Chemical Industry and Engineering Progress ›› 2019, Vol. 38 ›› Issue (05): 2285-2293.DOI: 10.16085/j.issn.1000-6613.2018-1299
• Materials science and technology • Previous Articles Next Articles
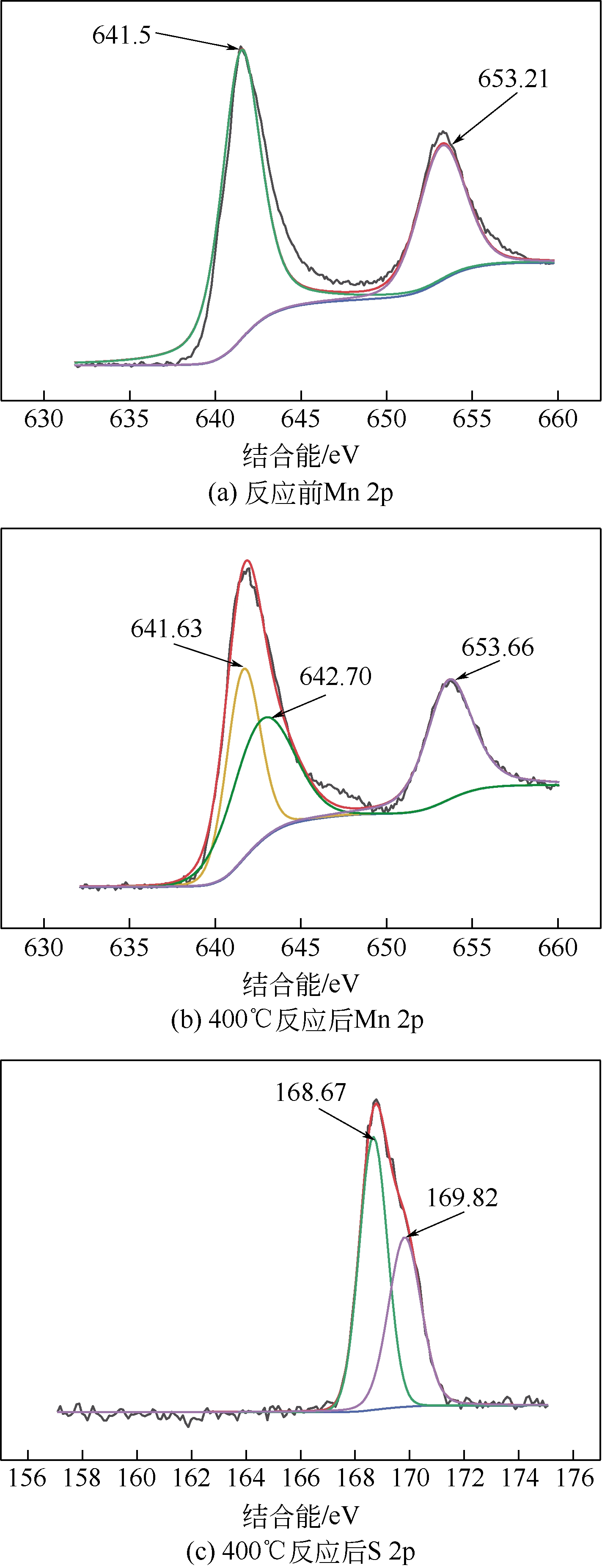
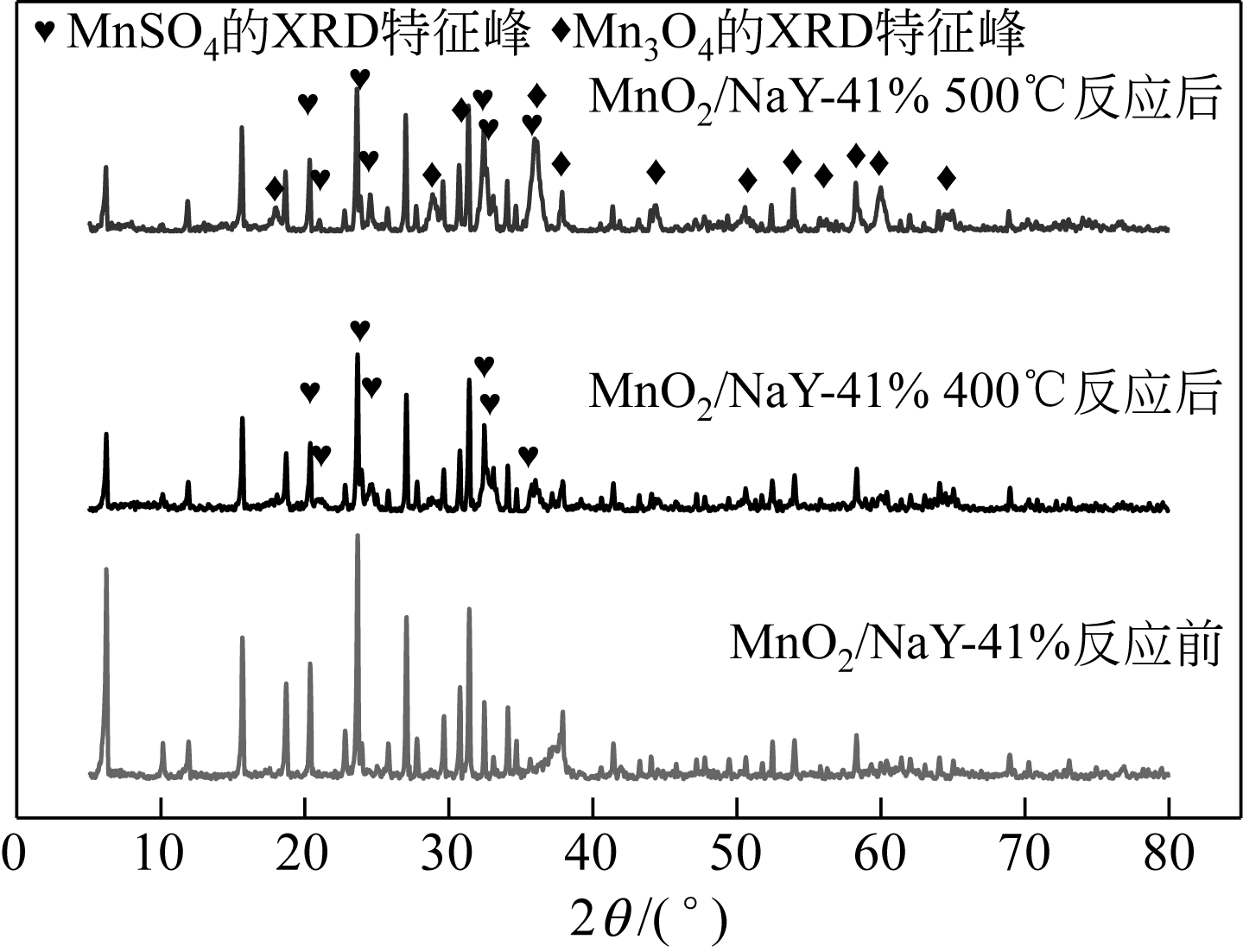
Preparation of MnO2/NaY composite and its performance inremoving SO2
Lintao CHEN1,2,5,6( ),Yugo OSAKA3,Xuecheng LIU1,4,5,6,Zhaohong HE1,5,6,Xing LI1,5,6,Hongyu HUANG1,5,6(
),Yugo OSAKA3,Xuecheng LIU1,4,5,6,Zhaohong HE1,5,6,Xing LI1,5,6,Hongyu HUANG1,5,6( )
)
- 1. Guangzhou Institute of Energy Conversion,Chinese Academy of Sciences,Guangzhou 510640, Guangdong, China
2. University of Chinese Academy of Sciences,Beijing 100049,China
3. Kanazawa University,Kanazawa 9201192,Japan
4. Chongqing Technology and Business University,Chongqing 400000,China
5. CAS Key Laboratory of Renewable Energy,Guangzhou 510640,Guangdong, China
6. Guangdong Provincial Key Laboratory of New and Renewable Energy Research and Development,Guangzhou 510640,Guangdong, China
-
Received:2018-06-22Revised:2018-12-13Online:2019-05-05Published:2019-05-05 -
Contact:Hongyu HUANG
MnO2/NaY复合材料的制备及其对SO2脱除性能
陈林涛1,2,5,6( ),大坂侑吾3,刘学成1,4,5,6,何兆红1,5,6,李兴1,5,6,黄宏宇1,5,6(
),大坂侑吾3,刘学成1,4,5,6,何兆红1,5,6,李兴1,5,6,黄宏宇1,5,6( )
)
- 1. 中国科学院广州能源研究所,广东 广州 510640
2. 中国科学院大学,北京 100049
3. 日本金泽大学,日本;金泽 9201192
4. 重庆工商大学,重庆 400000
5. 中国科学院可再生能源重点实验室,广东 广州 510640
6. 广东省新能源和可再生能源研究开发与应用重点实验室,广东 广州 510640
-
通讯作者:黄宏宇 -
作者简介:<named-content content-type="corresp-name">陈林涛</named-content>(1994—),男,硕士研究生,研究方向为干式脱硫技术。E-mail:<email>chenlt@ms.giec.ac.cn</email>。|黄宏宇,研究员,博士生导师,研究方向为节能与环保。E-mail:<email>huanghy@ms.giec.ac.cn</email>。 -
基金资助:中国科学院前沿科学重点研究项目(QYZDY-SSW-JSC038);广东省省级科技计划(2016A050502040);广东省新能源和可再生能源研究开发与应用重点实验室项目(Y807S21001)
CLC Number:
Cite this article
Lintao CHEN, Yugo OSAKA, Xuecheng LIU, Zhaohong HE, Xing LI, Hongyu HUANG. Preparation of MnO2/NaY composite and its performance inremoving SO2[J]. Chemical Industry and Engineering Progress, 2019, 38(05): 2285-2293.
