Chemical Industry and Engineering Progress ›› 2019, Vol. 38 ›› Issue (01): 692-706.DOI: 10.16085/j.issn.1000-6613.2018-0993
• Resources and environmental engineering • Previous Articles Next Articles
Research advances on adsorption of heavy metals by biochar
Chongqing WANG1( ),Hui WANG2,Xiaoyan JIANG1,Rong HUANG1,Yijun CAO1(
),Hui WANG2,Xiaoyan JIANG1,Rong HUANG1,Yijun CAO1( )
)
- 1. School of Chemical Engineering and Energy, Zhengzhou University, Zhengzhou 450001, Henan, China
2. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, Hunan, China
-
Received:2018-05-14Revised:2018-07-05Online:2019-01-05Published:2019-01-05 -
Contact:Yijun CAO
生物炭吸附重金属离子的研究进展
- 1. 郑州大学化工与能源学院,河南 郑州 450001
2. 中南大学化学化工学院,湖南 长沙 410083
-
通讯作者:曹亦俊 -
作者简介:王重庆(1990—),男,博士,讲师,研究方向为固体废弃物资源化和废水处理。E-mail:<email>zilangwang@126.com</email>。|曹亦俊,博士,教授,博士生导师,研究方向为微细粒浮选理论及工艺。E-mail:<email>yijuncao@126.com</email>。 -
基金资助:中国博士后科学基金项目(2018M630838);河南省高等学校重点科研项目(19B530002);中国博士后科学基金项目(2018M630838);河南省高等学校重点科研项目(19B530002)。
CLC Number:
Cite this article
Chongqing WANG, Hui WANG, Xiaoyan JIANG, Rong HUANG, Yijun CAO. Research advances on adsorption of heavy metals by biochar[J]. Chemical Industry and Engineering Progress, 2019, 38(01): 692-706.
王重庆, 王晖, 江小燕, 黄荣, 曹亦俊. 生物炭吸附重金属离子的研究进展[J]. 化工进展, 2019, 38(01): 692-706.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2018-0993
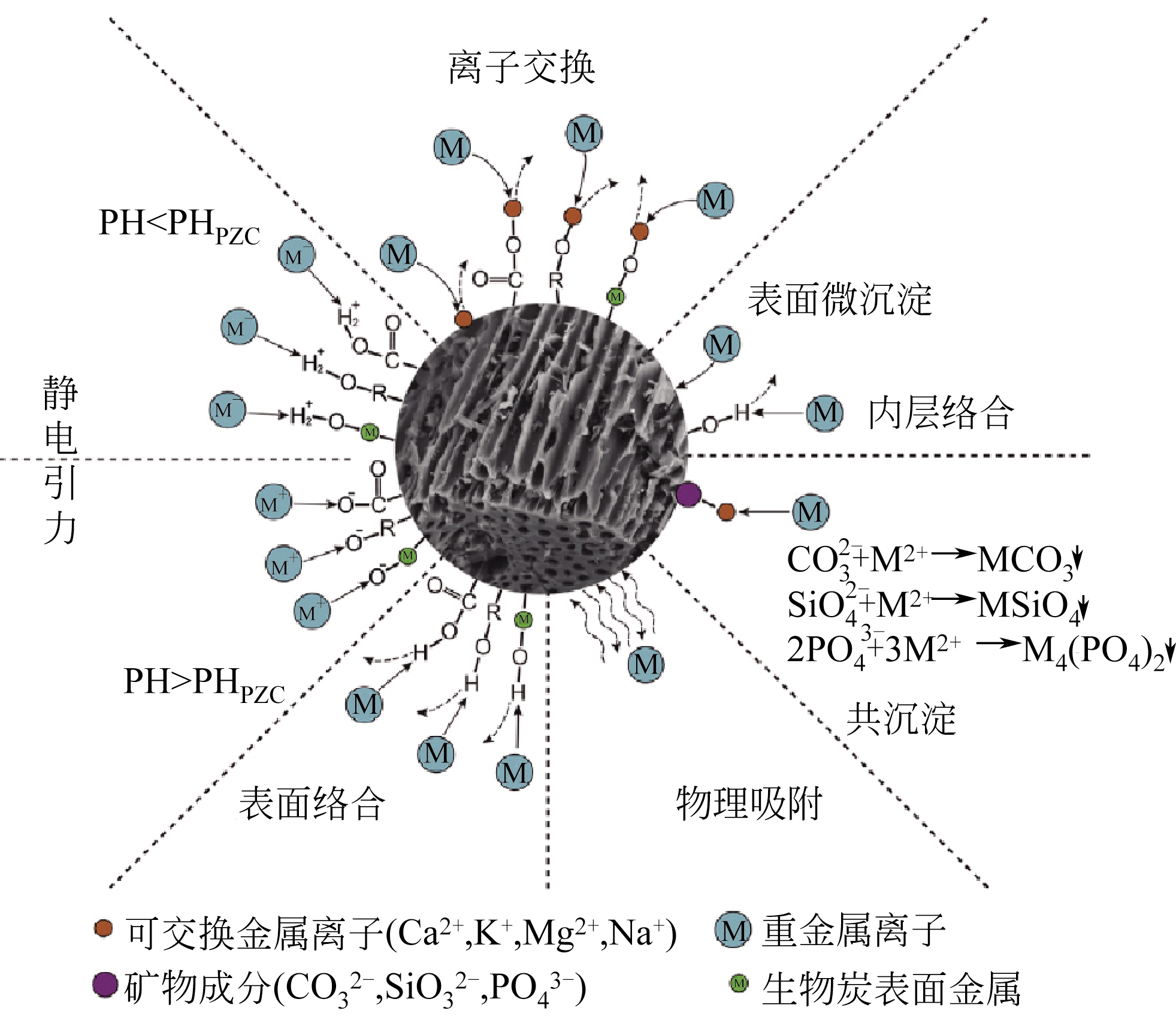
| 用途 | 作用 | 优点 | 缺点 |
|---|---|---|---|
| 土壤修复 | 碳封存、土壤改良 | 廉价、可持续性资源、保留水分和养分、降低肥料消费、减少温室气体释放 | 重金属和多环芳烃污染风险 |
| 吸附剂 | 吸附土壤和水体中的重金属和有机污染物 | 廉价、丰富的可持续资源、表面基团有利于 吸附 | 吸附性能随前体的不同变化较大、对有机/无机污染物修复效率不稳定 |
| 生物质废弃物管理 | 碳封存、减少废弃物排放 | 实现废弃生物质资源化、减少温室气体释放 | 存在污染的潜在风险 |
| 燃料电池 | 燃料电池燃料 | 可再生能源 | 高灰分、电压和能量输出较低 |
| 储存材料 | 二氧化碳封存、氢气储存 | 廉价、丰富的可持续资源 | 需要表面处理 |
| 催化剂 | 负载活性组分、催化作用 | 共催化、廉价、易于回收负载的金属 | 效率低、耐磨性差 |
| 用途 | 作用 | 优点 | 缺点 |
|---|---|---|---|
| 土壤修复 | 碳封存、土壤改良 | 廉价、可持续性资源、保留水分和养分、降低肥料消费、减少温室气体释放 | 重金属和多环芳烃污染风险 |
| 吸附剂 | 吸附土壤和水体中的重金属和有机污染物 | 廉价、丰富的可持续资源、表面基团有利于 吸附 | 吸附性能随前体的不同变化较大、对有机/无机污染物修复效率不稳定 |
| 生物质废弃物管理 | 碳封存、减少废弃物排放 | 实现废弃生物质资源化、减少温室气体释放 | 存在污染的潜在风险 |
| 燃料电池 | 燃料电池燃料 | 可再生能源 | 高灰分、电压和能量输出较低 |
| 储存材料 | 二氧化碳封存、氢气储存 | 廉价、丰富的可持续资源 | 需要表面处理 |
| 催化剂 | 负载活性组分、催化作用 | 共催化、廉价、易于回收负载的金属 | 效率低、耐磨性差 |
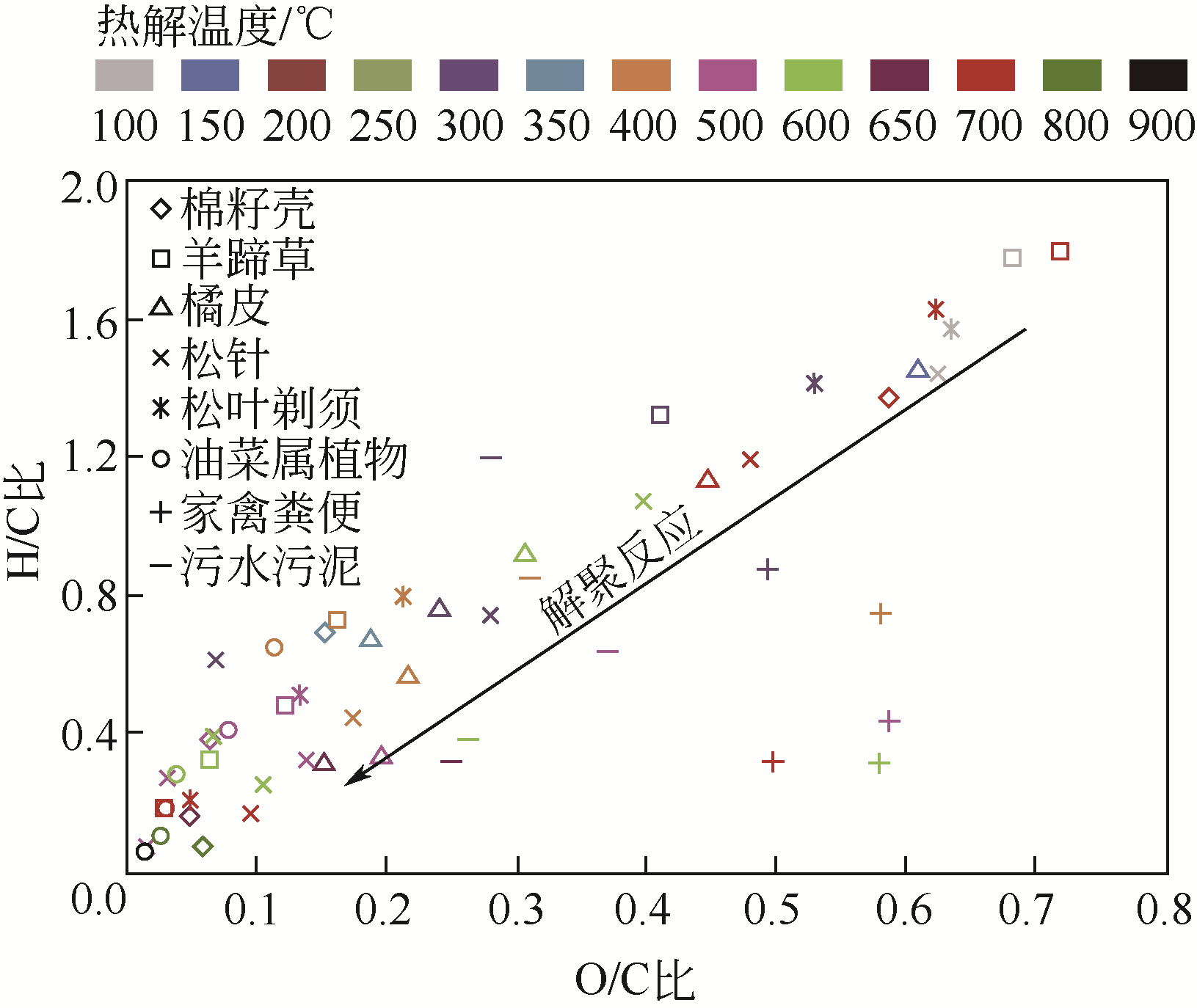
| 工艺 | 温度 /℃ | 加热速率 /℃·min-1 | 停留时间 | 目标产物 | 生物炭产率/% |
|---|---|---|---|---|---|
| 快速热解 | 400~600 | 约60000 | 数秒 | 生物油 | 10~20 |
| 慢速热解 | 350~800 | <10 | 数分到数小时 | 生物炭 | 20~40 |
| 气化 | 700~1500 | >100 | 数秒到数分 | 合成气 | 约10 |
| 水热炭化 | 175~250 | <10 | 数小时 | 水热炭 | 30~60 |
| 烘焙 | 200~300 | <10 | 数分到数小时 | 坚硬生物炭 | 67~84 |
| 工艺 | 温度 /℃ | 加热速率 /℃·min-1 | 停留时间 | 目标产物 | 生物炭产率/% |
|---|---|---|---|---|---|
| 快速热解 | 400~600 | 约60000 | 数秒 | 生物油 | 10~20 |
| 慢速热解 | 350~800 | <10 | 数分到数小时 | 生物炭 | 20~40 |
| 气化 | 700~1500 | >100 | 数秒到数分 | 合成气 | 约10 |
| 水热炭化 | 175~250 | <10 | 数小时 | 水热炭 | 30~60 |
| 烘焙 | 200~300 | <10 | 数分到数小时 | 坚硬生物炭 | 67~84 |
| 动力学模型 | 线性方程 | 线性拟合 | 特征 参数 |
|---|---|---|---|
| 准一级动力学 | | ln(Q e-Qt )对t | Q e,k 1 |
| 准二级动力学 | | t/Qt 对t | Q e,k 2 |
| 内扩散动力学 | | Qt 对t 0.5 | k |
| 液膜扩散动力学 | | ln(1-Qt /Q e)对t | k |
| Elovich动力学 | | Qt 对lnt | α,β |
| 动力学模型 | 线性方程 | 线性拟合 | 特征 参数 |
|---|---|---|---|
| 准一级动力学 | | ln(Q e-Qt )对t | Q e,k 1 |
| 准二级动力学 | | t/Qt 对t | Q e,k 2 |
| 内扩散动力学 | | Qt 对t 0.5 | k |
| 液膜扩散动力学 | | ln(1-Qt /Q e)对t | k |
| Elovich动力学 | | Qt 对lnt | α,β |
| 模型 | 非线性方程 | 线性方程 | 线性拟合 | 特征参数 |
|---|---|---|---|---|
| Langmuir | | | C e/Q e对C e | Q m,b,R L |
| Freundlich | | | lgQ e对lgC e | n,K F |
