Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (5): 2834-2845.DOI: 10.16085/j.issn.1000-6613.2024-1834
• CO2 emission reduction and utilization • Previous Articles
Synthetic biology enables efficient carbon conservation and fixation in yeasts
SUN Tao( ), WANG Xin, SUN Meili, WANG Kaifeng, JI Xiaojun(
), WANG Xin, SUN Meili, WANG Kaifeng, JI Xiaojun( )
)
- State Key Laboratory of Materials-Oriented Chemical Engineering, College of Biotechnology and Pharmaceutical Engineering, Nanjing Tech University, Nanjing 211816, Jiangsu, China
-
Received:2024-11-10Revised:2025-02-04Online:2025-05-20Published:2025-05-25 -
Contact:JI Xiaojun
合成生物学优化酵母代谢过程中的碳保存和碳固定
- 南京工业大学生物与制药工程学院,材料化学工程全国重点实验室,江苏 南京 211816
-
通讯作者:纪晓俊 -
作者简介:孙涛(1996—),男,博士研究生,研究方向为微生物代谢工程及合成生物技术。E-mail:taosun@njtech.edu.cn。 -
基金资助:国家自然科学基金面上项目(22178173)
CLC Number:
Cite this article
SUN Tao, WANG Xin, SUN Meili, WANG Kaifeng, JI Xiaojun. Synthetic biology enables efficient carbon conservation and fixation in yeasts[J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2834-2845.
孙涛, 汪鑫, 孙美莉, 王凯峰, 纪晓俊. 合成生物学优化酵母代谢过程中的碳保存和碳固定[J]. 化工进展, 2025, 44(5): 2834-2845.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-1834
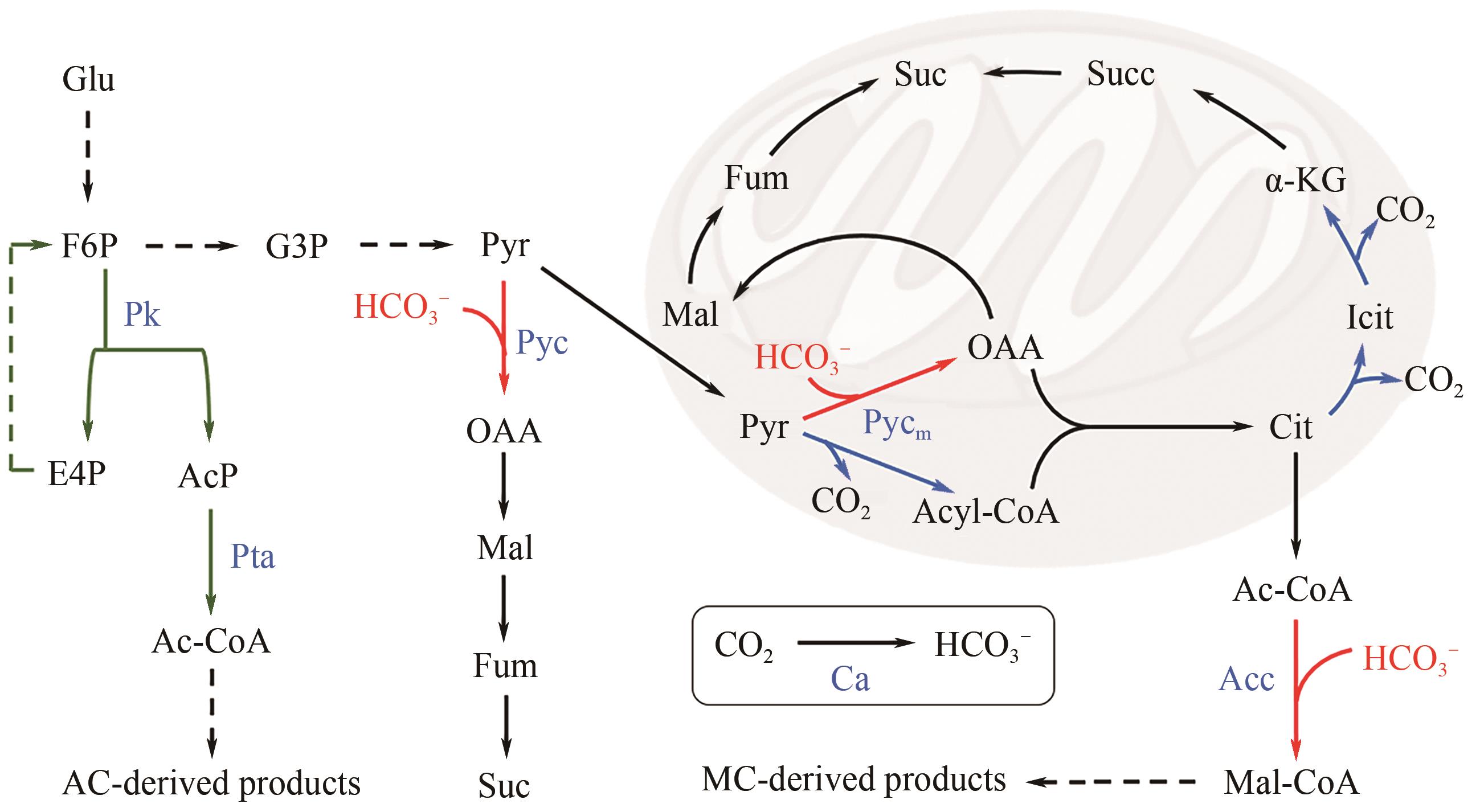
| 菌株 | 碳保存系统 | 改造策略 | 有益效果 | 参考文献 |
|---|---|---|---|---|
| 酿酒酵母 CEN.PK2-1C | NOG途径 | 表达LmPK、CkPTA | β-法尼烯得率提升了25% | [ |
| 酿酒酵母 JS-BE | NOG途径 | 表达LmPK、CkPTA、ZWF1、PPGI1-CTRPGI | β-胡萝卜产量提升了80%,番茄红素产量提升了353% | [ |
| 酿酒酵母IMX581 | GATHCYC途径 | 表达XfsPK*2、BlPTA、SHB17,敲除TKL1,2、TAL、NQM1、PHO13 | 3-羟基丙酸产量提升了109% | [ |
| 酿酒酵母 CEN.PK113-1A | Pyc羧化 | 表达CjFPS1、OpGDH、ScDAK1、ScMDH3、RoFUM、TbFRDg、ScPYC2,敲除GUT1 | 丁二酸产量达到35g/L,得率达到0.6g/g,相当于理论得率的47.1%。 | [ |
| 酿酒酵母 PYC2oe | Pyc羧化 | 敲除MPC3、SDH1 | 丁二酸产量达到45.5g/L,得率达到0.66g/g | [ |
| 酿酒酵母 CEN.PK113-11C | Acc羧化 | 表达ACC1S659A,S1157A | 脂肪酸、脂肪酸乙酯和3-羟基丙酸产量分别提高了0.65倍、3倍和3.5倍 | [ |
| 酿酒酵母 CEN.PK113-11C | Acc羧化 | 表达ACC1S659A,S1157A、NCE103、SUL1,游离质粒筛选标记URA3替换为HIS3 | 3-羟基丙酸产量达到11.25g/L,得率达到0.5625g/g、相当于理论得率的89.3% | [ |
| 酿酒酵母 CEN.PK2-1D | 3-HP分支途径 | 表达CaMCR、CaMCH、MsACR、StHPCD、SePREP、RsMCL、ACH1 | 中康酸产量达到90.78mg/L | [ |
| 解脂耶氏酵母AD | NOG途径 | 表达LmPK、CkPTA | 油脂含量提升了16.4% | [ |
| 解脂耶氏酵母YB-392 | NOG途径 | 表达CaPK*3、TsPTA,敲除PFK | 油脂含量提升了16% | [ |
| 解脂耶氏酵母FY14 | NOG途径 | 表达AnPK*2、BsPTA*2,敲除PFK | β-法尼烯产量提升了87.1% | [ |
| 解脂耶氏酵母PGC91 | Pyc羧化 | 表达TbFRD*2、EcFUM、YlMDH1、YlPYC、YlMDH2、YlFUM | 丁二酸产量达到111.9g/L,得率达到0.79g/g,生产强度达到1.79g/(L·h) | [ |
| 解脂耶氏酵母Po1g | Acc羧化 | 表达ACC1 | 油脂含量增加了2倍 | [ |
| 解脂耶氏酵母Po1g | Acc羧化 | 表达CgBC、HmCA | 脂肪酸含量提高了1.5倍,达到10.7g/L | [ |
| 毕赤酵母CY902 | Pyc羧化 | 表达AsPYC、AsMDH3、SpVHT1、EcPPC、EcSTHA,敲除MAE1 | 苹果酸产量达到199.4g/L,得率达到0.94g/g,生产强度达到1.86g/(L·h) | [ |
| 菌株 | 碳保存系统 | 改造策略 | 有益效果 | 参考文献 |
|---|---|---|---|---|
| 酿酒酵母 CEN.PK2-1C | NOG途径 | 表达LmPK、CkPTA | β-法尼烯得率提升了25% | [ |
| 酿酒酵母 JS-BE | NOG途径 | 表达LmPK、CkPTA、ZWF1、PPGI1-CTRPGI | β-胡萝卜产量提升了80%,番茄红素产量提升了353% | [ |
| 酿酒酵母IMX581 | GATHCYC途径 | 表达XfsPK*2、BlPTA、SHB17,敲除TKL1,2、TAL、NQM1、PHO13 | 3-羟基丙酸产量提升了109% | [ |
| 酿酒酵母 CEN.PK113-1A | Pyc羧化 | 表达CjFPS1、OpGDH、ScDAK1、ScMDH3、RoFUM、TbFRDg、ScPYC2,敲除GUT1 | 丁二酸产量达到35g/L,得率达到0.6g/g,相当于理论得率的47.1%。 | [ |
| 酿酒酵母 PYC2oe | Pyc羧化 | 敲除MPC3、SDH1 | 丁二酸产量达到45.5g/L,得率达到0.66g/g | [ |
| 酿酒酵母 CEN.PK113-11C | Acc羧化 | 表达ACC1S659A,S1157A | 脂肪酸、脂肪酸乙酯和3-羟基丙酸产量分别提高了0.65倍、3倍和3.5倍 | [ |