陈林涛, 大坂侑吾, 刘学成, 何兆红, 李兴, 黄宏宇. MnO2/NaY复合材料的制备及其对SO2脱除性能[J]. 化工进展, 2019, 38(05): 2285-2293.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2018-1299
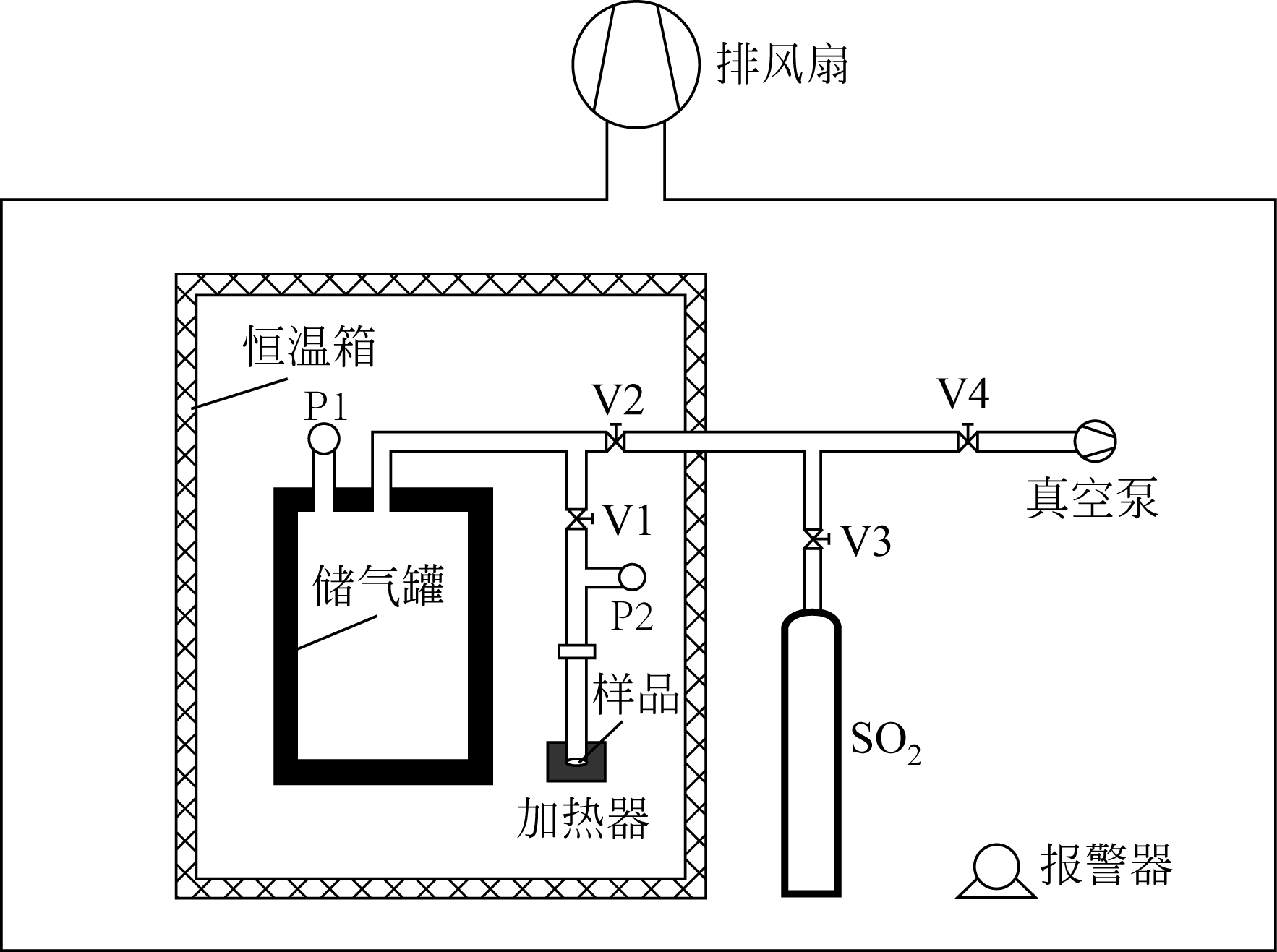
| 控制变量 | 数值 |
|---|---|
| 恒温室的温度/K | 308.15 |
| 样品的反应温度/K | 473.15~773.15 |
| 储气罐压力/Pa | 37~41 |
| 样品质量/g | 0.07~0.11 |
| 样品颗粒大小/目 | ≤100 |
| 控制变量 | 数值 |
|---|---|
| 恒温室的温度/K | 308.15 |
| 样品的反应温度/K | 473.15~773.15 |
| 储气罐压力/Pa | 37~41 |
| 样品质量/g | 0.07~0.11 |
| 样品颗粒大小/目 | ≤100 |
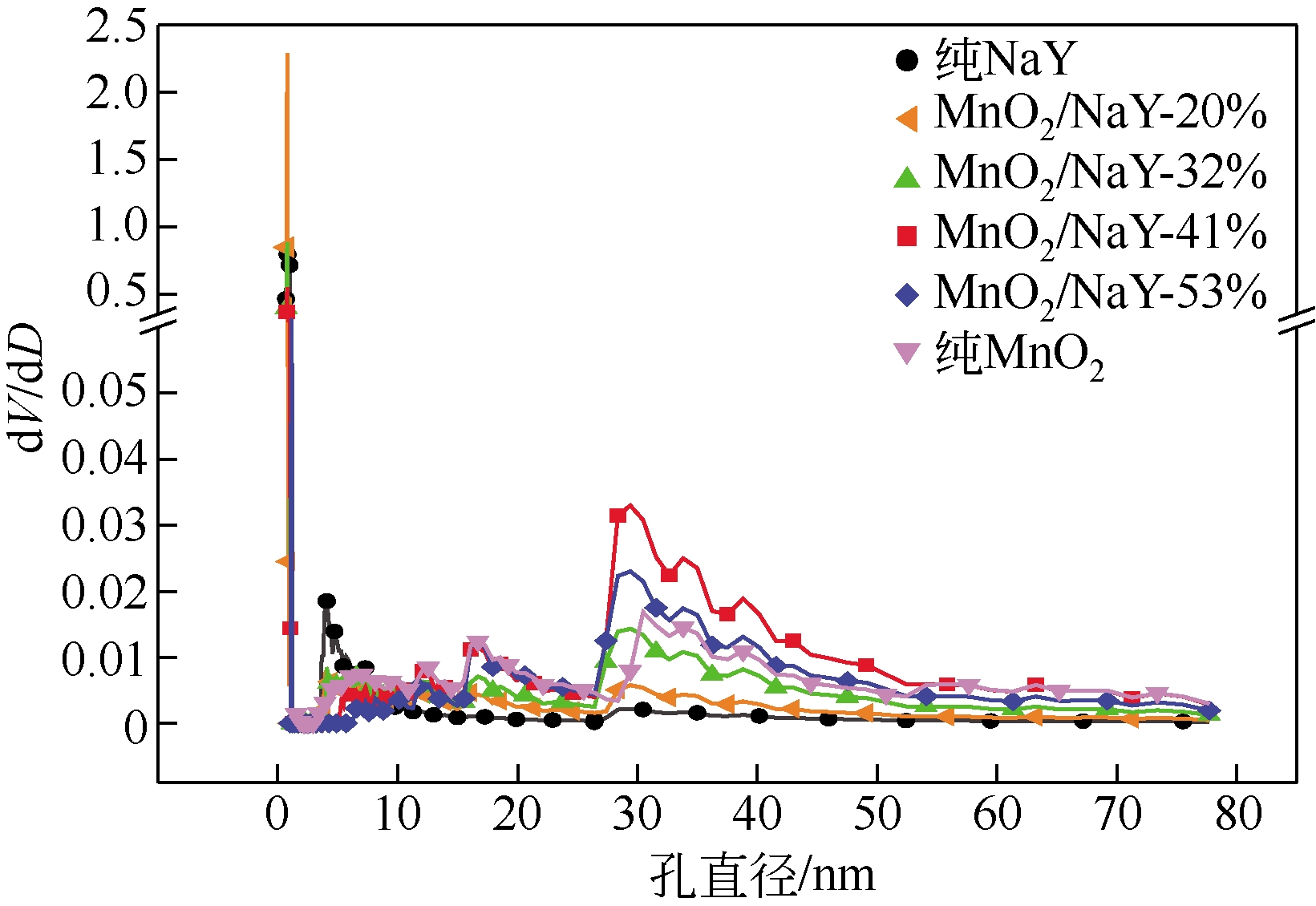
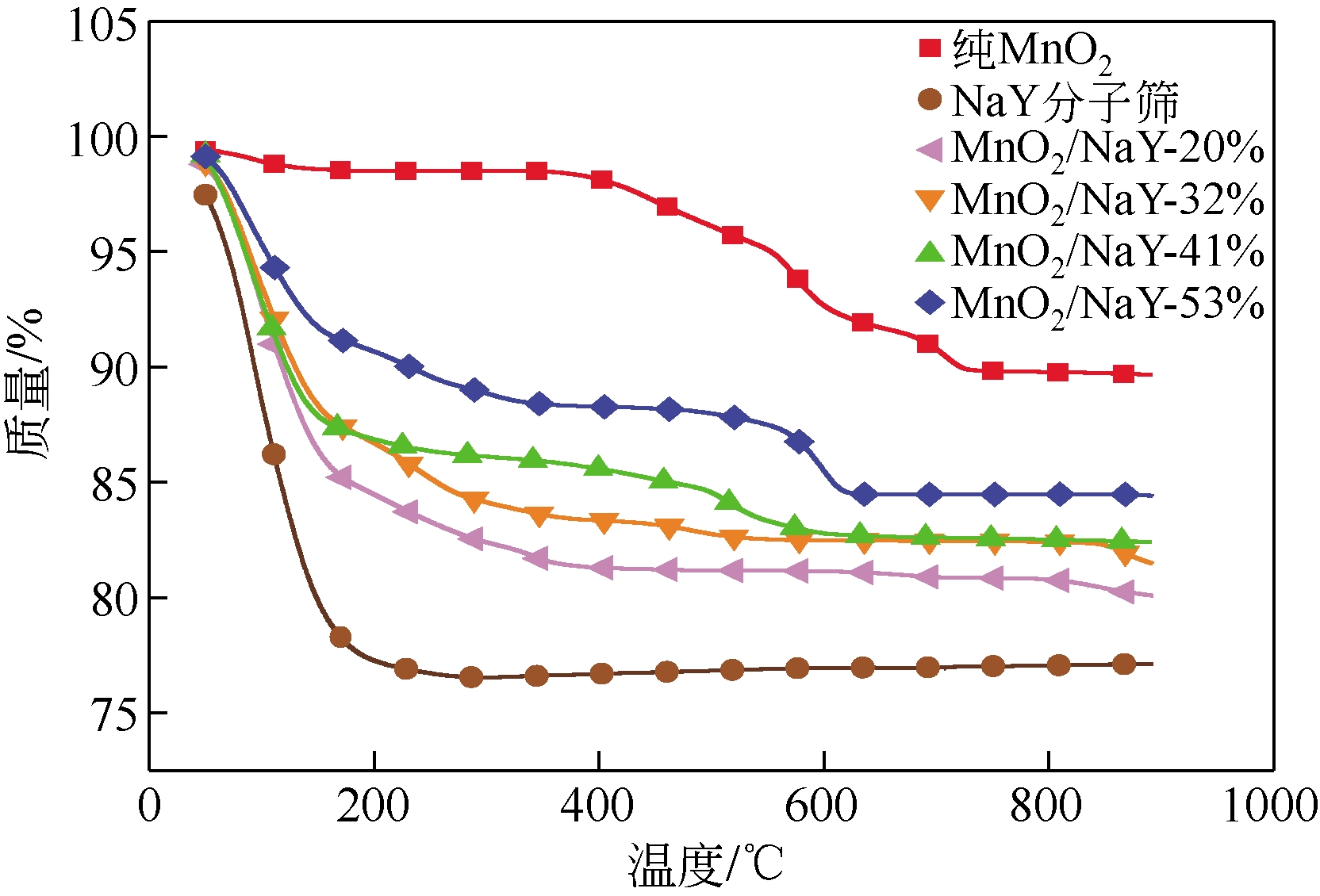
| 样品 | BET比表面积 /m2?g-1 | 孔容/mL?g-1 | 平均孔径/nm |
|---|---|---|---|
| 纯MnO2 | 91.75 | 0.5768 | 2.5148 |
| NaY分子筛 | 897.72 | 0.4459 | 1.9866 |
| MnO2/NaY-20% | 690.18 | 0.4511 | 2.6146 |
| MnO2/NaY-32% | 569.64 | 0.6048 | 4.2472 |
| MnO2/NaY-41% | 499.70 | 0.9660 | 7.7326 |
| MnO2/NaY-53% | 389.68 | 0.7166 | 7.3555 |
| 样品 | BET比表面积 /m2?g-1 | 孔容/mL?g-1 | 平均孔径/nm |
|---|---|---|---|
| 纯MnO2 | 91.75 | 0.5768 | 2.5148 |
| NaY分子筛 | 897.72 | 0.4459 | 1.9866 |
| MnO2/NaY-20% | 690.18 | 0.4511 | 2.6146 |
| MnO2/NaY-32% | 569.64 | 0.6048 | 4.2472 |
| MnO2/NaY-41% | 499.70 | 0.9660 | 7.7326 |
| MnO2/NaY-53% | 389.68 | 0.7166 | 7.3555 |
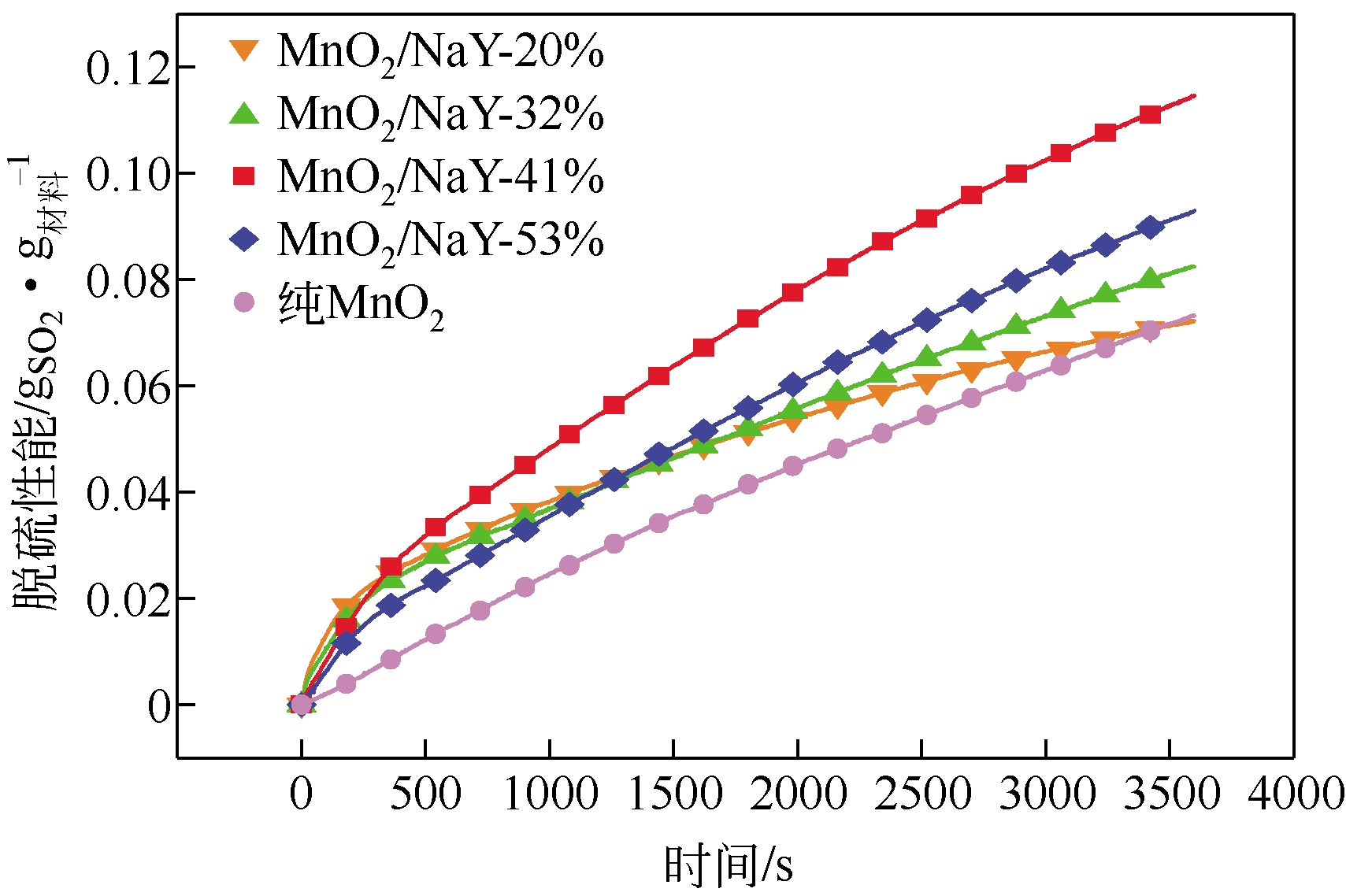
| 样品 | 第1h平均脱硫 速率( /mg | 脱硫容量 (吸附平衡) /mg | MnO2 转化率/% |
|---|---|---|---|
| 纯MnO2 | 73.37 | 198.51 | 26.99 |
| MnO2/NaY-20% | 72.11 | 104.63 | 71.11 |
| MnO2/NaY-32% | 82.69 | 155.74 | 66.16 |
| MnO2/NaY-41% | 114.56 | 206.11 | 68.33 |
| MnO2/NaY-53% | 92.78 | 215.74 | 55.34 |
| 样品 | 第1h平均脱硫 速率( /mg | 脱硫容量 (吸附平衡) /mg | MnO2 转化率/% |
|---|---|---|---|
| 纯MnO2 | 73.37 | 198.51 | 26.99 |
| MnO2/NaY-20% | 72.11 | 104.63 | 71.11 |
| MnO2/NaY-32% | 82.69 | 155.74 | 66.16 |
| MnO2/NaY-41% | 114.56 | 206.11 | 68.33 |
| MnO2/NaY-53% | 92.78 | 215.74 | 55.34 |
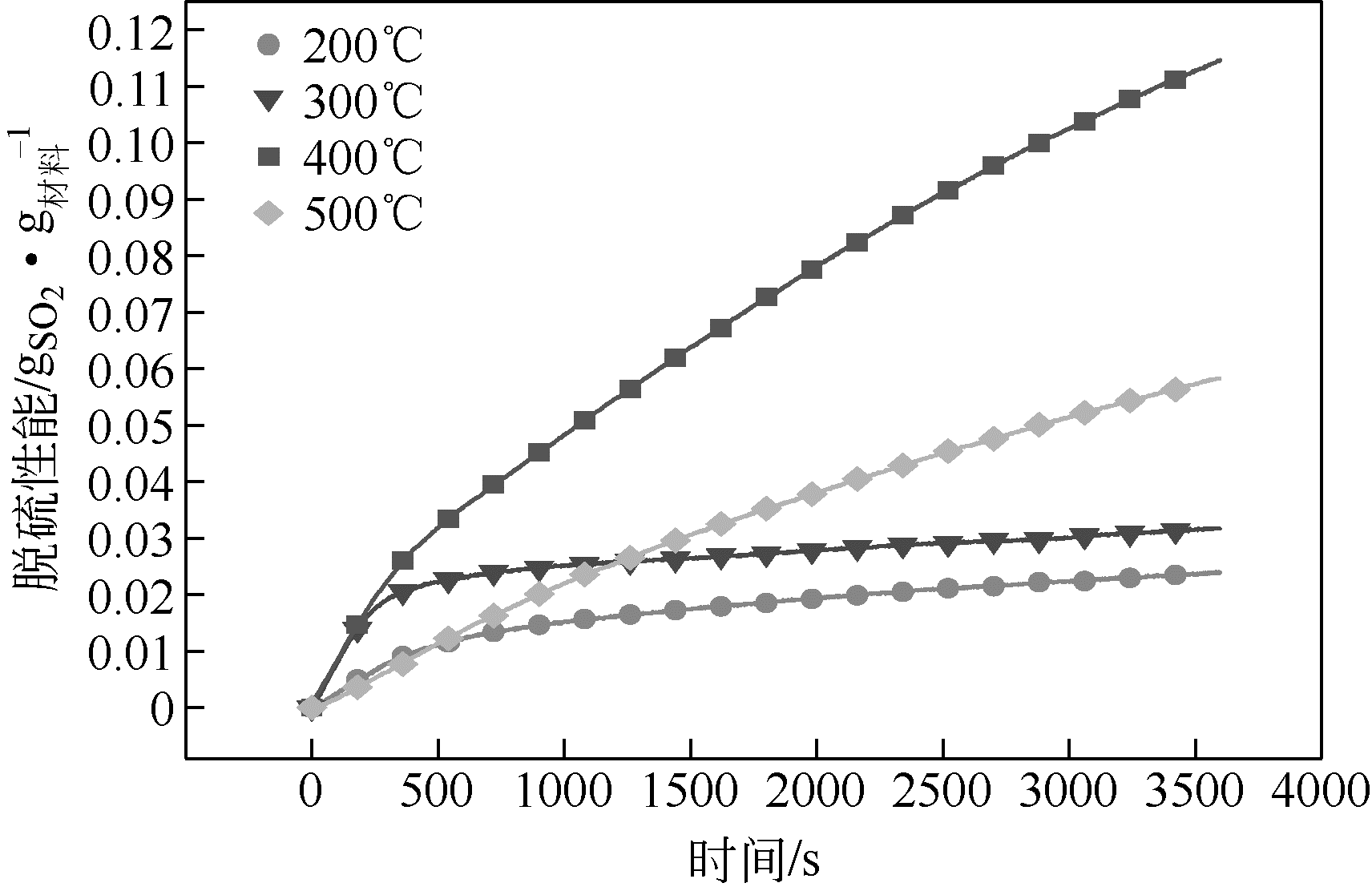
| 温度/℃ | 第1h平均脱硫 速率( /mg | 脱硫容量 (吸附平衡) /mg | MnO2转化率/% |
