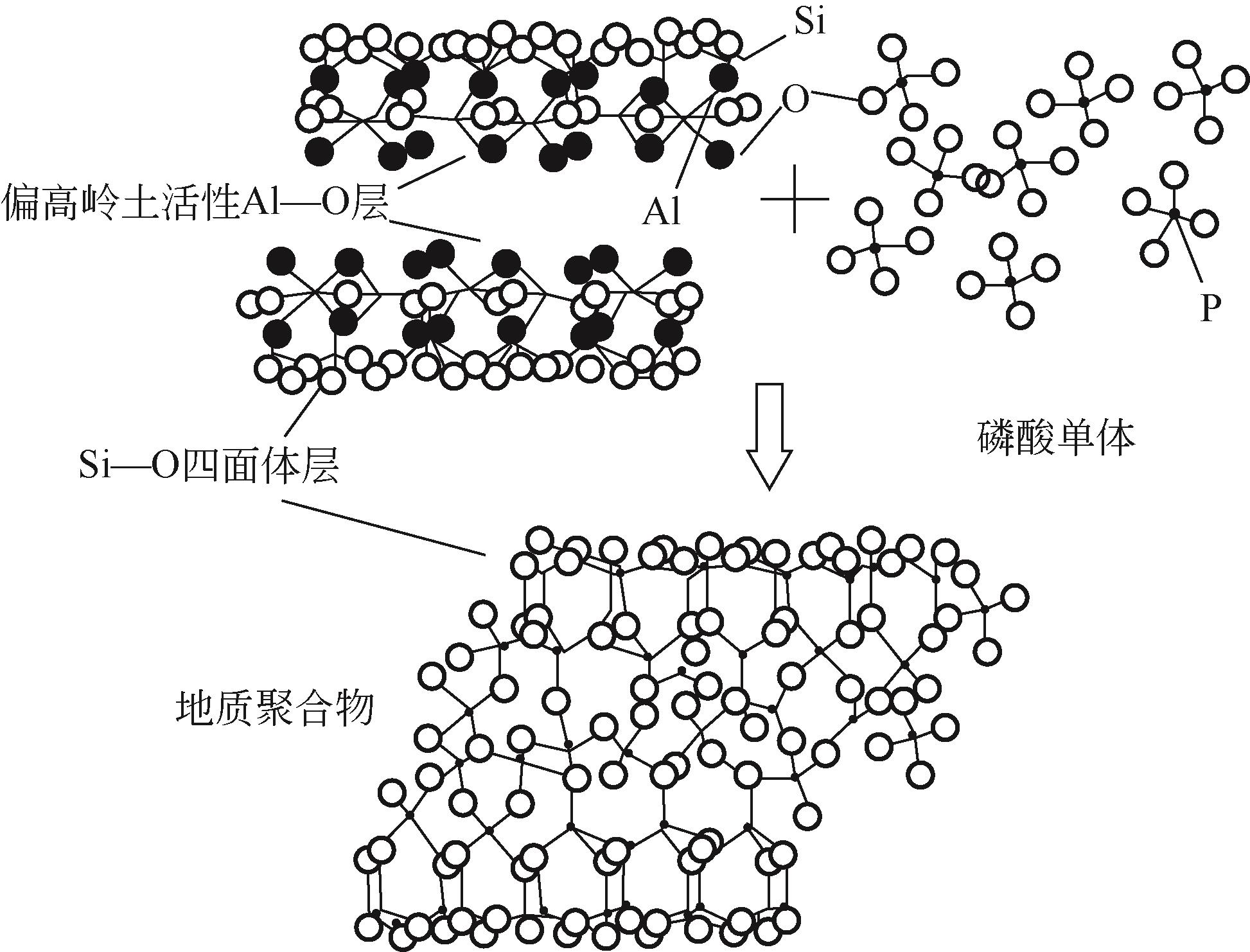
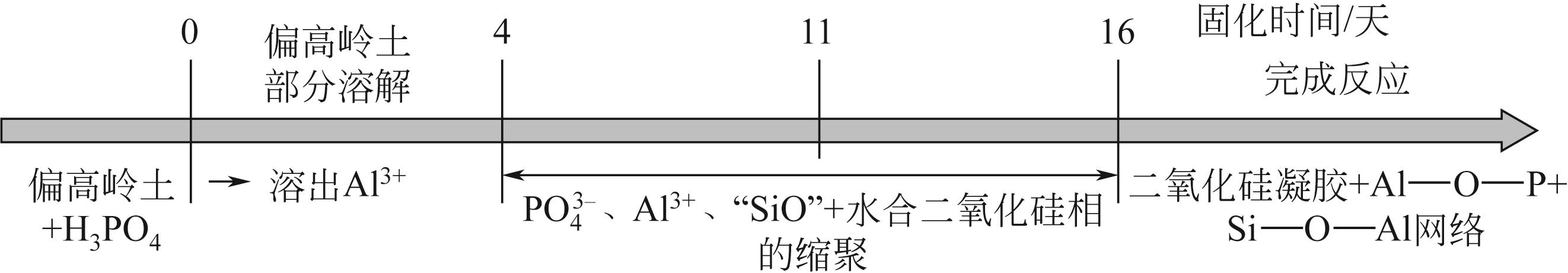
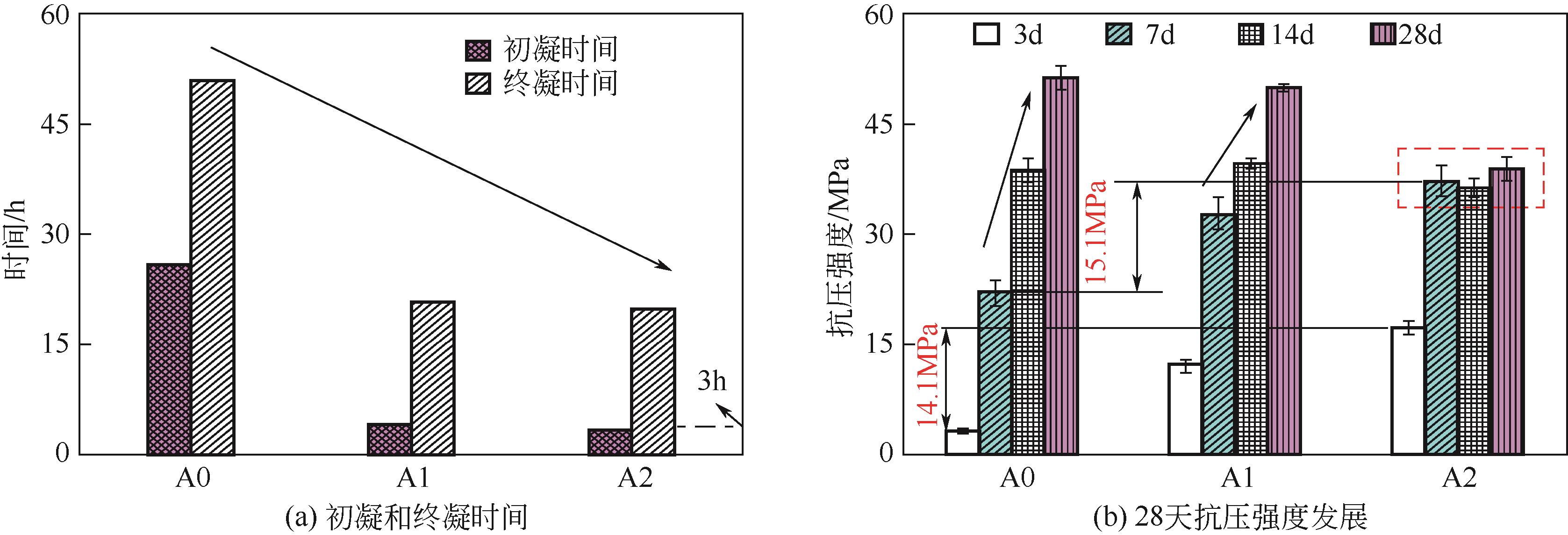
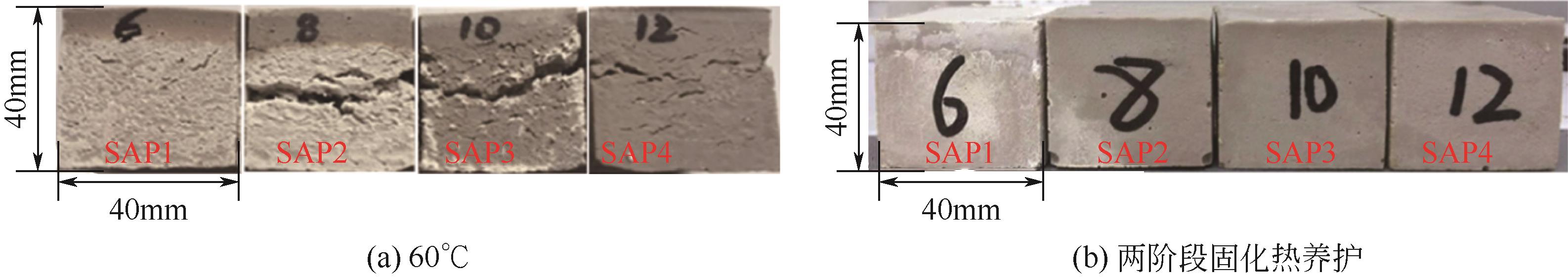
Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (3): 1411-1425.DOI: 10.16085/j.issn.1000-6613.2022-0918
• Materials science and technology • Previous Articles Next Articles
Recent progress on the fabrication and properties of phosphobase geopolymer
GAO Jiangyu1( ), ZHANG Yaojun1(
), ZHANG Yaojun1( ), HE Panyang2, LIU Licai1, ZHANG Fengye1
), HE Panyang2, LIU Licai1, ZHANG Fengye1
- 1.Materials Science and Engineering, Xi’an University of Architecture and Technology, Xi’an 710055, Shaanxi, China
2.Resource Engineering, Xi’an University of Architecture and Technology, Xi’an 710055, Shaanxi, China
-
Received:2022-05-17Revised:2022-06-27Online:2023-04-10Published:2023-03-15 -
Contact:ZHANG Yaojun
磷酸基地质聚合物的制备及其性能研究进展
高江雨1( ), 张耀君1(
), 张耀君1( ), 贺攀阳2, 刘礼才1, 张枫烨1
), 贺攀阳2, 刘礼才1, 张枫烨1
- 1.西安建筑科技大学材料科学与工程学院,陕西 西安 710055
2.西安建筑科技大学资源工程学院,陕西 西安 710055
-
通讯作者:张耀君 -
作者简介:高江雨(1998—),男,硕士研究生,研究方向为固体废弃物资源化。E-mail:gaojiangyu@xauat.edu.cn。 -
基金资助:国家自然科学基金(21676209)
CLC Number:
Cite this article
GAO Jiangyu, ZHANG Yaojun, HE Panyang, LIU Licai, ZHANG Fengye. Recent progress on the fabrication and properties of phosphobase geopolymer[J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1411-1425.
高江雨, 张耀君, 贺攀阳, 刘礼才, 张枫烨. 磷酸基地质聚合物的制备及其性能研究进展[J]. 化工进展, 2023, 42(3): 1411-1425.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-0918
| 养护温度制度 | 养护环境 | 磷酸质量分数 /% | 水用量 | 磷酸用量 | 原料性质 | 抗压强度 /MPa | 参考文献 |
|---|---|---|---|---|---|---|---|
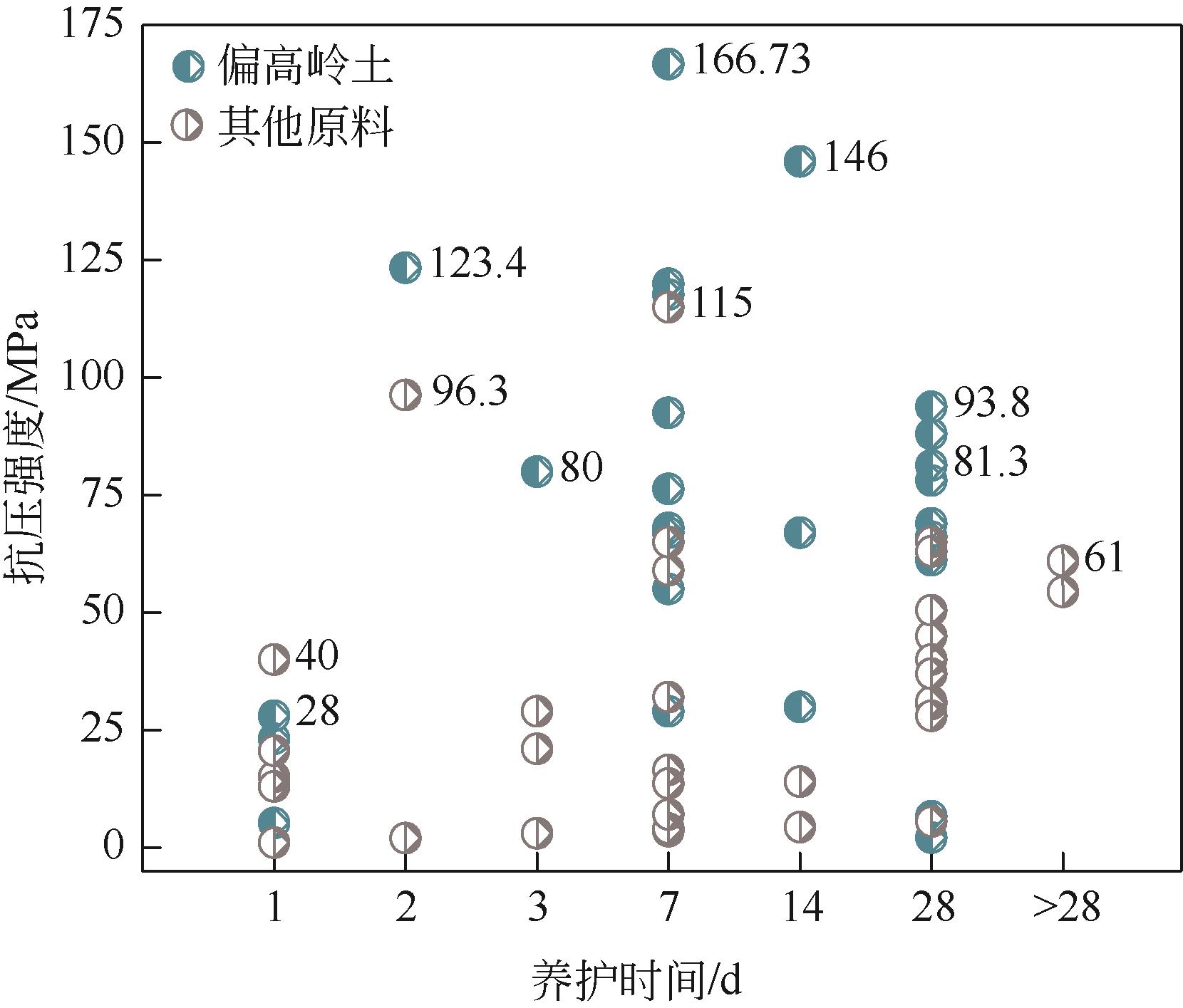
| 50℃(7d) | 潮湿/干燥 | 85 | W/B=0.3 | P/Al=0.6~1.0 | — | 40~117.7 | [ |
| 60℃(24h)、RT(21d) | — | 85 | W/B=0.25 | Si/P=2.25~3.50 | d≤125μm | 最大37 | [ |
| 60℃(24h)、RT(28d) | — | 85 | — | Si/P=2.75 | d≤125μm、100μm、80μm、63μm | 39~54 | [ |
| RT(24h)、60℃(24h)、RT(28d) | 密封 | 4~14mol/L | L/S=0.8 | — | — | 36.4~93.8 | [ |
| RT(5d)、60℃(24h)、RT(28d) | 密封 | 10mol/L | L/S=0.95 | — | 不同三水铝石含量,d≤90μm | 最大54.41 | [ |
| RT(3~28d) | 潮湿 | 85 | H2O/偏高岭土(MK)=0.4 | Si/P=2.75 | — | 最大51.3 | [ |
| 20℃、40℃和70℃ | 密封 | 85 | Al/H2O=0.15~0.62 | Al/P=1或4 | Si/Al=0.85~1.02,d50=10~6μm | 44~120 | [ |
| 60℃或RT(15d) | 密封 | 85 | L/S=1 | P/Al=1 | — | 29.9或20.7 | [ |
| 60℃(24h)、RT(28d) | 密封 | 85 | P/Al=0.5~2 | — | 最大2.1 | [ | |
| 80℃(24h)、60℃(3d) | 密封 | — | H2O/Al2O3=4∶1 | H3PO4/Al2O3=1 | SiO2/Al2O3=1,d≤45μm | 最大89.3 | [ |
| 80℃(24h)、60℃(3d) | 密封 | — | H2O/Al2O3=2∶1 | H3PO4/Al2O3=1 | SiO2/Al2O3=3/1,200~1000℃(2h) | 最大60 | [ |
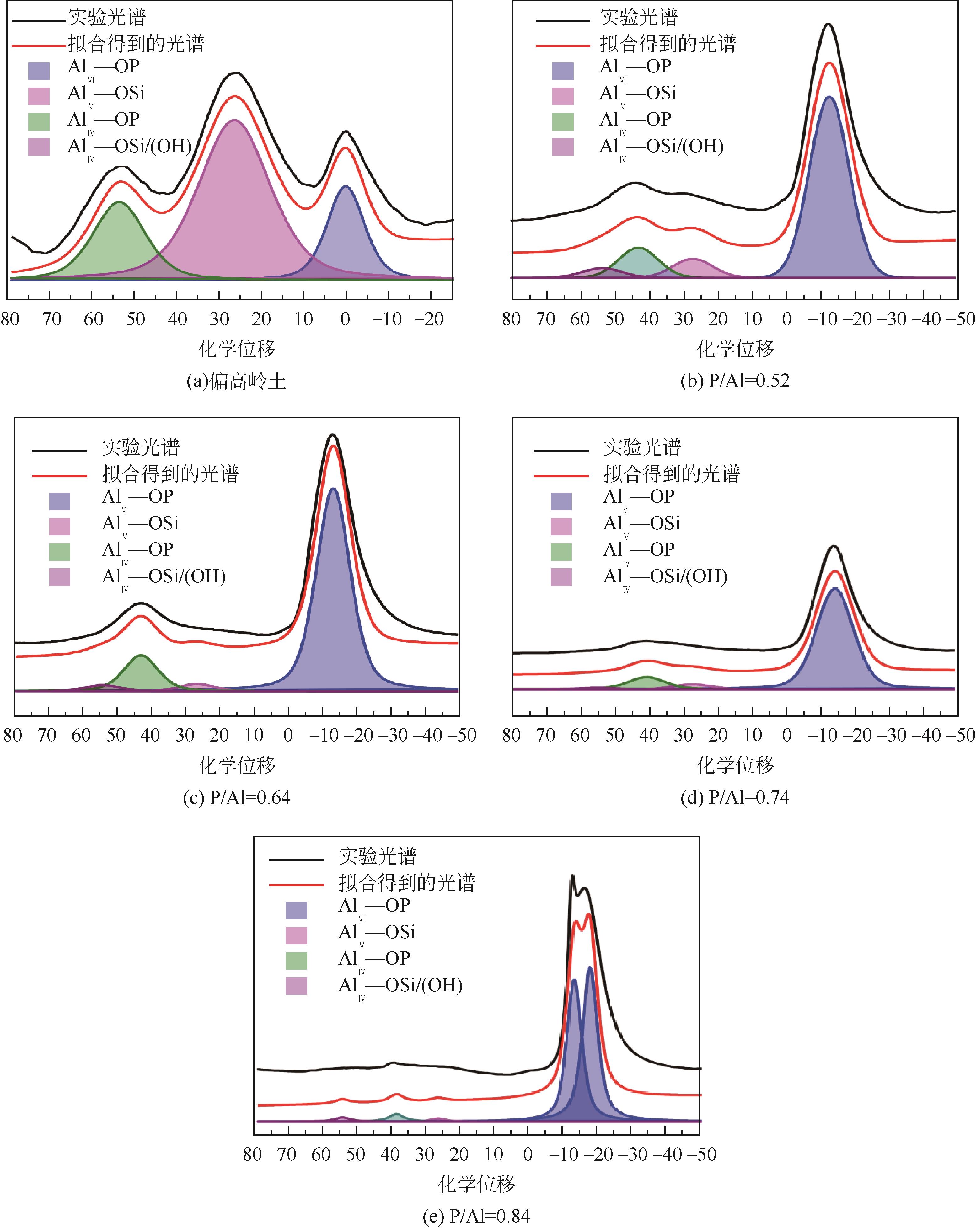
| 40℃(24h)、60℃(24h) | 密封 | 85 | L/S=1 | P/Al=0.52~0.84 | Si/Al=0.96 | 23.1~123.4 | [ |
| 40℃(24h)、80℃(24h) | 密封 | 85 | L/S=1 | P/Al=0.52~0.84 | Si/Al=0.96 | 21.6~96.8 | [ |
| 60℃(7d) | — | 68 | L/S=1.60~1.75 | — | — | 最大46.3 | [ |
| 70℃(72h) | 密封 | 85 | 0.45%~0.68% | Al/P=0.9~1.8 | Si/Al=1 | 80~11 | [ |
| RT(7d) | 密封 | 85 | L/S=1 | — | SiO2/Al2O3=1.0~2.2 | 最大13.59 | [ |
| 60℃(24h)、RT(7d、14d、28d) | — | 85 | L/S=0.67或1.25 | P/Al=1 | 热活化或机械研磨 | 最大5.5 | [ |
| RT(7d) | 密封 | 85 | L/S=1 | H3PO4/Al2O3=1.0~1.4 | — | 29 | [ |
| 60℃(24h)、RT(28d) | 干燥 | 10mol/L | L/S=1~1.2 | Al/P=0.8~1.0 | Si/Al=1.6,700~900℃(2h) | 最大67 | [ |
| 养护温度制度 | 养护环境 | 磷酸质量分数 /% | 水用量 | 磷酸用量 | 原料性质 | 抗压强度 /MPa | 参考文献 |
|---|---|---|---|---|---|---|---|
| 50℃(7d) | 潮湿/干燥 | 85 | W/B=0.3 | P/Al=0.6~1.0 | — | 40~117.7 | [ |
| 60℃(24h)、RT(21d) | — | 85 | W/B=0.25 | Si/P=2.25~3.50 | d≤125μm | 最大37 | [ |
| 60℃(24h)、RT(28d) | — | 85 | — | Si/P=2.75 | d≤125μm、100μm、80μm、63μm | 39~54 | [ |
| RT(24h)、60℃(24h)、RT(28d) | 密封 | 4~14mol/L | L/S=0.8 | — | — | 36.4~93.8 | [ |
| RT(5d)、60℃(24h)、RT(28d) | 密封 | 10mol/L | L/S=0.95 | — | 不同三水铝石含量,d≤90μm | 最大54.41 | [ |
| RT(3~28d) | 潮湿 | 85 | H2O/偏高岭土(MK)=0.4 | Si/P=2.75 | — | 最大51.3 | [ |
