Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (5): 2475-2488.DOI: 10.16085/j.issn.1000-6613.2024-1869
• Synthetic biomanufacturing • Previous Articles
Progress on yeast cell factory for lignocellulose biotransformation
NI Xin1,2( ), GAO Jiaoqi1, ZHOU Yongjin1(
), GAO Jiaoqi1, ZHOU Yongjin1( )
)
- 1.Dalian Institute of Chemical Physics, CAS, Dalian 116023, Liaoning, China
2.School of Biological Engineering, Dalian Polytechnic University, Dalian 116023, Liaoning, China
-
Received:2024-11-13Revised:2024-12-25Online:2025-05-20Published:2025-05-25 -
Contact:ZHOU Yongjin
酵母细胞工厂用于木质纤维素生物转化研究进展
- 1.中国科学院大连化学物理研究所生物技术研究部,辽宁 大连 116023
2.大连工业大学生物工程学院,辽宁 大连 116023
-
通讯作者:周雍进 -
作者简介:倪新(1998—),男,博士研究生,研究方向为合成生物学。E-mail:nixin@dicp.ac.cn。 -
基金资助:国家自然科学基金(22478382)
CLC Number:
Cite this article
NI Xin, GAO Jiaoqi, ZHOU Yongjin. Progress on yeast cell factory for lignocellulose biotransformation[J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2475-2488.
倪新, 高教琪, 周雍进. 酵母细胞工厂用于木质纤维素生物转化研究进展[J]. 化工进展, 2025, 44(5): 2475-2488.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-1869
| 改造策略 | 菌株 | 靶点基因 | 效果 | 参考文献 |
|---|---|---|---|---|
| 强化木糖转运蛋白 | S.cerevisiae | AN25 | 木糖消耗速度显著提升 | [ |
| S. cerevisiae | HXT2 | [ | ||
| S. cerevisiae | CYC8 | 木糖消耗速度提高了1.5倍 | [ | |
| S. cerevisiae | HXT7(F79S) | 木糖消耗速度提高了2倍 | [ | |
| S. cerevisiae | Cs4130 | 木糖消耗速度增加了30% | [ | |
| S. cerevisiae | SpXUT1 | 木糖消耗速度提高了25% | [ | |
| 强化木糖代谢 | S. cerevisiae | PMR1 | [ | |
| S. cerevisiae | ΔPHO13, TAL1 | 木糖消耗速度提高3.4倍 | [ | |
| S. cerevisiae | Hxt7m, CpXI, PsXK, PsTAL1, PsTKL1, | 在木糖中的生长速度提高了72% | [ | |
| S. cerevisiae | ΔYPR1, ΔGRE3, ΔASK10 | |||
| Y.lipolytica | SsXYL1, ssXYL2 | 木糖消耗速度显著提升 | [ | |
| Y. lipolytica | SsXYL1, ssXYL2, ylXYL3, Δpax1-6, Δtgl4, GPD1, DHA2 | 木糖消耗速度显著提升 | [ | |
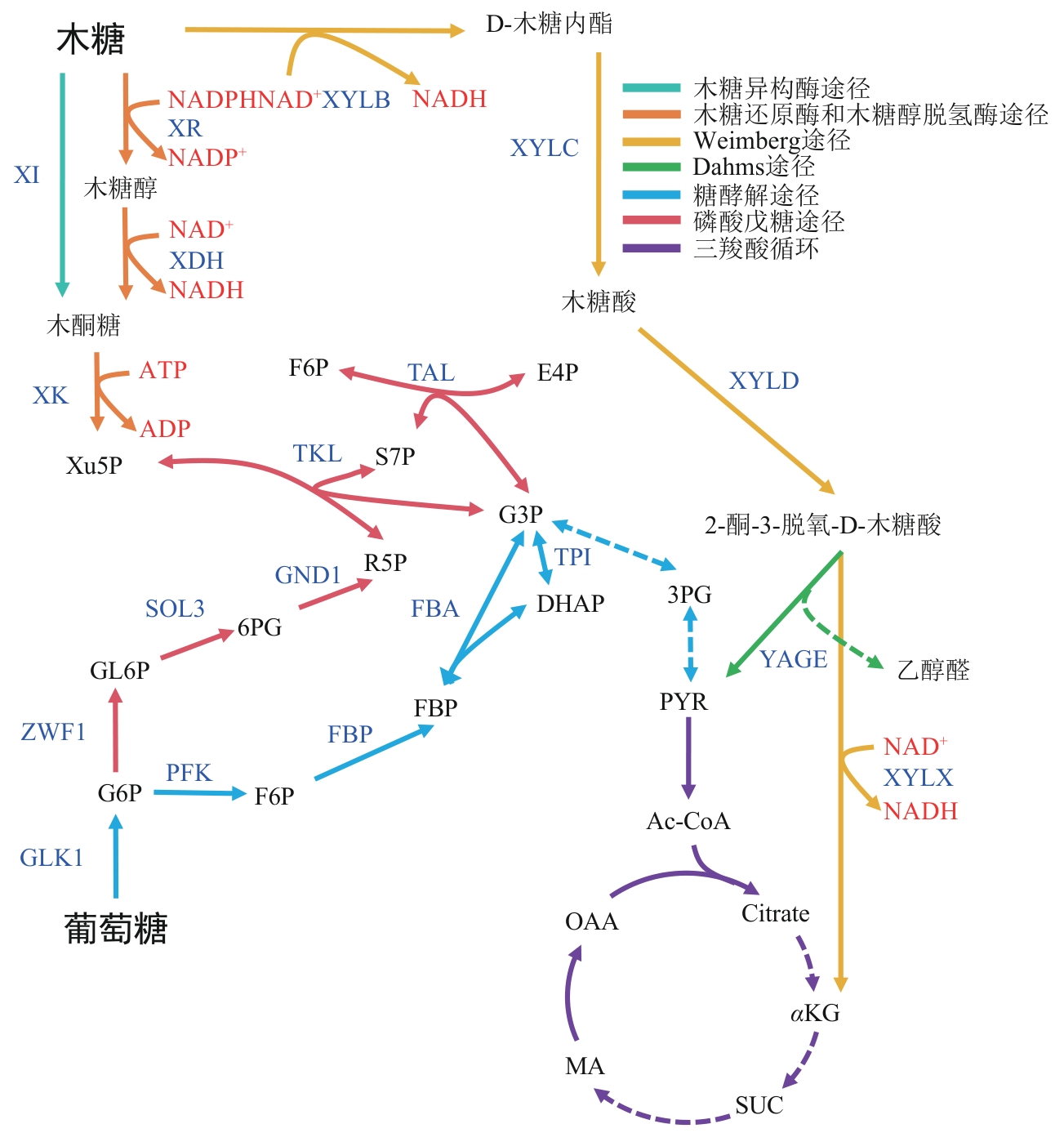
| Y. lipolytica | ylXDH, ylXR, ylXK, anXPKA, anACK | 木糖消耗速度显著提升,工程菌株能够在96h内同步消耗共计45g/L的葡萄糖和木糖 | [ | |
| 转录因子调控 | Y. lipolytica | Δpex10∷DGA1, XylA, XK | 木糖消耗速度显著提升,工程菌株能够同步利用芒草水解液中的67.9g/L葡萄糖和49.2g/L木糖 | [ |
| P.pastoris | PsXYL1 | 可以将半纤维素水解物中的木糖转化为木糖醇 | [ | |
| S.cerevisiae | ΔGCR2 | 木糖消耗速度提高2.1倍 | [ | |
| S. cerevisiae | ΔTHI2 | 木糖消耗速度提高1.27倍 | [ | |
| S. cerevisiae | ΔSNF6, ΔRGT1, ΔCAT8, ΔMSN4 | 木糖消耗速度显著提升 | [ |
| 改造策略 | 菌株 | 靶点基因 | 效果 | 参考文献 |
|---|---|---|---|---|
| 强化木糖转运蛋白 | S.cerevisiae | AN25 | 木糖消耗速度显著提升 | [ |
| S. cerevisiae | HXT2 | [ | ||
| S. cerevisiae | CYC8 | 木糖消耗速度提高了1.5倍 | [ | |
| S. cerevisiae | HXT7(F79S) | 木糖消耗速度提高了2倍 | [ | |
| S. cerevisiae | Cs4130 | 木糖消耗速度增加了30% | [ | |
| S. cerevisiae | SpXUT1 | 木糖消耗速度提高了25% | [ | |
| 强化木糖代谢 | S. cerevisiae | PMR1 | [ | |
| S. cerevisiae | ΔPHO13, TAL1 | 木糖消耗速度提高3.4倍 | [ | |
| S. cerevisiae | Hxt7m, CpXI, PsXK, PsTAL1, PsTKL1, | 在木糖中的生长速度提高了72% | [ | |
| S. cerevisiae | ΔYPR1, ΔGRE3, ΔASK10 | |||
| Y.lipolytica | SsXYL1, ssXYL2 | 木糖消耗速度显著提升 | [ | |
| Y. lipolytica | SsXYL1, ssXYL2, ylXYL3, Δpax1-6, Δtgl4, GPD1, DHA2 | 木糖消耗速度显著提升 | [ | |
| Y. lipolytica | ylXDH, ylXR, ylXK, anXPKA, anACK | 木糖消耗速度显著提升,工程菌株能够在96h内同步消耗共计45g/L的葡萄糖和木糖 | [ | |
| 转录因子调控 | Y. lipolytica | Δpex10∷DGA1, XylA, XK | 木糖消耗速度显著提升,工程菌株能够同步利用芒草水解液中的67.9g/L葡萄糖和49.2g/L木糖 | [ |
| P.pastoris | PsXYL1 | 可以将半纤维素水解物中的木糖转化为木糖醇 | [ | |
| S.cerevisiae | ΔGCR2 | 木糖消耗速度提高2.1倍 | [ | |
| S. cerevisiae | ΔTHI2 | 木糖消耗速度提高1.27倍 | [ | |
| S. cerevisiae | ΔSNF6, ΔRGT1, ΔCAT8, ΔMSN4 | 木糖消耗速度显著提升 | [ |
| 改造策略 | 菌株 | 靶点基因 | 效果 | 参考文献 |
|---|---|---|---|---|
| 增强木糖转运 | O. polymorpha | HXT1 N358A | 木糖消耗率提高1.73倍 | [ |
| 转录因子全局调控 | O. polymorpha | ΔCAT8 | 木糖转化为乙醇的收率提高了50% | [ |
| 转录因子全局调控 | O. polymorpha | ΔATG13 | 木糖的消耗显著提高 | [ |
| 增强木糖转运 | K. marxianus | CiGXF1 | 木糖醇生产效率显著提高 | [ |
| 增强木糖转运和代谢 | O. polymorpha | HXT1*, PsPXI*, XK | 同步利用木质纤维素水解液中的57g/L 木糖和111g/L葡萄糖生产7g/L的脂肪酸 | [ |
| 转录因子全局调控 | O. polymorpha | ΔMig1, ΔMig2, ΔTup1, ΔHap4 | 促进了葡萄糖和木糖的共利用 | [ |
| 转录因子全局调控 | S. stipitis | ΔHXK1 | 促进了葡萄糖和木糖的共利用 | [ |
| 转录因子全局调控及木糖代谢的强化 | K. marxianus | ΔKmSNF1, ΔKmHXK1, NcXYL1, ScGAL2(N376F) | 高效同步利用玉米芯水解液中的葡萄糖和木糖生产木糖醇 | [ |
| 改造策略 | 菌株 | 靶点基因 | 效果 | 参考文献 |
|---|---|---|---|---|
| 增强木糖转运 | O. polymorpha | HXT1 N358A | 木糖消耗率提高1.73倍 | [ |
| 转录因子全局调控 | O. polymorpha | ΔCAT8 | 木糖转化为乙醇的收率提高了50% | [ |
| 转录因子全局调控 | O. polymorpha | ΔATG13 | 木糖的消耗显著提高 | [ |
| 增强木糖转运 | K. marxianus | CiGXF1 | 木糖醇生产效率显著提高 | [ |
| 增强木糖转运和代谢 | O. polymorpha | HXT1*, PsPXI*, XK | 同步利用木质纤维素水解液中的57g/L 木糖和111g/L葡萄糖生产7g/L的脂肪酸 | [ |
| 转录因子全局调控 | O. polymorpha | ΔMig1, ΔMig2, ΔTup1, ΔHap4 | 促进了葡萄糖和木糖的共利用 | [ |
| 转录因子全局调控 | S. stipitis | ΔHXK1 | 促进了葡萄糖和木糖的共利用 | [ |
| 转录因子全局调控及木糖代谢的强化 | K. marxianus | ΔKmSNF1, ΔKmHXK1, NcXYL1, ScGAL2(N376F) | 高效同步利用玉米芯水解液中的葡萄糖和木糖生产木糖醇 | [ |
| 菌株 | 底物 | 靶点基因 | 效果 | 参考文献 |
|---|---|---|---|---|
| S. cerevisiae | 木糖 | CCR4, TIF1 | 木糖发酵的比生长速率分别提高了23%和14% | [ |
| S. cerevisiae | 木糖 | PHO13 | 木糖发酵的比生长速率提高了8.2倍 | [ |
| S. cerevisiae | 木糖 | HasXI的拷贝数增加 | 进化菌株同步利用了玉米芯水解液中的180g/L 葡萄糖和70g/L木糖生产了72g/L的乙醇 | [ |
| K. marxianus | 木糖 | 进化菌株的木糖利用速率提高了3倍 | [ | |
| Y. lipolytica | 木糖和葡萄糖类似物 2-脱氧葡萄糖 | YALI0_E23287g, YALI0_E15488g HXT2.4 | 进化的菌株可以共代谢木糖和葡萄糖 | [ |
| S. passalidarum | 木糖和葡萄糖类似物 2-脱氧葡萄糖 | STD1, PMC1, SMF2 | 进化菌株可以同时利用葡萄糖和木糖 | [ |
| S. stipitis | 木糖和葡萄糖类似物 2-脱氧葡萄糖 | SNF5, GPD1, ADH6, CCH1 | 进化菌株可以同时利用葡萄糖和木糖 | [ |