| 酿酒酵母 CEN.PK113-11C | Acc羧化 | 表达ACC1S659A,S1157A、NCE103、SUL1,游离质粒筛选标记URA3替换为HIS3 | 3-羟基丙酸产量达到11.25g/L,得率达到0.5625g/g、相当于理论得率的89.3% | [ |
| 酿酒酵母 CEN.PK2-1D | 3-HP分支途径 | 表达CaMCR、CaMCH、MsACR、StHPCD、SePREP、RsMCL、ACH1 | 中康酸产量达到90.78mg/L | [ |
| 解脂耶氏酵母AD | NOG途径 | 表达LmPK、CkPTA | 油脂含量提升了16.4% | [ |
| 解脂耶氏酵母YB-392 | NOG途径 | 表达CaPK*3、TsPTA,敲除PFK | 油脂含量提升了16% | [ |
| 解脂耶氏酵母FY14 | NOG途径 | 表达AnPK*2、BsPTA*2,敲除PFK | β-法尼烯产量提升了87.1% | [ |
| 解脂耶氏酵母PGC91 | Pyc羧化 | 表达TbFRD*2、EcFUM、YlMDH1、YlPYC、YlMDH2、YlFUM | 丁二酸产量达到111.9g/L,得率达到0.79g/g,生产强度达到1.79g/(L·h) | [ |
| 解脂耶氏酵母Po1g | Acc羧化 | 表达ACC1 | 油脂含量增加了2倍 | [ |
| 解脂耶氏酵母Po1g | Acc羧化 | 表达CgBC、HmCA | 脂肪酸含量提高了1.5倍,达到10.7g/L | [ |
| 毕赤酵母CY902 | Pyc羧化 | 表达AsPYC、AsMDH3、SpVHT1、EcPPC、EcSTHA,敲除MAE1 | 苹果酸产量达到199.4g/L,得率达到0.94g/g,生产强度达到1.86g/(L·h) | [ |
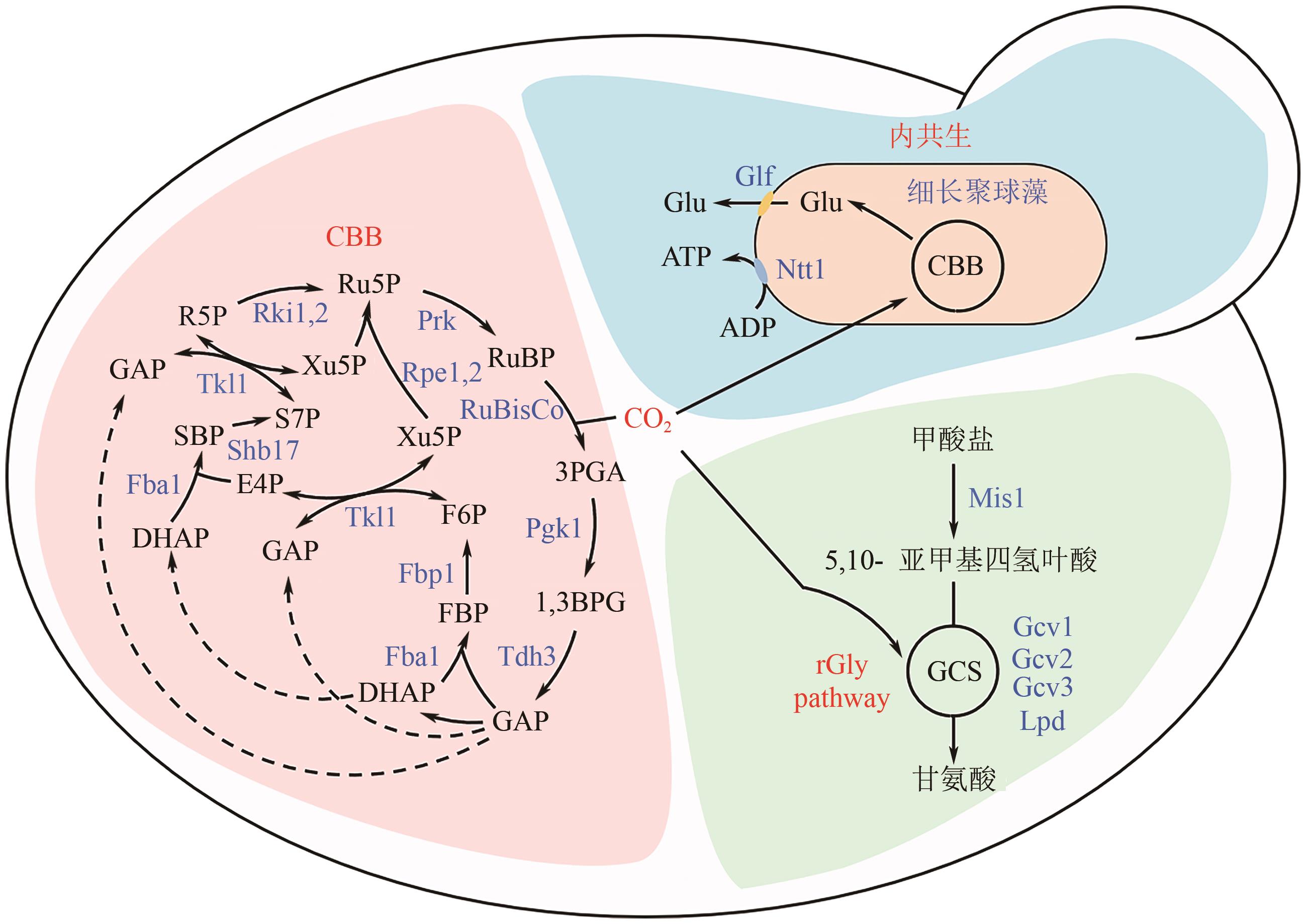
| 菌株 | 碳固定系统 | 改造策略 | 有益效果 | 参考文献 |
|---|---|---|---|---|
| 酿酒酵母 CEN.PK102-3A | RuBisCo 分支途径 | 表达TdCBBM、SoPRK、EcGROEL/S | 乙醇产量提升了11%,甘油产量降低了90% | [ |
| 酿酒酵母IMX581 | RuBisCo 分支途径 | 表达TdCBBM*9、PDAN1-SoPRK、EcGROEL/S、RPE1、TKL1,2、TAL1、NQM1、RKI1,敲除GPD2 | 乙醇得率提升了15%,甘油得率降低了86% | [ |
| 酿酒酵母IMX581 | RuBisCo 分支途径 | 表达TdCBBM*2、PANB1-SoPRK、EcGROEL/S、RPE1、TKL1,2、TAL1、NQM1、RKI1,敲除GPD2 | 乙醛、乙酸和甘油产量分别降低了79%、40%和72% | [ |
| 酿酒酵母 2593 | RuBisCo 分支途径 | 表达TdCBBM*2、PANB1-SoPRK-19aa、EcGROEL/S、RPE1、TKL1,2、TAL1、NQM1、RKI1、ECUT,敲除GPD2、ALD6 | 乙醇得率提升了6%,甘油得率降低了55% | [ |
| 酿酒酵母 SR8 | RuBisCo 分支途径 | 表达RrCBBM*2、SoPRK、EcGROEL/S*2 | 乙醇得率从0.324g/g增加到0.336g/g,回用了7%的CO2 | [ |
| 酿酒酵母 YS58 | RuBisCo 分支途径 | 表达SsXYL1、SsXYL2、XKS1、XYL1R276H、ReCBBL1-CBBS1、ReCFXP1、HSP60-HSP10 | 乙醇得率提升了15%,CO2固定效率达到336.6~436.3mg/L/h | [ |
| 酿酒酵母 DY02 | RuBisCo 分支途径 | 表达ReCBBL1-CBBS1*10、ReCFXP1*2、HSP60-HSP10*2 | 乙醇得率提升了16%,回用了7%的CO2 | [ |
| 酿酒酵母 cox2-60 | 内共生体 系统 | 细长聚球藻SynJEC0:表达SeNTT1、CtINCA、CT_813 | 细长聚球藻/酿酒酵母内共生体可以繁殖15~20代 | [ |
| 酿酒酵母 cox2-60 | 内共生体 系统 | 细长聚球藻SynJEC0:表达SeNTT1、CtINCA、CT_813、ZmGLF、ZmINVA | 细长聚球藻/酿酒酵母内共生体以HCO3-为底物生成柠檬烯 | [ |
| 酿酒酵母 YUW1 | rGly途径 | 表达GCV1,2,3、MIS1 | 工程菌株以甲酸盐和CO2为共底物积累生物量 | [ |
| 酿酒酵母 S288c | rGly途径 | 表达GCV1,2,3、MIS1、EcSHMT,敲除AGX1、GLY1、SER1,适应性进化 | 工程菌株以甲酸盐和CO2为共底物积累生物量 | [ |
| 酿酒酵母BY4741 | MFORG 途径 | 表达PpAOXPTS1、PpFDHPTS1、PpFLDPTS1、PpFGHPTS1、PpMIS1、PpGCV1,2,3、SHM1 | 工程菌株以甲醇和CO2为共底物分别合成了0.07g/L L-乳酸和1.67mg/L 5-氨基乙酰丙酸 | [ |
| 毕赤酵母CBS7435 | CBB循环 | 表达TdCBBM、SoPRK、EcGROEL/S、OpPGK1、OpTDH3、OpTPI1、OpTKL1,敲除DAS1,2、AOX1,适应性进化 | 工程菌株以CO2为底物积累生物量,最大生长速率达到0.018h-1 | [ |
| 毕赤酵母CBS7435 | CBB循环 | 表达TdCBBM、SoPRK、EcGROEL/S、OpPGK1、OpTDH3、OpTPI1、OpTKL1,敲除DAS1,2、AOX1 | 工程菌株以CO2为底物分别合成了2g/L 衣康酸和600mg/L L-乳酸 | [ |
| 毕赤酵母CBS7435 | rGly途径 | 敲除DAS1,2、SHM1 | 工程菌株以甲醇或甲酸盐和CO2为共底物积累生物量 | [ |
| 毕赤酵母 GS115 | MFORG 途径 | 表达GCV1,2,3、MIS1、AOXPTS1、FLDPTS1、FGHPTS1、SHM1,敲除DAS1-2 | 工程菌株以甲醇和CO2为共底物分别合成了0.21g/L L-乳酸和0.71mg/L 5-氨基乙酰丙酸 | [ |
| 菌株 | 碳固定系统 | 改造策略 | 有益效果 | 参考文献 |
|---|---|---|---|---|
| 酿酒酵母 CEN.PK102-3A | RuBisCo 分支途径 | 表达TdCBBM、SoPRK、EcGROEL/S | 乙醇产量提升了11%,甘油产量降低了90% | [ |
| 酿酒酵母IMX581 | RuBisCo 分支途径 | 表达TdCBBM*9、PDAN1-SoPRK、EcGROEL/S、RPE1、TKL1,2、TAL1、NQM1、RKI1,敲除GPD2 | 乙醇得率提升了15%,甘油得率降低了86% | [ |
| 酿酒酵母IMX581 | RuBisCo 分支途径 | 表达TdCBBM*2、PANB1-SoPRK、EcGROEL/S、RPE1、TKL1,2、TAL1、NQM1、RKI1,敲除GPD2 | 乙醛、乙酸和甘油产量分别降低了79%、40%和72% | [ |
| 酿酒酵母 2593 | RuBisCo 分支途径 | 表达TdCBBM*2、PANB1-SoPRK-19aa、EcGROEL/S、RPE1、TKL1,2、TAL1、NQM1、RKI1、ECUT,敲除GPD2、ALD6 | 乙醇得率提升了6%,甘油得率降低了55% | [ |