| 20℃、40℃和70℃ | 密封 | 85 | Al/H2O=0.15~0.62 | Al/P=1或4 | Si/Al=0.85~1.02,d50=10~6μm | 44~120 | [ |
| 60℃或RT(15d) | 密封 | 85 | L/S=1 | P/Al=1 | — | 29.9或20.7 | [ |
| 60℃(24h)、RT(28d) | 密封 | 85 | P/Al=0.5~2 | — | 最大2.1 | [ | |
| 80℃(24h)、60℃(3d) | 密封 | — | H2O/Al2O3=4∶1 | H3PO4/Al2O3=1 | SiO2/Al2O3=1,d≤45μm | 最大89.3 | [ |
| 80℃(24h)、60℃(3d) | 密封 | — | H2O/Al2O3=2∶1 | H3PO4/Al2O3=1 | SiO2/Al2O3=3/1,200~1000℃(2h) | 最大60 | [ |
| 40℃(24h)、60℃(24h) | 密封 | 85 | L/S=1 | P/Al=0.52~0.84 | Si/Al=0.96 | 23.1~123.4 | [ |
| 40℃(24h)、80℃(24h) | 密封 | 85 | L/S=1 | P/Al=0.52~0.84 | Si/Al=0.96 | 21.6~96.8 | [ |
| 60℃(7d) | — | 68 | L/S=1.60~1.75 | — | — | 最大46.3 | [ |
| 70℃(72h) | 密封 | 85 | 0.45%~0.68% | Al/P=0.9~1.8 | Si/Al=1 | 80~11 | [ |
| RT(7d) | 密封 | 85 | L/S=1 | — | SiO2/Al2O3=1.0~2.2 | 最大13.59 | [ |
| 60℃(24h)、RT(7d、14d、28d) | — | 85 | L/S=0.67或1.25 | P/Al=1 | 热活化或机械研磨 | 最大5.5 | [ |
| RT(7d) | 密封 | 85 | L/S=1 | H3PO4/Al2O3=1.0~1.4 | — | 29 | [ |
| 60℃(24h)、RT(28d) | 干燥 | 10mol/L | L/S=1~1.2 | Al/P=0.8~1.0 | Si/Al=1.6,700~900℃(2h) | 最大67 | [ |
| 原料 | 激发剂 | 氧化物 | 反应生成物 | 最大抗压强度/MPa | 参考文献 |
|---|---|---|---|---|---|
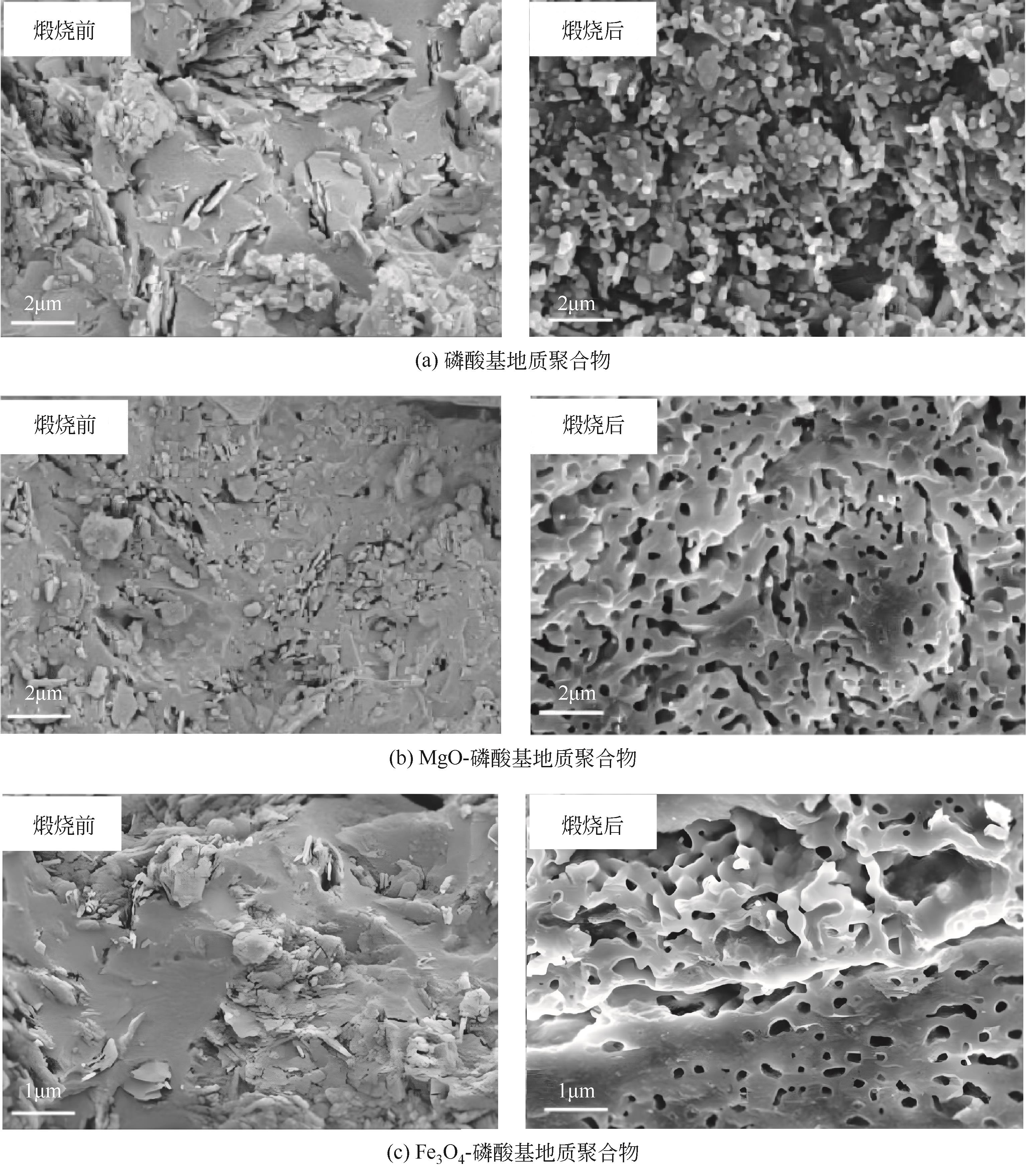
| 偏高岭土 | Al(H2PO4)3 | MgO(镁砂) | 磷镁石(MgHPO4·3H2O) | 53.07 | [ |
| 偏高岭土 | Al(H2PO4)3 | MgO(镁砂) | 磷镁石(MgHPO4·3H2O) | 53.07 | [ |
| 偏高岭土 | H3PO4(10mol/L) | Fe2O3(矿物相) | P—O—Si—O—Fe—O | 56.4 | [ |
| 富铁红土 | H3PO4(10mol/L) | Fe2O3(矿物相) | 无定形磷酸铁相 | 65 | [ |
| 偏高岭土 | Al(H2PO4)3 | MgO(重烧氧化镁) | 镁磷石和无定形磷酸铝镁 | 13.6 | [ |
| 偏高岭土 | H3PO4(85%) | MgO | 无定形凝胶相,掺量多则产生镁磷石 | 58.03 | [ |
| 偏高岭土 | H3PO4(85%) | Fe3O4 | 无定形凝胶 | 62.81 | [ |
| 偏高岭土 | Al(H2PO4)3 | Al2O3(纳米氧化铝) | 非晶相磷酸铝 | 70.2 | [ |
| 原料 | 激发剂 | 氧化物 | 反应生成物 | 最大抗压强度/MPa | 参考文献 |
|---|---|---|---|---|---|
| 偏高岭土 | Al(H2PO4)3 | MgO(镁砂) | 磷镁石(MgHPO4·3H2O) | 53.07 | [ |
| 偏高岭土 | Al(H2PO4)3 | MgO(镁砂) | 磷镁石(MgHPO4·3H2O) | 53.07 | [ |
| 偏高岭土 | H3PO4(10mol/L) | Fe2O3(矿物相) | P—O—Si—O—Fe—O | 56.4 | [ |
| 富铁红土 | H3PO4(10mol/L) | Fe2O3(矿物相) | 无定形磷酸铁相 | 65 | [ |
| 偏高岭土 | Al(H2PO4)3 | MgO(重烧氧化镁) | 镁磷石和无定形磷酸铝镁 | 13.6 | [ |
| 偏高岭土 | H3PO4(85%) | MgO | 无定形凝胶相,掺量多则产生镁磷石 | 58.03 | [ |
| 偏高岭土 | H3PO4(85%) | Fe3O4 | 无定形凝胶 | 62.81 | [ |
| 偏高岭土 | Al(H2PO4)3 | Al2O3(纳米氧化铝) | 非晶相磷酸铝 | 70.2 | [ |
| 原料 | 激发剂 | 纤维 | 最佳掺量 | 抗压强度/MPa | 抗弯强度/MPa | 参考文献 |
|---|---|---|---|---|---|---|
| 偏高岭土 | 磷酸铝溶液 | 聚乙烯醇(PVA)短纤维 | 1.5% | — | 13.86 | [ |
| 偏高岭土 | 磷酸铝溶液 | 碳纤维片材 | 3层 | — | 31.32 | [ |
| 偏高岭土 | H3PO4(85%) | PI纤维(碳纳米管修饰) | 1.5% | 110.4 | 37.9 | [ |
| 偏高岭土 | H3PO4(85%) | 玄武岩纤维 | 0.5% | 3.4 | 0.7867 | [ |
| 偏高岭土 | H3PO4(85%) | 玻璃纤维 | 1.3% | 3.45 | 0.825 | [ |
| 偏高岭土 | H3PO4(85%) | 杉木纤维 | 10% | — | 13.7 | [ |
| 原料 | 激发剂 | 纤维 | 最佳掺量 | 抗压强度/MPa | 抗弯强度/MPa | 参考文献 |
|---|---|---|---|---|---|---|
| 偏高岭土 | 磷酸铝溶液 | 聚乙烯醇(PVA)短纤维 | 1.5% | — | 13.86 | [ |
| 偏高岭土 | 磷酸铝溶液 | 碳纤维片材 | 3层 | — | 31.32 | [ |
| 偏高岭土 | H3PO4(85%) | PI纤维(碳纳米管修饰) | 1.5% | 110.4 | 37.9 | [ |
| 偏高岭土 | H3PO4(85%) | 玄武岩纤维 | 0.5% | 3.4 | 0.7867 | [ |
| 偏高岭土 | H3PO4(85%) | 玻璃纤维 | 1.3% | 3.45 | 0.825 | [ |
| 偏高岭土 | H3PO4(85%) | 杉木纤维 | 10% | — | 13.7 | [ |
| 发泡剂 | 总孔隙率/% | 热导率/W·m-1·K-1 | 抗压强度/MPa | 体积密度/g·cm-3 | 高温试验温度/℃ | 参考文献 |
|---|---|---|---|---|---|---|
| 石灰石粉 | 30.5~32.1 | 0.133 | 最大6 | 1.316~1.274 | 1000 | [ |
| H2O2(MnO2) | 42~56 | 0.17 | 2.09 | 0.66~0.76 | 1100 | [ |
| 铝粉 | 40~83 | — | 6~13.7 | — | 1450 | [ |
| Triton X-100 | 78.3 | — | 0.64 | 0.43 | 1100 | [ |
| H2O2(MnO2) | 42~56 | 最小0.17 | 最大2.73 | 0.68~0.73 | 1100 | [ |
| H2O2 | 55~64 | — | 最大1.17 | 最小0.336 | 1100 | [ |
| 石灰石粉 | 69~76 | 0.07~0.09 | — | 0.6~0.73 | 1000 | [ |
| 发泡剂 | 总孔隙率/% | 热导率/W·m-1·K-1 | 抗压强度/MPa | 体积密度/g·cm-3 | 高温试验温度/℃ | 参考文献 |
|---|---|---|---|---|---|---|
| 石灰石粉 | 30.5~32.1 | 0.133 | 最大6 | 1.316~1.274 | 1000 | [ |
| H2O2(MnO2) | 42~56 | 0.17 | 2.09 | 0.66~0.76 | 1100 | [ |
| 铝粉 | 40~83 | — | 6~13.7 | — | 1450 | [ |
