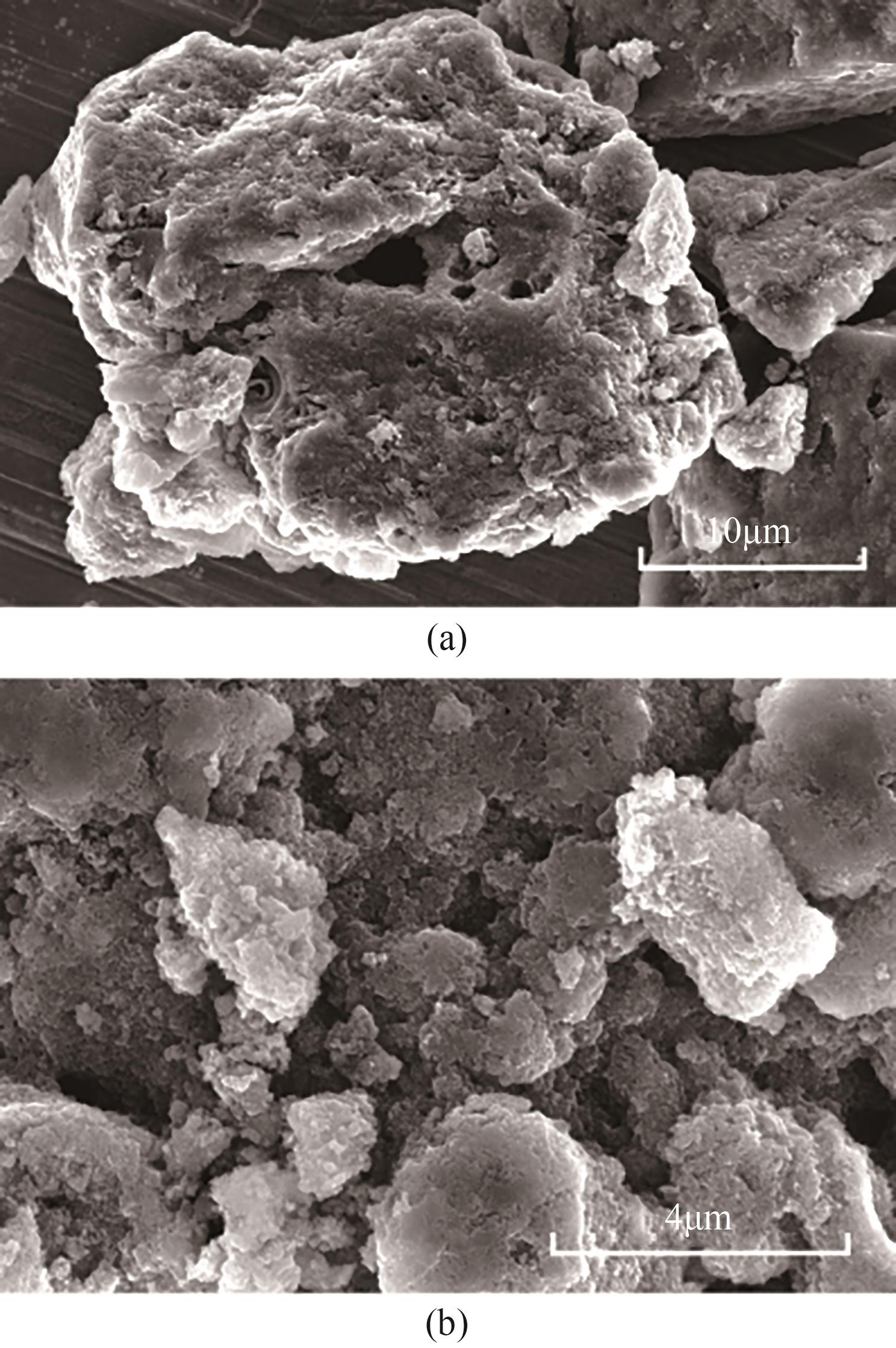
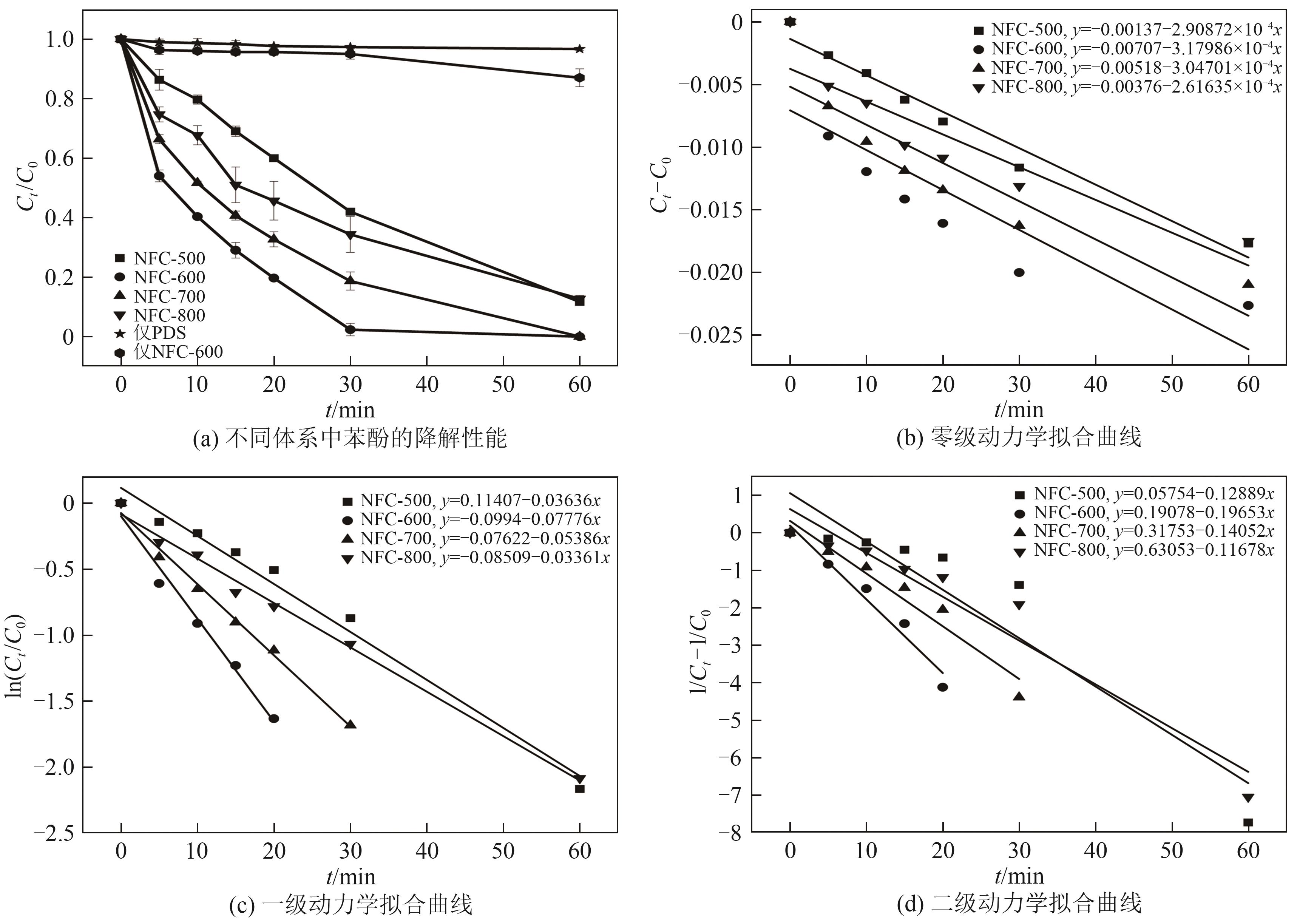
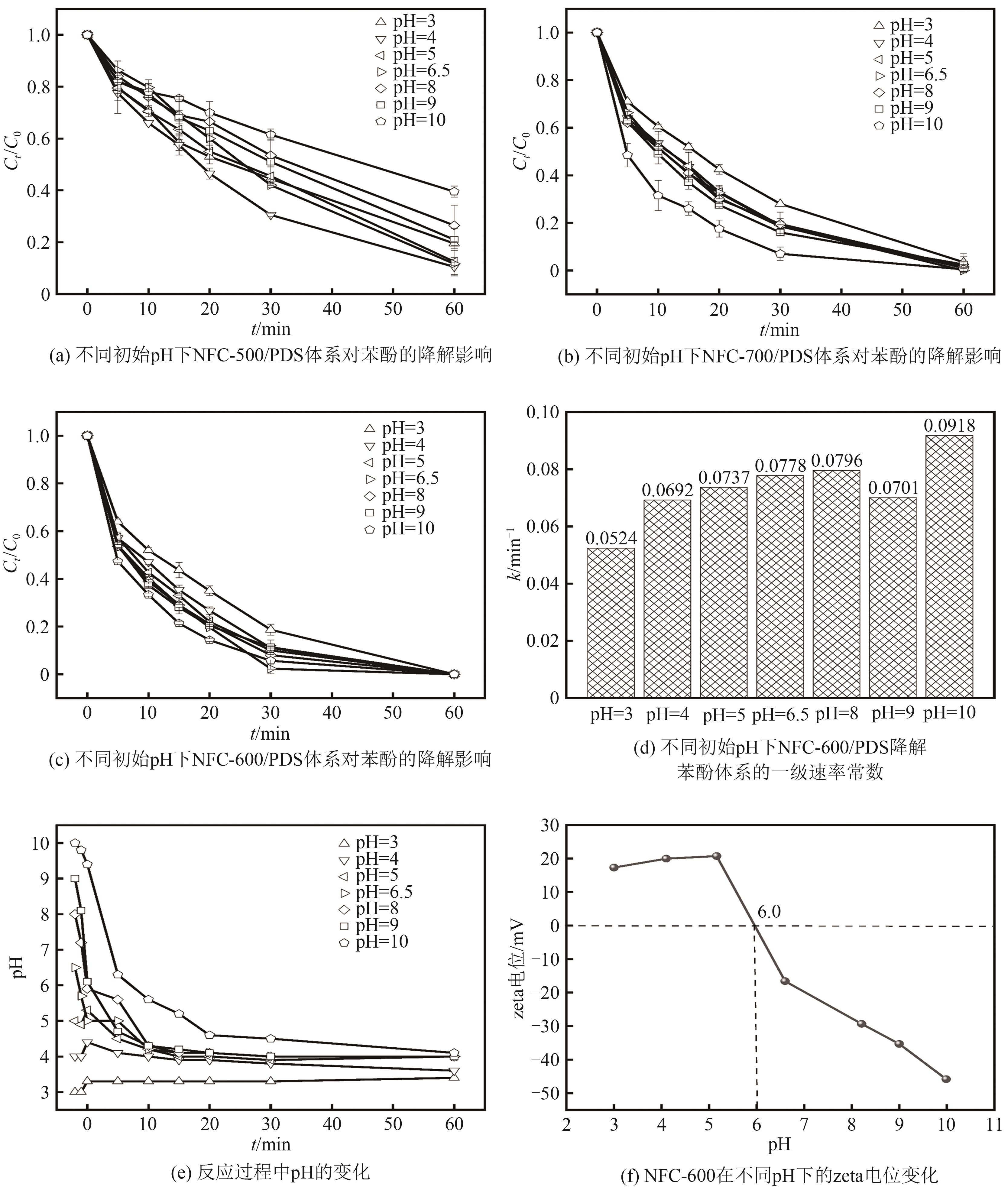
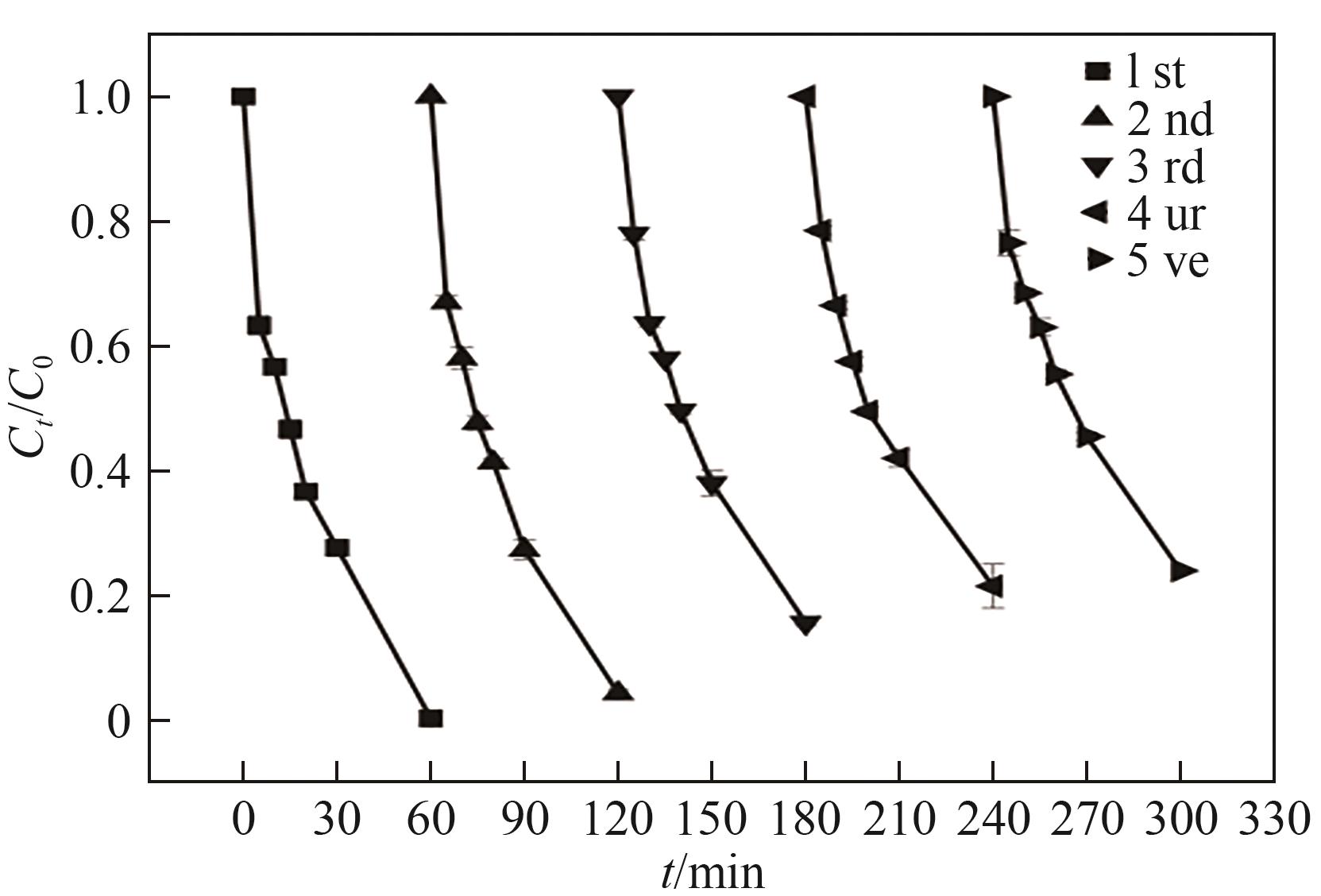
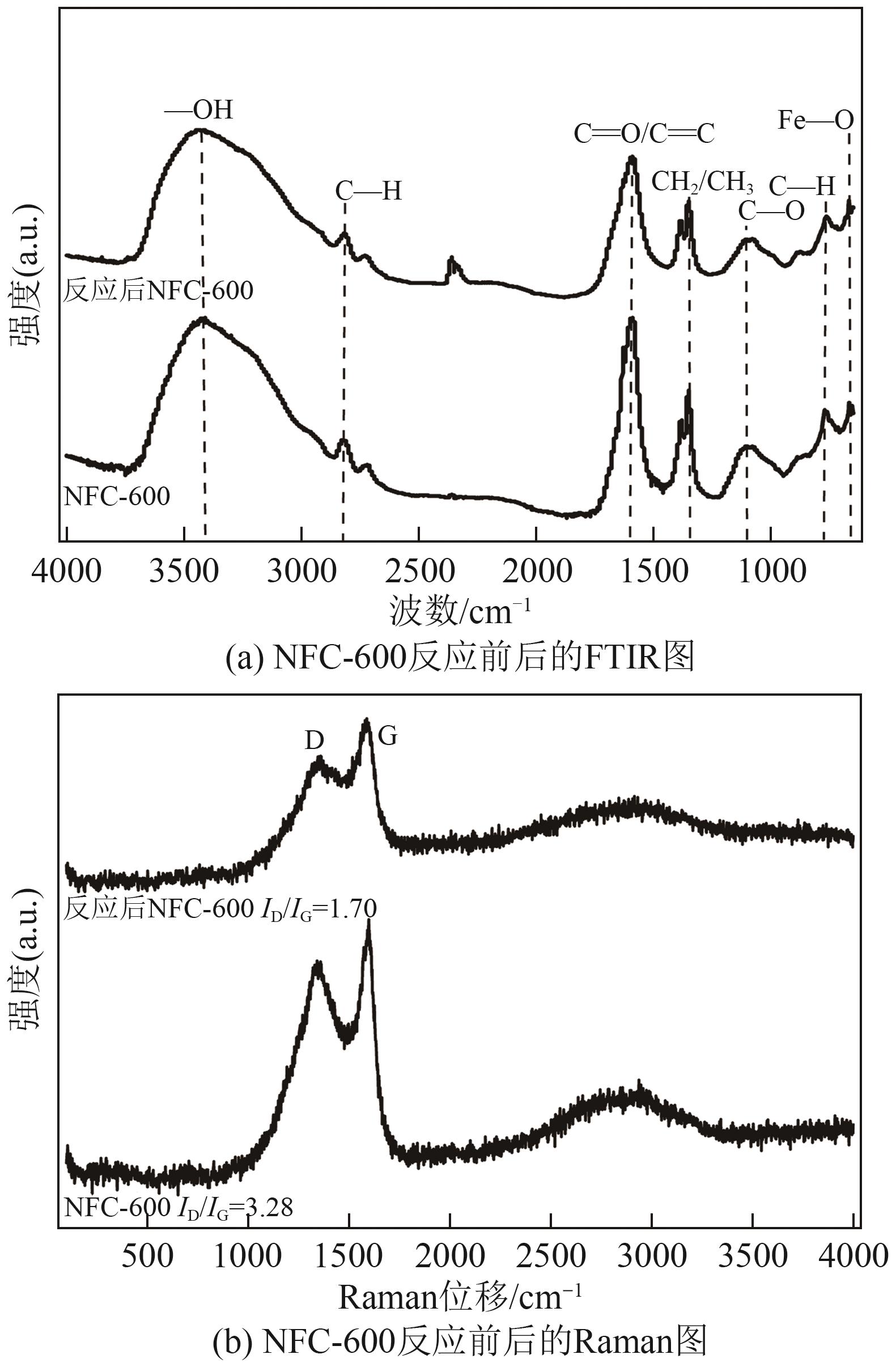
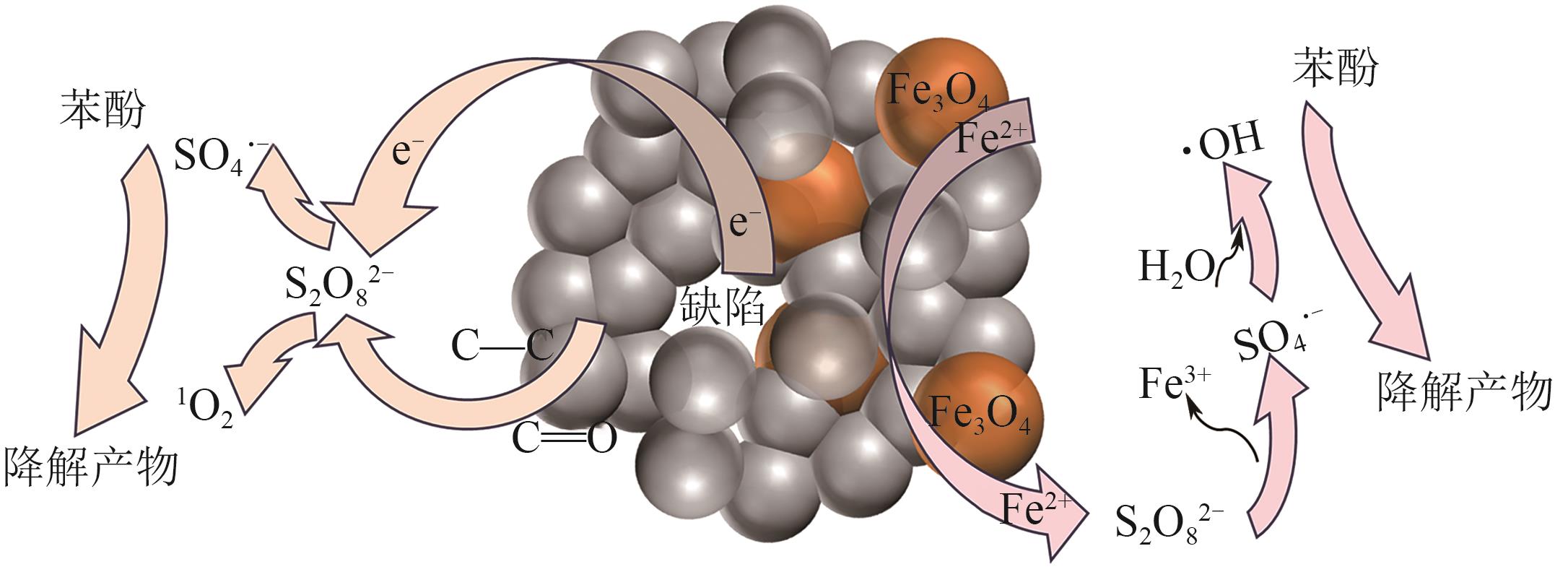
| [1] |
FAN Xinfei, LI Shanshan, SUN Menghan, et al. Degradation of phenol by coal-based carbon membrane integrating sulfate radicals-based advanced oxidation processes[J]. Ecotoxicology and Environmental Safety, 2019, 185: 109662.
