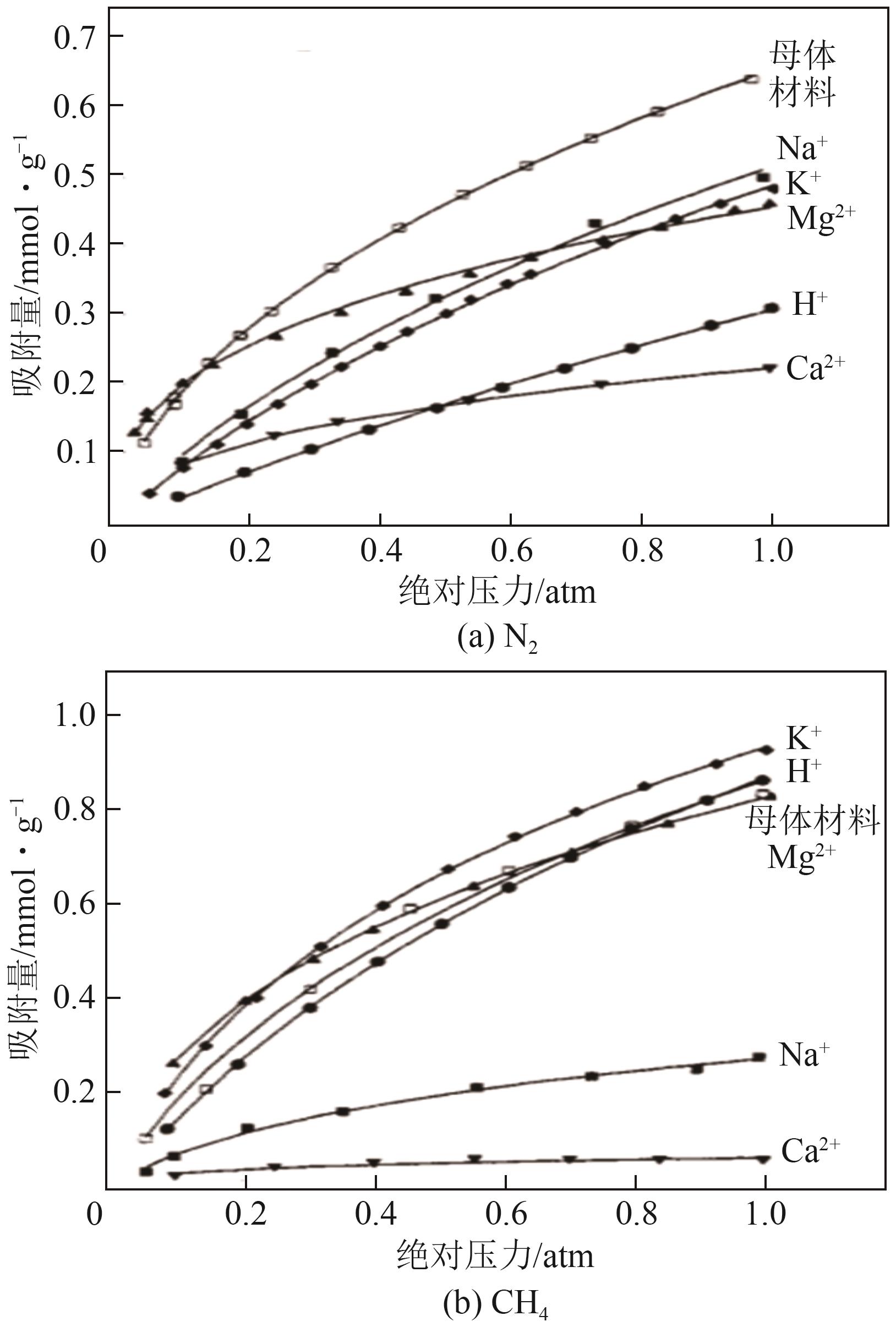
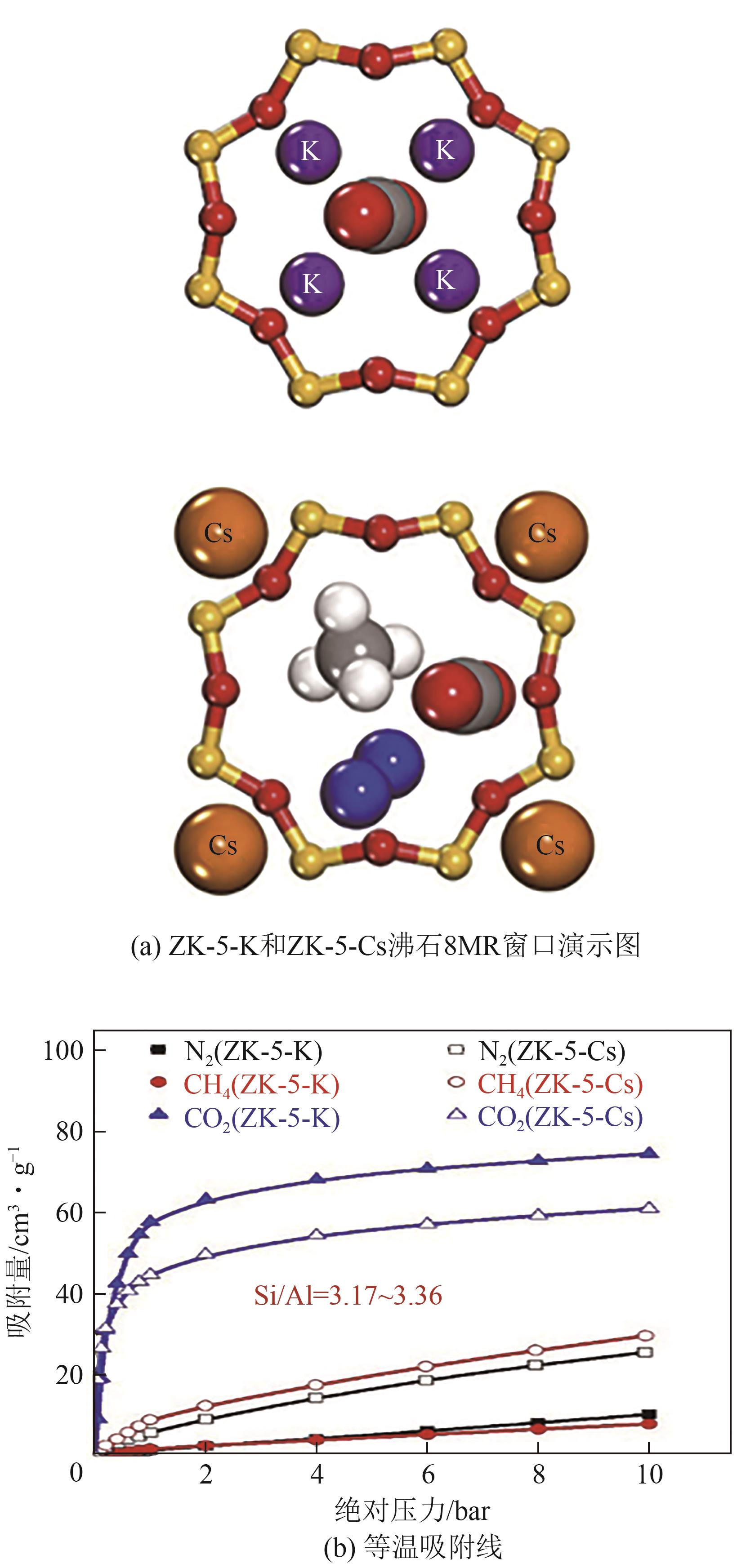
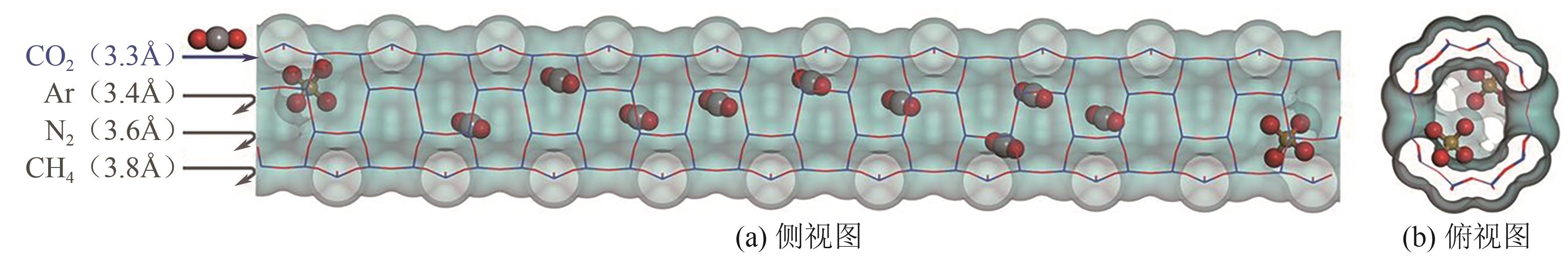
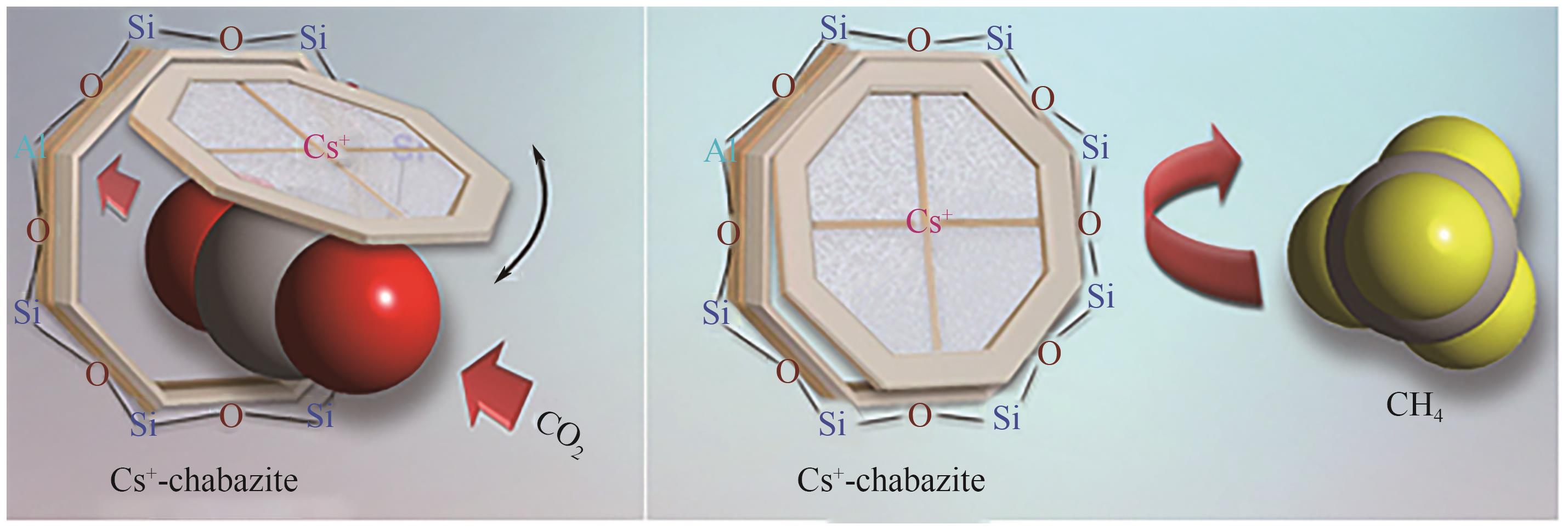
Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (7): 3938-3949.DOI: 10.16085/j.issn.1000-6613.2024-0803
• Materials science and technology • Previous Articles
Research progress on zeolite for CO2-N2-CH4 sieving separation
TANG Xuan1,2( ), BAI Xiaowei1,2, ZHANG Feifei1,2, LI Jinping1,2, YANG Jiangfeng1,2(
), BAI Xiaowei1,2, ZHANG Feifei1,2, LI Jinping1,2, YANG Jiangfeng1,2( )
)
- 1.College of Chemical Engineering and Technology, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
