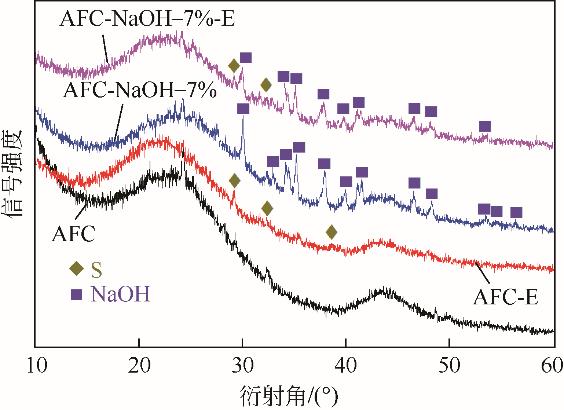
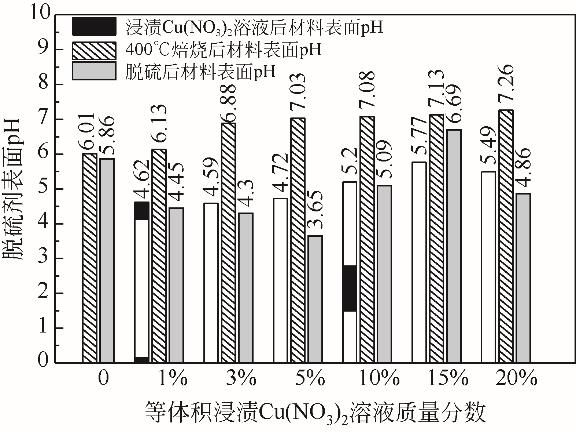
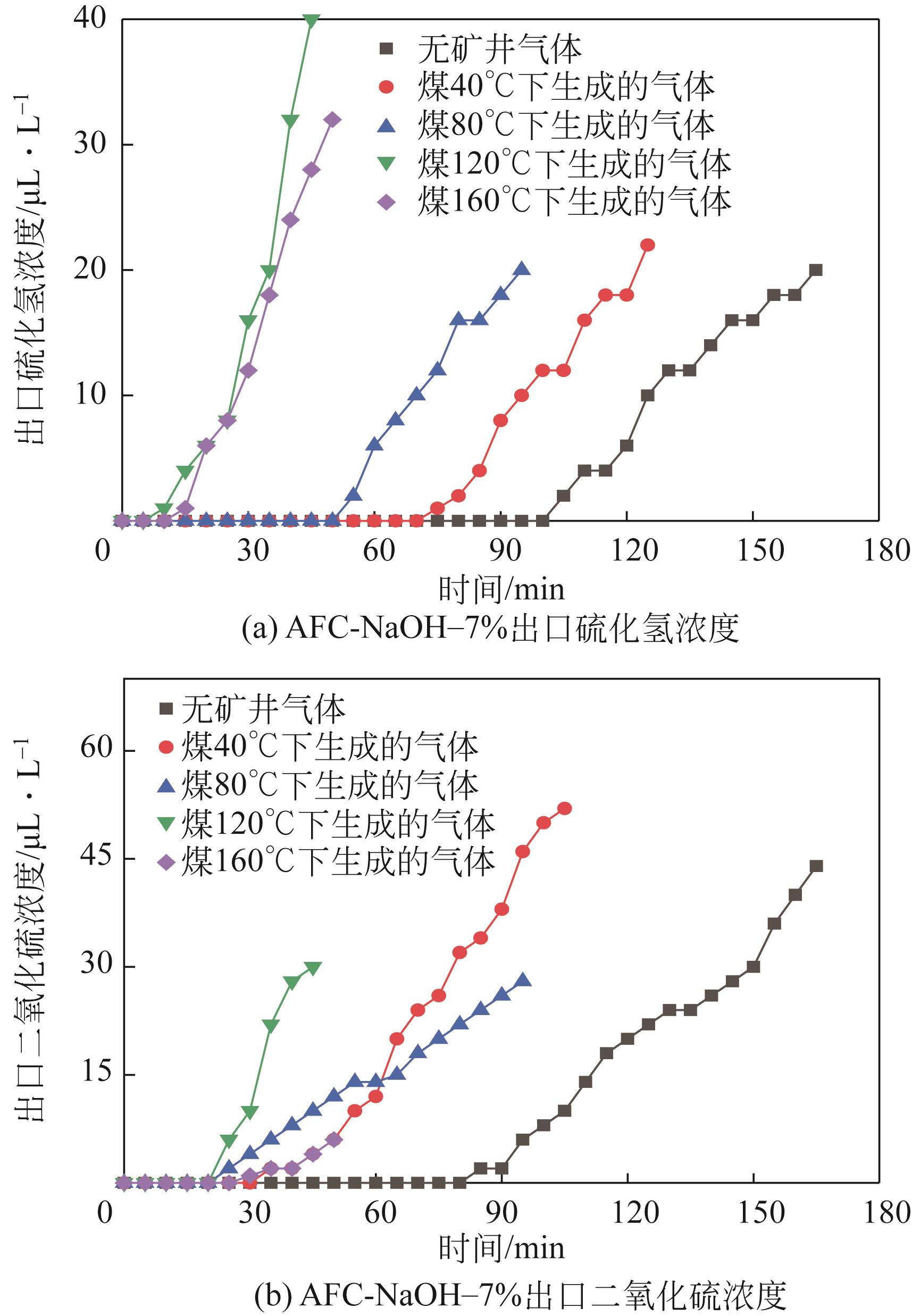
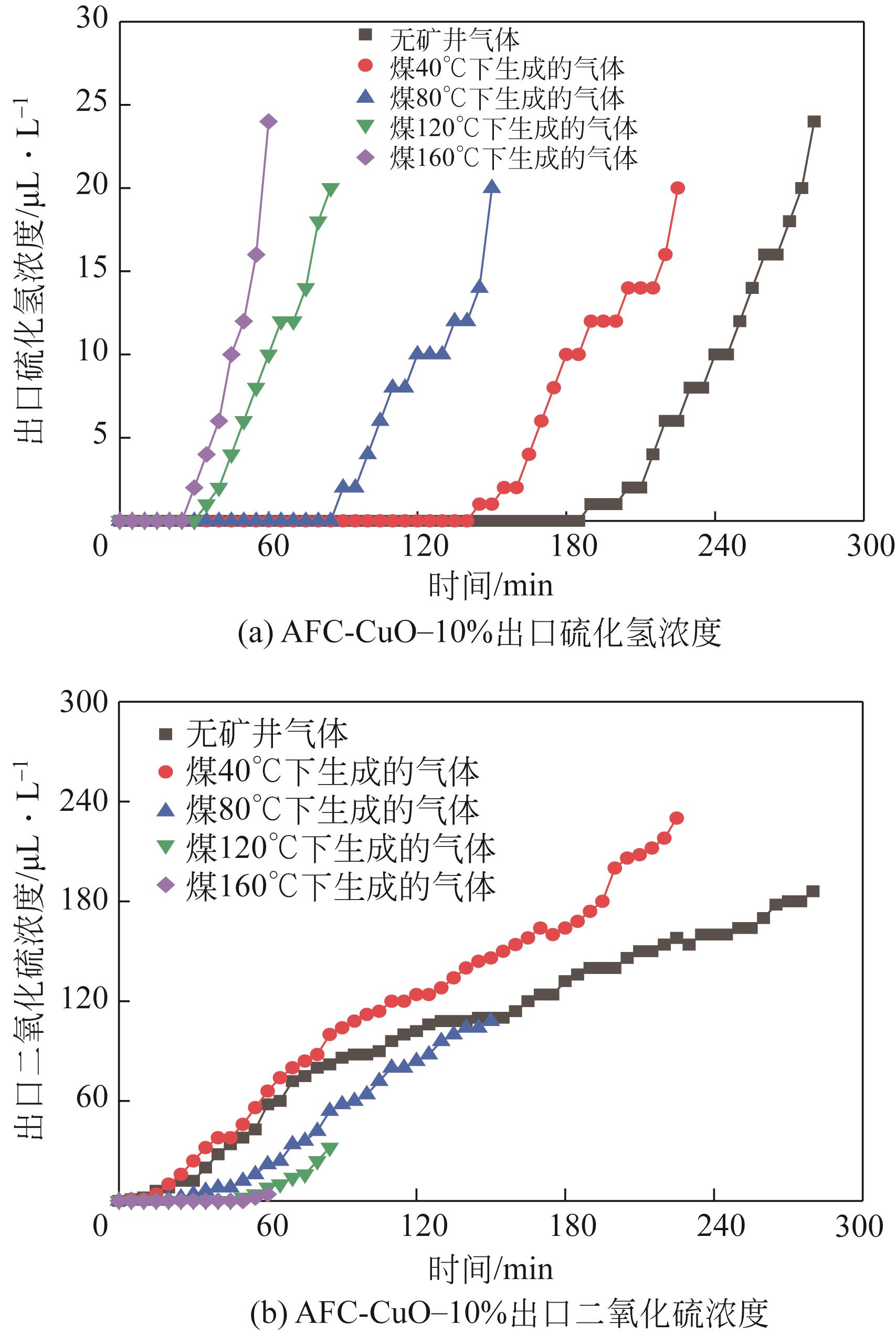
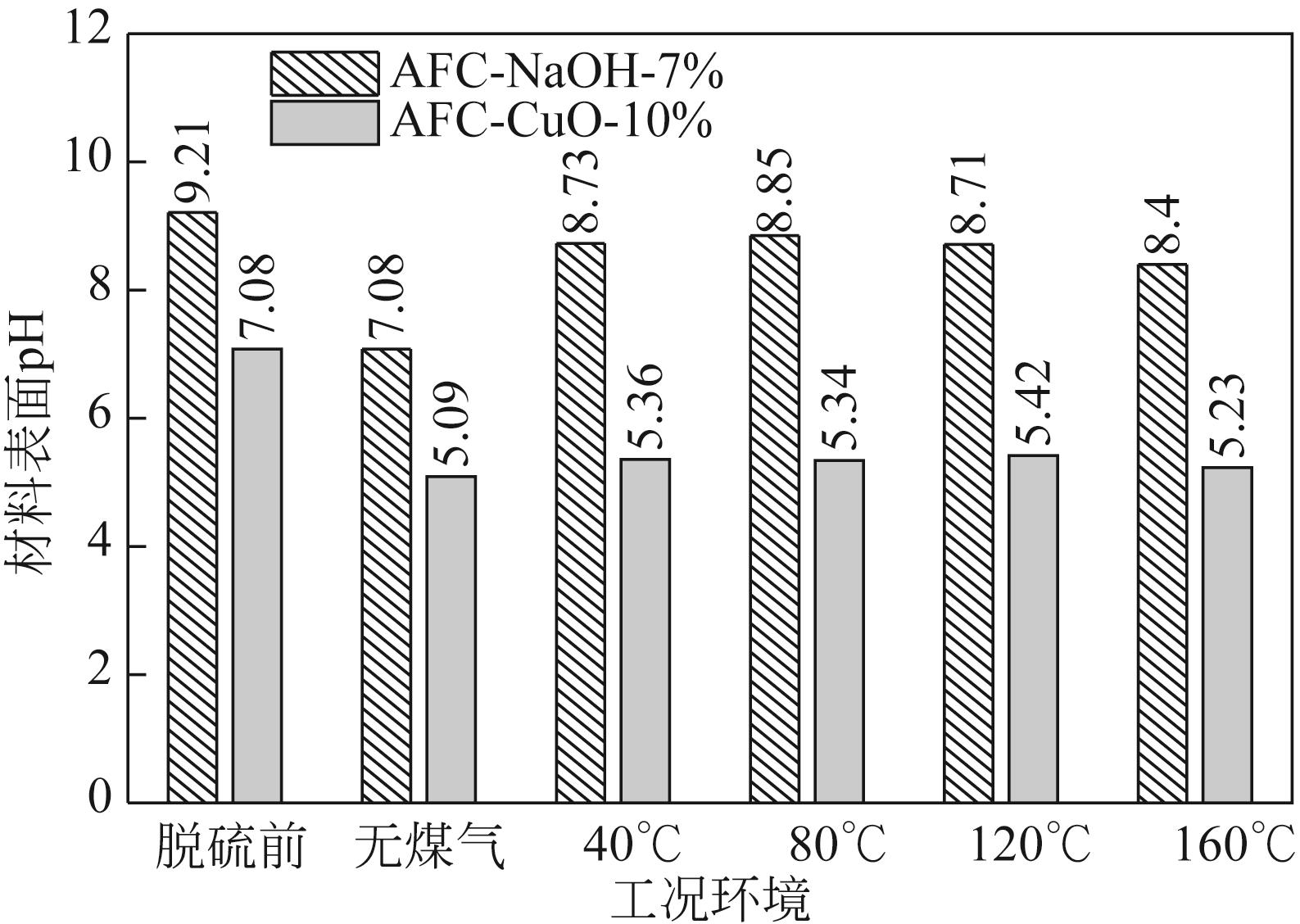
Chemical Industry and Engineering Progress ›› 2022, Vol. 41 ›› Issue (S1): 415-423.DOI: 10.16085/j.issn.1000-6613.2022-0780
• Materials science and technology • Previous Articles Next Articles
Application of activated carbon fiber supported desulfurizer in mine gas environment
ZHANG Xinhai( ), ZHAO Sichen(
), ZHAO Sichen( ), ZHU Hui, WANG Kai, ZHANG Shoushi
), ZHU Hui, WANG Kai, ZHANG Shoushi
- College of Safety Science and Engineering, Xi'an University of Science and Technology, Xi'an 710054, Shaanxi, China
-
Received:2022-04-28Revised:2022-05-31Online:2022-11-10Published:2022-10-20 -
Contact:ZHAO Sichen
活性碳纤维负载型脱硫剂在矿井气体环境条件下的应用
- 西安科技大学安全科学与工程学院,陕西 西安 710054
-
通讯作者:赵思琛 -
作者简介:张辛亥(1971—),男,博士,教授,研究方向为安全工程、煤矿防灭火技术。E-mail:Zhangxinh71@126.com。
CLC Number:
Cite this article
ZHANG Xinhai, ZHAO Sichen, ZHU Hui, WANG Kai, ZHANG Shoushi. Application of activated carbon fiber supported desulfurizer in mine gas environment[J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 415-423.
张辛亥, 赵思琛, 朱辉, 王凯, 张首石. 活性碳纤维负载型脱硫剂在矿井气体环境条件下的应用[J]. 化工进展, 2022, 41(S1): 415-423.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-0780
| 原料 | 供方公司名 | 型号 |
|---|---|---|
| 活性碳纤维粉 | 江苏科净 | KJF1500型 |
| 去离子水 | 江苏沐阳 | — |
| 氢氧化钠 | 天津致远 | 分析纯(AR) |
| 硝酸铜 | 北辰方正 | 分析纯(AR) |
| 气体采样袋 | 上海顺潮 | 铝箔复合膜气体采样袋 |
| 原料 | 供方公司名 | 型号 |
|---|---|---|
| 活性碳纤维粉 | 江苏科净 | KJF1500型 |
