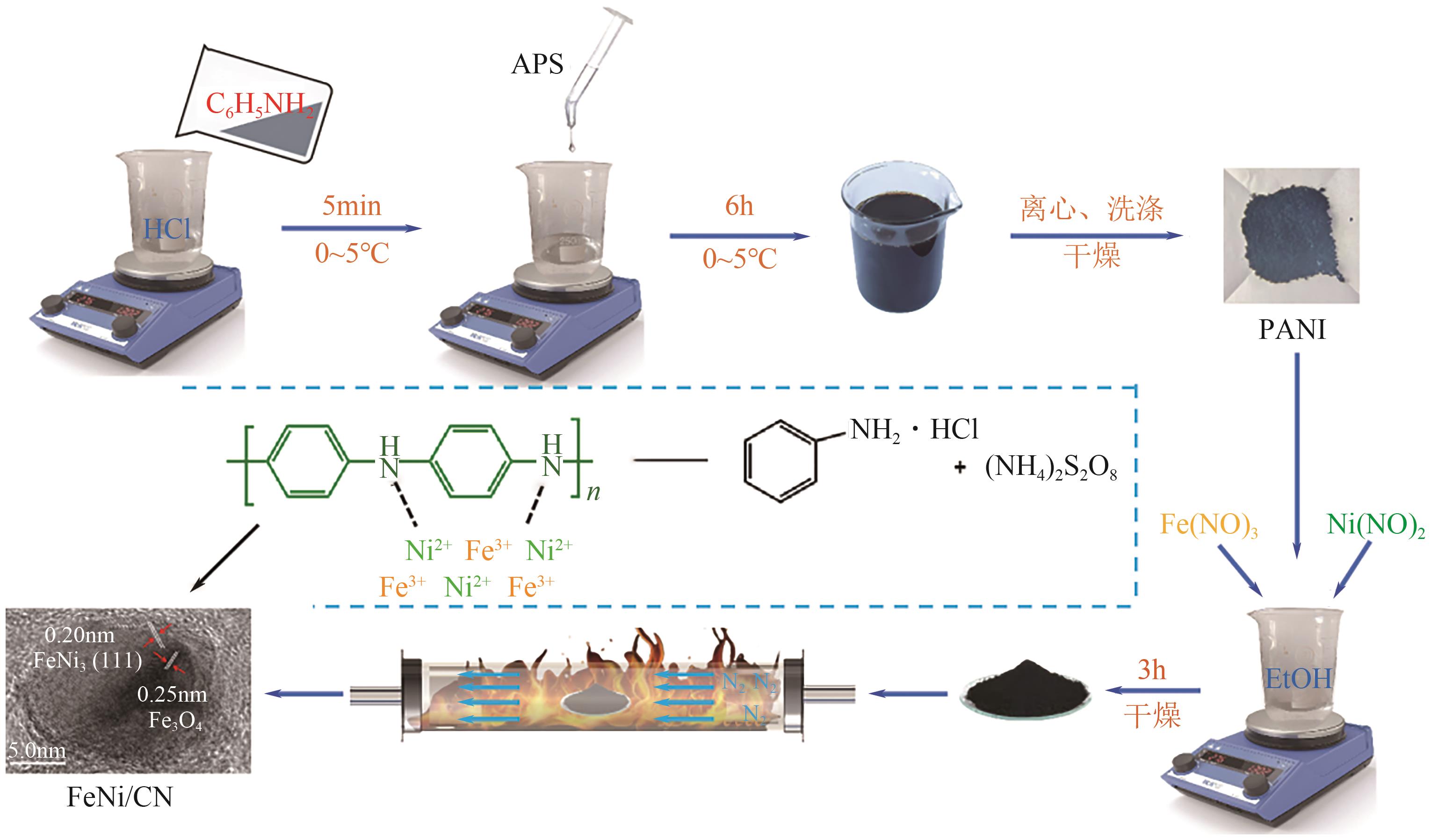
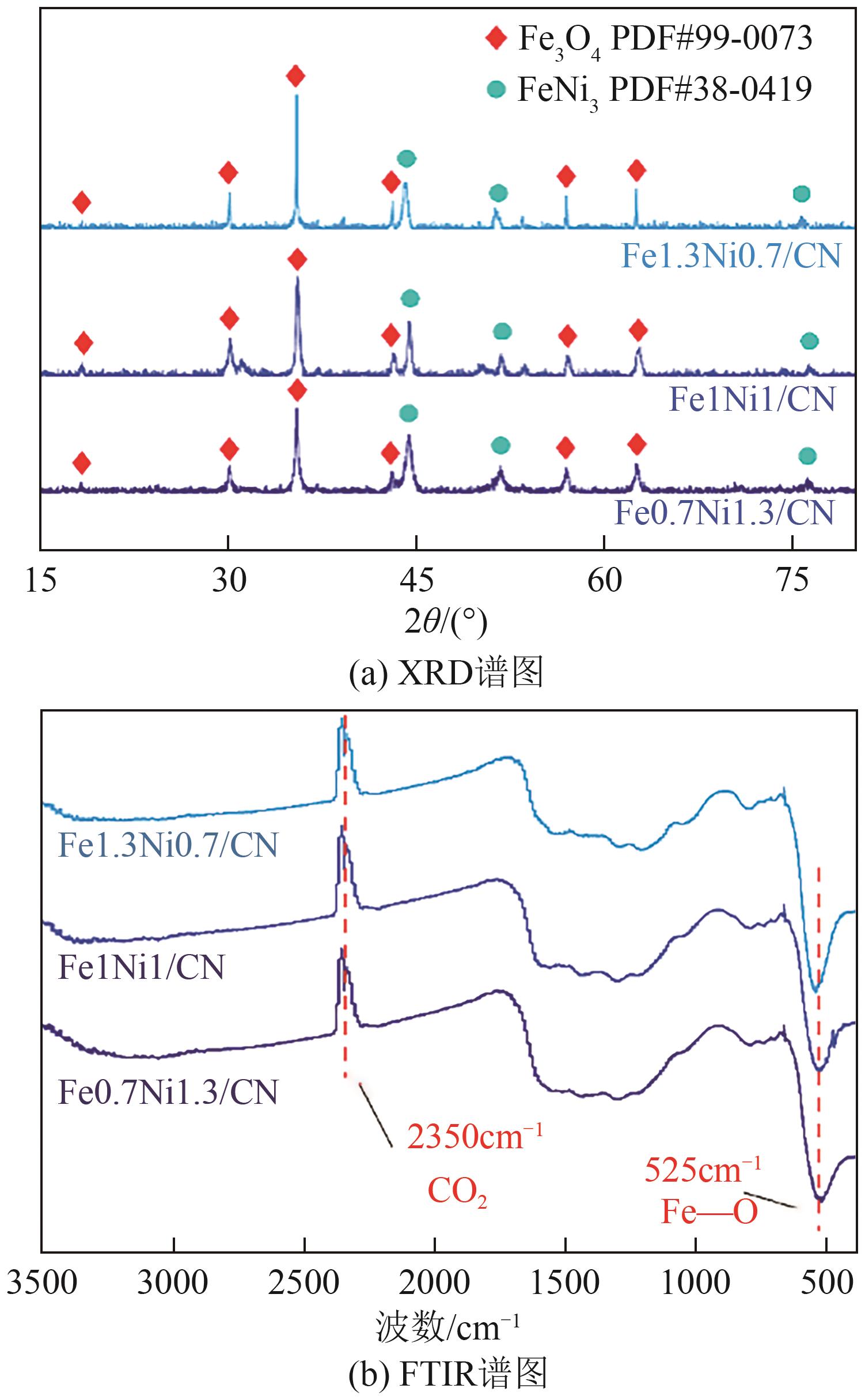
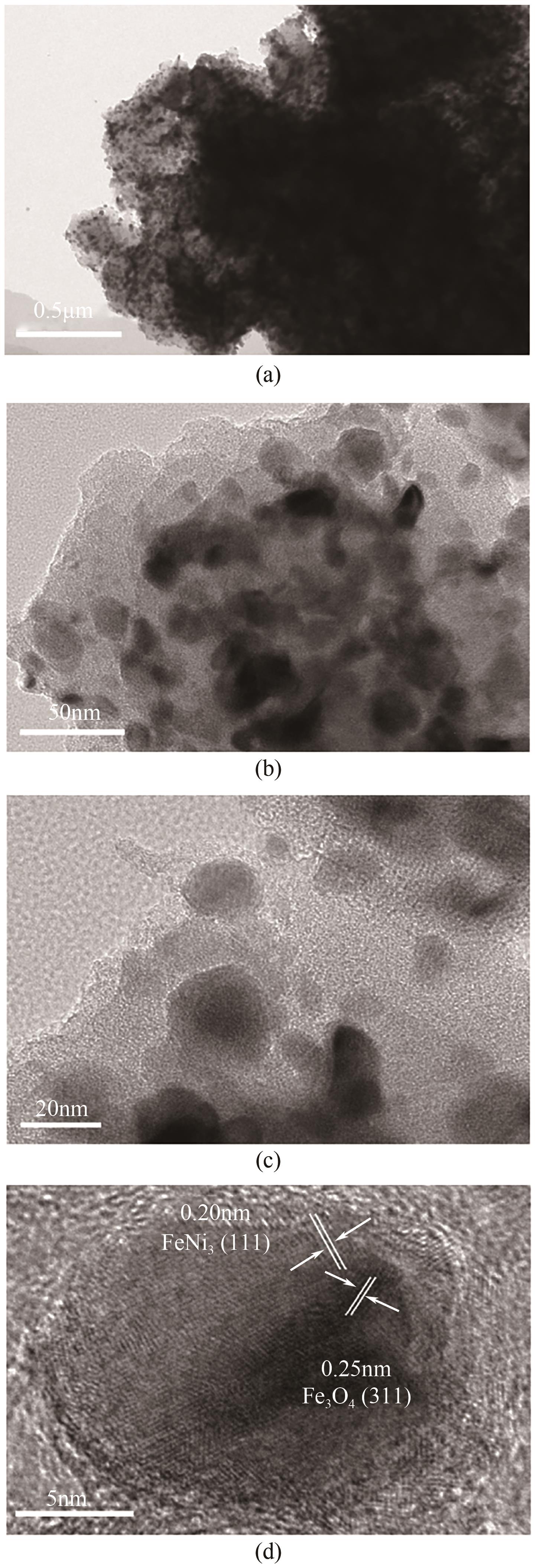
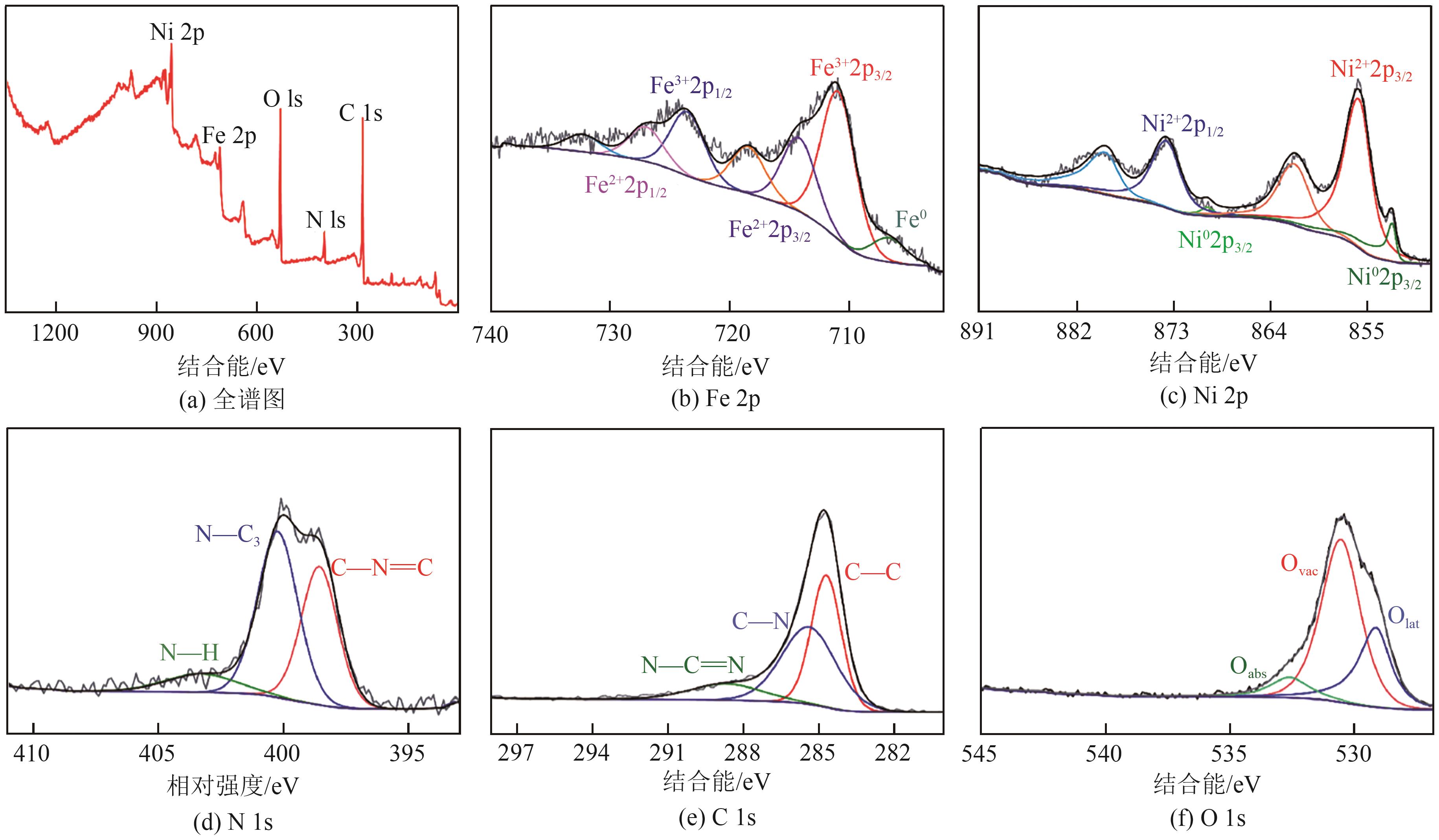
Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (7): 3928-3937.DOI: 10.16085/j.issn.1000-6613.2024-0966
• Industrial catalysis • Previous Articles
Preparation of FeNi3-Fe3O4/CN electrocatalysts and their electrocatalytic oxygen evolution performance