2.Shanxi Key Laboratory of Gas Energy Efficient and Clean Utilization, Taiyuan 030024, Shanxi, China
-
Received:2024-05-13Revised:2024-08-05Online:2025-08-04Published:2025-07-25 -
Contact:YANG Jiangfeng
沸石分子筛用于CO2-N2-CH4筛分分离的研究进展
唐轩1,2( ), 白晓炜1,2, 张飞飞1,2, 李晋平1,2, 杨江峰1,2(
), 白晓炜1,2, 张飞飞1,2, 李晋平1,2, 杨江峰1,2( )
)
- 1.太原理工大学化学工程与技术学院,山西 太原 030024
2.气体能源高效清洁利用山西省重点实验室,山西 太原 030024
-
通讯作者:杨江峰 -
作者简介:唐轩(1993—),男,博士研究生,研究方向为分子筛的合成与气体吸附分离。E-mail:tangxuanchina@163.com。 -
基金资助:国家自然科学基金(U20B6004);国家自然科学基金(22378287)
CLC Number:
Cite this article
TANG Xuan, BAI Xiaowei, ZHANG Feifei, LI Jinping, YANG Jiangfeng. Research progress on zeolite for CO2-N2-CH4 sieving separation[J]. Chemical Industry and Engineering Progress, 2025, 44(7): 3938-3949.
