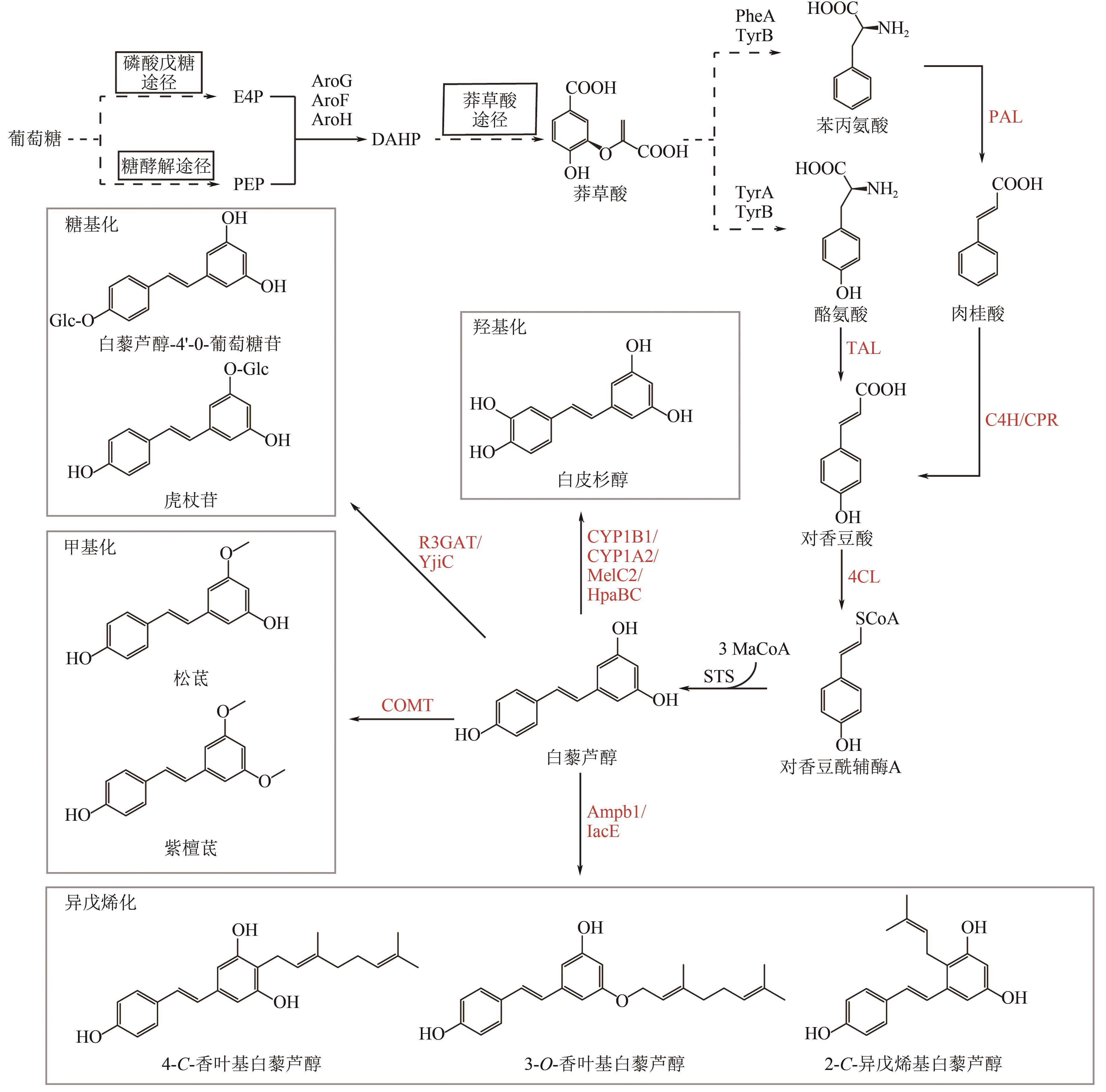
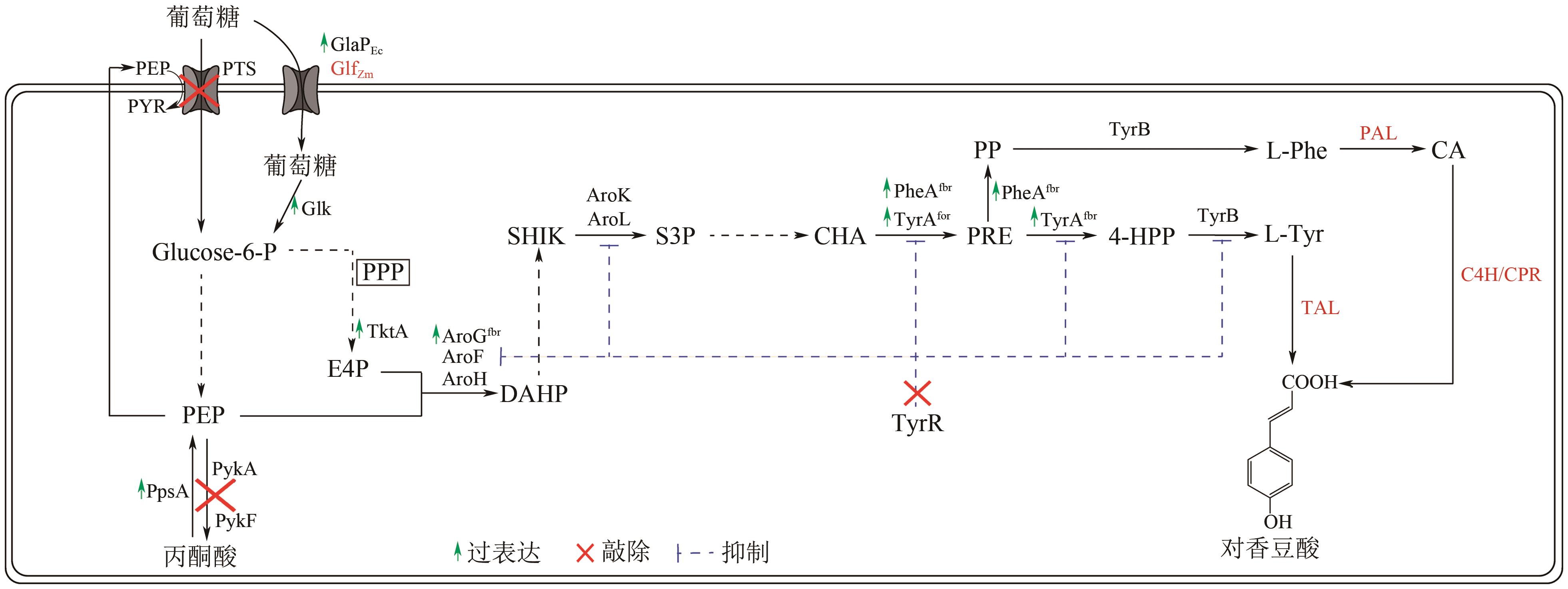
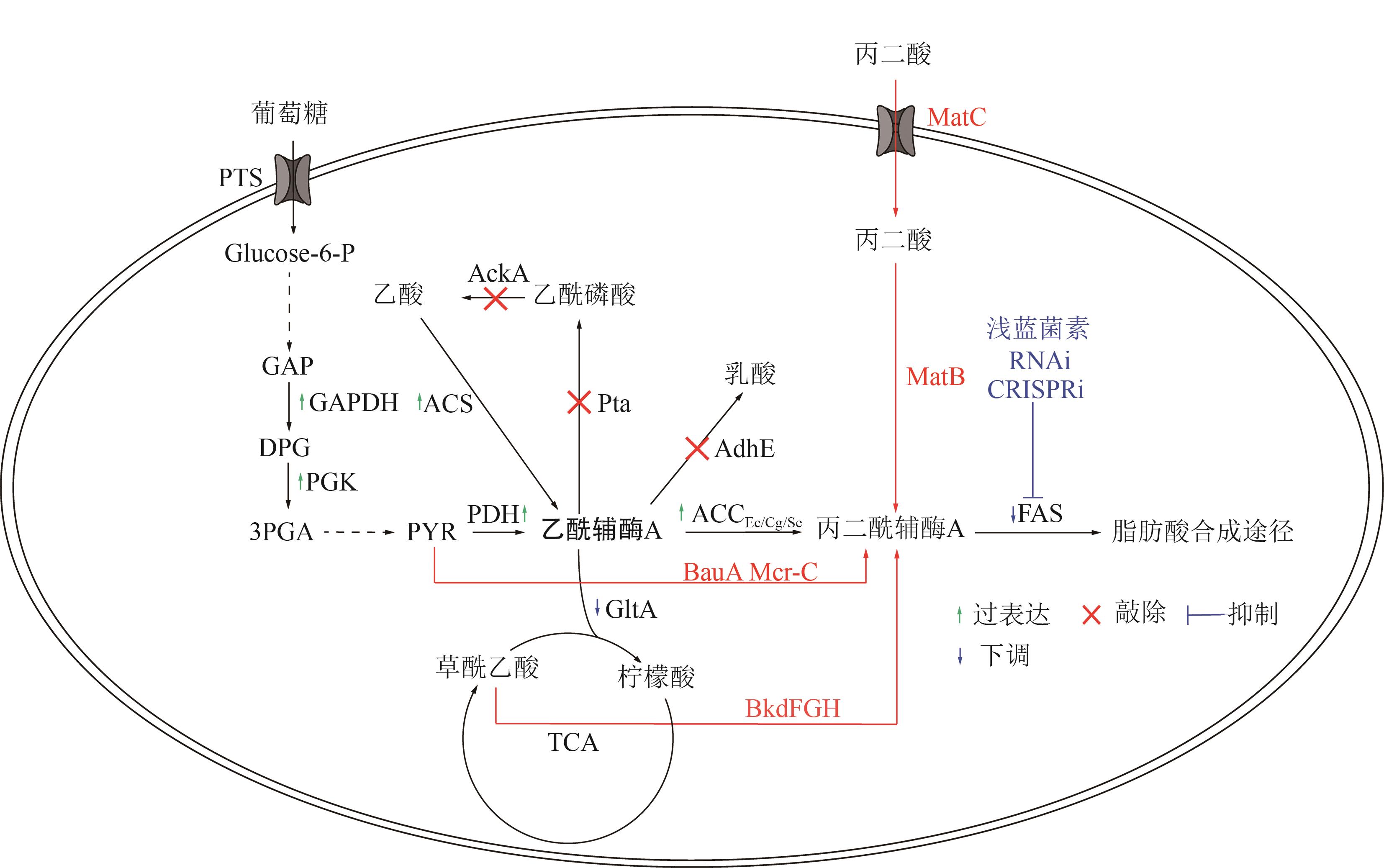
Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (5): 2463-2474.DOI: 10.16085/j.issn.1000-6613.2024-1830
• Synthetic biomanufacturing • Previous Articles Next Articles
Microbial synthesis of resveratrol and its derivatives
SHENG Huakang( ), ZHANG Bo, SHEN Xiaolin, SUN Xinxiao, WANG Jia(
), ZHANG Bo, SHEN Xiaolin, SUN Xinxiao, WANG Jia( ), YUAN Qipeng(
), YUAN Qipeng( )
)
- State Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029, China
-
Received:2024-11-10Revised:2025-02-16Online:2025-05-20Published:2025-05-25 -
Contact:WANG Jia, YUAN Qipeng
微生物合成白藜芦醇及其衍生物
盛华康( ), 张博, 申晓林, 孙新晓, 王佳(
), 张博, 申晓林, 孙新晓, 王佳( ), 袁其朋(
), 袁其朋( )
)
- 北京化工大学化工资源有效利用全国重点实验室,北京 100029
-
通讯作者:王佳,袁其朋 -
作者简介:盛华康(1996—),男,博士研究生,研究方向为合成生物学及代谢工程。E-mail:2020400255@buct.edu.cn。 -
基金资助:国家自然科学基金(22378016)
CLC Number:
Cite this article
SHENG Huakang, ZHANG Bo, SHEN Xiaolin, SUN Xinxiao, WANG Jia, YUAN Qipeng. Microbial synthesis of resveratrol and its derivatives[J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2463-2474.