CHEN Dongjian1,2( ), SUN Yuqian1, YIN Fengxiang1,2(
), SUN Yuqian1, YIN Fengxiang1,2( )
)
- 1.School of Petrochemical Engineering, Changzhou University, Changzhou 213164, Jiangsu, China
2.Jiangsu Key Laboratory of Advanced Catalytic Materials and Technology, Changzhou University, Changzhou 213164, Jiangsu, China
-
Received:2024-06-16Revised:2024-09-29Online:2025-08-04Published:2025-07-25 -
Contact:YIN Fengxiang
FeNi3-Fe3O4/CN催化剂的制备及其电催化析氧性能
- 1.常州大学石油化工学院,江苏 常州 213164
2.常州大学江苏省先进催化材料与技术重点实验室,江苏 常州 213164
-
通讯作者:银凤翔 -
作者简介:陈东健(1999—),男,硕士研究生,研究方向为电催化。E-mail:1970485350@qq.com。 -
基金资助:国家自然科学基金(22078027)
CLC Number:
Cite this article
CHEN Dongjian, SUN Yuqian, YIN Fengxiang. Preparation of FeNi3-Fe3O4/CN electrocatalysts and their electrocatalytic oxygen evolution performance[J]. Chemical Industry and Engineering Progress, 2025, 44(7): 3928-3937.
