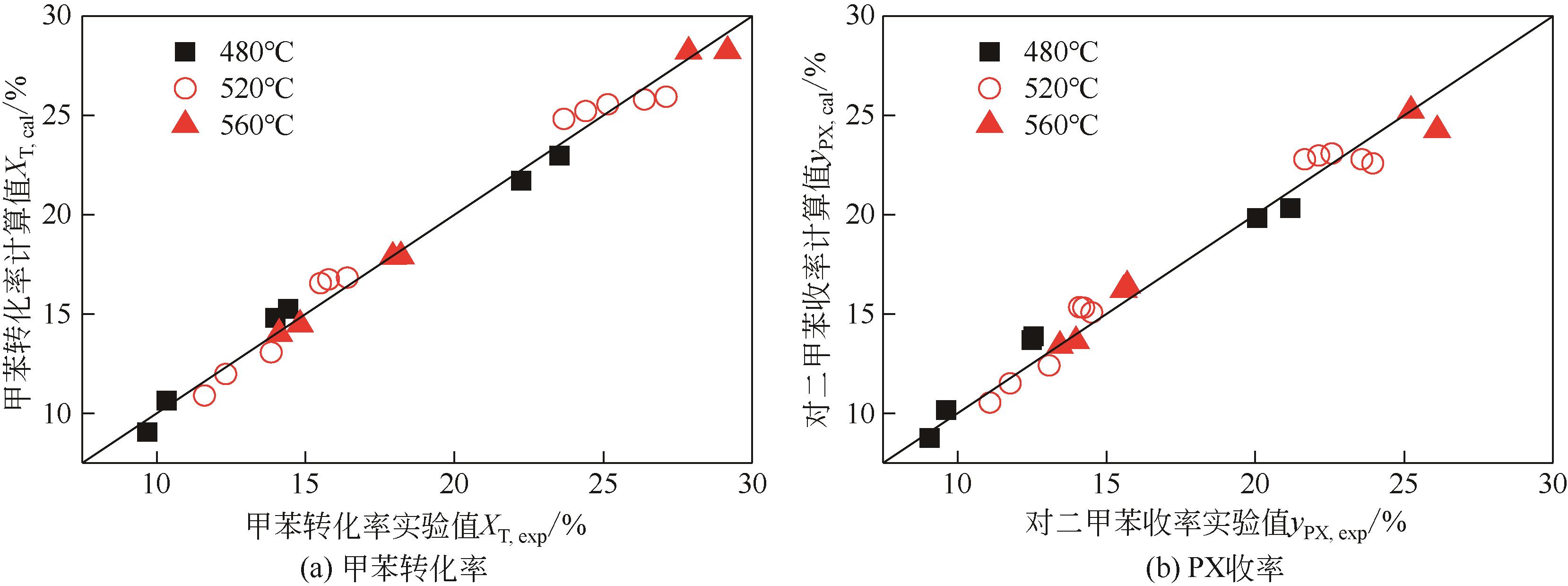
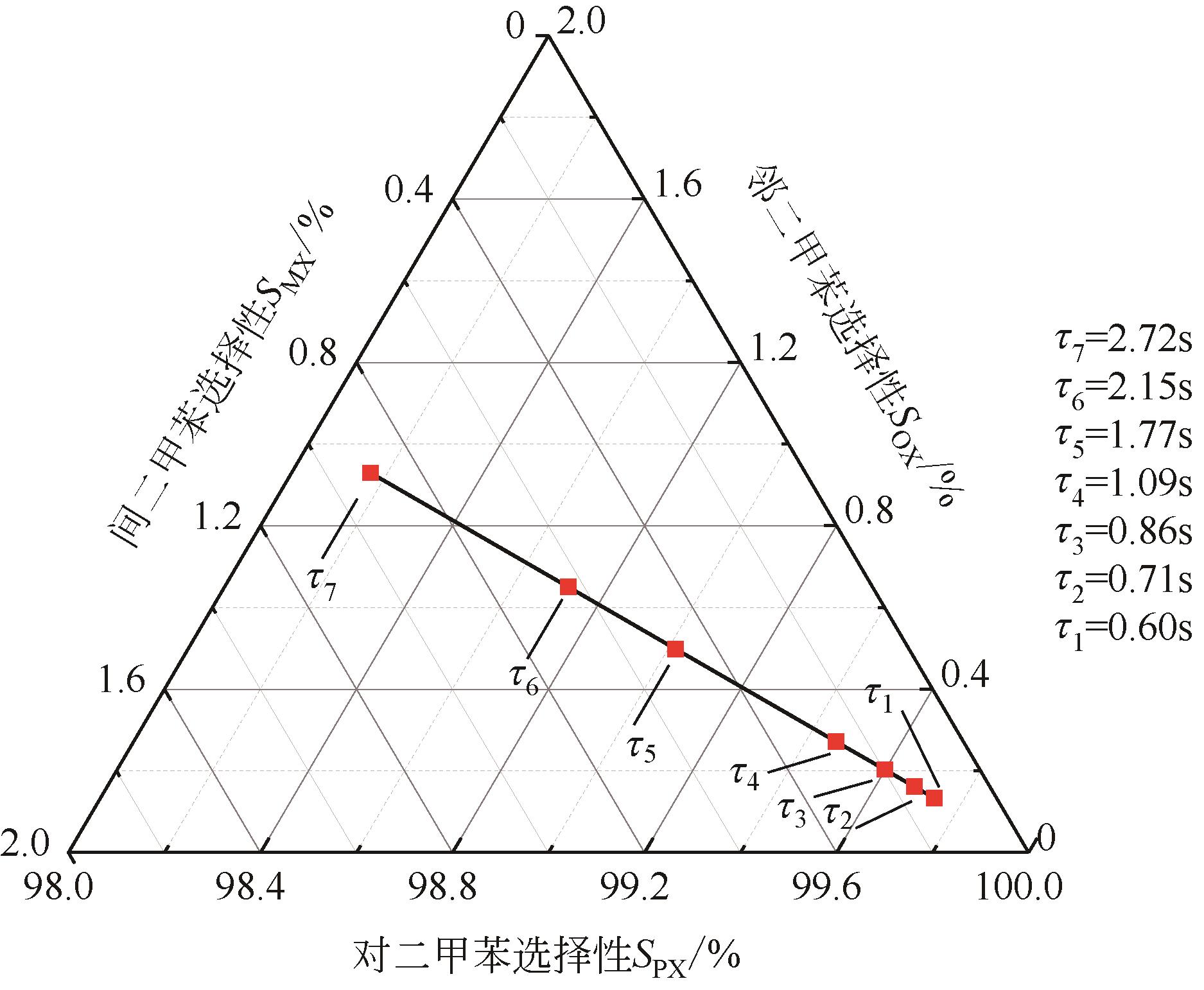
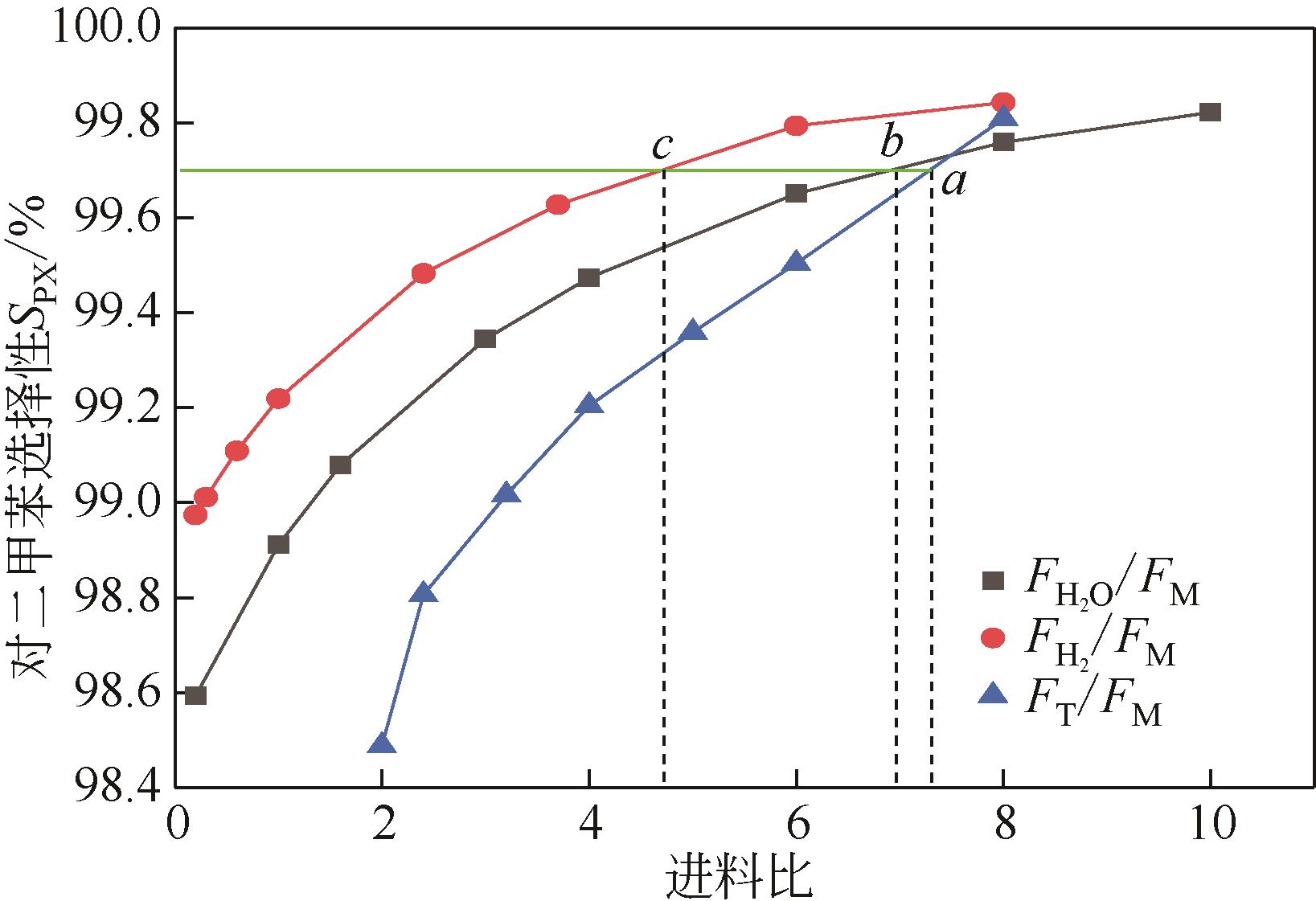
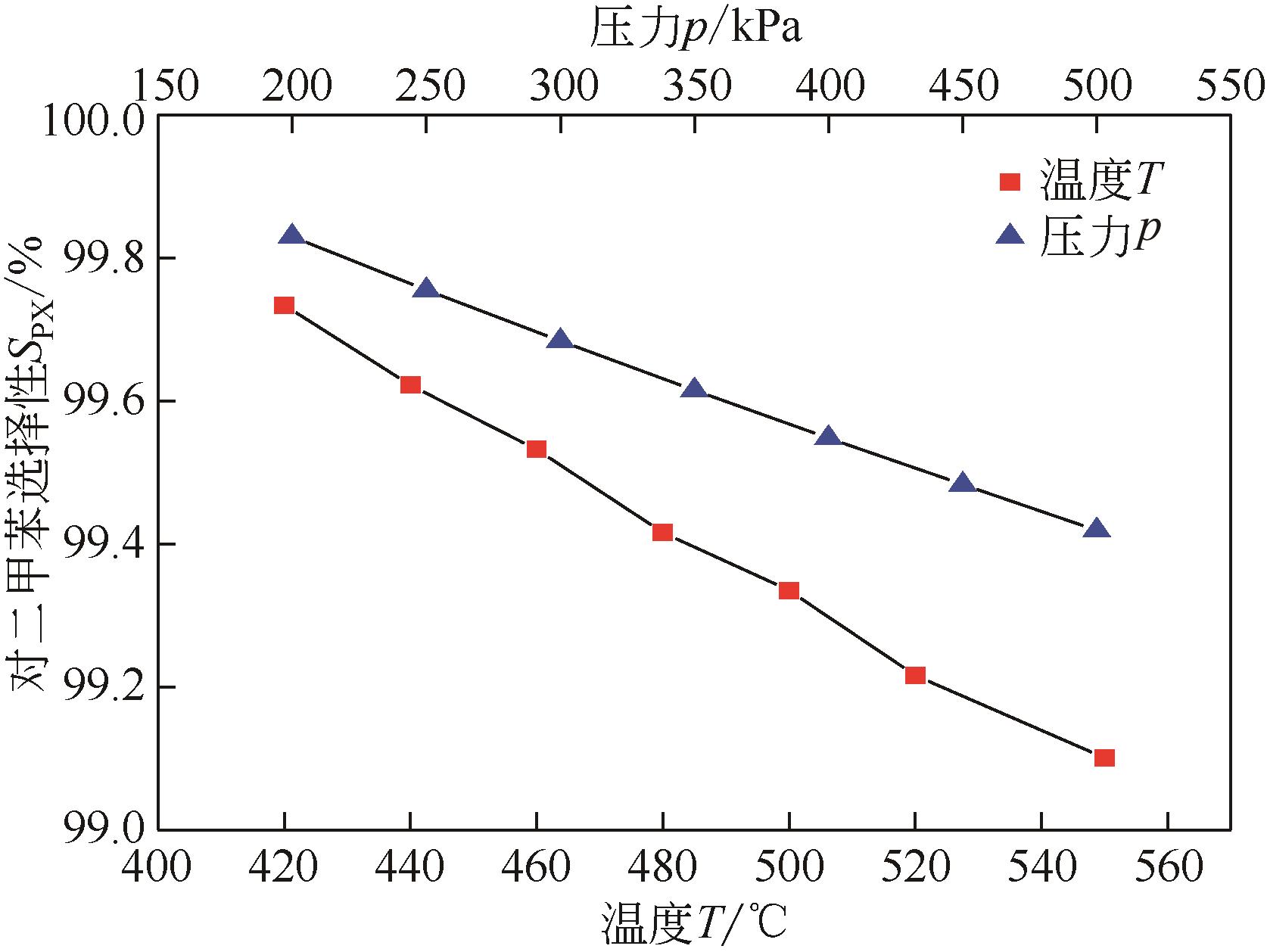
Chemical Industry and Engineering Progress ›› 2022, Vol. 41 ›› Issue (6): 2939-2947.DOI: 10.16085/j.issn.1000-6613.2021-1405
• Chemical processes and equipment • Previous Articles Next Articles
A novel PX production shortcut through PX selectivity intensification in toluene and methanol methylation
LI Guixian1,2( ), ZHANG Junqiang1,2, YANG Yong1,2, FAN Xueying3, WANG Dongliang1,2(
), ZHANG Junqiang1,2, YANG Yong1,2, FAN Xueying3, WANG Dongliang1,2( )
)
- 1.School of Petrochemical Engineering, Lanzhou University of Technology, Lanzhou 730050, Gansu, China
2.Key Laboratory of Low Carbon Energy and Chemical Engineering of Gansu Province, Lanzhou 730050, Gansu, China
3.Automation Institute, PetroChina Lanzhou Petrochemical Company, Lanzhou 730060, Gansu, China
-
Received:2021-07-05Revised:2021-11-11Online:2022-06-21Published:2022-06-10 -
Contact:WANG Dongliang
基于PX选择性强化的短流程甲苯甲醇甲基化PX生产新工艺
李贵贤1,2( ), 张军强1,2, 杨勇1,2, 范学英3, 王东亮1,2(
), 张军强1,2, 杨勇1,2, 范学英3, 王东亮1,2( )
)
- 1.兰州理工大学石油化工学院,甘肃 兰州 730050
2.甘肃省低碳能源化工重点实验室,甘肃 兰州 730050
3.中国石油兰州石化公司自动化研究院,甘肃 兰州 730060
-
通讯作者:王东亮 -
作者简介:李贵贤(1966—),男,博士,教授,博士生导师,研究方向为催化反应工程。E-mail:lgxwyf@163.com 。 -
基金资助:甘肃省科技重大专项(19ZD2GD001);甘肃省高等学校产业支撑计划(2020C-06)
CLC Number:
Cite this article
LI Guixian, ZHANG Junqiang, YANG Yong, FAN Xueying, WANG Dongliang. A novel PX production shortcut through PX selectivity intensification in toluene and methanol methylation[J]. Chemical Industry and Engineering Progress, 2022, 41(6): 2939-2947.
李贵贤, 张军强, 杨勇, 范学英, 王东亮. 基于PX选择性强化的短流程甲苯甲醇甲基化PX生产新工艺[J]. 化工进展, 2022, 41(6): 2939-2947.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2021-1405
| 序号 | 反应方程式 | 反应速率表达式 | 指前因子 | 活化能 |
|---|---|---|---|---|
| R1 | C7H8+CH3OH | 5.66×105 | 76.66 | |
| R2 | p-C8H10 | 5.85×10-2 | 19.24 | |
| R3 | p-C8H10 | 7.71×10-2 | 16.80 | |
| R4 | p-C8H10+CH3OH | 1.16×104 | 57.47 | |
| R5 | o-C8H10+CH3OH | 1.16×104 | 57.47 | |
| R6 | m-C8H10+CH3OH | 1.16×104 | 57.47 | |
| R7 | 2CH3OH | 1.73×104 | 44.94 |
| 序号 | 反应方程式 | 反应速率表达式 | 指前因子 | 活化能 |