| Dubinin-Radushkevich | | | lnQ e对ε2 | Β,ε,E |
| Temkin | | | Q e对lnC e | b,A |
| Flory–Huggins | | | lg | θ,K |
| Hill | | | lg | K , n |
| Sips | | | ln | K,a,β |
| Toth | | | ln | K,a. ,n |
| 模型 | 非线性方程 | 线性方程 | 线性拟合 | 特征参数 |
|---|---|---|---|---|
| Langmuir | | | C e/Q e对C e | Q m,b,R L |
| Freundlich | | | lgQ e对lgC e | n,K F |
| Dubinin-Radushkevich | | | lnQ e对ε2 | Β,ε,E |
| Temkin | | | Q e对lnC e | b,A |
| Flory–Huggins | | | lg | θ,K |
| Hill | | | lg | K , n |
| Sips | | | ln | K,a,β |
| Toth | | | ln | K,a. ,n |
| 1 | XIE T , REDDY K R , WANG C , et al . Characteristics and applications of biochar for environmental remediation: a review[J]. Critical Reviews in Environmental Science and Technology, 2015, 45(9): 939-969. |
| 2 | LEHMANN J , JOSEPH S . Biochar for environmental management: science, technology and implementation [M]. 2nd ed. London: Routledge, 2015. |
| 3 | VERHEIJEN F , JEFFERY S , BASTOS A C , et al . Biochar application to soils, a critical scientific review of effects on soil properties , processes and functions[R]. European Commission, Italy, 2010. |
| 4 | SHACKLEY S , CARTER S , KNOWLES T , et al . Sustainable gasification-biochar systems? A case-study of rice-husk gasification in Cambodia. Part Ⅰ: Context, chemical properties, environmental and health and safety issues[J]. Energy Policy, 2012, 42: 49-58. |
| 5 | International Biochar Initiative . Standardized product definition and product testing guidelines for biochar that is used in soil [EB/ OL]. . |
| 6 | LIBRA J A , RO K S , KAMMANN C , et al . Hydrothermal carbonization of biomass residuals: a comparative review of the chemistry, processes and applications of wet and dry pyrolysis[J]. Biofuels, 2011, 2(1): 71-106. |
| 7 | WOOLF D , AMONETTE J E , STREET-PERROTT F A , et al . Sustainable biochar to mitigate global climate change[J]. Nature Communications, 2010, 56: 1-9. |
| 8 | ROBERTS K G , GLOY B A , JOSEPH S , et al . Life cycle assessment of biochar systems: estimating the energetic, economic, and climate change potential[J]. Environmental Science & Technology, 2009, 44(2): 827-833. |
| 9 | QIAN K , KUMAR A , ZHANG H , et al . Recent advances in utilization of biochar[J]. Renewable and Sustainable Energy Reviews, 2015, 42: 1055-1064. |
| 10 | LAIRD D , FLEMING P , WANG B , et al . Biochar impact on nutrient leaching from a Midwestern agricultural soil[J]. Geoderma, 2010, 158(3/4): 436-442. |
| 11 | WARNOCK D D , LEHMANN J , KUYPER T W , et al . Mycorrhizal responses to biochar in soil-concepts and mechanisms[J]. Plant and Soil, 2007, 300(1/2): 9-20. |
| 12 | YU X Y , MU C L , GU C , et al . Impact of woodchip biochar amendment on the sorption and dissipation of pesticide acetamiprid in agricultural soils[J]. Chemosphere, 2011, 85(8): 1284-1289. |
| 13 | SINGH B P , COWIE A L , SMERNIK R J . Biochar carbon stability in a clayey soil as a function of feedstock and pyrolysis temperature[J]. Environmental Science & Technology, 2012, 46(21): 11770-11778. |
| 14 | LAIRD D A , BROWN R C , AMONETTE J E , et al . Review of the pyrolysis platform for coproducing bio‐oil and biochar[J]. Biofuels, Bioproducts and Biorefining, 2009, 3(5): 547-562. |
| 15 | LEHMANN J , GAUNT J , RONDON M . Bio-char sequestration in terrestrial ecosystems—A review[J]. Mitigation and Adaptation Strategies for Global Change, 2006, 11(2): 403-427. |
| 16 | BOLAN N S , THANGARAJAN R , SESHADRI B , et al . Landfills as a biorefinery to produce biomass and capture biogas[J]. Bioresource Technology, 2013, 135: 578-587. |