|---|---|---|---|
| 200 | 23.87 | 33.92 | 11.25 |
| 300 | 31.82 | 45.62 | 15.13 |
| 400 | 114.56 | 206.11 | 68.34 |
| 500 | 58.27 | 132.38 | 43.89 |
| 温度/℃ | 第1h平均脱硫 速率( /mg | 脱硫容量 (吸附平衡) /mg | MnO2转化率/% |
|---|---|---|---|
| 200 | 23.87 | 33.92 | 11.25 |
| 300 | 31.82 | 45.62 | 15.13 |
| 400 | 114.56 | 206.11 | 68.34 |
| 500 | 58.27 | 132.38 | 43.89 |
| 材料 | 比表面积/m2?g-1 | 脱硫容量/mg | 反应条件 | 最佳反应温度/℃ | |
|---|---|---|---|---|---|
| SO2浓度/μL?L-1 | 平衡气 | ||||
| CaO[ | 10 | 36 | 250 | 空气 | 325 |
| Ca(OH)2 [ | 16.4 | 32 | 250 | 空气 | 325 |
| MgO[ | 143 | 20 | 250 | 空气 | 325 |
| ZrO2 [ | 95.7 | 16 | 250 | 空气 | 325 |
| TiO2 [ | 120 | 36 | 常压 纯SO2 | 无 | 室温 |
| CuO-CeO2 [31] | 165 | 27 | 3600 | 氮气 | 500 |
| CuO/AC[32] | 844 | 44 | 200 | 氮气 | 250 |
| CuO/γ-Al2O3 [33] | 166 | 115 | 1500 | 氩气 | 400 |
| CuO/Y[34] | — | 160 | 3400 | 空气 | 450 |
| MnO2/AC[16] | 278 | 65.6 | 500 | 氮气 | 200 |
| MnO2/NaY-41%[本文] | 500 | 206 | 40Pa 纯SO2 | 无 | 400 |
| 材料 | 比表面积/m2?g-1 | 脱硫容量/mg | 反应条件 | 最佳反应温度/℃ | |
|---|---|---|---|---|---|
| SO2浓度/μL?L-1 | 平衡气 | ||||
| CaO[ | 10 | 36 | 250 | 空气 | 325 |
| Ca(OH)2 [ | 16.4 | 32 | 250 | 空气 | 325 |
| MgO[ | 143 | 20 | 250 | 空气 | 325 |
| ZrO2 [ | 95.7 | 16 | 250 | 空气 | 325 |
| TiO2 [ | 120 | 36 | 常压 纯SO2 | 无 | 室温 |
| CuO-CeO2 [31] | 165 | 27 | 3600 | 氮气 | 500 |
| CuO/AC[32] | 844 | 44 | 200 | 氮气 | 250 |
| CuO/γ-Al2O3 [33] | 166 | 115 | 1500 | 氩气 | 400 |
| CuO/Y[34] | — | 160 | 3400 | 空气 | 450 |
| MnO2/AC[16] | 278 | 65.6 | 500 | 氮气 | 200 |
| MnO2/NaY-41%[本文] | 500 | 206 | 40Pa 纯SO2 | 无 | 400 |
| 1 | LI L Y , KING D L . High-capacity sulfur dioxide absorbents for diesel emissions control[J]. Ind. Eng. Chem. Res., 2005, 44(1): 168-177. |
| 2 | BUENO-LÓPEZ A , GARCÍA-MARTÍNEZ J , GARCÍA-GARCÍA A , et al . Regenerable CaO sorbents for SO2 retention: carbonaceous versus inorganic dispersants[J]. Fuel, 2002, 81(18): 2435-2438. |
