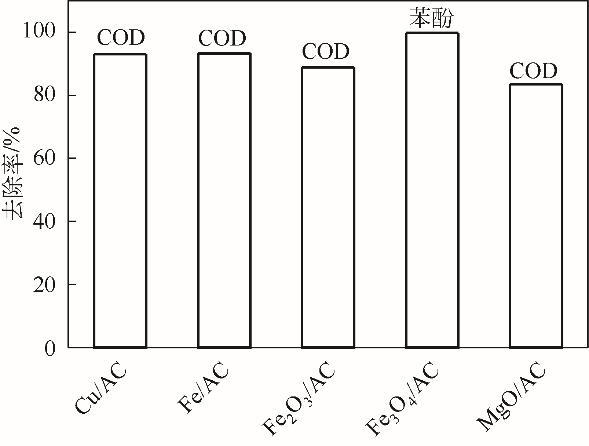
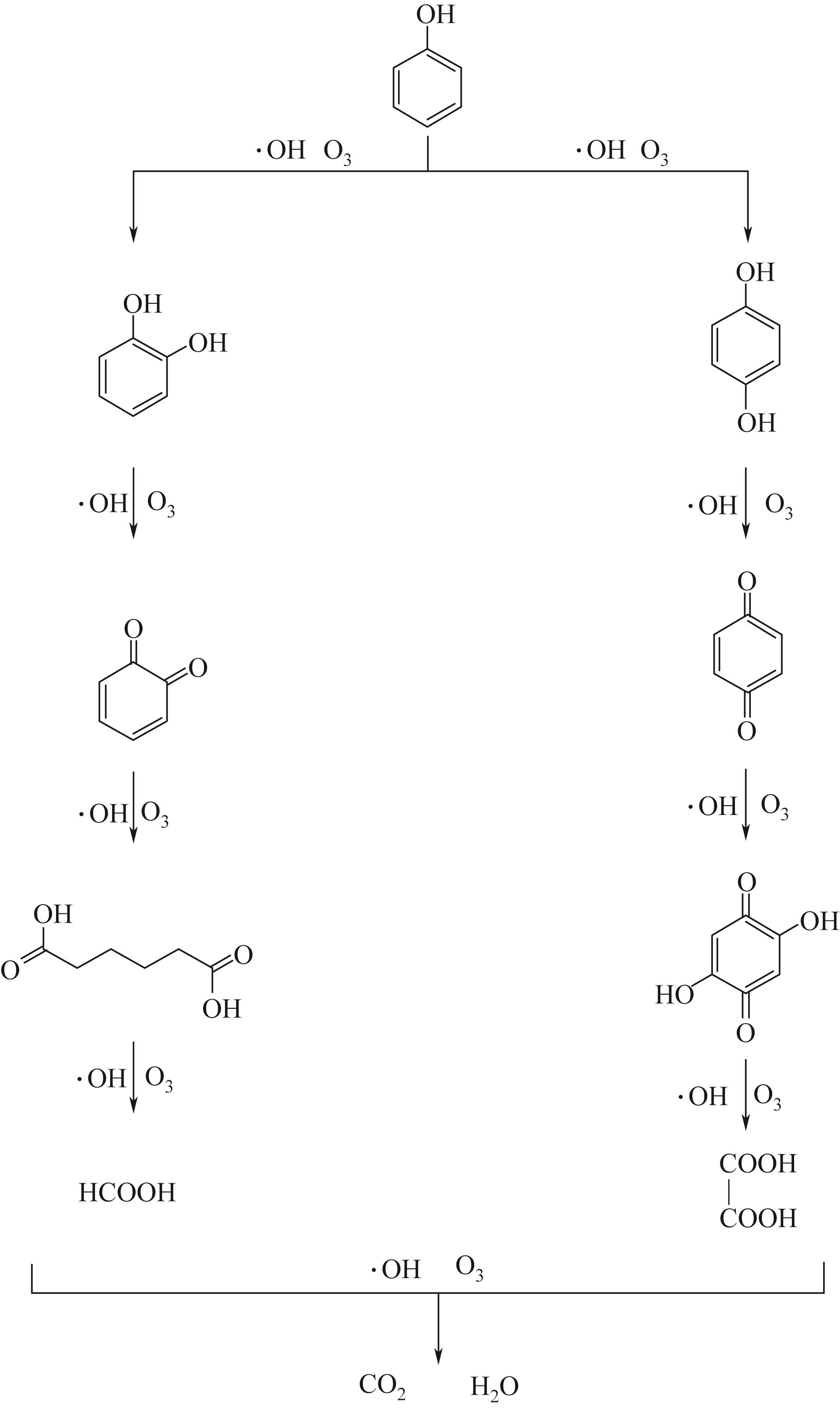
Chemical Industry and Engineering Progress ›› 2022, Vol. 41 ›› Issue (S1): 545-555.DOI: 10.16085/j.issn.1000-6613.2022-0725
• Resources and environmental engineering • Previous Articles Next Articles
Research advance and mechanism analysis of catalytic ozonation of phenolic compounds
ZHU Hao1,2( ), LIU Hanfei1, JI Yufan1, LI Shuangtao1, HUANG Yiping1, GAO Yuan1, WEI Zhenhao1, ZHU Kai1, HAN Weiqing2, WEI Kajia2
), LIU Hanfei1, JI Yufan1, LI Shuangtao1, HUANG Yiping1, GAO Yuan1, WEI Zhenhao1, ZHU Kai1, HAN Weiqing2, WEI Kajia2
- 1.China Construction Industrial & Energy Engineering Group Co. , Ltd. , Nanjing 210000, Jiangsu, China
2.School of Environmental and Biological Engineering, Nanjing University of Science and Technology, Nanjing 210094, Jiangsu, China
-
Received:2022-04-22Revised:2022-06-23Online:2022-11-10Published:2022-10-20 -
Contact:ZHU Hao
催化臭氧化处理酚类化合物的研究进展与机理解析
朱昊1,2( ), 刘汉飞1, 季雨凡1, 李双涛1, 黄益平1, 高源1, 魏振浩1, 主凯1, 韩卫清2, 魏卡佳2
), 刘汉飞1, 季雨凡1, 李双涛1, 黄益平1, 高源1, 魏振浩1, 主凯1, 韩卫清2, 魏卡佳2
- 1.中建安装集团有限公司,江苏 南京 210046
2.南京理工大学环境与生物工程学院,江苏 南京 210094
-
通讯作者:朱昊 -
作者简介:朱昊(1992—),男,博士,研究方向化工废水处理及资源化利用。E-mail: zhu-hao@cscec.com。 -
基金资助:中建安装2020年度科技研发计划(AZ-2020-05);中国建筑股份有限公司2022年度科技研发课题(CSCEC-2022-Z-60);南京市建设系统科技计划(Ks2116)
CLC Number:
Cite this article
ZHU Hao, LIU Hanfei, JI Yufan, LI Shuangtao, HUANG Yiping, GAO Yuan, WEI Zhenhao, ZHU Kai, HAN Weiqing, WEI Kajia. Research advance and mechanism analysis of catalytic ozonation of phenolic compounds[J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 545-555.
朱昊, 刘汉飞, 季雨凡, 李双涛, 黄益平, 高源, 魏振浩, 主凯, 韩卫清, 魏卡佳. 催化臭氧化处理酚类化合物的研究进展与机理解析[J]. 化工进展, 2022, 41(S1): 545-555.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-0725
催化剂 类型 | 技术指标 | 去除率/% | 规律 | 参考 文献 |
|---|---|---|---|---|
| Al2O3 | 双酚A对应的TOC | 90.0 | 同颗粒态相比,粉末态Al2O3使系统的TOC去除率由44.0%提高至90.0% | [ |
| β-MnO2 | 苯酚 | 91.6 | 4种MnO2按苯酚去除率从大到小排列顺序:β-MnO2>δ-MnO2>γ-MnO2>α-MnO2 | [ |
| α-MnO2 | 对硝基苯酚 | 98.4 | 优化制备条件:溴化十六烷基三甲铵的摩尔浓度0.02mol/L,焙烧温度500℃,焙烧时间3h | [ |
| FeOOH | 苯酚 | 66.5 | 当反应时间为30min时,催化臭氧化系统苯酚质量浓度降低值为66.5 mg/L,高于臭氧氧化系统的49.5mg/L | [ |
| α-Fe2O3 | 苯酚对应的COD | 97 | 当反应时间为30min时,催化臭氧化系统苯酚COD去除率达到97%以上 | [ |
| 零价铁 | COD | 79 | 催化剂连续使用5次后,废水COD去除率仍达70%以上 | [ |
| ZnO | 苯酚 | — | 当反应时间为60min时,催化臭氧化系统苯酚质量浓度剩余值为10.5 mg/L,低于臭氧氧化系统的27.7mg/L | [ |
| MgO | 苯酚 | — | MgO催化臭氧化处理苯酚的表观反应速率常数与MgO投量、臭氧投量、溶液pH值和温度呈正相关,与苯酚初始质量浓度呈负相关 | [ |
| MgO | 对氯苯酚 | 89.8 | 当反应时间为15min时,MgO催化臭氧化系统中对氯苯酚去除率最高为89.8% | [ |
