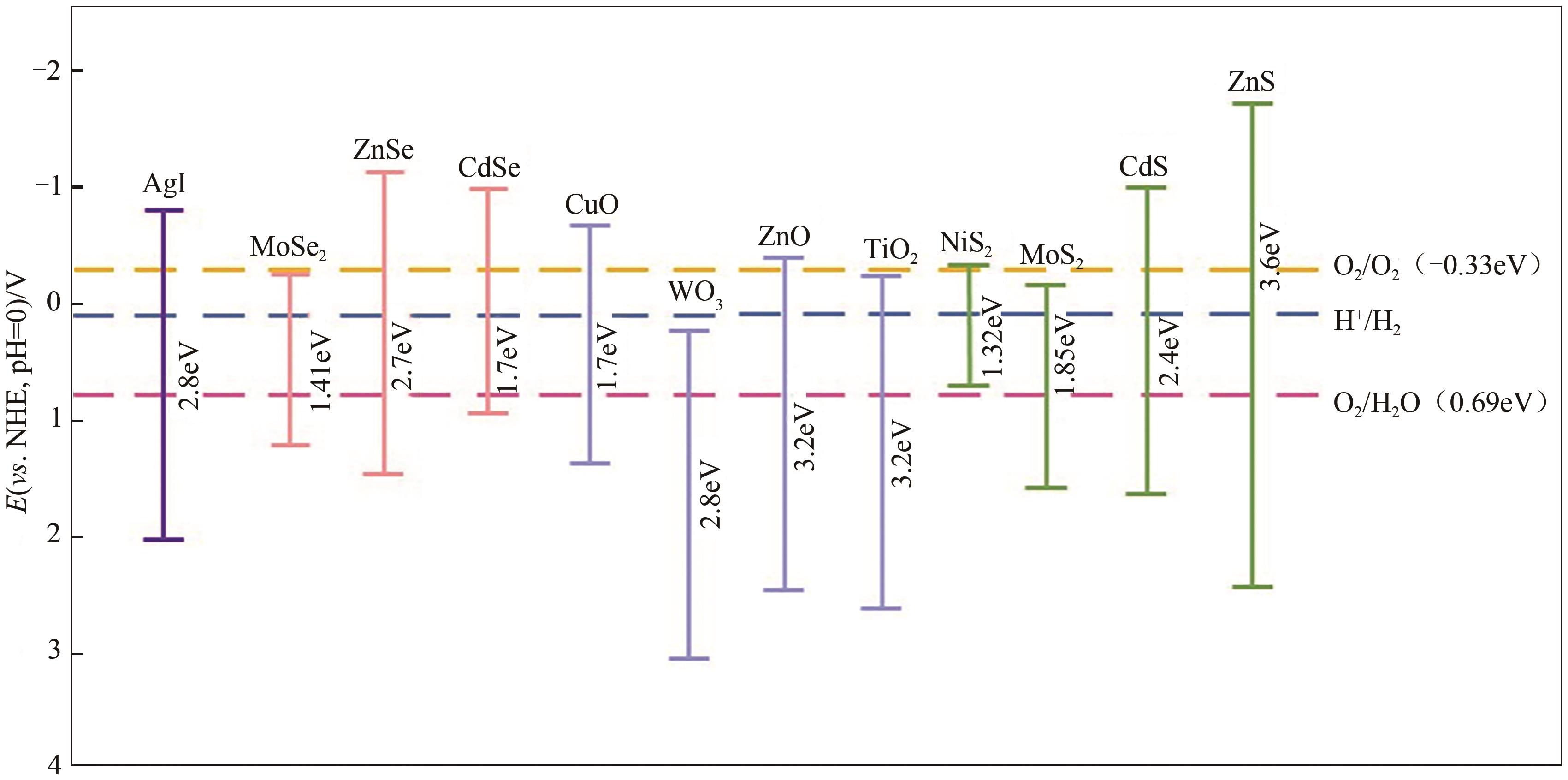
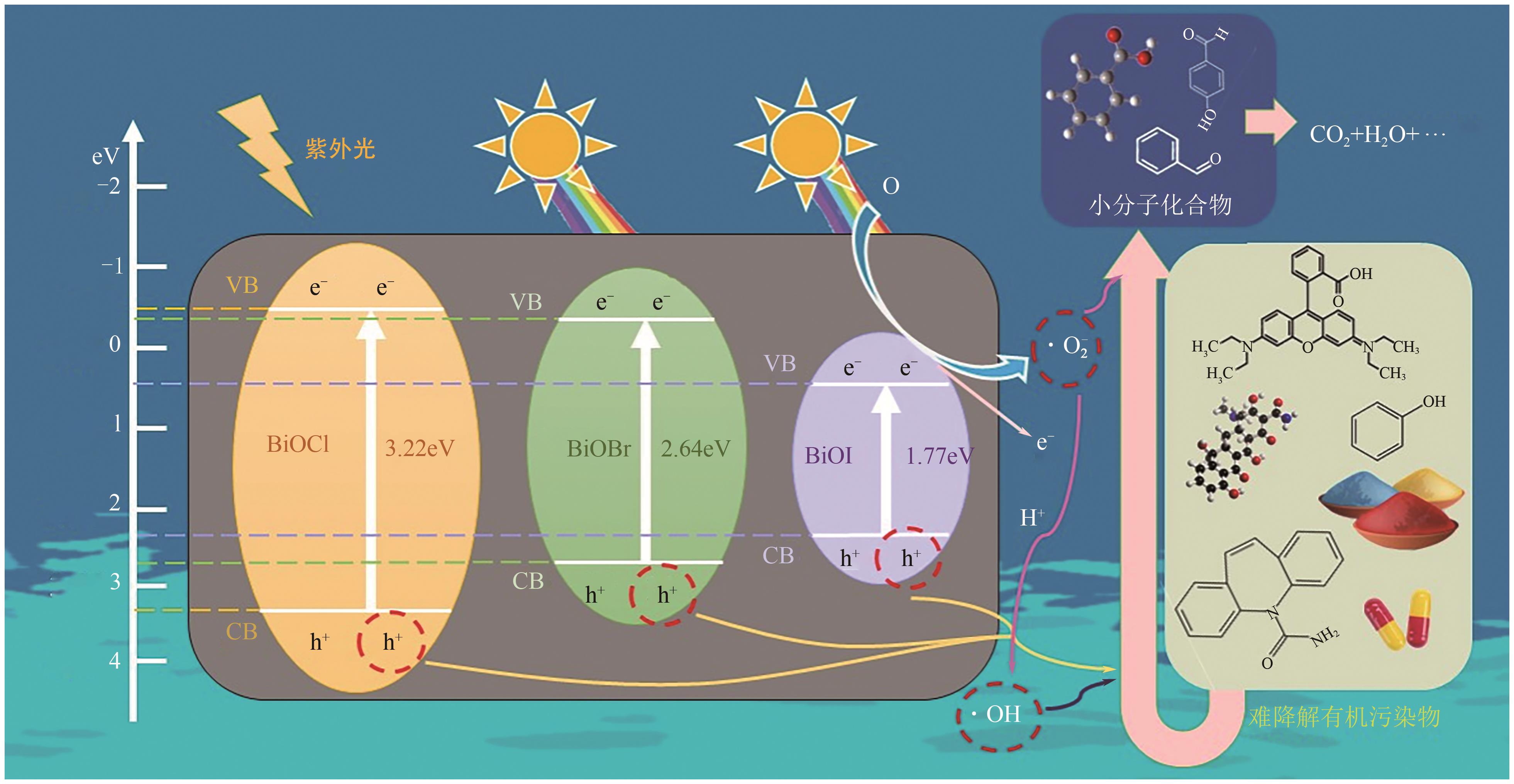
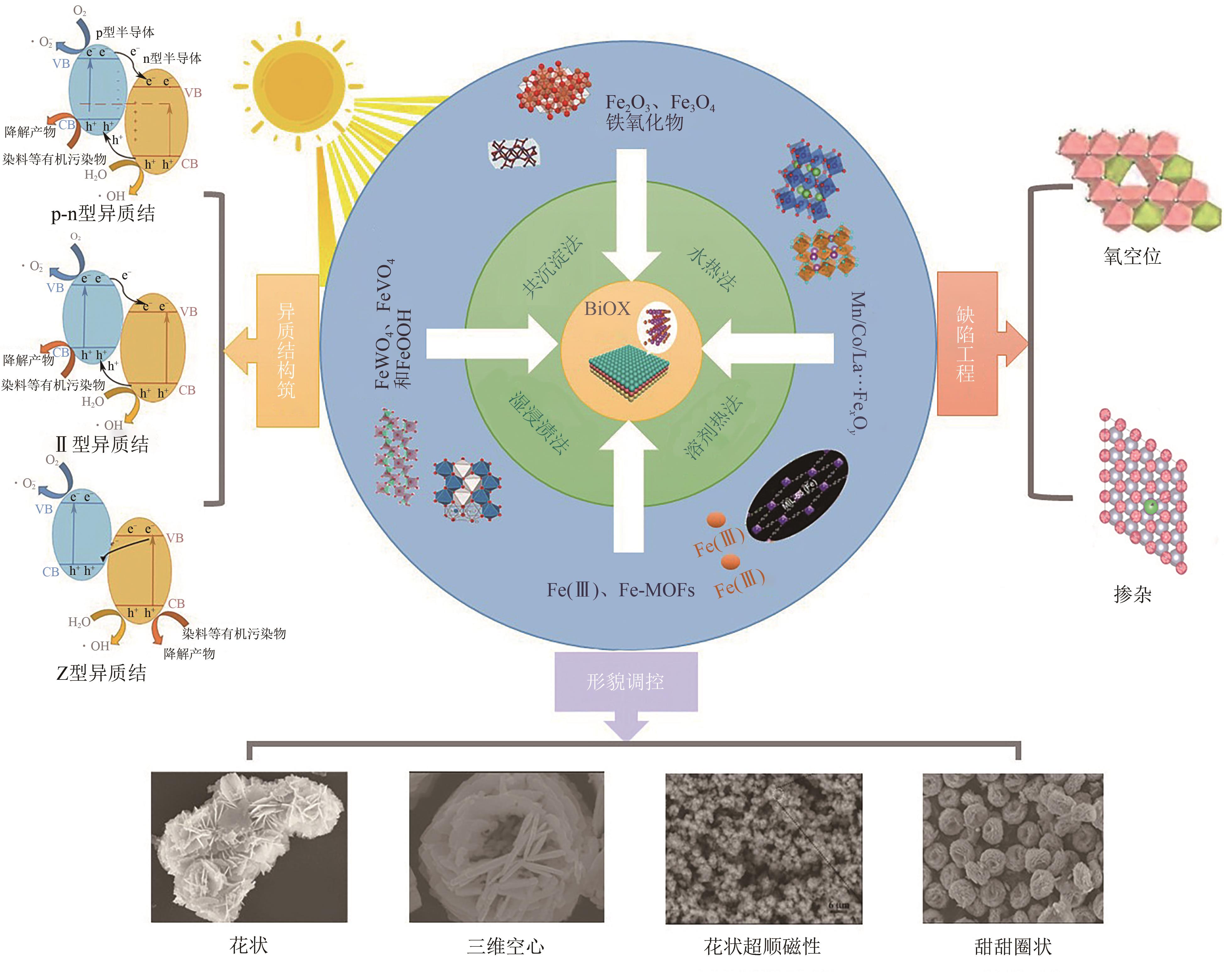
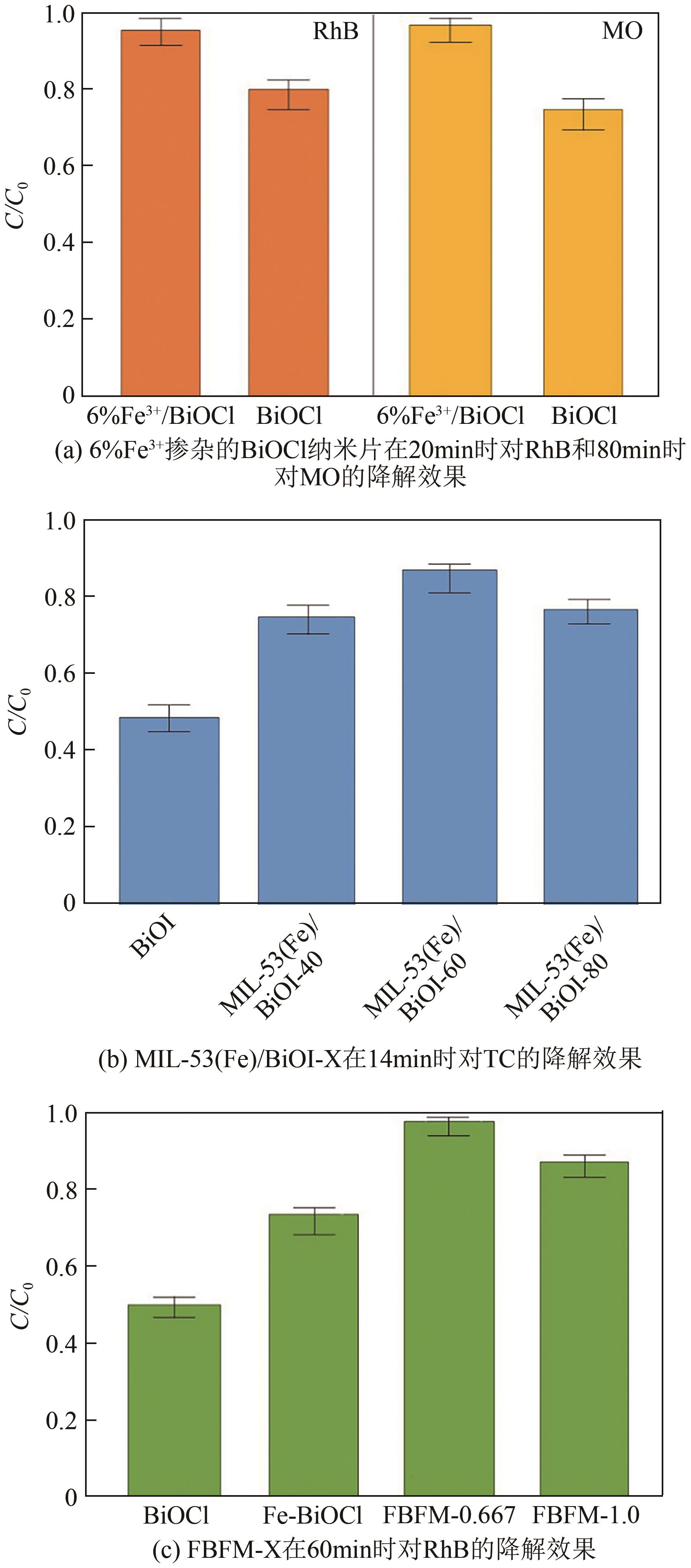
Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (4): 2258-2273.DOI: 10.16085/j.issn.1000-6613.2024-0501
• Resources and environmental engineering • Previous Articles Next Articles
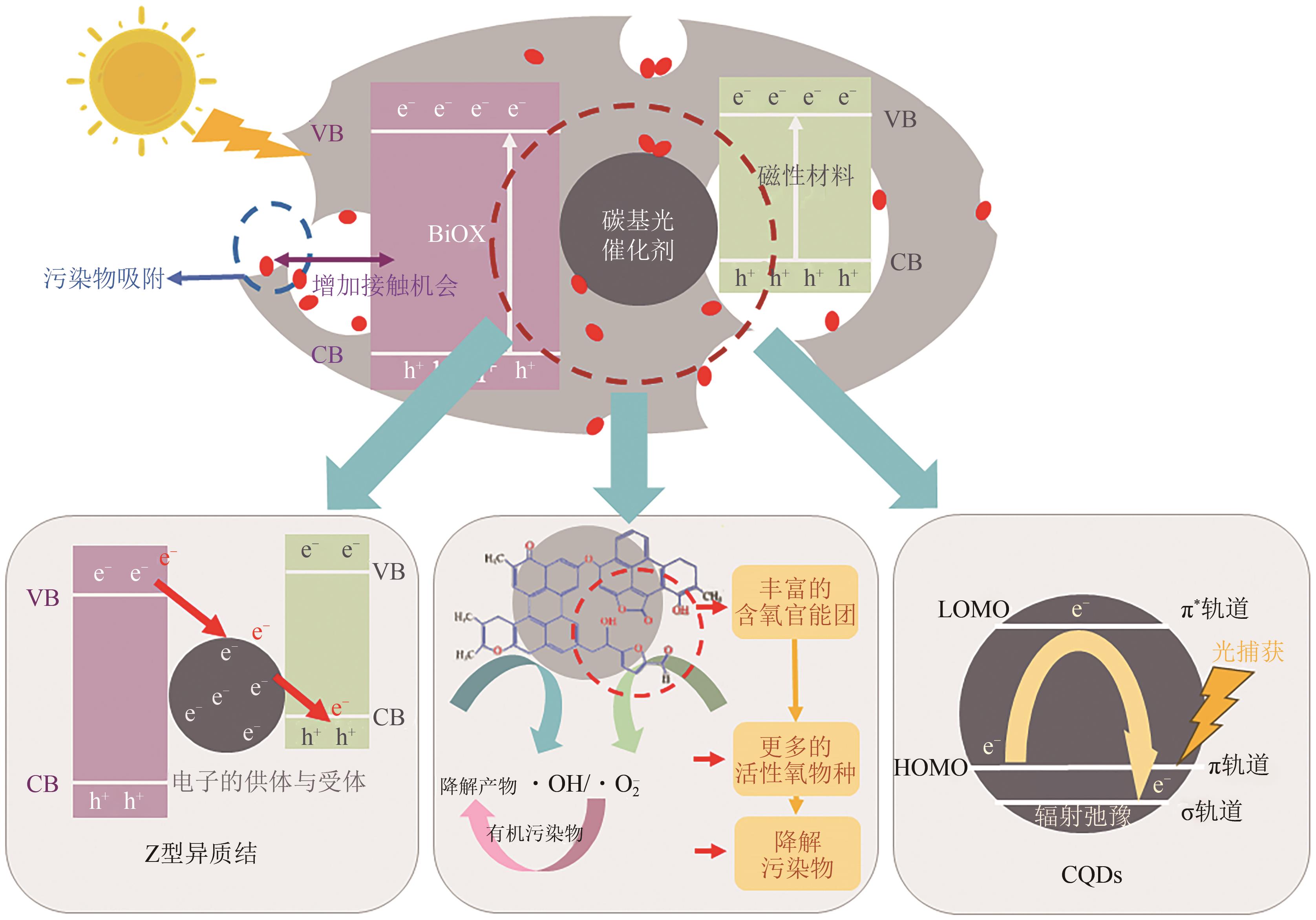
Research progress on iron-based composite bismuth oxyhalide magnetic materials for enhanced visible light catalytic treatment of refractory organic wastewater
ZHANG Yiru1( ), HAN Dongmei2, MA Weifang1(
), HAN Dongmei2, MA Weifang1( )
)
- 1.College of Environmental Science and Engineering, Beijing Forestry University, Beijing 100083, China
2.Beijing Energy Conservation and Environmental Protection Center, Beijing 100029, China
-
Received:2024-03-27Revised:2024-05-25Online:2025-05-07Published:2025-04-25 -
Contact:MA Weifang
铁基复合卤氧化铋磁性材料强化可见光催化处理难降解有机废水研究进展
- 1.北京林业大学环境科学与工程学院,北京 100083
2.北京节能环保中心,北京 100029
-
通讯作者:马伟芳 -
作者简介:张绎如(2001—),女,硕士研究生,研究方向为光催化。E-mail:3607612564@qq.com。 -
基金资助:北京市科技计划(Z141100000914009)
CLC Number:
Cite this article
ZHANG Yiru, HAN Dongmei, MA Weifang. Research progress on iron-based composite bismuth oxyhalide magnetic materials for enhanced visible light catalytic treatment of refractory organic wastewater[J]. Chemical Industry and Engineering Progress, 2025, 44(4): 2258-2273.