| 3 | LIU X C , OSAKA Y , HUANG H Y , et al . Development of high-performance SO2 trap materials in the low-temperature region for diesel exhaust emission control[J]. Sep. Purif. Technol. , 2016, 162: 127-133. |
| 4 | SINHA A K , SUZUKI K , TAKAHARA M , et al . Mesostructured manganese oxide/gold nanoparticle composites for extensive air purification[J]. Angew. Chem. , 2007, 46(16): 2949-2952. |
| 5 | LUO Y , LI D . Experimental study of nanometer TiO2 for use as an adsorbent for SO2 removal[J]. Dev. Chem. Eng. Mineral Process , 2002, 10(3/4): 443-457. |
| 6 | WANG S Q , LIU M Z , SUN L L , et al . Study on the mechanism of desulfurization and denitrification catalyzed by TiO2 in the combustion with biomass and coal[J]. Korean J. Chem. Eng., 2017, 34(6): 1882-1888. |
| 7 | KYLHAMMAR L , CARLSSON P A , INGELSTEN H H , et al . Regenerable ceria-based SO x traps for sulfur removal in lean exhausts[J]. Appl. Catal. B-Environ. , 2008, 84(1): 268-276. |
| 8 | YAN Z , WANG J P , ZOU R Q , et al . Hydrothermal synthesis of CeO2 nanoparticles on activated carbon with enhanced desulfurization activity[J]. Energy. Fuels. , 2012, 26(9): 5879-5886. |
| 9 | COOPER D A . Exhaust emissions from ships at berth[J]. Atmos. Environ. , 2003, 37(27): 3817-3830. |
| 10 | OSAKA Y , YAMADA K , TSUJIGUCHI T , et al . Study on the optimized design of deSO x filter operating at low temperature in diesel exhaust[J]. J. Chem. Eng. Jpn. , 2014, 47(7): 555-560. |
| 11 | MATHIEU Y , TZANIS L , SOULARD M , et al . Adsorption of SO x by oxide materials: a review[J]. Fuel Process Technol. , 2013, 114(3): 81-100. |
| 12 | WAQIF M , SAUR O , LAVALLEY J C , et al . Nature and mechanism of formation of sulfate species on copper/alumina sorbent-catalysts for SO2 removal[J]. J. Phys. Chem. , 1991, 22(33): 4051-4058. |
| 13 | PAYLISH J H , SONDREAL E A , MANN M D , et al . Status review of mercury control options for coal-fired power plants[J]. Fuel Process Technol. , 2003, 82(2): 89-165. |