| MgO | 苯酚 | — | NaCl含量对MgO催化臭氧化处理苯酚没有负面影响 | [ |
催化剂 类型 | 技术指标 | 去除率/% | 规律 | 参考 文献 |
|---|---|---|---|---|
| Al2O3 | 双酚A对应的TOC | 90.0 | 同颗粒态相比,粉末态Al2O3使系统的TOC去除率由44.0%提高至90.0% | [ |
| β-MnO2 | 苯酚 | 91.6 | 4种MnO2按苯酚去除率从大到小排列顺序:β-MnO2>δ-MnO2>γ-MnO2>α-MnO2 | [ |
| α-MnO2 | 对硝基苯酚 | 98.4 | 优化制备条件:溴化十六烷基三甲铵的摩尔浓度0.02mol/L,焙烧温度500℃,焙烧时间3h | [ |
| FeOOH | 苯酚 | 66.5 | 当反应时间为30min时,催化臭氧化系统苯酚质量浓度降低值为66.5 mg/L,高于臭氧氧化系统的49.5mg/L | [ |
| α-Fe2O3 | 苯酚对应的COD | 97 | 当反应时间为30min时,催化臭氧化系统苯酚COD去除率达到97%以上 | [ |
| 零价铁 | COD | 79 | 催化剂连续使用5次后,废水COD去除率仍达70%以上 | [ |
| ZnO | 苯酚 | — | 当反应时间为60min时,催化臭氧化系统苯酚质量浓度剩余值为10.5 mg/L,低于臭氧氧化系统的27.7mg/L | [ |
| MgO | 苯酚 | — | MgO催化臭氧化处理苯酚的表观反应速率常数与MgO投量、臭氧投量、溶液pH值和温度呈正相关,与苯酚初始质量浓度呈负相关 | [ |
| MgO | 对氯苯酚 | 89.8 | 当反应时间为15min时,MgO催化臭氧化系统中对氯苯酚去除率最高为89.8% | [ |
| MgO | 苯酚 | — | NaCl含量对MgO催化臭氧化处理苯酚没有负面影响 | [ |
催化剂 类型 | 技术指标 | 去除率/% | 规律 | 参考 文献 |
|---|---|---|---|---|
| Fe2O3/SBA-15 | 苯酚对应的COD | 65.0 | 催化剂连续使用500h,含酚废水COD去除率保持65%以上,催化剂活性不低于83% | [ |
| MnO2/SBA-15 | 苯酚对应的TOC | 35.0 | 高比表面积的有序纳米棒有助于催化剂表面的传质和活性位暴露,从而提高催化效率 | [ |
| CeO x /SBA-16 | 双酚A对应的TOC | 60.9 | 介孔催化剂比表面积大,孔分布均匀,有利于催化剂的表面传质 | [ |
| Ce/ZSM-5 | 苯酚对应的COD | 85.7 | Ce/ZSM-5催化臭氧化处理苯酚活性较高,稳定性较强 | [ |
| Co-Fe/ZSM-5 | 苯酚 | 92.9 | 碱改性的ZSM-5作为Co和Fe的载体,催化臭氧化处理苯酚效果显著 | [ |
| Cu/沸石 | COD | 72.4 | Cu/沸石催化臭氧化处理煤化工废水效果良好,对应的COD去除率为72.4% | [ |
| MnO x /泡沫陶瓷 | 苯酚 | 89.6 | 当Mn(NO3)2的摩尔浓度为0.5 mol/L时,制备的催化剂稳定性最佳 | [ |
| CoAl2O4/蜂窝陶瓷 | 对苯二酚 | 81.2 | 采用涂覆法制备的催化剂呈尖晶石结构,比表面积、孔径和孔容较大 | [ |
| Mn-Co/蜂窝陶瓷 | 对苯二酚 | 78.0 | 采用涂覆盖制备的催化剂主要活性组分为Mn3O4和CoO | [ |
催化剂 类型 | 技术指标 | 去除率/% | 规律 | 参考 文献 |
|---|---|---|---|---|
| Fe2O3/SBA-15 | 苯酚对应的COD | 65.0 | 催化剂连续使用500h,含酚废水COD去除率保持65%以上,催化剂活性不低于83% | [ |
| MnO2/SBA-15 | 苯酚对应的TOC | 35.0 | 高比表面积的有序纳米棒有助于催化剂表面的传质和活性位暴露,从而提高催化效率 | [ |
| CeO x /SBA-16 | 双酚A对应的TOC | 60.9 | 介孔催化剂比表面积大,孔分布均匀,有利于催化剂的表面传质 | [ |
| Ce/ZSM-5 | 苯酚对应的COD | 85.7 | Ce/ZSM-5催化臭氧化处理苯酚活性较高,稳定性较强 | [ |
| Co-Fe/ZSM-5 | 苯酚 | 92.9 | 碱改性的ZSM-5作为Co和Fe的载体,催化臭氧化处理苯酚效果显著 | [ |
| Cu/沸石 | COD | 72.4 | Cu/沸石催化臭氧化处理煤化工废水效果良好,对应的COD去除率为72.4% | [ |
| MnO x /泡沫陶瓷 | 苯酚 | 89.6 | 当Mn(NO3)2的摩尔浓度为0.5 mol/L时,制备的催化剂稳定性最佳 | [ |
| CoAl2O4/蜂窝陶瓷 | 对苯二酚 | 81.2 | 采用涂覆法制备的催化剂呈尖晶石结构,比表面积、孔径和孔容较大 | [ |
| Mn-Co/蜂窝陶瓷 | 对苯二酚 | 78.0 | 采用涂覆盖制备的催化剂主要活性组分为Mn3O4和CoO | [ |
| 催化剂类型 | 技术指标 | 去除率/% | 规律发现 | 参考文献 |
|---|---|---|---|---|
| Mn/Al2O3 | 苯酚 | 82.7 | 当Al2O3表面负载4%的Mn时,苯酚的催化臭氧化效率最高 | [ |
| Mn/Al2O3 | 苯酚 | 76.3 | Mn/Al2O3优化制备条件为:Mn负载量10%,焙烧温度500℃,焙烧时间5h | [ |
| Ce/Al2O3 | 苯酚 | 98.3 | Ce/Al2O3优化制备条件为:Ce负载量1%,焙烧温度500℃,焙烧时间1h | [ |
| Cu/Al2O3 | COD | 50.0 | Cu/Al2O3晶型良好,比表面积大,中孔结构丰富 | [ |