| 去离子水 | 江苏沐阳 | — |
| 氢氧化钠 | 天津致远 | 分析纯(AR) |
| 硝酸铜 | 北辰方正 | 分析纯(AR) |
| 气体采样袋 | 上海顺潮 | 铝箔复合膜气体采样袋 |
煤温 /℃ | O2 /% | N2 /% | CO /μL·L-1 | CO2 /μL·L-1 | CH4 /μL·L-1 | C2H6 /μL·L-1 | C2H4 /μL·L-1 |
|---|---|---|---|---|---|---|---|
| 40.00 | 20.22 | 73.36 | 179 | 2562 | 1.06 | 2.41 | 0 |
| 80.00 | 17.45 | 75.6 | 1349 | 17854 | 4.75 | 3.028 | 0.641 |
| 120.00 | 13.14 | 78.23 | 3376 | 22764 | 10.88 | 6.87 | 1.74 |
| 160.00 | 12.91 | 79.12 | 13174 | 26909 | 20.21 | 15.035 | 6.78 |
煤温 /℃ | O2 /% | N2 /% | CO /μL·L-1 | CO2 /μL·L-1 | CH4 /μL·L-1 | C2H6 /μL·L-1 | C2H4 /μL·L-1 |
|---|---|---|---|---|---|---|---|
| 40.00 | 20.22 | 73.36 | 179 | 2562 | 1.06 | 2.41 | 0 |
| 80.00 | 17.45 | 75.6 | 1349 | 17854 | 4.75 | 3.028 | 0.641 |
| 120.00 | 13.14 | 78.23 | 3376 | 22764 | 10.88 | 6.87 | 1.74 |
| 160.00 | 12.91 | 79.12 | 13174 | 26909 | 20.21 | 15.035 | 6.78 |
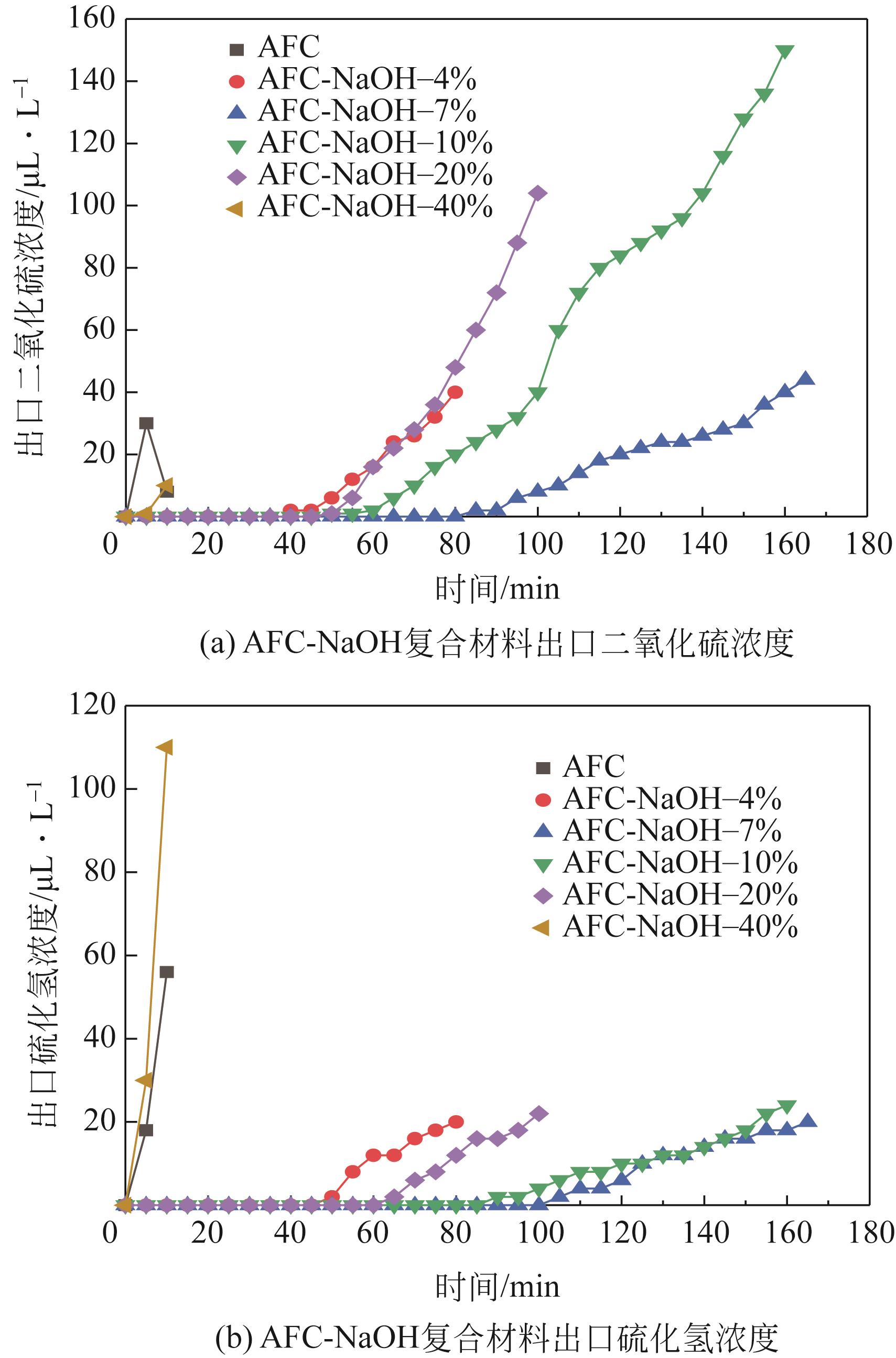
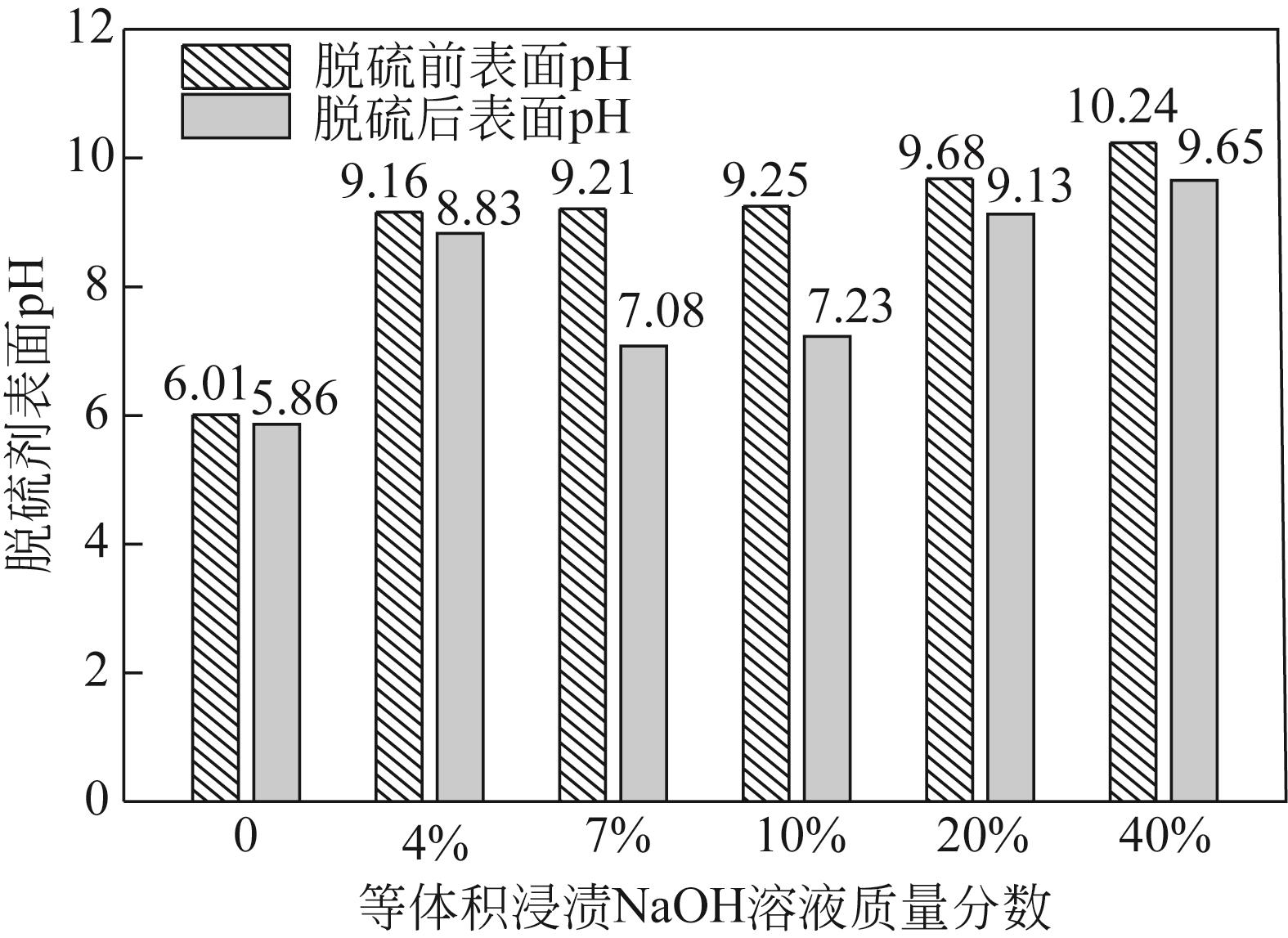
| 脱硫剂名称 | H2S出现时间/min | SO2出现时间/min | 穿透时间 /min | 穿透硫容 /g-S·g-1 |
|---|---|---|---|---|
| AFC | 5 | 5 | 10 | 0.0033 |
| AFC-NaOH-4% | 50 | 40 | 80 | 0.0278 |
| AFC-NaOH-7% | 105 | 85 | 165 | 0.0572 |
| AFC-NaOH-10% | 90 | 50 | 155 | 0.0505 |
| AFC-NaOH-20% | 65 | 50 | 100 | 0.0337 |
| AFC-NaOH-40% | 5 | 5 | 5 | 0.0017 |
| 脱硫剂名称 | H2S出现时间/min | SO2出现时间/min | 穿透时间 /min | 穿透硫容 /g-S·g-1 |
|---|---|---|---|---|
| AFC | 5 | 5 | 10 | 0.0033 |
| AFC-NaOH-4% | 50 | 40 | 80 | 0.0278 |
| AFC-NaOH-7% | 105 | 85 | 165 | 0.0572 |
| AFC-NaOH-10% | 90 | 50 | 155 | 0.0505 |
| AFC-NaOH-20% | 65 | 50 | 100 | 0.0337 |