| S. cerevisiae | 葡萄糖和木糖的切换 | 进化菌株在木糖上的生长速度提高了3.6倍 | [ |
| 菌株 | 底物 | 靶点基因 | 效果 | 参考文献 |
|---|---|---|---|---|
| S. cerevisiae | 木糖 | CCR4, TIF1 | 木糖发酵的比生长速率分别提高了23%和14% | [ |
| S. cerevisiae | 木糖 | PHO13 | 木糖发酵的比生长速率提高了8.2倍 | [ |
| S. cerevisiae | 木糖 | HasXI的拷贝数增加 | 进化菌株同步利用了玉米芯水解液中的180g/L 葡萄糖和70g/L木糖生产了72g/L的乙醇 | [ |
| K. marxianus | 木糖 | 进化菌株的木糖利用速率提高了3倍 | [ | |
| Y. lipolytica | 木糖和葡萄糖类似物 2-脱氧葡萄糖 | YALI0_E23287g, YALI0_E15488g HXT2.4 | 进化的菌株可以共代谢木糖和葡萄糖 | [ |
| S. passalidarum | 木糖和葡萄糖类似物 2-脱氧葡萄糖 | STD1, PMC1, SMF2 | 进化菌株可以同时利用葡萄糖和木糖 | [ |
| S. stipitis | 木糖和葡萄糖类似物 2-脱氧葡萄糖 | SNF5, GPD1, ADH6, CCH1 | 进化菌株可以同时利用葡萄糖和木糖 | [ |
| S. cerevisiae | 葡萄糖和木糖的切换 | 进化菌株在木糖上的生长速度提高了3.6倍 | [ |
| 产品 | 产量/g·L-1 | 底物 | 菌株 | 参考文献 |
|---|---|---|---|---|
| 乙醇 | 45.1 | 小麦秸秆水解物 | S. cerevisiae | [ |
| 68.8 | 玉米秸秆水解物 | S. cerevisiae | [ | |
| 60.8 | 玉米秸秆水解物 | S. cerevisiae | [ | |
| 101.4 | 小麦秸秆水解物 | S. cerevisiae | [ | |
| 26.7 | 木糖 | Pichia stipitis | [ | |
| 1.6 | 木糖 | H. polymorpha | [ | |
| 8.2 | 芦苇水解物 | S. stipitis | [ | |
| 36.2 | 小麦秸秆水解物 | K. marxianus | [ | |
| 脂质 | 20.1 | 木糖 | Y. lipolytica | [ |
| 16.5 | 龙舌兰蔗渣水解物 | Y. lipolytica | [ | |
| 12.0 | 芒水解物 | Y. lipolytica | [ | |
| 5.3 | 木糖 | R. toruloides | [ | |
| 39.5 | 小麦秸秆水解物 | R. toruloides | [ | |
| 2.8 | 桦木水解物 | R. toruloides | [ | |
| 15.1 | 木糖 | L. starkeyi | [ | |
| 9.6 | 玉米秸秆水解物 | L. starkeyi | [ | |
| 脂肪酸 | 38.2 | 葡萄糖和木糖 | O. polymorpha | [ |
| 7.0 | 玉米秸秆水解物 | O. polymorpha | [ | |
| 脂肪醇 | 1.2 | 木糖 | S. cerevisiae | [ |
| 0.7 | 柳枝稷和高粱水解物 | S. cerevisiae | [ | |
| 0.5 | 木糖 | L. starkeyi | [ |
| 产品 | 产量/g·L-1 | 底物 | 菌株 | 参考文献 |
|---|---|---|---|---|
| 乙醇 | 45.1 | 小麦秸秆水解物 | S. cerevisiae | [ |
| 68.8 | 玉米秸秆水解物 | S. cerevisiae | [ | |
| 60.8 | 玉米秸秆水解物 | S. cerevisiae | [ | |
| 101.4 | 小麦秸秆水解物 | S. cerevisiae | [ | |
| 26.7 | 木糖 | Pichia stipitis | [ | |
| 1.6 | 木糖 | H. polymorpha | [ | |
| 8.2 | 芦苇水解物 | S. stipitis | [ | |
| 36.2 | 小麦秸秆水解物 | K. marxianus | [ | |
| 脂质 | 20.1 | 木糖 | Y. lipolytica | [ |
| 16.5 | 龙舌兰蔗渣水解物 | Y. lipolytica | [ | |
| 12.0 | 芒水解物 | Y. lipolytica | [ | |
| 5.3 | 木糖 | R. toruloides | [ | |
| 39.5 | 小麦秸秆水解物 | R. toruloides | [ | |
| 2.8 | 桦木水解物 | R. toruloides | [ | |
| 15.1 | 木糖 | L. starkeyi | [ | |
| 9.6 | 玉米秸秆水解物 | L. starkeyi | [ | |
| 脂肪酸 | 38.2 | 葡萄糖和木糖 | O. polymorpha | [ |
| 7.0 | 玉米秸秆水解物 | O. polymorpha | [ | |
| 脂肪醇 | 1.2 | 木糖 | S. cerevisiae | [ |
| 0.7 | 柳枝稷和高粱水解物 | S. cerevisiae | [ | |
| 0.5 | 木糖 | L. starkeyi | [ |
| 产品 | 产量/g·L-1 | 底物 | 菌株 | 参考文献 |
|---|---|---|---|---|
| 木糖醇 | 178.0 | 葡萄糖和木糖 | S. cerevisiae | [ |
| 30.0 | 玉米芯水解物 | S. cerevisiae | [ | |
| 38.0 | 稻草水解物 | S. cerevisiae | [ | |
| 119.0 | 玉米芯水解物 | K. marxianus | [ | |
| 33.0 | 玉米芯水解物 | C. tropicalis | [ | |
| 赤藓糖醇 | 15.0 | 豆渣水解物 | Y. lipolytica | [ |
| 阿拉伯糖醇 | 43.0 | 大豆粉和豆皮水解物 | D. hansenii | [ |
| 12.0 | 小麦秸秆水解物 | D. hansenii | [ | |
| 乳酸 | 49.1 | 木糖 | S. cerevisiae | [ |
| 33.8 | 葡萄糖和木糖 | S. cerevisiae | [ | |
| 3-羟基丙酸 | 7.4 | 木糖 | S. cerevisiae | [ |
| 79.6 | 葡萄糖和木糖 | O. polymorpha | [ | |
| 琥珀酸 | 28.2 | 葡萄糖和木糖 | Y. lipolytica | [ |
| α-酮戊二酸 | 8.3 | 木质纤维素水解液 | Y. lipolytica | [ |
| 柠檬酸 | 42.4 | 木质纤维素水解液 | Y. lipolytica | [ |
| 2,3-丁二醇 | 96.8 | 木糖 | S. cerevisiae | [ |
| 对香豆酸 | 0.2 | 木糖 | S. cerevisiae | [ |
| 聚酮三乙酸内酯 | 23.9 | 木糖和乙酸 | S. cerevisiae | [ |
| 3.6 | 半纤维素水解物 | S. cerevisiae | [ |
| 产品 | 产量/g·L-1 | 底物 | 菌株 | 参考文献 |
|---|---|---|---|---|
| 木糖醇 | 178.0 | 葡萄糖和木糖 | S. cerevisiae | [ |
| 30.0 | 玉米芯水解物 | S. cerevisiae | [ | |
| 38.0 | 稻草水解物 | S. cerevisiae | [ | |
| 119.0 | 玉米芯水解物 | K. marxianus | [ | |
| 33.0 | 玉米芯水解物 | C. tropicalis | [ | |
| 赤藓糖醇 | 15.0 | 豆渣水解物 | Y. lipolytica | [ |
| 阿拉伯糖醇 | 43.0 | 大豆粉和豆皮水解物 | D. hansenii | [ |
| 12.0 | 小麦秸秆水解物 | D. hansenii | [ | |
| 乳酸 | 49.1 | 木糖 | S. cerevisiae | [ |
| 33.8 | 葡萄糖和木糖 | S. cerevisiae | [ | |
| 3-羟基丙酸 | 7.4 | 木糖 | S. cerevisiae | [ |
| 79.6 | 葡萄糖和木糖 | O. polymorpha | [ | |
| 琥珀酸 | 28.2 | 葡萄糖和木糖 | Y. lipolytica | [ |
| α-酮戊二酸 | 8.3 | 木质纤维素水解液 | Y. lipolytica | [ |
| 柠檬酸 | 42.4 | 木质纤维素水解液 | Y. lipolytica | [ |
| 2,3-丁二醇 | 96.8 | 木糖 | S. cerevisiae | [ |
| 对香豆酸 | 0.2 | 木糖 | S. cerevisiae | [ |
| 聚酮三乙酸内酯 | 23.9 | 木糖和乙酸 | S. cerevisiae | [ |
| 3.6 | 半纤维素水解物 | S. cerevisiae | [ |
| 1 | DECHAMPS Pierre. The IEA World Energy Outlook 2022—A brief analysis and implications[J]. The European Energy and Climate Journal, 2023, 11(3): 100-103. |
| 2 | YOLCAN Oguz Ozan. World energy outlook and state of renewable energy: 10-Year evaluation[J]. Innovation and Green Development, 2023, 2(4): 100070. |
| 3 | 袁振宏, 罗文, 吕鹏梅, 等. 生物质能产业现状及发展前景[J]. 化工进展, 2009, 28(10): 1687-1692. |
| YUAN Zhenhong, LUO Wen, Pengmei LYU, et al. Status and prospect of biomass energy industry[J]. Chemical Industry and Engineering Progress, 2009, 28(10):1687-1692. | |
| 4 | FIELD Christopher B, Elliott CAMPBELL J, LOBELL David B. Biomass energy: The scale of the potential resource[J]. Trends in Ecology & Evolution, 2008, 23(2): 65-72. |
| 5 | LIU Yang, HUANG Yihan. Assessing the interrelationship between fossil fuels resources and the biomass energy market for achieving a sustainable and green economy[J]. Resources Policy, 2024, 88: 104397. |
| 6 | 肖军, 段菁春, 王华, 等. 生物质利用现状[J]. 安全与环境工程, 2003, 10(1): 11-14. |