| Ni/Al2O3 | 对苯二酚 | 78.0 | Ni/Al2O3优化制备条件为:50%硝酸盐浸渍载体8h,焙烧温度600℃,焙烧时间3h | [ |
| Mn-Fe-Ce/Al2O3 | 苯酚 | 88.0 | 催化剂循环使用5次后仍具有较高的催化活性 | [ |
| 催化剂类型 | 技术指标 | 去除率/% | 规律发现 | 参考文献 |
|---|---|---|---|---|
| Mn/Al2O3 | 苯酚 | 82.7 | 当Al2O3表面负载4%的Mn时,苯酚的催化臭氧化效率最高 | [ |
| Mn/Al2O3 | 苯酚 | 76.3 | Mn/Al2O3优化制备条件为:Mn负载量10%,焙烧温度500℃,焙烧时间5h | [ |
| Ce/Al2O3 | 苯酚 | 98.3 | Ce/Al2O3优化制备条件为:Ce负载量1%,焙烧温度500℃,焙烧时间1h | [ |
| Cu/Al2O3 | COD | 50.0 | Cu/Al2O3晶型良好,比表面积大,中孔结构丰富 | [ |
| Ni/Al2O3 | 对苯二酚 | 78.0 | Ni/Al2O3优化制备条件为:50%硝酸盐浸渍载体8h,焙烧温度600℃,焙烧时间3h | [ |
| Mn-Fe-Ce/Al2O3 | 苯酚 | 88.0 | 催化剂循环使用5次后仍具有较高的催化活性 | [ |
| 1 | 石岩, 邓淑仪, 许丹宇, 等. 臭氧催化氧化法处理含酚废水的研究进展[J]. 环境工程, 2015, 33(3): 17-20. |
| SHI Yan, DENG Shuyi, XU Danyu, et al. Research progress of phenolic wastewater treatment by catalytic ozonation[J]. Environmental Engineering, 2015, 33(3): 17-20. | |
| 2 | CHENG Ning, WANG Bing, WU Pan, et al. Adsorption of emerging contaminants from water and wastewater by modified biochar: A review[J]. Environmental Pollution, 2021, 273: 116448. |
| 3 | AKINPELU A A, ALI M E, JOHAN M R, et al. Polycyclic aromatic hydrocarbons extraction and removal from wastewater by carbon nanotubes: A review of the current technologies, challenges and prospects[J]. Process Safety and Environmental Protection, 2019, 122: 68-82. |
| 4 | LI Yajie, WANG Mengyan, QIAN Jingli, et al. Enhanced degradation of phenolic compounds in coal gasification wastewater by an iron-carbon micro-electric field coupled with anaerobic co-digestion[J]. Science of the Total Environment, 2022, 819: 151991. |
| 5 | WANG Jianlong, CHEN Hai. Catalytic ozonation for water and wastewater treatment: Recent advances and perspective[J]. Science of the Total Environment, 2020, 704: 135249. |
| 6 | ZENG Zequan, ZOU Haikui, LI Xin, et al. Ozonation of acidic phenol wastewater with O3/Fe(II) in a rotating packed bed reactor: Optimization by response surface methodology[J]. Chemical Engineering and Processing: Process Intensification, 2012, 60: 1-8. |
| 7 | 金鑫, 许建军, 解明媛, 等. 五氯酚的臭氧催化氧化特性[J]. 环境化学, 2013, 32(4): 572-576. |
| JIN Xin, XU Jianjun, XIE Mingyuan, et al. The characteristics of pentachlorophenol decomposition by catalytic ozonation[J]. Environmental Chemistry, 2013, 32(4): 572-576. | |
| 8 | 陈磊, 杨长河. 非均相催化臭氧化催化剂载体研究进展[J]. 精细石油化工, 2021, 38(3): 69-74. |
| CHEN Lei, YANG Changhe. Research progress of heterogeneous catalytic ozonation carrier[J]. Speciality Petrochemicals, 2021, 38(3): 69-74. | |