盛华康, 张博, 申晓林, 孙新晓, 王佳, 袁其朋. 微生物合成白藜芦醇及其衍生物[J]. 化工进展, 2025, 44(5): 2463-2474.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-1830
| 产物 | 宿主 | 外源酶 | 宿主改造 | 前体 | 发酵方式 | 转化率 /mol·mol-1 | 生产水平 /mg·L-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| 白藜芦醇 | E. coli | At4CL, VvSTS | 对香豆酸 | 全细胞催化 | 0.683 | 2340 | [ | |
| E. coli | At4CL, VvSTS | △fumC, Pgk↑, GapA↑, AceF↑, AceF↑, LpdA↑ | 对香豆酸 | 全细胞催化 | 0.467 | 1600 | [ | |
| E. coli | TcTAL, Pc4CL, VvSTS, RtMatB, RtMatC | FabF↓, FabI↓, FabB↓, FabD↓, AroGfbr↑, TyrA fbr↑ | 葡萄糖 | 摇瓶发酵 | 0.048 | 304.5 | [ | |
| S. cerevisiae | HaTAL, At4CL, VvSTS | ARO4K229L↑, ARO7G141S↑, ACC1S659A/S1157A ↑ | 葡萄糖/ 乙醇 | 1L发酵罐流加发酵 | 0.011/ 0.004 | 415.65/ 531.41 | [ | |
| S. cerevisiae | AtPAL2, AtC4H, AtATR2, At4CL2, VvVST1, EcAroL, SeACSL641P | CYB5↑, ARO4K229L↑, ARO7G141S↑, △ARO10, ACC1S659A/S1157A ↑ | 葡萄糖 | 1L发酵罐流加发酵 | 0.005 | 812 | [ | |
| S. cerevisiae | RtPAL/TAL, AtC4H, AtCPR1, VvSTS, Pc4CL, EcAroL, | △PHA2, ARO4K229L↑, ARO7G141S↑, ArRO2↑, ACC1S659A/S1157A ↑ | 葡萄糖 | 3L发酵罐流加发酵 | 0.013 | 4100 | [ | |
| Y. lipolytica | FjTAL, At4CL1, VvVST, VvPAL, AtC4H | 甘油 | 5L发酵罐分批发酵 | 0.002 | 430 | [ | ||
| Y. lipolytica | FjTAL, At4CL, VvSTS | ARO4K221L↑, ARO7G139S ↑ | 葡萄糖 | 1L发酵罐流加发酵 | 0.042 | 12400 | [ | |
| Y. lipolytica | FjTAL, Pc4CL1, VvSTS, AtPAL, AtC4H, AtATR2, CaFPK. BsPTA | ARO4K221L↑, ARO7G139S ↑, ARO1↑, CYB5↑, ARO3K225L↑, △DGA1 | 葡萄糖 | 5L发酵罐流加发酵 | 0.018 | 22500 | [ | |
| 白皮杉醇 | E. coli | PaHpaBC | 白藜芦醇 | 全细胞催化 | 0.767 | 5200 | [ | |
| E. coli | EcHpaBC | 白藜芦醇 | 全细胞催化 | 0.748 | 1200 | [ | ||
| E. coli | EcHpaBC G209F | 白藜芦醇 | 全细胞催化 | 0.806 | 2010 | [ | ||
| E. coli | Pc4CL, VvSTS, EcHpaBC, StcMatB, StcMatC | 对香豆酸 | 摇瓶发酵 | 0.508 | 124 | [ | ||
| 虎杖苷 | S. cerevisiae | PcR3GAT, AtPAL, AtC4H, At4CL, VvSTS, AtATR2 | CYB5↑, ARO4K229L↑, ARO7G141S↑ | 葡萄糖 | 5L发酵罐分批发酵 | 0.010 | 545 | [ |
| Y. lipolytica | RgTAL, At4CL, VvSTS, PcR3GAT, EcAroGfbr, EcTyrAfbr, BbPK, BsPTA | △MHY1, △ACE, △DGA1, △BGL2, △EXG1 | 葡萄糖 | 摇瓶发酵 | 0.003 | 6880 | [ | |
| 紫檀芪 | C. glutamicum | Pc4CL, AhSTS, VvOMT | 对香豆酸 | 摇瓶发酵 | 0.032 | 42 | [ | |
| E. coli | RgTALS9N/A11T/E518V, At4CL, VvSTS, VvROMT | △RppH, △YgdT, △MutH, △YgdQ, △YgdR, △TAS, △LplT, △Aas, △OmrB, △PtsP, △GalR | 葡萄糖 | 摇瓶发酵 | 0.004 | 80.04 | [ | |
| 2-C-异戊二烯基白藜芦醇 | E. coli | PfIacE, At4CL, VvSTS, SaHMGS, SaHMGR, SacMK, SacPMK, SacPMD, EcIDI | GltA↓, AcpS↓, AcpT↓ | 对香豆酸 | 摇瓶发酵 | 0.577 | 68.4 | [ |
| 产物 | 宿主 | 外源酶 | 宿主改造 | 前体 | 发酵方式 | 转化率 /mol·mol-1 | 生产水平 /mg·L-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| 白藜芦醇 | E. coli | At4CL, VvSTS | 对香豆酸 | 全细胞催化 | 0.683 | 2340 | [ | |
| E. coli | At4CL, VvSTS | △fumC, Pgk↑, GapA↑, AceF↑, AceF↑, LpdA↑ | 对香豆酸 | 全细胞催化 | 0.467 | 1600 | [ | |
| E. coli | TcTAL, Pc4CL, VvSTS, RtMatB, RtMatC | FabF↓, FabI↓, FabB↓, FabD↓, AroGfbr↑, TyrA fbr↑ | 葡萄糖 | 摇瓶发酵 | 0.048 | 304.5 | [ | |
| S. cerevisiae | HaTAL, At4CL, VvSTS | ARO4K229L↑, ARO7G141S↑, ACC1S659A/S1157A ↑ | 葡萄糖/ 乙醇 | 1L发酵罐流加发酵 | 0.011/ 0.004 | 415.65/ 531.41 | [ | |
| S. cerevisiae | AtPAL2, AtC4H, AtATR2, At4CL2, VvVST1, EcAroL, SeACSL641P | CYB5↑, ARO4K229L↑, ARO7G141S↑, △ARO10, ACC1S659A/S1157A ↑ | 葡萄糖 | 1L发酵罐流加发酵 | 0.005 | 812 | [ | |
| S. cerevisiae | RtPAL/TAL, AtC4H, AtCPR1, VvSTS, Pc4CL, EcAroL, | △PHA2, ARO4K229L↑, ARO7G141S↑, ArRO2↑, ACC1S659A/S1157A ↑ | 葡萄糖 | 3L发酵罐流加发酵 | 0.013 | 4100 | [ | |
| Y. lipolytica | FjTAL, At4CL1, VvVST, VvPAL, AtC4H | 甘油 | 5L发酵罐分批发酵 | 0.002 | 430 | [ | ||
| Y. lipolytica | FjTAL, At4CL, VvSTS | ARO4K221L↑, ARO7G139S ↑ | 葡萄糖 | 1L发酵罐流加发酵 | 0.042 | 12400 | [ | |
| Y. lipolytica | FjTAL, Pc4CL1, VvSTS, AtPAL, AtC4H, AtATR2, CaFPK. BsPTA | ARO4K221L↑, ARO7G139S ↑, ARO1↑, CYB5↑, ARO3K225L↑, △DGA1 | 葡萄糖 | 5L发酵罐流加发酵 | 0.018 | 22500 | [ | |
| 白皮杉醇 | E. coli | PaHpaBC | 白藜芦醇 | 全细胞催化 | 0.767 | 5200 | [ | |
| E. coli | EcHpaBC | 白藜芦醇 | 全细胞催化 | 0.748 | 1200 | [ | ||
| E. coli | EcHpaBC G209F | 白藜芦醇 | 全细胞催化 | 0.806 | 2010 | [ | ||
| E. coli | Pc4CL, VvSTS, EcHpaBC, StcMatB, StcMatC | 对香豆酸 | 摇瓶发酵 | 0.508 | 124 | [ | ||
| 虎杖苷 | S. cerevisiae | PcR3GAT, AtPAL, AtC4H, At4CL, VvSTS, AtATR2 | CYB5↑, ARO4K229L↑, ARO7G141S↑ | 葡萄糖 | 5L发酵罐分批发酵 | 0.010 | 545 | [ |
| Y. lipolytica | RgTAL, At4CL, VvSTS, PcR3GAT, EcAroGfbr, EcTyrAfbr, BbPK, BsPTA | △MHY1, △ACE, △DGA1, △BGL2, △EXG1 | 葡萄糖 | 摇瓶发酵 | 0.003 | 6880 | [ | |
| 紫檀芪 | C. glutamicum | Pc4CL, AhSTS, VvOMT | 对香豆酸 | 摇瓶发酵 | 0.032 | 42 | [ | |
| E. coli | RgTALS9N/A11T/E518V, At4CL, VvSTS, VvROMT | △RppH, △YgdT, △MutH, △YgdQ, △YgdR, △TAS, △LplT, △Aas, △OmrB, △PtsP, △GalR | 葡萄糖 | 摇瓶发酵 | 0.004 | 80.04 | [ | |
| 2-C-异戊二烯基白藜芦醇 | E. coli | PfIacE, At4CL, VvSTS, SaHMGS, SaHMGR, SacMK, SacPMK, SacPMD, EcIDI | GltA↓, AcpS↓, AcpT↓ | 对香豆酸 | 摇瓶发酵 | 0.577 | 68.4 | [ |