唐轩, 白晓炜, 张飞飞, 李晋平, 杨江峰. 沸石分子筛用于CO2-N2-CH4筛分分离的研究进展[J]. 化工进展, 2025, 44(7): 3938-3949.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-0803
| 气体分子 | 分子量 | 沸点/K | 极化率/cm3 | 偶极矩/esu·cm | 四极矩/esu·cm2 | 动力学直径/Å |
|---|---|---|---|---|---|---|
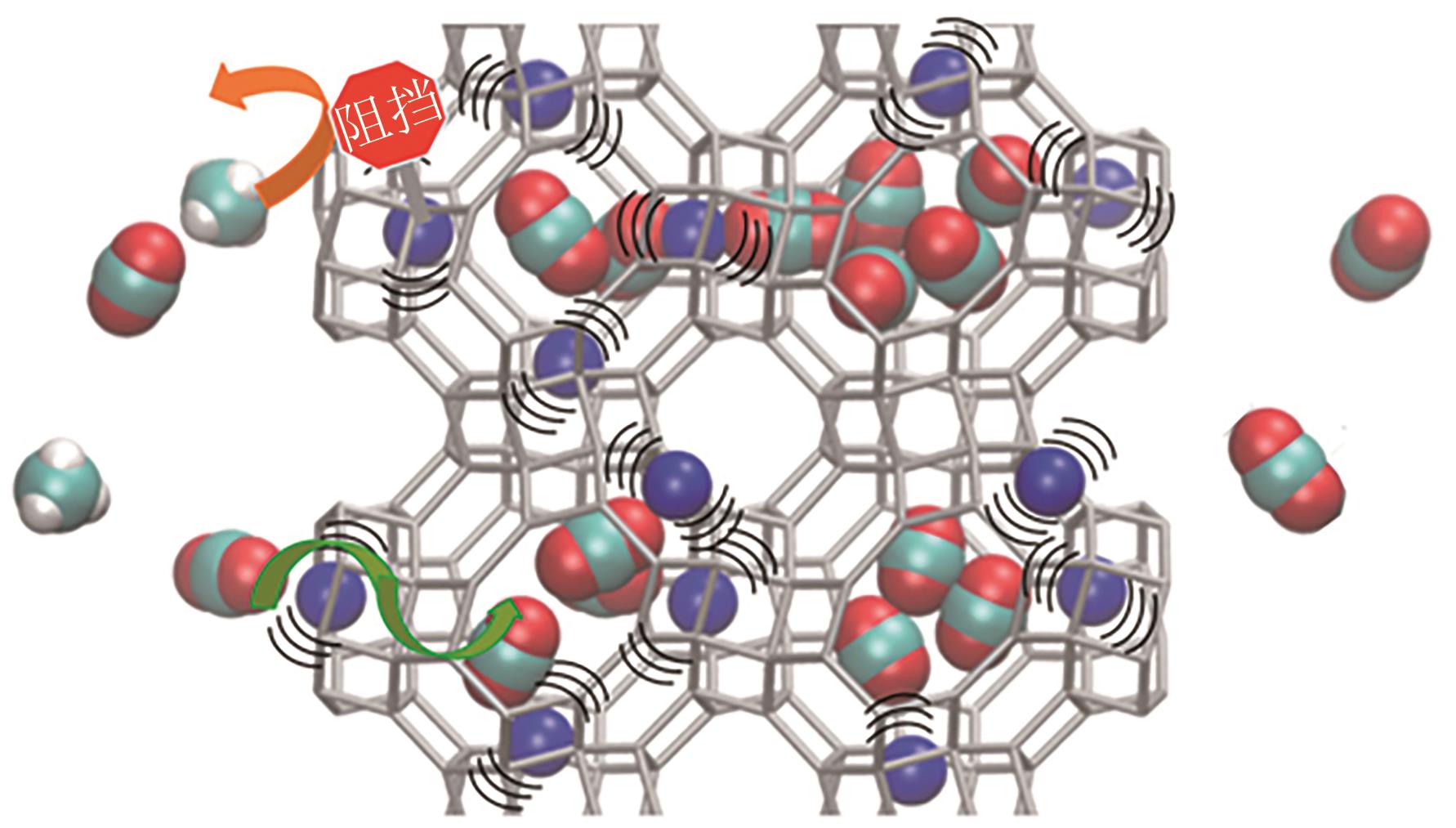
| CO2 | 44 | 216.6 | 29.11×10-25 | 0 | 4.30×10-26 | 3.30 |
| N2 | 28 | 77.3 | 17.40×10-25 | 0 | 1.52×10-26 | 3.64 |
| CH4 | 16 | 111.7 | 25.93×10-25 | 0 | 0 | 3.80 |
| 气体分子 | 分子量 | 沸点/K | 极化率/cm3 | 偶极矩/esu·cm | 四极矩/esu·cm2 | 动力学直径/Å |
|---|---|---|---|---|---|---|
| CO2 | 44 | 216.6 | 29.11×10-25 | 0 | 4.30×10-26 | 3.30 |
| N2 | 28 | 77.3 | 17.40×10-25 | 0 | 1.52×10-26 | 3.64 |
| CH4 | 16 | 111.7 | 25.93×10-25 | 0 | 0 | 3.80 |
| 筛分机制 | 窗口灵活性 | 特点 | 最大窗口环数 | 拓扑结构 | 骨架密度 | 典型材料 |
|---|---|---|---|---|---|---|
| 尺寸筛分机制 | 刚性 | 孔隙阻塞基团的类型、数量和位置 | 8 | KFI | 15.0T/1000Å3(1.49g/cm3) | K-ZK-5 |
| 10 | HEU | 17.5T/1000Å3(1.75g/cm3) | Na+和Ca2+型斜发沸石 | |||
| 12 | MOR | 17.0T/1000Å3(1.69g/cm3) | 铁掺杂的丝光沸石 | |||
| 分子陷阱门机制 | 刚性 | 客体分子和孔隙阻塞基团相互作用的差异 | 8 | CHA | 15.1T/1000Å3(1.50g/cm3) | K-chabazite和Cs-chabazite |
| MWF | 16.1T/1000Å3(1.60g/cm3) | NaTEA-ZSM-25和K-ZSM-25 | ||||
| LTA | 14.2T/1000Å3(1.41g/cm3) | |Na10.2KCs0.8|-LTA和NaK-ZK-4 | ||||
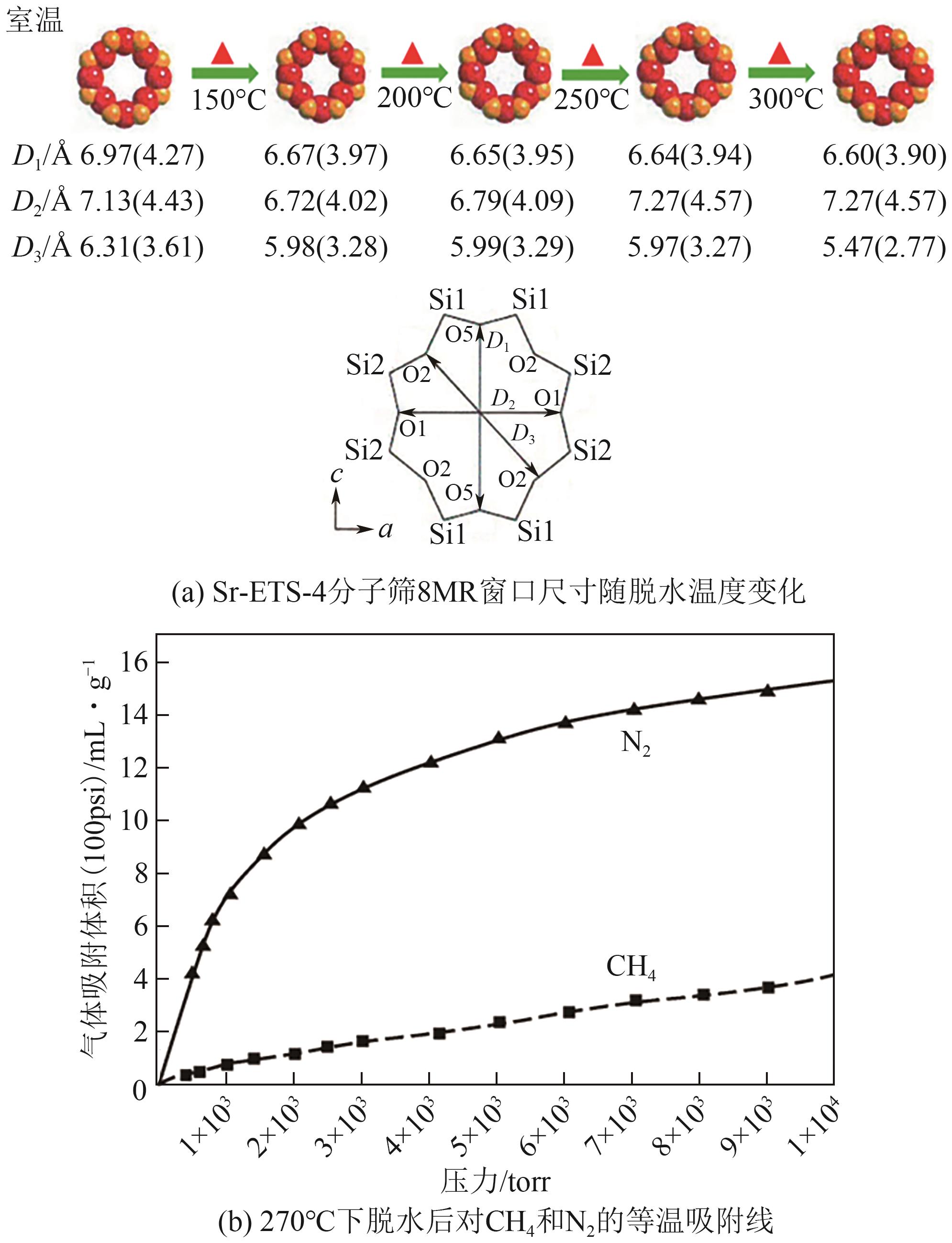
| 骨架呼吸-门控阳离子协同机制 | 柔性 | 柔性窗口协同孔隙阻塞基团迁移 | 8 | — | 2.20g/cm3 | Sr-ETS-4和Ba-ETS-4 |
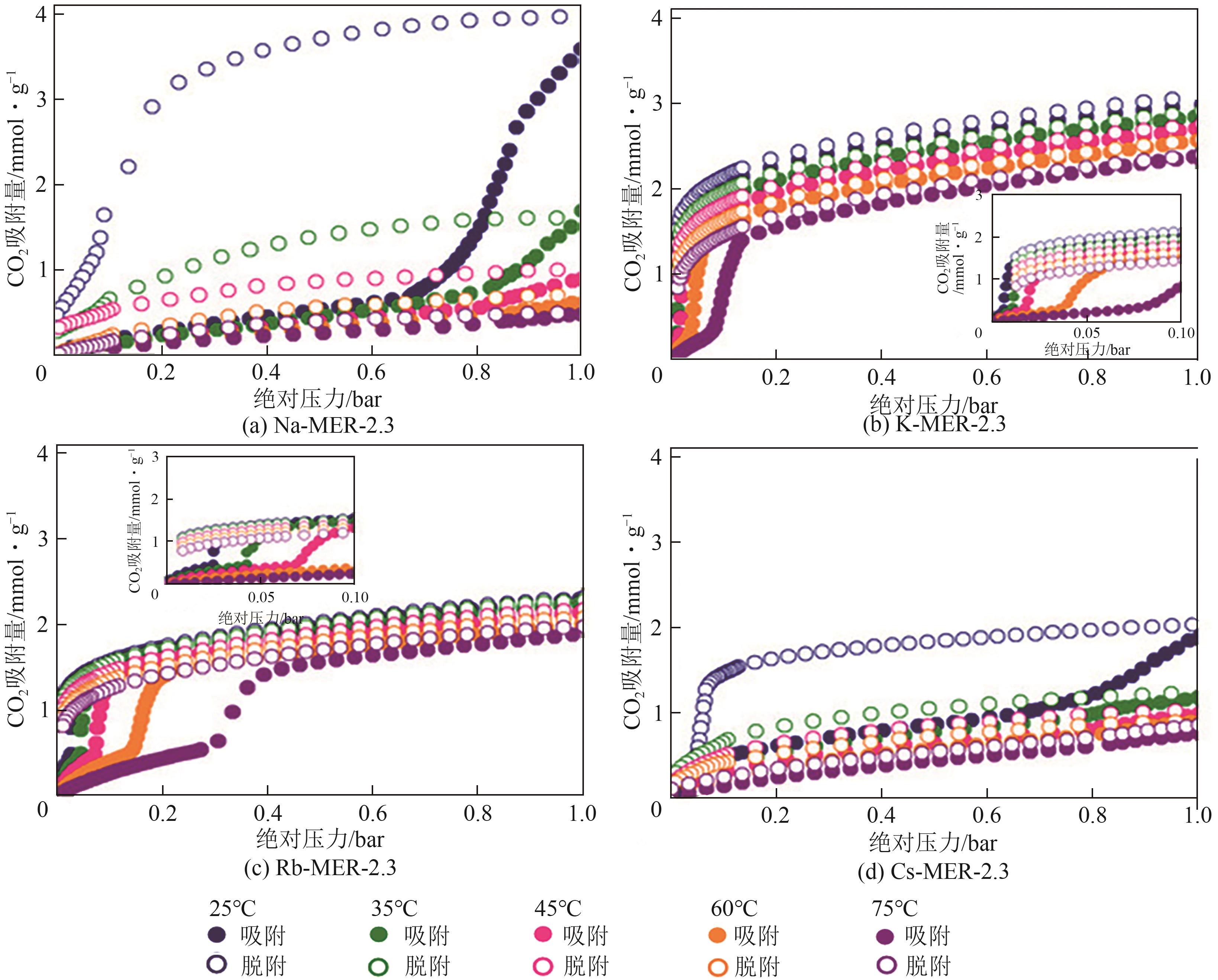
| MER | 16.4T/1000Å3(1.63g/cm3) | Na-MER-2.3、K-MER-2.3和Rb-MER-2.3 | ||||
| GIS | 16.4T/1000Å3(1.64g/cm3) | Na-GIS-2.8和Na-GIS-3.0 | ||||
| PHI | 16.4T/1000Å3(1.63g/cm3) | Cs-PHI-2.5 | ||||
| RHO | 14.5T/1000Å3(1.44g/cm3) | Na-RHO、K-RHO和Cs-RHO |
| 筛分机制 | 窗口灵活性 | 特点 | 最大窗口环数 | 拓扑结构 | 骨架密度 | 典型材料 |
|---|---|---|---|---|---|---|
| 尺寸筛分机制 | 刚性 | 孔隙阻塞基团的类型、数量和位置 | 8 | KFI | 15.0T/1000Å3(1.49g/cm3) | K-ZK-5 |
| 10 | HEU | 17.5T/1000Å3(1.75g/cm3) | Na+和Ca2+型斜发沸石 | |||
| 12 | MOR | 17.0T/1000Å3(1.69g/cm3) | 铁掺杂的丝光沸石 | |||
| 分子陷阱门机制 | 刚性 | 客体分子和孔隙阻塞基团相互作用的差异 | 8 | CHA | 15.1T/1000Å3(1.50g/cm3) | K-chabazite和Cs-chabazite |
| MWF | 16.1T/1000Å3(1.60g/cm3) | NaTEA-ZSM-25和K-ZSM-25 | ||||
| LTA | 14.2T/1000Å3(1.41g/cm3) | |Na10.2KCs0.8|-LTA和NaK-ZK-4 | ||||
| 骨架呼吸-门控阳离子协同机制 | 柔性 | 柔性窗口协同孔隙阻塞基团迁移 | 8 | — | 2.20g/cm3 | Sr-ETS-4和Ba-ETS-4 |
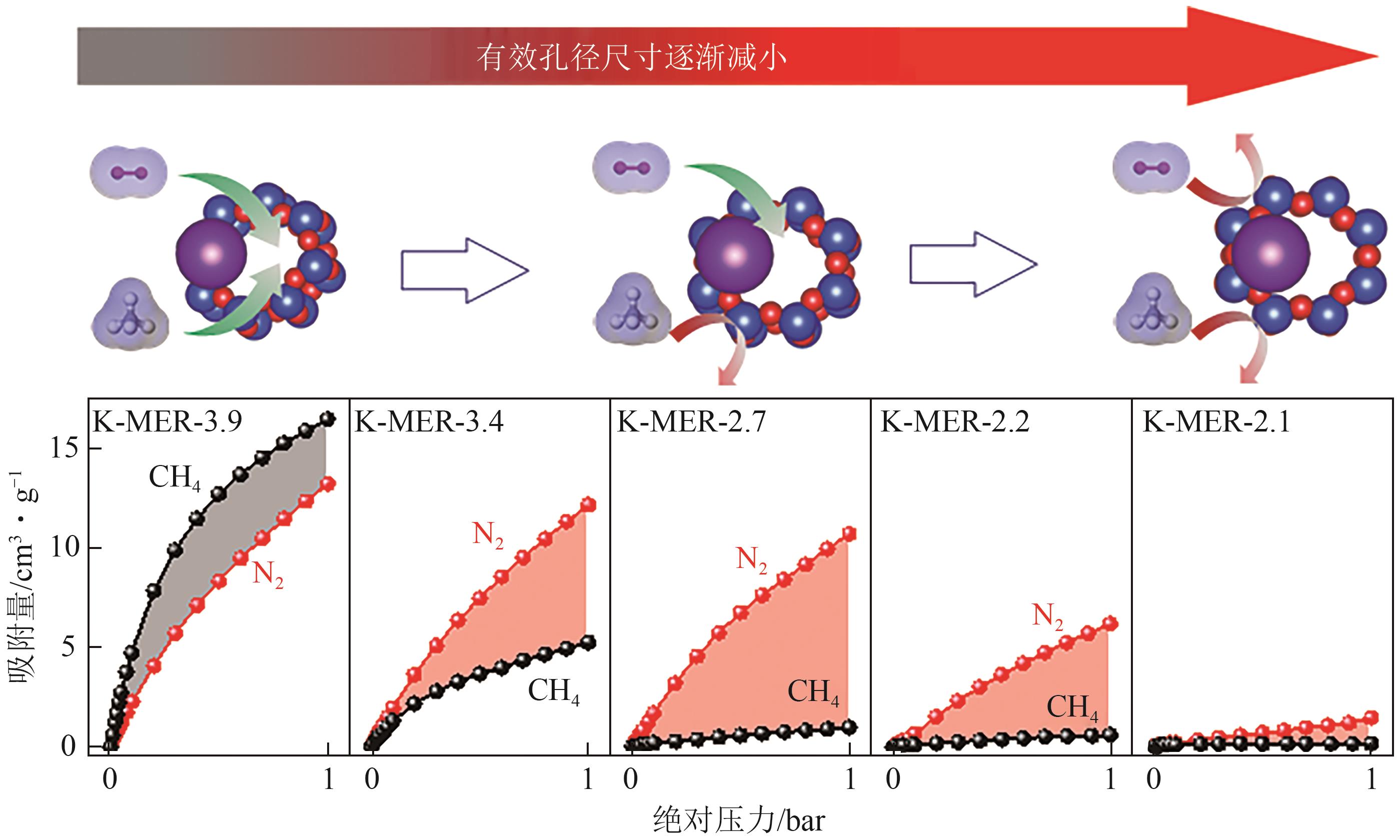
| MER | 16.4T/1000Å3(1.63g/cm3) | Na-MER-2.3、K-MER-2.3和Rb-MER-2.3 | ||||
| GIS | 16.4T/1000Å3(1.64g/cm3) | Na-GIS-2.8和Na-GIS-3.0 | ||||
| PHI | 16.4T/1000Å3(1.63g/cm3) | Cs-PHI-2.5 | ||||
| RHO | 14.5T/1000Å3(1.44g/cm3) | Na-RHO、K-RHO和Cs-RHO |
| [1] | GAO Wanlin, LIANG Shuyu, WANG Rujie, et al. Industrial carbon dioxide capture and utilization: State of the art and future challenges[J]. Chemical Society Reviews, 2020, 49(23): 8584-8686. |
| [2] | WELSBY Dan, PRICE James, Steve PYE, et al. Unextractable fossil fuels in a 1.5℃ world[J]. Nature, 2021, 597(7875): 230-234. |
| [3] | DEAN Joshua F, MIDDELBURG Jack J, Thomas RÖCKMANN, et al. Methane feedbacks to the global climate system in a warmer world[J]. Reviews of Geophysics, 2018, 56(1): 207-250. |
| [4] | SHOLL David S, LIVELY Ryan P. Seven chemical separations to change the world[J]. Nature, 2016, 532(7600): 435-437. |
| [5] | CUI Wengang, HU Tongliang, BU Xianhe. Metal-organic framework materials for the separation and purification of light hydrocarbons[J]. Advanced Materials, 2020, 32(3): 1806445. |
