer materials [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3012-3028. |
| [15] | ZHANG Chenyu, WANG Ning, XU Hongtao, LUO Zhuqing. Performance evaluation of the multiple layer latent heat thermal energy storage unit combined with nanoparticle for heat transfer enhancement [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2332-2342. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||