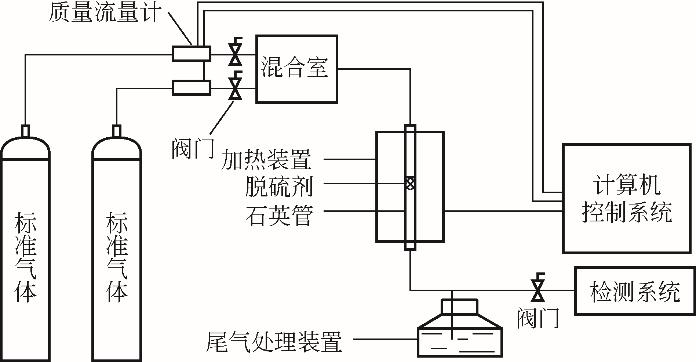
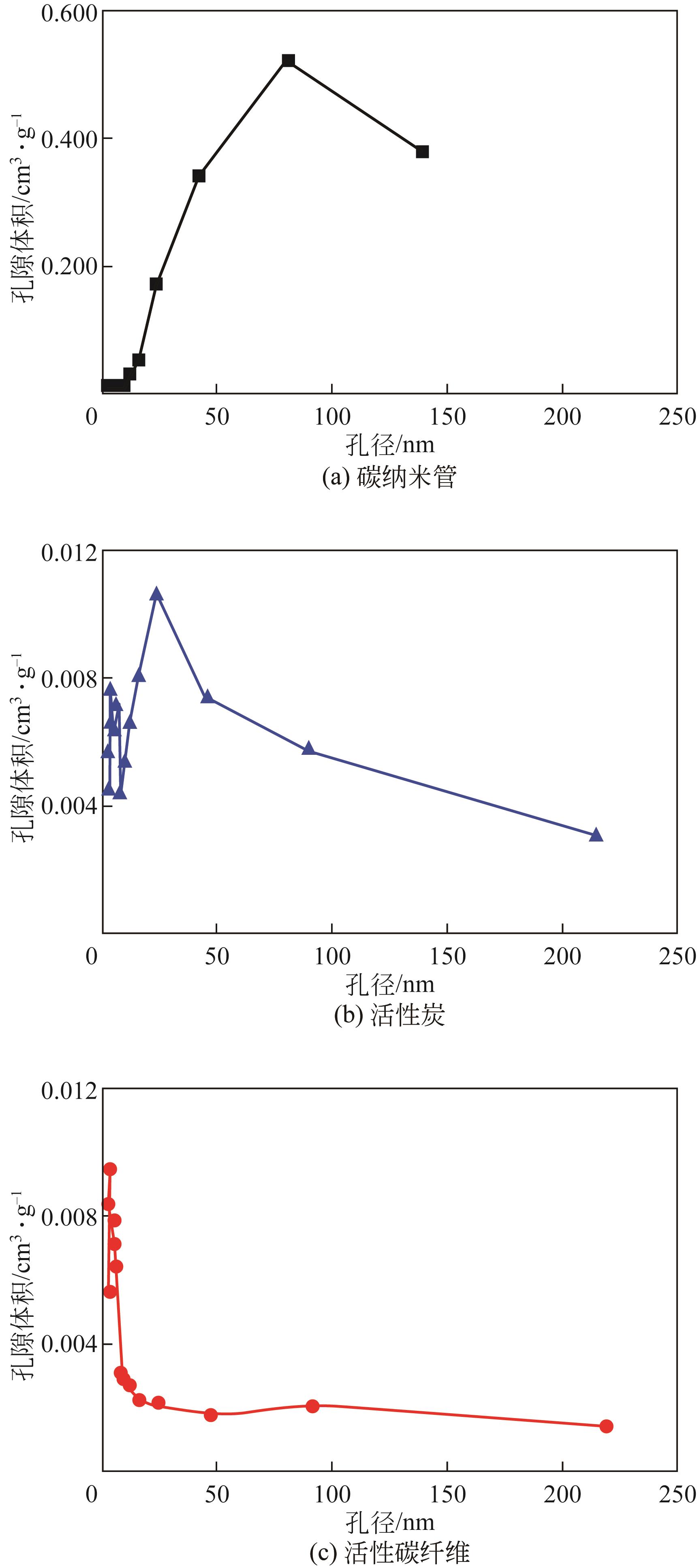
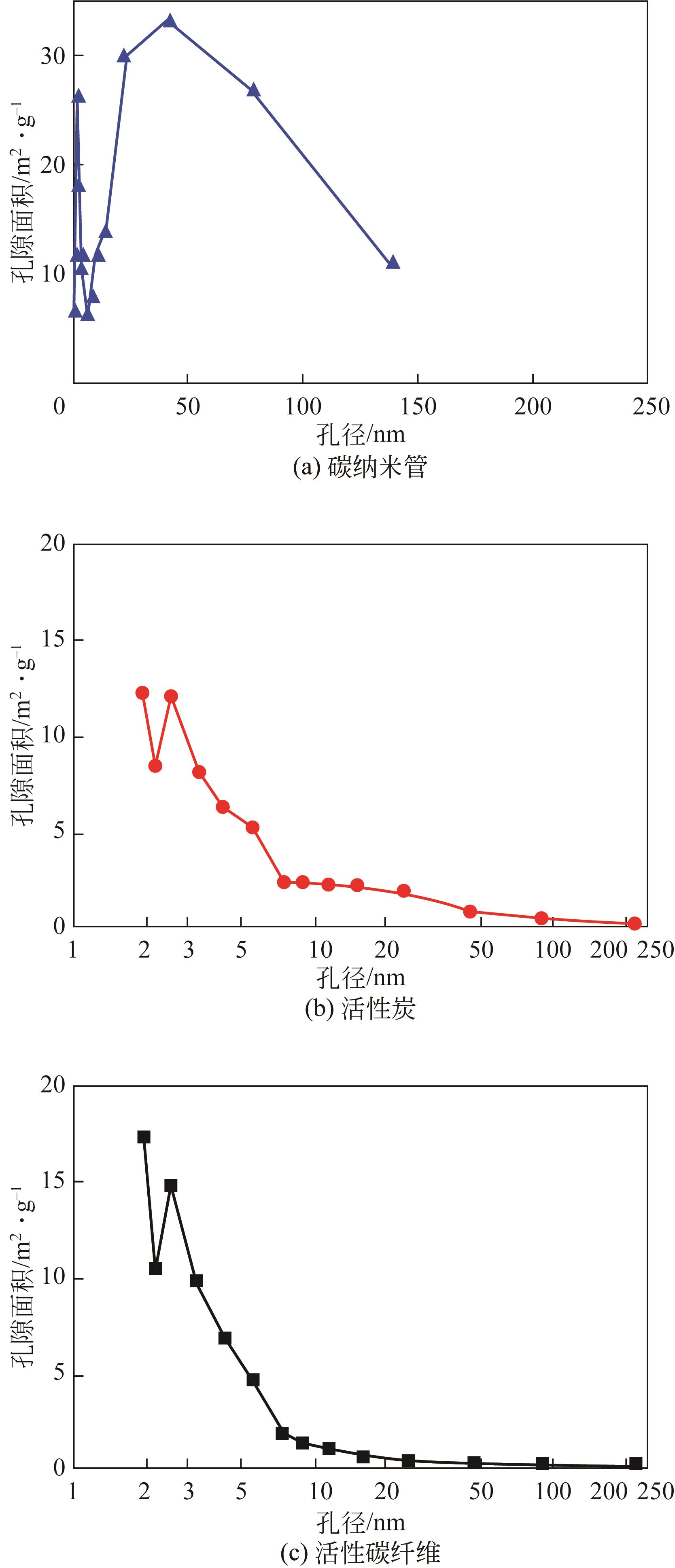
Chemical Industry and Engineering Progress ›› 2022, Vol. 41 ›› Issue (S1): 424-435.DOI: 10.16085/j.issn.1000-6613.2022-0781
• Materials science and technology • Previous Articles Next Articles
Comparative study on desulfurization performance of various carbon materials combined with sodium carbonate
ZHANG Xinhai( ), ZHAO Sichen(
), ZHAO Sichen( ), ZHU Hui, ZHANG Shoushi, WANG Kai
), ZHU Hui, ZHANG Shoushi, WANG Kai
- College of Safety Science and Engineering, Xi'an University of Science and Technology, Xi'an 710054, Shaanxi, China
-
Received:2022-04-28Revised:2022-05-31Online:2022-11-10Published:2022-10-20 -
Contact:ZHAO Sichen
多种碳材料与碳酸钠复合后脱硫性能对比
- 西安科技大学安全科学与工程学院,陕西 西安 710054
-
通讯作者:赵思琛 -
作者简介:张辛亥(1971-),男,教授,研究方向为安全工程、煤矿防灭火技术等。E-mail: zhangxinhai@126.com。
CLC Number:
Cite this article
ZHANG Xinhai, ZHAO Sichen, ZHU Hui, ZHANG Shoushi, WANG Kai. Comparative study on desulfurization performance of various carbon materials combined with sodium carbonate[J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 424-435.