张绎如, 韩东梅, 马伟芳. 铁基复合卤氧化铋磁性材料强化可见光催化处理难降解有机废水研究进展[J]. 化工进展, 2025, 44(4): 2258-2273.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-0501
| 高级氧化技术 | 反应原理 | 反应条件 | 降解率/% | 反应时间 | 运行费用 |
|---|---|---|---|---|---|
| 臭氧氧化法 | (pH低时,O3直接氧化为主) (pH高时,·OH自由基氧化为主) | — | 30~90 | ≤60min | 10~1000CNY/m3 |
湿式氧化法 (WAO) | ①链引发: ②链传递: ③链终止: (RH为有机物,M为催化剂) | ①环境温度:123~320℃ ②压力:0.5~10.0MPa ③催化剂:富氧气体、O2 | 75~90 | 15~20min | 10~1000CNY/m3 |
| 芬顿氧化法 | 总反应: | ①合理控制H2O2 及铁盐投放量 ②一般要求酸性条件 | 40~90 | 1~2h | 10~1000CNY/m3 |
超临界水氧化法 (SCWO) | 有机化合物 | ①水的温度374.3℃ ②压力>22.1MPa | >99.99 | <1min | 100~10000CNY/m3 |
| 光催化氧化法 | 光催化剂 有机污染物 (hv为光子的能量,h+和e-为空穴和电子) | — | 50~100 | 由光催化剂决定 | 由光催化剂决定 |
| 高级氧化技术 | 反应原理 | 反应条件 | 降解率/% | 反应时间 | 运行费用 |
|---|---|---|---|---|---|
| 臭氧氧化法 | (pH低时,O3直接氧化为主) (pH高时,·OH自由基氧化为主) | — | 30~90 | ≤60min | 10~1000CNY/m3 |
湿式氧化法 (WAO) | ①链引发: ②链传递: ③链终止: (RH为有机物,M为催化剂) | ①环境温度:123~320℃ ②压力:0.5~10.0MPa ③催化剂:富氧气体、O2 | 75~90 | 15~20min | 10~1000CNY/m3 |
| 芬顿氧化法 | 总反应: | ①合理控制H2O2 及铁盐投放量 ②一般要求酸性条件 | 40~90 | 1~2h | 10~1000CNY/m3 |
超临界水氧化法 (SCWO) | 有机化合物 | ①水的温度374.3℃ ②压力>22.1MPa | >99.99 | <1min | 100~10000CNY/m3 |
| 光催化氧化法 | 光催化剂 有机污染物 (hv为光子的能量,h+和e-为空穴和电子) | — | 50~100 | 由光催化剂决定 | 由光催化剂决定 |
| 种类 | 材料 | 合成方法 | 结合后结构 | 废水种类 | 目标污染物 | 剂量 /g·L-1 | 污染物 浓度/mg·L-1 | 反应时间 /min | 降解率/% | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|---|
| Fe掺杂BiOX (X=Cl、Br、I)、铁基金属有机骨架与BiOX(X=Cl、Br、I)结合 | Fe3+/BiOCl | 溶剂热法 | 陷阱能级 | 印染废水 | RhB MO | 1 | 5 | RhB:20 MO:80 | RhB:99 MO:99 | [ |
| Fe(Ⅲ)@BOC NS | 溶剂热法 | — | 印染废水 | RhB | 0.1 | 约9.6 | 15 | 99.27 | [ | |
| Fe-BiOCl | 水热法 | 陷阱能级 | 制药废水 | 左氧氟沙星 (LXV) | 0.5 | 15 | 60 | >95 | [ | |
| Fe-BiOCl@ Fe-MOF | 溶剂热法 | 环形、 Ⅰ型异质结 | 印染废水 制药废水 | RhB TC | 0.2 | RhB:5 TC:10 | 90 | RhB:99 TC:90.4 | [ | |
| MIL-53(Fe)/ BiOI | 水热和简易 共沉淀法 | Ⅱ型异质结 | 制药废水 | TC | 0.2 | 25 | 14 | 86.21 | [ | |
| 铁氧化物与 BiOX(X=Cl、 Br、I)结合 | BiOCl/Fe3O4 | 共沉淀法 | Ⅰ型异质结 | 制药废水 | 卡马西平(CBZ) | 0.6 | 80 | 60 | 90.3 | [ |
| Fe3O4/BiOCl | 共沉淀法 | 超顺磁花状 | 印染废水 | RhB | — | 40 | 30 | 98 | [ | |
| BiOCl@Fe3O4 | 共沉淀法 | — | 制药废水 | 阿替洛尔(ATL) | 0.4 | 2.5 | 40 | 100 | [ | |
| Fe3O4/BiOBr | 溶剂热法 | 微球状 | 印染废水 | 刚果红(CR) | 1.4 | 20 | 240 | 91.18 | [ | |
| BiOBr/ Fe3O4 | 溶剂热法 | p-n型异质结 | 农业废水 | 草甘膦(PMG) | 4 | 100 | 60 | 97 | [ | |
| Fe3O4/BiOI | 共沉淀法 | n-p型异质结 | 印染废水 制药废水 | MO 磺胺嘧啶钠盐 (SD-Na) | 1 | 20 | 120 | MO:81 SD-Na:79 | [ | |
| Fe3O4/BiOI | 共沉淀法 | 中空的三维微球、 异质结 | 有机化工废水 印染废水 | BPA RhB | 1 | BPA:20 RhB:— | BPA:90 RhB:180 | BPA:100 RhB:95.1 | [ | |
| 磁性双金属 氧化物和 多铁性质的 氧化物与 BiOX(X=Cl、 Br、I)结合 | LaFeO3/BiOBr | 水热法 | Z型异质结 | 印染废水 | RhB | 1 | 5 | 30 | 98.2 | [ |
| BiOBr/CoFe2O4/GO | 水热法 | Z型异质结 | 印染废水 | RhB | 0.2 | 30 | 120 | 100 | [ | |
| ZnFe2O4/BiOI | 水热法 | Ⅱ型异质结 | 印染废水 | RhB | 0.5 | 10 | 70 | 86.54 | [ | |
| BiOBr/MnFe2O4 | 无表面活性剂 水热合成法 | Z型异质结 | 农业废水 印染废水 | 2,4-D RhB | 1 | 2,4-D:20 RhB:20 | 80 | 2,4-D:89.3 RhB:96.5 | [ | |
| BiFeO3/BiOI | 湿浸渍法 | Ⅱ型异质结 | 印染废水 有机化工废水 | RhB BPA | 1 | 约3.6 | RhB:60 BPA:120 | RhB:约100 BPA:约71 | [ | |
| FeWO4、FeVO4、FeOOH与 BiOX(X=Cl、Br、I) 结合 | BiOBr/FeWO4 | 水热法 | Ⅱ型异质结 | 制药废水 | DOX | 1 | — | 60 | 90.4 | [ |
| α-FeOOH/BiOI | 沉淀法 | Ⅱ型异质结 | 印染废水 | RhB | 0.5 | 20 | 20 | 100 | [ | |
| FeVO4/BiOCl | 湿浸渍法 | Z型异质结 | 印染废水 | RhB | 0.5 | 2 | 360 | 99.8 | [ |
| 种类 | 材料 | 合成方法 | 结合后结构 | 废水种类 | 目标污染物 | 剂量 /g·L-1 | 污染物 浓度/mg·L-1 | 反应时间 /min | 降解率/% | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|---|
| Fe掺杂BiOX (X=Cl、Br、I)、铁基金属有机骨架与BiOX(X=Cl、Br、I)结合 | Fe3+/BiOCl | 溶剂热法 | 陷阱能级 | 印染废水 | RhB MO | 1 | 5 | RhB:20 MO:80 | RhB:99 MO:99 | [ |
| Fe(Ⅲ)@BOC NS | 溶剂热法 | — | 印染废水 | RhB | 0.1 | 约9.6 | 15 | 99.27 | [ | |
| Fe-BiOCl | 水热法 | 陷阱能级 | 制药废水 | 左氧氟沙星 (LXV) | 0.5 | 15 | 60 | >95 | [ | |
| Fe-BiOCl@ Fe-MOF | 溶剂热法 | 环形、 Ⅰ型异质结 | 印染废水 制药废水 | RhB TC | 0.2 | RhB:5 TC:10 | 90 | RhB:99 TC:90.4 | [ | |
| MIL-53(Fe)/ BiOI | 水热和简易 共沉淀法 | Ⅱ型异质结 | 制药废水 | TC | 0.2 | 25 | 14 | 86.21 | [ | |
| 铁氧化物与 BiOX(X=Cl、 Br、I)结合 | BiOCl/Fe3O4 | 共沉淀法 | Ⅰ型异质结 | 制药废水 | 卡马西平(CBZ) | 0.6 | 80 | 60 | 90.3 | [ |
| Fe3O4/BiOCl | 共沉淀法 | 超顺磁花状 | 印染废水 | RhB | — | 40 | 30 | 98 | [ | |
| BiOCl@Fe3O4 | 共沉淀法 | — | 制药废水 | 阿替洛尔(ATL) | 0.4 | 2.5 | 40 | 100 | [ | |
| Fe3O4/BiOBr | 溶剂热法 | 微球状 | 印染废水 | 刚果红(CR) | 1.4 | 20 | 240 | 91.18 | [ | |
| BiOBr/ Fe3O4 | 溶剂热法 | p-n型异质结 | 农业废水 | 草甘膦(PMG) | 4 | 100 | 60 | 97 | [ | |
| Fe3O4/BiOI | 共沉淀法 | n-p型异质结 | 印染废水 制药废水 | MO 磺胺嘧啶钠盐 (SD-Na) | 1 | 20 | 120 | MO:81 SD-Na:79 | [ | |
| Fe3O4/BiOI | 共沉淀法 | 中空的三维微球、 异质结 | 有机化工废水 印染废水 | BPA RhB | 1 | BPA:20 RhB:— | BPA:90 RhB:180 | BPA:100 RhB:95.1 | [ | |
| 磁性双金属 氧化物和 多铁性质的 氧化物与 BiOX(X=Cl、 Br、I)结合 | LaFeO3/BiOBr | 水热法 | Z型异质结 | 印染废水 | RhB | 1 | 5 | 30 | 98.2 | [ |
| BiOBr/CoFe2O4/GO | 水热法 | Z型异质结 | 印染废水 | RhB | 0.2 | 30 | 120 | 100 | [ | |
| ZnFe2O4/BiOI | 水热法 | Ⅱ型异质结 | 印染废水 | RhB | 0.5 | 10 | 70 | 86.54 | [ | |
| BiOBr/MnFe2O4 | 无表面活性剂 水热合成法 | Z型异质结 | 农业废水 印染废水 | 2,4-D RhB | 1 | 2,4-D:20 RhB:20 | 80 | 2,4-D:89.3 RhB:96.5 | [ | |
| BiFeO3/BiOI | 湿浸渍法 | Ⅱ型异质结 | 印染废水 有机化工废水 | RhB BPA | 1 | 约3.6 | RhB:60 BPA:120 | RhB:约100 BPA:约71 | [ | |
| FeWO4、FeVO4、FeOOH与 BiOX(X=Cl、Br、I) 结合 | BiOBr/FeWO4 | 水热法 | Ⅱ型异质结 | 制药废水 | DOX | 1 | — | 60 | 90.4 | [ |
| α-FeOOH/BiOI | 沉淀法 | Ⅱ型异质结 | 印染废水 | RhB | 0.5 | 20 | 20 | 100 | [ | |
| FeVO4/BiOCl | 湿浸渍法 | Z型异质结 | 印染废水 | RhB | 0.5 | 2 | 360 | 99.8 | [ |
| 材料 | 结构 | 合成方法 | 废水种类 | 降解对象 | 剂量 /g·L-1 | 污染物浓度 /mg·L-1 | 反应时间 /min | 降解率/% | 参考 文献 |
|---|---|---|---|---|---|---|---|---|---|
| 三元 | |||||||||
| BiOI/rGO/Fe3O4 | 类花三维分层 异质结 | 共沉淀法 | 印染废水 | RhB | — | 20 | 180 | 98 | [ |
| GO/Fe3O4/BiOI | 牡丹状 | — | 印染废水 印染废水 | RhB MB | 1 | RhB:约4.8 MB:约3.2 | RhB:80 MB:120 | RhB:99.2 MB:91.4 | [ |
| Fe3O4@BiOI@AgI | 微球状 | 多步合成法 | 印染废水 有机化工废水 | RhB BPA | 0.5 | RhB:5 BPA:20 | 60 | RhB:90 BPA:>70 | [ |
| Fe3O4/BiOBr/BC | 异质结 | 水解法 | 制药废水 | CBZ | 1 | 10 | 180 | 95.51 | [ |
| CoFe2O4/BiOBr/GO | Z型异质结 | 水热法 | 印染废水 | RhB | 0.2 | 30 | 120 | 100 | [ |
| Fe3O4/BiOCl/BiOBr | Z型异质结 | 溶剂热法 | 有机化工废水 | TC | 0.4 | 40 | 90 | 85 | [ |
| BiOBr/BiOI/Fe3O4 | 三维花状、 异质结 | 共沉淀法 | 有机化工废水 | TBBPA | 1 | 40 | 90 | 98.5 | [ |
| Fe3O4/BiOBr/CQDs | Z型异质结 | 光诱导法/水解法 | 制药废水 | CBZ | 0.6 | 10 | 120 | 99.52 | [ |