| 9 | DONG Yuming, YANG Hongxiao, HE Kun, et al. Catalytic activity and stability of Y zeolite for phenol degradation in the presence of ozone[J]. Applied Catalysis B: Environmental, 2008, 82(3/4): 163-168. |
| 10 | 董玉明, 王光丽, 蒋平平, 等. 陶瓷粉体催化臭氧化降解水中苯酚[J]. 水处理技术, 2010, 36(10): 28-31. |
| DONG Yuming, WANG Guangli, JIANG Pingping, et al. Catalytic ozonation degradation of phenol in water by ceramic powder[J]. Technology of Water Treatment, 2010, 36(10): 28-31. | |
| 11 | ZHANG Fengzhen, WU Kaiyi, ZHOU Hongtao, et al. Ozonation of aqueous phenol catalyzed by biochar produced from sludge obtained in the treatment of coking wastewater[J]. Journal of Environmental Management, 2018, 224: 376-386. |
| 12 | KEYKAVOOS R, MANKIDY R, MA H, et al. Mineralization of bisphenol A by catalytic ozonation over alumina[J]. Separation and Purification Technology, 2013, 107: 310-317. |
| 13 | 占小翠. 低维MnO2基催化臭氧化降解含酚废水的效能与机制研究[D]. 徐州:中国矿业大学, 2019. |
| ZHAN Xiaocui. Efficiency and mechanism of low-dimensional MnO2 catalyzed ozonation for degradation of phenolic wastewater[D]. Xuzhou: China University of Mining and Technology, 2019. | |
| 14 | NAWAZ F, XIE Yongbing, CAO Hongbin, et al. Catalytic ozonation of 4-nitrophenol over an mesoporous α-MnO2 with resistance to leaching[J]. Catalysis Today, 2015, 258: 595-601. |
| 15 | 董玉明, 蒋平平, 张爱民. 介孔结构的α-FeOOH对苯酚的催化臭氧化降解[J]. 无机化学学报,2009, 25(9): 1595-1600. |
| DONG Yuming, JIANG Pingping, ZHANG Aimin. Catalytic ozonation degradation of phenol in water by mesoporous α-FeOOH[J]. Chinese Journal of Inorganic Chemistry, 2009, 25(9): 1595-1600. | |
| 16 | 王勇, 张耀宗, 毕莹莹, 等. α-Fe2O3催化臭氧氧化耦合陶瓷膜处理含酚废水[J]. 环境工程技术学报, 2022, . |
| WANG Yong, ZHANG Yaozong, BI Yingying, et al. α-Fe2O3 catalytic ozonation coupled with ceramic membrane for phenol wastewater treatment[J]. Journal of Environmental Engineering Technology, 2022, . | |
| 17 | 殷浩翔, 车坤, 张文超, 等. Fe0/O3体系预处理三硝基间苯二酚铅生产废水[J]. 工业水处理, 2022, 42(4): 119-124. |
| YIN Haoxiang, CHE Kun, ZHANG Wenchao, et al. Pretreatment of lead-2, 4, 6-trinitroresorcinate styphnate wastewater by Fe0/O3 system[J]. Industrial Water Treatment, 2022, 42(4): 119-124. | |
| 18 | DONG Yuming, WANG Guangli, JIANG Pingping, et al. Simple preparation and catalytic properties of ZnO for ozonation degradation of phenol in water[J]. Chinese Chemical Letters, 2011, 22(2): 209-212. |
| 19 | 王兵, 刘璞真, 任宏洋, 等. 非均相催化臭氧化降解水中苯酚动力学[J]. 环境工程学报, 2016, 10(7): 3427-3433. |
| WANG Bing, LIU Puzhen, REN Hongyang, et al. Degradation kinetics of catalytic ozone oxidation of phenol in water[J]. Chinese Journal of Environmental Engineering, 2016, 10(7): 3427-3433. | |