| [6] | WU Yaqi, WECKHUYSEN Bert M. Separation and purification of hydrocarbons with porous materials[J]. Angewandte Chemie International Edition, 2021, 60(35): 18930-18949. |
| [7] | BAI Ruobing, SONG Xiaowei, YAN Wenfu, et al. Low-energy adsorptive separation by zeolites[J]. National Science Review, 2022, 9(9): nwac064. |
| [8] | SIEGELMAN Rebecca L, KIM Eugene J, LONG Jeffrey R. Porous materials for carbon dioxide separations[J]. Nature Materials, 2021, 20(8): 1060-1072. |
| [9] | HUDSON Matthew R, QUEEN Wendy L, MASON Jarad A, et al. Unconventional, highly selective CO2 adsorption in zeolite SSZ-13[J]. Journal of the American Chemical Society, 2012, 134(4): 1970-1973. |
| [10] | VASUDEVAN Suraj, FAROOQ Shamsuzzaman, KARIMI Iftekhar A, et al. Energy penalty estimates for CO2 capture: Comparison between fuel types and capture-combustion modes[J]. Energy, 2016, 103: 709-714. |
| [11] | CHOI Sunho, DRESE Jeffrey H, JONES Christopher W. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources[J]. ChemSusChem, 2009, 2(9): 796-854. |
| [12] | PENG Qilong, CHEN Yu, FANG Diyi, et al. Enhancing size-selective adsorption of CO2/CH4 on ETS-4 via ion-exchange coupled with thermal treatment[J]. Industrial & Engineering Chemistry Research, 2023, 62(23): 9313-9324. |
| [13] | ZHOU Sheng, SHEKHAH Osama, Adrian RAMÍREZ, et al. Asymmetric pore windows in MOF membranes for natural gas valorization[J]. Nature, 2022, 606(7915): 706-712. |
| [14] | SHANG Jin, LI Gang, GU Qinfen, et al. Temperature controlled invertible selectivity for adsorption of N2 and CH4 by molecular trapdoor chabazites[J]. Chemical Communications, 2014, 50(35): 4544-4546. |
| [15] | HU Peng, WANG Hao, XIONG Chao, et al. Probing the node chemistry of a metal-organic framework to achieve ultrahigh hydrophobicity and highly efficient CO2/CH4 separation[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(47): 15897-15907. |
| [16] | CHEN Zhenhe, DENG Shubo, WEI Haoran, et al. Activated carbons and amine-modified materials for carbon dioxide capture—A review[J]. Frontiers of Environmental Science & Engineering, 2013, 7(3): 326-340. |
| [17] | DING Meili, LIU Xi, MA Pan, et al. Porous materials for capture and catalytic conversion of CO2 at low concentration[J]. Coordination Chemistry Reviews, 2022, 465: 214576. |
| [18] | LIU Jiaqi, TANG Xuan, LIANG Xiaowu, et al. Superhydrophobic zeolitic imidazolate framework with suitable SOD cage for effective CH4/N2 adsorptive separation in humid environments[J]. AIChE Journal, 2022, 68(5): e17589. |
| [19] | XU Shuang, LI Wencui, WANG Chengtong, et al. Self-pillared ultramicroporous carbon nanoplates for selective separation of CH4/N2 [J]. Angewandte Chemie International Edition, 2021, 60(12): 6339-6343. |
| [20] | WANG Shaomin, WU Pengchao, FU Jianwei, et al. Heteroatom-doped porous carbon microspheres with ultramicropores for efficient CH4/N2 separation with ultra-high CH4 uptake[J]. Separation and Purification Technology, 2021, 274: 119121. |
| [21] | DU Shengjun, WU Ying, WANG Xingjie, et al. Facile synthesis of ultramicroporous carbon adsorbents with ultra-high CH4 uptake by in situ ionic activation[J]. AIChE Journal, 2020, 66(7): e16231. |