陈东健, 孙雨倩, 银凤翔. FeNi3-Fe3O4/CN催化剂的制备及其电催化析氧性能[J]. 化工进展, 2025, 44(7): 3928-3937.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-0966
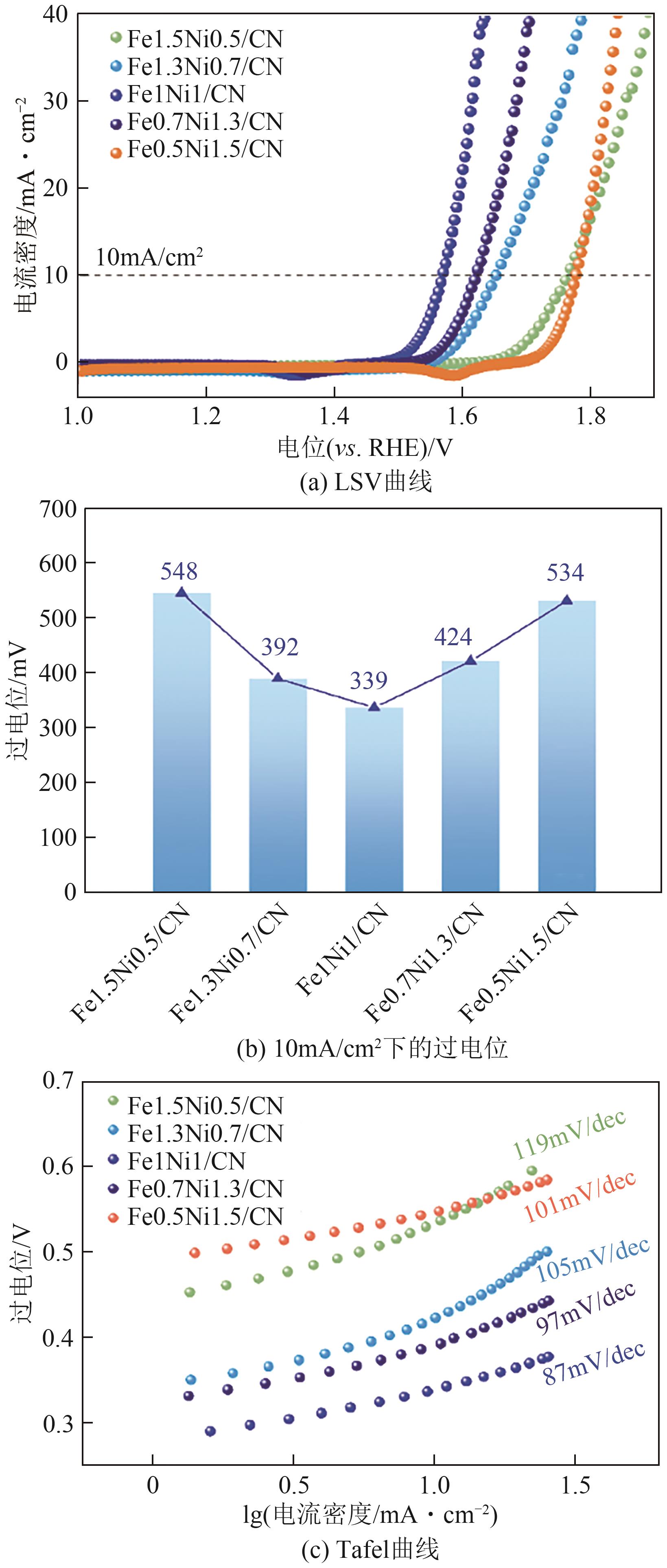
| 催化剂 | 电解液 | η10/mV | Tafel斜率/mV·dec-1 | 参考文献 |
|---|---|---|---|---|
| Fe1Ni1/CN | 1mol/L KOH | 339 | 87 | 本工作 |
| Fe3O4/FeS2 | 1mol/L KOH | 253 | 48 | [ |
| FeNi3N-Ni3S2 | 1mol/L KOH | 230 | 39 | [ |
| FeNiO x /C | 1mol/L KOH | 310 | 58 | [ |
| Fe3O4@N-HCNT | 1mol/L KOH | 500 | 64 | [ |
| Fe-NiO | 1mol/L KOH | 380 | 42 | [ |
| Ni3FeN | 0.1mol/L KOH | 355 | 70 | [ |
| NiFe-LDH/CNT | 0.1mol/L KOH | 350 | 54 | [ |
| NiFe2O x | 1mol/L KOH | 356 | 57 | [ |
| Ni9S8 | 1mol/L KOH | 480 | 56 | [ |
| Fe x Ni1-x Co2O4 | 1mol/L KOH | 350 | 27 | [ |
| 催化剂 | 电解液 | η10/mV | Tafel斜率/mV·dec-1 | 参考文献 |
|---|---|---|---|---|
| Fe1Ni1/CN | 1mol/L KOH | 339 | 87 | 本工作 |
| Fe3O4/FeS2 | 1mol/L KOH | 253 | 48 | [ |
| FeNi3N-Ni3S2 | 1mol/L KOH | 230 | 39 | [ |
| FeNiO x /C | 1mol/L KOH | 310 | 58 | [ |
| Fe3O4@N-HCNT | 1mol/L KOH | 500 | 64 | [ |
| Fe-NiO | 1mol/L KOH | 380 | 42 | [ |
| Ni3FeN | 0.1mol/L KOH | 355 | 70 | [ |
| NiFe-LDH/CNT | 0.1mol/L KOH | 350 | 54 | [ |
| NiFe2O x | 1mol/L KOH | 356 | 57 | [ |
| Ni9S8 | 1mol/L KOH | 480 | 56 | [ |
| Fe x Ni1-x Co2O4 | 1mol/L KOH | 350 | 27 | [ |
| [1] | 梁严, 吴璇, 王军, 等. “双碳”背景下天然气制氢先进技术及应用场景[J]. 当代化工研究, 2023(16): 101-103. |
| LIANG Yan, WU Xuan, WANG Jun, et al. Advanced technology and application scenarios of natural gas to hydrogen in the context of carbon peaking and carbon neutrality[J]. Modern Chemical Research, 2023(16): 101-103. | |
| [2] | 我国首个甲醇制氢加氢一体站投用[J]. 中国环境监察, 2023(S1): 5. |
| China’s first integrated methanol hydrogen production and hydrogenation station put into use[J]. China Environment Supervision, 2023(S1): 5. | |
| [3] | 解寅珑, 云红红, 白韡, 等. 电解水制氢气用催化剂制备及应用性能[J]. 化学工程师, 2023, 37(8): 106-111. |
| XIE Yinlong, YUN Honghong, BAI Wei, et al. Preparation and application performance of catalyst for hydrogen production from electrolytic water[J]. Chemical Engineer, 2023, 37(8): 106-111. | |
| [4] | CHENG Guishi, YANG Yihao, XIU Taichun, et al. Analysis of hydrogen production potential from water electrolysis in China[J]. Energy & Fuels, 2023, 37(13): 9220-9232. |
| [5] | YAO Dongxue, GU Lingling, ZUO Bin, et al. A strategy for preparing high-efficiency and economical catalytic electrodes toward overall water splitting[J]. Nanoscale, 2021, 13(24): 10624-10648. |
| [6] | 符淑瑢, 王丽娜, 王东伟, 等. 析氧助催化剂增强光阳极光电催化分解水性能研究进展[J]. 化工进展, 2023, 42(5): 2353-2370. |
| FU Shurong, WANG Lina, WANG Dongwei, et al. Oxygen evolution cocatalyst enhancing the photoanode performances for photoelectrochemical water splitting[J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2353-2370. | |
| [7] | 胡志豪, 张皓景, 周叶, 等. 高效镍基有序多孔电极气泡行为可视化观测及性能影响[J]. 化工进展, 2024, 43(2): 680-687. |
| HU Zhihao, ZHANG Haojing, ZHOU Ye, et al. Visualization observation of bubble behavior and performance impact analysis in efficient nickel based ordered porous electrodes[J]. Chemical Industry and Engineering Progress, 2024, 43(2): 680-687. | |
| [8] | ZHU Mengshu, AI Xiaomeng, FANG Jiakun, et al. Optimal integration of electrolysis, gasification and reforming for stable hydrogen production[J]. Energy Conversion and Management, 2023, 292: 117400. |
