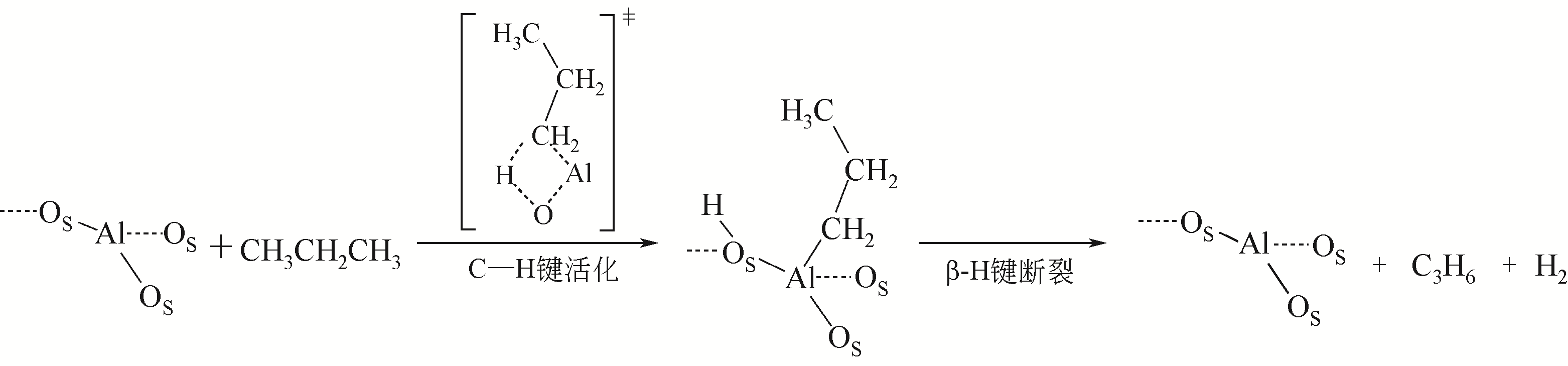
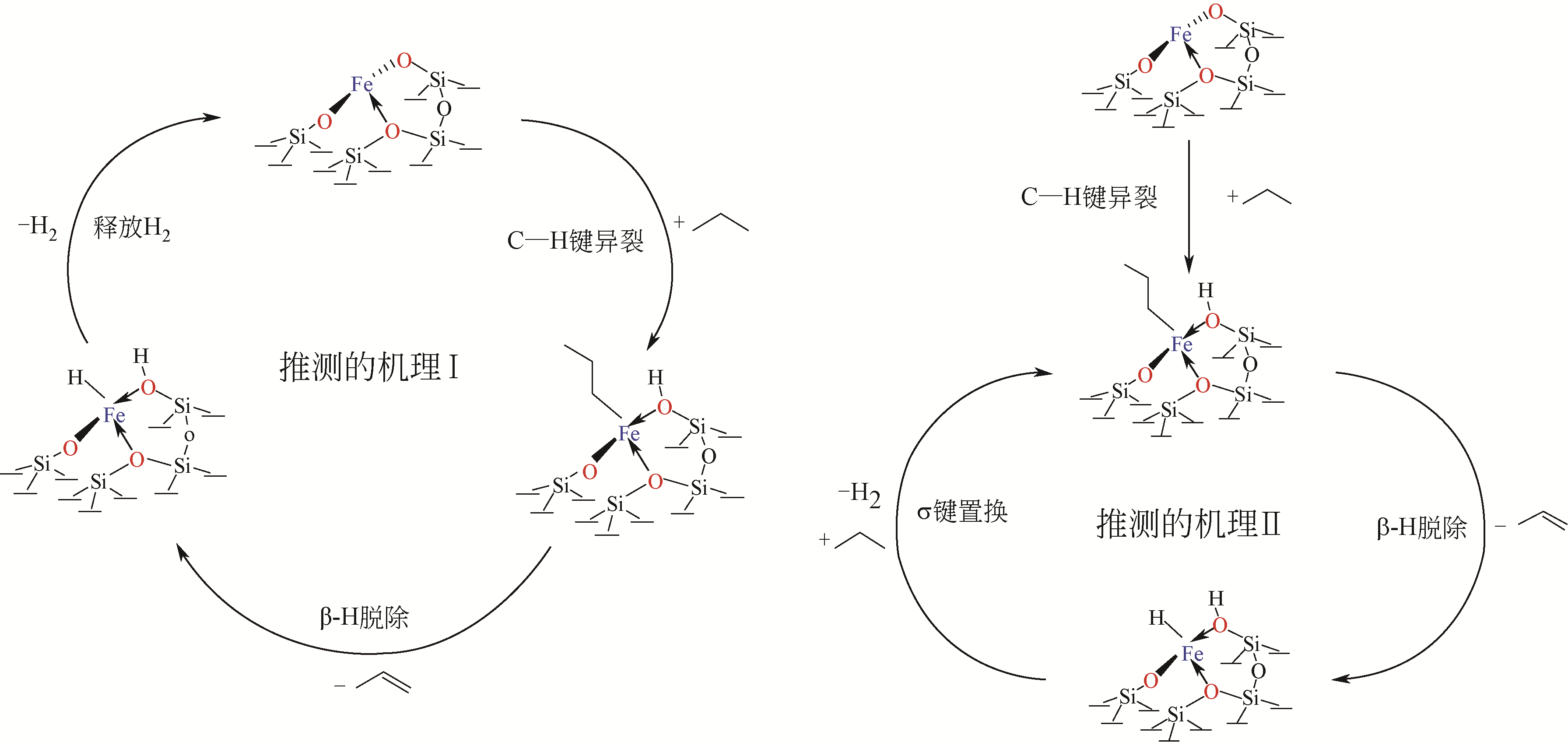
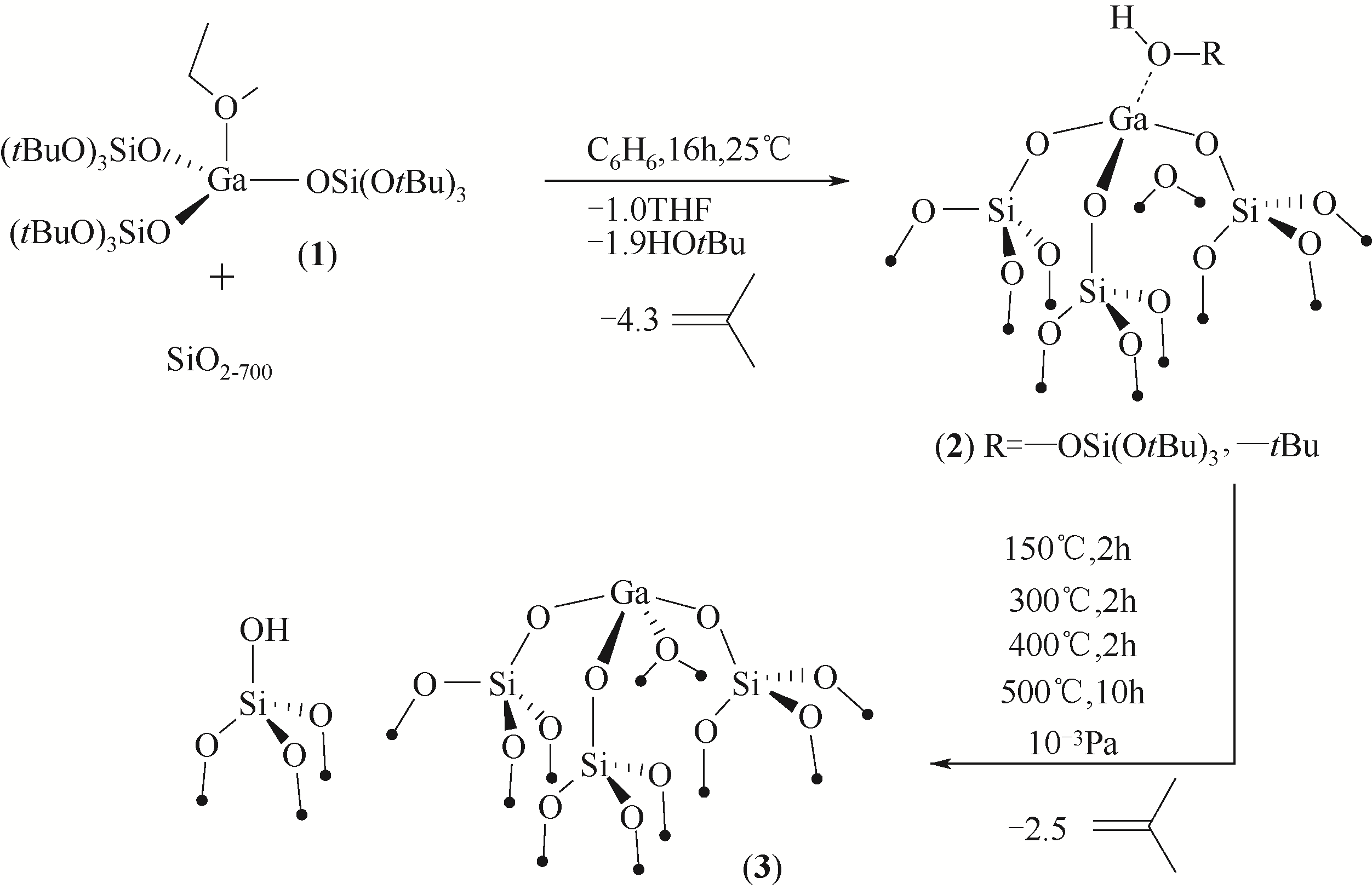
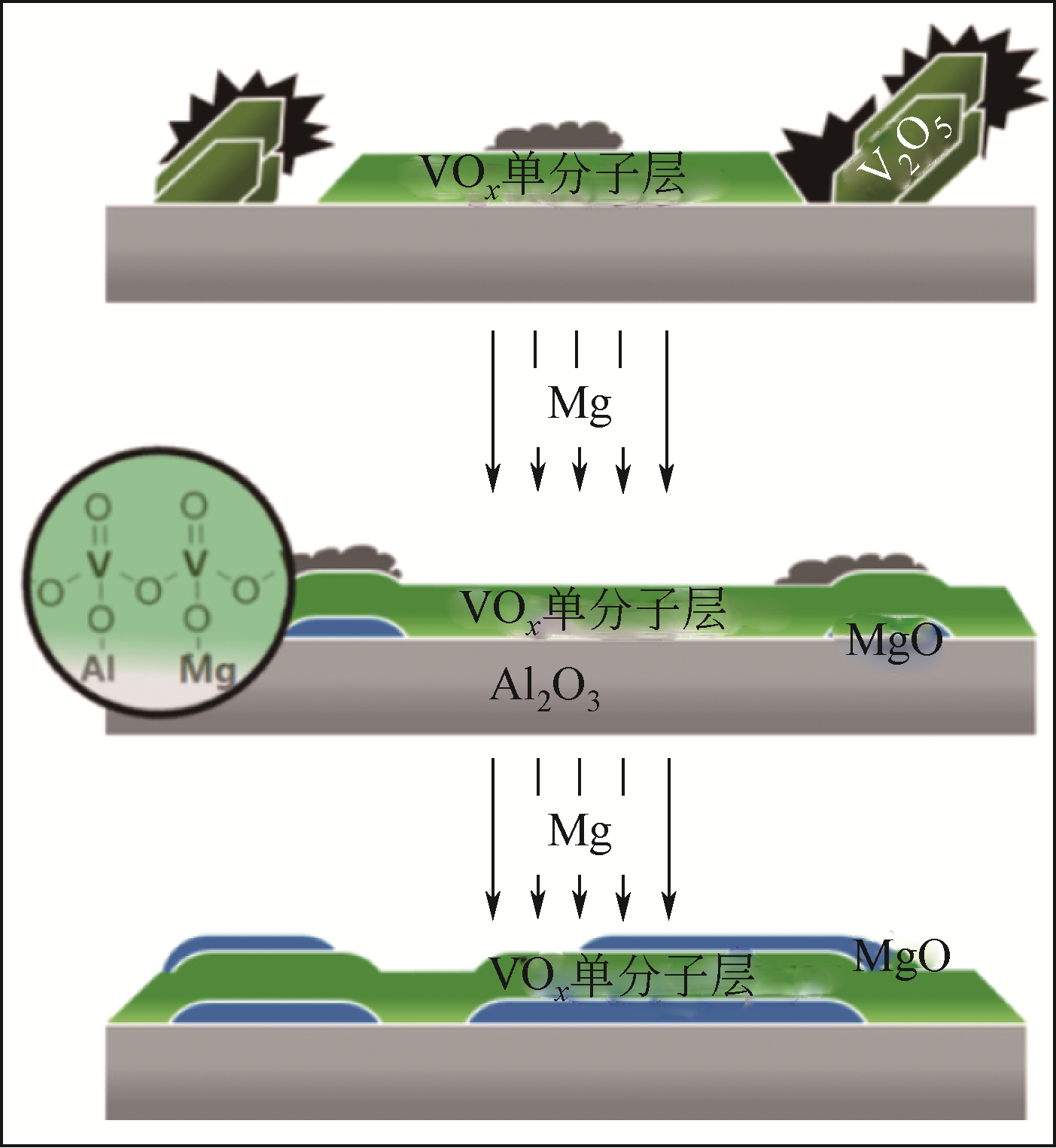
Chemical Industry and Engineering Progress ›› 2021, Vol. 40 ›› Issue (4): 1893-1916.DOI: 10.16085/j.issn.1000-6613.2020-1990
• Special column:Industrial catalysis • Previous Articles Next Articles
Advances in catalysts for propane dehydrogenation to propylene
XU Zhikang( ), HUANG Jialu, WANG Tinghai, YUE Yuanyuan, BAI Zhengshuai, BAO Xiaojun, ZHU Haibo(
), HUANG Jialu, WANG Tinghai, YUE Yuanyuan, BAI Zhengshuai, BAO Xiaojun, ZHU Haibo( )
)
- College of Chemical Engineering, Fuzhou University, Fuzhou 350108, Fujian, China
-
Received:2020-09-30Online:2021-04-14Published:2021-04-05 -
Contact:ZHU Haibo
丙烷脱氢制丙烯催化剂的研究进展
徐志康( ), 黄佳露, 王廷海, 岳源源, 白正帅, 鲍晓军, 朱海波(
), 黄佳露, 王廷海, 岳源源, 白正帅, 鲍晓军, 朱海波( )
)
- 福州大学石油化工学院,福建 福州 350108
-
通讯作者:朱海波 -
作者简介:徐志康(1993—),男,博士研究生,研究方向为丙烷脱氢。E-mail:1392098485@qq.com 。 -
基金资助:国家自然科学基金(21878050);高等学校学科创新引智计划(“111计划”)(D17005)
CLC Number:
Cite this article
XU Zhikang, HUANG Jialu, WANG Tinghai, YUE Yuanyuan, BAI Zhengshuai, BAO Xiaojun, ZHU Haibo. Advances in catalysts for propane dehydrogenation to propylene[J]. Chemical Industry and Engineering Progress, 2021, 40(4): 1893-1916.