| AFC-NaOH-40% | 5 | 5 | 5 | 0.0017 |
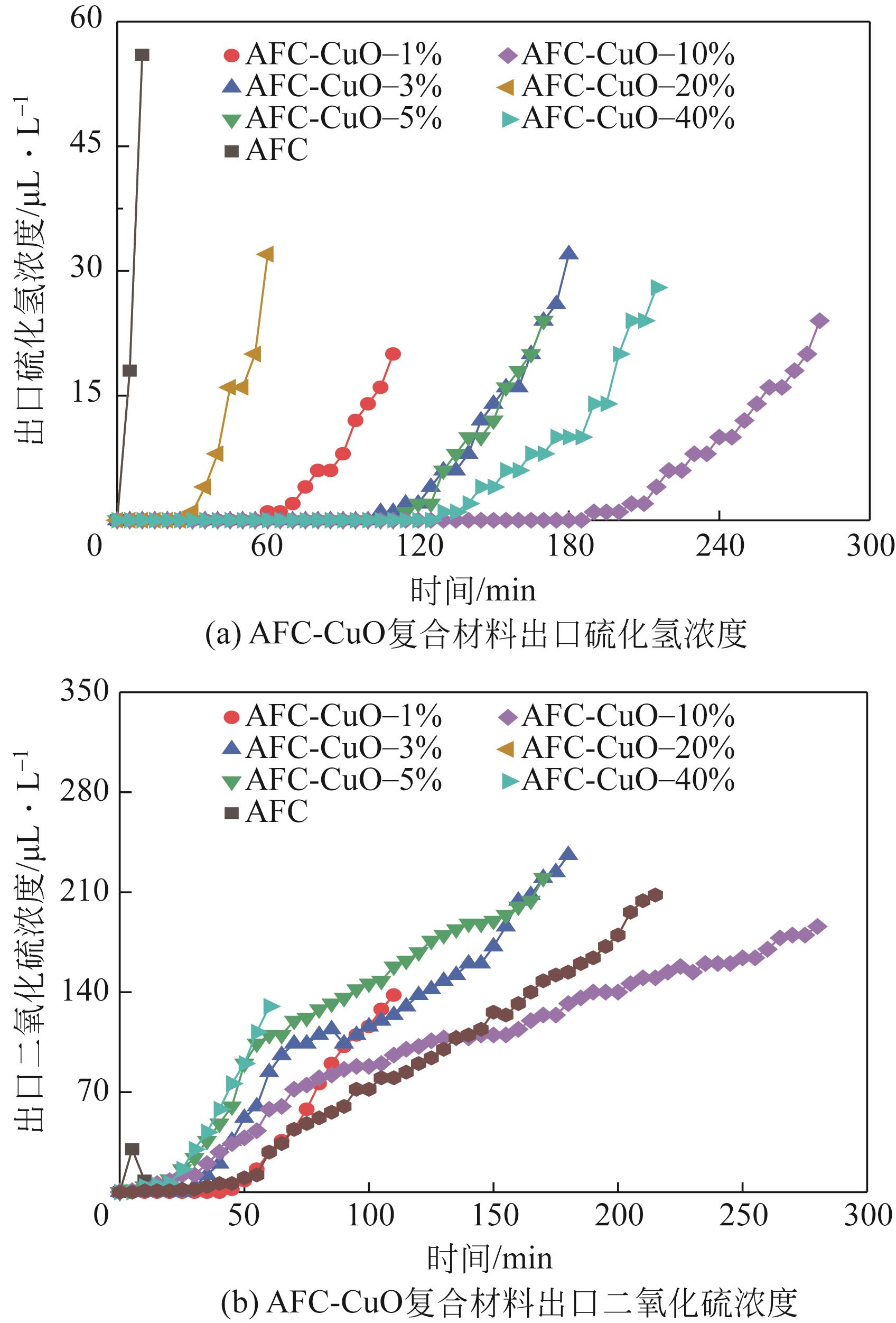
| 脱硫剂名称 | H2S出现 时间/min | SO2出现 时间/min | 穿透时间 /min | 穿透硫容 /g-S·g-1 |
|---|---|---|---|---|
| AFC | 5 | 5 | 10 | 0.0033 |
| AFC-CuO-1% | 60 | 45 | 110 | 0.0357 |
| AFC-CuO-3% | 105 | 30 | 165 | 0.0476 |
| AFC-CuO-5% | 115 | 5 | 165 | 0.0451 |
| AFC-CuO-10% | 190 | 5 | 275 | 0.0779 |
| AFC-CuO-20% | 30 | 10 | 55 | 0.0180 |
| AFC-CuO-40% | 130 | 10 | 200 | 0.0605 |
| 脱硫剂名称 | H2S出现 时间/min | SO2出现 时间/min | 穿透时间 /min | 穿透硫容 /g-S·g-1 |
|---|---|---|---|---|
| AFC | 5 | 5 | 10 | 0.0033 |
| AFC-CuO-1% | 60 | 45 | 110 | 0.0357 |
| AFC-CuO-3% | 105 | 30 | 165 | 0.0476 |
| AFC-CuO-5% | 115 | 5 | 165 | 0.0451 |
| AFC-CuO-10% | 190 | 5 | 275 | 0.0779 |
| AFC-CuO-20% | 30 | 10 | 55 | 0.0180 |
| AFC-CuO-40% | 130 | 10 | 200 | 0.0605 |
| 脱硫剂名称 | H2S出现 时间/min | SO2出现 时间/min | 穿透时间 /min | 穿透硫容 /g-S·g-1 |
|---|---|---|---|---|
| 无矿煤气 | ||||
| AFC-NaOH-7% | 105 | 85 | 165 | 0.0571 |
| AFC-CuO-10% | 190 | 5 | 275 | 0.0779 |
| 煤40℃生成气体 | ||||
| AFC-NaOH-7% | 75 | 35 | 125 | 0.0420 |
| AFC-CuO-10% | 145 | 5 | 225 | 0.0619 |
| 煤80℃生成气体 | ||||
| AFC-NaOH-7% | 55 | 25 | 95 | 0.0328 |
| AFC-CuO-10% | 90 | 20 | 150 | 0.0484 |
| 煤120℃生成气体 | ||||
| AFC-NaOH-7% | 10 | 25 | 35 | 0.0122 |
| AFC-CuO-10% | 35 | 50 | 85 | 0.0296 |
| 煤160℃生成气体 | ||||
| AFC-NaOH-7% | 15 | 30 | 40 | 0.0141 |
| AFC-CuO-10% | 30 | 60 | 55 | 0.0212 |
| 脱硫剂名称 | H2S出现 时间/min | SO2出现 时间/min | 穿透时间 /min | 穿透硫容 /g-S·g-1 |
|---|---|---|---|---|
| 无矿煤气 | ||||
| AFC-NaOH-7% | 105 | 85 | 165 | 0.0571 |
| AFC-CuO-10% | 190 | 5 | 275 | 0.0779 |