| XIAO Jun, DUAN Jingchun, WANG Hua, et al. The present situation of using biomass[J]. Safety and Environmental Engineering, 2003, 10(1): 11-14. | |
| 7 | 谢光辉. 论我国非粮生物质原料的非粮属性[J]. 中国农业大学学报, 2013, 18(6): 1-5. |
| XIE Guanghui. Nature of non-food for the non-food biomass feedstock in China[J]. Journal of China Agricultural University, 2013, 18(6): 1-5. | |
| 8 | 周中仁. 生物质能概念界定与属性划分的探讨[J]. 安徽农业科学, 2009, 37(25): 12085-12086, 12094. |
| ZHOU Zhongren. Discussion on the definition and attributes division of biomass energy[J]. Journal of Anhui Agricultural Sciences, 2009, 37(25): 12085-12086, 12094. | |
| 9 | WANG Tong, ZHOU Tuo, LI Chaoran, et al. Development status and prospects of biomass energy in China[J]. Energies, 2024, 17(17): 4484. |
| 10 | Sara SALDARRIAGA-HERNÁNDEZ, Carolina VELASCO-AYALA, LEAL-ISLA FLORES Paulina, et al. Biotransformation of lignocellulosic biomass into industrially relevant products with the aid of fungi-derived lignocellulolytic enzymes[J]. International Journal of Biological Macromolecules, 2020, 161: 1099-1116. |
| 11 | BAJPAI Pratima, BAJPAI P. Structure of lignocellulosic biomass[M]//SpringerBriefs in molecular science. Singapore: Springer Singapore, 2016: 7-12. |
| 12 | HONG Kuk-Ki, NIELSEN Jens. Metabolic engineering of Saccharomyces cerevisiae: A key cell factory platform for future biorefineries[J]. Cellular and Molecular Life Sciences, 2012, 69(16): 2671-2690. |
| 13 | Ja Kyong KO, LEE Jae Hoon, JUNG Je Hyeong, et al. Recent advances and future directions in plant and yeast engineering to improve lignocellulosic biofuel production[J]. Renewable and Sustainable Energy Reviews, 2020, 134: 110390. |
| 14 | KUMAR Amit, GAUTAM Archana, DUTT Dharm. Biotechnological transformation of lignocellulosic biomass in to industrial products: An overview[J]. Advances in Bioscience and Biotechnology, 2016, 7(3): 149-168. |
| 15 | LU Hedong, YADAV Vivek, BILAL Muhammad, et al. Bioprospecting microbial hosts to valorize lignocellulose biomass–Environmental perspectives and value-added bioproducts[J]. Chemosphere, 2022, 288: 132574. |
| 16 | 刘新, 李静, 晏飞来, 等. 木质纤维素预处理工艺的研究现状[J]. 黑龙江农业科学, 2010 (2): 112-114. |
| LIU Xin, LI Jing, YAN Feilai, et al. Research progress in pretreatment of lignocellulose[J]. Heilongjiang Agricultural Sciences, 2010 (2):112-114. | |
| 17 | CHEN Xiangxue, YUAN Xinchuan, CHEN Sitong, et al. Densifying Lignocellulosic biomass with alkaline Chemicals (DLC) pretreatment unlocks highly fermentable sugars for bioethanol production from corn stover[J]. Green Chemistry, 2021, 23(13): 4828-4839. |
| 18 | 祝其丽, 何明雄, 谭芙蓉, 等. 木质纤维素生物质预处理研究现状[J]. 生物技术进展, 2015, 5(6): 414-419. |
| ZHU Qili, HE Mingxiong, TAN Furong, et al. Progress on pretreatment technologies of lignocellulosic biomass[J]. Current Biotechnology, 2015, 5(6): 414-419. | |
| 19 | YUAN Xinchuan, SHEN Guannan, CHEN Sitong, et al. Elucidating the mechanism of densifying lignocellulosic biomass with acidic chemicals (DLC) for lignocellulosic biorefinery[J]. Green Chemistry, 2023, 25(17): 6759-6773. |
| 20 | KUMAR Parveen, BARRETT Diane M, DELWICHE Michael J, et al. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production[J]. Industrial & Engineering Chemistry Research, 2009, 48(8): 3713-3729. |
| 21 | GU Tingyue, HELD Michael A, FAIK Ahmed. Supercritical CO2 and ionic liquids for the pretreatment of lignocellulosic biomass in bioethanol production[J]. Environmental Technology, 2013, 34(13/14/15/16): 1735-1749. |
| 22 | ISROI, MILLATI Ria, SYAMSIAH Siti, et al. Biological pretreatment of lignocelluloses with white-rot fungi and its applications: A review[J]. BioResources, 2011, 6(4): 5224-5259. |
| 23 | SINGH Rajendra, KUMAR Manoj, MITTAL Anshumali, et al. Microbial enzymes: Industrial progress in 21st century[J]. 3 Biotech, 2016, 6(2): 174. |
| 24 | SHIRKAVAND Ehsan, BAROUTIAN Saeid, GAPES Daniel J, et al. Combination of fungal and physicochemical processes for lignocellulosic biomass pretreatment-A review[J]. Renewable and Sustainable Energy Reviews, 2016, 54: 217-234. |
| 25 | PALMQUIST Eva, Bärbel HAHN-HÄGERDAL. Fermentation of lignocellulosic hydrolysates.Ⅰ:Inhibition and detoxification[J]. Bioresource Technology, 2000, 74(1): 17-24. |
| 26 | PALMQUIST Eva, Bärbel HAHN-HÄGERDAL. Fermentation of lignocellulosic hydrolysates.Ⅱ: Inhibitors and mechanisms of inhibition[J]. Bioresource Technology, 2000, 74(1): 25-33. |
| 27 | JÖNSSON Leif J, ALRIKSSON Björn, NILVEBRANT Nils-Olof. Bioconversion of lignocellulose: inhibitors and detoxification[J]. Biotechnology for Biofuels, 2013, 6(1): 16. |
| 28 | MUSSATTO Solange Inês, ROBERTO Inês Conceição. Alternatives for detoxification of diluted-acid lignocellulosic hydrolyzates for use in fermentative processes: A review[J]. Bioresource Technology, 2004, 93(1): 1-10. |
| 29 | ZHA Jian, YUWEN Miaomiao, QIAN Weidong, et al. Yeast-based biosynthesis of natural products from xylose[J]. Frontiers in Bioengineering and Biotechnology, 2021, 9: 634919. |
| 30 | 王明,栾韬,赵建志,等.酿酒酵母转化木糖生产化学品的研究进展[J].生物工程学报, 2021, 37(3): 1042-1057. |
| WANG Ming, LUAN Tao, ZHAO Jianzhi, et al. Progress in studies on production of chemicals from xylose by Saccharomyces cerevisiae [J]. Chinese Journal of Biotechnology, 2021, 37(3): 1042-1057. | |