徐志康, 黄佳露, 王廷海, 岳源源, 白正帅, 鲍晓军, 朱海波. 丙烷脱氢制丙烯催化剂的研究进展[J]. 化工进展, 2021, 40(4): 1893-1916.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2020-1990
| 催化剂 | 反应温度 /℃ | 反应原料比 | 总流速 /mL·min-1 | 质量空速WHSV | 丙烷始-末 转化率/% | 丙烯始-末 选择性/% | 反应时间 TOS/h | 失活速率Kd/h-1 | 制备方法 | 参考 文献 |
|---|---|---|---|---|---|---|---|---|---|---|
| Pt0Gaδ+/SiO2 | 550 | C3H8/Ar=1∶4 | 50 | 98.3 | 31.9~18.2 | 约99 | 20 | 0.04 | 表面化学法 | [ |
| PtSn/TS-1 | 590 | C3H8∶H2∶N2=1∶1∶4 | — | 3 | 53~47.5 | 92.5~93 | 7 | 0.03 | 水热+浸渍法 | [ |
| Pt/Al2O3 | 520 | C3H8/H2/N2=4∶4∶17 | 50 | 4.0 | 5.8~2.6 | 70~68 | 12 | 0.070 | 浸渍法 | [ |
| PtCu/Al2O3 | 520 | C3H8/H2/N2=4∶4∶17 | 50 | 4.0 | 13.1~12.4 | 87~89 | 120 | 0.0005 | 浸渍法 | [ |
| PtGa-Pb/SiO2 | 600 | C3H8/H2/He=5∶3.9∶40 | — | 30.6 | 27~31 | >99.6 | 50 | 0.0038 | 共沉淀法 | [ |
| PtSn/Mg(Al)O-sv | 550 | C3H8=20 | — | 1.13 | 27.7~27.8 | >96.6 | 5 | 0.001 | 浸渍法 | [ |
| PtIn/Mg(Al)O-4 | 620 | C3H8/H2/Ar=8∶7∶35 | — | 3.3 | 45~61.3 | 95.3~96.2 | 12 | — | 浸渍法 | [ |
| PtSn3/Al2O3 | 550 | C3H8/H2/N2=1∶1∶8 | 80 | 11.8 | 40~38 | >99.5 | 72 | 0.0012 | 表面化学法 | [ |
| Pt0Znδ+/SiO2 | 550 | C3H8/Ar=1∶4 | 50 | 32 | 35.3~26.6 | 97.6~96.3 | 30 | 0.014 | 表面化学法 | [ |
| Pt0Znδ+/SiO2 | 550 | C3H8/Ar=1∶4 | 50 | 75 | 30.2~16.2 | 98.1~95 | 30 | 0.027 | 表面化学法 | [ |
| PtSn/Al2O3片 | 590 | C3H8/H2/N2=1∶1.25∶8 | — | 9.4 | 48.7~44.6 | 约99.1 | 24 | 0.007 | 浸渍法 | [ |
| Pt-Sn/SiO2(1073K H2) | 500 | C3H8/N2=4∶1 | 100 | 47 | 27~12 | 90.9~99.5 | 3 | 0.33 | 浸渍法 | [ |
| Pt/Mg(In)(Al)O | 550 | C3H8=20 | — | 1.57 | 24.2~17.5 | >98.2 | 5 | 0.081 | 共沉淀法 | [ |
| Pt/Mg(Sn)(Al)O | 550 | C3H8/H2/N2=1∶0.5∶2 | — | 14 | 29.4~27.8 | 93.7~99.2 | 240 | 0.0067 | 浸渍法 | [ |
| Pt/Mg(Sn)(Al)O | 600 | C3H8/H2/N2=1∶0.5∶2 | — | 14 | 48.3~43.0 | 86.4~98.1 | 48 | 0.11 | 浸渍法 | [ |
| Pt/Sn2-Beta | 580 | C3H8/H2/N2=1∶1∶8 | 60 | 141 | 50~44 | 95~98 | 48 | 0.006 | 表面化学法 | [ |
| PtZnx@S-1-H | 550 | C3H8/N2=10∶30 | 40 | 3.6 | 47.4~40.4 | 93.2~99.2 | 217 | 0.001 | 原位合成法 | [ |
| PtZn@S-1 | 550 | C3H8/N2=11∶19 | 30 | 6.5 | 45.3~41.5 | >99 | 60 | 0.002 | 原位合成法 | [ |
| K-PtSn@MFI | 650 | C3H8/N2=5∶16 | 21 | 29.5 | 54~37 | >99 | 70 | 0.099 | 原位合成法 | [ |
| K-PtSn@MFI-(600H2-22h) | 600 | C3H8/N2=5∶16.5 | 21.5 | 29.5 | 38.7~31.9 | >97 | 25 | 0.012 | 原位合成法 | [ |
| 催化剂 | 反应温度 /℃ | 反应原料比 | 总流速 /mL·min-1 | 质量空速WHSV | 丙烷始-末 转化率/% | 丙烯始-末 选择性/% | 反应时间 TOS/h | 失活速率Kd/h-1 | 制备方法 | 参考 文献 |
|---|---|---|---|---|---|---|---|---|---|---|
| Pt0Gaδ+/SiO2 | 550 | C3H8/Ar=1∶4 | 50 | 98.3 | 31.9~18.2 | 约99 | 20 | 0.04 | 表面化学法 | [ |
| PtSn/TS-1 | 590 | C3H8∶H2∶N2=1∶1∶4 | — | 3 | 53~47.5 | 92.5~93 | 7 | 0.03 | 水热+浸渍法 | [ |
| Pt/Al2O3 | 520 | C3H8/H2/N2=4∶4∶17 | 50 | 4.0 | 5.8~2.6 | 70~68 | 12 | 0.070 | 浸渍法 | [ |
| PtCu/Al2O3 | 520 | C3H8/H2/N2=4∶4∶17 | 50 | 4.0 | 13.1~12.4 | 87~89 | 120 | 0.0005 | 浸渍法 | [ |
| PtGa-Pb/SiO2 | 600 | C3H8/H2/He=5∶3.9∶40 | — | 30.6 | 27~31 | >99.6 | 50 | 0.0038 | 共沉淀法 | [ |
| PtSn/Mg(Al)O-sv | 550 | C3H8=20 | — | 1.13 | 27.7~27.8 | >96.6 | 5 | 0.001 | 浸渍法 | [ |
| PtIn/Mg(Al)O-4 | 620 | C3H8/H2/Ar=8∶7∶35 | — | 3.3 | 45~61.3 | 95.3~96.2 | 12 | — | 浸渍法 | [ |
| PtSn3/Al2O3 | 550 | C3H8/H2/N2=1∶1∶8 | 80 | 11.8 | 40~38 | >99.5 | 72 | 0.0012 | 表面化学法 | [ |
| Pt0Znδ+/SiO2 | 550 | C3H8/Ar=1∶4 | 50 | 32 | 35.3~26.6 | 97.6~96.3 | 30 | 0.014 | 表面化学法 | [ |
| Pt0Znδ+/SiO2 | 550 | C3H8/Ar=1∶4 | 50 | 75 | 30.2~16.2 | 98.1~95 | 30 | 0.027 | 表面化学法 | [ |
| PtSn/Al2O3片 | 590 | C3H8/H2/N2=1∶1.25∶8 | — | 9.4 | 48.7~44.6 | 约99.1 | 24 | 0.007 | 浸渍法 | [ |
| Pt-Sn/SiO2(1073K H2) | 500 | C3H8/N2=4∶1 | 100 | 47 | 27~12 | 90.9~99.5 | 3 | 0.33 | 浸渍法 | [ |
| Pt/Mg(In)(Al)O | 550 | C3H8=20 | — | 1.57 | 24.2~17.5 | >98.2 | 5 | 0.081 | 共沉淀法 | [ |
| Pt/Mg(Sn)(Al)O | 550 | C3H8/H2/N2=1∶0.5∶2 | — | 14 | 29.4~27.8 | 93.7~99.2 | 240 | 0.0067 | 浸渍法 | [ |
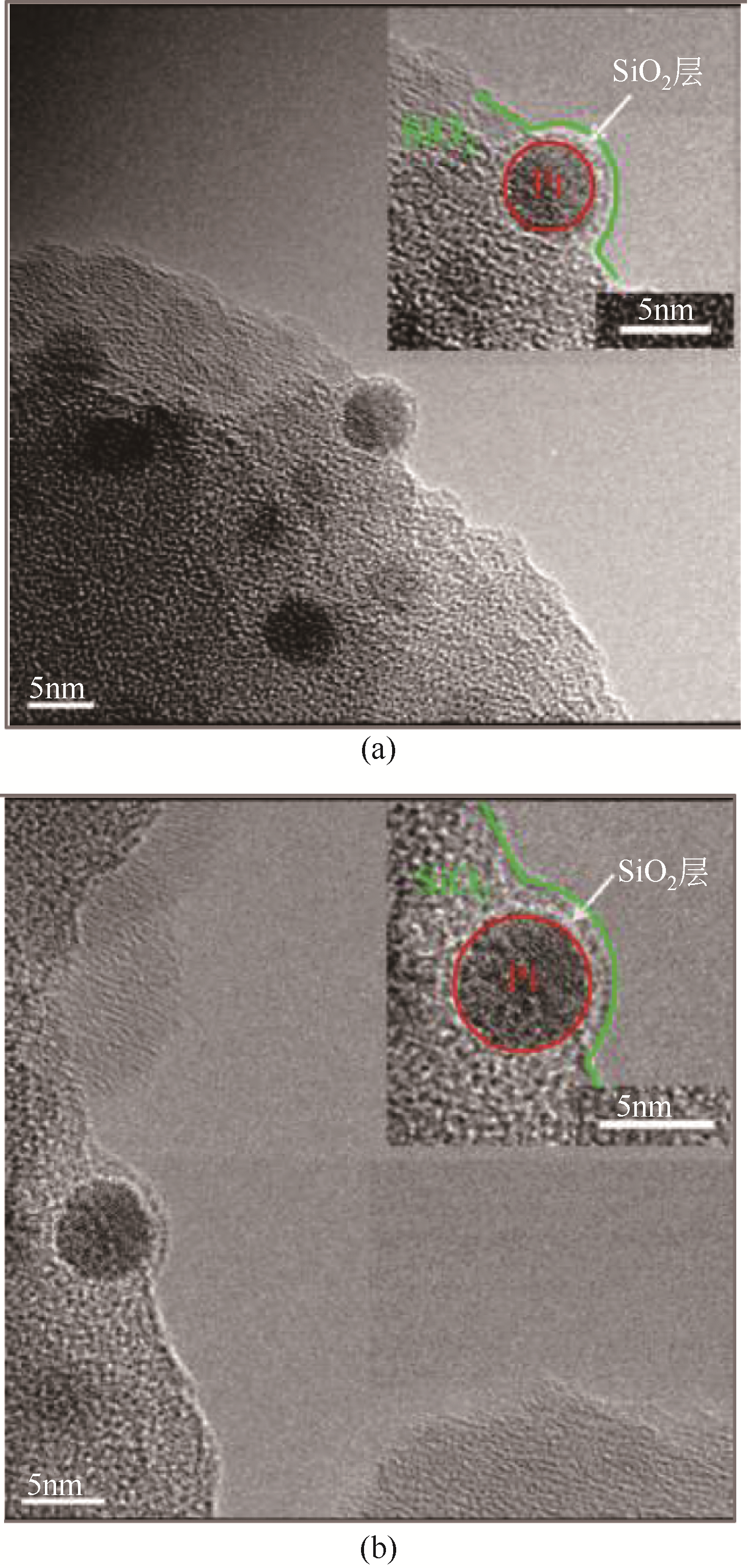
| Pt/Mg(Sn)(Al)O | 600 | C3H8/H2/N2=1∶0.5∶2 | — | 14 | 48.3~43.0 | 86.4~98.1 | 48 | 0.11 | 浸渍法 | [ |
| Pt/Sn2-Beta | 580 | C3H8/H2/N2=1∶1∶8 | 60 | 141 | 50~44 | 95~98 | 48 | 0.006 | 表面化学法 | [ |
| PtZnx@S-1-H | 550 | C3H8/N2=10∶30 | 40 | 3.6 | 47.4~40.4 | 93.2~99.2 | 217 | 0.001 | 原位合成法 | [ |
| PtZn@S-1 | 550 | C3H8/N2=11∶19 | 30 | 6.5 | 45.3~41.5 | >99 | 60 | 0.002 | 原位合成法 | [ |
| K-PtSn@MFI | 650 | C3H8/N2=5∶16 | 21 | 29.5 | 54~37 | >99 | 70 | 0.099 | 原位合成法 | [ |
| K-PtSn@MFI-(600H2-22h) | 600 | C3H8/N2=5∶16.5 | 21.5 | 29.5 | 38.7~31.9 | >97 | 25 | 0.012 | 原位合成法 | [ |
| 催化剂 | 反应温度 /℃ | 反应原料比 | 总流速 /mL·min-1 | 质量空速WHSV | 丙烷始-末 转化率/% | 丙烯始-末选择性/% | 反应时间TOS/h | 失活速率Kd /h-1 | 制备方法 | 参考 文献 |
|---|---|---|---|---|---|---|---|---|---|---|
| VOx/ZrO2 | 550 | C3H8/H2/N2=7∶36∶7 | 50 | 2.1 | 25~10 | 85~88 | 2 | 0.549 | 浸渍法 | [ |
| VOx/Al2O3 | 600 | C3H8/H2/N2=7∶7∶11 | 25 | 3 | 32~15 | 约94 | 4 | 0.245 | 浸渍法 | [ |
| VOx/Al2O3 | 600 | C3H8/N2=7∶18 | 25 | 8 | 25~15 | 70~75 | 约1.5 | 0.423 | 浸渍法 | [ |
| Ga8Al2O15 | 500 | C3H8/N2=1∶39 | — | — | 49.7~33.1 | 91.7~98 | 8 | 0.086 | 共沉淀法 | [ |
| [Fe]ZSM-5(MFI) | 530 | — | — | — | 约7.2 | 约78 | 3 | — | 原位合成法 | [ |
| Co/Si-Beta | 600 | C3H8/N2=5∶95 | 20 | 0.4 | 85~40 | 1~97 | 6 | 0.344 | 浸渍法 | [ |
| Sn-HMS | 600 | C3H8=5 | — | 0.4 | 约40 | 约90 | 170 | — | 水热法 | [ |
| SnO2/SiO2(ws) | 600 | C3H8=5 | — | 0.65 | 约30 | 约85 | 5 | — | 浸渍法 | [ |