张辛亥, 赵思琛, 朱辉, 张首石, 王凯. 多种碳材料与碳酸钠复合后脱硫性能对比[J]. 化工进展, 2022, 41(S1): 424-435.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-0781
| 原料 | 提供方 | 型号 |
|---|---|---|
| 活性碳 纤维粉 | 江苏科净碳纤维有限公司 | KJF1500型,孔隙以微孔为主,具有大的比表面积 |
| 活性炭 | 苏州旋风活性炭有限公司 | 椰壳炭粉,具有丰富的微孔以及中孔结构 |
| 碳纳米管 | 苏州碳丰石墨烯科技有限公司 | 单壁碳纳米管,孔隙结构以中孔和大孔为主 |
| 去离子水 | 江苏沐阳环境工程科技有限公司 | — |
| 碳酸钠 | 天津致远化学试剂有限公司 | 分析纯(AR) |
| 原料 | 提供方 | 型号 |
|---|---|---|
| 活性碳 纤维粉 | 江苏科净碳纤维有限公司 | KJF1500型,孔隙以微孔为主,具有大的比表面积 |
| 活性炭 | 苏州旋风活性炭有限公司 | 椰壳炭粉,具有丰富的微孔以及中孔结构 |
| 碳纳米管 | 苏州碳丰石墨烯科技有限公司 | 单壁碳纳米管,孔隙结构以中孔和大孔为主 |
| 去离子水 | 江苏沐阳环境工程科技有限公司 | — |
| 碳酸钠 | 天津致远化学试剂有限公司 | 分析纯(AR) |
| 碳材料 | 称重 /g | 加入水 质量/g | 上层滤液质量/g | 滤纸吸出水质量/g | 吸水 质量/g | 吸水率/% |
|---|---|---|---|---|---|---|
| 活性碳纤维 | 3 | 13 | 7.3 | 0.6 | 5.1 | 176 |
| 椰壳活性炭 | 3 | 11.1 | 3.7 | 1.2 | 6.5 | 216 |
| 碳纳米管 | 1 | 30.47 | 11.88 | 8.01 | 10.58 | 1058 |
| 碳材料 | 称重 /g | 加入水 质量/g | 上层滤液质量/g | 滤纸吸出水质量/g | 吸水 质量/g | 吸水率/% |
|---|---|---|---|---|---|---|
| 活性碳纤维 | 3 | 13 | 7.3 | 0.6 | 5.1 | 176 |
| 椰壳活性炭 | 3 | 11.1 | 3.7 | 1.2 | 6.5 | 216 |
| 碳纳米管 | 1 | 30.47 | 11.88 | 8.01 | 10.58 | 1058 |
| 样品 | 比表面积BET /m2·g-1 | 微孔面积 T-Plot/m2·g-1 | 平均吸附孔径 BET/nm |
|---|---|---|---|
| AFC | 978.8741 | 797.2340 | 1.9113 |
| AC | 1378.5892 | 1091.8004 | 1.7756 |
| SWNT | 223.2513 | 11.3447 | 29.7342 |
| 样品 | 比表面积BET /m2·g-1 | 微孔面积 T-Plot/m2·g-1 | 平均吸附孔径 BET/nm |
|---|---|---|---|
| AFC | 978.8741 | 797.2340 | 1.9113 |
| AC | 1378.5892 | 1091.8004 | 1.7756 |
| SWNT | 223.2513 | 11.3447 | 29.7342 |
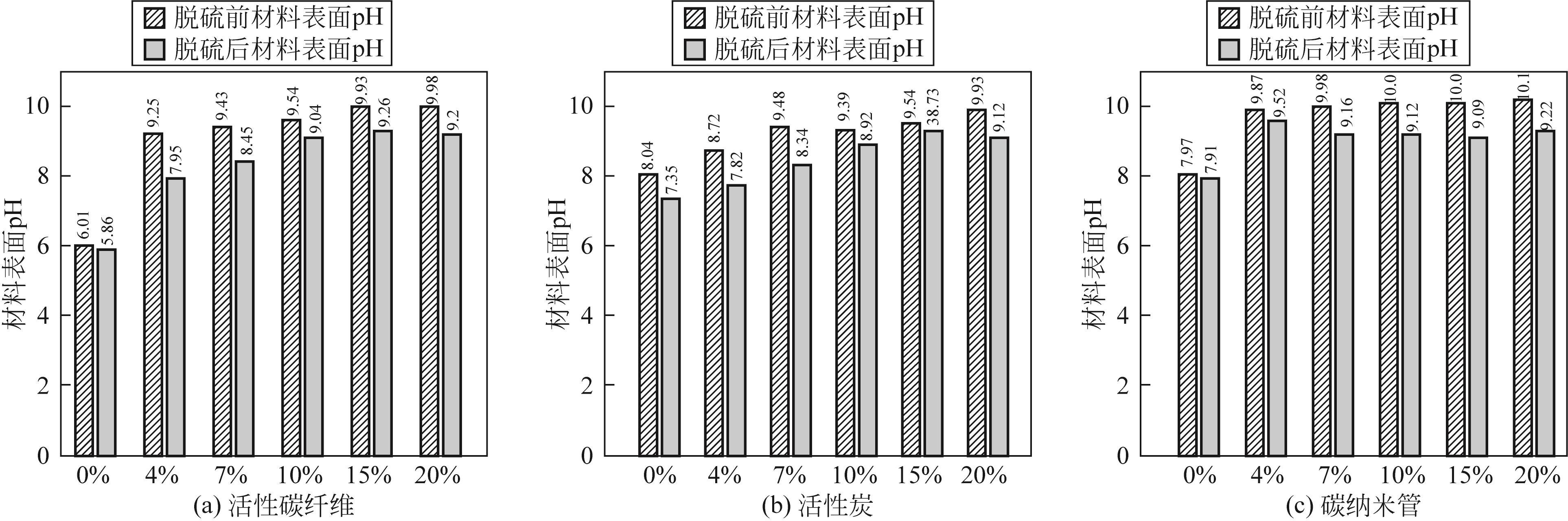
| 材料 | pH |
|---|---|
| AFC | 6.01 |
| AC | 8.04 |
| SWNT | 7.97 |
| 材料 | pH |
|---|---|
| AFC | 6.01 |
| AC | 8.04 |
| SWNT | 7.97 |
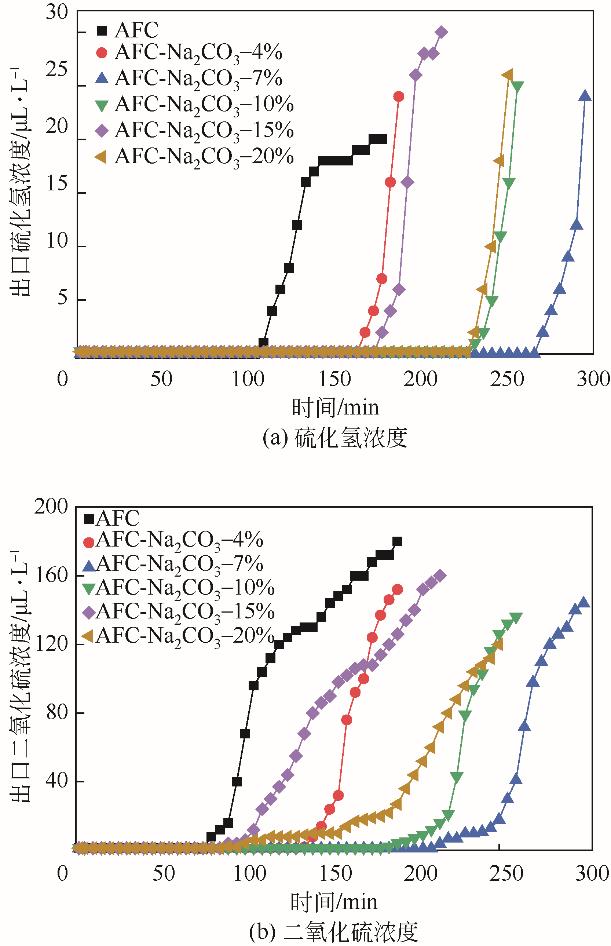
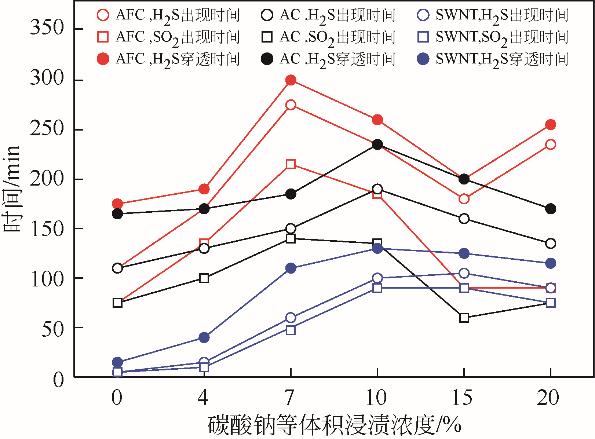
| 脱硫剂名称 | H2S出现 时间/min | SO2出现 时间/min | 穿透时间 /min | 穿透硫容 /g-S·g-1 |
|---|---|---|---|---|
| AFC | 110 | 75 | 175 | 0.0272 |
| AFC-Na2CO3-4% | 170 | 135 | 190 | 0.0330 |