| 14 | LIU S H , YAN N Q , LIU Z R , et al . Using bromine gas to enhance mercury removal from flue gas of coal-fired power plants[J]. Environ. Sci. Technol. , 2007, 41(4): 1405-1412. |
| 15 | HUTSON N D , ATTWOOD B C , SCHECKEL K G . XAS and XPS characterization of mercury binding on brominated activated carbon[J]. Environ. Sci. Technol. , 2007, 41(5): 1747-1752. |
| 16 | LIU X C , OSAKA Y , HUANG H Y , et al . Development of low-temperature desulfurization performance of a MnO2/AC composite for a combined SO2 trap for diesel exhaust[J]. RSC Adv., 2016, 6(98): 96367-96375. |
| 17 | RICHER M , BERNDERT H , ECKELT R , et al . Zeolite-mediated removal of NO x by NH3 from exhaust streams at low temperature[J]. Catal. Today , 1999, 54(4): 531-545. |
| 18 | RICHTER M , TRUNSCHKE A , BENTRUP U , et al . Selective catalytic reduction of nitric oxide by ammonia over egg-shell MnO x /NaY composite catalysts[J]. J. Catal. , 2002, 206(1): 98-113. |
| 19 | SCHRIMPF G , SCHLENKRICH M , BRICKMANN J , et al . Molecular dynamics simulation of zeolite NaY. A study of structure, dynamics, and thermalization of sorbates[J]. J. Phys. Chem. , 1992, 96(18): 7404-7410. |
| 20 | 赵文江, 刘靖, 朱金红, 等 . 纳米NaY分子筛的合成[J]. 工业催化, 2004, 12(4): 50-53. |
| ZHAO W J , LIU J , ZHU J H , et al . Synthesis of nanoparticle NaY molecular sieve[J]. Ind. Catal. , 2004, 12(4): 50-53. | |
| 21 | DRAGAN G . The individual adsorption of carbon dioxide and sulphur dioxide by Y zeolites[J]. Rev. Chim-Bucharest. , 2010, 61(9): 897-902. |
| 22 | MARCU I C , SANDULESCU I . Study of sulfur dioxide adsorption on Y zeolite[J]. J. Serb. Chem. Soc. , 2004, 69(7): 563-569. |
| 23 | JIA M L , BAI H F , ZHAO R G T , et al . Preparation of Au/CeO2 catalyst and its catalytic performance for HCHO oxidation[J]. J. Rare Earths , 2008, 26(4): 528-531. |
| 24 | 姜健准, 刘红梅, 张明森 . Ni/ZrO2催化剂的制备及甲烷分步水蒸气重整反应性能[J]. 化工进展, 2018, 37(1): 112-118. |