| 煤40℃生成气体 | ||||
| AFC-NaOH-7% | 75 | 35 | 125 | 0.0420 |
| AFC-CuO-10% | 145 | 5 | 225 | 0.0619 |
| 煤80℃生成气体 | ||||
| AFC-NaOH-7% | 55 | 25 | 95 | 0.0328 |
| AFC-CuO-10% | 90 | 20 | 150 | 0.0484 |
| 煤120℃生成气体 | ||||
| AFC-NaOH-7% | 10 | 25 | 35 | 0.0122 |
| AFC-CuO-10% | 35 | 50 | 85 | 0.0296 |
| 煤160℃生成气体 | ||||
| AFC-NaOH-7% | 15 | 30 | 40 | 0.0141 |
| AFC-CuO-10% | 30 | 60 | 55 | 0.0212 |
| 1 | 中华人民共和国国家卫生健康委员会. 中华人民共和国国家职业卫生标准: [S]. 北京: 中国标准出版社, 2019. |
| National Health Commission of the People's Republic of China. National occupational health standards of the People’s Republic of China: [S]. Beijing: Standards Press of China, 2019. | |
| 2 | 黄新, 朱道平. 硫化氢脱除方法述评[J]. 化学工业与工程技术, 2004, 25(5): 47-49, 62. |
| HUANG Xin, ZHU Daoping. Review on removal technology of H2S[J]. Journal of Chemical Industry & Engineering, 2004, 25(5): 47-49, 62. | |
| 3 | DALRYMPLE D A, TROFE T W, EVANS J M. An overview of liquid redox sulfur recovery [J]. Chemical Engineering Progress, 1989, 85(3): 5966572. |
| 4 | LIU Xinpeng, WANG Rui. H2S removal by peroxo heteropoly compound/ionic liquid solution[J]. Fuel Processing Technology, 2017, 160: 78-85. |
| 5 | 梁锋, 徐丙根, 施小红, 等. 湿式氧化法脱硫的技术进展[J]. 现代化工, 2003, 23(5): 21-24. |
| LIANG Feng, XU Binggen, SHI Xiaohong, et al. Advances in desulfurization with wet oxidation process[J]. Modern Chemical Industry, 2003, 23(5): 21-24. | |
| 6 | 李石雷, 张冬冬, 宁平, 等. 液相催化氧化法脱除硫化氢的研究进展[J]. 广州化学, 2017, 42(5): 57-64. |
| LI Shilei, ZHANG Dongdong, NING Ping, et al. Progress of liquid phase catalytic oxidation removing hydrogen sulfide[J]. Guangzhou Chemistry, 2017, 42(5): 57-64. | |