| 17 | MOHAN D , PITTMAN C U , STEELE P H . Pyrolysis of wood/biomass for bio-oil: a critical review[J]. Energy & Fuels, 2006, 20(3): 848-889. |
| 18 | WOOLF D . Biochar as a soil amendment: a review of the environmental implications[R]. 2008. http://orgprints.org/13268/1/Biochar_as_a_soil_amendment_-_a_review.pdf. |
| 19 | MOHAN D , SARSWAT A , OK Y S , et al . Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review[J]. Bioresource Technology, 2014, 160: 191-202. |
| 20 | YAN Q , WAN C , LIU J , et al . Iron nanoparticles in situ encapsulated in biochar-based carbon as an effective catalyst for the conversion of biomass-derived syngas to liquid hydrocarbons[J]. Green Chemistry, 2013, 15(6): 1631-1640. |
| 21 | KASTNER J R , MILLER J , GELLER D P , et al . Catalytic esterification of fatty acids using solid acid catalysts generated from biochar and activated carbon[J]. Catalysis Today, 2012, 190(1): 122-132. |
| 22 | QIN J , CHEN Q , SUN M , et al . Pyrolysis temperature-induced changes in the catalytic characteristics of rice husk-derived biochar during 1,3-dichloropropene degradation[J]. Chemical Engineering Journal, 2017, 330: 804-812. |
| 23 | MIAN M M , LIU G . Recent progress in biochar-supported photocatalysts: synthesis, role of biochar, and applications[J]. RSC Advances, 2018, 8(26): 14237-14248. |
| 24 | 王艳, 李春花, 龚畏, 等 . Fe/生物炭活化过硫酸盐降解偶氮染料金橙Ⅱ[J]. 应用化工, 2017, 46(12): 2328-2330. |
| WANG Y , LI C H , GONG W , et al . Study on azo dyes Orange Ⅱ, removal using Fe/BC activated persulfate [J]. Applied Chemical Industry, 2017, 46(12): 2328-2330. | |
| 25 | KHATAEE A , KAYAN B , GHOLAMI P , et al . Sonocatalytic degradation of Reactive Yellow 39 using synthesized ZrO2 nanoparticles on biochar[J]. Ultrasonics Sonochemistry, 2017, 39: 540-549. |
| 26 | PARK J H , WANG J J , XIAO R , et al . Degradation of Orange G by Fenton-like reaction with Fe-impregnated biochar catalyst[J]. Bioresource Technology, 2018, 249: 368-376. |
| 27 | HOUBEN D , EVRARD L , SONNET P . Mobility, bioavailability and pH-dependent leaching of cadmium, zinc and lead in a contaminated soil amended with biochar[J]. Chemosphere, 2013, 92(11): 1450-1457. |
| 28 | XU R , XIAO S , YUAN J , et al . Adsorption of methyl violet from aqueous solutions by the biochars derived from crop residues[J]. Bioresource Technology, 2011, 102(22): 10293-10298. |
| 29 | CANTRELL K B , HUNT P G , UCHIMIYA M , et al . Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar[J]. Bioresource Technology, 2012, 107: 419-428. |
| 30 | KEILUWEIT M , NICO P S , JOHNSON M G , et al . Dynamic molecular structure of plant biomass-derived black carbon (biochar)[J]. Environmental Science & Technology, 2010, 44(4): 1247-1253. |
| 31 | UCHIMIYA M , CHANG S C , KLASSON K T . Screening biochars for heavy metal retention in soil: role of oxygen functional groups[J]. Journal of Hazardous Materials, 2011, 190(1/2/3): 432-441. |
| 32 | AHMAD M , RAJAPAKSHA A U , LIM J E , et al . Biochar as a sorbent for contaminant management in soil and water: a review[J]. Chemosphere, 2014, 99: 19-33. |
| 33 | XU X , CAO X , ZHAO L . Comparison of rice husk-and dairy manure-derived biochars for simultaneously removing heavy metals from aqueous solutions: role of mineral components in biochars[J]. Chemosphere, 2013, 92(8): 955-961. |
| 34 | DING W , DONG X , IME I M , et al . Pyrolytic temperatures impact lead sorption mechanisms by bagasse biochars[J]. Chemosphere, 2014, 105: 68-74. |