| JIANG J Z , LIU H M , ZHANG M S . Preparation of Ni/ZrO2 catalyst and its performance in the reaction of stepwise steam reforming of methane[J]. Chemical Industry and Engineering Progress , 2018, 37(1): 112-118. | |
| 25 | 侯影飞, 李力军, 蒋驰, 等 . 活性炭负载磷钨酸催化剂的制备及其催化氧化脱硫性能[J]. 化工进展, 2017, 36(11): 4072-4079. |
| HOU Y F , LI L J , JIANG C , et al . Preparation and performance of phosphotungstic acid/activated carbon catalyst for catalytic oxidative desulfurization[J]. Chemical Industry and Engineering Progress , 2017, 36(11): 4072-4079. | |
| 26 | 智佳 . 铈改性分子筛的制备及对水蒸气吸脱附性能的研究[D]. 重庆: 重庆大学, 2015. |
| ZHI J . Preparation and study on the performance of water vapor adsorption and desorption on modified molecular sieves with Ce as modifier[D]. Chongqing: Chongqing University, 2015. | |
| 27 | TSANG C , KIM J, MANTHIRAM A . Synthesis of manganese oxides by reduction of KMnO4 with KBH4 in aqueous solution[J]. J.Solid. State. Chem. , 1998, 137(1): 28-32. |
| 28 | WU Y , LU Y , SONG C , et al . A novel redox-precipitation method for the preparation of α-MnO2 with a high surface Mn4+ concentration and its activity toward complete catalytic oxidation of o-xylene[J]. Catal. Today , 2013, 201(1): 32-39. |
| 29 | XIA Y , MENG L , JIANG Y , et al . Facile preparation of MnO2 functionalized baker’s yeast composites and their adsorption mechanism for Cadmium[J]. Chem. Eng. J. , 2015, 259(1): 927-935. |
| 30 | SMIRNOV M Y , KALINKIN A V , PASHIS A V , et al . Comparative XPS study of Al2O3 and CeO2 sulfation in reactions with SO2, SO2+O2, SO2+H2O and SO2+O2+H2O[J]. Kinet. Catal. , 2003, 44(4): 575-583. |
| 31 | RODAS-GRAPAÍN A , ARENAS-ALATORRE J , GÓMEZ-CORTÉS A , et al . Catalytic properties of a CuO-CeO2 sorbentcatalyst for deSO x reaction[J]. Catal. Today , 2005, 107/108(15): 168-174. |
| 32 | TSENG H H , WEY M Y, FU C H . Carbon materials as catalyst supports for SO2 oxidation: catalytic activity of CuO-AC[J]. Carbon, 2003, 41(1): 139-149. |