| 20 | CHEN Jun, TIAN Shuanghong, LU Jiang, et al. Catalytic performance of MgO with different exposed crystal facets towards the ozonation of 4-chlorophenol[J]. Applied Catalysis A: General, 2015, 506: 118-125. |
| 21 | MOUSSAVI G, KHAVANIN A, ALIZADEH R. The integration of ozonation catalyzed with MgO nanocrystals and the biodegradation for the removal of phenol from saline wastewater[J]. Applied Catalysis B: Environmental, 2010, 97(1/2): 160-167. |
| 22 | 刘俊逸, 李倩, 李杰, 等. 介孔Fe2O3/SBA-15催化臭氧氧化含酚废水[J]. 化工进展, 2019, 38(11): 5158-5164. |
| LIU Junyi, LI Qian, LI Jie, et al. Catalytic oxidation of phenolic wastewater by O3 over mesoporous Fe2O3/SBA-15[J]. Chemical Industry and Engineering Progress, 2019, 38(11): 5158-5164. | |
| 23 | ZHANG Jianlin, ZHUANG Tao, LIU Shanjun, et al. Catalytic ozonation of phenol enhanced by mesoporous MnO2 prepared through nanocasting method with SBA-15 as template[J]. Journal of Environmental Chemical Engineering, 2020, 8(4): 103967. |
| 24 | MU Jiaxin, LI Shangyi, WANG Jing, et al. Efficient catalytic ozonation of bisphenol A by three-dimensional mesoporous CeO x -loaded SBA-16[J]. Chemosphere, 2021, 278: 130412. |
| 25 | 崔福旭, 张晶, 张波, 等. 金属负载ZSM⁃5分子筛催化臭氧化苯酚废水[J]. 辽宁石油化工大学学报, 2019, 39(2): 10-14. |
| CUI Fuxu, ZHANG Jing, ZHANG Bo, et al. Metal loaded ZSM⁃5 molecular sieve catalyzed ozonation of phenol wastewater[J]. Journal of Liaoning Shihua University, 2019, 39(2): 10-14. | |
| 26 | 徐增益, 余金鹏, 郝敏, 等. Co-Fe/ZSM-5催化臭氧化降解废水中苯酚的性能研究[J]. 肥料与健康, 2020,47(6): 39-45. |
| XU Zengyi, YU Jinpeng, HAO Min, et al. Study on the performance of Co-Fe/ZSM-5 catalytic ozonation to degrade phenol in wastewater[J]. Fertilizer & Health, 2020,47(6): 39-45. | |
| 27 | 李帅, 孙文全, 孙永军, 等. Cu/人造沸石催化剂催化臭氧氧化煤化工废水的性能研究[J]. 南京工业大学学报(自然科学版), 2022,44(2): 221-228. |
| LI Shuai, SUN Wenquan, SUN Yongjun, et al. Performance of Cu/artificial zeolite catalysts for catalytic ozone oxidation [J]. Journal of Nanjing Tech University (Natural Science Edition), 2022, 44(2): 221-228. | |
| 28 | 廖润华, 李月明, 成岳, 等. 泡沫陶瓷负载锰氧化物的制备及催化臭氧化苯酚研究[J]. 工业水处理, 2014, 34(12): 25-27. |
| LIAO Runhua, LI Yueming, CHENG Yue, et al. Preparation of manganese oxide coated foam ceramic and research on catalytic ozonation phenol[J]. Industrial Water Treatment, 2014, 34(12): 25-27. | |
| 29 | 张兰河, 高伟围, 陈子成, 等. CoAl2O4/蜂窝陶瓷催化剂的制备及其催化臭氧化性能[J]. 无机化学学报, 2017, 33(6): 985-992. |
| ZHANG Lanhe, GAO Weiwei, CHEN Zicheng, et al. CoAl2O4/ceramic honeycomb catalyst: Preparation and performance on catalytic ozonation in wastewater treatment[J]. Chinese Journal of Inorganic Chemistry, 2017, 33(6): 985-992. | |