| Triton X-100 | 78.3 | — | 0.64 | 0.43 | 1100 | [ |
| H2O2(MnO2) | 42~56 | 最小0.17 | 最大2.73 | 0.68~0.73 | 1100 | [ |
| H2O2 | 55~64 | — | 最大1.17 | 最小0.336 | 1100 | [ |
| 石灰石粉 | 69~76 | 0.07~0.09 | — | 0.6~0.73 | 1000 | [ |
| 69 | 帅勤. 磷酸基地聚合物多孔材料的制备及性能研究[D]. 绵阳: 西南科技大学, 2020. |
| SHUAI Qin. Preparation and properties of phosphoric acid-based geopolymer foams[D]. Mianyang: Southwest University of Science and Technology, 2020. | |
| 70 | 禤小欣. 磷酸基地聚物杉木纤维复合材料的制备及其性能研究[D]. 南宁: 南宁师范大学, 2020. |
| XUAN Xiaoxin. Study on preparation and properties of phosphoric acid-based geopolymers Chinese fir fiber composites[D]. Nanning: Nanning Normal University, 2020. | |
| 71 | LIU Leping, CUI Xuemin, QIU Shuheng, et al. Preparation of phosphoric acid-based porous geopolymers[J]. Applied Clay Science, 2010, 50(4): 600-603. |
| 72 | SELLAMI M, BARRE M, TOUMI M. Synthesis, thermal properties and electrical conductivity of phosphoric acid-based geopolymer with metakaolin[J]. Applied Clay Science, 2019, 180: 105192. |
| 73 | NOBOUASSIA BEWA C, TCHAKOUTÉ H K, FOTIO D, et al. Water resistance and thermal behavior of metakaolin-phosphate-based geopolymer cements[J]. Journal of Asian Ceramic Societies, 2018, 6(3): 271-283. |
| 74 | BAI C Y, CONTE A, COLOMBO P. Open-cell phosphate-based geopolymer foams by frothing[J]. Materials Letters, 2017, 188: 379-382. |
| 75 | CELERIER H, JOUIN J, GHARZOUNI A, et al. Relation between working properties and structural properties from 27Al, 29Si and 31P NMR and XRD of acid-based geopolymers from 25 to 1000℃[J]. Materials Chemistry and Physics, 2019, 228: 293-302. |
| 76 | 安然, 徐中慧, 帅勤, 等. 磷酸基地聚合物多孔材料的制备及其隔热防火性能研究[J]. 硅酸盐通报, 2021, 40(4): 1258-1265. |
| AN Ran, XU Zhonghui, SHUAI Qin, et al. Preparation of phosphoric acid-based geopolymer foams and its fire-resistance[J]. Bulletin of the Chinese Ceramic Society, 2021, 40(4): 1258-1265. | |
| 77 | SHUAI Qin, XU Zhonghui, YAO Zhengzhen, et al. Fire resistance of phosphoric acid-based geopolymer foams fabricated from metakaolin and hydrogen peroxide[J]. Materials Letters, 2020, 263: 127228. |
| 78 | LASSINANTTI GUALTIERI M, ROMAGNOLI M, GUALTIERI A F. Preparation of phosphoric acid-based geopolymer foams using limestone as pore forming agent - thermal properties by in situ XRPD and Rietveld refinements[J]. Journal of the European Ceramic Society, 2015, 35(11): 3167-3178. |
| 79 | 牛丽丽, 李玉香, 马雪. 磷酸基地质聚合物抗侵蚀性研究[J]. 西南科技大学学报, 2020, 35(3): 31-36. |
| NIU Lili, LI Yuxiang, MA Xue. Study on the corrosion resistance of phosphoric acid-based geopolymer[J]. Journal of Southwest University of Science and Technology, 2020, 35(3): 31-36. | |
| 80 | 牛丽丽. 磷酸基地质聚合物的制备及固化模拟中放α废液的研究[D]. 绵阳: 西南科技大学, 2020. |
| NIU Lili. Study on preparation of phosphoric acid-based geopolymer and immobilization of simulation medium level alpha liquid waste[D]. Mianyang: Southwest University of Science and Technology, 2020. | |
| 81 | 何流. 磷酸基地质聚合物的结构演变及固化模拟核素研究[D]. 绵阳: 西南科技大学, 2019. |
| HE Liu. Study on structural evolution and solidification of simulated nuclide of phosphoric acid-based geopolymer[D]. Mianyang: Southwest University of Science and Technology, 2019. | |
| 82 | PU Shaoyun, ZHU Zhiduo, SONG Weilong, et al. A novel acidic phosphoric-based geopolymer binder for lead solidification/stabilization[J]. Journal of Hazardous Materials, 2021, 415: 125659. |
| 83 | DONG Teng, XIE Shuibo, WANG Jingsong, et al. Solidification and stabilization of spent TBP/OK organic liquids in a phosphate acid-based geopolymer[J]. Science and Technology of Nuclear Installations, 2020, 2020: 8094205. |
| 84 | CUI Xuemin, LIU Leping, HE Yan, et al. A novel aluminosilicate geopolymer material with low dielectric loss[J]. Materials Chemistry and Physics, 2011, 130(1/2): 1-4. |