| 酿酒酵母 SR8 | RuBisCo 分支途径 | 表达RrCBBM*2、SoPRK、EcGROEL/S*2 | 乙醇得率从0.324g/g增加到0.336g/g,回用了7%的CO2 | [ |
| 酿酒酵母 YS58 | RuBisCo 分支途径 | 表达SsXYL1、SsXYL2、XKS1、XYL1R276H、ReCBBL1-CBBS1、ReCFXP1、HSP60-HSP10 | 乙醇得率提升了15%,CO2固定效率达到336.6~436.3mg/L/h | [ |
| 酿酒酵母 DY02 | RuBisCo 分支途径 | 表达ReCBBL1-CBBS1*10、ReCFXP1*2、HSP60-HSP10*2 | 乙醇得率提升了16%,回用了7%的CO2 | [ |
| 酿酒酵母 cox2-60 | 内共生体 系统 | 细长聚球藻SynJEC0:表达SeNTT1、CtINCA、CT_813 | 细长聚球藻/酿酒酵母内共生体可以繁殖15~20代 | [ |
| 酿酒酵母 cox2-60 | 内共生体 系统 | 细长聚球藻SynJEC0:表达SeNTT1、CtINCA、CT_813、ZmGLF、ZmINVA | 细长聚球藻/酿酒酵母内共生体以HCO3-为底物生成柠檬烯 | [ |
| 酿酒酵母 YUW1 | rGly途径 | 表达GCV1,2,3、MIS1 | 工程菌株以甲酸盐和CO2为共底物积累生物量 | [ |
| 酿酒酵母 S288c | rGly途径 | 表达GCV1,2,3、MIS1、EcSHMT,敲除AGX1、GLY1、SER1,适应性进化 | 工程菌株以甲酸盐和CO2为共底物积累生物量 | [ |
| 酿酒酵母BY4741 | MFORG 途径 | 表达PpAOXPTS1、PpFDHPTS1、PpFLDPTS1、PpFGHPTS1、PpMIS1、PpGCV1,2,3、SHM1 | 工程菌株以甲醇和CO2为共底物分别合成了0.07g/L L-乳酸和1.67mg/L 5-氨基乙酰丙酸 | [ |
| 毕赤酵母CBS7435 | CBB循环 | 表达TdCBBM、SoPRK、EcGROEL/S、OpPGK1、OpTDH3、OpTPI1、OpTKL1,敲除DAS1,2、AOX1,适应性进化 | 工程菌株以CO2为底物积累生物量,最大生长速率达到0.018h-1 | [ |
| 毕赤酵母CBS7435 | CBB循环 | 表达TdCBBM、SoPRK、EcGROEL/S、OpPGK1、OpTDH3、OpTPI1、OpTKL1,敲除DAS1,2、AOX1 | 工程菌株以CO2为底物分别合成了2g/L 衣康酸和600mg/L L-乳酸 | [ |
| 毕赤酵母CBS7435 | rGly途径 | 敲除DAS1,2、SHM1 | 工程菌株以甲醇或甲酸盐和CO2为共底物积累生物量 | [ |
| 毕赤酵母 GS115 | MFORG 途径 | 表达GCV1,2,3、MIS1、AOXPTS1、FLDPTS1、FGHPTS1、SHM1,敲除DAS1-2 | 工程菌株以甲醇和CO2为共底物分别合成了0.21g/L L-乳酸和0.71mg/L 5-氨基乙酰丙酸 | [ |
| 1 | WEGAT Vanessa, FABARIUS Jonathan T, SIEBER Volker. Synthetic methylotrophic yeasts for the sustainable fuel and chemical production[J]. Biotechnology for Biofuels and Bioproducts, 2022, 15(1): 113. |
| 2 | TERRER César, PHILLIPS Naiya R, HUNGATE Bruce A, et al. A trade-off between plant and soil carbon storage under elevated CO2 [J]. Nature, 2021, 591(7851): 599-603. |
| 3 | FABARIUS Jonathan Thomas, WEGAT Vanessa, ROTH Arne, et al. Synthetic methylotrophy in yeasts: Towards a circular bioeconomy[J]. Trends in Biotechnology, 2021, 39(4): 348-358. |
| 4 | JEONG Deokyeol, PARK Heeyoung, JANG Byeong-Kwan, et al. Recent advances in the biological valorization of citrus peel waste into fuels and chemicals[J]. Bioresource Technology, 2021, 323: 124603. |
| 5 | GAO Huaxiao, WANG Qian, QI Qingsheng. Synthetic biology optimizes carbon conservation and carbon fixation during microbial carbon metabolism[J]. Chinese Science Bulletin, 2023, 68(19): 2446-2456. |
| 6 | Sze Mun ONN, Gui Jen KOH, Wei Hsum YAP, et al. Recent advances in genetic engineering of microalgae: Bioengineering strategies, regulatory challenges and future perspectives[J]. Journal of Applied Phycology, 2024: 1-18. |
| 7 | KIM Soo Rin, KIM Soo-Jung, KIM Sun-Ki, et al. Yeast metabolic engineering for carbon dioxide fixation and its application[J]. Bioresource Technology, 2022, 346: 126349. |
| 8 | 孙涛, 孙美莉, 陆然, 等. 合成生物学改造酵母驱动丁二酸绿色生物制造[J]. 化工学报, 2024, 75(4): 1382-1393. |
| SUN Tao, SUN Meili, LU Ran, et al. Synthetic biology of yeasts drives green biomanufacturing of succinic acid[J]. CIESC Journal, 2024, 75(4): 1382-1393. | |
| 9 | TRAN Vinh G, MISHRA Somesh, BHAGWAT Sarang S, et al. An end-to-end pipeline for succinic acid production at an industrially relevant scale using Issatchenkia orientalis [J]. Nature Communications, 2023, 14(1): 6152. |
| 10 | MEADOWS Adam L, HAWKINS Kristy M, TSEGAYE Yoseph, et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production[J]. Nature, 2016, 537(7622): 694-697. |