| FeⅡ/SiO2 | 650 | C3H8/Ar=3∶97 | 55 | 0.38 | 4.9~6.3 | >99 | 18 | — | 表面化学法 | [ |
| Co-Al2O3-HT | 590 | C3H8/H2/N2=1∶0.8∶3.2 | — | 2.9 | 23~22 | 约97 | 5 | 0.011 | 水热法 | [ |
| VOx/meso-Al2O3 | 510 | C3H8/N2=4∶1 | 30 | 2.8 | 70~30 | 约85 | 10 | 0.169 | 浸渍法 | [ |
| GaOx/SiO2 | 580 | C3H8/N2=1∶10 | 16.5 | 0.59 | 65~45 | 约90 | 6 | 0.136 | 原位合成法 | [ |
| ZnO/Beta | 600 | C3H8/N2=5∶95 | 20 | 0.4 | 52~38 | 约93 | 6 | 0.095 | 离子交换法 | [ |
| 催化剂 | 反应温度 /℃ | 反应原料比 | 总流速 /mL·min-1 | 质量空速WHSV | 丙烷始-末 转化率/% | 丙烯始-末选择性/% | 反应时间TOS/h | 失活速率Kd /h-1 | 制备方法 | 参考 文献 |
|---|---|---|---|---|---|---|---|---|---|---|
| VOx/ZrO2 | 550 | C3H8/H2/N2=7∶36∶7 | 50 | 2.1 | 25~10 | 85~88 | 2 | 0.549 | 浸渍法 | [ |
| VOx/Al2O3 | 600 | C3H8/H2/N2=7∶7∶11 | 25 | 3 | 32~15 | 约94 | 4 | 0.245 | 浸渍法 | [ |
| VOx/Al2O3 | 600 | C3H8/N2=7∶18 | 25 | 8 | 25~15 | 70~75 | 约1.5 | 0.423 | 浸渍法 | [ |
| Ga8Al2O15 | 500 | C3H8/N2=1∶39 | — | — | 49.7~33.1 | 91.7~98 | 8 | 0.086 | 共沉淀法 | [ |
| [Fe]ZSM-5(MFI) | 530 | — | — | — | 约7.2 | 约78 | 3 | — | 原位合成法 | [ |
| Co/Si-Beta | 600 | C3H8/N2=5∶95 | 20 | 0.4 | 85~40 | 1~97 | 6 | 0.344 | 浸渍法 | [ |
| Sn-HMS | 600 | C3H8=5 | — | 0.4 | 约40 | 约90 | 170 | — | 水热法 | [ |
| SnO2/SiO2(ws) | 600 | C3H8=5 | — | 0.65 | 约30 | 约85 | 5 | — | 浸渍法 | [ |
| FeⅡ/SiO2 | 650 | C3H8/Ar=3∶97 | 55 | 0.38 | 4.9~6.3 | >99 | 18 | — | 表面化学法 | [ |
| Co-Al2O3-HT | 590 | C3H8/H2/N2=1∶0.8∶3.2 | — | 2.9 | 23~22 | 约97 | 5 | 0.011 | 水热法 | [ |
| VOx/meso-Al2O3 | 510 | C3H8/N2=4∶1 | 30 | 2.8 | 70~30 | 约85 | 10 | 0.169 | 浸渍法 | [ |
| GaOx/SiO2 | 580 | C3H8/N2=1∶10 | 16.5 | 0.59 | 65~45 | 约90 | 6 | 0.136 | 原位合成法 | [ |
| ZnO/Beta | 600 | C3H8/N2=5∶95 | 20 | 0.4 | 52~38 | 约93 | 6 | 0.095 | 离子交换法 | [ |
| 催化剂 | 反应温度 /℃ | 反应原料比 | 总流速 /mL·min-1 | 质量空速WHSV | 丙烷始-末转化率 /% | 丙烯始-末选择性 /% | 反应时间TOS/h | 失活速率Kd /h-1 | 制备方法 | 参考 文献 |
|---|---|---|---|---|---|---|---|---|---|---|
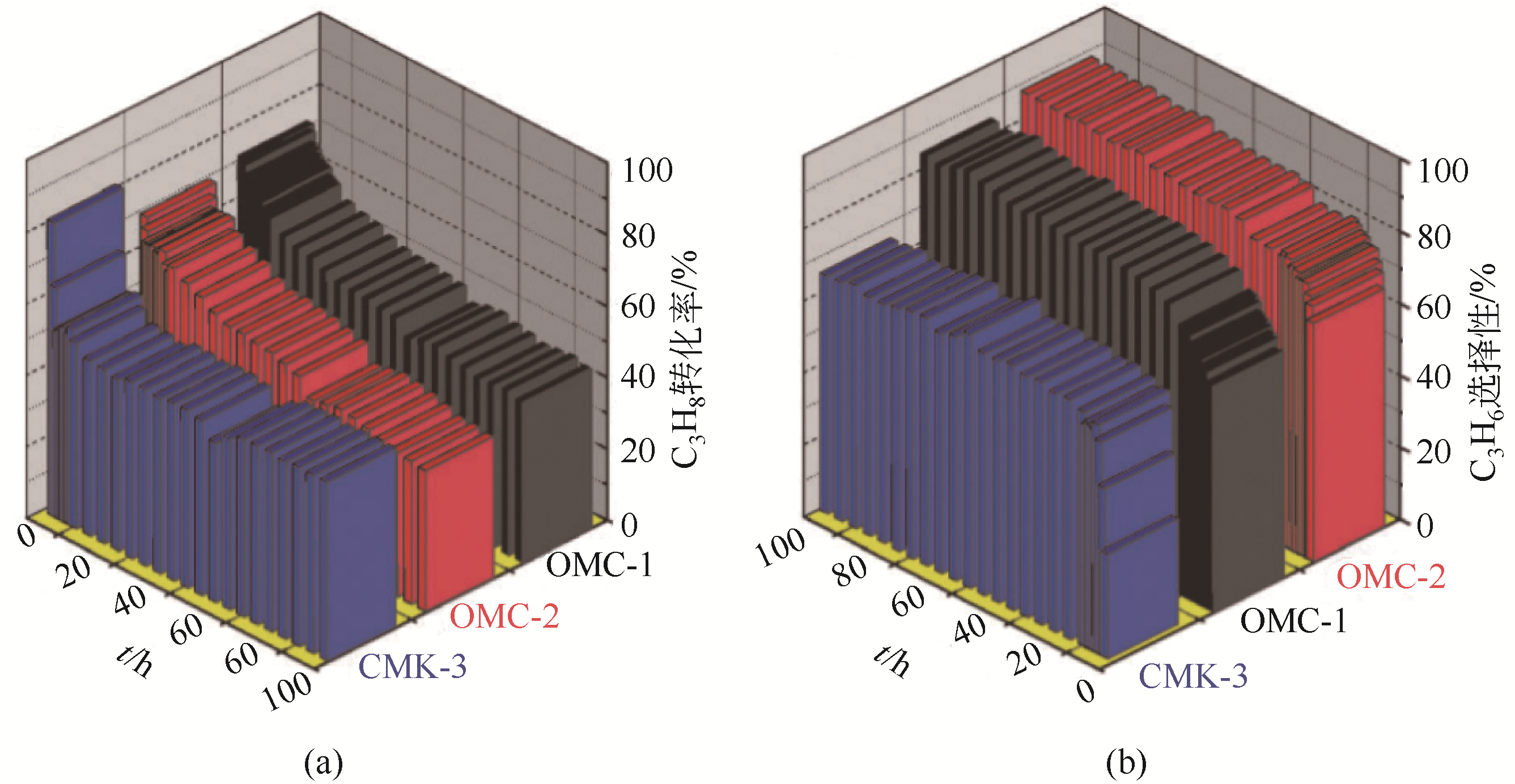
| OMC-1 | 600 | C3H8/N2=1∶19 | 40 | 0.59 | 69.3~44.5 | 62.2~85.1 | 100 | 0.01 | 有机自组装法 | [ |
| OMC-2 | 600 | C3H8/N2=1∶19 | 40 | 0.59 | 65.7~39.3 | 70.6~88.6 | 100 | 0.01 | 有机自组装法 | [ |
| CMK-3 | 550 | C3H8/N2=1∶19 | 40 | 0.59 | 54.3~49.8 | 27.6~68.0 | 100 | 0.018 | 硬膜版法 | [ |
| MCO | 600 | C3H8/N2=1∶19 | 40 | 0.59 | 32.9~21.2 | 66.3~86.6 | 50 | 0.012 | 酸洗法 | [ |
| P/CMK-3 | 600 | C3H8/N2=1∶19 | 20 | 0.59 | 约28 | 约88 | 24 | — | 杂原子掺杂法 | [ |
| ND-1000 | 550 | C3H8/He=1∶49 | 15 | — | 约10.6 | 约90 | 8 | — | 高温退火法 | [ |
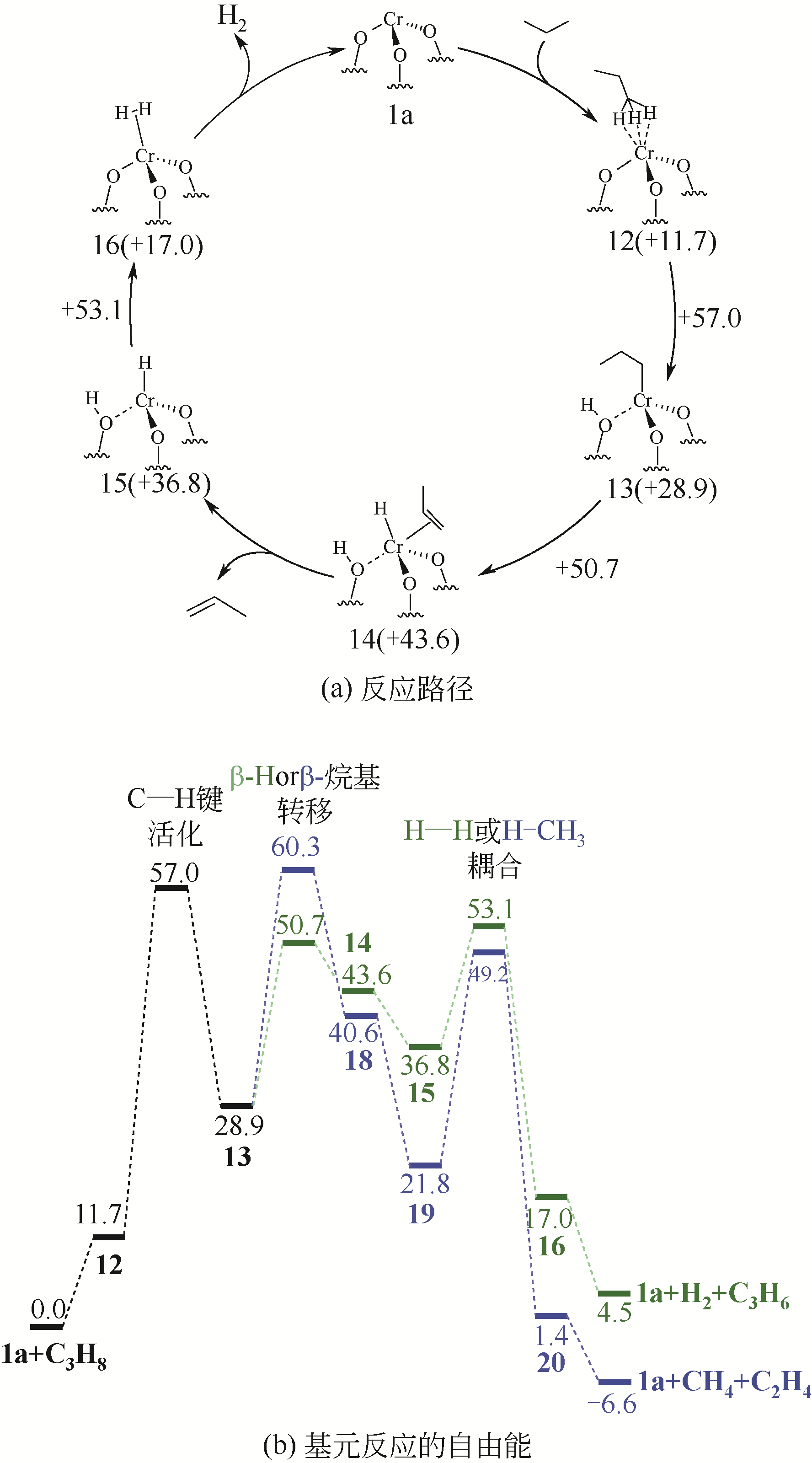
| HOMC | 600 | C3H8/N2=1∶19 | 40 | 0.59 | 20.1~10.3 | 66.1~78.5 | 50 | 0.016 | 水热法 | [ |
| COMC | 600 | C3H8/N2=1∶19 | 40 | 0.59 | 22.6~12.1 | 89~95.1 | 50 | 0.015 | 酸洗法 | [ |
| MC-2-600 | 600 | C3H8/N2=1∶19 | 20 | 0.59 | 37~31 | 85~89 | 10 | 0.027 | 水热法 | [ |
| so-MWCNTs | 600 | C3H8/N2=1∶19 | 20 | 0.59 | 11.2~5.8 | 92.1~87.9 | 4 | 0.179 | 酸洗+热处理法 | [ |
| BDAC-700 | 600 | C3H8/N2=1∶19 | 20 | 0.59 | 41.3~24.7 | 88.6~93.5 | 50 | 0.015 | 碱洗法 | [ |
| BDAC-800 | 600 | C3H8/N2=1∶19 | 20 | 0.59 | 42.5~29 | 89.2~91.3 | 50 | 0.012 | 碱洗法 | [ |
| PT-MCNs | 600 | C3H8/N2=1∶19 | 20 | 0.59 | 32.3~18 | 约90 | 10 | 0.077 | 水热法 | [ |
| PMCNs | 600 | C3H8/N2=1∶19 | 20 | 0.59 | 26~16 | 约88 | 10 | 0.061 | 水热法 | [ |
| 催化剂 | 反应温度 /℃ | 反应原料比 | 总流速 /mL·min-1 | 质量空速WHSV | 丙烷始-末转化率 /% | 丙烯始-末选择性 /% | 反应时间TOS/h | 失活速率Kd /h-1 | 制备方法 | 参考 文献 |
|---|---|---|---|---|---|---|---|---|---|---|
| OMC-1 | 600 | C3H8/N2=1∶19 | 40 | 0.59 | 69.3~44.5 | 62.2~85.1 | 100 | 0.01 | 有机自组装法 | [ |
| OMC-2 | 600 | C3H8/N2=1∶19 | 40 | 0.59 | 65.7~39.3 | 70.6~88.6 | 100 | 0.01 | 有机自组装法 | [ |
| CMK-3 | 550 | C3H8/N2=1∶19 | 40 | 0.59 | 54.3~49.8 | 27.6~68.0 | 100 | 0.018 | 硬膜版法 | [ |
| MCO | 600 | C3H8/N2=1∶19 | 40 | 0.59 | 32.9~21.2 | 66.3~86.6 | 50 | 0.012 | 酸洗法 | [ |
| P/CMK-3 | 600 | C3H8/N2=1∶19 | 20 | 0.59 | 约28 | 约88 | 24 | — | 杂原子掺杂法 | [ |
| ND-1000 | 550 | C3H8/He=1∶49 | 15 | — | 约10.6 | 约90 | 8 | — | 高温退火法 | [ |
| HOMC | 600 | C3H8/N2=1∶19 | 40 | 0.59 | 20.1~10.3 | 66.1~78.5 | 50 | 0.016 | 水热法 | [ |
| COMC | 600 | C3H8/N2=1∶19 | 40 | 0.59 | 22.6~12.1 | 89~95.1 | 50 | 0.015 | 酸洗法 | [ |
| MC-2-600 | 600 | C3H8/N2=1∶19 | 20 | 0.59 | 37~31 | 85~89 | 10 | 0.027 | 水热法 | [ |
| so-MWCNTs | 600 | C3H8/N2=1∶19 | 20 | 0.59 | 11.2~5.8 | 92.1~87.9 | 4 | 0.179 | 酸洗+热处理法 | [ |
| BDAC-700 | 600 | C3H8/N2=1∶19 | 20 | 0.59 | 41.3~24.7 | 88.6~93.5 | 50 | 0.015 | 碱洗法 | [ |
| BDAC-800 | 600 | C3H8/N2=1∶19 | 20 | 0.59 | 42.5~29 | 89.2~91.3 | 50 | 0.012 | 碱洗法 | [ |
| PT-MCNs | 600 | C3H8/N2=1∶19 | 20 | 0.59 | 32.3~18 | 约90 | 10 | 0.077 | 水热法 | [ |
| PMCNs | 600 | C3H8/N2=1∶19 | 20 | 0.59 | 26~16 | 约88 | 10 | 0.061 | 水热法 | [ |
| 1 | SU D S, PERATHONER S, CENTI G. ChemInform abstract: nanocarbons for the development of advanced catalysts[J]. Chemical Reviews, 2013, 113(8): 5782-5816. |
| 2 | RAZAVIAN M, FATEMI S. Synthesis and application of ZSM-5/SAPO-34 and SAPO-34/ZSM-5 composite systems for propylene yield enhancement in propane dehydrogenation process[J]. Microporous & Mesoporous Materials, 2015, 201: 176-189. |
| 3 | QI Wei, YAN Pengqiang, SU Dangsheng. Oxidative dehydrogenation on nanocarbon: insights into the reaction mechanism and kinetics viain situ experimental methods[J]. Accounts of Chemical Research, 2018, 51(3): 640-648. |