| 35 | KUMAR S , LOGANATHAN V A , GUPTA R B , et al . An assessment of U(Ⅵ) removal from groundwater using biochar produced from hydrothermal carbonization[J]. Journal of Environmental Management, 2011, 92(10): 2504-2512. |
| 36 | 常皓, 柴立元, 王云燕, 等 . Cu2+-H2O 系羟合配离子配位平衡研究[J]. 矿冶工程, 2007, 27(6): 37-40. |
| CHANG H , CHAI L Y , WANG Y Y , et al . Study on equilibrium of hydroxyl complex ions in Cu2+H2O system [J]. Mining and Metallurgical Engineering, 2007, 27(6): 37-40. | |
| 37 | LU H , ZHANG W , YANG Y , et al . Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar[J]. Water Research, 2012, 46(3): 854-862. |
| 38 | ZHANG W , MAO S , CHEN H , et al . Pb(Ⅱ) and Cr(Ⅵ) sorption by biochars pyrolyzed from the municipal wastewater sludge under different heating conditions[J]. Bioresource Technology, 2013, 147: 545-552. |
| 39 | KONG H , HE J , GAO Y , et al . Cosorption of phenanthrene and mercury (Ⅱ) from aqueous solution by soybean stalk-based biochar[J]. Journal of Agricultural and Food Chemistry, 2011, 59(22): 12116-12123. |
| 40 | TAN K L , HAMEED B H . Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions[J]. Journal of the Taiwan Institute of Chemical Engineers, 2017, 74: 25-48. |
| 41 | FEBRIANTO J , KOSASIH A N , SUNARSO J , et al . Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: a summary of recent studies[J]. Journal of Hazardous Materials, 2009, 162(2/3): 616-645. |
| 42 | FAROOQ U , KOZINSKI J A , KHAN M A , et al . Biosorption of heavy metal ions using wheat based biosorbents—A review of the recent literature[J]. Bioresource Technology, 2010, 101(14): 5043-5053. |
| 43 | FOO K Y , HAMEED B H . Insights into the modeling of adsorption isotherm systems[J]. Chemical Engineering Journal, 2010, 156(1): 2-10. |
| 44 | SUD D , MAHAJAN G , KAUR M P . Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions—A review[J]. Bioresource Technology, 2008, 99(14): 6017-6027. |
| 45 | TAN X , LIU Y , ZENG G , et al . Application of biochar for the removal of pollutants from aqueous solutions[J]. Chemosphere, 2015, 125: 70-85. |
| 46 | DONG X , MA L Q, LI Y . Characteristics and mechanisms of hexavalent chromium removal by biochar from sugar beet tailing[J]. Journal of Hazardous Materials, 2011, 190(1/2/3): 909-915. |
| 47 | CAO X , MA L, GAO B , et al . Dairy-manure derived biochar effectively sorbs lead and atrazine[J]. Environmental Science & Technology, 2009, 43(9): 3285-3291. |
| 48 | DING W , DONG X , IME I M , et al . Pyrolytic temperatures impact lead sorption mechanisms by bagasse biochars[J]. Chemosphere, 2014, 105: 68-74. |
| 49 | SIZMUR T , FRESNO T , AKGÜL G , et al . Biochar modification to enhance sorption of inorganics from water[J]. Bioresource Technology, 2017, 246: 34-47. |
| 50 | HO S H , ZHU S , CHANG J S . Recent advances in nanoscale-metal assisted biochar derived from waste biomass used for heavy metals removal[J]. Bioresource Technology, 2017, 246: 123-134. |
| 51 | SHIM T , YOO J , RYU C , et al . Effect of steam activation of biochar produced from a giant Miscanthus on copper sorption and toxicity[J]. Bioresource Technology, 2015, 197: 85-90. |
| 52 | HO S H , YANG Z , NAGARAJAN D , et al . High-efficiency removal of lead from wastewater by biochar derived from anaerobic digestion sludge[J]. Bioresource Technology, 2017, 246: 142-149. |