| 31 | ALMEIDA João RM, MODIG Tobias, PETERSSON Anneli, et al. Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae [J]. Journal of Chemical Technology & Biotechnology, 2007, 82(4): 340-349. |
| 32 | SWINNEN Steve, HENRIQUES Sílvia F, SHRESTHA Ranjan, et al. Improvement of yeast tolerance to acetic acid through Haa1 transcription factor engineering: Towards the underlying mechanisms[J]. Microbial Cell Factories, 2017, 16(1): 7. |
| 33 | PETERSSON Anneli, ALMEIDA João R M, MODIG Tobias, et al. A 5-hydroxymethyl furfural reducing enzyme encoded by the Saccharomyces cerevisiae ADH6 gene conveys HMF tolerance[J]. Yeast, 2006, 23(6): 455-464. |
| 34 | KUMARI Rajni, PRAMANIK Krishna. Improvement of multiple stress tolerance in yeast strain by sequential mutagenesis for enhanced bioethanol production[J]. Journal of Bioscience and Bioengineering, 2012, 114(6): 622-629. |
| 35 | MENEGON Yasmine Alves, GROSS Jeferson, JACOBUS Ana Paula. How adaptive laboratory evolution can boost yeast tolerance to lignocellulosic hydrolyses[J]. Current Genetics, 2022, 68(3/4): 319-342. |
| 36 | ZHANG Yiwen, YANG Junjie, QIAN Fenghui, et al. Engineering a xylose fermenting yeast for lignocellulosic ethanol production[J]. Nature Chemical Biology, 2024: 1-8. |
| 37 | LI Xiaowei, WANG Yanyan, LI Gang, et al. Metabolic network remodelling enhances yeast’s fitness on xylose using aerobic glycolysis[J]. Nature Catalysis, 2021, 4: 783-796. |
| 38 | CHEN Sitong, XU Zhaoxian, DING Boning, et al. Big data mining, rational modification, and ancestral sequence reconstruction inferred multiple xylose isomerases for biorefinery[J]. Science Advances, 2023, 9(5): eadd8835. |
| 39 | VALDEHUESA Kris Niño G, RAMOS Kristine Rose M, NISOLA Grace M, et al. Everyone loves an underdog: Metabolic engineering of the xylose oxidative pathway in recombinant microorganisms[J]. Applied Microbiology and Biotechnology, 2018, 102(18): 7703-7716. |
| 40 | DE PAULA Renato Graciano, ANTONIÊTO Amanda Cristina Campos, RIBEIRO Liliane Fraga Costa, et al. Engineered microbial host selection for value-added bioproducts from lignocellulose[J]. Biotechnology Advances, 2019, 37(6): 107347. |
| 41 | STEPHENS Craig, CHRISTEN Beat, FUCHS Thomas, et al. Genetic analysis of a novel pathway for D-xylose metabolism in Caulobacter crescentus [J]. Journal of Bacteriology, 2007, 189(5): 2181-2185. |
| 42 | KIM Soo Rin, PARK Yong-Cheol, JIN Yongsu, et al. Strain engineering of Saccharomyces cerevisiae for enhanced xylose metabolism[J]. Biotechnology Advances, 2013, 31(6): 851-861. |
| 43 | WU Yufen, XU Shuo, GAO Xiao, et al. Enhanced protopanaxadiol production from xylose by engineered Yarrowia lipolytica [J]. Microbial Cell Factories, 2019, 18(1): 83. |
| 44 | Celina BORGSTRÖM, WASSERSTROM Lisa, ALMQVIST Henrik, et al. Identification of modifications procuring growth on xylose in recombinant Saccharomyces cerevisiae strains carrying the Weimberg pathway[J]. Metabolic Engineering, 2019, 55: 1-11. |
| 45 | KWAK Suryang, JIN Yongsu. Production of fuels and chemicals from xylose by engineered Saccharomyces cerevisiae: A review and perspective[J]. Microbial Cell Factories, 2017, 16(1): 82. |
| 46 | CAI Peng, WU Xiaoyan, DENG Jun, et al. Methanol biotransformation toward high-level production of fatty acid derivatives by engineering the industrial yeast Pichia pastoris [J]. Proceedings of the National Academy of Sciences, 2022, 119(29): e2201711119. |
| 47 | 汪城墙, 李洪兴, 徐丽丽, 等. 酿酒酵母戊糖转运蛋白及C6/C5共代谢菌株的研究进展[J]. 生物工程学报, 2018, 34(10): 1543-1555. |
| WANG Chengqiang, LI Hongxing, XU Lili, et al. Progress in research of pentose transporters and C6/C5 co-metabolic strains in Saccharomyces cerevisiae [J]. Chinese Journal of Biotechnology, 2018, 34(10): 1543-1555. | |
| 48 | WANG Meng, YU Chenzhao, ZHAO Huimin. Directed evolution of xylose specific transporters to facilitate glucose-xylose co-utilization[J]. Biotechnology and Bioengineering, 2016, 113(3): 484-491. |
| 49 | SHIN Hyun Yong, NIJLAND Jeroen G, DE WAAL Paul P, et al. The amino-terminal tail of Hxt11 confers membrane stability to the Hxt2 sugar transporter and improves xylose fermentation in the presence of acetic acid[J]. Biotechnology and Bioengineering, 2017, 114(9): 1937-1945. |
| 50 | NIJLAND Jeroen G, SHIN Hyun Yong, BOENDER Leonie G M, et al. Improved xylose metabolism by a CYC8 mutant of Saccharomyces cerevisiae [J]. Applied and Environmental Microbiology, 2017, 83(11): e00095-17. |
| 51 | BUENO João Gabriel Ribeiro, BORELLI Guilherme, CORRÊA Thamy Livia Ribeiro, et al. Novel xylose transporter Cs4130 expands the sugar uptake repertoire in recombinant Saccharomyces cerevisiae strains at high xylose concentrations[J]. Biotechnology for Biofuels, 2020, 13: 145. |
| 52 | KNYCHALA Marilia M, DOS SANTOS Angela A, KRETZER Leonardo G, et al. Strategies for efficient expression of heterologous monosaccharide transporters in Saccharomyces cerevisiae [J]. Journal of Fungi, 2022, 8(1): 84. |
| 53 | KONISHI Jin, FUKUDA Akira, MUTAGUCHI Kozue, et al. Xylose fermentation by Saccharomyces cerevisiae using endogenous xylose-assimilating genes[J]. Biotechnology Letters, 2015, 37(8): 1623-1630. |