| 11 | XUE Zhixiong, SHARPE Pamela L, HONG Seung-Pyo, et al. Production of omega-3 eicosapentaenoic acid by metabolic engineering of Yarrowia lipolytica [J]. Nature Biotechnology, 2013, 31(8): 734-740. |
| 12 | DU Fei, LI Zijia, LI Xin, et al. Optimizing multicopy chromosomal integration for stable high-performing strains[J]. Nature Chemical Biology, 2024, 20(12): 1670-1679. |
| 13 | CASTRO Evelyn Vásquez, MEMARI Golnaz, Özge ATA, et al. Carbon efficient production of chemicals with yeasts[J]. Yeast, 2023, 40(12): 583-593. |
| 14 | MA Jingbo, GU Yang, MARSAFARI Monireh, et al. Synthetic biology, systems biology, and metabolic engineering of Yarrowia lipolytica toward a sustainable biorefinery platform[J]. Journal of Industrial Microbiology & Biotechnology, 2020, 47(9/10): 845-862. |
| 15 | WANG Kaifeng, SHI Tianqiong, LIN Lu, et al. Engineering Yarrowia lipolytica to produce tailored chain-length fatty acids and their derivatives[J]. ACS Synthetic Biology, 2022, 11(8): 2564-2577. |
| 16 | BOGORAD Igor W, LIN Tzu-Shyang, LIAO James C. Synthetic non-oxidative glycolysis enables complete carbon conservation[J]. Nature, 2013, 502(7473): 693-697. |
| 17 | BAPTISTA Sara L, COSTA Carlos E, CUNHA Joana T, et al. Metabolic engineering of Saccharomyces cerevisiae for the production of top value chemicals from biorefinery carbohydrates[J]. Biotechnology Advances, 2021, 47: 107697. |
| 18 | FAN Jian, ZHANG Yang, LI Wenhao, et al. Multidimensional optimization of Saccharomyces cerevisiae for carotenoid overproduction[J]. Biodesign Research, 2024, 6: 0026. |
| 19 | HELLGREN John, GODINA Alexei, NIELSEN Jens, et al. Promiscuous phosphoketolase and metabolic rewiring enables novel non-oxidative glycolysis in yeast for high-yield production of acetyl-CoA derived products[J]. Metabolic Engineering, 2020, 62: 150-160. |
| 20 | QIAO Kangjian, WASYLENKO Thomas M, ZHOU Kang, et al. Lipid production in Yarrowia lipolytica is maximized by engineering cytosolic redox metabolism[J]. Nature Biotechnology, 2017, 35(2): 173-177. |
| 21 | KAMINENI Annapurna, SHAW Joe. Engineering triacylglycerol production from sugars in oleaginous yeasts[J]. Current Opinion in Biotechnology, 2020, 62: 239-247. |
| 22 | KAMINENI Annapurna, CONSIGLIO Andrew L, MACEWEN Kyle, et al. Increasing lipid yield in Yarrowia lipolytica through phosphoketolase and phosphotransacetylase expression in a phosphofructokinase deletion strain[J]. Biotechnology for Biofuels, 2021, 14(1): 113. |
| 23 | BI Haoran, XU Chenchen, BAO Yufei, et al. Enhancing precursor supply and modulating metabolism to achieve high-level production of β-farnesene in Yarrowia lipolytica [J]. Bioresource Technology, 2023, 382: 129171. |
| 24 | YANG Yaping, LIN Yuheng, LI Lingyun, et al. Regulating malonyl-CoA metabolism via synthetic antisense RNAs for enhanced biosynthesis of natural products[J]. Metabolic Engineering, 2015, 29: 217-226. |
| 25 | YEH L A, SONG C S, KIM K H. Coenzyme A activation of acetyl-CoA carboxylase[J]. Journal of Biological Chemistry, 1981, 256(5): 2289-2296. |
| 26 | LI Jian, MU Xin, DONG Wenyue, et al. A non-carboxylative route for the efficient synthesis of central metabolite malonyl-CoA and its derived products[J]. Nature Catalysis, 2024, 7: 361-374. |