|
| [2] |
PRABHU Shweta, MOLATH Aleena, CHOKSI Hinal, et al. Classifications of polyphenols and their potential application in human health and diseases[J]. International Journal of Physiology, Nutrition and Physical Education, 2021, 6(1): 293-301.
|
| [3] |
CHAE Yooeun, KIM Lia, KIM Dokyung, et al. Deriving hazardous concentrations of phenol in soil ecosystems using a species sensitivity distribution approach[J]. Journal of Hazardous Materials, 2020, 399: 123036.
|
| [4] |
VAIANO V, MATARANGOLO M, MURCIA J J, et al. Enhanced photocatalytic removal of phenol from aqueous solutions using ZnO modified with Ag[J]. Applied Catalysis B: Environmental, 2018, 225: 197-206.
|
| [5] |
ZAGHLOUL Ghada Y, MOHAMEDEIN Lamiaa I, KELANY Mahmoud S, et al. Impact of total phenolic compounds on ecological and health risks of water and sediments from Timsah Lake, Suez Canal, Egypt[J]. Environmental Science and Pollution Research, 2024, 31(33): 45667-45682.
|
| [6] |
XIN Ke, XUN Sun, ZHENG Yan, et al. Distributions, compositions, and ecological risk assessment of typical pollutants in surface sediment of Xihe river, China[J]. Research Square, 2021, 152: 110923.
|
| [7] |
SUN Jianfei, MU Qin, KIMURA Hideo, et al. Oxidative degradation of phenols and substituted phenols in the water and atmosphere: A review[J]. Advanced Composites and Hybrid Materials, 2022, 5(2): 627-640.
|
| [8] |
MACÍAS-QUIROGA Iván F, HENAO-AGUIRRE Paula A, Alexander MARÍN-FLÓREZ, et al. Bibliometric analysis of advanced oxidation processes (AOPs) in wastewater treatment: Global and Ibero-American research trends[J]. Environmental Science and Pollution Research, 2021, 28(19): 23791-23811.
|
| [9] |
HUANG Yunxin, ZHAO Shouyan, CHEN Keyu, et al. A review of persulfate-based advanced oxidation system for decontaminating organic wastewater via non-radical regime[J]. Frontiers of Environmental Science & Engineering, 2024, 18(11): 134.