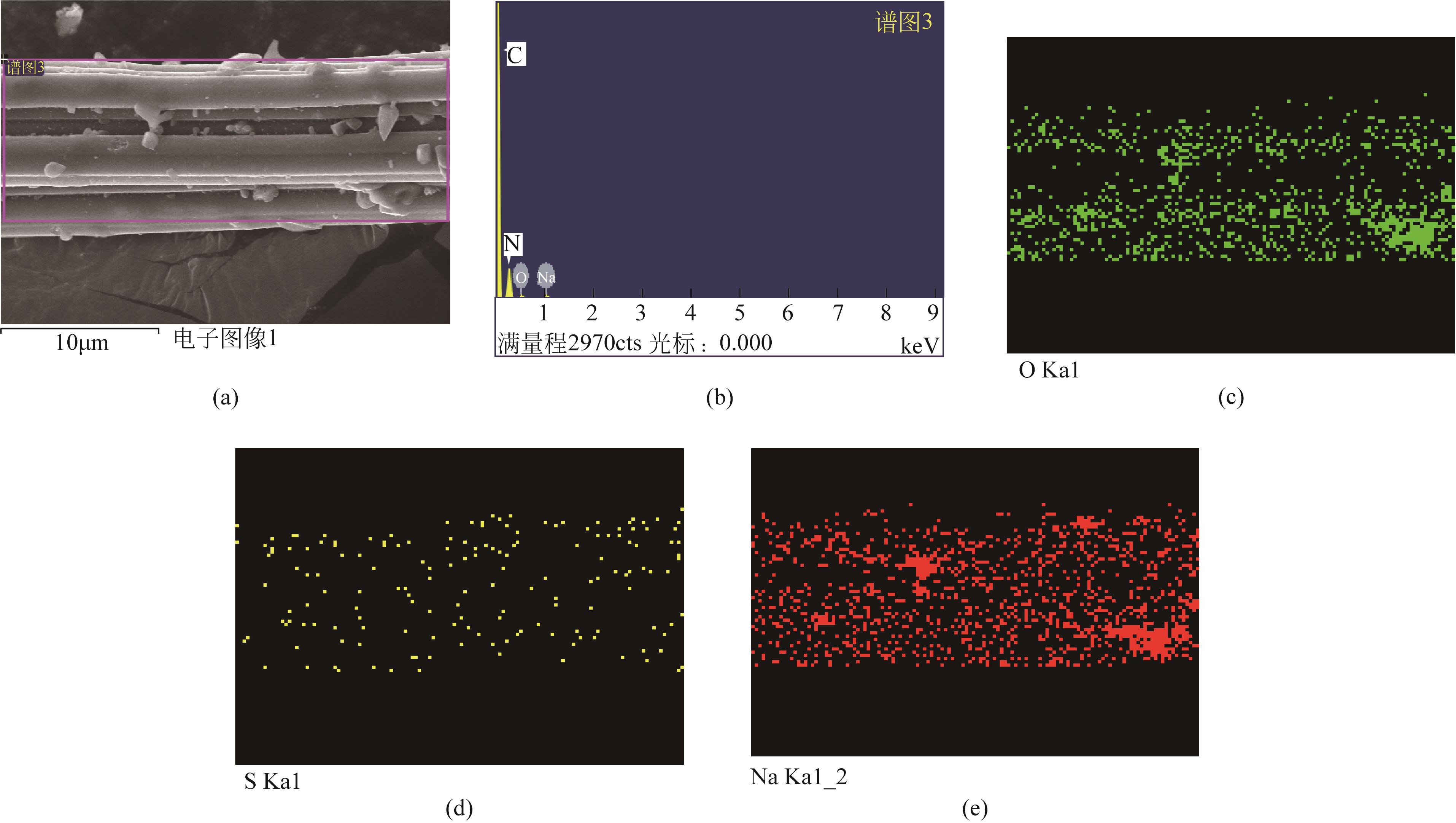
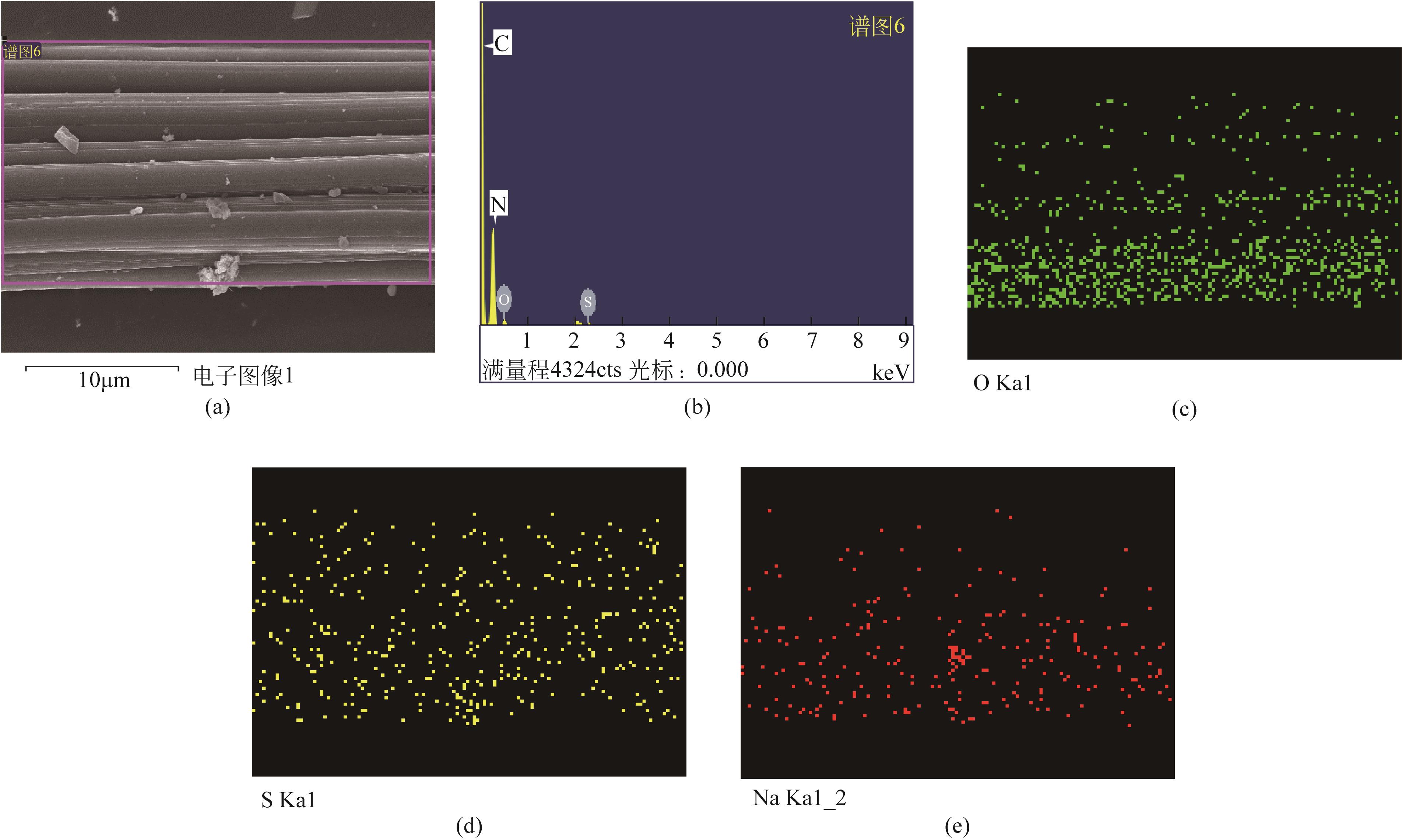
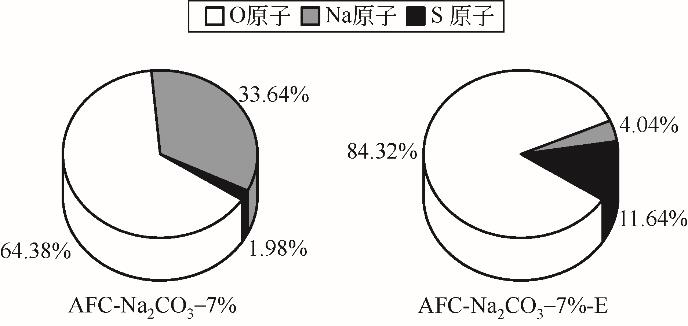
| AFC-Na2CO3-7% | 275 | 215 | 300 | 0.0523 |
| AFC-Na2CO3-10% | 235 | 185 | 260 | 0.0456 |
| AFC-Na2CO3-15% | 180 | 90 | 200 | 0.0335 |
| AFC-Na2CO3-20% | 235 | 90 | 255 | 0.0439 |
| 脱硫剂名称 | H2S出现 时间/min | SO2出现 时间/min | 穿透时间 /min | 穿透硫容 /g-S·g-1 |
|---|---|---|---|---|
| AFC | 110 | 75 | 175 | 0.0272 |
| AFC-Na2CO3-4% | 170 | 135 | 190 | 0.0330 |
| AFC-Na2CO3-7% | 275 | 215 | 300 | 0.0523 |
| AFC-Na2CO3-10% | 235 | 185 | 260 | 0.0456 |
| AFC-Na2CO3-15% | 180 | 90 | 200 | 0.0335 |
| AFC-Na2CO3-20% | 235 | 90 | 255 | 0.0439 |
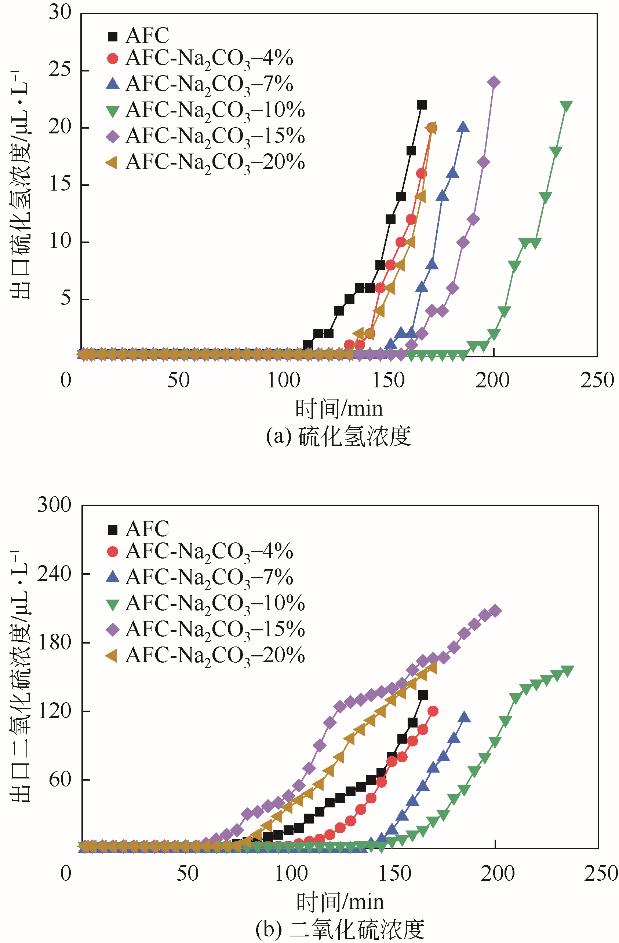
| 脱硫剂名称 | H2S出现 时间/min | SO2出现 时间/min | 穿透时间 /min | 穿透硫容 /g-S·g-1 |
|---|---|---|---|---|
| AC | 110 | 75 | 165 | 0.0278 |
| AC-Na2CO3-4% | 130 | 100 | 170 | 0.0290 |
| AC-Na2CO3-7% | 150 | 140 | 185 | 0.0320 |
| AC-Na2CO3-10% | 190 | 135 | 235 | 0.0393 |
| AC-Na2CO3-15% | 160 | 60 | 200 | 0.0301 |
| AC-Na2CO3-20% | 135 | 75 | 170 | 0.0276 |
| 脱硫剂名称 | H2S出现 时间/min | SO2出现 时间/min | 穿透时间 /min | 穿透硫容 /g-S·g-1 |
|---|---|---|---|---|
| AC | 110 | 75 | 165 | 0.0278 |
| AC-Na2CO3-4% | 130 | 100 | 170 | 0.0290 |
| AC-Na2CO3-7% | 150 | 140 | 185 | 0.0320 |
| AC-Na2CO3-10% | 190 | 135 | 235 | 0.0393 |
| AC-Na2CO3-15% | 160 | 60 | 200 | 0.0301 |
| AC-Na2CO3-20% | 135 | 75 | 170 | 0.0276 |
| 脱硫剂名称 | H2S出现 时间/min | SO2出现 时间/min | 穿透时间 /min | 穿透硫容 /g-S·g-1 |
|---|---|---|---|---|
| SWNT | 5 | 5 | 15 | 0.0021 |
| SWNT-Na2CO3-4% | 15 | 10 | 40 | 0.0067 |
| SWNT-Na2CO3-7% | 60 | 50 | 110 | 0.0190 |
| SWNT-Na2CO3-10% | 100 | 90 | 130 | 0.0228 |
| SWNT-Na2CO3-15% | 105 | 90 | 125 | 0.0219 |
| SWNT-Na2CO3-20% | 90 | 75 | 115 | 0.0200 |
| 脱硫剂名称 | H2S出现 时间/min | SO2出现 时间/min | 穿透时间 /min | 穿透硫容 /g-S·g-1 |
|---|---|---|---|---|
| SWNT | 5 | 5 | 15 | 0.0021 |
| SWNT-Na2CO3-4% | 15 | 10 | 40 | 0.0067 |
| SWNT-Na2CO3-7% | 60 | 50 | 110 | 0.0190 |