| 1 | TIAN Bingren, LIU Jiayue. Resveratrol: A review of plant sources, synthesis, stability, modification and food application[J]. Journal of the Science of Food and Agriculture, 2020, 100(4): 1392-1404. |
| 2 | CHU Mingyu, ALMAGRO Lorena, CHEN Baihong, et al. Recent trends and comprehensive appraisal for the biotechnological production of trans-resveratrol and its derivatives[J]. Phytochemistry Reviews, 2018, 17(3): 491-508. |
| 3 | BENSA Maja, VOVK Irena, GLAVNIK Vesna. Resveratrol food supplement products and the challenges of accurate label information to ensure food safety for consumers[J]. Nutrients, 2023, 15(2): 474. |
| 4 | COSTA Carlos E, Aloia ROMANÍ, DOMINGUES Lucília. Overview of resveratrol properties, applications, and advances in microbial precision fermentation[J]. Critical Reviews in Biotechnology, 2024: 1-17. |
| 5 | 树林一, 赵航, 黄雯莉, 等. 白藜芦醇及衍生物的研究进展[J]. 河北医药, 2019, 41(13): 2043-2048. |
| SHU Linyi, ZHAO Hang, HUANG Wenli, et al. Research progress on resveratrol and resveratrol derivants[J]. Hebei Medical Journal, 2019, 41(13): 2043-2048. | |
| 6 | 朱小桂, 李立, 苏慧珊, 等. 白藜芦醇衍生物的结构与活性综述[J]. 科学咨询(科技·管理), 2021(1): 50-53. |
| ZHU Xiaogui, LI Li, SU Huishan, et al. Review on the structure-activity relationship of resveratrol derivatives[J]. Technology & Management, 2021(1): 50-53. | |
| 7 | LIN Weisheng, LELAND Jane Valorie, Chi-Tang HO, et al. Occurrence, bioavailability, anti-inflammatory, and anticancer effects of pterostilbene[J]. Journal of Agricultural and Food Chemistry, 2020, 68(46): 12788-12799. |
| 8 | KARAMI Ahmad, FAKHRI Sajad, KOOSHKI Leila, et al. Polydatin: Pharmacological mechanisms, therapeutic targets, biological activities, and health benefits[J]. Molecules, 2022, 27(19): 6474. |
| 9 | KISELEV Konstantin V. Perspectives for production and application of resveratrol[J]. Applied Microbiology and Biotechnology, 2011, 90(2): 417-425. |
| 10 | 王崑仑, 赵修华, 祖元刚,等. 白藜芦醇在几种植物中各部位的分布[J]. 植物研究, 2015, 35(4): 638-640. |
| WANG Kunlun, ZHAO Xiuhua, ZU Yuangang, et al. Distribution of resveratrol in different parts of several plants[J]. Bulletin of Botanical Research, 2015, 35(4): 638-640. | |
| 11 | BRAGA A, FERREIRA P, OLIVEIRA J, et al. Heterologous production of resveratrol in bacterial hosts: Current status and perspectives[J]. World Journal of Microbiology and Biotechnology, 2018, 34(8): 122. |
| 12 | FAN Enguo, ZHANG Kai, ZHU Mingzhao, et al. Obtaining resveratrol: From chemical synthesis to biotechnological production[J]. Mini-Reviews in Organic Chemistry, 2010, 7(4): 272-281. |
| 13 | YAN Wenlong, CAO Zhibei, DING Mingzhu, et al. Design and construction of microbial cell factories based on systems biology[J]. Synthetic and Systems Biotechnology, 2023, 8(1): 176-185. |
| 14 | LIU Xiaonan, DING Wentao, JIANG Huifeng. Engineering microbial cell factories for the production of plant natural products: From design principles to industrial-scale production[J]. Microbial Cell Factories, 2017, 16(1): 125. |
| 15 | LIU Mengsu, WANG Chao, REN Xuefeng, et al. Remodelling metabolism for high-level resveratrol production in Yarrowia lipolytica [J]. Bioresource Technology, 2022, 365: 128178. |
| 16 | Chin Giaw LIM, FOWLER Zachary L, HUELLER Thomas, et al. High-yield resveratrol production in engineered Escherichia coli [J]. Applied and Environmental Microbiology, 2011, 77(10): 3451-3460. |
| 17 | BHAN Namita, XU Peng, KHALIDI Omar, et al. Redirecting carbon flux into malonyl-CoA to improve resveratrol titers: Proof of concept for genetic interventions predicted by OptForce computational framework[J]. Chemical Engineering Science, 2013, 103: 109-114. |
| 18 | WU Junjun, ZHOU Peng, ZHANG Xia, et al. Efficient de novo synthesis of resveratrol by metabolically engineered Escherichia coli [J]. Journal of Industrial Microbiology and Biotechnology, 2017, 44(7): 1083-1095. |
| 19 | LI Mingji, KILDEGAARD Kanchana R, CHEN Yun, et al. De novo production of resveratrol from glucose or ethanol by engineered Saccharomyces cerevisiae [J]. Metabolic Engineering, 2015, 32: 1-11. |
| 20 | LI Mingji, SCHNEIDER Konstantin, KRISTENSEN Mette, et al. Engineering yeast for high-level production of stilbenoid antioxidants[J]. Scientific Reports, 2016, 6: 36827. |
| 21 | MENG Lijun, DIAO Mengxue, WANG Qingyan, et al. Efficient biosynthesis of resveratrol via combining phenylalanine and tyrosine pathways in Saccharomyces cerevisiae [J]. Microbial Cell Factories, 2023, 22(1): 46. |
| 22 | HE Qin, Patrycja SZCZEPAŃSKA, YUZBASHEV Tigran, et al. De novo production of resveratrol from glycerol by engineering different metabolic pathways in Yarrowia lipolytica [J]. Metabolic Engineering Communications, 2020, 11: e00146. |