| [22] | WANG Shaomin, SHIVANNA Mohana, YANG Qingyuan. Nickel-based metal-organic frameworks for coal-bed methane purification with record CH4/N2 selectivity[J]. Angewandte Chemie International Edition, 2022, 61(15): e202201017. |
| [23] | NIU Zheng, CUI Xili, PHAM Tony, et al. A metal-organic framework based methane nano-trap for the capture of coal-mine methane[J]. Angewandte Chemie International Edition, 2019, 58(30): 10138-10141. |
| [24] | JARAMILLO David E, REED Douglas A, JIANG Henry Z H, et al. Selective nitrogen adsorption via backbonding in a metal-organic framework with exposed vanadium sites[J]. Nature Materials, 2020, 19(5): 517-521. |
| [25] | YOON Ji Woong, CHANG Hyunju, LEE Seung Joon, et al. Selective nitrogen capture by porous hybrid materials containing accessible transition metal ion sites[J]. Nature Materials, 2017, 16(5): 526-531. |
| [26] | SADEGHI POUYA Ehsan, FARMAHINI Amir H, SADEGHI Paria, et al. Improving separation of CH4 and N2 by adsorption on zeolite Y ion-exchanged with ammonium cations: An experimental and Grand-Canonical Monte Carlo (GCMC) simulation investigation[J]. Chemical Engineering Science, 2024, 289: 119819. |
| [27] | TANG Xuan, WANG Yugao, WEI Mengni, et al. Synthesis of nanosized IM-5 zeolite and its CH4/N2 adsorption and separation[J]. Separation and Purification Technology, 2023, 318: 124003. |
| [28] | YANG Jiangfeng, LIU Jiaqi, LIU Puxu, et al. K-chabazite zeolite nanocrystal aggregates for highly efficient methane separation[J]. Angewandte Chemie International Edition, 2022, 61(8): e202116850. |
| [29] | ZHANG Qiang, YU Jihong, CORMA Avelino. Applications of zeolites to C1 chemistry: Recent advances, challenges, and opportunities[J]. Advanced Materials, 2020, 32(44): 2002927. |
| [30] | ZHOU Yu, ZHANG Jianlin, WANG Lei, et al. Self-assembled iron-containing mordenite monolith for carbon dioxide sieving[J]. Science, 2021, 373(6552): 315-320. |
| [31] | CHAI Yuchao, HAN Xue, LI Weiyao, et al. Control of zeolite pore interior for chemoselective alkyne/olefin separations[J]. Science, 2020, 368(6494): 1002-1006. |
| [32] | LI Yi, YU Jihong. Emerging applications of zeolites in catalysis, separation and host-guest assembly[J]. Nature Reviews Materials, 2021, 6(12): 1156-1174. |
| [33] | FARAMAWY S, ZAKI T, SAKR A A E. Natural gas origin, composition, and processing: A review[J]. Journal of Natural Gas Science and Engineering, 2016, 34: 34-54. |
| [34] | LI Jianrong, KUPPLER Ryan J, ZHOU Hongcai. Selective gas adsorption and separation in metal-organic frameworks[J]. Chemical Society Reviews, 2009, 38(5): 1477-1504. |
| [35] | ACKLEY Mark W, YANG Ralph T. Adsorption characteristics of high-exchange clinoptilolites[J]. Industrial & Engineering Chemistry Research, 1991, 30(12): 2523-2530. |
| [36] | ACKLEY Mark W, YANG Ralph T. Diffusion in ion-exchanged clinoptilolites[J]. AIChE Journal, 1991, 37(11): 1645-1656. |
| [37] | KENNEDY D A, KHANAFER M, TEZEL F H. The effect of Ag+ cations on the micropore properties of clinoptilolite and related adsorption separation of CH4 and N2 gases[J]. Microporous and Mesoporous Materials, 2019, 281: 123-133. |
| [38] | YANG Jiangfeng, SHANG Hua, KRISHNA Rajamani, et al. Adjusting the proportions of extra-framework K+ and Cs+ cations to construct a “molecular gate” on ZK-5 for CO2 removal[J]. Microporous and Mesoporous Materials, 2018, 268: 50-57. |