| [9] | 田锦锐, 田浩, 戴佳玮, 等. 自支撑电极电解水制氢应用研究进展[J]. 化学试剂, 2023, 45(6): 78-87. |
| TIAN Jinrui, TIAN Hao, DAI Jiawei, et al. Progress of hydrogen production application by water electrolysis with self-supported electrode[J]. Chemical Reagents, 2023, 45(6): 78-87. | |
| [10] | 张静, 贺业亨, 王晶晶, 等. 电沉积法制备碱性电解水镍基析氧电极的研究进展[J]. 化工进展, 2023, 42(12): 6239-6250. |
| ZHANG Jing, HE Yeheng, WANG Jingjing, et al. Research progress on nickel-based oxygen evolution electrode prepared by electrodeposition for alkaline water electrolysis[J]. Chemical Industry and Engineering Progress, 2023, 42(12): 6239-6250. | |
| [11] | JAMESH Mohammed-Ibrahim, SUN Xiaoming. Recent progress on earth abundant electrocatalysts for oxygen evolution reaction (OER) in alkaline medium to achieve efficient water splitting—A review[J]. Journal of Power Sources, 2018, 400: 31-68. |
| [12] | TAMILARASI B, JITHUL K P, PANDEY J. Non-noble metal-based electro-catalyst for the oxygen evolution reaction (OER): Towards an active & stable electro-catalyst for PEM water electrolysis[J]. International Journal of Hydrogen Energy, 2024, 58: 556-582. |
| [13] | WU Zhipeng, LU Xuefeng, ZANG Shuangquan, et al. Non-noble-metal-based electrocatalysts toward the oxygen evolution reaction[J]. Advanced Functional Materials, 2020, 30(15): 1910274. |
| [14] | MALEK Abdul, XUE Yanrong, LU Xu. Dynamically restructuring Ni x Cr y O electrocatalyst for stable oxygen evolution reaction in real seawater[J]. Angewandte Chemie International Edition, 2023, 62(40): e202309854. |
| [15] | CHEN Qiao, USMAN Zahid, WEI Jie, et al. Efficient O-O coupling at catalytic interface to assist kinetics optimization on concerted and sequential proton-electron transfer for water oxidation[J]. ACS Nano, 2023, 17(13): 12278-12289. |
| [16] | GONG Lanqian, YANG Huan, DOUKA Abdoulkader Ibro, et al. Recent progress on NiFe-based electrocatalysts for alkaline oxygen evolution[J]. Advanced Sustainable Systems, 2021, 5(1): 2000136. |
| [17] | DENG Chen, WU Kuang-Hsu, SCOTT Jason, et al. Core/shell NiFe nanoalloy with a discrete N-doped graphitic carbon cover for enhanced water oxidation[J]. ChemElectroChem, 2018, 5(5): 732-736. |
| [18] | 韩银凤, 张瑞林. 以泡沫镍为基底的纳米片层状Ni2+-Fe3+-V3+-LDHs的制备及其电催化析氧性能研究[J]. 化学试剂, 2020, 42(1): 8-12. |
| HAN Yinfeng, ZHANG Ruilin. Preparation and electrolytic oxygen evolution performance characteration of Ni2+-Fe3+-V3+ LDHs with nanosheet structure based on foam nickel[J]. Chemical Reagents, 2020, 42(1): 8-12. | |
| [19] | ZHENG Dong, YU Linhai, LIU Wenxian, et al. Structural advantages and enhancement strategies of heterostructure water-splitting electrocatalysts[J]. Cell Reports Physical Science, 2021, 2(6): 100443. |
| [20] | MA Hancheng, DING Yao, LI Jianqi, et al. Approaching high oxygen evolution reaction performance by synergetic dual-ion leaching[J]. Nano Research, 2024, 17(9): 7975-7983. |
| [21] | LI Yichuan, TANG Guoqiang, WANG Yu, et al. Interfacial engineering of a phase-controlled heterojunction for high-efficiency HER, OER, and ORR trifunctional electrocatalysis[J]. ACS Omega, 2022, 7(16): 13687-13696. |
| [22] | PAN Jiaqi, CHEN Zhanfen, WANG Panhong, et al. The overall water splitting of CdS/Ti3+-SrTiO3 core-shell heterojunction via OER enhancement of MnO x nanoparticles[J]. Chemical Engineering Journal, 2021, 424: 130357. |