| 54 | REIDER APEL Amanda, OUELLET Mario, Heather SZMIDT-MIDDLETON, et al. Evolved hexose transporter enhances xylose uptake and glucose/xylose co-utilization in Saccharomyces cerevisiae [J]. Scientific Reports, 2016, 6: 19512. |
| 55 | 王倩, 高教琪, 周雍进. 葡萄糖和木糖高效共利用代谢工程研究进展[J]. 生物工程学报, 2024, 40(8): 2710-2730. |
| WANG Qian, GAO Jiaoqi, ZHOU Yongjin. Metabolic engineering for the efficient co-utilization of glucose and xylose[J]. Chinese Journal of Biotechnology, 2024, 40(8): 2710-2730. | |
| 56 | VERHOEVEN Maarten D, LEE Misun, KAMOEN Lycka, et al. Mutations in PMR1 stimulate xylose isomerase activity and anaerobic growth on xylose of engineered Saccharomyces cerevisiae by influencing manganese homeostasis[J]. Scientific Reports, 2017, 7: 46155. |
| 57 | XU Haiqing, KIM Sooah, SOREK Hagit, et al. PHO13 deletion-induced transcriptional activation prevents sedoheptulose accumulation during xylose metabolism in engineered Saccharomyces cerevisiae [J]. Metabolic Engineering, 2016, 34: 88-96. |
| 58 | LI Haibo, ALPER Hal S. Enabling xylose utilization in Yarrowia lipolytica for lipid production[J]. Biotechnology Journal, 2016, 11(9): 1230-1240. |
| 59 | Rodrigo LEDESMA-AMARO, LAZAR Zbigniew, RAKICKA Magdalena, et al. Metabolic engineering of Yarrowia lipolytica to produce chemicals and fuels from xylose[J]. Metabolic Engineering, 2016, 38: 115-124. |
| 60 | NIEHUS Xochitl, Anne-Marie CRUTZ-LE COQ, SANDOVAL Georgina, et al. Engineering Yarrowia lipolytica to enhance lipid production from lignocellulosic materials[J]. Biotechnology for Biofuels, 2018, 11: 11. |
| 61 | YOOK Sang Do, KIM Jiwon, GONG Gyeongtack, et al. High-yield lipid production from lignocellulosic biomass using engineered xylose-utilizing Yarrowia lipolytica [J]. GCB Bioenergy, 2020, 12(9): 670-679. |
| 62 | LOUIE Tai Man, LOUIE Kailin, DENHARTOG Samuel, et al. Production of bio-xylitol from D-xylose by an engineered Pichia pastoris expressing a recombinant xylose reductase did not require any auxiliary substrate as electron donor[J]. Microbial Cell Factories, 2021, 20(1): 50. |
| 63 | SHIN Minhye, KIM Soo Rin. Metabolic changes induced by deletion of transcriptional regulator GCR2 in xylose-fermenting Saccharomyces cerevisiae [J]. Microorganisms, 2020, 8(10): 1499. |
| 64 | WEI Shan, BAI Penggang, LIU Yanan, et al. A Thi2p regulatory network controls the post-glucose effect of xylose utilization in Saccharomyces cerevisiae [J]. Frontiers in Microbiology, 2019, 10: 1649. |
| 65 | GAO Jiaoqi, GAO Ning, ZHAI Xiaoxin, et al. Recombination machinery engineering for precise genome editing in methylotrophic yeast Ogataea polymorpha [J]. iScience, 2021, 24(3):102168. |
| 66 | BAPTISTA Marlene, DOMINGUES Lucília. Kluyveromyces marxianus as a microbial cell factory for lignocellulosic biomass valorisation[J]. Biotechnology Advances, 2022, 60: 108027. |
| 67 | 张亚云, 丁长河, 李里特, 等. 树干毕赤酵母发酵木糖生产燃料乙醇[J]. 酿酒, 2009, 36(1): 23-26. |
| ZHANG Yayun, DING Changhe, LI Lite, et al. Fermentation of D-xylose for ethanol production by Pichia stipitis [J]. Liquor Making, 2009, 36(1): 23-26. | |
| 68 | VASYLYSHYN Roksolana, KURYLENKO Olena, RUCHALA Justyna, et al. Engineering of sugar transporters for improvement of xylose utilization during high-temperature alcoholic fermentation in Ogataea polymorpha yeast[J]. Microbial Cell Factories, 2020, 19(1): 96. |
| 69 | RUCHALA Justyna, KURYLENKO Olena O, SOONTORNGUN Nitnipa, et al. Transcriptional activator Cat8 is involved in regulation of xylose alcoholic fermentation in the thermotolerant yeast Ogataea (Hansenula) polymorpha [J]. Microbial Cell Factories, 2017, 16(1): 36. |
| 70 | DMYTRUK Kostyantyn V, RUCHALA Justyna, Dorota GRABEK-LEJKO, et al. Autophagy-related gene ATG13 is involved in control of xylose alcoholic fermentation in the thermotolerant methylotrophic yeast Ogataea polymorpha [J]. FEMS Yeast Research, 2018, 18(2): 4847886. |
| 71 | ZHANG Jia, ZHANG Biao, WANG Dongmei, et al. Improving xylitol production at elevated temperature with engineered Kluyveromyces marxianus through over-expressing transporters[J]. Bioresource Technology, 2015, 175: 642-645. |
| 72 | HAN Ji-Hye, PARK Ju-Yong, YOO Kye Sang, et al. Effect of glucose on xylose utilization in Saccharomyces cerevisiae harboring the xylose reductase gene[J]. Archives of Microbiology, 2011, 193(5): 335-340. |
| 73 | 蔡艳青, 齐显尼, 齐奇, 等. 敲除MIG1和SNF1基因对酿酒酵母共利用葡萄糖和木糖的影响[J]. 生物工程学报, 2018, 34(1): 54-67. |
| CAI Yanqing, QI Xianni, QI Qi, et al. Effect of MIG1 and SNF1 deletion on simultaneous utilization of glucose and xylose by Saccharomyces cerevisiae [J]. Chinese Journal of Biotechnology, 2018, 34(1): 54-67. | |
| 74 | 李燕军, 赵岩, 黄龙辉, 等. 微生物同步利用葡萄糖和木糖代谢工程概述[J]. 发酵科技通讯, 2017, 46(1): 54-59. |
| LI Yanjun, ZHAO Yan, HUANG Longhui, et al. Overview of metabolic engineering on simultaneous utilization of glucose and xylose by microbes[J]. Bulletin of Fermentation Science and Technology, 2017, 46(1): 54-59. | |
| 75 | GAO Jiaoqi, YU Wei, LI Yunxia, et al. Engineering co-utilization of glucose and xylose for chemical overproduction from lignocellulose[J]. Nature Chemical Biology, 2023, 19(12): 1524-1531. |