|---|---|---|---|---|
| R1 | C7H8+CH3OH | 5.66×105 | 76.66 | |
| R2 | p-C8H10 | 5.85×10-2 | 19.24 | |
| R3 | p-C8H10 | 7.71×10-2 | 16.80 | |
| R4 | p-C8H10+CH3OH | 1.16×104 | 57.47 | |
| R5 | o-C8H10+CH3OH | 1.16×104 | 57.47 | |
| R6 | m-C8H10+CH3OH | 1.16×104 | 57.47 | |
| R7 | 2CH3OH | 1.73×104 | 44.94 |
| 工艺参数 | 优化前 | 优化后 |
|---|---|---|
| 温度/℃ | 493.00 | 470.00 |
| 压力/kPa | 300.00 | 350.00 |
| 空时/g·h·mol-1 | 1.37 | 1.20 |
| 甲苯甲醇进料比 | 6.72 | 8.10 |
| 对二甲苯选择性/% | 99.62 | 99.71 |
| 甲苯转化率/% | 14.57 | 12.00 |
| 甲醇转化率/% | 97.24 | 92.12 |
| 工艺参数 | 优化前 | 优化后 |
|---|---|---|
| 温度/℃ | 493.00 | 470.00 |
| 压力/kPa | 300.00 | 350.00 |
| 空时/g·h·mol-1 | 1.37 | 1.20 |
| 甲苯甲醇进料比 | 6.72 | 8.10 |
| 对二甲苯选择性/% | 99.62 | 99.71 |
| 甲苯转化率/% | 14.57 | 12.00 |
| 甲醇转化率/% | 97.24 | 92.12 |
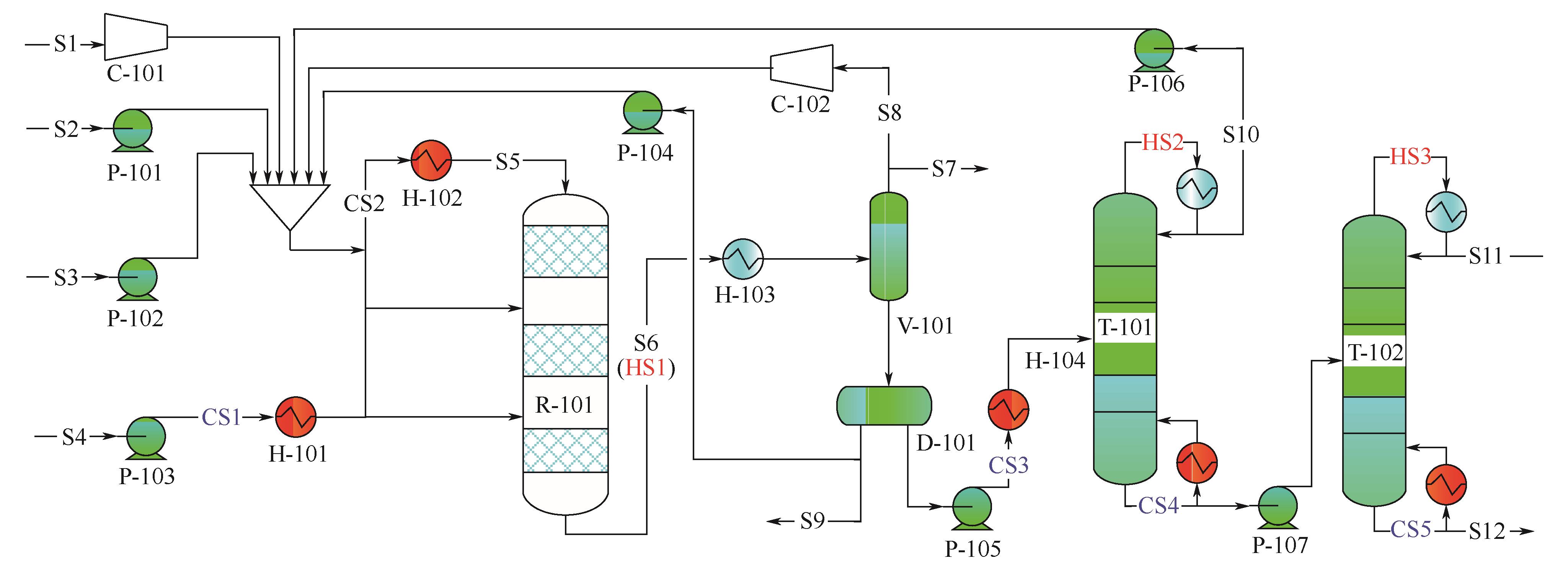
| 设备编号 | 模块名称 | 设备名称 | 工艺参数 |
|---|---|---|---|
| C-101和C-102 | Compr | 单级压缩机 | 级数:单级;出口压力400kPa,效率0.80 |
| H-101 | Heater | 预热器 | 冷物流出口温度90℃ |
| H-102 | Heater | 加热器 | 冷物流出口温度470°C |
| H-103 | Heater | 冷却器 | 热物流出口温度40℃ |
| H-104 | Heater | 预热器 | 冷物流出口温度150℃ |
| P-101~P-107 | Pump | 进料泵/循环泵 | 泵出口压力400kPa,效率0.90 |
| R-101 | Rplug | 甲基化反应器 | 绝热操作,压力350kPa |
| 催化剂:改性高硅铝比HZSM-5 | |||
| V-101 | Flash2 | 闪蒸罐 | 温度40℃,压力300kPa |
| D-101 | Decanter | 析水器 | 绝热操作,压力300kPa |
| T-101 | Radfrac | 甲苯精馏塔 | 操作压力200kPa;塔顶馏出物与进料比0.88 |
| 塔顶冷凝器类型:部分汽-液 | |||
| 理论板数80;进料板位置40;回流比1.55 | |||
| T-102 | Radfrac | 对二甲苯精馏塔 | 操作压力200kPa;塔顶馏出物与进料比0.977 |
| 塔顶冷凝器类型:全回流 | |||
| 理论板数103;进料板位置12;回流比4.129 | |||
| S-101 | FSplit | 分流器 | 弛放分流比0.1 |
| S-102 | FSplit | 分流器 | 弛放分流比0.36 |
| 设备编号 | 模块名称 | 设备名称 | 工艺参数 |
|---|---|---|---|
| C-101和C-102 | Compr | 单级压缩机 | 级数:单级;出口压力400kPa,效率0.80 |
| H-101 | Heater | 预热器 | 冷物流出口温度90℃ |
| H-102 | Heater | 加热器 | 冷物流出口温度470°C |
| H-103 | Heater | 冷却器 | 热物流出口温度40℃ |
| H-104 | Heater | 预热器 | 冷物流出口温度150℃ |
| P-101~P-107 | Pump | 进料泵/循环泵 | 泵出口压力400kPa,效率0.90 |
| R-101 | Rplug | 甲基化反应器 | 绝热操作,压力350kPa |
| 催化剂:改性高硅铝比HZSM-5 | |||
| V-101 | Flash2 | 闪蒸罐 | 温度40℃,压力300kPa |
| D-101 | Decanter | 析水器 | 绝热操作,压力300kPa |
| T-101 | Radfrac | 甲苯精馏塔 | 操作压力200kPa;塔顶馏出物与进料比0.88 |
| 塔顶冷凝器类型:部分汽-液 | |||
| 理论板数80;进料板位置40;回流比1.55 | |||