| 30 | 张兰河, 高伟围, 陈子成, 等. Mn-Co/蜂窝陶瓷催化剂制备及催化臭氧化对苯二酚效能[J]. 环境科学, 2018, 39(7): 3194-3202. |
| ZHANG Lanhe, GAO Weiwei, CHEN Zicheng, et al. Preparation of Mn-Co/ceramic honeycomb catalyst and its performance on catalytic ozonation of hydroquinone[J]. Environmental Science, 2018, 39(7): 3194-3202. | |
| 31 | 王利平, 刘兵昌, 蔡华, 等. 多相催化臭氧氧化法处理甲萘酚废水[J]. 中国给水排水, 2009, 25(23): 111-113. |
| WANG Liping, LIU Bingchang, CAI Hua, et al. Heterogeneous catalytic ozonation process for treatment of wastewater from 1-naphthol production[J]. China Water & Waste Water, 2009, 25(23): 111-113. | |
| 32 | 洪浩峰, 潘湛昌, 许磊, 等. 臭氧催化氧化处理苯酚废水研究[J]. 环境科学与技术, 2010, 33(S1): 301-304. |
| HONG Haofeng, PAN Zhanchang, XU Lei, et al. Degradation of aqueous phenol by catalytic ozonation[J]. Environmental Science & Technology, 2010, 33(S1): 301-304. | |
| 33 | 雷利荣, 党中煦, 李友明. 活性炭负载Fe2O3催化臭氧降解丁香酚[J]. 华南理工大学学报(自然科学版), 2018, 46(10): 132-140. |
| LEI Lirong, DANG Zhongxu, LI Youming. Catalytic ozonation of eugenol in the presence of Fe2O3 supported by activated carbon[J]. Journal of South China University of Technology(Natural Science Edition), 2018, 46(10): 132-140. | |
| 34 | 鲁涛, 李华昌, 陈滢, 等. 磁性活性炭催化臭氧氧化苯酚废水[J]. 四川环境, 2018, 37(5): 40-44. |
| LU Tao, LI Huachang, CHEN Ying, et al. Treatment of phenol containing wastewater by ozone catalyzed by magnetic activated carbon[J]. Sichuan Environment, 2018, 37(5): 40-44. | |
| 35 | ZHOU Lilong, ZHANG Shanshan, LI Zhengjie, et al. Efficient degradation of phenol in aqueous solution by catalytic ozonation over MgO/AC[J]. Journal of Water Process Engineering, 2020, 36: 101168. |
| 36 | BAO Q, HUI K S, DUH J G. Promoting catalytic ozonation of phenol over graphene through nitrogenation and Co3O4 compositing[J]. Journal of Environmental Sciences, 2016, 50: 38-48. |
| 37 | 冯林强, 罗汉金, 方伟. 纳米二氧化锰/还原态氧化石墨烯复合材料催化臭氧降解苯酚的研究[J]. 环境工程, 2016, 34(7): 56-60. |
| FENG Linqiang, LUO Hanjin, FANG Wei. Study on catalytic ozonation of phenol over nanosized MnO2 supported on reduced graphene oxide[J]. Environmental Engineering, 2016, 34(7): 56-60. | |
| 38 | LI Gang, LU Yongtao, LU Cheng, et al. Efficient catalytic ozonation of bisphenol—A over reduced graphene oxide modified sea urchin-like α-MnO2 architectures[J]. Journal of Hazardous Materials, 2015, 294: 201-208. |
| 39 | WANG Ye, YANG Wenzhong, YIN Xiaoshuang, et al. The role of Mn-doping for catalytic ozonation of phenol using Mn/γ-Al2O3 nanocatalyst: Performance and mechanism[J]. Journal of Environmental Chemical Engineering, 2016, 4: 3415-3425. |