| 7 | 刘岱. 氧化铜基脱硫剂低温脱硫与再生性能研究[D]. 大连: 大连理工大学, 2017. |
| LIU Dai. Low temperature desulfurization and regeneration performance of CuO-based adsorbents[D]. Dalian: Dalian University of Technology, 2017. | |
| 8 | XUE Mei, CHITRAKAR Ramesh, SAKANE Kohji, et al. Screening of adsorbents for removal of H2S at room temperature[J]. Green Chemistry, 2003, 5(5): 529-534. |
| 9 | JIANG Dahao, SU Lianghu, MA Lei, et al. Cu-Zn-Al mixed metal oxides derived from hydroxycarbonate precursors for H2S removal at low temperature[J]. Applied Surface Science, 2010, 256(10): 3216-3223. |
| 10 | DHAGE Priyanka, SAMOKHVALOV A, REPALA Divya, et al. Copper-promoted ZnO/SiO2 regenerable sorbents for the room temperature removal of H2S from reformate gas streams[J]. Industrial & Engineering Chemistry Research, 2010, 49: 8388-8396. |
| 11 | 颜杰, 李红, 刘科财, 等. 干法脱除硫化氢技术研究进展[J]. 四川化工, 2011, 14(5): 27-31. |
| YAN Jie, LI Hong, LIU Kecai, et al. Research progress of removing H2S by dry method[J]. Sichuan Chemical Industry, 2011, 14(5): 27-31. | |
| 12 | 常化振, 赵朝成. 浸渍铜盐改性活性炭吸附/催化氧化低浓度H2S[J]. 石油化工, 2008, 37(11): 1195-1200. |
| CHANG Huazhen, ZHAO Chaocheng. Adsorption & catalytic oxidation of low concentration H2S on activated carbon modified by copper salts[J]. Petrochemical Technology, 2008, 37(11): 1195-1200. | |
| 13 | 陈勇, 赖小林. 氧化铁/活性炭负载型硫化氢脱除剂制备及性能评价[J]. 工业催化, 2014, 22(9): 680-682. |
| CHEN Yong, LAI Xiaolin. Preparation and performance evaluation of iron oxide/activated carbon supported hydrogen sulfide scavenger[J]. Industrial Catalysis, 2014, 22(9): 680-682. | |