|
| [10] |
LE MINH TRI Nguyen, THANG Phan Quang, TAN Lam Van, et al. Removal of phenolic compounds from wastewaters by using synthesized Fe-nano zeolite[J]. Journal of Water Process Engineering, 2020, 33: 101070.
|
| [11] |
WU Liying, LI Zhuoyu, CHENG Pingtong, et al. Efficient activation of peracetic acid by mixed sludge derived biochar: Critical role of persistent free radicals[J]. Water Research, 2022, 223: 119013.
|
| [12] |
ZHANG Tianfu, LIU Wei, HAN Junwei, et al. Selective separation of calcium from zinc-rich neutralization sludge by sulfidation roasting and HCl leaching[J]. Separation and Purification Technology, 2021, 259: 118064.
|
| [13] |
SHI Chunhong, ZHANG Yuqi, ZHOU Shuo, et al. Status of research on the resource utilization of stainless steel pickling sludge in China: A review[J]. Environmental Science and Pollution Research International, 2023, 30(39): 90223-90242.
|
| [14] |
苍大强, 张玲玲, 刘洋, 等. 国内外钢铁工业固相二次资源利用现状、存在问题与对策[J]. 过程工程学报, 2022, 22(10): 1418-1424.
|
|
CANG Daqiang, ZHANG Lingling, LIU Yang, et al. Secondary resources utilization, problems and countermeasures of the domestic and oversea steel industry[J]. The Chinese Journal of Process Engineering, 2022, 22(10): 1418-1424.
|
| [15] |
HOU Meifang, GUO Yuanyuan, CHEN Xiaoyang, et al. Preparation, characterization and catalytic performance of paper mill sludge and municipal wastewater treatment sludge-based catalysts for Fenton-like oxidation of Rhodamine B[J]. Desalination and Water Treatment, 2017, 84: 190-198.
|
| [16] |
HUANG Baocheng, JIANG Jun, HUANG Guixiang, et al. Sludge biochar-based catalysts for improved pollutant degradation by activating peroxymonosulfate[J]. Journal of Materials Chemistry A, 2018, 6(19): 8978-8985.
|
| [17] |
WU Jingqi, WANG Tongshuai, LIU Yuyan, et al. Norfloxacin adsorption and subsequent degradation on ball-milling tailored N-doped biochar[J]. Chemosphere, 2022, 303: 135264.
|
| [18] |
徐皓普, 汤波. 碱改性生物炭处理含Cd2+废水效果对比研究[J]. 环境科学与管理, 2022, 47(6): 82-85.
|
|
XU Haopu, TANG Bo. Study on adsorption mechanism of Cd2+ wastewater by modified biochar[J]. Environmental Science and Management, 2022, 47(6): 82-85.
|
| [19] |
JIANG Qun, JIANG Simeng, LI Hui, et al. A stable biochar supported S-nZVI to activate persulfate for effective dichlorination of atrazine[J]. Chemical Engineering Journal, 2022, 431: 133937.
|
| [20] |
SU Pei, FU Wenyang, DU Xuedong, et al. Confined Fe0@CNTs for highly efficient and super stable activation of persulfate in wide pH ranges: Radicals and non-radical co-catalytic mechanism[J]. Chemical Engineering Journal, 2021, 420: 129446.
|
| [21] |
SONG Xiangru, LIU Jia, JIANG Qing, et al. Enhanced electron transfer and methane production from low-strength wastewater using a new granular activated carbon modified with nano-Fe3O4 [J]. Chemical Engineering Journal, 2019, 374: 1344-1352.
|
| [22] |
徐东卫, 张明举, 申志豪, 等. 氮掺杂碳纳米管原位封装磁性粒子异质结构(Fe3O4@NCNTs)及其轻质宽频吸波性能[J]. 材料研究学报, 2024, 38(6): 430-436.
|
|
XU Dongwei, ZHANG Mingju, SHEN Zhihao, et al. Microwave absorption performance of encapsulated magnetic particles with nitrogen-doped carbon nanotubes Fe3O4@NCNTs[J]. Chinese Journal of Materials Research, 2024, 38(6): 430-436.
|
| [23] |
INDRAYANA I P T, TJUANA L A, TUNY M T, et al. Nanostructure and optical properties of Fe3O4: Effect of calcination temperature and dwelling time[J]. Journal of Physics: Conference Series, 2019, 1341(8): 082044.
|
| [24] |
KARUME Ibrahim, BBUMBA Simon, TEWOLDE Simon, et al. Impact of carbonization conditions and adsorbate nature on the performance of activated carbon in water treatment[J]. BMC Chemistry, 2023, 17(1): 162.
|
| [25] |
WU Wei, ZHU Shishu, HUANG Xiaochen, et al. Mechanisms of persulfate activation on biochar derived from two different sludges: Dominance of their intrinsic compositions[J]. Journal of Hazardous Materials, 2021, 408: 124454.