| 76 | KURYLENKO Olena, RUCHALA Justyna, KRUK Barbara, et al. The role of Mig1, Mig2, Tup1 and Hap4 transcription factors in regulation of xylose and glucose fermentation in the thermotolerant yeast Ogataea polymorpha [J]. FEMS Yeast Research, 2021, 21(4): foab029. |
| 77 | DASHTBAN Mehdi, WEN Xin, BAJWA Paramjit K, et al. Deletion of hxk1 gene results in derepression of xylose utilization in Scheffersomyces stipitis [J]. Journal of Industrial Microbiology & Biotechnology, 2015, 42(6): 889-896. |
| 78 | HUA Yan, WANG Jichao, ZHU Yelin, et al. Release of glucose repression on xylose utilization in Kluyveromyces marxianus to enhance glucose-xylose co-utilization and xylitol production from corncob hydrolysate[J]. Microbial Cell Factories, 2019, 18(1): 24. |
| 79 | MAVROMMATI Maria, DASKALAKI Alexandra, PAPANIKOLAOU Seraphim, et al. Adaptive laboratory evolution principles and applications in industrial biotechnology[J]. Biotechnology Advances, 2022, 54: 107795. |
| 80 | DRAGOSITS Martin, MATTANOVICH Diethard. Adaptive laboratory evolution—Principles and applications for biotechnology[J]. Microbial Cell Factories, 2013, 12: 64. |
| 81 | SANDBERG Troy E, SALAZAR Michael J, WENG Liam L, et al. The emergence of adaptive laboratory evolution as an efficient tool for biological discovery and industrial biotechnology[J]. Metabolic Engineering, 2019, 56: 1-16. |
| 82 | PROMDONKOY Peerada, MHUANTONG Wuttichai, CHAMPREDA Verawat, et al. Improvement in d-xylose utilization and isobutanol production in S. cerevisiae by adaptive laboratory evolution and rational engineering[J]. Journal of Industrial Microbiology and Biotechnology, 2020, 47(6/7): 497-510. |
| 83 | LEE Sunmi, JELLISON Taylor, ALPER Hal S. Systematic and evolutionary engineering of a xylose isomerase-based pathway in Saccharomyces cerevisiae for efficient conversion yields[J]. Biotechnology for Biofuels, 2014, 7(1): 122. |
| 84 | SHARMA Nilesh Kumar, BEHERA Shuvashish, ARORA Richa, et al. Evolutionary adaptation of Kluyveromyces marxianus NIRE-K3 for enhanced xylose utilization[J]. Frontiers in Energy Research, 2017, 5: 32. |
| 85 | ZHOU Linlin, WEN Zhiqiang, WANG Zedi, et al. Evolutionary engineering improved D-glucose/xylose cofermentation of Yarrowia lipolytica [J]. Industrial & Engineering Chemistry Research, 2020, 59(39): 17113-17123. |
| 86 | TRICHEZ Débora, STEINDORFF Andrei S, DE MORAIS JÚNIOR Wilson G, et al. Identification of traits to improve co-assimilation of glucose and xylose by adaptive evolution of Spathaspora passalidarum and Scheffersomyces stipitis yeasts[J]. Applied Microbiology and Biotechnology, 2023, 107(4): 1143-1157. |
| 87 | 张子晨, 宋元达. 木质纤维素材料综合利用生物技术研究进展[J]. 广东农业科学, 2021, 48(1): 150-159. |
| ZHANG Zichen, SONG Yuanda. Research progress in biotechnology for comprehensive utilization of lignocellulosic materials[J]. Guangdong Agricultural Sciences, 2021, 48(1): 150-159. | |
| 88 | 唐瑞琪, 熊亮, 程诚, 等. 纤维素乙醇生产重组酿酒酵母菌株的构建与优化研究进展[J]. 化工进展, 2018, 37(8): 3119-3128. |
| TANG Ruiqi, XIONG Liang, CHENG Cheng, et al. Progress of research on construction and optimization of recombinant Saccharomyces cerevisiae strains for cellulosic ethanol production[J]. Chemical Industry and Engineering Progress, 2018, 37(8): 3119-3128. | |
| 89 | DEMEKE Mekonnen M, DIETZ Heiko, LI Yingying, et al. Development of a D-xylose fermenting and inhibitor tolerant industrial Saccharomyces cerevisiae strain with high performance in lignocellulose hydrolysates using metabolic and evolutionary engineering[J]. Biotechnology for Biofuels, 2013, 6(1): 89. |
| 90 | ZHU Jiaqing, QIN Lei, LI Bingzhi, et al. Simultaneous saccharification and co-fermentation of aqueous ammonia pretreated corn stover with an engineered Saccharomyces cerevisiae SyBE005[J]. Bioresource Technology, 2014, 169: 9-18. |
| 91 | LIU Zhihua, CHEN Hongzhang. Simultaneous saccharification and co-fermentation for improving the xylose utilization of steam exploded corn stover at high solid loading[J]. Bioresource Technology, 2016, 201: 15-26. |
| 92 | LIU Gang, ZHANG Qiang, LI Hongxing, et al. Dry biorefining maximizes the potentials of simultaneous saccharification and co-fermentation for cellulosic ethanol production[J]. Biotechnology and Bioengineering, 2018, 115(1): 60-69. |
| 93 | SILVA J P A, MUSSATTO S I, ROBERTO I C, et al. Ethanol production from xylose by Pichia stipitis NRRL Y-7124 in a stirred tank bioreactor[J]. Brazilian Journal of Chemical Engineering, 2011, 28(1): 151-156. |
| 94 | ISHCHUK Olena P, VORONOVSKY Andriy Y, STASYK Oleh V, et al. Overexpression of pyruvate decarboxylase in the yeast Hansenula polymorpha results in increased ethanol yield in high-temperature fermentation of xylose[J]. FEMS Yeast Research, 2008, 8(7): 1164-1174. |
| 95 | SCORDIA Danilo, COSENTINO Salvatore L, LEE Jae-Won, et al. Bioconversion of giant reed (Arundo donax L.) hemicellulose hydrolysate to ethanol by Scheffersomyces stipitis CBS6054[J]. Biomass and Bioenergy, 2012, 39: 296-305. |
| 96 | TOMÁS-PEJÓ E, OLIVA J M, GONZÁLEZ A, et al. Bioethanol production from wheat straw by the thermotolerant yeast Kluyveromyces marxianus CECT 10875 in a simultaneous saccharification and fermentation fed-batch process[J]. Fuel, 2009, 88(11): 2142-2147. |