| 14 | 刘孝坤, 刘永军. 活性炭的改性条件及其对硫化氢吸附性能的影响[J]. 化工进展, 2012, 31(3): 676-680. |
| LIU Xiaokun, LIU Yongjun. Activated carbon modification condition and its influence on the performance of the adsorption of hydrogen sulfide[J]. Chemical Industry and Engineering Progress, 2012, 31(3): 676-680. | |
| 15 | 罗佳妮. 活性炭纳米纤维的功能改性及性能研究[D]. 武汉: 武汉纺织大学, 2020. |
| LUO Jiani. Study on functional modification and properties of activated carbon nanofibers[D]. Wuhan: Wuhan Textile University, 2020. | |
| 16 | 费金彪. 煤自燃阶段判定理论与分级预警方法研究[D]. 西安: 西安科技大学, 2019. |
| FEI Jinbiao. Study on stage determination theory and classified early warning method for spontaneous combustion of coal[D]. Xi'an: Xi'an University of Science and Technology, 2019. | |
| 17 | 鲍勇强. 活性炭纤维负载掺铜BiVO4光催化剂降解车内甲醛的研究[D]. 重庆: 重庆大学, 2018. |
| BAO Yongqiang. Study on the degradation of formaldehyde in car by activated carbon fiber loaded with copper-doped BiVO4 composite photocatalyst[D]. Chongqing: Chongqing University, 2018. | |
| 18 | 刘飞. 活性炭纤维负载金属氧化物复合材料的制备及其性能研究[D]. 成都: 西南交通大学, 2019. |
| LIU Fei. Preparation and properties of activated carbon fiber supported metal oxide composites[D]. Chengdu: Southwest Jiaotong University, 2019. | |
| 19 | BAIRD Thomas, DENNY Patrick J, HOYLE Robert, et al. Modified zinc oxide absorbents for low-temperature gas desulfurisation[J]. Journal of the Chemical Society, Faraday Transactions, 1992, 88(22): 3375-3382. |