| 4 | FAN Zeyun, ZHANG Zhixiang, FANG Wenjian, et al. Low-temperature catalytic oxidation of formaldehyde over Co3O4 catalysts prepared using various precipitants[J]. Chinese Journal of Catalysis, 2016, 37(6): 947-954. |
| 5 | 中国产业信息网.中国丙烯市场供需现状分析及预测[EB/OL]. . |
| Web of China Industry Information. The analysis and forecast of China’s propylene market supply and demand status[EB/OL]. . | |
| 6 | SERRANO-RUIZ J C, SEPÚLVEDA-ESCRIBANO A, RODRÍGUEZ-REINOSO F. Bimetallic PtSn/C catalysts promoted by ceria: application in the nonoxidative dehydrogenation of isobutane[J]. Journal of Catalysis, 2007, 246(1): 158-165. |
| 7 | ZHANG J, LIU X, BLUME R, et al. Surface-modified carbon nanotubes catalyze oxidative dehydrogenation of n-Butane[J]. Science, 2008, 322(5898): 73-77. |
| 8 | ZHAO Huahua, SONG Huanling, XU Leilei, et al. Isobutane dehydrogenation over the mesoporous Cr2O3/Al2O3 catalysts synthesized from a metal-organic framework MIL-101[J]. Applied Catalysis A: General, 2013, 456: 188-196. |
| 9 | YARULINA I, DE WISPELAERE K, BAILLEUL S, et al. Structure-performance descriptors and the role of Lewis acidity in the methanol-to-propylene process[J]. Nature Chemistry, 2018, 10(8): 804-812. |
| 10 | TASBIHI M, FEYZI F, AMLASHI M A, et al. Effect of the addition of potassium and lithium in Pt-Sn/Al2O3 catalysts for the dehydrogenation of isobutane[J]. Fuel Processing Technology, 2007, 88(9): 883-889. |
| 11 | ATANGA M A, REZAEI F, JAWAD A, et al. Oxidative dehydrogenation of propane to propylene with carbon dioxide[J]. Applied Catalysis B: Environmental, 2018, 220: 429-445. |
| 12 | CAVANI F, BALLARINI N, CERICOLA A. Oxidative dehydrogenation of ethane and propane: how far from commercial implementation?[J]. Catalysis Today, 2007, 127(1/2/3/4): 113-131. |
| 13 | 盖希坤, 田原宇, 夏道宏. 丙烷催化脱氢制丙烯工艺分析[J]. 炼油技术与工程, 2012, 40(12): 27-32. |
| GAI Xikun, TIAN Yuanyu, XIA Daohong. Study on technology of propane dehydrogenation for propylene production[J]. Petroleum Refinery Engineering, 2012, 40(12): 27-32. | |
| 14 | STEPHANIE S, SABBE M K, GALVITA V V. Positive effect of Ga-promoting on the catalytic dehydrogenation of propane over a Pt catalyst[C]//18th Netherlands’ Catalysis and Chemistry Conference (NCCC), 2017. |
| 15 | JIANG Guiyuan, ZHANG Li, ZHAO Zhen, et al. Highly effective P-modified HZSM-5 catalyst for the cracking of C4 alkanes to produce light olefins[J]. Applied Catalysis A: General, 2008, 340(2): 176-182. |
| 16 | CHEN De, HOLMEN A, Zhijun SUI, et al. Carbon mediated catalysis: a review on oxidative dehydrogenation[J]. Chinese Journal of Catalysis, 2014, 35(6): 824-841. |
| 17 | KAINTHLA I, BHANUSHALI J T, KERI R, et al. Activity studies of vanadium, iron, carbon and mixed oxides based catalysts for the oxidative dehydrogenation of ethylbenzene to styrene: a review[J]. Catalysis Science & Technology, 2015, 5(12): 5062-5076. |
| 18 | FENG Jing, ZHANG Mingsen, KE Li, et al. Research progress in propane dehydrogenation catalysts[J]. Industrial Catalysis, 2011, 19(3): 8-14. |
| 19 | ZHANG Lingfeng, LIU Yalu, HU Zhongpan, et al. Advance in catalysts for propane dehydrogenation to propylene[J]. Acta Petrol Sinica, 2015, 31(2): 400-417. |
| 20 | GRUNERT W. Principles and practice of heterogeneous catalysis[J]. Focus on Catalysts, 2015, 7: 7. |
| 21 | ZHOU Shijian, ZHOU Yuming, ZHANG Yiwei, et al. The synthesis of new coke-resistant support and its application in propane dehydrogenation to propene[J]. Journal of Chemical Technology & Biotechnology, 2016, 91(4): 1072-1081. |
| 22 | YU Changlin, XU Hengyong, GE Qingjie, et al. Properties of the metallic phase of zinc-doped platinum catalysts for propane dehydrogenation[J]. Journal of Molecular Catalysis A: Chemical, 2007, 266(1/2): 80-87. |
| 23 | SATTLER J J, GONZALEZ-JIMENEZ I D, MENS A M, et al. Operando UV-vis spectroscopy of a catalytic solid in a pilot-scale reactor: deactivation of a CrOx/Al2O3 propane dehydrogenation catalyst[J]. Chemical Communications, 2013, 49(15): 1518-1520. |
| 24 | SEARLES K, CHAN Kawing, BURAK J A M, et al. Highly productive propane dehydrogenation catalyst using silica-supported Ga-Pt nanoparticles generated from single-sites[J]. Journal of the American Chemical Society, 2018, 140(37): 11674-11679. |
| 25 | LI Jiacheng, LI Jianmei, ZHAO Zhen, et al. Size effect of TS-1 supports on the catalytic performance of PtSn/TS-1 catalysts for propane dehydrogenation[J]. Journal of Catalysis, 2017, 352: 361-370. |
| 26 | SOKOLOV S, STOYANOVA M, RODEMERCK U, et al. Comparative study of propane dehydrogenation over V-, Cr-, and Pt-based catalysts: time on-stream behavior and origins of deactivation[J]. Journal of Catalysis, 2012, 293: 67-75. |
| 27 | XIONG Haifeng, LIN Sen, GOETZE J, et al. Thermally stable and regenerable platinum-tin clusters for propane dehydrogenation prepared by atom trapping on ceria[J]. Angewandte Chemie International Edition, 2017, 56(31): 8986-8991. |
| 28 | ZHOU Shijian, ZHOU Yuming, ZHANG Yiwei, et al. The synthesis of new coke-resistant support and its application in propane dehydrogenation to propene[J]. Journal of Chemical Technology & Biotechnology, 2016, 91(4): 1072-1081. |