| 33 | XIE G , LIU Z , ZHU Z , et al . Reductive regeneration of sulfated CuO/Al2O3 catalyst-sorbent in ammonia[J]. Appl. Catal. B-Environ. , 2003, 45(3): 213-221. |
| 34 | SUB S C, NIIYAMA H . Oxidative sorption of SO2 by Cu/zeolite[J]. Sekiyu Gakkaishi. , 1988, 31(2): 147-153. |
| [1] | WANG Shengyan, DENG Shuai, ZHAO Ruikai. Research progress on carbon dioxide capture technology based on electric swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 233-245. |
| [2] | WANG Yungang, JIAO Jian, DENG Shifeng, ZHAO Qinxin, SHAO Huaishuang. Experimental analysis of condensation heat transfer and synergistic desulfurization [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4230-4237. |
| [3] | WANG Xiaohan, ZHOU Yasong, YU Zhiqing, WEI Qiang, SUN Jinxiao, JIANG Peng. Synthesis and hydrocracking performance of Y molecular sieves with different crystal sizes [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4283-4295. |
| [4] | LU Yang, ZHOU Jinsong, ZHOU Qixin, WANG Tang, LIU Zhuang, LI Bohao, ZHOU Lingtao. Leaching mechanism of Hg-absorption products on CeO2/TiO2 sorbentsin syngas [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3875-3883. |
| [5] | HAN Hengwen, HAN Wei, LI Mingfeng. Research progress in olefin hydration process and the catalysts [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3489-3500. |
| [6] | WANG Darui, SUN Hongmin, XUE Mingwei, WANG Yiyan, LIU Wei, YANG Weimin. Efficient synthesis of fully crystalline ZSM-5 zeolite catalyst by microwave method and its catalytic performance [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3582-3588. |
| [7] | WANG Baowen, LIU Tongqing, ZHANG Gang, LI Weiguang, LIN Deshun, WANG Mengjia, MA Jingjing. Reaction characteristics of CuFe2O4 modified desulfurization slag oxygen carrier with lignite [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2884-2894. |