| [1] | ZHANG Xinhai, ZHAO Sichen, ZHU Hui, ZHANG Shoushi, WANG Kai. Comparative study on desulfurization performance of various carbon materials combined with sodium carbonate [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 424-435. |
| [2] | QI Yuan, XU Xinrong, RUAN Wei, WU Hao, WU Ke, ZHOU Yaming, YANG Hongmin. Characterization of aniline adsorption by modified activated carbon fiber [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 622-630. |
| [3] | ZHOU Yongquan, ZHANG Ai, LIU Yanan, WANG Zheng. Removal of glucocorticoids from aqueous solution by plasma jet combined with activated carbon fiber [J]. Chemical Industry and Engineering Progress, 2022, 41(4): 2209-2215. |
| [4] | Xinghui ZHANG,Jiaojiao YANG,Yaru DU. Desulfurization performance of activated carbon fiber after oxidation modification [J]. Chemical Industry and Engineering Progress, 2019, 38(11): 5151-5157. |
| [5] | LI Cancan, ZHU Jiamei, REN Ting, GUO Bin, YAN Hongfang. CO2 adsorption performance of modified activated carbon fibers [J]. Chemical Industry and Engineering Progress, 2018, 37(09): 3520-3527. |
| [6] | LIN Hao, ZHAO Jinyun, HU Jiapeng, LIU Ruilai, RAO Ruiye. A review on preparation and application of activated carbon nanofibers via electrospinning [J]. Chemical Industry and Engineering Progress, 2017, 36(08): 2986-2993. |
| [7] | LU Kang, WEI Jiana, XIONG Liang. Research on preparation and antibacterial properties of ZnO-activated carbon fiber [J]. Chemical Industry and Engineering Progree, 2015, 34(1): 208-211. |
| [8] | XUE Bei, ZHANG Xiaoping, LI Nan, ZHANG Pei. Adsorption characteristics of pyridine on rayon-based activated carbon fiber [J]. Chemical Industry and Engineering Progree, 2015, 34(07): 2055-2059. |