| 97 | LIU Zihe, MORADI Hamideh, SHI Shuobo, et al. Yeasts as microbial cell factories for sustainable production of biofuels[J]. Renewable and Sustainable Energy Reviews, 2021, 143: 110907. |
| 98 | BEOPOULOS Athanasios, CHARDOT Thierry, NICAUD Jean-Marc. Yarrowia lipolytica: A model and a tool to understand the mechanisms implicated in lipid accumulation[J]. Biochimie, 2009, 91(6): 692-696. |
| 99 | LIN Jintao, LI Shuangyue, SUN Mingzhong, et al. Microbial lipid production by oleaginous yeast in d-xylose solution using a two-stage culture mode[J]. RSC Advances, 2014, 4(66): 34944-34949. |
| 100 | Teresa DÍAZ, FILLET Sandy, CAMPOY Sonia, et al. Combining evolutionary and metabolic engineering in Rhodosporidium toruloides for lipid production with non-detoxified wheat straw hydrolysates[J]. Applied Microbiology and Biotechnology, 2018, 102(7): 3287-3300. |
| 101 | MATSAKAS Leonidas, NOVAK Katharina, ENMAN Josefine, et al. Acetate-detoxification of wood hydrolysates with alkali tolerant Bacillus sp. as a strategy to enhance the lipid production from Rhodosporidium toruloides [J]. Bioresource Technology, 2017, 242: 287-294. |
| 102 | JUANSSILFERO Ario B, KAHAR Prihardi, AMZA Rezky L, et al. Effect of inoculum size on single-cell oil production from glucose and xylose using oleaginous yeast Lipomyces starkeyi [J]. Journal of Bioscience and Bioengineering, 2018, 125(6): 695-702. |
| 103 | CALVEY Christopher H, SU Yikai, WILLIS Laura B, et al. Nitrogen limitation, oxygen limitation, and lipid accumulation in Lipomyces starkeyi [J]. Bioresource Technology, 2016, 200: 780-788. |
| 104 | GUO Weihua, SHENG Jiayuan, ZHAO Huimin, et al. Metabolic engineering of Saccharomyces cerevisiae to produce 1-hexadecanol from xylose[J]. Microbial Cell Factories, 2016, 15: 24. |
| 105 | Leo D’ESPAUX, GHOSH Amit, RUNGUPHAN Weerawat, et al. Engineering high-level production of fatty alcohols by Saccharomyces cerevisiae from lignocellulosic feedstocks[J]. Metabolic Engineering, 2017, 42: 115-125. |
| 106 | MCNEIL Bonnie A, STUART David T. Optimization of C16 and C18 fatty alcohol production by an engineered strain of Lipomyces starkeyi [J]. Journal of Industrial Microbiology & Biotechnology, 2018, 45(1): 1-14. |
| 107 | BAPTISTA Sara L, COSTA Carlos E, CUNHA Joana T, et al. Metabolic engineering of Saccharomyces cerevisiae for the production of top value chemicals from biorefinery carbohydrates[J]. Biotechnology Advances, 2021, 47: 107697. |
| 108 | FELIPE HERNÁNDEZ-PÉREZ Andrés, DE ARRUDA Priscila Vaz, SENE Luciane, et al. Xylitol bioproduction: State-of-the-art, industrial paradigm shift, and opportunities for integrated biorefineries[J]. Critical Reviews in Biotechnology, 2019, 39(7): 924-943. |
| 109 | KIM Hyoju, LEE Hyun-Soo, PARK Haeseong, et al. Enhanced production of xylitol from xylose by expression of Bacillus subtilis Arabinose: H+ symporter and Scheffersomyces stipitis xylose reductase in recombinant Saccharomyces cerevisiae [J]. Enzyme and Microbial Technology, 2017, 107: 7-14. |
| 110 | BAPTISTA Sara L, CUNHA Joana T, Aloia ROMANÍ, et al. Xylitol production from lignocellulosic whole slurry corn cob by engineered industrial Saccharomyces cerevisiae PE-2[J]. Bioresource Technology, 2018, 267: 481-491. |
| 111 | GUIRIMAND Gregory, SASAKI Kengo, INOKUMA Kentaro, et al. Cell surface engineering of Saccharomyces cerevisiae combined with membrane separation technology for xylitol production from rice straw hydrolysate[J]. Applied Microbiology and Biotechnology, 2016, 100(8): 3477-3487. |
| 112 | Kubra ERYASAR-ORER, Seda KARASU-YALCIN. Optimization of activated charcoal detoxification and concentration of chestnut shell hydrolysate for xylitol production[J]. Biotechnology Letters, 2021, 43(6): 1195-1209. |
| 113 | ERIAN Anna Maria, SAUER Michael. Utilizing yeasts for the conversion of renewable feedstocks to sugar alcohols-a review[J]. Bioresource Technology, 2022, 346: 126296. |
| 114 | LIU Xiaoyan, YU Xinjun, XIA Jun, et al. Erythritol production by Yarrowia lipolytica from okara pretreated with the in-house enzyme pools of fungi[J]. Bioresource Technology, 2017, 244: 1089-1095. |
| 115 | LOMAN Abdullah A, ISLAM S M, JU Lu-Kwang. Production of Arabitol from enzymatic hydrolysate of soybean flour by Debaryomyces hansenii fermentation[J]. Applied Microbiology and Biotechnology, 2018, 102(2): 641-653. |
| 116 | YAMAKAWA Celina K, KASTELL Laura, MAHLER Mikkel R, et al. Exploiting new biorefinery models using non-conventional yeasts and their implications for sustainability[J]. Bioresource Technology, 2020, 309: 123374. |
| 117 | YIN Xian, LI Jianghua, SHIN Hyun-dong, et al. Metabolic engineering in the biotechnological production of organic acids in the tricarboxylic acid cycle of microorganisms: Advances and prospects[J]. Biotechnology Advances, 2015, 33(6): 830-841. |
| 118 | TURNER Timothy L, ZHANG Guochang, KIM Soo Rin, et al. Lactic acid production from xylose by engineered Saccharomyces cerevisiae without PDC or ADH deletion[J]. Applied Microbiology and Biotechnology, 2015, 99(19): 8023-8033. |
| 119 | KILDEGAARD Kanchana R, WANG Zheng, CHEN Yun, et al. Production of 3-hydroxypropionic acid from glucose and xylose by metabolically engineered Saccharomyces cerevisiae [J]. Metabolic Engineering Communications, 2015, 2: 132-136. |