| 23 | Javier SÁEZ-SÁEZ, WANG Guokun, MARELLA Eko Roy, et al. Engineering the oleaginous yeast Yarrowia lipolytica for high-level resveratrol production[J]. Metabolic Engineering, 2020, 62: 51-61. |
| 24 | FURUYA Toshiki, KINO Kuniki. Regioselective synthesis of piceatannol from resveratrol: Catalysis by two-component flavin-dependent monooxygenase HpaBC in whole cells[J]. Tetrahedron Letters, 2014, 55(17): 2853-2855. |
| 25 | LIN Yuheng, YAN Yajun. Biotechnological production of plant-specific hydroxylated phenylpropanoids[J]. Biotechnology and Bioengineering, 2014, 111(9): 1895-1899. |
| 26 | ZHANG Qianchao, ZHANG Zhiwei, CHEN Yong, et al. Engineering of 4-hydroxyphenylacetate-3-hydroxylase from Escherichia coli for efficient biosynthesis of piceatannol[J]. Process Biochemistry, 2023, 135: 33-39. |
| 27 | SHRESTHA Anil, PANDEY Ramesh Prasad, POKHREL Anaya Raj, et al. Modular pathway engineering for resveratrol and piceatannol production in engineered Escherichia coli [J]. Applied Microbiology and Biotechnology, 2018, 102(22): 9691-9706. |
| 28 | LIU Tian, LIU Yuqian, LI Lan, et al. De novo biosynthesis of polydatin in Saccharomyces cerevisiae [J]. Journal of Agricultural and Food Chemistry, 2021, 69(21): 5917-5925. |
| 29 | SHANG Yanzhe, ZHANG Ping, WEI Wenping, et al. Metabolic engineering for the high-yield production of polydatin in Yarrowia lipolytica [J]. Bioresource Technology, 2023, 381: 129129. |
| 30 | KALLSCHEUER Nicolai, VOGT Michael, BOTT Michael, et al. Functional expression of plant-derived O-methyltransferase, flavanone 3-hydroxylase, and flavonol synthase in Corynebacterium glutamicum for production of pterostilbene, kaempferol, and quercetin[J]. Journal of Biotechnology, 2017, 258: 190-196. |
| 31 | YAN Zhibo, LIANG Jinglong, NIU Fuxing, et al. Enhanced production of pterostilbene in Escherichia coli through directed evolution and host strain engineering[J]. Frontiers in Microbiology, 2021, 12: 710405. |
| 32 | WANG Haijiao, ZHOU Ting, LIU Hui, et al. Heterologous biosynthesis of prenylated resveratrol through multiplex metabolic engineering in Escherichia coli [J]. Green Chemistry, 2024, 26(8): 4792-4802. |
| 33 | CUI Di, DENG Aihua, BAI Hua, et al. Molecular basis for feedback inhibition of tyrosine-regulated 3-deoxy-d-arabino-heptulosonate-7-phosphate synthase from Escherichia coli [J]. Journal of Structural Biology, 2019, 206(3): 322-334. |
| 34 | RODRIGUEZ Alberto, MARTÍNEZ Juan A, FLORES Noemí, et al. Engineering Escherichia coli to overproduce aromatic amino acids and derived compounds[J]. Microbial Cell Factories, 2014, 13(1): 126. |
| 35 | PITTARD James, CAMAKARIS Helen, YANG Ji. The TyrR regulon[J]. Molecular Microbiology, 2005, 55(1): 16-26. |
| 36 | CHÁVEZ BÉJAR María I, BALDERAS-HERNANDEZ Victor E, Aída GUTIÉRREZ-ALEJANDRE, et al. Metabolic engineering of Escherichia coli to optimize melanin synthesis from glucose[J]. Microbial Cell Factories, 2013, 12: 108. |
| 37 | YUAN Shuofu, YI Xiunan, JOHNSTON Trevor G, et al. De novo resveratrol production through modular engineering of an Escherichia coli-Saccharomyces cerevisiae co-culture[J]. Microbial Cell Factories, 2020, 19(1): 143. |
| 38 | LIU Xiaozhen, NIU Hao, LI Qiang, et al. Metabolic engineering for the production of l-phenylalanine in Escherichia coli [J]. 3 Biotech, 2019, 9(3): 85. |
| 39 | GOSSET Guillermo. Improvement of Escherichia coli production strains by modification of the phosphoenolpyruvate:Sugar phosphotransferase system[J]. Microbial Cell Factories, 2005, 4(1): 14. |
| 40 | SIEDLER Solvej, BRINGER Stephanie, BLANK Lars M, et al. Engineering yield and rate of reductive biotransformation in Escherichia coli by partial cyclization of the pentose phosphate pathway and PTS-independent glucose transport[J]. Applied Microbiology and Biotechnology, 2012, 93(4): 1459-1467. |
| 41 | LIU Yongfei, XU Yiran, DING Dongqin, et al. Genetic engineering of Escherichia coli to improve L-phenylalanine production[J]. BMC Biotechnology, 2018, 18(1): 5. |
| 42 | CHANDRAN Sunil S, YI Jian, DRATHS K M, et al. Phosphoenolpyruvate availability and the biosynthesis of shikimic acid[J]. Biotechnology Progress, 2003, 19(3): 808-814. |
| 43 | TOYA Yoshihiro, ISHII Nobuyoshi, NAKAHIGASHI Kenji, et al. 13C-metabolic flux analysis for batch culture of Escherichia coli and its Pyk and Pgi gene knockout mutants based on mass isotopomer distribution of intracellular metabolites[J]. Biotechnology Progress, 2010, 26(4): 975-992. |
| 44 | EMMERLING Marcel, DAUNER Michael, PONTI Aaron, et al. Metabolic flux responses to pyruvate kinase knockout in Escherichia coli [J]. Journal of Bacteriology, 2002, 184(1): 152-164. |