| T-102 | Radfrac | 对二甲苯精馏塔 | 操作压力200kPa;塔顶馏出物与进料比0.977 |
| 塔顶冷凝器类型:全回流 | |||
| 理论板数103;进料板位置12;回流比4.129 | |||
| S-101 | FSplit | 分流器 | 弛放分流比0.1 |
| S-102 | FSplit | 分流器 | 弛放分流比0.36 |
| 项目 | 物流 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | |
| 温度/℃ | 25.00 | 25.00 | 25.00 | 25.00 | 470.00 | 478.40 | 40.00 | 40.00 | 27.00 | 135.50 | 165.65 | 224.53 |
| 压力/kPa | 101.33 | 101.33 | 101.33 | 101.33 | 350.00 | 350.00 | 300.00 | 300.00 | 300.00 | 200.00 | 200.00 | 400.00 |
| 气相分率 | 1.00 | 0 | 0 | 0 | 1.00 | 1.00 | 1.00 | 1.00 | 0 | 1.00 | 0 | 0 |
| 总摩尔流量/kmol·h-1 | 90.00 | 2371.00 | 185.50 | 187.00 | 9448.66 | 9579.56 | 95.09 | 855.80 | 2555.18 | 1347.72 | 179.13 | 4.12 |
| 组分摩尔流量/kmol·h-1 | ||||||||||||
| H2 | 90.00 | 0 | 0 | 0 | 907.68 | 907.68 | 90.00 | 810.01 | 0 | 7.67 | 0 | 0 |
| C2H4 | 0 | 0 | 0 | 0 | 0.06 | 0.06 | 0 | 0.04 | 0 | 0.02 | 0 | 0 |
| M | 0 | 0 | 0 | 187.00 | 70.90 | 14.82 | 0.02 | 0.17 | 0 | 14.63 | 0 | 0 |
| T | 0 | 0 | 185.50 | 0 | 1523.41 | 1340.26 | 2.20 | 19.76 | 0 | 1318.15 | 0.15 | 0 |
| OX | 0 | 0 | 0 | 0 | 0 | 0.24 | 0 | 0 | 0 | 0 | 0.12 | 0.12 |
| PX | 0 | 0 | 0 | 0 | 0.56 | 179.39 | 0.06 | 0.55 | 0 | 0.01 | 178.59 | 0.18 |
| MX | 0 | 0 | 0 | 0 | 0 | 0.27 | 0 | 0 | 0 | 0 | 0.27 | 0 |
| C9H12 | 0 | 0 | 0 | 0 | 0 | 3.82 | 0 | 0 | 0 | 0 | 0 | 3.82 |
| H2O | 0 | 2371.00 | 0 | 0 | 6946.05 | 7133.03 | 2.81 | 25.27 | 2555.18 | 7.24 | 0 | 0 |
| 项目 | 物流 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | |
| 温度/℃ | 25.00 | 25.00 | 25.00 | 25.00 | 470.00 | 478.40 | 40.00 | 40.00 | 27.00 | 135.50 | 165.65 | 224.53 |
| 压力/kPa | 101.33 | 101.33 | 101.33 | 101.33 | 350.00 | 350.00 | 300.00 | 300.00 | 300.00 | 200.00 | 200.00 | 400.00 |
| 气相分率 | 1.00 | 0 | 0 | 0 | 1.00 | 1.00 | 1.00 | 1.00 | 0 | 1.00 | 0 | 0 |
| 总摩尔流量/kmol·h-1 | 90.00 | 2371.00 | 185.50 | 187.00 | 9448.66 | 9579.56 | 95.09 | 855.80 | 2555.18 | 1347.72 | 179.13 | 4.12 |
| 组分摩尔流量/kmol·h-1 | ||||||||||||
| H2 | 90.00 | 0 | 0 | 0 | 907.68 | 907.68 | 90.00 | 810.01 | 0 | 7.67 | 0 | 0 |
| C2H4 | 0 | 0 | 0 | 0 | 0.06 | 0.06 | 0 | 0.04 | 0 | 0.02 | 0 | 0 |
| M | 0 | 0 | 0 | 187.00 | 70.90 | 14.82 | 0.02 | 0.17 | 0 | 14.63 | 0 | 0 |