|
| [26] |
GONG Chuangxin, LIN Wei, DING Xiuxiang, et al. A three-stage process of Mn(Ⅶ)-Fe(Ⅲ)/PDS system for enhancing sludge dewaterability: Effective driving of Fe(Ⅱ)/Fe(Ⅲ) cycle and adequate assurance of ROS[J]. Separation and Purification Technology, 2024, 330: 125377.
|
| [27] |
GUO Qianqian, QIAO Shixuan, ZHANG Dongming, et al. A comparison of hydrothermal carbonization versus pyrolysis-activation for sludge-derived carbon materials on physiochemical properties and electrochemical performance[J]. Biomass and Bioenergy, 2024, 182: 107079.
|
| [28] |
YANG Haiping, YAN Rong, CHEN Hanping, et al. Characteristics of hemicellulose, cellulose and lignin pyrolysis[J]. Fuel, 2007, 86(12/13): 1781-1788.
|
| [29] |
LIANG Jun, XU Xiaoyun, ZHONG Qijun, et al. Roles of the mineral constituents in sludge-derived biochar in persulfate activation for phenol degradation[J]. Journal of Hazardous Materials, 2020, 398: 122861.
|
| [30] |
DUAN Ran, MA Shuanglong, XU Shengjun, et al. Soybean straw biochar activating peroxydisulfate to simultaneously eliminate tetracycline and tetracycline resistance bacteria: Insights on the mechanism[J]. Water Research, 2022, 218: 118489.
|
| [31] |
FU Haichao, ZHAO Peng, XU Shengjun, et al. Fabrication of Fe3O4 and graphitized porous biochar composites for activating peroxymonosulfate to degrade p-hydroxybenzoic acid: Insights on the mechanism[J]. Chemical Engineering Journal, 2019, 375: 121980.
|
| [32] |
HU Peidong, SU Hanrui, CHEN Zhenyu, et al. Selective degradation of organic pollutants using an efficient metal-free catalyst derived from carbonized polypyrrole via peroxymonosulfate activation[J]. Environmental Science & Technology, 2017, 51(19): 11288-11296.
|
| [33] |
冯琪瑞, 唐玉朝, 王坤, 等. UV/乙酰丙酮高级氧化体系降解甲基橙的反应机理研究[J]. 环境科学与技术, 2023, 46(12): 29-38.
|
|
FENG Qirui, TANG Yuchao, WANG Kun, et al. Study on treatment of methyl orange printing/dyeing wastewater by advnaced UV/acetylacetone system[J]. Environmental Science & Technology, 2023, 46(12): 29-38.
|
| [34] |
ZHAO Xin, AN Qingda, XIAO Zuoyi, et al. One-step preparation of Fe x O y /N-GN/CNTs heterojunctions as a peroxymonosulfate activator for relatively highly-efficient methylene blue degradation[J]. Chinese Journal of Catalysis, 2018, 39(11): 1842-1853.
|
| [35] |
YU Qinghui, TANG Mengshuang, LIU Guotao, et al. Phenol removal by activation of persulfate using magnetic carbon generated from sludge and steel converter slag[J]. Chemical Engineering and Processing - Process Intensification, 2023, 186: 109313.
|
| [36] |
LI Maolin, CHEN Guofang. Revisiting catalytic model reaction p-nitrophenol/NaBH4 using metallic nanoparticles coated on polymeric spheres[J]. Nanoscale, 2013, 5(23): 11919-11927.
|
| [37] |
ZHENG Wan, XIAO Xin, CHEN Baoliang. A nonradical reaction-dominated phenol degradation with peroxydisulfate catalyzed by nitrogen-doped graphene[J]. Science of the Total Environment, 2019, 667: 287-296.
|
| [38] |
LU Li, TANG Diyong, LUO Zhipeng, et al. Water hyacinth derived hierarchical porous biochar absorbent: Ideal peroxydisulfate activator for efficient phenol degradation via an electron-transfer pathway[J]. Environmental Research, 2024, 242: 117773.
|
| [39] |
AHMAD Mushtaque, TEEL Amy L, WATTS Richard J. Mechanism of persulfate activation by phenols[J]. Environmental Science & Technology, 2013, 47(11): 5864-5871.
|
| [40] |
REN Wei, XIONG Liangliang, NIE Gang, et al. Insights into the electron-transfer regime of peroxydisulfate activation on carbon nanotubes: The role of oxygen functional groups[J]. Environmental Science & Technology, 2020, 54(2): 1267-1275.
|
| [41] |
FAN Zhixuan, FENG Tao, WU Si, et al. Chitin-derived biochar with nitrogen doping to activate persulfate for phenol degradation: Application potential and electron transfer pathway in system[J]. Chemosphere, 2023, 330: 138641.
|
| [42] |
王佩佩, 杨岚, 魏学锋, 等. 氮掺杂碳负载Fe3O4活化过硫酸盐降解苯酚[J]. 化工环保, 2023, 43(3): 339-345.