| 85 | DOUIRI H, LOUATI S, BAKLOUTI S, et al. Structural and dielectric comparative studies of geopolymers prepared with metakaolin and Tunisian natural clay[J]. Applied Clay Science, 2017, 139: 40-44. |
| 86 | SELLAMI M, BARRE M, TOUMI M. The new challenge of acid-based geopolymers synthesized with the incorporation of lithium ions as cathode materials for lithium-ion batteries[J]. Journal of Inorganic and Organometallic Polymers and Materials, 2020, 30(8): 3126-3131. |
| 1 | DAVIDOVITS J. Geopolymers and geopolymeric materials[J]. Journal of Thermal Analysis, 1989, 35(2): 429-441. |
| 2 | 张耀君, 杨梦阳, 康乐, 等. 一类新型碱激发胶凝材料催化剂的研究进展[J]. 无机材料学报, 2016, 31(3): 225-233. |
| ZHANG Yaojun, YANG Mengyang, KANG Le, et al. Research progresses of new type alkali-activated cementitious material catalyst[J]. Journal of Inorganic Materials, 2016, 31(3): 225-233. | |
| 3 | 翁履谦, 宋申华. 新型地质聚合物胶凝材料[J]. 材料导报, 2005, 19(2): 67-68. |
| WENG Lyuqian, SONG Shenhua. Development of novel cementious geopolymers[J]. Materials Review, 2005, 19(2): 67-68, 80. | |
| 4 | 孙道胜, 王爱国, 胡普华. 地质聚合物的研究与应用发展前景[J]. 材料导报, 2009, 23(7): 61-65. |
| SUN Daosheng, WANG Aiguo, HU Puhua. Research of geopolymer and its applications and development prospects[J]. Materials Review, 2009, 23(7): 61-65. | |
| 5 | AMRAN Y H M, ALYOUSEF R, ALABDULJABBAR H, et al. Clean production and properties of geopolymer concrete: a review[J]. Journal of Cleaner Production, 2020, 251: 119679. |
| 6 | YU Haoyang, XU Mengxue, CHEN Chaoni, et al. A review on the porous geopolymer preparation for structural and functional materials applications[J]. International Journal of Applied Ceramic Technology, 2022, 19(4): 1793-1813. |
| 7 | ZHANG Xiaolong, ZHANG Shiyu, LIU Hui, et al. Disposal of mine tailings via geopolymerization[J]. Journal of Cleaner Production, 2021, 284: 124756. |
| 8 | COPPOLA B, TULLIANI J M, ANTONACI P, et al. Role of natural stone wastes and minerals in the alkali activation process: a review[J]. Materials, 2020, 13(10): E2284. |
| 9 | WAGH A S. Introduction to chemically bonded ceramics[M]// Chemically Bonded Phosphate Ceramics. Amsterdam: Elsevier, 2004: 1-13. |
| 87 | PENGOU M, NGOUNÉ B, TCHAKOUTÉ H K, et al. Correction to: utilization of geopolymer cements as supercapacitors: influence of the hardeners on their properties[J]. SN Applied Sciences, 2020, 2(8): 1. |
| 88 | PENGOU M, NGASSA G B P, BOUTIANALA M, et al. Geopolymer cement-modified carbon paste electrode: application to electroanalysis of traces of lead(II) ions in aqueous solution[J]. Journal of Solid State Electrochemistry, 2021, 25(4): 1183-1195. |
| 10 | COSTATO M. Acid-base cements. Their biomedical and industrial applications[J]. Il Nuovo Cimento D, 1995, 17(5): 545. |
| 11 | TCHAKOUTÉ H K, RÜSCHER C H. Mechanical and microstructural properties of metakaolin-based geopolymer cements from sodium waterglass and phosphoric acid solution as hardeners: a comparative study[J]. Applied Clay Science, 2017, 140: 81-87. |
| 12 | LIU Leping, CUI Xuemin, HE Yan, et al. Study on the dielectric properties of phosphoric acid-based geopolymers[J]. Materials Science Forum, 2010, 663/664/665: 538-541. |
| 13 | PERERA D S, HANNA J V, DAVIS J, et al. Relative strengths of phosphoric acid-reacted and alkali-reacted metakaolin materials[J]. Journal of Materials Science, 2008, 43(19): 6562-6566. |
| 14 | WANG Yanshuai, ALREFAEI Yazan, DAI Jianguo. Silico-aluminophosphate and alkali-aluminosilicate geopolymers: a comparative review[J]. Frontiers in Materials, 2019, 6: 106. |
| 15 | ZRIBI M, BAKLOUTI S. Phosphate-based geopolymers: a critical review[J]. Polymer Bulletin, 2022, 79(9): 6827-6855. |
| 16 | 曹德光, 苏达根, 路波, 等. 偏高岭石-磷酸基矿物键合材料的制备与结构特征[J]. 硅酸盐学报, 2005, 33(11): 1385-1389. |