| T | 0 | 0 | 185.50 | 0 | 1523.41 | 1340.26 | 2.20 | 19.76 | 0 | 1318.15 | 0.15 | 0 |
| OX | 0 | 0 | 0 | 0 | 0 | 0.24 | 0 | 0 | 0 | 0 | 0.12 | 0.12 |
| PX | 0 | 0 | 0 | 0 | 0.56 | 179.39 | 0.06 | 0.55 | 0 | 0.01 | 178.59 | 0.18 |
| MX | 0 | 0 | 0 | 0 | 0 | 0.27 | 0 | 0 | 0 | 0 | 0.27 | 0 |
| C9H12 | 0 | 0 | 0 | 0 | 0 | 3.82 | 0 | 0 | 0 | 0 | 0 | 3.82 |
| H2O | 0 | 2371.00 | 0 | 0 | 6946.05 | 7133.03 | 2.81 | 25.27 | 2555.18 | 7.24 | 0 | 0 |
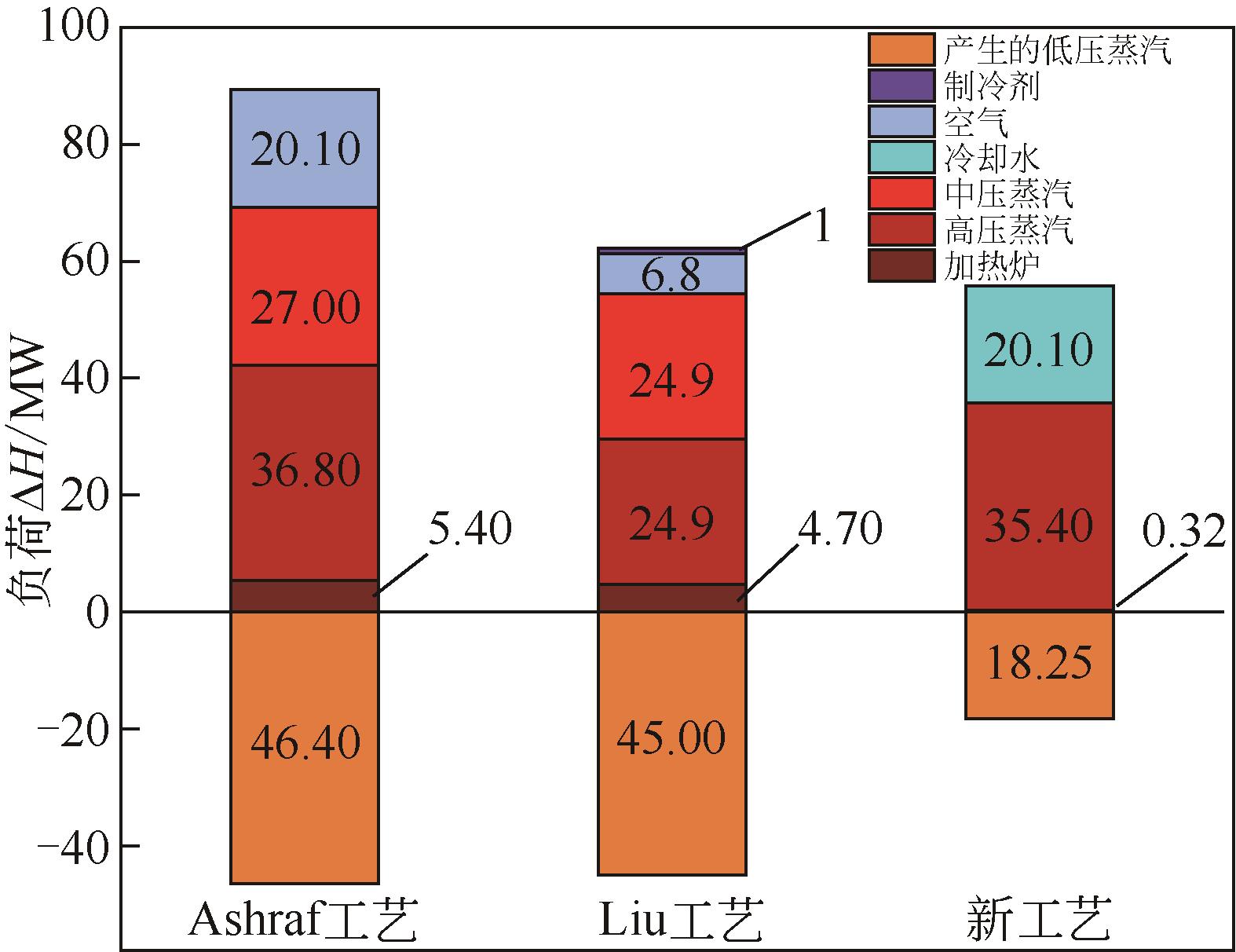
| 工艺 | 原料/kmol·h-1 | 对二甲苯 /kmol·h-1 | 甲苯 有效利用率/% | 甲醇 有效利用率/% | |
|---|---|---|---|---|---|
| 甲苯 | 甲醇 | ||||
| Ashraf工艺[ | 215.24 | 393.65 | 178.59 | 82.97 | 45.36 |
| Liu工艺[ | 215.24 | 393.00 | 178.37 | 82.87 | 45.38 |
| 新工艺 | 185.50 | 187.00 | 178.59 | 96.27 | 95.50 |
| 工艺 | 原料/kmol·h-1 | 对二甲苯 /kmol·h-1 | 甲苯 有效利用率/% | 甲醇 有效利用率/% | |
|---|---|---|---|---|---|
| 甲苯 | 甲醇 | ||||
| Ashraf工艺[ | 215.24 | 393.65 | 178.59 | 82.97 | 45.36 |
| Liu工艺[ | 215.24 | 393.00 | 178.37 | 82.87 | 45.38 |
| 新工艺 | 185.50 | 187.00 | 178.59 | 96.27 | 95.50 |
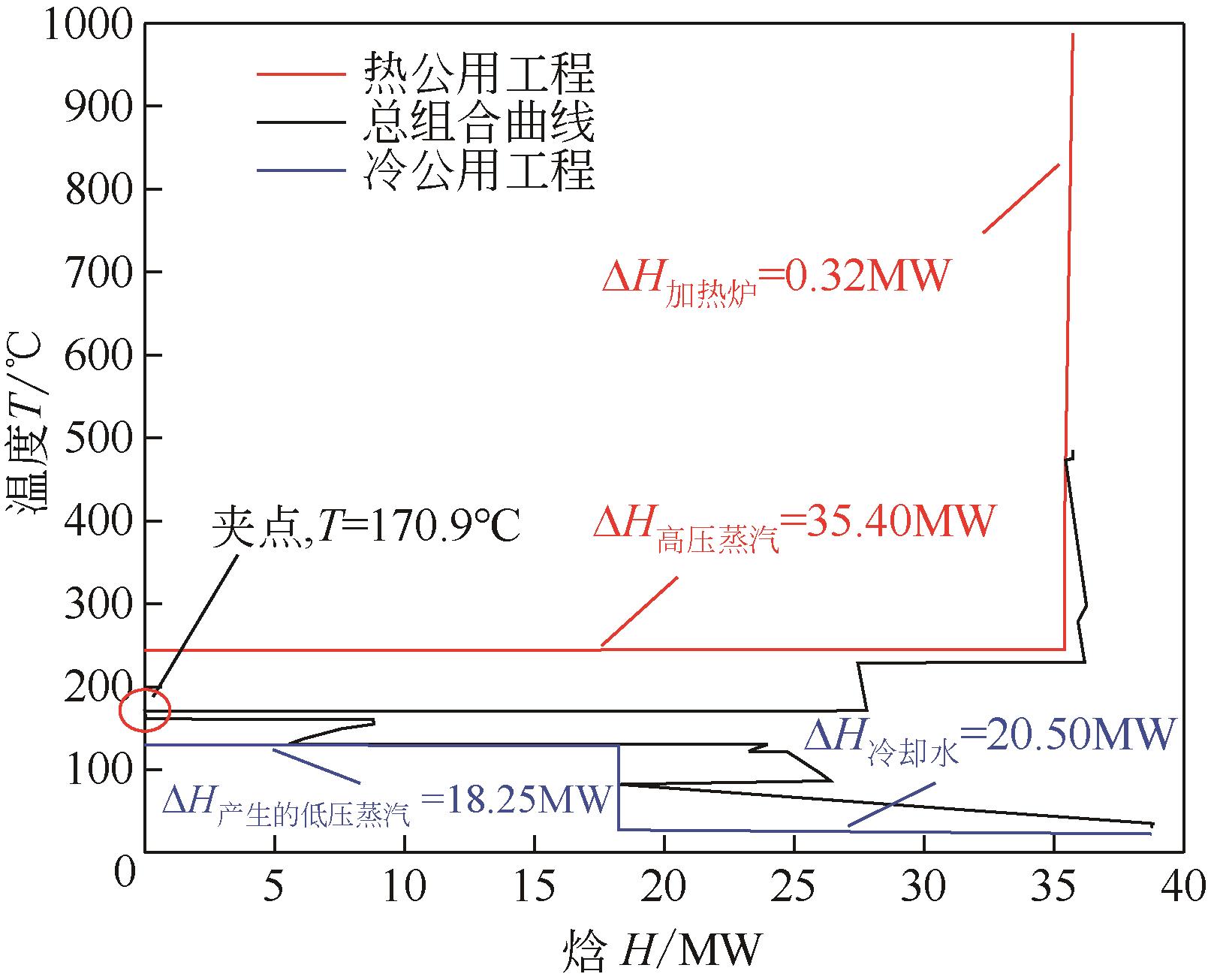
| 流股名称 | 流股位置 | 起始温度 /℃ | 目标温度 /℃ | 热负荷/MW |
|---|---|---|---|---|
| CS1 | 甲醇进料预热 | 25.00 | 90.00 | 0.39 |
| CS2 | 反应器进料 | 77.00 | 470.00 | 147.20 |
| CS3 | H-104进料预热器 | 28.90 | 150.00 | 11.06 |
| CS4 | T-101塔釜再沸器 | 165.90 | 166.10 | 27.80 |
| CS5 | T-102塔釜再沸器 | 224.00 | 224.50 | 8.74 |
| HS1 | 反应器出口 | 479.00 | 40.00 | 171.20 |
| HS2 | T-101塔顶冷凝器 | 136.10 | 135.50 | 18.46 |
| HS3 | T-102塔顶冷凝器 | 166.20 | 165.70 | 8.73 |
| 流股名称 | 流股位置 | 起始温度 /℃ | 目标温度 /℃ | 热负荷/MW |
|---|---|---|---|---|
| CS1 | 甲醇进料预热 | 25.00 | 90.00 | 0.39 |
| CS2 | 反应器进料 | 77.00 | 470.00 | 147.20 |
| CS3 | H-104进料预热器 | 28.90 | 150.00 | 11.06 |
| CS4 | T-101塔釜再沸器 | 165.90 | 166.10 | 27.80 |
| CS5 | T-102塔釜再沸器 | 224.00 | 224.50 | 8.74 |
| HS1 | 反应器出口 | 479.00 | 40.00 | 171.20 |
| HS2 | T-101塔顶冷凝器 | 136.10 | 135.50 | 18.46 |
| HS3 | T-102塔顶冷凝器 | 166.20 | 165.70 | 8.73 |
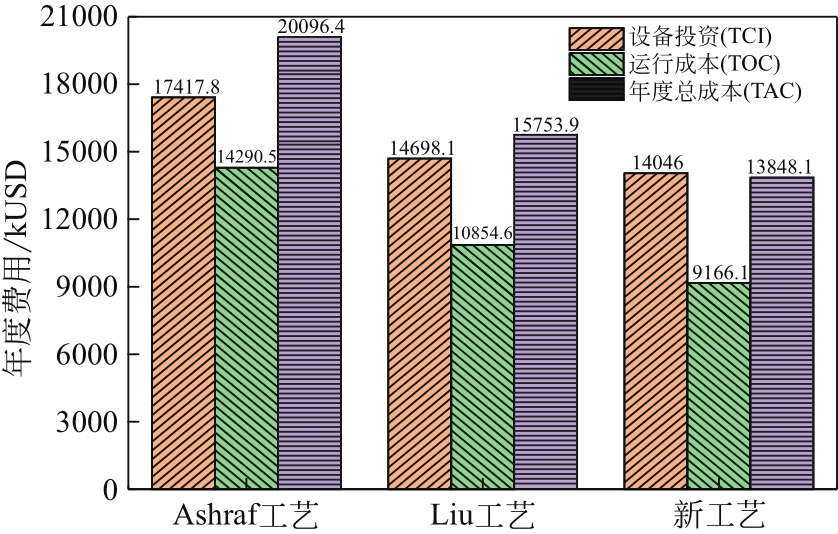
| 项目 | 费用 |
|---|---|
| TCI | |
| 塔壳成本/kUSD | |
| 塔盘成本/kUSD | |
| 塔径D/m | Aspen tray sizing |
| 塔高H/m | |
| 换热器成本/USD | |
| 换热面积A/m2 | |
| TOC | |
| 加热炉/USD·MW-1·a-1 | 17.1 |
| 急冷水/USD·MW-1·a-1 | 0.36 |
| 电费/USD·(kW·h·a)-1 | 0.132 |
| 高压蒸汽/USD·MW-1·a-1 | 35.6 |