| [39] | SHANG Jin, LI Gang, SINGH Ranjeet, et al. Discriminative separation of gases by a “molecular trapdoor” mechanism in chabazite zeolites[J]. Journal of the American Chemical Society, 2012, 134(46): 19246-19253. |
| [40] | DE BAERDEMAEKER Trees, DE VOS Dirk. Trapdoors in zeolites[J]. Nature Chemistry, 2013, 5(2): 89-90. |
| [41] | SHANG Jin, LI Gang, SINGH Ranjeet, et al. Determination of composition range for “molecular trapdoor” effect in chabazite zeolite[J]. The Journal of Physical Chemistry C, 2013, 117(24): 12841-12847. |
| [42] | SMITH Luis J, ECKERT Hellmut, CHEETHAM Anthony K. Site preferences in the mixed cation zeolite, Li, Na-chabazite: A combined solid-state NMR and neutron diffraction study[J]. Journal of the American Chemical Society, 2000, 122(8): 1700-1708. |
| [43] | SAXTON Carl G, KRUTH Angela, CASTRO Maria, et al. Xenon adsorption in synthetic chabazite zeolites[J]. Microporous and Mesoporous Materials, 2010, 129(1/2): 68-73. |
| [44] | CALLIGARIS M, NARDIN G. Cation site location in hydrated chabazites. Crystal structure of barium- and cadmium- exchanged chabazites[J]. Zeolites, 1982, 2(3): 200-204. |
| [45] | Javier TORRES F, CIVALLERI Bartolomeo, TERENTYEV Alexander, et al. Theoretical study of molecular hydrogen adsorption in Mg-exchanged chabazite[J]. The Journal of Physical Chemistry C, 2007, 111(5): 1871-1873. |
| [46] | MORTIER W J, PLUTH J J, SMITH J V. Positions of cations and molecules in zeolites with the chabazite framework Ⅰ. Dehydrated Ca-exchanged chabazite[J]. Materials Research Bulletin, 1977, 12(1): 97-102. |
| [47] | CALLIGARIS M, NARDIN G, RANDACCIO L, et al. Cation-site location in a natural chabazite[J]. Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry, 1982, 38(2): 602-605. |
| [48] | GUO Peng, SHIN Jiho, GREENAWAY Alex G, et al. A zeolite family with expanding structural complexity and embedded isoreticular structures[J]. Nature, 2015, 524(7563): 74-78. |
| [49] | ZHAO Jianhua, MOUSAVI Seyed Hesam, XIAO Gongkui, et al. Nitrogen rejection from methane via a “trapdoor” K-ZSM-25 zeolite[J]. Journal of the American Chemical Society, 2021, 143(37): 15195-15204. |
| [50] | ZHAO Jianhua, XIE Ke, SINGH Ranjeet, et al. Li+/ZSM-25 zeolite as a CO2 capture adsorbent with high selectivity and improved adsorption kinetics, showing CO2-induced framework expansion[J]. The Journal of Physical Chemistry C, 2018, 122(33): 18933-18941. |
| [51] | CHEUNG Ocean, WARDECKI Dariusz, BACSIK Zoltán, et al. Highly selective uptake of carbon dioxide on the zeolite |Na10.2KCs0.8|-LTA-a possible sorbent for biogas upgrading[J]. Physical Chemistry Chemical Physics, 2016, 18(24): 16080-16083. |
| [52] | CHEUNG Ocean, BACSIK Zoltán, Nicolas FIL, et al. Selective adsorption of CO2 on zeolites NaK-ZK-4 with Si/Al of 1.8—2.8[J]. ACS Omega, 2020, 5(39): 25371-25380. |
| [53] | KUZNICKI S M, BELL V A, NAIR S, et al. A titanosilicate molecular sieve with adjustable pores for size-selective adsorption of molecules[J]. Nature, 2001, 412(6848): 720-724. |
| [54] | NAIR Sankar, TSAPATSIS Michael, TOBY Brian H, et al. A study of heat-treatment induced framework contraction in strontium-ETS-4 by powder neutron diffraction and vibrational spectroscopy[J]. Journal of the American Chemical Society, 2001, 123(51): 12781-12790. |