| 53 | SUN K , TANG J , GONG Y , et al . Characterization of potassium hydroxide (KOH) modified hydrochars from different feedstocks for enhanced removal of heavy metals from water[J]. Environmental Science and Pollution Research, 2015, 22(21): 16640-16651. |
| 54 | PENG H , GAO P , CHU G , et al . Enhanced adsorption of Cu(Ⅱ) and Cd(Ⅱ) by phosphoric acid-modified biochars[J]. Environmental Pollution, 2017, 229: 846-853. |
| 55 | ZHAO N , ZHAO C , LV Y , et al . Adsorption and coadsorption mechanisms of Cr(Ⅰ) and organic contaminants on H3PO4 treated biochar[J]. Chemosphere, 2017, 186: 422-429. |
| 56 | ZHAO T , YAO Y , LI D , et al . Facile low-temperature one-step synthesis of pomelo peel biochar under air atmosphere and its adsorption behaviors for Ag(Ⅰ) and Pb(Ⅱ)[J]. Science of the Total Environment, 2018, 640: 73-79. |
| 57 | ZHOU N , CHEN H , FENG Q , et al . Effect of phosphoric acid on the surface properties and Pb(Ⅱ) adsorption mechanisms of hydrochars prepared from fresh banana peels[J]. Journal of Cleaner Production, 2017, 165: 221-230. |
| 58 | WANG Q , WANG B , LEE X , et al . Sorption and desorption of Pb(Ⅱ) to biochar as affected by oxidation and pH[J]. Science of the Total Environment, 2018, 634: 188-194. |
| 59 | WANG Y , LIU R . H2O2 treatment enhanced the heavy metals removal by manure biochar in aqueous solutions[J]. Science of the Total Environment, 2018, 628: 1139-1148. |
| 60 | CIBATI A , FOEREID B , BISSESSUR A , et al . Assessment of Miscanthus× giganteus derived biochar as copper and zinc adsorbent: study of the effect of pyrolysis temperature, pH and hydrogen peroxide modification[J]. Journal of Cleaner Production, 2017, 162: 1285-1296. |
| 61 | HO S H , WANG D , WEI Z , et al . Lead removal by a magnetic biochar derived from persulfate-ZVI treated sludge together with one-pot pyrolysis[J]. Bioresource Technology, 2018, 247: 463-470. |
| 62 | ZHANG S , ZHANG H , CAI J , et al . Evaluation and prediction of cadmium removal from aqueous solution by phosphate-modified activated bamboo biochar[J]. Energy & Fuels, 2017, 32(4): 4469-4477. |
| 63 | TAN G , SUN W , XU Y , et al . Sorption of mercury (Ⅱ) and atrazine by biochar, modified biochars and biochar based activated carbon in aqueous solution[J]. Bioresource Technology, 2016, 211: 727-735. |
| 64 | WANG S , GAO B , ZIMMERMAN A R , et al . Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite[J]. Bioresource Technology, 2015, 175: 391-395. |
| 65 | HAN Y , CAO X , OUYANG X , et al . Adsorption kinetics of magnetic biochar derived from peanut hull on removal of Cr(Ⅵ) from aqueous solution: effects of production conditions and particle size[J]. Chemosphere, 2016, 145: 336-341. |
| 66 | BAIG S A , ZHU J , MUHAMMAD N , et al . Effect of synthesis methods on magnetic Kans grass biochar for enhanced As (Ⅲ, Ⅴ) adsorption from aqueous solutions[J]. Biomass and Bioenergy, 2014, 71: 299-310. |
| 67 | REDDY D H K , LEE S M . Magnetic biochar composite: facile synthesis, characterization, and application for heavy metal removal[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2014, 454: 96-103. |
| 68 | RUTHIRAAN M , ABDULLAH E C , MUBARAK N M , et al . A promising route of magnetic based materials for removal of cadmium and methylene blue from waste water[J]. Journal of Environmental Chemical Engineering, 2017, 5(2): 1447-1455. |
| 69 | GUPTA P L , JUNG H , TIWARI D , et al . Insight into the mechanism of Cd(Ⅱ) and Pb(Ⅱ) removal by sustainable magnetic biosorbent precursor to Chlorella vulgaris [J]. Journal of the Taiwan Institute of Chemical Engineers, 2017, 71: 206-213. |