| 40 | 郭二亮. MnO2/γ-Al2O3催化剂的制备及催化臭氧氧化苯酚废水[D]. 天津: 天津科技大学, 2017. |
| GUO Erliang. Preparation of MnO2/γ-Al2O3 catalyst and catalytic ozonation degradation of phenol effluent[D]. Tianjin: Tianjin University of Science & Technology, 2017. | |
| 41 | 李钰. 活性氧化铝及载铈活性氧化铝催化臭氧氧化处理废水[D]. 杭州: 浙江大学, 2016. |
| LI Yu. Catalytic ozonation of wastewater by activated alumina or cerium supported activated alumina[D]. Hangzhou: Zhejiang University, 2016. | |
| 42 | 冯思慧. Al2O3负载CuO催化臭氧氧化含酚污水[D]. 哈尔滨: 哈尔滨工程大学, 2016. |
| FENG Sihui. Catalytic ozonation of phenolic wastewater by CuO loaded on Al2O3 [D]. Harbin: Harbin Engineering Technology, 2016. | |
| 43 | 李欣欣. Al2O3负载金属氧化物催化臭氧化水中苯酚[D]. 大连: 大连理工大学, 2014. |
| LI Xinxin. The ozone decomposition of phenol over Al2O3 supported transition metal oxide catalysts[D]. Dalian: Dalian University of Technology, 2014. | |
| 44 | 张曼宁. 过渡金属基催化剂的制备及催化臭氧氧化苯酚研究[D]. 长沙: 湖南师范大学, 2021. |
| ZHANG Manning. Preparation of transition metal-based catalyst and study on catalytic ozonation of phenol[D]. Changsha: Hunan Normal University, 2021. | |
| 45 | 杨文玲, 王坦. 臭氧催化剂催化机理及其应用研究进展[J]. 应用化工, 2020, 49(11): 2936-2940. |
| YANG Wenling, WANG Tan. Research progress in catalytic mechanism and application of ozone catalyst[J]. Applied Chemical Industry, 2020, 49(11): 2936-2940. | |
| 46 | NAWROCKI J, KASPRZYK-HORDERN B. The efficiency and mechanisms of catalytic ozonation[J]. Applied Catalysis B: Environmental, 2010, 99(1/2): 27-42. |
| 47 | 王勇, 杜明辉, 张宁, 等. α-Fe2O3催化臭氧氧化处理苯酚废水效果及机理[J]. 环境科学研究, 2022, 35(8): 1818-1826. |
| WANG Yong, DU Minghui, ZHANG Ning, et al. Effect and mechanism study on the degradation of phenol wastewater by α-Fe2O3 catalytic ozone oxidation treatment[J]. Research of Environmental Sciences, 2022, 35(8): 1818-1826. | |
| 48 | 陈炜. 磁性氧化镱催化臭氧化降解苯三唑和愈创木酚的研究[D]. 杭州: 浙江工业大学, 2015. |
| CHEN Wei. The study on catalytic ozonation for the degradation of 1H-benzotrizaole and guaiacol by magnetic ytterbium oxide[D]. Hangzhou: Zhejiang University of Technology, 2015. |
| [1] | ZHANG Mingyan, LIU Yan, ZHANG Xueting, LIU Yake, LI Congju, ZHANG Xiuling. Research progress of non-noble metal bifunctional catalysts in zinc-air batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 276-286. |
| [2] | SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298. |
| [3] | XIE Luyao, CHEN Songzhe, WANG Laijun, ZHANG Ping. Platinum-based catalysts for SO2 depolarized electrolysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 299-309. |