| SWNT-Na2CO3-10% | 100 | 90 | 130 | 0.0228 |
| SWNT-Na2CO3-15% | 105 | 90 | 125 | 0.0219 |
| SWNT-Na2CO3-20% | 90 | 75 | 115 | 0.0200 |
| 1 | 中华人民共和国国家职业卫生标准 [S]. 北京: 中国标准出版社, 2019. |
| National occupational health standards of the People’s Republic of China [S]. Beijing: Standards Press of China, 2019. | |
| 2 | 黄新, 朱道平. 硫化氢脱除方法述评[J]. 化学工业与工程技术, 2004, 25(5): 47-49, 62. |
| HUANG Xin, ZHU Daoping. Review on removal technology of H2S[J]. Journal of Chemical Industry & Engineering, 2004, 25(5): 47-49, 62. | |
| 3 | DALRYMPLE D A, TROFE T W, EVANS J M. An overview of liquid redox sulfur recovery[J]. Chemical Engineering Progress, 1989, 85. |
| 4 | LIU Xinpeng, WANG Rui. H2S removal by peroxo heteropoly compound/ionic liquid solution[J]. Fuel Processing Technology, 2017, 160: 78-85. |
| 5 | 梁锋, 徐丙根, 施小红, 等. 湿式氧化法脱硫的技术进展[J]. 现代化工, 2003, 23(5): 21-24. |
| LIANG Feng, XU Binggen, SHI Xiaohong, et al. Advances in desulfurization with wet oxidation process[J]. Modern Chemical Industry, 2003, 23(5): 21-24. | |
| 6 | 李石雷, 张冬冬, 宁平, 等. 液相催化氧化法脱除硫化氢的研究进展[J]. 广州化学, 2017, 42(5): 57-64. |
| LI Shilei, ZHANG Dongdong, NING Ping, et al. Progress of liquid phase catalytic oxidation removing hydrogen sulfide[J]. Guangzhou Chemistry, 2017, 42(5): 57-64. | |
| 7 | 刘岱. 氧化铜基脱硫剂低温脱硫与再生性能研究[D]. 大连: 大连理工大学, 2017. |
| LIU Dai. Low temperature desulfurization and regeneration performance of CuO-based adsorbents[D]. Dalian: Dalian University of Technology, 2017. | |
| 8 | XUE Mei, CHITRAKAR Ramesh, SAKANE Kohji, et al. Screening of adsorbents for removal of H2S at room temperature[J]. Green Chemistry, 2003, 5(5): 529-534. |
| 9 | JIANG Dahao, SU Lianghu, MA Lei, et al. Cu-Zn-Al mixed metal oxides derived from hydroxycarbonate precursors for H2S removal at low temperature[J]. Applied Surface Science, 2010, 256(10): 3216-3223. |