| Fe3O4/BiOBr@HC | 微球状 | 水热法 | 制药废水 | CBZ | 0.6 | 10 | 40 | 100 | [ |
| BiOBr@SiO2@Fe3O4 | 微球状 | 多步合成法 | 有机化工废水 | BPA | 1 | 20 | 50 | 87 | [ |
| Fe3O4/Bi2S3/BiOBr | 纳米球/ 纳米棒/ 纳米片状、 异质结 | 一锅溶剂热法 | 制药废水 制药废水 | 双氯芬酸 (DCF) 布洛芬 (IBU) | 0.6 0.2 | DCF:10 IBU:10 | DCF:40 IBU:30 | DCF:93.81 IBU:96.78 | [ |
| BiOBr/Fe3O4/RGO | n型肖特基结 | 水热法 | 印染废水 | RhB | 0.45 | 20 | 60 | 96 | [ |
| 四元 | |||||||||
| BiOCl/g-C3N4/ Cu2O/Fe3O4 | p-n-p型异 质结 | 共沉淀法 | 制药废水 | SME | 0.2 | 约25 | Xe灯:60 自然光:120 | Xe灯:99.5 自然光:92.1 | [ |
| Fe3O4@SiO2@Co3O4@BiOCl | 三维花状 | 水热法、油浴、 离子交换法 | 印染废水 | RhB | 0.3077 | 10 | 50 | 98.41 | [ |
| Fe3O4/SiO2/ MnO2/BiOBr-Bi | 三维花状 | 水热法 | 印染废水 | MB | 0.2308 | 10 | 150 | 95.23 | [ |
| 材料 | 结构 | 合成方法 | 废水种类 | 降解对象 | 剂量 /g·L-1 | 污染物浓度 /mg·L-1 | 反应时间 /min | 降解率/% | 参考 文献 |
|---|---|---|---|---|---|---|---|---|---|
| 三元 | |||||||||
| BiOI/rGO/Fe3O4 | 类花三维分层 异质结 | 共沉淀法 | 印染废水 | RhB | — | 20 | 180 | 98 | [ |
| GO/Fe3O4/BiOI | 牡丹状 | — | 印染废水 印染废水 | RhB MB | 1 | RhB:约4.8 MB:约3.2 | RhB:80 MB:120 | RhB:99.2 MB:91.4 | [ |
| Fe3O4@BiOI@AgI | 微球状 | 多步合成法 | 印染废水 有机化工废水 | RhB BPA | 0.5 | RhB:5 BPA:20 | 60 | RhB:90 BPA:>70 | [ |
| Fe3O4/BiOBr/BC | 异质结 | 水解法 | 制药废水 | CBZ | 1 | 10 | 180 | 95.51 | [ |
| CoFe2O4/BiOBr/GO | Z型异质结 | 水热法 | 印染废水 | RhB | 0.2 | 30 | 120 | 100 | [ |
| Fe3O4/BiOCl/BiOBr | Z型异质结 | 溶剂热法 | 有机化工废水 | TC | 0.4 | 40 | 90 | 85 | [ |
| BiOBr/BiOI/Fe3O4 | 三维花状、 异质结 | 共沉淀法 | 有机化工废水 | TBBPA | 1 | 40 | 90 | 98.5 | [ |
| Fe3O4/BiOBr/CQDs | Z型异质结 | 光诱导法/水解法 | 制药废水 | CBZ | 0.6 | 10 | 120 | 99.52 | [ |
| Fe3O4/BiOBr@HC | 微球状 | 水热法 | 制药废水 | CBZ | 0.6 | 10 | 40 | 100 | [ |
| BiOBr@SiO2@Fe3O4 | 微球状 | 多步合成法 | 有机化工废水 | BPA | 1 | 20 | 50 | 87 | [ |
| Fe3O4/Bi2S3/BiOBr | 纳米球/ 纳米棒/ 纳米片状、 异质结 | 一锅溶剂热法 | 制药废水 制药废水 | 双氯芬酸 (DCF) 布洛芬 (IBU) | 0.6 0.2 | DCF:10 IBU:10 | DCF:40 IBU:30 | DCF:93.81 IBU:96.78 | [ |
| BiOBr/Fe3O4/RGO | n型肖特基结 | 水热法 | 印染废水 | RhB | 0.45 | 20 | 60 | 96 | [ |
| 四元 | |||||||||
| BiOCl/g-C3N4/ Cu2O/Fe3O4 | p-n-p型异 质结 | 共沉淀法 | 制药废水 | SME | 0.2 | 约25 | Xe灯:60 自然光:120 | Xe灯:99.5 自然光:92.1 | [ |
| Fe3O4@SiO2@Co3O4@BiOCl | 三维花状 | 水热法、油浴、 离子交换法 | 印染废水 | RhB | 0.3077 | 10 | 50 | 98.41 | [ |
| Fe3O4/SiO2/ MnO2/BiOBr-Bi | 三维花状 | 水热法 | 印染废水 | MB | 0.2308 | 10 | 150 | 95.23 | [ |
| 材料 | 可处理废水种类 | 反应活性物种 | 降解率/% | 降解时长 | 材料成本 | 循环次数 |
|---|---|---|---|---|---|---|
| 卤氧化铋 BiOX(X=Cl、Br、I) | 印染废水 制药废水 | h+、∙O | 50~90 | 1~6h | 0.8~14.0CNY/g | 重复利用性差, 几乎无法循环 |
| 二元铁基复合卤 氧化铋磁性材料 | 印染废水 制药废水 有机化工废水 农业废水 | h+、∙O | 70~100 | 15min~6h | 根据复合材料种类决定 | 3~6次 超顺磁性:20次 |
| 多元铁基复合卤 氧化铋磁性材料 | 印染废水 制药废水 有机化工废水 | h+、∙O | 70~100 | 40min~3h | 根据复合材料种类决定 | 3~5次 |
| 材料 | 可处理废水种类 | 反应活性物种 | 降解率/% | 降解时长 | 材料成本 | 循环次数 |
|---|---|---|---|---|---|---|
| 卤氧化铋 BiOX(X=Cl、Br、I) | 印染废水 制药废水 | h+、∙O | 50~90 | 1~6h | 0.8~14.0CNY/g | 重复利用性差, 几乎无法循环 |
| 二元铁基复合卤 氧化铋磁性材料 | 印染废水 制药废水 有机化工废水 农业废水 | h+、∙O | 70~100 | 15min~6h | 根据复合材料种类决定 | 3~6次 超顺磁性:20次 |
| 多元铁基复合卤 氧化铋磁性材料 | 印染废水 制药废水 有机化工废水 | h+、∙O | 70~100 | 40min~3h | 根据复合材料种类决定 | 3~5次 |
| 1 | 唐乾, 段东霞, 张显峰, 等. 废水电芬顿处理技术研究进展[J]. 四川环境, 2023, 42(2): 305-311. |
| TANG Qian, DUAN Dongxia, ZHANG Xianfeng, et al. Recent research progress on electro-Fenton process for wastewater treatment[J]. Sichuan Environment, 2023, 42(2): 305-311. | |
| 2 | 王锐, 刘宪华, 王勇, 等. Fenton氧化处理难降解有机废水研究进展[J]. 工业水处理, 2022, 42(5): 58-66. |
| WANG Rui, LIU Xianhua, WANG Yong, et al. Research progress of Fenton oxidation treatment refractory organic wastewater[J]. Industrial Water Treatment, 2022, 42(5): 58-66. | |
| 3 | 于佳. 某工业园区污水有效处理技术研究[J]. 能源与环保, 2023, 45(1): 228-233. |
| YU Jia. Research on effective treatment technology of sewage in an industrial park[J]. China Energy and Environmental Protection, 2023, 45(1): 228-233. | |
| 4 | 王朱良, 曾泽亭, 杨杰, 等. 高级氧化技术处理染料废水研究进展[J]. 日用化学品科学, 2023, 46(7): 57-60. |
| WANG Zhuliang, ZENG Zeting, YANG Jie, et al. Research progress of advanced oxidation processes for treatment of dye wastewater[J]. Detergent & Cosmetics, 2023, 46(7): 57-60. | |
| 5 | OTURAN Mehmet A, AARON Jean-Jacques. Advanced oxidation processes in water/wastewater treatment: Principles and applications. A review[J]. Critical Reviews in Environmental Science and Technology, 2014, 44(23): 2577-2641. |
| 6 | SARAVANAN A, DEIVAYANAI V C, Senthil KUMAR P, et al. A detailed review on advanced oxidation process in treatment of wastewater: Mechanism, challenges and future outlook[J]. Chemosphere, 2022, 308: 136524. |
| 7 | KRISHNAN Radhakrishnan Yedhu, MANIKANDAN Sivasubramanian, SUBBAIYA Ramasamy, et al. Removal of emerging micropollutants originating from pharmaceuticals and personal care products (PPCPs) in water and wastewater by advanced oxidation processes: A review[J]. Environmental Technology & Innovation, 2021, 23: 101757. |
| 8 | 赵明, 王俊萍, 武慧敏, 等. 掺杂ZnO应用领域与表征方法研究进展[J]. 中国无机分析化学, 2022, 12(3): 75-80. |
| ZHAO Ming, WANG Junping, WU Huimin, et al. Review of application and characterization method of doped ZnO[J]. Chinese Journal of Inorganic Analytical Chemistry, 2022, 12(3): 75-80. | |
| 9 | CUI Xiaofeng, WANG Yajun, JIANG Guiyuan, et al. The encapsulation of CdS in carbon nanotubes for stable and efficient photocatalysis[J]. Journal of Materials Chemistry A, 2014, 2(48): 20939-20946. |
| 10 | LI Zhe, YAN Qinghua, JIANG Qian, et al. Oxygen vacancy mediated Cu y Co3- y Fe1O x mixed oxide as highly active and stable toluene oxidation catalyst by multiple phase interfaces formation and metal doping effect[J]. Applied Catalysis B: Environmental, 2020, 269: 118827. |
| 11 | CHAKHTOUNA Hanane, BENZEID Hanane, ZARI Nadia, et al. Recent progress on Ag/TiO2 photocatalysts: Photocatalytic and bactericidal behaviors[J]. Environmental Science and Pollution Research International, 2021, 28(33): 44638-44666. |
| 12 | GAO Peng, YANG Yuning, YIN Ze, et al. A critical review on bismuth oxyhalide based photocatalysis for pharmaceutical active compounds degradation: Modifications, reactive sites, and challenges[J]. Journal of Hazardous Materials, 2021, 412: 125186. |