| 70 | HE R , PENG Z , LYU H , et al . Synthesis and characterization of an iron-impregnated biochar for aqueous arsenic removal[J]. Science of the Total Environment, 2018, 612: 1177-1186. |
| 71 | SON E B , POO K M , CHANG J S , et al . Heavy metal removal from aqueous solutions using engineered magnetic biochars derived from waste marine macro-algal biomass[J]. Science of the Total Environment, 2018, 615: 161-168. |
| 72 | ZHOU Y , LIU X , XIANG Y , et al . Modification of biochar derived from sawdust and its application in removal of tetracycline and copper from aqueous solution: adsorption mechanism and modelling[J]. Bioresource Technology, 2017, 245: 266-273. |
| 73 | KARUNANAYAKE A G , TODD O A , CROWLEY M , et al . Lead and cadmium remediation using magnetized and nonmagnetized biochar from Douglas fir[J]. Chemical Engineering Journal, 2018, 331: 480-491. |
| 74 | ZHOU Q , LIAO B , LIN L , et al . Adsorption of Cu(Ⅱ) and Cd(Ⅱ) from aqueous solutions by ferromanganese binary oxide-biochar composites[J]. Science of the Total Environment, 2018, 615: 115-122. |
| 75 | YANG F , ZHANG S , LI H , et al . Corn straw-derived biochar impregnated with α-FeOOH nanorods for highly effective copper removal[J]. Chemical Engineering Journal, 2018, 348: 191-201. |
| 76 | GAN C , LIU Y , TAN X , et al . Effect of porous zinc–biochar nanocomposites on Cr(Ⅵ ) adsorption from aqueous solution[J]. RSC Advances, 2015, 5(44): 35107-35115. |
| 77 | YU J , JIANG C , GUAN Q , et al . Enhanced removal of Cr(Ⅵ) from aqueous solution by supported ZnO nanoparticles on biochar derived from waste water hyacinth[J]. Chemosphere, 2018, 195: 632-640 |
| 78 | WAN S , WU J , ZHOU S , et al . Enhanced lead and cadmium removal using biochar-supported hydrated manganese oxide (HMO) nanoparticles: behavior and mechanism[J]. Science of the Total Environment, 2018, 616: 1298-1306. |
| 79 | LI B , YANG L , WANG C , et al . Adsorption of Cd(Ⅱ) from aqueous solutions by rape straw biochar derived from different modification processes[J]. Chemosphere, 2017, 175: 332-340. |
| 80 | JUNG K W , LEE S Y , LEE Y J . Hydrothermal synthesis of hierarchically structured birnessite-type MnO2/biochar composites for the adsorptive removal of Cu(Ⅱ ) from aqueous media[J]. Bioresource Technology, 2018, 260: 204-212. |
| 81 | LIANG J , LI X , YU Z , et al . Amorphous MnO2 modified biochar derived from aerobically composted swine manure for adsorption of Pb(Ⅱ) and Cd(Ⅱ)[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(6): 5049-5058. |
| 82 | LIU C M , DIAO Z H , HUO W Y , et al . Simultaneous removal of Cu2+ and bisphenol A by a novel biochar-supported zero valent iron from aqueous solution: synthesis, reactivity and mechanism[J]. Environmental Pollution, 2018, 239: 698-705. |
| 83 | QIAN L , ZHANG W , YAN J , et al . Nanoscale zero-valent iron supported by biochars produced at different temperatures: synthesis mechanism and effect on Cr(Ⅵ) removal[J]. Environmental Pollution, 2017, 223: 153-160. |
| 84 | ZUO W Q , CHEN C , CUI H J , et al . Enhanced removal of Cd(Ⅱ) from aqueous solution using CaCO3 nanoparticle modified sewage sludge biochar[J]. RSC Advances, 2017, 7(26): 16238-16243. |
| 85 | WANG T , LI C , WANG C , et al . Biochar/MnAl-LDH composites for Cu(Ⅱ) removal from aqueous solution[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2018, 538: 443-450. |