|
|
WANG Peipei, YANG Lan, WEI Xuefeng, et al. Activation of persulfate by nitrogen-doped carbon supported Fe3O4 for phenol degradation[J]. Environmental Protection of Chemical Industry, 2023, 43(3): 339-345.
|
| [43] |
CAO Bo, QU Jianhua, CHU Yingyu, et al. One-step self-assembly of Fe-biochar composite for enhanced persulfate activation to phenol degradation: Different active sites-induced radical/non-radical mechanism[J]. Chemosphere, 2023, 322: 138168.
|
| [44] |
QU Jianhua, XU Yuan, ZHANG Xiubo, et al. Ball milling-assisted preparation of N-doped biochar loaded with ferrous sulfide as persulfate activator for phenol degradation: Multiple active sites-triggered radical/non-radical mechanism[J]. Applied Catalysis B: Environmental, 2022, 316: 121639.
|
| [45] |
BAO Kai, YAN Chongchong, NIU Deli, et al. Persulfate oxidation enhanced extraction to improve the removal of high concentration phenol wastewater[J]. Environmental Science: Water Research & Technology, 2022, 8(5): 981-997.
|
| [46] |
DIAO Zenghui, XU Xiangrong, CHEN Hui, et al. Simultaneous removal of Cr(Ⅵ) and phenol by persulfate activated with bentonite-supported nanoscale zero-valent iron: Reactivity and mechanism[J]. Journal of Hazardous Materials, 2016, 316: 186-193.
|
| [47] |
ZHANG Jun, LI Shiyao, XIANG Haotian, et al. Exploring the peroxydisulfate activation by carbon nanotubes for pollutants degradation: Identification of the primary reaction mechanism and key molecular descriptor[J]. Journal of Environmental Chemical Engineering, 2024, 12(2): 112495.
|
| [48] |
ZHU Shishu, JIN Chao, DUAN Xiaoguang, et al. Nonradical oxidation in persulfate activation by graphene-like nanosheets (GNS): Differentiating the contributions of singlet oxygen (1O2) and sorption-dependent electron transfer[J]. Chemical Engineering Journal, 2020, 393: 124725.
|
| [49] |
DONG Hao, ZHANG Siyu, GUO Chunli, et al. Amorphous carbon from coal slime for efficient degradation of phenol by non-free radical activation of peroxymonosulfate[J]. Fuel, 2024, 373: 132285.
|
| [50] |
WEI Yu, LEI Xuefei, LUO Shaohua, et al. Effect of pore-forming agent on degradation of phenol by iron tailings based porous ceramics[J]. Ceramics International, 2024, 50(18): 33791-33801.
|
| [51] |
XIA Qixing, JIANG Zhaohua, LI Dongqi, et al. Green synthesis of a dendritic Fe3O4@FeO composite modified with polar C-groups for Fenton-like oxidation of phenol[J]. Journal of Alloys and Compounds, 2018, 746: 453-461.
|
| [52] |
SANG Wenjiao, XU Xinyang, ZHAN Cheng, et al. Recent advances of antibiotics degradation in different environment by iron-based catalysts activated persulfate: A review[J]. Journal of Water Process Engineering, 2022, 49: 103075.
|
| [53] |
MIAO Xiaozeng, CHEN Xiliang, WU Wenhao, et al. Intrinsic defects enhanced biochar/peroxydisulfate oxidation capacity through electron-transfer regime[J]. Chemical Engineering Journal, 2022, 438: 135606.
|
| [54] |
CHEN Hongliang, LONG Qian, WEI Fuhua. Heterogeneous activation of peroxide via acid-modified red mud for the degradation of phenol[J]. Desalination and Water Treatment, 2023, 283: 209-221.
|
| [55] |
OTHMAN Israa, HISHAM ZAIN Jerina, HAIJA Mohammad ABU, et al. Catalytic activation of peroxymonosulfate using CeVO4 for phenol degradation: An insight into the reaction pathway[J]. Applied Catalysis B: Environmental, 2020, 266: 118601.
|
| [56] |
SUN Zhiming, ZHANG Xinchao, YANG Zhongqing, et al. Efficient peroxymonosulfate activation of immobilized Fe-N-C catalyst on ceramsite for the continuous flow removal of phenol[J]. Chemosphere, 2022, 307: 136149.
|
 ), ZHONG Guanghong1,2,3,4, LI Xitong5, GAO Xiaoya1,2,3,4, ZHU Wenjie1,2,3,4(
), ZHONG Guanghong1,2,3,4, LI Xitong5, GAO Xiaoya1,2,3,4, ZHU Wenjie1,2,3,4( ), LUO Yongming2,3,4
), LUO Yongming2,3,4
 ), 钟广宏1,2,3,4, 李曦同5, 高晓亚1,2,3,4, 朱文杰1,2,3,4(
), 钟广宏1,2,3,4, 李曦同5, 高晓亚1,2,3,4, 朱文杰1,2,3,4( ), 罗永明2,3,4
), 罗永明2,3,4