| 13 | KANDAVELU V, KASTIEN H, Ravindranathan THAMPI K. Photocatalytic degradation of isothiazolin-3-ones in water and emulsion paints containing nanocrystalline TiO2 and ZnO catalysts[J]. Applied Catalysis B: Environmental, 2004, 48(2): 101-111. |
| 14 | WANG Mengye, CAI Lejuan, WANG Yi, et al. Graphene-draped semiconductors for enhanced photocorrosion resistance and photocatalytic properties[J]. Journal of the American Chemical Society, 2017, 139(11): 4144-4151. |
| 15 | Julius KNÖPPEL, ZHANG Siyuan, SPECK Florian D, et al. Time-resolved analysis of dissolution phenomena in photoelectrochemistry—A case study of WO3 photocorrosion[J]. Electrochemistry Communications, 2018, 96: 53-56. |
| 16 | MA Mingliang, CHEN Yan, TONG Zhouyu, et al. Research progress of magnetic bismuth-based materials in photocatalysis: A review[J]. Journal of Alloys and Compounds, 2021, 886: 161096. |
| 17 | 张喜. 新型卤化氧铋BiOX(X=Cl、Br、I)光催化剂的合成、表征及催化性能研究[D]. 武汉: 华中师范大学, 2010. |
| ZHANG Xi. Synthesis, characterization and photocatalytic activity of BiOX (X=Cl, Br and I) photocatalysts[D]. Wuhan: Central China Normal University, 2010. | |
| 18 | 胡泽书. 铋基复合氧化物的制备及对有机废水的光催化性能研究[D]. 武汉: 武汉理工大学, 2019. |
| HU Zeshu. Preparation of ruthenium-based composite oxides and their photocatalytic properties for organic wastewater[D]. Wuhan: Wuhan University of Technology, 2019. | |
| 19 | 陈庆国. 一维铋基氧化物、卤氧化物异质结的合成及其光催化性能研究[D]. 芜湖: 安徽师范大学, 2015. |
| CHEN Qingguo. Synthesis and the photocatalytic performances of one-dimensional bismuth based oxide, halogen oxide heterojunction[D]. Wuhu: Anhui Normal University, 2015. | |
| 20 | ZHANG Kelei, LIU Cunming, HUANG Fuqiang, et al. Study of the electronic structure and photocatalytic activity of the BiOCl photocatalyst[J]. Applied Catalysis B: Environmental, 2006, 68(3/4): 125-129. |
| 21 | RAN Zhao, WANG Xinjiang, LI Yuwei, et al. Bismuth and antimony-based oxyhalides and chalcohalides as potential optoelectronic materials[J]. NPJ Computational Materials, 2018, 4: 14. |
| 22 | WANG Ziwei, CHEN Ming, HUANG Danlian, et al. Multiply structural optimized strategies for bismuth oxyhalide photocatalysis and their environmental application[J]. Chemical Engineering Journal, 2019, 374: 1025-1045. |
| 23 | ZHAO Lijun, ZHANG Xiaochao, FAN Caimei, et al. First-principles study on the structural, electronic and optical properties of BiOX (X=Cl, Br, I) crystals[J]. Physica B: Condensed Matter, 2012, 407(17): 3364-3370. |
| 24 | ZHANG Xi, AI Zhihui, JIA Falong, et al. Generalized one-pot synthesis, characterization, and photocatalytic activity of hierarchical BiOX (X=Cl, Br, I) nanoplate microspheres[J]. The Journal of Physical Chemistry C, 2008, 112(3): 747-753. |
| 25 | YANG Yang, ZHANG Chen, LAI Cui, et al. BiOX (X=Cl, Br, I) photocatalytic nanomaterials: Applications for fuels and environmental management[J]. Advances in Colloid and Interface Science, 2018, 254: 76-93. |
| 26 | 刘家琴, 吴玉程. 基于BiOX(X=Cl、Br、I)新型高性能光催化材料的最新研究进展[J]. 无机材料学报, 2015, 30(10): 1009-1017. |
| LIU Jiaqin, WU Yucheng. Recent advances in the high performance BiOX (X=Cl, Br, I) based photo-catalysts[J]. Journal of Inorganic Materials, 2015, 30(10): 1009-1017. | |
| 27 | YAO Ling, YANG Hui, CHEN Zhongshan, et al. Bismuth oxychloride-based materials for the removal of organic pollutants in wastewater[J]. Chemosphere, 2021, 273: 128576. |
| 28 | SUDARSANAM Putla, ZHONG Ruyi, VAN DEN BOSCH Sander, et al. Functionalised heterogeneous catalysts for sustainable biomass valorisation[J]. Chemical Society Reviews, 2018, 47(22): 8349-8402. |
| 29 | WU Wei, JIANG Changzhong, ROY Vellaisamy A L. Recent progress in magnetic iron oxide-semiconductor composite nanomaterials as promising photocatalysts[J]. Nanoscale, 2015, 7(1): 38-58. |
| 30 | ZHAO Guoqing, ZOU Jiao, CHEN Xiaoqing, et al. Iron-based catalysts for persulfate-based advanced oxidation process: Microstructure, property and tailoring[J]. Chemical Engineering Journal, 2021, 421: 127845. |
| 31 | LUO Hongwei, ZENG Yifeng, HE Dongqin, et al. Application of iron-based materials in heterogeneous advanced oxidation processes for wastewater treatment: A review[J]. Chemical Engineering Journal, 2021, 407: 127191. |
| 32 | LIU Yong, LI Jia, LI Jiayao, et al. Active magnetic Fe3+-doped BiOBr micromotors as efficient solar photo-Fenton catalyst[J]. Journal of Cleaner Production, 2020, 252: 119573. |
| 33 | LIU Yang, HU Zhuofeng, YU Jimmy C. Fe enhanced visible-light-driven nitrogen fixation on BiOBr nanosheets[J]. Chemistry of Materials, 2020, 32(4): 1488-1494. |
| 34 | MI Yan, WEN Liaoyong, WANG Zhijie, et al. Fe(Ⅲ) modified BiOCl ultrathin nanosheet towards high-efficient visible-light photocatalyst[J]. Nano Energy, 2016, 30: 109-117. |
| 35 | AI Lunhong, ZHANG Caihong, LI Lili, et al. Iron terephthalate metal-organic framework: Revealing the effective activation of hydrogen peroxide for the degradation of organic dye under visible light irradiation[J]. Applied Catalysis B: Environmental, 2014, 148/149: 191-200. |
| 36 | Huanli LYU, ZHAO Hongying, CAO Tongcheng, et al. Efficient degradation of high concentration azo-dye wastewater by heterogeneous Fenton process with iron-based metal-organic framework[J]. Journal of Molecular Catalysis A: Chemical, 2015, 400: 81-89. |
| 37 | ZHANG Caihong, AI Lunhong, JIANG Jing. Solvothermal synthesis of MIL-53(Fe) hybrid magnetic composites for photoelectrochemical water oxidation and organic pollutant photodegradation under visible light[J]. Journal of Materials Chemistry A, 2015, 3(6): 3074-3081. |
| 38 | LAURIER Katrien G M, VERMOORTELE Frederik, AMELOOT Rob, et al. Iron(Ⅲ)-based metal-organic frameworks as visible light photocatalysts[J]. Journal of the American Chemical Society, 2013, 135(39): 14488-14491. |
| 39 | WU Qiangshun, YANG Hanpei, KANG Li, et al. Fe-based metal-organic frameworks as Fenton-like catalysts for highly efficient degradation of tetracycline hydrochloride over a wide pH range: Acceleration of Fe(Ⅱ)/Fe(Ⅲ) cycle under visible light irradiation[J]. Applied Catalysis B: Environmental, 2020, 263: 118282. |