| 29 | ZHANG Yiwei, ZHOU Yuming, QIU Anding, et al. Propane dehydrogenation on PtSn/ZSM-5 catalyst: effect of tin as a promoter[J]. Catalysis Communications, 2006, 7(11): 860-866. |
| 30 | SATTLWER J J H B, MENS A M, WECKHUYSEN B M. Real-time quantitative operando raman spectroscopy of a CrOx/Al2O3 propane dehydrogenation catalyst in a pilot-scale reactor[J]. ChemCatChem, 2014, 6(11): 3139-3145. |
| 31 | MCGREGOR J, HUANG Zhenyu, PARROTT E P J, et al. Active coke: carbonaceous materials as catalysts for alkane dehydrogenation[J]. Journal of Catalysis, 2010, 269(2): 329-339. |
| 32 | LIAN Zan, ALI S, LIU Tianfu, et al. Revealing Janus character of the coke precursor in the propane direct dehydrogenation on Pt catalysts from a kMC simulation[J]. ACS Catalysis, 2018, 8(5): 4694-4704. |
| 33 | AIRAKSINEN S M K, KRAUSE A O I. In situ characterisation of carbon-containing species formed on chromia/alumina during propane dehydrogenation[J]. Journal of Catalysis, 2005, 230(2): 507-513. |
| 34 | CASPARY K J, GEHRKE H, HEINRITZ-ADRIAN M. Handbook of heterogeneous catalysis[M]. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA, 2008. |
| 35 | LI Qing, Zhijun SUI, ZHOU Xinggui, et al. Coke formation on Pt-Sn/Al2O3 catalyst in propane dehydrogenation: coke characterization and kinetic study[J]. Topics in Catalysis, 2011, 54(13/14/15): 888-896. |
| 36 | SHAN Yuling, WANG Ting, Zhijun SUI, et al. Hierarchical MgAl2O4 supported Pt-Sn as a highly thermostable catalyst for propane dehydrogenation[J]. Catalysis Communications, 2016, 84(5): 85-88. |
| 37 | SHAN Yuling, ZHU Yian, SUN Zhijun, et al. Insights into the effects of steam on propane dehydrogenation over a Pt/Al2O3 catalyst[J]. Catalysis Science & Technology, 2015, 5(8): 3991-4000. |
| 38 | HAUSRE A W, HORN P R, HEAD-GORDON M, et al. A systematic study on Pt based, subnanometer-sized alloy cluster catalysts for alkane dehydrogenation: effects of intermetallic interaction[J]. Physical Chemistry Chemical Physics, 2016, 18(16): 10906-109017. |
| 39 | YANG Minglei, ZHU Yian, FAN Chen, et al. DFT study of propane dehydrogenation on Pt catalyst: effects of step sites[J]. Physical Chemistry Chemical Physics, 2011, 13(8): 3257-3267. |
| 40 | ZHU Jun, YANG Minglei, ZHU Yian. Size-dependent reaction mechanism and kinetics for propane dehydrogenation over Pt catalysts[J]. ACS Catalysis, 2015, 5(11): 6310-6319. |
| 41 | SUN Guodong, ZHAO Zhi Jian, MU Rentao, et al. Breaking the scaling relationship via thermally stable Pt/Cu single atom alloys for catalytic dehydrogenation[J]. Nature Communications, 2018, 9(1): 4454-4462. |
| 42 | NAKAYA Y, HIRAYAMA J, YAMAZOE S, et al. Single-atom Pt in intermetallics as an ultrastable and selective catalyst for propane dehydrogenation[J]. Nature Communications, 2020, 11(1):2838-2845. |
| 43 | VU B K, SONG M B, AHN I Y, et al. Propane dehydrogenation over Pt-Sn/rare-earth-doped Al2O3: influence of La, Ce, or Y on the formation and stability of Pt-Sn alloys[J]. Catalysis Today, 2011, 164(1): 214-220. |
| 44 | SRISAKWATTANA T, SURIYE K, PRASERTHDAM P, et al. Preparation of aluminum magnesium oxide by different methods for use as PtSn catalyst supports in propane dehydrogenation[J]. Catalysis Today, 2020, 358: 90-99. |
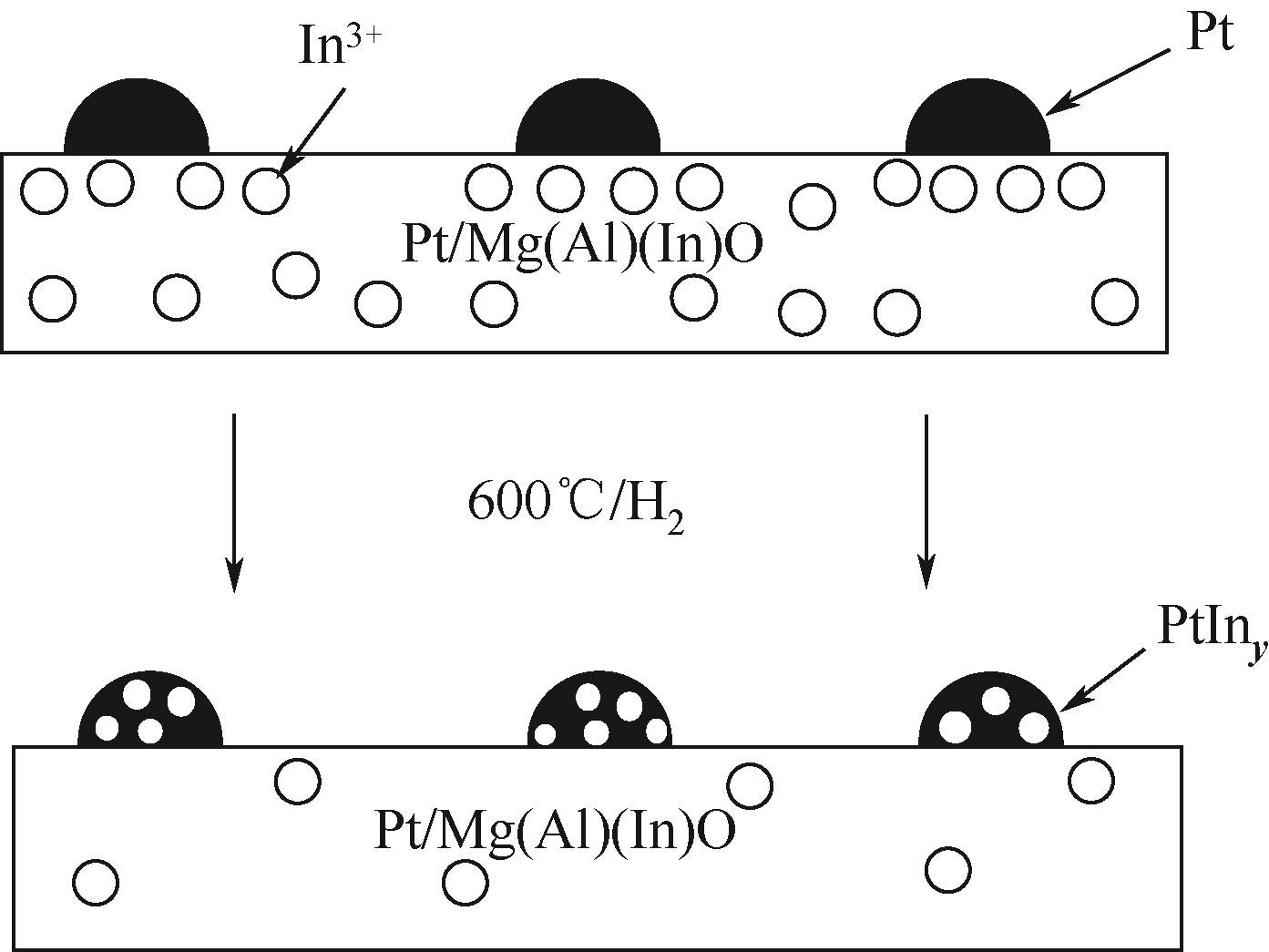
| 45 | XIA Ke, LANG Wanzhong, LI Peipei, et al. The influences of Mg/Al molar ratio on the properties of PtIn/Mg(Al)O-x catalysts for propane dehydrogenation reaction[J]. Chemical Engineering Journal, 2016, 284(15): 1068-1079. |
| 46 | XU Zhikang, XU Rui, YUE Yuanyuan, et al. Bimetallic Pt-Sn nanocluster from the hydrogenolysis of a well-defined surface compound consisting of [(Al≡O)Pt(COD)Me] and [(Al≡O)SnPh3] fragments for propane dehydrogenation[J]. Journal of Catalysis, 2019, 374: 391-400. |
| 47 | ROCHLITZ L, SEARLES K, ALFKE J, et al. Silica-supported, narrowly distributed, subnanometric Pt-Zn particles from single sites with high propane dehydrogenation performance[J]. Chemical Science, 2020, 11(6): 1549-1555. |
| 48 | NANDA K, SAHU S, BEHERA S. Liquid-drop model for the size-dependent melting of low-dimensional systems[J]. Physical Review A, 66(1): 013208. |
| 49 | MORGAN K, GOGUET A, HARDACRE C. Metal redispersion strategies for recycling of supported metal catalysts: a perspective[J]. ACS Catalysis, 2015, 5(6): 3430-3445. |
| 50 | BARTHOLOMEW C H. Mechanisms of catalyst deactivation[J]. Applied Catalysis A: General, 2001, 212(1): 17-60. |
| 51 | HATANAKA M, TAKAHASHI N, TAKAHASHI N, et al. Reversible changes in the Pt oxidation state and nanostructure on a ceria-based supported Pt[J]. Journal of Catalysis, 2009, 266(2): 182-190. |
| 52 | TAUSTER S J, FUNG S C. Strong metal-support interactions: occurrence among the binary oxides of groups ⅡA—VB[J]. Journal of Catalysis, 1978, 55(1): 29-35. |
| 53 | TAUSTER S J, FUNG S C, GARTEN R L. Strong metal-support interactions. group 8 noble metals supported on TiO2[J]. Chemischer Informationsdienst, 1978, 9(16): 170-175. |