| 27 | PEREIRA Humberto, AZEVEDO Flávio, DOMINGUES Lucília, et al. Expression of Yarrowia lipolytica acetyl-CoA carboxylase in Saccharomyces cerevisiae and its effect on in-vivo accumulation of Malonyl-CoA[J]. Computational and Structural Biotechnology Journal, 2022, 20: 779-787. |
| 28 | VAN ROSSUM Harmen M, KOZAK Barbara U, NIEMEIJER Matthijs S, et al. Alternative reactions at the interface of glycolysis and citric acid cycle in Saccharomyces cerevisiae [J]. FEMS Yeast Research, 2016, 16(3): fow017. |
| 29 | 李娇娇, 祁庆生. 琥珀酸的生物制造: 细菌还是酵母?[J]. 生物产业技术, 2016(1): 49-53. |
| LI Jiaojiao, QI Qingsheng. Bioproduction of succinic acid: Bacteria or yeast?[J]. Biotechnology & Business, 2016(1): 49-53. | |
| 30 | XI Yongyan, XU Hongtao, ZHAN Tao, et al. Metabolic engineering of the acid-tolerant yeast Pichia kudriavzevii for efficient L-malic acid production at low pH[J]. Metabolic Engineering, 2023, 75: 170-180. |
| 31 | MALUBHOY Zahabiya, BAHIA Frederico Mendonça, DE VALK Sophie Claire, et al. Carbon dioxide fixation via production of succinic acid from glycerol in engineered Saccharomyces cerevisiae [J]. Microbial Cell Factories, 2022, 21(1): 102. |
| 32 | Toni RENDULIĆ, PERPELEA Andreea, ORTIZ Juan Paulo Ragas, et al. Mitochondrial membrane transporters as attractive targets for the fermentative production of succinic acid from glycerol in Saccharomyces cerevisiae [J]. FEMS Yeast Research, 2024, 24: foae009. |
| 33 | GUO Qi, YANG Yuxin, LI Dongxun, et al. Advances in multi-enzyme co-localization strategies for the construction of microbial cell factory[J]. Biotechnology Advances, 2024, 77: 108453. |
| 34 | CUI Zhiyong, ZHONG Yutao, SUN Zhijie, et al. Reconfiguration of the reductive TCA cycle enables high-level succinic acid production by Yarrowia lipolytica [J]. Nature Communications, 2023, 14(1): 8480. |
| 35 | WANG Jinpeng, YU Xiao, WANG Kaifeng, et al. Reprogramming the fatty acid metabolism of Yarrowia lipolytica to produce the customized omega-6 polyunsaturated fatty acids[J]. Bioresource Technology, 2023, 383: 129231. |
| 36 | SHI Shuobo, CHEN Yun, SIEWERS Verena, et al. Improving production of malonyl coenzyme A-derived metabolites by abolishing Snf1-dependent regulation of Acc1[J]. mBio, 2014, 5(3): e01130-14. |
| 37 | QIN Ning, LI Lingyun, WAN Xiaozhen, et al. Increased CO2 fixation enables high carbon-yield production of 3-hydroxypropionic acid in yeast[J]. Nature Communications, 2024, 15(1): 1591. |
| 38 | TAI Mitchell, STEPHANOPOULOS Gregory. Engineering the push and pull of lipid biosynthesis in oleaginous yeast Yarrowia lipolytica for biofuel production[J]. Metabolic Engineering, 2013, 15: 1-9. |
| 39 | YOU Seung Kyou, PARK Hyeon Min, LEE Myeong-Eun, et al. Non-photosynthetic CO2 utilization to increase fatty acid production in Yarrowia lipolytica [J]. Journal of Agricultural and Food Chemistry, 2021, 69(40): 11912-11918. |
| 40 | LIU Zihe, WANG Kai, CHEN Yun, et al. Third-generation biorefineries as the means to produce fuels and chemicals from CO2 [J]. Nature Catalysis, 2020, 3: 274-288. |
| 41 | XU Shijie, QIAO Weibo, WANG Zuanwen, et al. Exploiting a heterologous construction of the 3-hydroxypropionic acid carbon fixation pathway with mesaconate as an indicator in Saccharomyces cerevisiae [J]. Bioresources and Bioprocessing, 2023, 10(1): 33. |