| 40 | ZHONG Xin, ZHANG Kaixin, WU Di, et al. Enhanced photocatalytic degradation of levofloxacin by Fe-doped BiOCl nanosheets under LED light irradiation[J]. Chemical Engineering Journal, 2020, 383: 123148. |
| 41 | RAMESHBABU R, PECCHI Gina, DELGADO Eduardo J, et al. BiOCl ultrathin nanosheets modified with Fe3+ for enhanced visible light driven photocatalytic activity[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2021, 411: 113211. |
| 42 | MA Yuhan, LI Mingyu, JIANG Jingjing, et al. In-situ prepared MIL-53(Fe)/BiOI photocatalyst for efficient degradation of tetracycline under visible-light driven photo-Fenton system: Investigation of performance and mechanism[J]. Journal of Alloys and Compounds, 2021, 870: 159524. |
| 43 | SHI Yunxin, WANG Liying, MIAO Xin, et al. In situ synthesis of donut-like Fe-doped-BiOCl@Fe-MOF composites using for excellent performance photodegradation of dyes and tetracycline[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2023, 442: 114704. |
| 44 | YANG Xiaoli, SUN Shaodong, YE Lin, et al. One-pot integration of S-doped BiOCl and ZnO into type-Ⅱ photocatalysts: Simultaneously boosting bulk and surface charge separation for enhanced antibiotic removal[J]. Separation and Purification Technology, 2022, 299: 121725. |
| 45 | HAO Lin, HUANG Hongwei, ZHANG Yihe, et al. Oxygen vacant semiconductor photocatalysts[J]. Advanced Functional Materials, 2021, 31(25): 2100919. |
| 46 | LI Hao, SHANG Jian, YANG Zhiping, et al. Oxygen vacancy associated surface Fenton chemistry: Surface structure dependent hydroxyl radicals generation and substrate dependent reactivity[J]. Environmental Science & Technology, 2017, 51(10): 5685-5694. |
| 47 | AN Weijia, HU Shining, YANG Tao, et al. Oxygen vacancies enhance Fe-doped BiOCl photocatalysis-Fenton synergy degradation of phenol[J]. Materials Letters, 2022, 322: 132466. |
| 48 | ZHANG Gaoke, GAO Yuanyuan, ZHANG Yalei, et al. Fe2O3-pillared rectorite as an efficient and stable Fenton-like heterogeneous catalyst for photodegradation of organic contaminants[J]. Environmental Science & Technology, 2010, 44(16): 6384-6389. |
| 49 | GUO Yadan, ZHANG Gaoke, LIU Jin, et al. Hierarchically structured α-Fe2O3/Bi2WO6 composite for photocatalytic degradation of organic contaminants under visible light irradiation[J]. RSC Advances, 2013, 3(9): 2963-2970. |
| 50 | LI Kai, LIANG Yujun, YANG Jian, et al. Controllable synthesis of{001} facet dependent foursquare BiOCl nanosheets: A high efficiency photocatalyst for degradation of methyl orange[J]. Journal of Alloys and Compounds, 2017, 695: 238-249. |
| 51 | BAGHERI Samira, JULKAPLI Nurhidayatullaili Muhd. Magnetite hybrid photocatalysis: Advance environmental remediation[J]. Reviews in Inorganic Chemistry, 2016, 36(3): 1-16. |
| 52 | Maryam SHEKOFTEH-GOHARI, Aziz HABIBI-YANGJEH. Fabrication of novel magnetically separable visible-light-driven photocatalysts through photosensitization of Fe3O4/ZnO with CuWO4 [J]. Journal of Industrial and Engineering Chemistry, 2016, 44: 174-184. |
| 53 | AKHUNDI Anise, Aziz HABIBI-YANGJEH. Facile preparation of novel quaternary g-C3N4/Fe3O4/AgI/Bi2S3 nanocomposites: Magnetically separable visible-light-driven photocatalysts with significantly enhanced activity[J]. RSC Advances, 2016, 6(108): 106572-106583. |
| 54 | GAO Shengwang, GUO Changsheng, Jiapei LYU, et al. A novel 3D hollow magnetic Fe3O4/BiOI heterojunction with enhanced photocatalytic performance for bisphenol A degradation[J]. Chemical Engineering Journal, 2017, 307: 1055-1065. |
| 55 | HUNGE Y M, YADAV A A, KHAN Sovann, et al. Photocatalytic degradation of bisphenol A using titanium dioxide@nanodiamond composites under UV light illumination[J]. Journal of Colloid and Interface Science, 2021, 582: 1058-1066. |
| 56 | WANG Wei, HE Mingyi, ZHANG Huan, et al. Facile synthesis of flower-like superparamagnetic Fe3O4/BiOCl nanocomposites as high effective magnetic recyclable photocatalyst under visible light[J]. Journal of Magnetics, 2016, 21(2): 179-182. |
| 57 | RUSEVOVA Klara, Roberto KÖFERSTEIN, Mònica ROSELL, et al. LaFeO3 and BiFeO3 perovskites as nanocatalysts for contaminant degradation in heterogeneous Fenton-like reactions[J]. Chemical Engineering Journal, 2014, 239: 322-331. |
| 58 | Jin-Chung SIN, Sze-Mun LAM, ZENG Honghu, et al. Z-scheme heterojunction nanocomposite fabricated by decorating magnetic MnFe2O4 nanoparticles on BiOBr nanosheets for enhanced visible light photocatalytic degradation of 2,4-dichlorophenoxyacetic acid and rhodamine B[J]. Separation and Purification Technology, 2020, 250: 117186. |
| 59 | DONG Pengyu, HOU Guihua, XI Xinguo, et al. WO3-based photocatalysts: Morphology control, activity enhancement and multifunctional applications[J]. Environmental Science: Nano, 2017, 4(3): 539-557. |
| 60 | ZHOU Yawen, FANG Shanshan, ZHOU Man, et al. Fabrication of novel ZnFe2O4/BiOI nanocomposites and its efficient photocatalytic activity under visible-light irradiation[J]. Journal of Alloys and Compounds, 2017, 696: 353-361. |
| 61 | GAO Jiacong, GAO Yushu, SUI Zhenying, et al. Hydrothermal synthesis of BiOBr/FeWO4 composite photocatalysts and their photocatalytic degradation of doxycycline[J]. Journal of Alloys and Compounds, 2018, 732: 43-51. |
| 62 | JIANG Saihua, ZHOU Keqing, SHI Yongqian, et al. In situ synthesis of hierarchical flower-like Bi2S3/BiOCl composite with enhanced visible light photocatalytic activity[J]. Applied Surface Science, 2014, 290: 313-319. |
| 63 | SAMANTA Subhajyoti, SRIVASTAVA Rajendra. Thermal catalysis vs. photocatalysis: A case study with FeVO4/g-C3N4 nanocomposites for the efficient activation of aromatic and benzylic C—H bonds to oxygenated products[J]. Applied Catalysis B: Environmental, 2017, 218: 621-636. |
| 64 | CHACHVALVUTIKUL Auttaphon, KAOWPHONG Sulawan. Direct Z-scheme FeVO4/BiOCl heterojunction as a highly efficient visible-light-driven photocatalyst for photocatalytic dye degradation and Cr(Ⅵ) reduction[J]. Nanotechnology, 2020, 31(14): 145704. |