| 45 | GONZALEZ Jacqueline E, LONG Christopher P, ANTONIEWICZ Maciek R. Comprehensive analysis of glucose and xylose metabolism in Escherichia coli under aerobic and anaerobic conditions by 13C metabolic flux analysis[J]. Metabolic Engineering, 2017, 39: 9-18. |
| 46 | 孙薇, 丁冬芹, 柏丹阳, 等. 芳香族氨基酸及其衍生物的细胞工厂构建策略[J]. 合成生物学, 2021, 2(6): 982-999. |
| SUN Wei, DING Dongqin, BAI Danyang, et al. Strategies of cell factory construction for the production of aromatic amino acids and their derivatives[J]. Synthetic Biology Journal, 2021, 2(6): 982-999. | |
| 47 | AN Ning, XIE Chong, ZHOU Shubin, et al. Establishing a growth-coupled mechanism for high-yield production of β-arbutin from glycerol in Escherichia coli [J]. Bioresource Technology, 2023, 369: 128491. |
| 48 | YAKANDAWALA N, ROMEO T, FRIESEN A D, et al. Metabolic engineering of Escherichia coli to enhance phenylalanine production[J]. Applied Microbiology and Biotechnology, 2008, 78(2): 283-291. |
| 49 | SANTOS Christine Nicole S, KOFFAS Mattheos, STEPHANOPOULOS Gregory. Optimization of a heterologous pathway for the production of flavonoids from glucose[J]. Metabolic Engineering, 2011, 13(4): 392-400. |
| 50 | ZHOU Shenghu, LIU Peiran, CHEN Jian, et al. Characterization of mutants of a tyrosine ammonia-lyase from Rhodotorula glutinis [J]. Applied Microbiology and Biotechnology, 2016, 100(24): 10443-10452. |
| 51 | KANEKO Masafumi, HWANG Eui Il, OHNISHI Yasuo, et al. Heterologous production of flavanones in Escherichia coli: Potential for combinatorial biosynthesis of flavonoids in bacteria[J]. Journal of Industrial Microbiology and Biotechnology, 2003, 30(8): 456-461. |
| 52 | QIU Chong, WANG Xiaoge, ZUO Jiaojiao, et al. Systems engineering Escherichia coli for efficient production p-coumaric acid from glucose[J]. Biotechnology and Bioengineering, 2024, 121(7): 2147-2162. |
| 53 | LIU Quanli, YU Tao, LI Xiaowei, et al. Rewiring carbon metabolism in yeast for high level production of aromatic chemicals[J]. Nature Communications, 2019, 10(1): 4976. |
| 54 | KYNDT J A, MEYER T E, CUSANOVICH M. A, et al. Characterization of a bacterial tyrosine ammonia lyase, a biosynthetic enzyme for the photoactive yellow protein[J]. FEBS Letters, 2002, 512(1/2/3): 240-244. |
| 55 | GURUPRASAD R, DEEPASHREEH R, MANASA B, et al. Tyrosine ammonia lyase extracted from Clitoria ternatea Linn.—Its important role in metabolism of humans and reaction with different metal ions[J]. International Journal of Pharma and Bio Sciences, 2014, 5(1): B76-B82. |
| 56 | WU Junjun, ZHOU Lin, DUAN Xuguo, et al. Applied evolution: Dual dynamic regulations-based approaches in engineering intracellular malonyl-CoA availability[J]. Metabolic Engineering, 2021, 67: 403-416. |
| 57 | ZHA Wenjuan, RUBIN-PITEL Sheryl B, SHAO Zengyi, et al. Improving cellular malonyl-CoA level in Escherichia coli via metabolic engineering[J]. Metabolic Engineering, 2009, 11(3): 192-198. |
| 58 | ZHANG Qian, YU Shiqin, Yunbin LYU, et al. Systematically engineered fatty acid catabolite pathway for the production of (2S)-naringenin in Saccharomyces cerevisiae [J]. ACS Synthetic Biology, 2021, 10(5): 1166-1175. |
| 59 | WU Junjun, ZHANG Xia, XIA Xiudong, et al. A systematic optimization of medium chain fatty acid biosynthesis via the reverse beta-oxidation cycle in Escherichia coli [J]. Metabolic Engineering, 2017, 41: 115-124. |
| 60 | SCHWANEMANN Tobias, OTTO Maike, WYNANDS Benedikt, et al. A Pseudomonas taiwanensis malonyl-CoA platform strain for polyketide synthesis[J]. Metabolic Engineering, 2023, 77: 219-230. |
| 61 | ZHOU Shenghu, YUAN Shuofu, NAIR Priya H, et al. Development of a growth coupled and multi-layered dynamic regulation network balancing malonyl-CoA node to enhance (2S)-naringenin biosynthesis in Escherichia coli [J]. Metabolic Engineering, 2021, 67: 41-52. |
| 62 | PRICE Allen C, CHOI Keum-Hwa, HEATH Richard J, et al. Inhibition of β-ketoacyl-acyl carrier protein synthases by thiolactomycin and cerulenin STRUCTURE AND MECHANISM[J]. Journal of Biological Chemistry, 2001, 276(9): 6551-6559. |
| 63 | YANG Yaping, LIN Yuheng, LI Lingyun, et al. Regulating malonyl-CoA metabolism via synthetic antisense RNAs for enhanced biosynthesis of natural products[J]. Metabolic Engineering, 2015, 29: 217-226. |
| 64 | WU Junjun, DU Guocheng, ZHOU Jingwen, et al. Metabolic engineering of Escherichia coli for (2S)-pinocembrin production from glucose by a modular metabolic strategy[J]. Metabolic Engineering, 2013, 16: 48-55. |
| 65 | WU Xia, LIU Jingyi, LIU Dan, et al. Biosynthesis of eriodictyol from tyrosine by Corynebacterium glutamicum [J]. Microbial Cell Factories, 2022, 21(1): 86. |
| 66 | LIU Bo, ZHANG Yuwei, CUI Qianqian, et al. An alternative malonyl-CoA producing pathway in nature[J]. bioRxiv, 2022. |