| 54 | LEE J, JANG E J, OH D G, et al. Morphology and size of Pt on Al2O3: the role of specific metal-support interactions between Pt and Al2O3[J]. Journal of Catalysis, 2020, 385: 204-212. |
| 55 | KWAK J H, HU Jianzhi, MEI Donghai, et al. Coordinatively unsaturated Al3+ centers as binding sites for active catalyst phases of platinum on gamma-Al2O3[J]. Science, 2009, 325(5948): 1670-1673. |
| 56 | SHI Lei, DENG Gaoming, LI Wencui, et al. Al2O3 nanosheets rich in pentacoordinate Al3+ ions stabilize Pt-Sn clusters for propane dehydrogenation[J]. Angewandte Chemie: International Edition, 2016, 54(47): 13994-13998. |
| 57 | ZHANG Yiwei, ZHOU Yuming, SHI Junjun, et al. Comparative study of bimetallic Pt-Sn catalysts supported on different supports for propane dehydrogenation[J]. Journal of Molecular Catalysis A: Chemical, 2014, 381: 138-147. |
| 58 | DENG Lidan, MIURA H, TETSUYA S, et al. Strong metal-support interaction between Pt and SiO2 following high-temperature reduction: a catalytic interface for propane dehydrogenation[J]. Chemical Communications, 2017, 53(51): 6937-6940. |
| 59 | TOLEK W, SURIYE K, PRASERTHDAM P, et al. Effect of preparation method on the Pt-In modified Mg(Al)O catalysts over dehydrogenation of propane[J]. Catalysis Today, 2020, 358: 100-108. |
| 60 | LIU Jie, YUE Yuanyuan, LIU Hongyang, et al. Origin of the robust catalytic performance of nanodiamond-graphene-supported Pt nanoparticles used in the propane dehydrogenation reaction[J]. ACS Catalysis, 2017, 7(5): 3349-3355. |
| 61 | LIU Jie, LI Jiaquan, RONG Junfeng, et al. Defect-driven unique stability of Pt/carbon nanotubes for propane dehydrogenation[J]. Applied Surface Science, 2019, 464(15): 146-152. |
| 62 | ZHU Yanru, AN Zhe, SONG Hongyan, et al. Lattice-confined Sn (Ⅳ/Ⅱ) stabilizing raft-like Pt clusters: high selectivity and durability in propane dehydrogenation[J]. ACS Catalysis, 2017, 7(10): 6973-6978. |
| 63 | XU Zhikang, YUE Yuanyuan, BAO Xiaojun, et al. Propane dehydrogenation over Pt clusters localized at the Sn single-site in zeolite framework[J]. ACS Catalysis, 2020, 10: 818-828. |
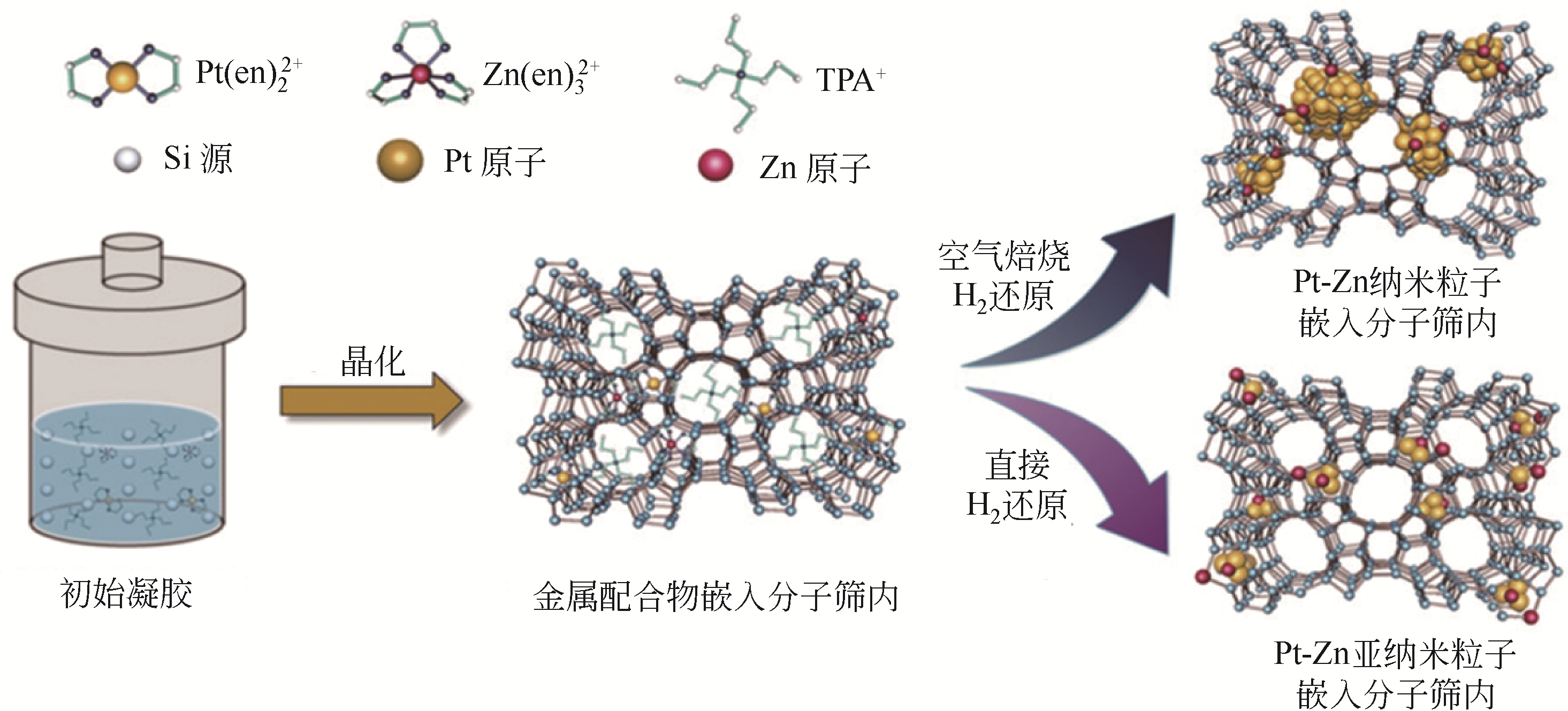
| 64 | SUN Qiming, WANG Ning, FAN Qiyuan, et al. Subnanometer bimetallic platinum-zinc clusters in zeolites for propane dehydrogenation[J]. Angewandte Chemie: International Edition, 2020, 59(44): 19450-19459. |
| 65 | WANG Yansu, HU ZhongPan, YUAN Zhongyong. Ultrasmall PtZn bimetallic nanoclusters encapsulated in silicalite-1 zeolite with superior performance for propane dehydrogenation[J]. Journal of Catalysis, 2020, 385: 61-69. |
| 66 | LIU Lichen, LOPEZ-HARO M, LOPES C W, et al. Atomic-level understanding on the evolution behavior of subnanometric Pt and Sn species during high-temperature treatments for generation of dense PtSn clusters in zeolites[J]. Journal of Catalysis, 2020, 391: 11-24. |
| 67 | LIU Lichen, LOPEZ-HARO M, LOPES C W, et al. Structural modulation and direct measurement of subnanometric bimetallic PtSn clusters confined in zeolites[J]. Nature Catalysis, 2020, 3: 628-638. |
| 68 | ZHU Jie, RYOTA O, RYO I. Ultrafast encapsulation of metal nanoclusters into MFI zeolite in the course of its crystallization: catalytic application for propane dehydrogenation[J]. Angewandte Chemie International Edition, 2020, 59(44): 19669-19674. |
| 69 | WANG Haizhi, SUN Lili, Zhijun SUI, et al. Coke formation on Pt-Sn/Al2O3 catalyst for propane dehydrogenation[J]. Industrial & Engineering Chemistry Research, 2018, 57(26): 8647-8654. |
| 70 | ZIMMER H, DOBROVOLSZKY M, TETENY P, et al. Hydrogen control of platinum-catalyzed skeletal reactions of alkanes: selectivity and surface species[J]. The Journal of Physical Chemistry, 1986, 90(20): 4758-4764. |
| 71 | LARSSON M, MAGNUS H, BLEKKAN E A, et al. The effect of reaction conditions and time on stream on the coke formed during propane dehydrogenation[J]. Journal of Catalysis, 1996, 164(1): 44-53. |
| 72 | YANG Minglei, ZHU Yian, FAN Chen, et al. Density functional study of the chemisorption of C1, C2 and C3 intermediates in propane dissociation on Pt(111)[J]. Journal of Molecular Catalysis A: Chemical, 2010, 321(1/2): 42-49. |
| 73 | SABBE M K, CANDUELA-RODRIGUEZ G, REYNIERS M F, et al. DFT-based modeling of benzene hydrogenation on Pt at industrially relevant coverage[J]. Journal of Catalysis, 2015, 330: 406-422. |
| 74 | LI Qing, Zhijun SUI, ZHOU Xinggui. Kinetics of propane dehydrogenation over Pt-Sn/Al2O3 catalyst[J]. Applied Catalysis A: General, 2011, 398(1/2): 18-26. |
| 75 | SAERENS S, SABBE M, GALVITA V, et al. Positive effect of Ga-promoting on the catalytic dehydrogenation of propane over a Pt catalyst[J]. ACS Cataysis, 2017, 7: 7495-7508. |
| 76 | ZAERA F, CHRYSOSTOMOU D. Propylene on Pt(111) Ⅱ. Hydrogenation, dehydrogenation, and H-D exchange[J]. Surface Science, 2000, 457(1/2): 71-88. |
| 77 | SIDDIQI G, SUN Pingping, GALVITA V, et al. Catalyst performance of novel Pt/Mg(Ga)(Al)O catalysts for alkane dehydrogenation[J]. Journal of Catalysis, 2010, 274(2): 200-206. |
| 78 | ZANGENEH F T, MEHRAZMA S, SAHEBDELFAR S. The influence of solvent on the performance of Pt-Sn/θ-Al2O3 propane dehydrogenation catalyst prepared by co-impregnation method[J]. Fuel Processing Technology, 2013, 109: 118-123. |