| 65 | PAN Zihan, WEI Yan, WANG Qinyu, et al. A highly efficient visible light driven Fenton catalyst α-FeOOH/BiOI to degrade RhB[J]. Journal of Environmental Chemical Engineering, 2021, 9(6): 106627. |
| 66 | CHEN Hongche, WANG Xiaoning, BI Wenlong, et al. Photodegradation of carbamazepine with BiOCl/Fe3O4 catalyst under simulated solar light irradiation[J]. Journal of Colloid and Interface Science, 2017, 502: 89-99. |
| 67 | SHI Yahong, CHEN Hongche, WU Yanlin, et al. Degradation of atenolol via heterogeneous activation of persulfate by using BiOCl@Fe3O4 catalyst under simulated solar light irradiation[J]. Environmental Science and Pollution Research International, 2018, 25(1): 693-703. |
| 68 | JIANG Ru, ZHU Huayue, JIANG Shentao, et al. Magnetically separable Fe3O4/BiOBr microspheres: Synthesis, characterization, and photocatalytic performance for removal of anionic azo dye[J]. Environmental Engineering Science, 2019, 36(4): 466-477. |
| 69 | CAO Lidong, MA Dukang, ZHOU Zhaolu, et al. Efficient photocatalytic degradation of herbicide glyphosate in water by magnetically separable and recyclable BiOBr/Fe3O4 nanocomposites under visible light irradiation[J]. Chemical Engineering Journal, 2019, 368: 212-222. |
| 70 | XIE Xin, LIU Yonggang, DONG Xiaoxia, et al. Synthesis and characterization of Fe3O4/BiOI n-p heterojunction magnetic photocatalysts[J]. Applied Surface Science, 2018, 455: 742-747. |
| 71 | GUAN Sitao, YANG Hua, SUN Xiaofeng, et al. Preparation and promising application of novel LaFeO3/BiOBr heterojunction photocatalysts for photocatalytic and photo-Fenton removal of dyes[J]. Optical Materials, 2020, 100: 109644. |
| 72 | LI Meirun, SONG Chi, WU Ying, et al. Novel Z-scheme visible-light photocatalyst based on CoFe2O4/BiOBr/graphene composites for organic dye degradation and Cr(Ⅵ) reduction[J]. Applied Surface Science, 2019, 478: 744-753. |
| 73 | MALATHI A, ARUNACHALAM Prabhakarn, KIRANKUMAR V S, et al. An efficient visible light driven bismuth ferrite incorporated bismuth oxyiodide (BiFeO3/BiOI) composite photocatalytic material for degradation of pollutants[J]. Optical Materials, 2018, 84: 227-235. |
| 74 | LI Houfen, YU Hongtao, QUAN Xie, et al. Uncovering the key role of the Fermi level of the electron mediator in a Z-scheme photocatalyst by detecting the charge transfer process of WO 3 - metal-gC3N4 (metal=Cu, Ag, Au)[J]. ACS Applied Materials & Interfaces, 2016, 8(3): 2111-2119. |
| 75 | KAGESHIMA Yosuke, GOMYO Yui, MATSUOKA Hikaru, et al. Z-scheme overall water splitting using Zn x Cd1– x Se particles coated with metal cyanoferrates as hydrogen evolution photocatalysts[J]. ACS Catalysis, 2021, 11(13): 8004-8014. |
| 76 | WANG Yuanyuan, WEI Yan, SONG Wenjing, et al. Photocatalytic hydrodehalogenation for the removal of halogenated aromatic contaminants[J]. ChemCatChem, 2019, 11(1): 258-268. |
| 77 | LI Shan, MA Qipu, CHEN Lei, et al. Hydrochar-mediated photocatalyst Fe3O4/BiOBr@HC for highly efficient carbamazepine degradation under visible LED light irradiation[J]. Chemical Engineering Journal, 2022, 433: 134492. |
| 78 | LI Shan, WANG Zhaowei, ZHAO Xiating, et al. Insight into enhanced carbamazepine photodegradation over biochar-based magnetic photocatalyst Fe3O4/BiOBr/BC under visible LED light irradiation[J]. Chemical Engineering Journal, 2019, 360: 600-611. |
| 79 | XIE Xiaoyun, LI Shan, QI Kemin, et al. Photoinduced synthesis of green photocatalyst Fe3O4/BiOBr/CQDs derived from corncob biomass for carbamazepine degradation: The role of selectively more CQDs decoration and Z-scheme structure[J]. Chemical Engineering Journal, 2021, 420: 129705. |
| 80 | WANG Jing, GAO Minmin, Ghim Wei HO. Bidentate-complex-derived TiO2/carbon dot photocatalysts: In situ synthesis, versatile heterostructures, and enhanced H2 evolution[J]. Journal of Materials Chemistry A, 2014, 2(16): 5703-5709. |
| 81 | MIAO Ran, LUO Zhu, ZHONG Wei, et al. Mesoporous TiO2 modified with carbon quantum dots as a high-performance visible light photocatalyst[J]. Applied Catalysis B: Environmental, 2016, 189: 26-38. |
| 82 | LIANG Zhao, HOU Huilin, FANG Zhi, et al. Hydrogenated TiO2 nanorod arrays decorated with carbon quantum dots toward efficient photoelectrochemical water splitting[J]. ACS Applied Materials & Interfaces, 2019, 11(21): 19167-19175. |
| 83 | TYAGI Himanshi, CHAWLA Harshita, BHANDARI Hema, et al. Recent-enhancements in visible-light photocatalytic degradation of organochlorines pesticides: A review[J]. Materials Today: Proceedings, 2022, 49: 3289-3305. |
| 84 | KUMAR Ajay, KUMAR Amit, SHARMA Gaurav, et al. Quaternary magnetic BiOCl/g-C3N4/Cu2O/Fe3O4 nano-junction for visible light and solar powered degradation of sulfamethoxazole from aqueous environment[J]. Chemical Engineering Journal, 2018, 334: 462-478. |
| 85 | CHEN Yan, SU Xuewei, MA Mingliang, et al. Constructing 3D magnetic flower-like Fe3O4@SiO2@Co3O4@BiOCl heterojunction photocatalyst for degrading rhodamine B[J]. Environmental Science and Pollution Research International, 2022, 29(58): 87310-87318. |
| 86 | LI Tian bao, CHEN Gang, ZHOU Chao, et al. New photocatalyst BiOCl/BiOI composites with highly enhanced visible light photocatalytic performances[J]. Dalton Transactions, 2011, 40(25): 6751-6758. |
| 87 | DONG Fan, SUN Yanjuan, FU Min, et al. Room temperature synthesis and highly enhanced visible light photocatalytic activity of porous BiOI/BiOCl composites nanoplates microflowers[J]. Journal of Hazardous Materials, 2012, 219/220: 26-34. |
| 88 | XIAO Xin, HAO Rong, LIANG Min, et al. One-pot solvothermal synthesis of three-dimensional (3D) BiOI/BiOCl composites with enhanced visible-light photocatalytic activities for the degradation of bisphenol-A[J]. Journal of Hazardous Materials, 2012, 233/234: 122-130. |