| 42 | 陶雨萱, 郭亮, 高聪, 等. 代谢工程改造微生物固定二氧化碳研究进展[J]. 化工进展, 2023, 42(1): 40-52. |
| TAO Yuxuan, GUO Liang, GAO Cong, et al. Progress in metabolic engineering of microorganisms for CO2 fixation[J]. Chemical Industry and Engineering Progress, 2023, 42(1): 40-52. | |
| 43 | XIA Qi, YANG Junzhu, HU Liangwei, et al. Biotransforming CO2 into valuable chemicals[J]. Journal of Cleaner Production, 2024, 434: 140185. |
| 44 | FENG Jia, MA Ding, GAO Siyuan, et al. Recent advances in engineering heterotrophic microorganisms for reinforcing CO2 fixation based on Calvin-Benson-bassham cycle[J]. ACS Sustainable Chemistry & Engineering, 2023, 11(26): 9509-9522. |
| 45 | BLOMBERG Anders, ADLER Lennart. Physiology of osmotolerance in fungi[J]. Advances in Microbial Physiology, 1992, 33: 145-212. |
| 46 | FRANÇOIS Jean Marie, LACHAUX Cléa, MORIN Nicolas. Synthetic biology applied to carbon conservative and carbon dioxide recycling pathways[J]. Frontiers in Bioengineering and Biotechnology, 2020, 7: 446. |
| 47 | Víctor GUADALUPE-MEDINA, WOUTER WISSELINK H, LUTTIK Marijke Ah, et al. Carbon dioxide fixation by Calvin-Cycle enzymes improves ethanol yield in yeast[J]. Biotechnology for Biofuels, 2013, 6(1): 125. |
| 48 | PAPAPETRIDIS Ioannis, GOUDRIAAN Maaike, VITALI María Vázquez, et al. Optimizing anaerobic growth rate and fermentation kinetics in Saccharomyces cerevisiae strains expressing Calvin-cycle enzymes for improved ethanol yield[J]. Biotechnology for Biofuels, 2018, 11: 17. |
| 49 | VAN AALST Aafke C A, JANSEN Mickel L A, MANS Robert, et al. Quantification and mitigation of byproduct formation by low-glycerol-producing Saccharomyces cerevisiae strains containing Calvin-cycle enzymes[J]. Biotechnology for Biofuels and Bioproducts, 2023, 16(1): 81. |
| 50 | MEDINA Víctor Guadalupe, ALMERING Marinka J H, VAN MARIS Antonius J A, et al. Elimination of glycerol production in anaerobic cultures of a Saccharomyces cerevisiae strain engineered to use acetic acid as an electron acceptor[J]. Applied and Environmental Microbiology, 2010, 76(1): 190-195. |
| 51 | VAN AALST Aafke C A, GERAATS Ellen H, JANSEN Mickel L A, et al. Optimizing the balance between heterologous acetate- and CO2-reduction pathways in anaerobic cultures of Saccharomyces cerevisiae strains engineered for low-glycerol production[J]. FEMS Yeast Research, 2023, 23: foad048. |
| 52 | OREB Mislav, DIETZ Heiko, FARWICK Alexander, et al. Novel strategies to improve co-fermentation of pentoses with D-glucose by recombinant yeast strains in lignocellulosic hydrolysates[J]. Bioengineered, 2012, 3(6): 347-351. |
| 53 | KARHUMAA Kaisa, SANCHEZ Rosa Garcia, Bärbel HAHN-HÄGERDAL, et al. Comparison of the xylose reductase-xylitol dehydrogenase and the xylose isomerase pathways for xylose fermentation by recombinant Saccharomyces cerevisiae [J]. Microbial Cell Factories, 2007, 6: 5. |
| 54 | XIA Pengfei, ZHANG Guochang, WALKER Berkley, et al. Recycling carbon dioxide during xylose fermentation by engineered Saccharomyces cerevisiae [J]. ACS Synthetic Biology, 2017, 6(2): 276-283. |
| 55 | LI Yunjie, WANG Miaomiao, CHEN Yawei, et al. Engineered yeast with a CO2-fixation pathway to improve the bio-ethanol production from xylose-mixed sugars[J]. Scientific Reports, 2017, 7: 43875. |