| [23] | PAN Jiaqi, WANG Panhong, WANG Peipei, et al. The photocatalytic overall water splitting hydrogen production of g-C3N4/CdS hollow core-shell heterojunction via the HER/OER matching of Pt/MnO x [J]. Chemical Engineering Journal, 2021, 405: 126622. |
| [24] | WU Dongting, KONG Aiqun, LI Wei, et al. Facile synthesis of bimetallic Ni-Fe phosphide as robust electrocatalyst for oxygen evolution reaction in alkaline media[J]. International Journal of Hydrogen Energy, 2021, 46(80): 39844-39854. |
| [25] | WANG Minjie, ZHENG Xingqun, SONG Lele, et al. Fe3O4/FeS2 heterostructures enable efficient oxygen evolution reaction[J]. Journal of Materials Chemistry A, 2020, 8(28): 14145-14151. |
| [26] | LIANG Shuqin, JING Meizan, PERVAIZ Erum, et al. Nickel-iron nitride-nickel sulfide composites for oxygen evolution electrocatalysis[J]. ACS Applied Materials & Interfaces, 2020, 12(37): 41464-41470. |
| [27] | BANJAR Mohd Faizar, ABEDIN Fatin Najwa Joynal, FIZAL Ahmad Noor Syimir, et al. Synthesis and characterization of a novel nanosized polyaniline[J]. Polymers, 2023, 15(23): 4565. |
| [28] | WANG Jie, ZHAO Shuo, WANG Jin, et al. In-situ embedding Co9S8 nanoparticles in polyaniline-based carbon nanotubes for enhanced lithium storage[J]. Journal of Alloys and Compounds, 2022, 919: 165819. |
| [29] | FENG Juan, ZONG Yan, SUN Yong, et al. Optimization of porous FeNi3/N-GN composites with superior microwave absorption performance[J]. Chemical Engineering Journal, 2018, 345: 441-451. |
| [30] | HAN Cong’ai, ZHANG Haiyan, ZHANG Danfeng, et al. Ultrafine FeNi3 nanocrystals embedded in 3D honeycomb-like carbon matrix for high-performance microwave absorption[J]. Nanomaterials, 2020, 10(4): 598. |
| [31] | WANG Lili, LI Na, ZHAO Tiqi, et al. Magnetic properties of FeNi3 nanoparticle modified Pinus radiata wood nanocomposites[J]. Polymers, 2019, 11(3): 421. |
| [32] | GAO Qiuyue, LI Guoru, KOFIE Gideon, et al. Ionic liquid/ZIF-67 derived Co9S8-SNC catalyst for oxygen reduction reaction in alkaline electrolyte[J]. Carbon Letters, 2024, 34(3): 951-960. |
| [33] | LIU Yang, ZHANG Shiqing, MA Shaokai, et al. Electronic structure modification of MnO2 nanosheet arrays with enhanced water oxidation activity and stability by nitrogen plasma[J]. ACS Applied Materials & Interfaces, 2024, 16(28): 36498-36508. |
| [34] | ZHENG Lingxia, YE Weiqing, ZHAO Yijian, et al. Defect-induced atomic arrangement in CoFe bimetallic heterostructures with boosted oxygen evolution activity[J]. Small, 2023, 19(9): 2205092. |
| [35] | SUEN Nian-Tzu, HUNG Sung-Fu, QUAN Quan, et al. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives[J]. Chemical Society Reviews, 2017, 46(2): 337-365. |
| [36] | CHEN Hui, HUANG Xiaoxi, ZHOU Lijing, et al. Electrospinning synthesis of bimetallic nickel-iron oxide/carbon composite nanofibers for efficient water oxidation electrocatalysis[J]. ChemCatChem, 2016, 8(5): 992-1000. |
| [37] | SHI Kun, ZHENG Man, LIU Jiaxian, et al. Novel Fe3O4 nanoparticles encapsulated in and loaded on hollow carbon nanotubes wrapped dendritic carbon layers architecture for water decomposition[J]. International Journal of Hydrogen Energy, 2024, 51: 1303-1317. |