| 中压蒸汽/USD·MW-1·a-1 | 29.6 |
| 低压蒸汽/USD·MW-1·a-1 | 28.0 |
| 总年度成本TAC/kUSD·a-1 |
| 项目 | 费用 |
|---|---|
| TCI | |
| 塔壳成本/kUSD | |
| 塔盘成本/kUSD | |
| 塔径D/m | Aspen tray sizing |
| 塔高H/m | |
| 换热器成本/USD | |
| 换热面积A/m2 | |
| TOC | |
| 加热炉/USD·MW-1·a-1 | 17.1 |
| 急冷水/USD·MW-1·a-1 | 0.36 |
| 电费/USD·(kW·h·a)-1 | 0.132 |
| 高压蒸汽/USD·MW-1·a-1 | 35.6 |
| 中压蒸汽/USD·MW-1·a-1 | 29.6 |
| 低压蒸汽/USD·MW-1·a-1 | 28.0 |
| 总年度成本TAC/kUSD·a-1 |
| 1 | 顾祥万. 对二甲苯市场分析及发展建议[J]. 化工进展, 2014, 33(6): 1628-1631. |
| GU Xiangwan. Paraxylene market analysis and development proposal[J]. Chemical Industry and Engineering Progress, 2014, 33(6):1628-1631. | |
| 2 | ZHANG Jingui, QIAN Weizhong, KONG Chuiyan, et al. Increasing para-xylene selectivity in making aromatics from methanol with a surface-modified Zn/P/ZSM-5 catalyst[J]. ACS Catalysis, 2015, 5(5): 2982-2988. |
| 3 | 陈嵩嵩, 张国帅, 霍锋, 等. 煤基大宗化学品市场及产业发展趋势[J]. 化工进展, 2020, 39(12): 5009-5020. |
| CHEN Songsong, ZHANG Guoshuai, HUO Feng, et al. Market and technology development trends of coal-based bulk chemicals[J]. Chemical Industry and Engineering Progress, 2020, 39(12): 5009-5020. | |
| 4 | 于政锡, 徐庶亮, 张涛, 等. 对二甲苯生产技术研究进展及发展趋势[J]. 化工进展, 2020, 39(12): 4984-4992. |
| YU Zhengxi, XU Shuliang, ZHANG Tao, et al. Research progress and development trend in para-xylene production technology[J]. Chemical Industry and Engineering Progress, 2020, 39(12): 4984-4992. | |
| 5 | CHAKINALA Nandana, CHAKINALA Anand. Process design strategies to produce p-xylene via toluene methylation: a review[J]. Industrial & Engineering Chemistry Research, 2021, 60(15): 5331-5351. |
| 6 | 张玉黎, 徐庶亮, 叶茂. 甲醇甲苯烷基化流化床反应器的数值模拟[J]. 化工进展, 2020, 39(12): 5057-5065. |
| ZHANG Yuli, XU Shuliang, YE Mao. A numerical investigation on alkylation of toluene with methanol in fluidized bed reactor[J]. Chemical Industry and Engineering Progress, 2020, 39(12): 5057-5065. | |
| 7 | CHEN Qingteng, LIU Jian, YANG Bo. Identifying the key steps determining the selectivity of toluene methylation with methanol over HZSM-5[J]. Nature Communications, 2021, 12(1): 3725. |
| 8 | 代成义, 陈中顺, 杜康, 等. 甲醇制芳烃催化剂及相关工艺研究进展[J]. 化工进展, 2020, 39(12): 5029-5041. |