| 86 | YU C , WANG M , DONG X , et al . Removal of Cu( Ⅱ ) from aqueous solution using Fe3O4-alginate modified biochar microspheres[J]. RSC Advances, 2017, 7(84): 53135-53144. |
| 87 | WANG B , GAO B , WAN Y . Entrapment of ball-milled biochar in Ca-alginate beads for the removal of aqueous Cd(Ⅱ)[J]. Journal of Industrial and Engineering Chemistry, 2018, 61: 161-168. |
| 88 | WANG Y Y , LIU Y X , LU H H , et al . Competitive adsorption of Pb(Ⅱ), Cu(Ⅱ), and Zn(Ⅱ) ions onto hydroxyapatite-biochar nanocomposite in aqueous solutions[J]. Journal of Solid State Chemistry, 2018, 261: 53-61. |
| 89 | JUNG K W , LEE S Y , LEE Y J . Facile one-pot hydrothermal synthesis of cubic spinel-type manganese ferrite/biochar composites for environmental remediation of heavy metals from aqueous solutions[J]. Bioresource Technology, 2018, 261: 1-9. |
| 90 | WANG S , ZHOU Y , GAO B , et al . The sorptive and reductive capacities of biochar supported nanoscaled zero-valent iron (nZVI) in relation to its crystallite size[J]. Chemosphere, 2017, 186: 495-500. |
| 91 | WANG C , WANG H . Pb(Ⅱ) sorption from aqueous solution by novel biochar loaded with nano-particles[J]. Chemosphere, 2018, 192: 1-4. |
| 92 | SHI S , YANG J , LIANG S , et al . Enhanced Cr(Ⅵ) removal from acidic solutions using biochar modified by Fe3O4@SiO2-NH2 particles[J]. Science of the Total Environment, 2018, 628: 499-508. |
| 93 | LING L L , LIU W J , ZHANG S , et al . Magnesium oxide embedded nitrogen self-doped biochar composites: fast and high-efficiency adsorption of heavy metals in an aqueous solution[J]. Environmental Science & Technology, 2017, 51(17): 10081-10089. |
| 94 | ZHANG M , LIU Y , LI T , et al . Chitosan modification of magnetic biochar produced from Eichhornia crassipes for enhanced sorption of Cr(Ⅵ ) from aqueous solution[J]. RSC Advances, 2015, 5(58): 46955-46964. |
| 95 | HUANG X , LIU Y , LIU S , et al . Effective removal of Cr(Ⅵ) using βcyclodextrin-chitosan modified biochars with adsorption/ reduction bifuctional roles[J]. RSC Advances, 2016, 6(1): 94-104. |
| 96 | MA Y, LIU W J , ZHANG N , et al . Polyethylenimine modified biochar adsorbent for hexavalent chromium removal from the aqueous solution[J]. Bioresource Technology, 2014, 169: 403-408. |
| 97 | SHI Y , ZHANG T , REN H , et al . Polyethylene imine modified hydrochar adsorption for chromium (Ⅵ) and nickel (Ⅱ) removal from aqueous solution[J]. Bioresource Technology, 2018, 247: 370-379. |
| 98 | LUO M , LIN H , LI B , et al . A novel modification of lignin on corncob-based biochar to enhance removal of cadmium from water[J]. Bioresource Technology, 2018, 259: 312-318. |
| 99 | DENG J , LIU Y , LIU S , et al . Competitive adsorption of Pb(Ⅱ), Cd(Ⅱ) and Cu(Ⅱ) onto chitosan-pyromellitic dianhydride modified biochar[J]. Journal of Colloid and Interface Science, 2017, 506: 355-364. |
| 100 | YANG G X , JIANG H . Amino modification of biochar for enhanced adsorption of copper ions from synthetic wastewater[J]. Water Research, 2014, 48: 396-405. |
| 101 | YU W , LIAN F , CUI G , et al . N-doping effectively enhances the adsorption capacity of biochar for heavy metal ions from aqueous solution[J]. Chemosphere, 2018, 193: 8-16. |
| 102 | ZHANG Y , CAO B , ZHAO L , et al . Biochar-supported reduced graphene oxide composite for adsorption and coadsorption of atrazine and lead ions[J]. Applied Surface Science, 2018, 427: 147-155. |