| [38] | KIM Sang Jun, Seung Geun JO, LEE Eún Been, et al. Morphology-controlled nickel oxide and iron-nickel oxide for electrochemical oxygen evolution reaction[J]. ACS Applied Energy Materials, 2023, 6(16): 8360-8367. |
| [39] | FU Gengtao, CUI Zhiming, CHEN Yifan, et al. Hierarchically mesoporous nickel-iron nitride as a cost-efficient and highly durable electrocatalyst for Zn-air battery[J]. Nano Energy, 2017, 39: 77-85. |
| [40] | GONG Ming, LI Yanguang, WANG Hailiang, et al. An advanced Ni-Fe layered double hydroxide electrocatalyst for water oxidation[J]. Journal of the American Chemical Society, 2013, 135(23): 8452-8455. |
| [41] | CHERVIN Christopher N, DESARIO Paul A, PARKER Joseph F, et al. Aerogel architectures boost oxygen-evolution performance of NiFe2O x spinels to activity levels commensurate with nickel-rich oxides[J]. ChemElectroChem, 2016, 3(9): 1369-1375. |
| [42] | LI Hao, SHAO Youdong, SU Yantao, et al. Vapor-phase atomic layer deposition of nickel sulfide and its application for efficient oxygen-evolution electrocatalysis[J]. Chemistry of Materials, 2016, 28(4): 1155-1164. |
| [43] | YAN Kaili, SHANG Xiao, LI Zhen, et al. Ternary mixed metal Fe-doped NiCo2O4 nanowires as efficient electrocatalysts for oxygen evolution reaction[J]. Applied Surface Science, 2017, 416: 371-378. |
| [44] | TIEN Chien-Ping, LIANG Wuu-Jyh, KUO Pinglin, et al. Electric double layer capacitors with gelled polymer electrolytes based on poly(ethylene oxide) cured with poly(propylene oxide) diamines[J]. Electrochimica Acta, 2008, 53(13): 4505-4511. |
| [45] | ASHOK Venkatachalam, MATHI Selvam, SANGAMITHIRAI Muthukumaran, et al. Regulated bimetal-doped polyaniline: Amorphous-crumple-structured viable electrocatalyst for an efficient oxygen evolution reaction[J]. Energy & Fuels, 2022, 36(23): 14349-14360. |
| [46] | QIANG Chenchen, LIU Min, ZHANG Liang, et al. In situ growth of Ni-based metal-organic framework nanosheets on carbon nanotube films for efficient oxygen evolution reaction[J]. Inorganic Chemistry, 2021, 60(5): 3439-3446. |
| [47] | SUN Xuan, ZHANG Xiuxiu, LI Yuanli, et al. In situ construction of flexible V-Ni redox centers over Ni-based MOF nanosheet arrays for electrochemical water oxidation[J]. Small Methods, 2021, 5(10): e2100573. |
| [48] | TANG Lei, XIA Meihan, CAO Shiyu, et al. Operando identification of active sites in Co-Cr oxyhydroxide oxygen evolution electrocatalysts[J]. Nano Energy, 2022, 101: 107562. |
| [49] | LIN Yangming, YU Linhui, TANG Ling, et al. In situ identification and time-resolved observation of the interfacial state and reactive intermediates on a cobalt oxide nanocatalyst for the oxygen evolution reaction[J]. ACS Catalysis, 2022, 12(9): 5345-5355. |
| [50] | ZHOU Feng, CHEN Jiadong, YANG Yun, et al. Exceptionally active and stable RuO2 by constructing p-n heterojunction between Co3O4 and RuO2 for acidic water oxidation[J]. Applied Surface Science, 2023, 641: 158508. |
| [1] | ZHANG Wei, LIANG Yaocheng, WU Qiao, FU Yehao, YIN Yanshan, CHENG Shan, RUAN Min, LIU Tao, ZHOU Zhaoyi, ZHANG Kaikai, LI Dancong. Metal ion modified Cu-SSZ-13 catalyst for NH3-selective catalytic reduction of NO x [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 3879-3891. |