| 10 | DHAGE Priyanka, SAMOKHVALOV Alexander, REPALA Divya, et al. Copper-promoted ZnO/SiO2 regenerable sorbents for the room temperature removal of H2S from reformate gas streams[J]. Industrial & Engineering Chemistry Research, 2010, 49(18): 8388-8396. |
| 11 | 颜杰, 李红, 刘科财, 等. 干法脱除硫化氢技术研究进展[J]. 四川化工, 2011, 14(5): 27-31. |
| YAN Jie, LI Hong, LIU Kecai, et al. Research progress of removing H2S by dry method[J]. Sichuan Chemical Industry, 2011, 14(5): 27-31. | |
| 12 | 常化振, 赵朝成. 浸渍铜盐改性活性炭吸附/催化氧化低浓度H2S[J]. 石油化工, 2008, 37(11): 1195-1200. |
| CHANG Huazhen, ZHAO Chaocheng. Adsorption & catalytic oxidation of low concentration H2S on activated carbon modified by copper salts[J]. Petrochemical Technology, 2008, 37(11): 1195-1200. | |
| 13 | 陈勇, 赖小林. 氧化铁/活性炭负载型硫化氢脱除剂制备及性能评价[J]. 工业催化, 2014, 22(9): 680-682. |
| CHEN Yong, LAI Xiaolin. Preparation and performance evaluation of iron oxide/activated carbon supported hydrogen sulfide scavenger[J]. Industrial Catalysis, 2014, 22(9): 680-682. | |
| 14 | LI Yuran, LIN Yuting, XU Zhicheng, et al. Oxidation mechanisms of H2S by oxygen and oxygen-containing functional groups on activated carbon[J]. Fuel Processing Technology, 2019, 189: 110-119. |
| 15 | 鲍勇强. 活性碳纤维负载掺铜BiVO4光催化剂降解车内甲醛的研究[D]. 重庆: 重庆大学, 2018. |
| BAO Yongqiang. Study on the degradation of formaldehyde in car by activated carbon fiber loaded with copper-doped BiVO4 composite photocatalyst[D]. Chongqing: Chongqing University, 2018. | |
| 16 | 刘飞. 活性碳纤维负载金属氧化物复合材料的制备及其性能研究[D]. 成都: 西南交通大学, 2019. |
| LIU Fei. Preparation and properties of activated carbon fiber supported metal oxide composites[D]. Chengdu: Southwest Jiaotong University, 2019. | |
| 17 | 钱佳燕, 赵彤, 吴逸雨, 等. 改性活性炭的制备及其吸附解析效果研究[J]. 辽宁化工, 2022, 51(2): 160-162. |
| QIAN Jiayan, ZHAO Tong, WU Yiyu, et al. Study on preparation of modified activated carbon and its adsorption and resolution effect[J]. Liaoning Chemical Industry, 2022, 51(2): 160-162. | |
| 18 | 刘楠, 李伟, 张伟, 等. 碳纳米管在天然橡胶中的应用研究[J]. 中国橡胶, 2022, 38(1): 45-51. |