| [8] | WANG Zijian, KE Ming, SONG Zhaozheng, LI Jiahan, TONG Yanbing, SUN Jinru. Progress in alkylation of gasoline with molecular sieve catalyst for benzene reduction [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2371-2389. |
| [9] | HE Chuan, WU Guoxun, LI Ang, ZHANG Fajie, BIAN Zijun, LU Chengzheng, WANG Lipeng, ZHAO Min. Characteristics of calcium and magnesium deactivation and regeneration of waste incineration SCR catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2413-2420. |
| [10] | REN Zhongyuan, HE Jinlong, YUAN Qing. Research progress on intercrystalline defects control and remediation technologies for zeolite membranes [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2454-2463. |
| [11] | ZHAO Yao, ZHOU Zhihui, WU Hongdan, HU Chuanzhi, ZHANG Guochun, WU Ruipeng. Response surface analysis and optimization of membrane permeation vaporization by Silicalite-1 molecular sieve [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2586-2594. |
| [12] | ZHAO Chongyang, ZHAO Lei, SHI Xiangwen, HUANG Jun, LI Zhiyao, SHEN Kai, ZHANG Yaping. Effect of O2/H2O/SO2 on the adsorption of PbCl2 by modified iron-rich attapulgite at high temperature [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2190-2200. |
| [13] | NING Shuying, SU Yaxin, YANG Honghai, WEN Nini. Research progress on supported Cu-based zeolite catalysts for the selective catalytic reduction of NO x with hydrocarbons [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1308-1320. |
| [14] | ZHANG Chenguang, FENG Shuo, XING Yuye, SHEN Boxiong, SU Lichao. Research progress of isolated Cu2+ in copper based zeolite NH3-SCR catalyst for diesel vehicles [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1321-1331. |
| [15] | GUO Shuaishuai, CHEN Jinlu, JIN Liangchenglong, TAO Zui, CHEN Xiaoli, PENG Guowen. Research progress of porous aromatic frameworks based on uranium extraction from seawater [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1426-1436. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||