| [2] | LU Peng, ZHANG Di, LIU Yaoyao, YU Wanjin, LIU Wucan, ZHANG Jianjun. Research progress of catalysts for gas-phase dehydrofluorination to synthesize C2 hydrofluoroolefins [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 3907-3916. |
| [3] | GAO Jiaojiao, YAN Shiyu, YANG Taishun, XIE Shangzhi, YANG Yanjuan, XU Jing. Effect of alumina support crystal structure of Ru-based catalysts on polyethylene hydrogenolysis performance [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 3917-3927. |
| [4] | SHI Xiuding, WANG Yongquan, ZENG Jing, SU Chang, HONG Junming. Nanotubular Co-N-C activated percarbonate for tetracycline degradation [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3041-3052. |
| [5] | LI Peiyi, SUN Bolong, LIU Ruiyan, ZHOU Xinyao, LIU Ruilin, HU Yuanyuan, XU Gongtao, LI Xinping. Preparation of sodium alginate/titanium dioxide composite porous material and its application in oil-water separation [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3053-3061. |
| [6] | KONG Can, LIU Yuhan, SHENG Yu, LIU Fang, CHANG Huazhen. Polyaniline enhanced cuprous oxide for carbon dioxide reduction [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3144-3153. |
| [7] | YAO Ruwei, SONG Yueyin, NIU Qinqin, LI Congming. Na-S co-modified iron catalysts for CO2 hydrogenation to C2+ alcohols [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3154-3162. |
| [8] | CHEN Shaowei, CHEN Yi, NIU Jiangqi, LIU Tianqi, HUANG Jianguo, CHEN Huanhao, FAN Xiaolei. Research progress and application prospects of dielectric barrier discharge plasma catalytic reactors [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3175-3189. |
| [9] | WANG Jiahui, LI Peiya, YANG Fusheng, WANG Bin, FANG Tao. Research progress on the dehydrogenation of methylcyclohexane as a liquid organic hydrogen carrier [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3208-3223. |
| [10] | ZHANG Ying, ZHENG Xuemei, MA Aiyuan, TIAN Shihong. Research progress based on conventional and microwave pyrolysis behavior of polyethylene [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3224-3237. |
| [11] | LI Hongwei, XU Hanqiao, ZHAO Yan, LIU Yaozong, TENG Zhijun, JI Dong, LI Guixian. Research progress and prospect of platinum-based catalysts for electrocatalytic methanol oxidation [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3443-3456. |
| [12] | KONG Xiaoyang, LIU Zhentao, ZOU Yutong, WANG Dandan, DUAN Aijun, XU Chunming, WANG Xilong. Development in catalysts for hydrocracking of polycyclic aromatic hydrocarbons to BTX [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3468-3485. |
| [13] | LIU Shizhe. Advances in catalytic system for methylcyclohexane dehydrogenation [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3486-3496. |
| [14] | WU Yali, ZHANG Xiaolin, GAO Limin, HUANG Maocai, CAI Bin, ZHANG Jibing. Technical progress in resource utilization of straw powder/fiber [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3509-3523. |
| [15] | WANG Linyan, ZHOU Pingde, ZHANG Yiduo, LIU Yuxi, HAO Mingzheng, LIANG Yurong. Preparation and performance of high thermal conductivity boron nitride/natural rubber nanocomposites [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3541-3549. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||