| [9] | LI Li1,HUANG Huacun2,WEI Tengyou1,SUN Jianhua1,TONG Zhangfa1. Influence of cerium additive on selective catalytic reduction of NOx with MnOx/ACFN catalyst [J]. Chemical Industry and Engineering Progree, 2013, 32(11): 2655-2660. |
| [10] | ZHANG Pei1,ZHANG Xiaoping1,FANG Yimin,LAN Yonghui3. Adsorption characteristics of quinoline on activated carbon fiber [J]. Chemical Industry and Engineering Progree, 2013, 32(01): 209-213. |
| [11] | ZHANG Pei1,ZHANG Xiaoping1,LAN Yonghui2,WEI Chaohai1. Adsorption and treatment of simulated coking waste water by activated carbon fiber [J]. Chemical Industry and Engineering Progree, 2012, 31(12): 2786-2790. |
| [12] | HUANG Huacun. Research advance in mechanism of low-temperature SCR of NO on carbon materials [J]. Chemical Industry and Engineering Progree, 2011, 30(7): 1478-. |
| [13] | ZHOU Xuan,YI Honghong,TANG Xiaolong,DENG Hua. Progress in the removal of SO2,CO2 and NOx in coal-combustion gases by activated carbon fibers [J]. Chemical Industry and Engineering Progree, 2011, 30(12): 2764-. |
| [14] | HUANG Xin1,HUANG Bichun1,ZHANG Chunju1,YE Daiqi1,LUO Cuihong2. Plasma modification of V-ACF for NO SCR at low temperatures [J]. Chemical Industry and Engineering Progree, 2010, 29(11): 2187-. |
| [15] | LI Haiyan,CHEN Qiufei,ZHANG Xuejun. Dynamic adsorption of toluene on pitch-based activated carbon fibers [J]. Chemical Industry and Engineering Progree, 2009, 28(9): 1522-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||