| LIU Nan, LI Wei, ZHANG Wei, et al. Application of carbon nanotubes in natural rubber[J]. China Rubber, 2022, 38(1): 45-51. | |
| 19 | 包宗尧, 李永贵, 杨建忠, 等. 聚酰胺基碳纳米管复合纤维的研究现状与进展[J]. 丝绸, 2022, 59(2): 40-47. |
| BAO Zongyao, LI Yonggui, YANG Jianzhong, et al. Research progress of polyamide-based carbon nanotube composite fibers[J]. Journal of Silk, 2022, 59(2): 40-47. |
| [1] | ZHANG Yaojie, ZHANG Chuanxiang, SUN Yue, ZENG Huihui, JIA Jianbo, JIANG Zhendong. Application of coal-based graphene quantum dots in supercapacitors [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4340-4350. |
| [2] | ZHANG Lihong, JIN Yaoru, CHENG Fangqin. Resource utilization of coal gasification slag [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4447-4457. |
| [3] | YU Shan, DUAN Yuangang, ZHANG Yixin, TANG Chun, FU Mengyao, HUANG Jinyuan, ZHOU Ying. Research progress of catalysts for two-step hydrogen sulfide decomposition to produce hydrogen and sulfur [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3780-3790. |
| [4] | GONG Pengcheng, YAN Qun, CHEN Jinfu, WEN Junyu, SU Xiaojie. Properties and mechanism of eriochrome black T degradation by carbon nanotube-cobalt ferrite composites activated persulfate [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3572-3581. |
| [5] | XU Chunshu, YAO Qingda, LIANG Yongxian, ZHOU Hualong. Effects of graphene oxide/carbon nanotubes on the properties of several typical polymer materials [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3012-3028. |
| [6] | CHANG Zhankun, ZHANG Chi, SU Bingqin, ZHANG Congzheng, WANG Jian, QUAN Xiaohui. Effect of H2S gaseous substrate on sludge bioleaching treatment efficiency [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2733-2743. |
| [7] | GONG Chenjun, MEI Daofeng. Effects of tungsten decoration on the performance of a Ni-based oxygen carrier during chemical looping reforming of biogas for hydrogen generation [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2130-2141. |