| 56 | PARK Sujeong, PARK Bo-Ram, JEONG Deokyeol, et al. Functional expression of RuBisCO reduces CO2 emission during fermentation by engineered Saccharomyces cerevisiae [J]. Process Biochemistry, 2023, 134: 286-293. |
| 57 | FIELD Christopher B, BEHRENFELD Michael J, RANDERSON James T, et al. Primary production of the biosphere: Integrating terrestrial and oceanic components[J]. Science, 1998, 281(5374): 237-240. |
| 58 | Hannes RUßMAYER, BUCHETICS Markus, GRUBER Clemens, et al. Systems-level organization of yeast methylotrophic lifestyle[J]. BMC Biology, 2015, 13: 80. |
| 59 | GASSLER Thomas, SAUER Michael, GASSER Brigitte, et al. The industrial yeast Pichia pastoris is converted from a heterotroph into an autotroph capable of growth on CO2 [J]. Nature Biotechnology, 2020, 38(2): 210-216. |
| 60 | GASSLER Thomas, BAUMSCHABL Michael, SALLABERGER Jakob, et al. Adaptive laboratory evolution and reverse engineering enhances autotrophic growth in Pichia pastoris [J]. Metabolic Engineering, 2022, 69: 112-121. |
| 61 | BAUMSCHABL Michael, Özge ATA, MITIC Bernd M, et al. Conversion of CO2 into organic acids by engineered autotrophic yeast[J]. Proceedings of the National Academy of Sciences of the United States of America, 2022, 119(47): e2211827119. |
| 62 | GLEIZER Shmuel, Roee BEN-NISSAN, BAR-ON Yinon M, et al. Conversion of Escherichia coli to generate all biomass carbon from CO2 [J]. Cell, 2019, 179(6): 1255-1263. |
| 63 | COURNOYER Jason E, ALTMAN Sarah D, GAO Yangle, et al. Engineering artificial photosynthetic life-forms through endosymbiosis[J]. Nature Communications, 2022, 13(1): 2254. |
| 64 | GAO Yangle, COURNOYER Jason E, DE Bidhan C, et al. Introducing carbon assimilation in yeasts using photosynthetic directed endosymbiosis[J]. Nature Communications, 2024, 15(1): 5947. |
| 65 | Irene SÁNCHEZ-ANDREA, GUEDES Iame Alves, HORNUNG Bastian, et al. The reductive glycine pathway allows autotrophic growth of Desulfovibrio desulfuricans [J]. Nature Communications, 2020, 11(1): 5090. |
| 66 | CLAASSENS Nico J, HE Hai, Arren BAR-EVEN. Synthetic methanol and formate assimilation via modular engineering and selection strategies[J]. Current Issues in Molecular Biology, 2019, 33: 237-248. |
| 67 | DE LA CRUZ Jorge Gonzalez, MACHENS Fabian, MESSERSCHMIDT Katrin, et al. Core catalysis of the reductive glycine pathway demonstrated in yeast[J]. ACS Synthetic Biology, 2019, 8(5): 911-917. |
| 68 | BYSANI Viswanada R, ALAM Ayesha S, Arren BAR-EVEN, et al. Engineering and evolution of the complete Reductive Glycine Pathway in Saccharomyces cerevisiae for formate and CO2 assimilation[J]. Metabolic Engineering, 2024, 81: 167-181. |
| 69 | 郭峰, 张尚杰, 蒋羽佳, 等. 一碳资源在酵母中的利用与转化[J]. 化工进展, 2023, 42(1): 30-39. |
| GUO Feng, ZHANG Shangjie, JIANG Yujia, et al. Biotransformation of one-carbon resources by yeast[J]. Chemical Industry and Engineering Progress, 2023, 42(1): 30-39. | |
| 70 | MITIC Bernd M, TROYER Christina, LUTZ Lisa, et al. The oxygen-tolerant reductive glycine pathway assimilates methanol, formate and CO2 in the yeast Komagataella phaffii [J]. Nature Communications, 2023, 14(1): 7754. |
| 71 | GUO Yuanke, ZHANG Rui, WANG Jing, et al. Engineering yeasts to co-utilize methanol or formate coupled with CO2 fixation[J]. Metabolic Engineering, 2024, 84: 1-12. |