| CAO Deguang, SU Dagen, LU Bo, et al. Synthesis and structure characterization of geopolymeric material based on metakaolinite and phosphoric acid [J]. Journal of the Chinese Ceramic Society, 2005, 33(11): 1385-1389. | |
| 17 | 何流, 马雪, 李良锋, 等. Al2O3·nSiO2-mH3PO4磷酸基地质聚合物的制备与结构表征[J]. 人工晶体学报, 2018, 47(12): 2527-2533. |
| HE Liu, MA Xue, LI Liangfeng, et al. Preparation and structural characterization of Al2O3·nSiO2-mH3PO4 phosphoric acid-based geopolymer[J]. Journal of Synthetic Crystals, 2018, 47(12): 2527-2533. | |
| 18 | 周新涛, 苏达根, 钟明峰. 铝硅磷质胶凝材料的微观结构与性能[J]. 硅酸盐学报, 2007, 35(1): 105-108. |
| ZHOU Xintao, SU Dagen, ZHONG Mingfeng. Microstructure and performance of cementing material based on aluminosilicate and phosphate[J]. Journal of the Chinese Ceramic Society, 2007, 35(1): 105-108. | |
| 19 | 刘乐平. 磷酸基地质聚合物的反应机理与应用研究[D]. 南宁: 广西大学, 2012. |
| LIU Leping. Reaction mechanism and application study of phosphoric acid-based geopolymers[D]. Nanning: Guangxi University, 2012. | |
| 20 | 刘建, 刘派, 丁铸. 磷酸盐基矿聚物材料的制备与机理研究[J]. 深圳大学学报(理工版), 2020, 37(6): 597-603. |
| LIU Jian, LIU Pai, DING Zhu. Preparation and mechanism of phosphate based geopolymer[J]. Journal of Shenzhen University (Science and Engineering), 2020, 37(6): 597-603. | |
| 21 | LOUATI S, BAKLOUTI S, SAMET B. Acid based geopolymerization kinetics: effect of clay particle size[J]. Applied Clay Science, 2016, 132/133: 571-578. |
| 22 | DOUIRI H, LOUATI S, BAKLOUTI S, et al. Structural, thermal and dielectric properties of phosphoric acid-based geopolymers with different amounts of H3PO4 [J]. Materials Letters, 2014, 116: 9-12. |
| 23 | TCHAKOUTÉ H K, RÜSCHER C H, KAMSEU E, et al. Influence of the molar concentration of phosphoric acid solution on the properties of metakaolin-phosphate-based geopolymer cements[J]. Applied Clay Science, 2017, 147: 184-194. |
| 24 | TCHAKOUTÉ H K, RÜSCHER C H, KAMSEU E, et al. The influence of gibbsite in Kaolin and the formation of berlinite on the properties of metakaolin-phosphate-based geopolymer cements[J]. Materials Chemistry and Physics, 2017, 199: 280-288. |
| 25 | MORSY M S, RASHAD A M, SHOUKRY H, et al. Potential use of limestone in metakaolin-based geopolymer activated with H3PO4 for thermal insulation[J]. Construction and Building Materials, 2019, 229: 117088. |
| 26 | GUO Changming, WANG Kaituo, LIU Mengyi, et al. Preparation and characterization of acid-based geopolymer using metakaolin and disused polishing liquid[J]. Ceramics International, 2016, 42(7): 9287-9291. |
| 27 | LIU Leping, CUI Xuemin, HE Yan, et al. The phase evolution of phosphoric acid-based geopolymers at elevated temperatures[J]. Materials Letters, 2012, 66(1): 10-12. |
| 28 | DONG Teng, XIE Shuibo, WANG Jingsong, et al. Properties and characterization of a metakaolin phosphate acid-based geopolymer synthesized in a humid environment[J]. Journal of the Australian Ceramic Society, 2020, 56(1): 175-184. |
| 29 | MATHIVET V, JOUIN J, GHARZOUNI A, et al. Acid-based geopolymers: understanding of the structural evolutions during consolidation and after thermal treatments[J]. Journal of Non-Crystalline Solids, 2019, 512: 90-97. |
| 30 | ZRIBI M, BAKLOUTI S. Investigation of Phosphate based geopolymers formation mechanism[J]. Journal of Non-Crystalline Solids, 2021, 562: 120777. |
| 31 | LOUATI S, BAKLOUTI S, SAMET B. Geopolymers based on phosphoric acid and illito-kaolinitic clay[J]. Advances in Materials Science and Engineering, 2016, 2016: 2359759. |
| 32 | 邢书银. 利用粉煤灰制备地质聚合物的实验研究[D]. 西宁: 青海大学, 2016. |
| XING Shuyin. Study on preparation of geopolymer with fly ash[D]. Xining: Qinghai University, 2016. | |
| 33 | GUO Haozhe, YUAN Peng, ZHANG Baifa, et al. Realization of high-percentage addition of fly ash in the materials for the preparation of geopolymer derived from acid-activated metakaolin[J]. Journal of Cleaner Production, 2021, 285: 125430. |