| 67 | LI Jian, MU Xin, DONG Wenyue, et al. A non-carboxylative route for the efficient synthesis of central metabolite malonyl-CoA and its derived products[J]. Nature Catalysis, 2024, 7: 361-374. |
| 68 | BECKER John V W, ARMSTRONG Gareth O, VAN DER MERWE Marthinus J, et al. Metabolic engineering of Saccharomyces cerevisiae for the synthesis of the wine-related antioxidant resveratrol[J]. FEMS Yeast Research, 2003, 4(1): 79-85. |
| 69 | WU Junjun, LIU Peiran, FAN Yongming, et al. Multivariate modular metabolic engineering of Escherichia coli to produce resveratrol from L-tyrosine[J]. Journal of Biotechnology, 2013, 167(4): 404-411. |
| 70 | ZHANG Yansheng, LI Songzhe, LI Jia, et al. Using unnatural protein fusions to engineer resveratrol biosynthesis in yeast and Mammalian cells[J]. Journal of the American Chemical Society, 2006, 128(40): 13030-13031. |
| 71 | WANG Yechun, YI Hankuil, WANG Melissa, et al. Structural and kinetic analysis of the unnatural fusion protein 4-coumaroyl-CoA ligase::Stilbene synthase[J]. Journal of the American Chemical Society, 2011, 133(51): 20684-20687. |
| 72 | WANG Yechun, YU Oliver. Synthetic scaffolds increased resveratrol biosynthesis in engineered yeast cells[J]. Journal of Biotechnology, 2012, 157(1): 258-260. |
| 73 | CONRADO Robert J, WU Gabriel C, BOOCK Jason T, et al. DNA-guided assembly of biosynthetic pathways promotes improved catalytic efficiency[J]. Nucleic Acids Research, 2012, 40(4): 1879-1889. |
| 74 | AL-JABER Hala I, SHAKYA Ashok K, AL-QUDAH Mahmoud A, et al. Piceatannol, a comprehensive review of health perspectives and pharmacological aspects[J]. Arabian Journal of Chemistry, 2024, 17(9): 105939. |
| 75 | POTTER G A, PATTERSON L H, WANOGHO E,et al. The cancer preventative agent resveratrol is converted to the anticancer agent piceatannol by the cytochrome P450 enzyme CYP1B1[J]. British Journal of Cancer, 2002, 86(5): 774-778. |
| 76 | PIVER Bertrand, Maude FER, VITRAC Xavier, et al. Involvement of cytochrome P450 1A2 in the biotransformation of trans-resveratrol in human liver microsomes[J]. Biochemical Pharmacology, 2004, 68(4): 773-782. |
| 77 | KIM Dong-Hyun, Taeho AHN, JUNG Heung-Chae, et al. Generation of the human metabolite piceatannol from the anticancer-preventive agent resveratrol by bacterial cytochrome P450 BM3[J]. Drug Metabolism and Disposition, 2009, 37(5): 932-936. |
| 78 | LEE Nahum, KIM Eun Jung, KIM Byung-Gee. Regioselective hydroxylation of trans-resveratrol via inhibition of tyrosinase from Streptomyces avermitilis MA4680[J]. ACS Chemical Biology, 2012, 7(10): 1687-1692. |
| 79 | FURUYA Toshiki, Masahiko SAI, KINO Kuniki. Efficient monooxygenase-catalyzed piceatannol production: Application of cyclodextrins for reducing product inhibition[J]. Journal of Bioscience and Bioengineering, 2018, 126(4): 478-481. |
| 80 | PANDEY Ramesh Prasad, PARAJULI Prakash, SHIN Ju Yong, et al. Enzymatic biosynthesis of novel resveratrol glucoside and glycoside derivatives[J]. Applied and Environmental Microbiology, 2014, 80(23): 7235-7243. |
| 81 | CHOI Oksik, LEE Jae Kyoung, KANG Sun-Young, et al. Construction of artificial biosynthetic pathways for resveratrol glucoside derivatives[J]. Journal of Microbiology and Biotechnology, 2014, 24(5): 614-618. |
| 82 | CHAN Eric Wei Chiang. 3'-hydroxypterostilbene and pinostilbene: Their chemistry, sources, anticancer and other pharmacological properties, pharmacokinetics, and patents[J]. Journal of Applied Pharmaceutical Science, 2023, 13(9): 1-8. |
| 83 | WANG Pei, SANG Shengmin. Metabolism and pharmacokinetics of resveratrol and pterostilbene[J]. BioFactors, 2018, 44(1): 16-25. |
| 84 | JEONG Yu Jeong, AN Chul Han, Su Gyeong WOO, et al. Production of pinostilbene compounds by the expression of resveratrol O-methyltransferase genes in Escherichia coli [J]. Enzyme and Microbial Technology, 2014, 54: 8-14. |
| 85 | Kyung Taek HEO, KANG Sun-Young, HONG Young-Soo. De novo biosynthesis of pterostilbene in an Escherichia coli strain using a new resveratrol O-methyltransferase from Arabidopsis[J]. Microbial Cell Factories, 2017, 16(1): 30. |
| 86 | HERRERA Daniela P, CHÁNIQUE Andrea M, Ascensión MARTÍNEZ-MÁRQUEZ, et al. Rational design of resveratrol O-methyltransferase for the production of pinostilbene[J]. International Journal of Molecular Sciences, 2021, 22(9): 4345. |
| 87 | KANG Sun-Young, LEE Jae Kyoung, CHOI Oksik, et al. Biosynthesis of methylated resveratrol analogs through the construction of an artificial biosynthetic pathway in E. coli [J]. BMC Biotechnology, 2014, 14: 67. |
| 88 | RIMANDO Agnes M, PAN Zhiqiang, POLASHOCK James J, et al. In planta production of the highly potent resveratrol analogue pterostilbene via stilbene synthase and O-methyltransferase co-expression[J]. Plant Biotechnology Journal, 2012, 10(3): 269-283. |