| [55] | MARATHE R P, MANTRI K, SRINIVASAN M P, et al. Effect of ion exchange and dehydration temperature on the adsorption and diffusion of gases in ETS-4[J]. Industrial & Engineering Chemistry Research, 2004, 43(17): 5281-5290. |
| [56] | MARATHE R P, FAROOQ S, SRINIVASAN M P. Modeling gas adsorption and transport in small-pore titanium silicates[J]. Langmuir, 2005, 21(10): 4532-4546. |
| [57] | MAJUMDAR B, BHADRA S J, MARATHE R P, et al. Adsorption and diffusion of methane and nitrogen in barium exchanged ETS-4[J]. Industrial & Engineering Chemistry Research, 2011, 50(5): 3021-3034. |
| [58] | KUZNICKI Steven M, BELL Valerie A, PETROVIC Ivan, et al. Small-pored crystalline titanium molecular sieve zeolites and their use in gas separation processes: US6068682[P]. 2000-05-30. |
| [59] | CHOI Hyun June, Donghui JO, MIN Jung Gi, et al. The origin of selective adsorption of CO2 on merlinoite zeolites[J]. Angewandte Chemie International Edition, 2021, 60(8): 4307-4314. |
| [60] | TANG Xuan, WEI Mengni, BAI Xiaowei, et al. Precise pore size modulation of K-MER zeolites for N2 trapping[J]. Separation and Purification Technology, 2024, 339: 126601. |
| [61] | CHOI Hyun June, HONG Suk Bong. Effect of framework Si/Al ratio on the mechanism of CO2 adsorption on the small-pore zeolite gismondine[J]. Chemical Engineering Journal, 2022, 433: 133800. |
| [62] | CHOI Hyun June, MIN Jung Gi, Sang Hyun AHN, et al. Framework flexibility-driven CO2 adsorption on a zeolite[J]. Materials Horizons, 2020, 7(6): 1528-1532. |
| [63] | CHOI Hyun June, BRUCE Elliott L, KENCANA Kevin S, et al. Highly cooperative CO2 adsorption via a cation crowding mechanism on a cesium-exchanged phillipsite zeolite[J]. Angewandte Chemie International Edition, 2023, 62(36): e202305816. |
| [64] | LEE Hwajun, SHIN Jiho, CHOI Wanuk, et al. PST-29: A missing member of the RHO family of embedded isoreticular zeolites[J]. Chemistry of Materials, 2018, 30(19): 6619-6623. |
| [65] | BALESTRA Salvador R G, HAMAD Said, Rabdel RUIZ-SALVADOR A, et al. Understanding nanopore window distortions in the reversible molecular valve zeolite RHO[J]. Chemistry of Materials, 2015, 27(16): 5657-5667. |
| [66] | LOZINSKA Magdalena M, MANGANO Enzo, MOWAT John P S, et al. Understanding carbon dioxide adsorption on univalent cation forms of the flexible zeolite Rho at conditions relevant to carbon capture from flue gases[J]. Journal of the American Chemical Society, 2012, 134(42): 17628-17642. |
| [67] | LOZINSKA Magdalena M, MOWAT John P S, WRIGHT Paul A, et al. Cation gating and relocation during the highly selective “trapdoor” adsorption of CO2 on univalent cation forms of zeolite rho[J]. Chemistry of Materials, 2014, 26(6): 2052-2061. |
| [68] | COUDERT François-Xavier, KOHEN Daniela. Molecular insight into CO2 “trapdoor” adsorption in zeolite Na-RHO[J]. Chemistry of Materials, 2017, 29(7): 2724-2730. |
| [1] | ZHANG Pengfei, ZHAO Guangle, ZHAO Yang, MO Changyi, REN Liang. Distribution and molecular structure of nitrogen compounds in inferior gas oil, and their transformation rules during the hydrotreating process [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 3828-3837. |