| 34 | WANG Yanshuai, ALREFAEI Yazan, DAI Jianguo. Influence of coal fly ash on the early performance enhancement and formation mechanisms of silico-aluminophosphate geopolymer[J]. Cement and Concrete Research, 2020, 127: 105932. |
| 35 | 梁郁, 钱觉时, 王智. 磷酸激发粉煤灰的试验研究[J]. 粉煤灰综合利用, 2004, 17(3): 44-45. |
| LIANG Yu, QIAN Jueshi, WANG Zhi. Testing research on fly ash activated by phosphoric acid[J]. Fly Ash Comprehensive Utilization, 2004, 17(3): 44-45. | |
| 36 | DJON LI NDJOCK B I, ROBAYO-SALAZAR R A, DE GUTIÉRREZ R M, et al. Phosphoric acid activation of volcanic ashes: influence of the molar ratio R = (MgO + CaO)/P2O5 on reactivity of volcanic ash and strength of obtained cementitious material[J]. Journal of Building Engineering, 2021, 33: 101879. |
| 37 | DJON LI NDJOCK B I, BAENLA J, MBAH J B B, et al. Amorphous phase of volcanic ash and microstructure of cement product obtained from phosphoric acid activation[J]. SN Applied Sciences, 2020, 2(4): 1-10. |
| 38 | DJOBO J N Y, STEPHAN D, ELIMBI A. Setting and hardening behavior of volcanic ash phosphate cement[J]. Journal of Building Engineering, 2020, 31: 101427. |
| 39 | 刘梦怡. 失效磷酸基抛光液制备地质聚合物的合成、结构和性能表征[D]. 南宁: 广西大学, 2014. |
| LIU Mengyi. Preparation, synthesis, structure and performance characterization of invalid phosphoric acid-based polishing liquid geopolymer[D]. Nanning: Guangxi University, 2014. | |
| 40 | HAN Yaocong, CUI Xuemin, Xuesen LYU, et al. Preparation and characterization of geopolymers based on a phosphoric-acid-activated electrolytic manganese dioxide residue[J]. Journal of Cleaner Production, 2018, 205: 488-498. |
| 41 | WANG Yanshuai, PROVIS John L, DAI Jianguo. Role of soluble aluminum species in the activating solution for synthesis of silico-aluminophosphate geopolymers[J]. Cement and Concrete Composites, 2018, 93: 186-195. |
| 42 | 卢灿. 磷酸盐矿物键合材料的制备及其机理研究[D]. 深圳: 深圳大学, 2016. |
| LU Can. Mixproportion and mechanism analysis of chemically bonded phosphate ceramic material[D]. Shenzhen: Shenzhen University, 2016. | |
| 43 | CELERIER H, JOUIN J, TESSIER-DOYEN N, et al. Influence of various metakaolin raw materials on the water and fire resistance of geopolymers prepared in phosphoric acid[J]. Journal of Non-Crystalline Solids, 2018, 500: 493-501. |
| 44 | ZRIBI M, SAMET B, BAKLOUTI S. Effect of curing temperature on the synthesis, structure and mechanical properties of phosphate-based geopolymers[J]. Journal of Non-Crystalline Solids, 2019, 511: 62-67. |
| 45 | CELERIER H, JOUIN J, MATHIVET V, et al. Composition and properties of phosphoric acid-based geopolymers[J]. Journal of Non-Crystalline Solids, 2018, 493: 94-98. |
| 46 | 邢书银, 田亮亮, 王海霞, 等. 磷酸基偏高岭土地质聚合物研究[J]. 青海大学学报(自然科学版), 2015, 33(6): 30-35. |
| XING Shuyin, TIAN Liangliang, WANG Haixia, et al. Study on phosphoric acid-metakaolin based geopolymer[J]. Journal of Qinghai University (Natural Science Edition), 2015, 33(6): 30-35. | |
| 47 | ZRIBI M, SAMET B, BAKLOUTI S. Mechanical, microstructural and structural investigation of phosphate-based geopolymers with respect to P/Al molar ratio[J]. Journal of Solid State Chemistry, 2020, 281: 121025. |
| 48 | HE Yan, LIU Leping, HE Liping, et al. Characterization of chemosynthetic H3PO4-Al2O3-2SiO2 geopolymers[J]. Ceramics International, 2016, 42(9): 10908-10912. |
| 49 | LOUATI S, HAJJAJI W, BAKLOUTI S, et al. Structure and properties of new eco-material obtained by phosphoric acid attack of natural Tunisian clay[J]. Applied Clay Science, 2014, 101: 60-67. |
| 50 | LIN Hui, LIU Hui, LI Yue, et al. Properties and reaction mechanism of phosphoric acid activated metakaolin geopolymer at varied curing temperatures[J]. Cement and Concrete Research, 2021, 144: 106425. |