| [4] | YANG Xiazhen, PENG Yifan, LIU Huazhang, HUO Chao. Regulation of active phase of fused iron catalyst and its catalytic performance of Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 310-318. |
| [5] | GAO Yufei, LU Jinfeng. Mechanism of heterogeneous catalytic ozone oxidation:A review [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 430-438. |
| [6] | WANG Ying, HAN Yunping, LI Lin, LI Yanbo, LI Huili, YAN Changren, LI Caixia. Research status and future prospects of the emission characteristics of virus aerosols in urban wastewater treatment plants [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 439-446. |
| [7] | WANG Lele, YANG Wanrong, YAO Yan, LIU Tao, HE Chuan, LIU Xiao, SU Sheng, KONG Fanhai, ZHU Canghai, XIANG Jun. Influence of spent SCR catalyst blending on the characteristics and deNO x performance for new SCR catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 489-497. |
| [8] | GU Yongzheng, ZHANG Yongsheng. Dynamic behavior and kinetic model of Hg0 adsorption by HBr-modified fly ash [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 498-509. |
| [9] | ZHAO Jingchao, TAN Ming. Effect of surfactants on the reduction of industrial saline wastewater by electrodialysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 529-535. |
| [10] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [11] | WANG Fu'an. Consumption and emission reduction of the reactor of 300kt/a propylene oxide process [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 213-218. |
| [12] | CHENG Tao, CUI Ruili, SONG Junnan, ZHANG Tianqi, ZHANG Yunhe, LIANG Shijie, PU Shi. Analysis of impurity deposition and pressure drop increase mechanisms in residue hydrotreating unit [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4616-4627. |
| [13] | WANG Peng, SHI Huibing, ZHAO Deming, FENG Baolin, CHEN Qian, YANG Da. Recent advances on transition metal catalyzed carbonylation of chlorinated compounds [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4649-4666. |
| [14] | ZHANG Qi, ZHAO Hong, RONG Junfeng. Research progress of anti-toxicity electrocatalysts for oxygen reduction reaction in PEMFC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4677-4691. |
| [15] | GE Quanqian, XU Mai, LIANG Xian, WANG Fengwu. Research progress on the application of MOFs in photoelectrocatalysis [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4692-4705. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||