| [2] | GAO Jiaojiao, YAN Shiyu, YANG Taishun, XIE Shangzhi, YANG Yanjuan, XU Jing. Effect of alumina support crystal structure of Ru-based catalysts on polyethylene hydrogenolysis performance [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 3917-3927. |
| [3] | LIU Yongbing, WANG Yajun, GU Ping, ZHANG Yongmin, GUO Huaiyong, LIU Kai. A review on multiphase separation researches in slurry bed reactors [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3345-3363. |
| [4] | FU Yu, LI Xiaoyu, WU Yue, TAO Chunhui, DUAN Ran, ZHANG Wenxiang, MA Heping. Removal of trace NF3 impurities from electronic grade CF4 by adsorption [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3570-3578. |
| [5] | GAO Feng, WANG Chongyang, GAO Sheng, ZHANG Yahong, CHEN Tao, NIAN Zheng. Nitrous oxide production pathway and its regulation strategy in Anammox system [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3579-3591. |
| [6] | HAN Pei, LI Jinjian, KE Tian, ZHANG Zhiguo, BAO Zongbi, REN Qilong, YANG Qiwei. Advances in adsorption separation of sulfur hexafluoride/nitrogen by novel porous materials [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3592-3617. |
| [7] | WU Min, LIAO Yalong, JIA Xiaobao, YANG Shuangyu. Research progress on enhancing bacterial leaching methods of chalcopyrite [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3618-3629. |
| [8] | FU Yuanfeng, FAN Zhenzhong, ZANG Xin, TONG Qilei, LIU Jingang. Preparation of superhydrophilic underwater superoleophobic composite SSM and its application in oil-water separation [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3659-3670. |
| [9] | SHI Xiuding, WANG Yongquan, ZENG Jing, SU Chang, HONG Junming. Nanotubular Co-N-C activated percarbonate for tetracycline degradation [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3041-3052. |
| [10] | LI Peiyi, SUN Bolong, LIU Ruiyan, ZHOU Xinyao, LIU Ruilin, HU Yuanyuan, XU Gongtao, LI Xinping. Preparation of sodium alginate/titanium dioxide composite porous material and its application in oil-water separation [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3053-3061. |
| [11] | XIE Wuqiang, ZHANG Ling, HE Gang, JIANG Lifeng, ZHENG Xirui, ZHANG Hepeng. Electrocatalytic CO2 reduction to methane by CoTBrPP-PTAB-Cu catalyst [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3093-3100. |
| [12] | FU Jiang, SUN Jiaoxia, FU Junjie, ZHU Min, SONG Pinxue, ZHOU Yining, FAN Jianxin. Self-cleaning effect and oil-water separation performance of hydrophobic modified polyester fiber fabric [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3121-3131. |
| [13] | LEI Xueyan, ZHU Yichun, ZHANG Chao, HAO Wanting, CHEN Zilong, SONG Xianwei, HUANG Shuchang, DONG Shanyan. Efficient synchronous nitrogen and phosphorus removal in zero valent iron coupled anaerobic ammonia oxidation system [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3132-3143. |
| [14] | YAO Ruwei, SONG Yueyin, NIU Qinqin, LI Congming. Na-S co-modified iron catalysts for CO2 hydrogenation to C2+ alcohols [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3154-3162. |
| [15] | WANG Heng, LU Chunxi. Structural optimization and operational performance analysis of cyclone separators in a 3.6Mt/a catalytic cracking unit [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3238-3246. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||