| [1] | LI Yuzhen, HE Mingjing, WANG Haoming, MA Xiaoqing, LIU Licheng, LI Fuli. Current status of the third-generation carbon-one biorefinery using CO2 as raw material [J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2811-2824. |
| [2] | FENG Jiao, LIU Mingming, LIU Yao, WANG Xin, CHEN Kequan. Research progress in the biosynthesis of aliphatic short-chain diamines and diols from renewable feedstocks [J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2655-2666. |
| [3] | TANG Yongsheng, GU Ziyun, CHEN Xiulai. Regulation of microbial cell viability for succinic acid production [J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2489-2504. |
| [4] | SHENG Huakang, ZHANG Bo, SHEN Xiaolin, SUN Xinxiao, WANG Jia, YUAN Qipeng. Microbial synthesis of resveratrol and its derivatives [J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2463-2474. |
| [5] | WANG Yuanyuan, ZHANG Chong, HAN Shuangyan, XING Xinhui. Research progress on bioproduction of recombinant proteins by Pichia pastoris utilizing methanol [J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2441-2450. |
| [6] | WANG Wanying, ZHOU Yingzhe, LIU Huajuan, JIANG Guoqiang. Analysis of competitive advantages and disadvantages in China's biomanufacturing field [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 662-666. |
| [7] | XU Kai, CUI Jinna, LIU Zhanying. Research progress in the production of cellulosic ethanol via consolidated bioprocessing [J]. Chemical Industry and Engineering Progress, 2024, 43(12): 6873-6882. |
| [8] | YAO Lun, ZHOU Yongjin. Progress in microbial utilization of one-carbon feedstocks for biomanufacturing [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 16-29. |
| [9] | ZHAO Tongxin, ZHAO Lei, ZHANG Yanping, LI Yin. Research progress of formic acid biotransformation [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 67-72. |
| [10] | GUO Feng, ZHANG Shangjie, JIANG Yujia, JIANG Wankui, XIN Fengxue, ZHANG Wenming, JIANG Min. Biotransformation of one-carbon resources by yeast [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 30-39. |
| [11] | TAO Yuxuan, ZHANG Shangjie, JING Yiwen, XIN Fengxue, DONG Weiliang, ZHOU Jie, JIANG Yujia, ZHANG Wenming, JIANG Min. Recent advances in the construction strategy of methylotrophic Escherichia coli [J]. Chemical Industry and Engineering Progress, 2021, 40(7): 3932-3941. |
| [12] | GUO Liang, GAO Cong, ZHANG Li, CHEN Xiulai, LIU Liming. Advances in the suitability of artificial metabolic pathways [J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1252-1261. |
| [13] | WANG Ying, QU Junze, LIANG Nan, HAO He, YUAN Yingjin. Rapid construction and directed evolution of cell factories for carotenoid biosynthesis [J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1187-1201. |
| [14] | LIU Weibing, YE Bangce. Progress of synthetic biology research and biological manufacturing of actinomycetes polyketides [J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1226-1237. |
| [15] | SUN Wentao, LI Chun. Design and construction of microbial cell factory for biosynthesis of plant natural products [J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1202-1214. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||