| 51 | 董腾, 邹艺璇, 宋培, 等. 微波养护偏高岭土磷酸基地聚物的特征与表征[J]. 中国粉体技术, 2020, 26(4): 52-58. |
| DONG Teng, ZOU Yixuan, SONG Pei, et al. Characteristics and characterization of metakaolin phosphoric acid-based geopolymer cured by microwave[J]. China Powder Science and Technology, 2020, 26(4): 52-58. | |
| 52 | MATHIVET V, JOUIN J, PARLIER M, et al. Control of the alumino-silico-phosphate geopolymers properties and structures by the phosphorus concentration[J]. Materials Chemistry and Physics, 2021, 258: 123867. |
| 53 | GAO Li, XIA Chulin, HONG Xuecai, et al. Effect of SiO2/Al2O3 molar ratio on microstructure and properties of phosphoric acid-based metakaolin geopolymerss[C]// 2016 International Conference on Material Science and Civil Engineering. Guilin, China, 2017: 307-315. |
| 54 | ZRIBI M, SAMET B, BAKLOUTI S. Screening of factors influencing phosphate-based geopolymers consolidation time, using plackett-burman design[M]//Advances in Materials, Mechanics and Manufacturing. Cham: Springer, 2020: 115-122. |
| 55 | DEROUICHE R, BAKLOUTI S. Phosphoric acid based geopolymerization: effect of the mechanochemical and the thermal activation of the Kaolin[J]. Ceramics International, 2021, 47(10): 13446-13456. |
| 56 | BEWA C N, TCHAKOUTÉ H K, BANENZOUÉ C, et al. Acid-based geopolymers using waste fired brick and different metakaolins as raw materials[J]. Applied Clay Science, 2020, 198: 105813. |
| 57 | KAZE C R, LECOMTE-NANA G L, KAMSEU E, et al. Mechanical and physical properties of inorganic polymer cement made of iron-rich laterite and lateritic clay: a comparative study[J]. Cement and Concrete Research, 2021, 140: 106320. |
| 58 | ZHANG Baifa, GUO Haozhe, YUAN Peng, et al. Novel acid-based geopolymer synthesized from nanosized tubular halloysite: the role of precalcination temperature and phosphoric acid concentration[J]. Cement and Concrete Composites, 2020, 110: 103601. |
| 59 | LASSINANTTI GUALTIERI M, ROMAGNOLI M, POLLASTRI S, et al. Inorganic polymers from laterite using activation with phosphoric acid and alkaline sodium silicate solution: mechanical and microstructural properties[J]. Cement and Concrete Research, 2015, 67: 259-270. |
| 60 | GAO Li, ZHENG Youxiong, TANG Yan, et al. Effect of phosphoric acid content on the microstructure and compressive strength of phosphoric acid-based metakaolin geopolymers[J]. Heliyon, 2020, 6(4): e03853. |
| 61 | WANG Yanshuai, DAI Jianguo, DING Zhu, et al. Phosphate-based geopolymer: formation mechanism and thermal stability[J]. Materials Letters, 2017, 190: 209-212. |
| 62 | DOUIRI H, LOUATI S, BAKLOUTI S, et al. Enhanced dielectric performance of metakaolin-H3PO4 geopolymers[J]. Materials Letters, 2016, 164: 299-302. |
| 63 | 李浩天. 掺杂石墨基地质聚合物的制备及其导电性能研究[D]. 南宁: 广西大学, 2019. |
| LI Haotian. Study on preparation and conductive properties of doped graphite-based geopolymers[D]. Nanning: Guangxi University, 2019. | |
| 64 | MAJDOUBI H, HADDAJI Y, MANSOURI S, et al. Thermal, mechanical and microstructural properties of acidic geopolymer based on Moroccan kaolinitic clay[J]. Journal of Building Engineering, 2021, 35: 102078. |
| 65 | WANG Yanshuai, ALREFAEI Yazan, DAI Jianguo. Improvement of early-age properties of silico-aluminophosphate geopolymer using dead burnt magnesia[J]. Construction and Building Materials, 2019, 217: 1-11. |
| 66 | 姚正珍. 磷酸基地聚合物的制备及耐高温耐腐蚀性能研究[D]. 绵阳: 西南科技大学, 2020. |
| YAO Zhengzhen. Synthesis, high-temperature and corrosion ristance of phosphoric acid based geopolymer[D]. Mianyang: Southwest University of Science and Technology, 2020. | |
| 67 | 钟明峰, 张志杰, 周杰, 等. 纳米氧化铝增强铝硅磷质矿物键合材料的合成和性能[J]. 贵州大学学报(自然科学版), 2009, 26(3): 103-105. |
| ZHONG Mingfeng, ZHANG Zhijie, ZHOU Jie, et al. Synthesis and mechanical properties of nano-alumina reinforced acid-activated metakaolinite-based geopolymer[J]. Journal of Guizhou University(Natural Science Edition),2009, 26(3): 103-105. | |