| DAI Chengyi, CHEN Zhongshun, DU Kang, et al. Research progress of catalysts and related technologies for methanol to aromatics[J]. Chemical Industry and Engineering Progress, 2020, 39(12): 5029-5041. | |
| 9 | HUANG Xin, WANG Ruizhuang, PAN Xu, et al. Catalyst design strategies towards highly shape-selective HZSM-5 for para-xylene through toluene alkylation[J]. Green Energy & Environment, 2020, 5(4): 385-393. |
| 10 | ZHANG Chundong, KWAK Geunjae, LEE Yun-Jo, et al. Light hydrocarbons to BTEX aromatics over Zn-modified hierarchical ZSM-5 combined with enhanced catalytic activity and stability[J]. Microporous and Mesoporous Materials, 2019, 284: 316-326. |
| 11 | 张志萍, 赵岩, 吴宏宇, 等. 改性纳米HZSM-5催化剂上甲苯与甲醇的烷基化反应[J]. 催化学报, 2011, 32(7): 1280-1286. |
| ZHANG Zhiping, ZHAO Yan, WU Hongyu, et al. Shape-selective alkylation of toluene with methanol over modified nano-scale HZSM-5 zeolite[J]. Chinese Journal of Catalysis, 2011, 32(7): 1280-1286. | |
| 12 | ASHRAF Muhammad T, CHEBBI Rachid, DARWISH Naif A. Process of p-xylene production by highly selective methylation of toluene[J]. Industrial and Engineering Chemistry Research, 2013, 52(38): 13730-13737. |
| 13 | LIU Jing, YANG Yu, WEI Shun’an, et al. Intensified p-xylene production process through toluene and methanol alkylation[J]. Industrial & Engineering Chemistry Research, 2018, 57(38): 12829-12841. |
| 14 | BREEN John, BURCH Robbie, KULKARNI Manisha, et al. Enhanced para-xylene selectivity in the toluene alkylation reaction at ultralow contact time[J]. Journal of the American Chemical Society, 2005, 127(14): 5020-5021. |
| 15 | BREEN John, BURCH Robbie, COLLIER Paul, et al. Improved selectivity in the toluene alkylation reaction through understanding and optimising the process variables[J]. Applied Catalysis A: General, 2007, 316(1): 53-60. |
| 16 | FAN Zongliang, SU Xin, WANG Dongliang, et al. Simulation of catalytic toluene alkylation with methanol in fixed-bed reactors[J]. International Journal of Chemical Kinetics, 2021, 53(4): 558-568. |
| 17 | 谭远婷, 祝然, 张新平, 等. 甲苯甲醇烷基化制对二甲苯动力学模型[J]. 化学反应工程与工艺, 2016, 32(2): 120-128. |
| TAN Yuanting, ZHU Ran, ZHANG Xinping, et al. Kinetic model of toluene alkylation with methanol to para-xylene[J]. Chemical Reaction Engineering and Technology, 2016, 32(2): 120-128. | |