| 89 | CHONG Yoojin, LEE Hye Lim, SONG Jihyeon, et al. Biosynthesis of resveratrol derivatives and evaluation of their anti-inflammatory activity[J]. Applied Biological Chemistry, 2021, 64(1): 33. |
| 90 | 李宁, 杨媛媛, 曲彤, 等. 异戊烯基黄酮类化合物药理作用研究进展[J]. 中成药, 2024, 46(4): 1255-1262. |
| LI Ning, YANG Yuanyuan, QU Tong, et al. Research progress on pharmacological effects of isopentenyl flavonoids[J]. Chinese Traditional Patent Medicine, 2024, 46(4): 1255-1262. | |
| 91 | BO Shengtao, CHANG Sui Kiat, ZHOU Ting, et al. Heterologous biosynthesis of prenylated resveratrol and evaluation of antioxidant activity[J]. Food Chemistry, 2022, 378: 132118. |
| 92 | ZHOU Ting, YANG Bao. Novel strategy to produce prenylated resveratrol by prenyltransferase iacE and evaluation of neuroprotective mechanisms[J]. Biochemical and Biophysical Research Communications, 2022, 609: 127-133. |
| 93 | VADALI Ravishankar V, BENNETT George N, Ka-Yiu SAN. Cofactor engineering of intracellular CoA/acetyl-CoA and its effect on metabolic flux redistribution in Escherichia coli [J]. Metabolic Engineering, 2004, 6(2): 133-139. |
| 94 | SHARMA Anshula, GUPTA Gaganjot, AHMAD Tawseef, et al. Enzyme engineering: Current trends and future perspectives[J]. Food Reviews International, 2021, 37(2): 121-154. |
| [1] | SUN Tao, WANG Xin, SUN Meili, WANG Kaifeng, JI Xiaojun. Synthetic biology enables efficient carbon conservation and fixation in yeasts [J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2834-2845. |
| [2] | LI Yuzhen, HE Mingjing, WANG Haoming, MA Xiaoqing, LIU Licheng, LI Fuli. Current status of the third-generation carbon-one biorefinery using CO2 as raw material [J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2811-2824. |
| [3] | FENG Jiao, LIU Mingming, LIU Yao, WANG Xin, CHEN Kequan. Research progress in the biosynthesis of aliphatic short-chain diamines and diols from renewable feedstocks [J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2655-2666. |
| [4] | TANG Yongsheng, GU Ziyun, CHEN Xiulai. Regulation of microbial cell viability for succinic acid production [J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2489-2504. |
| [5] | NI Xin, GAO Jiaoqi, ZHOU Yongjin. Progress on yeast cell factory for lignocellulose biotransformation [J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2475-2488. |
| [6] | YAO Lu, MA Zengxin, ZHANG Cong, YANG Song, XING Xinhui. Recent advances in strengthening Methylobacterium chassis for the utilization of methanol from industrial and plant sources [J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2451-2462. |
| [7] | WANG Yuanyuan, ZHANG Chong, HAN Shuangyan, XING Xinhui. Research progress on bioproduction of recombinant proteins by Pichia pastoris utilizing methanol [J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2441-2450. |
| [8] | WU Mengqin, WANG Jiayao, XU Youqiang, WANG Yu. Progress in cascade conversion of CO2 to single cell protein through chemical and biological catalysis [J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2429-2440. |
| [9] | XU Kai, CUI Jinna, LIU Zhanying. Research progress in the production of cellulosic ethanol via consolidated bioprocessing [J]. Chemical Industry and Engineering Progress, 2024, 43(12): 6873-6882. |
| [10] | TAO Yuxuan, GUO Liang, GAO Cong, SONG Wei, CHEN Xiulai. Progress in metabolic engineering of microorganisms for CO2 fixation [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 40-52. |
| [11] | GUO Feng, ZHANG Shangjie, JIANG Yujia, JIANG Wankui, XIN Fengxue, ZHANG Wenming, JIANG Min. Biotransformation of one-carbon resources by yeast [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 30-39. |
| [12] | TAO Yuxuan, ZHANG Shangjie, JING Yiwen, XIN Fengxue, DONG Weiliang, ZHOU Jie, JIANG Yujia, ZHANG Wenming, JIANG Min. Recent advances in the construction strategy of methylotrophic Escherichia coli [J]. Chemical Industry and Engineering Progress, 2021, 40(7): 3932-3941. |
| [13] | LI Ling, YU Yong, HU Yonghong. Research progress in production of lipstatinfermentation [J]. Chemical Industry and Engineering Progress, 2021, 40(4): 2251-2257. |
| [14] | GUO Liang, GAO Cong, ZHANG Li, CHEN Xiulai, LIU Liming. Advances in the suitability of artificial metabolic pathways [J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1252-1261. |
| [15] | WANG Ying, QU Junze, LIANG Nan, HAO He, YUAN Yingjin. Rapid construction and directed evolution of cell factories for carotenoid biosynthesis [J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1187-1201. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||