| 120 | NOVY Vera, BRUNNER Bernd, Gerdt MÜLLER, et al. Toward “homolactic” fermentation of glucose and xylose by engineered Saccharomyces cerevisiae harboring a kinetically efficient l-lactate dehydrogenase within pdc1-pdc5 deletion background[J]. Biotechnology and Bioengineering, 2017, 114(1): 163-171. |
| 121 | Khai Lun ONG, LI Chong, LI Xiaotong, et al. Co-fermentation of glucose and xylose from sugarcane bagasse into succinic acid by Yarrowia lipolytica [J]. Biochemical Engineering Journal, 2019, 148: 108-115. |
| 122 | Seunghyun RYU, Nicole LABBÉ, TRINH Cong T. Simultaneous saccharification and fermentation of cellulose in ionic liquid for efficient production of α-ketoglutaric acid by Yarrowia lipolytica [J]. Applied Microbiology and Biotechnology, 2015, 99(10): 4237-4244. |
| 123 | LIU Xiaoyan, Jinshun LYU, ZHANG Tong, et al. Citric acid production from hydrolysate of pretreated straw cellulose by Yarrowia lipolytica SWJ-1b using batch and fed-batch cultivation[J]. Preparative Biochemistry & Biotechnology, 2015, 45(8): 825-835. |
| 124 | KIM Soo-Jung, Hee-Jin SIM, KIM Jin-Woo, et al. Enhanced production of 2,3-butanediol from xylose by combinatorial engineering of xylose metabolic pathway and cofactor regeneration in pyruvate decarboxylase-deficient Saccharomyces cerevisiae [J]. Bioresource Technology, 2017, 245: 1551-1557. |
| 125 | BORJA Gheorghe M, RODRIGUEZ Angelica, CAMPBELL Kate, et al. Metabolic engineering and transcriptomic analysis of Saccharomyces cerevisiae producing p-coumaric acid from xylose[J]. Microbial Cell Factories, 2019, 18(1): 191. |
| 126 | WEI Na, QUARTERMAN Josh, KIM Soo Rin, et al. Enhanced biofuel production through coupled acetic acid and xylose consumption by engineered yeast[J]. Nature Communications, 2013, 4: 2580. |
| [1] | SHENG Huakang, ZHANG Bo, SHEN Xiaolin, SUN Xinxiao, WANG Jia, YUAN Qipeng. Microbial synthesis of resveratrol and its derivatives [J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2463-2474. |
| [2] | WANG Yuanyuan, ZHANG Chong, HAN Shuangyan, XING Xinhui. Research progress on bioproduction of recombinant proteins by Pichia pastoris utilizing methanol [J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2441-2450. |
| [3] | SHU Gangwei, LIN Yucheng, ZHANG Weihong, ZHAO Shiqiang, ZHENG Xiaoyang, CHANG Chun. Research progress in biorefinery and high value application of xylose [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3798-3811. |
| [4] | CHEN Zhiqiang, XIA Mingwei, YANG Haiping, CHEN Yingquan, WANG Xianhua, CHEN Hanping. Research progress on synthesis and regulation of lignocellulose-based carbon quantum dots [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3100-3113. |
| [5] | BAI Yuli, BAI Fudong, ZHANG Lei, SUN Qimei, LI Xiuzheng. Preparation and process optimization of lignin-based phenolic resin [J]. Chemical Industry and Engineering Progress, 2024, 43(2): 1033-1038. |
| [6] | TAO Yuxuan, GUO Liang, GAO Cong, SONG Wei, CHEN Xiulai. Progress in metabolic engineering of microorganisms for CO2 fixation [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 40-52. |
| [7] | GUO Feng, ZHANG Shangjie, JIANG Yujia, JIANG Wankui, XIN Fengxue, ZHANG Wenming, JIANG Min. Biotransformation of one-carbon resources by yeast [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 30-39. |
| [8] | XU Lijie, LIU Haojie, XUE Rui, ZHOU Xiaoli, ZHOU Jie, QIAN Xiujuan, DONG Weiliang, JIANG Min. Interdisciplinary assistance for biological recycling of waste plastics [J]. Chemical Industry and Engineering Progress, 2022, 41(9): 5029-5036. |
| [9] | HUANG Yuefeng, MA Lisha, ZHANG Lili, WANG Zhiguo. Research progress on functional application of lignocellulose composite biomass film materials [J]. Chemical Industry and Engineering Progress, 2022, 41(9): 4840-4854. |
| [10] | HAN Mingyang, QIAO Hui, FU Jiaming, MA Zewen, WANG Yan, OUYANG Jia. Research progress of non-aqueous solvents on the pretreatment of lignocellulose [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4086-4097. |
| [11] | DUAN Zhengyang, HU Ningmeng, LI Tianguo. Preparation of xanthate-functionalized cross-linked baker's yeast and its adsorption characteristics for Pb(Ⅱ) [J]. Chemical Industry and Engineering Progress, 2022, 41(7): 3925-3937. |
| [12] | SHI Chengfeng, JIA Ranran, YAN Zhenli, HUI Fengli. Identification of a cold-adapted lipase-producing yeast and its enzyme characterization [J]. Chemical Industry and Engineering Progress, 2022, 41(10): 5541-5548. |
| [13] | BAO Wenjun, LI Zifu, WANG Xuemei, GAO Ruiling, CHENG Shikun, MEN Yu. Progress of oleaginous yeast utilizing low-cost substrates to synthesize lipids [J]. Chemical Industry and Engineering Progress, 2021, 40(5): 2484-2495. |
| [14] | LI Ling, YU Yong, HU Yonghong. Research progress in production of lipstatinfermentation [J]. Chemical Industry and Engineering Progress, 2021, 40(4): 2251-2257. |
| [15] | LI Yang, ZHU Chenhui, FAN Daidi. Green biological manufacture and application of recombinant collagen [J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1262-1275. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||