| 18 | CHOUDHARY Vasant, NAYAK Vikram, CHOUDHARY Tushar. Single-component sorption/diffusion of cyclic compounds from their bulk liquid phase in H-ZSM-5 zeolite[J]. Industrial & Engineering Chemistry Research, 1997, 36(5): 1812-1818. |
| 19 | ALABI Wahab, ATANDA Luqman, JERMY Rabindran, et al. Kinetics of toluene alkylation with methanol catalyzed by pure and hybridized HZSM-5 catalysts[J]. Chemical Engineering Journal, 2012, 195/196: 276-288. |
| 20 | 唐建远, 娄报华, 宁春利, 等. 改性的高硅铝比的HZSM-5催化剂上甲苯与甲醇的择形烷基化制对二甲苯[J]. 复旦学报(自然科学版), 2013, 52(1): 23-29. |
| TANG Jianyuan, LOU Baohua, NING Chunli, et al. Shape-selective alkylation of toluene and methanol to p-xylene over modified HZSM-5 catalyst with high Si/Al molar ratios[J]. Journal of Fudan University (Natural Science), 2013, 52(1): 23-29. | |
| 21 | 娄报华. 甲苯甲醇烷基化合成对二甲苯催化剂研究[D]. 上海: 华东理工大学, 2014. |
| LOU Baohua. Catalyst of toluene alkylation with methanol to p-xylene[D]. Shanghai: East China University of Science and Technology, 2014. |
| [1] | YANG Jianping. PSE for feedstock consumption reduction in reaction system of HPPO plant [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 21-32. |
| [2] | SUN Yuyu, CAI Xinlei, TANG Jihai, HUANG Jingjing, HUANG Yiping, LIU Jie. Optimization and energy-saving of a reactive distillation process for the synthesis of methyl methacrylate [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 56-63. |
| [3] | WANG Chen, BAI Haoliang, KANG Xue. Performance study of high power UV-LED heat dissipation and nano-TiO2 photocatalytic acid red 26 coupling system [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4905-4916. |
| [4] | LIU Xuanlin, WANG Yikai, DAI Suzhou, YIN Yonggao. Analysis and optimization of decomposition reactor based on ammonium carbamate in heat pump [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4522-4530. |
| [5] | DONG Jiayu, WANG Simin. Experimental on ultrasound enhancement of para-xylene crystallization characteristics and regulation mechanism [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4504-4513. |