| 103 | WANG T , SUN H , REN X , et al . Adsorption of heavy metals from aqueous solution by UV-mutant Bacillus subtilis loaded on biochars derived from different stock materials[J]. Ecotoxicology and Environmental Safety, 2018, 148: 285-292. |
| [1] | WANG Shengyan, DENG Shuai, ZHAO Ruikai. Research progress on carbon dioxide capture technology based on electric swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 233-245. |
| [2] | DAI Huantao, CAO Lingyu, YOU Xinxiu, XU Haoliang, WANG Tao, XIANG Wei, ZHANG Xueyang. Adsorption properties of CO2 on pomelo peel biochar impregnated by lignin [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 356-363. |
| [3] | CUI Shoucheng, XU Hongbo, PENG Nan. Simulation analysis of two MOFs materials for O2/He adsorption separation [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 382-390. |
| [4] | CHEN Chongming, CHEN Qiu, GONG Yunqian, CHE Kai, YU Jinxing, SUN Nannan. Research progresses on zeolite-based CO2 adsorbents [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 411-419. |
| [5] | LI Shilin, HU Jingze, WANG Yilin, WANG Qingji, SHAO Lei. Research progress in separation and extraction of high value components by electrodialysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 420-429. |
| [6] | XU Chunshu, YAO Qingda, LIANG Yongxian, ZHOU Hualong. Research progress on functionalization strategies of covalent organic frame materials and its adsorption properties for Hg(Ⅱ) and Cr(Ⅵ) [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 461-478. |
| [7] | GU Yongzheng, ZHANG Yongsheng. Dynamic behavior and kinetic model of Hg0 adsorption by HBr-modified fly ash [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 498-509. |
| [8] | GUO Qiang, ZHAO Wenkai, XIAO Yonghou. Numerical simulation of enhancing fluid perturbation to improve separation of dimethyl sulfide/nitrogen via pressure swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 64-72. |
| [9] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [10] | SHI Keke, LIU Muzi, ZHAO Qiang, LI Jinping, LIU Guang. Properties and research progress of magnesium based hydrogen storage materials [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4731-4745. |
| [11] | WANG Chen, BAI Haoliang, KANG Xue. Performance study of high power UV-LED heat dissipation and nano-TiO2 photocatalytic acid red 26 coupling system [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4905-4916. |
| [12] | SHAO Zhiguo, REN Wen, XU Shipei, NIE Fan, XU Yu, LIU Longjie, XIE Shuixiang, LI Xingchun, WANG Qingji, XIE Jiacai. Influence of final temperature on the distribution and characteristics of oil-based drilling cuttings pyrolysis products [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4929-4938. |
| [13] | LI Zhiyuan, HUANG Yaji, ZHAO Jiaqi, YU Mengzhu, ZHU Zhicheng, CHENG Haoqiang, SHI Hao, WANG Sheng. Characterization of heavy metals during co-pyrolysis of sludge with PVC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4947-4956. |
| [14] | YANG Ying, HOU Haojie, HUANG Rui, CUI Yu, WANG Bing, LIU Jian, BAO Weiren, CHANG Liping, WANG Jiancheng, HAN Lina. Coal tar phenol-based carbon nanosphere prepared by Stöber method for adsorption of CO2 [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 5011-5018. |
| [15] | WANG Haoran, YIN Quanyu, FANG Ming, HOU Jianlin, LI Jun, HE Bin, ZHANG Mingyue. Optimization of near critical-water treatment process of tobacco stems [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 5019-5027. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||