| 68 | YANG Tao, HAN Enlin, WANG Xiaodong, et al. Surface decoration of polyimide fiber with carbon nanotubes and its application for mechanical enhancement of phosphoric acid-based geopolymers[J]. Applied Surface Science, 2017, 416: 200-212. |
| [1] | WANG Jiaqing, SONG Guangwei, LI Qiang, GUO Shuaicheng, DAI Qingli. Rubber-concrete interface modification method and performance enhancement path [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 328-343. |
| [2] | LIU Muzi, SHI Keke, ZHAO Qiang, LI Jinping, LIU Guang. Research progress of solid hydrogen storage materials [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4746-4769. |
| [3] | XIANG Shuo, LU Peng, SHI Weinian, YANG Xin, HE Yan, ZHU Liye, KONG Xiangwei. Controllable and large-scale preparation of two-dimensional WS2 nanosheet and its tribological properties as lubricant additives in lithium grease [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4783-4790. |
| [4] | ZHANG Dailing, DING Yumei, ZUO Xiahua, LI Haowei, YANG Weimin, YAN Hua, AN Ying. Photothermal characteristics of waste toner nanofluids [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4791-4798. |
| [5] | LI Bogeng, LUO Yingwu, LIU Pingwei. Consideration on research content and method of polymer product engineering [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3905-3909. |
| [6] | TANG Lei, ZENG Desen, LING Ziye, ZHANG Zhengguo, FANG Xiaoming. Research progress of phase change materials and their application systems for cool storage [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4322-4339. |
| [7] | XU Guobin, LIU Honghao, LI Jie, GUO Jiaqi, WANG Qi. Preparation and properties of ZnO QDs water-based inkjet fluorescent ink [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3114-3122. |
| [8] | LYU Xuedong, LUO Faliang, LIN Haitao, SONG Danqing, LIU Yi, NIU Ruixue, ZHENG Liuchun. Recent progress of synthesis technology and gas barrier research of poly(butylene succinate) [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2546-2554. |
| [9] | XU Yuzhen, JIANG Dahua, LIU Jingtao, CHEN Pu. Preparation and properties of fly ash based phase change energy storage materials [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2595-2605. |
| [10] | YU Jie, ZHANG Wenlong. Development status and progress of lithium ion battery separator [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 1760-1768. |
| [11] | SHANG Xiaobiao, LI Guangchao, XIAO Liping, BAI Yongzhen, XIAO Renyou, LI Jiajian, ZHANG Zhihao. Wave transmission performance of zirconium aluminum silicate fiberboard under large temperature gradient [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1551-1561. |
| [12] | LI Guangwen, HUA Qucheng, HUANG Zuoxin, DA Zhijian. Progress on polymethacrylate as viscosity index improvers for lube oil [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1562-1571. |
| [13] | ZHANG Jianzhong, XU Sheng, FAN Jiashu, FEI Zhenyu, WANG Kun, HUANG Jian, CUI Fengbo, RAN Wenhua. Progress in characterization and analysis of glass fiber sizing [J]. Chemical Industry and Engineering Progress, 2023, 42(2): 821-838. |
| [14] | ZHU Qichen, WU Zhangyong, WANG Zhiqiang, JIANG Jiajun, LI Xiang. Sedimentation stability and viscosity properties of silicone oil-based magnetic nanofluid at low temperature [J]. Chemical Industry and Engineering Progress, 2023, 42(10): 5101-5110. |
| [15] | TIAN Yazhou, HU Yujing, LI Jiyou, REN Jiangyan, WANG Liwei, WANG Xiuli, DING Ying, CHENG Jue, ZHANG Junying. Synthesis, curing kinetics and properties of vanilla alcohol-based epoxy resin [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 477-484. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||