| [6] | LI Lanyu, HUANG Xinye, WANG Xiaonan, QIU Tong. Reflection and prospects on the intelligent transformation of chemical engineering research [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3325-3330. |
| [7] | WANG Junjie, PAN Yanqiu, NIU Yabin, YU Lu. Molecular level catalytic reforming model construction and application [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3404-3412. |
| [8] | XUE Kai, WANG Shuai, MA Jinpeng, HU Xiaoyang, CHONG Daotong, WANG Jinshi, YAN Junjie. Planning and dispatch of distributed integrated energy systems for industrial parks [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3510-3519. |
| [9] | GU Shiya, DONG Yachao, LIU Linlin, ZHANG Lei, ZHUANG Yu, DU Jian. Design and optimization of pipeline system for carbon capture considering intermediate nodes [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2799-2808. |
| [10] | LI Xue, WANG Yanjun, WANG Yuchao, TAO Shengyang. Recent advances in bionic surfaces for fog collection [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2486-2503. |
| [11] | GE Weitong, LIAO Yalong, LI Mingyuan, JI Guangxiong, XI Jiajun. Preparation and dechlorination kinetics of Pd-Fe/MWCNTs bimetallic catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 1885-1894. |
| [12] | SUN Xiao, ZHU Guangtao, PEI Aiguo. Industrialization and research progress of hydrogen liquefier [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1103-1117. |
| [13] | ZOU Yincai, LI Qingguo, WU Hui, ZHONG Xiaobing, CHEN Xianzhi. Heat transfer simulation and optimization of missile borne phase change heat sink [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1248-1256. |
| [14] | ZENG Siying, YANG Minbo, FENG Xiao. Machine learning-based prediction of coalbed methane composition and real-time optimization of liquefaction process [J]. Chemical Industry and Engineering Progress, 2023, 42(10): 5059-5066. |
| [15] | YAN Zihan, CHEN Qunyun, LI Zhuo, FU Rongbing, LI Yanwei, WU Zhigen. Numerical analysis and optimization of the performance of an improved soil crushing and mixing structure [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 72-80. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||