| [8] | XING Xianjun, LUO Tian, BU Yuzheng, MA Peiyong. Preparation of biochar from walnut shells activated by H3PO4 and its application in Cr(Ⅵ) adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1527-1539. |
| [9] | XUE Bo, YANG Tingting, WANG Xuefeng. Research progress of polyaniline/carbon nanotube gas sensing materials [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1448-1456. |
| [10] | YUAN Li, WANG Xueqian, LI Xiang, WANG Langlang, MA Yixing, NING ping, XIONG Yiran. Research advances on catalytic removal COS and H2S from by-product gas in iron and steel industry [J]. Chemical Industry and Engineering Progress, 2023, 42(10): 5147-5161. |
| [11] | LIU Yajuan. Research status of membrane fouling mitigation by PAC in submerged PAC-AMBRs [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 457-468. |
| [12] | LIU Nan, HU Yiming, YANG Ying, LI Hongjin, GAO Zhuqing, HAO Xiuli. Microwave assisted co-pyrolysis of waste polypropylene /activated carbon to produce combustible pyrolysis gas and light pyrolysis oil [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 150-159. |
| [13] | CHEN Xiaoyun, GUO Yadong, DI Lu, BI Dongmei, LI Kaikai, LIN Xiaona. Catalytic co-pyrolysis of biomass and plastic for aromatics production with boron doped activated carbon catalyst [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 199-209. |
| [14] | ZHANG Xinhai, ZHAO Sichen, ZHU Hui, WANG Kai, ZHANG Shoushi. Application of activated carbon fiber supported desulfurizer in mine gas environment [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 415-423. |
| [15] | QI Yuan, XU Xinrong, RUAN Wei, WU Hao, WU Ke, ZHOU Yaming, YANG Hongmin. Characterization of aniline adsorption by modified activated carbon fiber [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 622-630. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||