| 79 | JANG E J, LEE J J, JEONG H Y, et al. Controlling the acid-base properties of alumina for stable PtSn-based propane dehydrogenation catalysts[J]. Applied Catalysis A: General, 2019, 572: 1-8. |
| 80 | WANG Tuo, JIANG Feng, LIU Gang. Effects of Ga doping on Pt/CeO2‐Al2O3 catalysts for propane dehydrogenation[J]. AIChE Journal, 2016, 62(12): 4365-4376. |
| 81 | LONG Liuliu, XIA Ke, LANG Wanzhong, et al. The comparison and optimization of zirconia, alumina, and zirconia-alumina supported PtSnIn trimetallic catalysts for propane dehydrogenation reaction[J]. Journal of Industrial and Engineering Chemistry, 2017, 51(25): 271-280. |
| 82 | JIANG Feng, ZENG Liang, LI Shuirong, et al. Propane dehydrogenation over Pt/TiO2-Al2O3 catalysts[J]. ACS Catalysis, 2015, 5(1): 438-447. |
| 83 | ZHOU Hualan, GONG Jingjing, XU Bolian, et al. PtSnNa/SUZ-4: an efficient catalyst for propane dehydrogenation[J]. Chinese Journal of Catalysis, 2017, 38(3): 529-536. |
| 84 | LEE M H, NAGARAJA B M, NATARAJAN P, et al. Effect of potassium addition on bimetallic PtSn/θ-Al2O3 catalyst for dehydrogenation of propane to propylene[J]. Research on Chemical Intermediates, 2016, 42(1): 123-140. |
| 85 | DAUN Yongzheng, ZHOU Yuming, ZHANG Yiwei, et al. Effect of sodium addition to PtSn/AlSBA-15 on the catalytic properties in propane dehydrogenation[J]. Catalysis Letters, 2011, 141(1): 120-127. |
| 86 | LOBERA M P, HERGUIDO J, SCHUUMAN Y, et al. TAP studies of Pt-Sn-K/γ-Al2O3 catalyst for propane dehydrogenation[J]. Chemical Engineering Journal, 2011, 171(3): 1317-1323. |
| 87 | LI Bing, XU Zhenxin, JING Fangli, et al. Facile one-pot synthesized ordered mesoporous Mg-SBA-15 supported PtSn catalysts for propane dehydrogenation[J]. Applied Catalysis A: General, 2017, 533: 17-27. |
| 88 | BAI Linyang, ZHOU Yuming, ZHANG Yiwei, et al. Effect of magnesium addition to PtSnNa/ZSM-5 on the catalytic properties in the dehydrogenation of propane[J]. Industrial & Engineering Chemistry Research, 2009, 48(22): 9885-9891. |
| 89 | BAI Linyang, ZHOU Yuming, ZHANG Yiwei, et al. Influence of calcium addition on catalytic properties of PtSn/ZSM-5 catalyst for propane dehydrogenation[J]. Catalysis Letters, 2009, 129(3/4): 449-456. |
| 90 | ALY M, FORNERO E L, GALVITA V V, et al. Effect of boron promotion on coke formation during propane dehydrogenation over Pt/γ-Al2O3 catalysts[J]. ACS Catalysis, 2020, 10(9): 5208-5216. |
| 91 | ZHU Haibo, ANJUM D H, WANG Qingxiao, et al. Sn surface-enriched Pt-Sn bimetallic nanoparticles as a selective and stable catalyst for propane dehydrogenation[J]. Journal of Catalysis, 2014, 320: 52-62. |
| 92 | ZHANG Yiwei, ZHOU Yuming, HUANG Li, et al. Structure and catalytic properties of the Zn-modified ZSM-5 supported platinum catalyst for propane dehydrogenation[J]. Chemical Engineering Journal, 2015, 270: 352-361. |
| 93 | CAMACHO-BUNQUIN J, FERRANDON M S., SOHN H, et al. Atomically precise strategy to a PtZn alloy nano-cluster catalyst for the deep dehydrogenation of n-butane to 1,3-butadiene[J]. ACS Catalysis, 2018, 8(11): 10058-10063. |
| 94 | CYBULSKIS V J, BUKOWSKI B C, TSENG H T, et al. Zinc promotion of platinum for catalytic light alkane dehydrogenation: insights into geometric and electronic effects[J]. ACS Catalysis, 2017, 7(6): 4173-4181. |
| 95 | SUN Pingping, GEORGES S, WILLIAM C V. Novel Pt/Mg(In)(Al)O catalysts for ethane and propane dehydrogenation[J]. Journal of Catalysis, 2011, 282(1): 165-174. |
| 96 | LIU Xue, LANG Wanzhong, LONG Liuliu, et al. Improved catalytic performance in propane dehydrogenation of PtSn/γ-Al2O3 catalysts by doping indium[J]. Chemical Engineering Journal, 2014, 247: 183-192. |
| 97 | ZHANG Yiwei, ZHOU Yuming, SHI Junjun, et al. Propane dehydrogenation over PtSnNa/La-doped Al2O3 catalyst: effect of La content[J]. Fuel Processing Technology, 2013, 111(3): 94-104. |
| 98 | HAN Zhiping, LI Shuirong, JIANG Feng, et al. Propane dehydrogenation over Pt-Cu bimetallic catalysts: the nature of coke deposition and the role of copper[J]. Nanoscale, 2014, 6(17): 10000-10008. |
| 99 | FREY F E, HUPPKE W F. Equilibrium dehydrogenation of ethane, propane, and the butanes[J]. Industrial & Engineering Chemistry, 1933, 25(1): 54-59. |
| 100 | WECKHUYSEN B M, SCHOONHEYD T R A. Alkane dehydrogenation over supported chromium oxide catalysts[J]. Catalysis Today, 1967, 51(2): 223-232. |
| 101 | ADAMS C R. Catalytic oxidations with sulfur dioxide : Ⅰ. Exploratory studies[J]. Journal of Catalysis, 1968, 11(2): 96-112. |
| 102 | HARDCASTLE F D, WACHS I E. Raman spectroscopy of chromium oxide supported on Al2O3, TiO2 and SiO2: a comparative study[J]. Journal of Molecular Catalysis, 1988, 46(1/2/3): 173-186. |
| 103 | VUURMAN M A, WACHS I E. In situ Raman spectroscopy of alumina-supported metal oxide catalysts[J]. Journal of Physical Chemistry, 1992, 96(12): 5008-5016. |
| 104 |
DINES T J, INGLIS S. Raman spectroscopic study of supported chromium( ) oxide catalysts[J]. Physical Chemistry Chemical Physics, 2003, 5(6): 1320-1328. ) oxide catalysts[J]. Physical Chemistry Chemical Physics, 2003, 5(6): 1320-1328.
|
| 105 | WECKHUYSEN B M, VERBERCKMOES A A, DEBAERE J, et al. In-situ UV-vis diffuse reflectance spectroscopy-on line activity measurements of supported chromium oxide catalysts: relating isobutane dehydrogenation activity with Cr-speciation via experimental design[J]. Journal of Molecular Catalysis A: Chemical, 2000, 151(1/2): 115-131. |
| 106 | PUURUNEN R L, WECKHUYSEN B M. Spectroscopic study on the irreversible deactivation of chromia/alumina dehydrogenation catalysts[J]. Journal of Catalysis, 2002, 210(2): 418-430. |
| 107 | AIRAKSINEN S M K, OUTI A, KRAUSE I, et al. Reduction of chromia/alumina catalyst monitored by DRIFTS-mass spectrometry and TPR-Raman spectroscopy[J]. Physical Chemistry Chemical Physics, 2003, 5(20): 4371-4377. |
| 108 | CONLEY M P, DELLEY M F, COMAS-VIVES A, et al. Heterolytic activation of C—H bonds on CrⅢ—O surface sites is a key step in catalytic polymerization of ethylene and dehydrogenation of propane[J]. Inorganic Chemistry, 2015, 54(11): 5065-5078. |
| 109 | XIE Yufei, LUO Ran, SUN Guodong, et al. Facilitating the reduction of V—O bonds on VOx/ZrO2 catalysts for non-oxidative propane dehydrogenation[J]. Chemical Science, 2020, 11: 3845-3851. |
| 110 | LIU Gang, ZHAO Zhijian, WU Tengfang, et al. On the nature of active sites of VOx/Al2O3 catalysts for propane dehydrogenation[J]. ACS Catalysis, 2016, 6(8): 5207-5214. |
| 111 | GONG Jinlong, ZHAO Zhijian, WU Tengfang, et al. Hydroxyl-mediated non-oxidative propane dehydrogenation over VOx/γ-Al2O3 catalysts with improved stability[J]. Angewandte Chemie: International Edition, 2018, 57(23): 6791-6795. |
| 112 | KITAGAWA Hiroyoshi, SENDODA Yoko, Yoshio ONO. Transformation of propane into aromatic hydrocarbons over ZSM-5 zeolites[J]. Journal of Catalysis, 1986, 101(1): 12-18. |
| 113 | CYBULSKIS V J, PRADHAN S U, JUAN J L Q, et al. The nature of the isolated gallium active center for propane dehydrogenation on Ga/SiO2[J]. Catalysis Letters, 2017, 147(5): 1252-1262. |
| 114 | CHEN Miao, XU Jie, SU Fangzheng, et al. Dehydrogenation of propane over spinel-type gallia-alumina solid solution catalysts[J]. Journal of Catalysis, 2008, 256(2): 293-300. |
| 115 | WNAG Pengzhao, XU Zhikang, ZHU Haibo. Unmodified bulk alumina as an efficient catalyst for propane dehydrogenation[J]. Catalysis Science & Technology, 2020, 10: 3537-3541. |
| 116 | YUN Jangho, LOBO R F. Catalytic dehydrogenation of propane over iron-silicate zeolites[J]. Journal of Catalysis, 2014, 312(15): 263-270. |
| 117 | CHEN Chong, ZHANG Shoumin, WANG Zheng, et al. Ultrasmall Co confined in the silanols of dealuminated beta zeolite: a highly active and selective catalyst for direct dehydrogenation of propane to propylene[J]. Journal of Catalysis, 2020, 383: 77-87. |
| 118 | OTROSHCHENKO T, KONDRATENKO V A, RODEMERCK U, et al. ZrO2-based unconventional catalysts for non-oxidative propane dehydrogenation: factors determining catalytic activity[J]. Journal of Catalysis, 2017, 348: 282-290. |
| 119 | WANG Guowei, ZHANG Huanling, ZHU Qingqing, et al. Sn-containing hexagonal mesoporous silica (HMS) for catalytic dehydrogenation of propane: an efficient strategy to enhance stability[J]. Journal of Catalysis, 2017, 351: 90-94. |
| 120 | WANG Haoren, HUANG Huiwen, BASHIR K, et al. Isolated Sn on mesoporous silica as a highly stable and selective catalyst for the Propane dehydrogenation[J]. Applied Catalysis A: General, 2020, 590: 117291-117299. |
| 121 | YUE Yuanyuan FU Jing, WANG Chuanming, et al. Propane dehydrogenation catalyzed by single Lewis acid site in Sn-Beta zeolite[J]. Journal of Catalysis, 2021, 395: 155-167. |
| 122 | HU Bo, SCHWEITZER N M, ZHANG Guanghui, et al. Isolated FeⅡ on silica as a selective propane dehydrogenation catalyst[J]. ACS Catalysis, 2015, 5(6): 3494-3503. |
| 123 | SCHWEITZER N M, HU Bo, DAS U. Propylene hydrogenation and propane dehydrogenation by a single-site Zn2+ on silica catalyst[J]. ACS Catalysis, 2014, 4(4): 1091-1098. |
| 124 | HU Bo, GETSOIAN A B, SCHWEITZER N M, et al. Selective propane dehydrogenation with single-site CoⅡ on SiO2 by a non-redox mechanism[J]. Journal of Catalysis, 2015, 322: 24-37. |
| 125 | DAI Yihu. γ-Al2O3 sheet-stabilized isolate Co2+ for catalytic propane dehydrogenation[J]. Journal of Catalysis, 2019, 381: 482-492. |
| 126 | SHAO Chuntao, LANG Wanzhong, YAN Xi, et al. Catalytic performance of gallium oxide based-catalysts for the propane dehydrogenation reaction: effects of support and loading amount[J]. RSC Advances, 2017, 7(8): 4710-4723. |
| 127 | BAI Peng, MA Zhipeng, LI Tingting, et al. Relationship between surface chemistry and catalytic performance of mesoporous γ-Al2O3 supported VOx catalyst in catalytic dehydrogenation of propane[J]. ACS Applied Materials & Interfaces, 2016, 8(39): 25979-25990. |
| 128 | LANG Wanzhong, HU Changlong, CHU Lianfeng, et al. Hydrothermally prepared chromia-alumina (xCr/Al2O3) catalysts with hierarchical structure for propane dehydrogenation[J]. RSC Advances, 2014, 4(70): 37107-37113. |
| 129 | HU Ping, LANG Wanzhong, YAN Xi, et al. Influence of gelation and calcination temperature on the structure-performance of porous VOx-SiO2 solids in non-oxidative propane dehydrogenation[J]. Journal of Catalysis, 2018, 358: 108-117. |
| 130 | SEARLES K, GEORGES S, SAFONOVA O, et al. Silica-supported isolated gallium sites as highly active, selective and stable propane dehydrogenation catalysts[J]. Chemical Science, 2017, 8(4): 2661-2666. |
| 131 | HU Bo, KIM W G, SULMONETTI T P, et al. Mesoporous CoAl2O4 spinel catalyst for non-oxidative propane dehydrogenation[J]. Chemcatchem, 2017, 9(17): 3330-3337. |
| 132 | WU Tengfang, LIU Gang, ZENG Liang, et al. Structure and catalytic consequence of Mg-modified VOx/Al2O3 catalysts for propane dehydrogenation[J]. AIChE Journal, 2017, 63(11): 4911-4919. |
| 133 | KIM T H, GIM M Y, SONG J H, et al. Deactivation behavior of CrOy/Al2O3-ZrO2 catalysts in the dehydrogenation of propane to propylene by lattice oxygen[J]. Catalysis Communications, 2017, 97(5): 37-41. |
| 134 | WANG Guowei, SHAN Honghong, GAO Yanan, et al. Effect of sulfate addition on the performance of Co/Al2O3 catalysts in catalytic dehydrogenation of propane[J]. Catalysis Communications, 2015, 60: 42-45. |
| 135 | SOKOLOV S, STOYANOVA M, RODEMERCK U, et al. Effect of support on selectivity and on-stream stability of surface VOx species in non-oxidative propane dehydrogenation[J]. Catalysis Science & Technology, 2014, 4(5): 1323-1332. |
| 136 | KUMAR M S, HAMMER N, CHEN D. The nature of active chromium species in Cr-catalysts for dehydrogenation of propane: new insights by a comprehensive spectroscopic study[J]. Journal of Catalysis, 2009, 261(1): 116-128. |
| 137 | PIOVANO A, GROPPO E, BRAGLIA L, et al. Insights into Cr/SiO2 catalysts during dehydrogenation of propane: an operando XAS investigation[J]. Catalysis Science & Technology, 2017, 7(8): 1690-1700. |
| 138 | REN Yingjie, WANG Jie, HUA Weiming, et al. Ga2O3/HZSM-48 for dehydrogenation of propane: effect of acidity and pore geometry of support[J]. Journal of Industrial & Engineering Chemistry, 2012, 18(2): 731-736. |
| 139 | GONG Ting, QIN Lijun, LU Jian, et al. ZnO modified ZSM-5 and Y zeolites fabricated by atomic layer deposition for propane conversion[J]. Physical Chemistry Chemical Physics, 2016, 18(1): 601-614. |
| 140 | CHEN Chong, HU Zhongpan, REN Jintao, et al. ZnO nanoclusters supported on dealuminated zeolite β as a novel catalyst for direct dehydrogenation of propane to propylene[J]. ChemCatChem, 2019, 11(2): 868-877. |
| 141 | AUROUX A, MONACI R, ROMBI E, et al. Acid sites investigation of simple and mixed oxides by TPD and microcalorimetric techniques[J]. Thermochimica Acta, 2001, 379(1/2): 227-231. |
| 142 | ADAM W, ANNA R, BARBARA M, et al. High-performance Cr-Zr-O and Cr-Zr-K-O catalysts prepared by nanocasting for dehydrogenation of propane to propene[J]. Catalysis Science & Technology, 2017, 7(24): 6059-6068. |
| 143 | ROMBI E, GAZZOLI D, CUTRUFELLO M G, et al. Modifications induced by potassium addition on chromia/alumina catalysts and their influence on the catalytic activity for the oxidative dehydrogenation of propane[J]. Applied Surface Science, 2010, 256(17): 5576-5580. |
| 144 | CABRERA F, ARDISSONE D, GORRIZ O F. Dehydrogenation of propane on chromia/alumina catalysts promoted by tin[J]. Catalysis Today, 2008, 133: 800-804. |
| 145 | LIU Lei, DENG Qingfang, YUAN Zhongyong. Ordered mesoporous carbon catalyst for dehydrogenation of propane to propylene[J]. Catalysis Communications, 2011, 47(29): 8334-8336. |
| 146 | LIU Lei, DENG Qingfeng, YUAN Zhongyong. HNO3-activated mesoporous carbon catalyst for direct dehydrogenation of propane to propylene[J]. Catalysis Communications, 2011, 16(1): 81-85. |
| 147 | LI Lina, ZHU Wenliang, LIU Yong, et al. Phosphorous-modified ordered mesoporous carbon for catalytic dehydrogenation of propane to propylene[J]. RSC Advances, 2015, 5(69): 56304-56310. |
| 148 | WANG Rui, SUN Xiaoyan, ZHANG Bingsen, et al. Hybrid nanocarbon as a catalyst for direct dehydrogenation of propane: formation of an active and selective core-shell sp2/sp3 nanocomposite structure[J]. Chemistry-A European Journal, 2014, 20(21): 6324-6331. |
| 149 | LIU Lei, DENG Qingfang, REN Tiezhen, et al. Synthesis of ordered mesoporous carbon materials and their catalytic performance in dehydrogenation of propane to propylene[J]. Catalysis Today, 2012, 186(1): 35-41. |
| 150 | HU Zhongpan, REN Jintao, YANG Dandan, et al. Mesoporous carbons as metal-free catalysts for propane dehydrogenation: effect of the pore structure and surface property[J]. Chinese Journal of Catalysis, 2019, 40(9): 1385-1394. |
| 151 | HU Zhongpan, CHEN Chong, REN Jintao, et al. Direct dehydrogenation of propane to propylene on surface-oxidized multiwall carbon nanotubes[J]. Applied Catalysis A: General, 2018, 559: 85-93. |
| 152 | HU Zhongpan, ZHANG Lingfeng, WANG Zheng, et al. Bean dregs‐derived hierarchical porous carbons as metal‐free catalysts for efficient dehydrogenation of propane to propylene[J]. Journal of Chemical Technology & Biotechnology, 2018, 93(12): 3410-3417. |
| 153 | PAN Shangfa, YIN Jianglong, ZHU Xuelian, et al. P-modified microporous carbon nanospheres for direct propane dehydrogenation reactions[J]. Carbon, 2019, 152: 855-864. |
| [1] | ZHANG Mingyan, LIU Yan, ZHANG Xueting, LIU Yake, LI Congju, ZHANG Xiuling. Research progress of non-noble metal bifunctional catalysts in zinc-air batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 276-286. |
| [2] | SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298. |
| [3] | XIE Luyao, CHEN Songzhe, WANG Laijun, ZHANG Ping. Platinum-based catalysts for SO2 depolarized electrolysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 299-309. |
| [4] | YANG Xiazhen, PENG Yifan, LIU Huazhang, HUO Chao. Regulation of active phase of fused iron catalyst and its catalytic performance of Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 310-318. |
| [5] | WANG Lele, YANG Wanrong, YAO Yan, LIU Tao, HE Chuan, LIU Xiao, SU Sheng, KONG Fanhai, ZHU Canghai, XIANG Jun. Influence of spent SCR catalyst blending on the characteristics and deNO x performance for new SCR catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 489-497. |
| [6] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [7] | CHENG Tao, CUI Ruili, SONG Junnan, ZHANG Tianqi, ZHANG Yunhe, LIANG Shijie, PU Shi. Analysis of impurity deposition and pressure drop increase mechanisms in residue hydrotreating unit [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4616-4627. |
| [8] | WANG Peng, SHI Huibing, ZHAO Deming, FENG Baolin, CHEN Qian, YANG Da. Recent advances on transition metal catalyzed carbonylation of chlorinated compounds [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4649-4666. |
| [9] | ZHANG Qi, ZHAO Hong, RONG Junfeng. Research progress of anti-toxicity electrocatalysts for oxygen reduction reaction in PEMFC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4677-4691. |
| [10] | GE Quanqian, XU Mai, LIANG Xian, WANG Fengwu. Research progress on the application of MOFs in photoelectrocatalysis [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4692-4705. |
| [11] | WANG Weitao, BAO Tingyu, JIANG Xulu, HE Zhenhong, WANG Kuan, YANG Yang, LIU Zhaotie. Oxidation of benzene to phenol over aldehyde-ketone resin based metal-free catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4706-4715. |
| [12] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [13] | XIANG Yang, HUANG Xun, WEI Zidong. Recent progresses in the activity and selectivity improvement of electrocatalytic organic synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4005-4014. |
| [14] | WANG Yaogang, HAN Zishan, GAO Jiachen, WANG Xinyu, LI Siqi, YANG Quanhong, WENG Zhe. Strategies for regulating product selectivity of copper-based catalysts in electrochemical CO2 reduction [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4043-4057. |
| [15] | LIU Yi, FANG Qiang, ZHONG Dazhong, ZHAO Qiang, LI Jinping. Cu facets regulation of Ag/Cu coupled catalysts for electrocatalytic reduction of carbon dioxide [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4136-4142. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||