| 89 | LIN Li, HUANG Manhong, LONG Liping, et al. Fabrication of a three-dimensional BiOBr/BiOI photocatalyst with enhanced visible light photocatalytic performance[J]. Ceramics International, 2014, 40(8): 11493-11501. |
| 90 | CAO Jing, XU Benyan, LUO Bangde, et al. Novel BiOI/BiOBr heterojunction photocatalysts with enhanced visible light photocatalytic properties[J]. Catalysis Communications, 2011, 13(1): 63-68. |
| 91 | GAO Shengwang, GUO Changsheng, HOU Song, et al. Photocatalytic removal of tetrabromobisphenol A by magnetically separable flower-like BiOBr/BiOI/Fe3O4 hybrid nanocomposites under visible-light irradiation[J]. Journal of Hazardous Materials, 2017, 331: 1-12. |
| 92 | PREMALATHA N, MIRANDA Lima Rose. A magnetic separable 3D hierarchical BiOI/rGO/Fe3O4 catalyst for degradation of rhodamine B under visible light: Kinetic studies and mechanism of degradation[J]. Materials Science and Engineering: B, 2022, 276: 115576. |
| 93 | XIA Yamu, ZHANG Jiahong, XIA Meng, et al. Peony-like magnetic graphene oxide/Fe3O4/BiOI nanoflower as a novel photocatalyst for enhanced photocatalytic degradation of rhodamine B and methylene blue dyes[J]. Journal of Materials Science: Materials in Electronics, 2020, 31(3): 1996-2009. |
| 94 | LIANG Jialiang, LIU Fuyang, LI Mian, et al. Facile synthesis of magnetic Fe3O4@BiOI@AgI for water decontamination with visible light irradiation: Different mechanisms for different organic pollutants degradation and bacterial disinfection[J]. Water Research, 2018, 137: 120-129. |
| 95 | DANG Jingjing, ZHANG Jianxu, SHEN Yun, et al. Fabrication of magnetically recyclable Fe3O4/BiOCl/BiOBr nanocomposite with Z-scheme heterojunction for high-efficiency photocatalytic degradation of tetracycline[J]. Materials Science in Semiconductor Processing, 2023, 158: 107371. |
| 96 | ZHANG Ling, WANG Wenzhong, SUN Songmei, et al. Elimination of BPA endocrine disruptor by magnetic BiOBr@SiO2@Fe3O4 photocatalyst[J]. Applied Catalysis B: Environmental, 2014, 148/149: 164-169. |
| 97 | LI Shan, WANG Zirun, ZHANG Xiaoli, et al. Preparation of magnetic nanosphere/nanorod/nanosheet-like Fe3O4/Bi2S3/BiOBr with enhanced (001) and (110) facets to photodegrade diclofenac and ibuprofen under visible LED light irradiation[J]. Chemical Engineering Journal, 2019, 378: 122169. |
| 98 | ZHENG Mingkun, MA Xinguo, HU Jisong, et al. Novel recyclable BiOBr/Fe3O4/RGO composites with remarkable visible-light photocatalytic activity[J]. RSC Advances, 2020, 10(34): 19961-19973. |
| 99 | MA Mingliang, YANG Yuying, CHEN Yan, et al. Photocatalytic degradation of MB dye by the magnetically separable 3D flower-like Fe3O4/SiO2/MnO2/BiOBr-Bi photocatalyst[J]. Journal of Alloys and Compounds, 2021, 861: 158256. |
| 100 | 王俊清. BiOX(X=Br, I)光催化材料的制备及其光催化性能研究[D]. 沈阳: 沈阳师范大学, 2023. |
| WANG Junqing. Preparation and photocatalytic performance of BiOX (X=Br, I) photocatalytic materials[D]. Shenyang: Shenyang Normal University, 2023. | |
| 101 | 赵立业, 李恒, 王亮, 等. 卤素原子对卤氧化铋(BiOX, X=Cl、Br、I)光催化性能的影响[J]. 燃料化学学报, 2022, 50(1): 122-128. |
| ZHAO Liye, LI Heng, WANG Liang, et al. Effect of halogen atoms on photocatalytic activity of bismuth oxyhalide (BIOX, X=Cl, Br, I)[J]. Journal of Fuel Chemistry and Technology, 2022, 50(1): 122-128. |
| [1] | WANG Shuyuan, YIN Lingling, GAO Zhihua, HUANG Wei. Effect of intercalated Cu proportion on the structure and catalytic performance of CuZnAl-LDHs catalysts [J]. Chemical Industry and Engineering Progress, 2025, 44(4): 2036-2044. |
| [2] | ZHANG Pei, GAO Lining, DING Siqing, LI Li, ZHU Xiruo, HE Rui. Preparation of g-C3N4/TiO2 heterojunction catalyst and its photocatalytic NO degradation performance [J]. Chemical Industry and Engineering Progress, 2025, 44(4): 2045-2056. |
| [3] | NIU Jingwei, CHEN Xiaoyang, ZHANG Jian, ZHOU Yuzhi, CHEN Min. Activated persulfate-induced degradation of typical environmental endocrine disruptors in soil [J]. Chemical Industry and Engineering Progress, 2025, 44(4): 2285-2296. |
| [4] | CHEN Yuhang, LI Qiaoyan, LIANG Meisheng, SONG Tianyuan, WANG Yue, LI Simeng, ZHOU Yuxuan. Role of the Sn dopant on Cu/CeZrO2/γ-Al2O3 three-way catalyst: Enhancement of low-temperature activity and sulfur resistance [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1368-1377. |
| [5] | ZHANG Xin’er, PEI Liujun, ZHOU Yudie, JIN Kaili, WANG Jiping. Progress of TiO2-based photocatalysts for hydrogen production by water splitting with solar energy [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1298-1308. |
| [6] | LIU Junjie, WU Jianmin, SUN Qiwen, WANG Jiancheng, SUN Yan. Research of metallocene catalysts for linear α-olefins polymerization to obtain high molecular weight products [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1309-1322. |
| [7] | ZHU Guoyu, GE Qi, FU Mingli. Durability testing and life prediction of methanol reforming catalysts for hydrogen production [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1338-1346. |
| [8] | XIE Xinyao, WAN Fen, FU Xuanyu, FAN Yuting, CHEN Lingxiu, LI Peng. Catalytic performance and mechanism of CO2 electroreduction of Cu-Ag nanoclusters [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1387-1395. |
| [9] | ZUO Ji, LUO Li, XIE Yongkai, CHEN Wenyao, QIAN Gang, ZHOU Xinggui, DUAN Xuezhi. Effect of Cu catalyst particle size on methanol nonoxidative dehydrogenation to formaldehyde [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1347-1354. |
| [10] | BI Wentao, WANG Xuelin, QU Wei, WANG Congxin, TIAN Zhijian. Effect of Mg-modification on the catalytic performance of Pt/ZSM-22 with low Pt content in n-alkane hydroisomerization [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1355-1367. |
| [11] | ZHANG Maorun, SUN Weiru, MA Tianlin, XIN Zhiling. Anti-SO2 poisoning performance of Mo-modified MnCe/SiC in low-temperature SCR denitrification [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1378-1386. |
| [12] | ZHANG Qi, WANG Tao, ZHANG Xuebing, LI Weizhen, CHENG Meng, ZHANG Kui, LYU Yijun, MEN Zhuowu. Advances in Fe-based catalysts for conversion of syngas/CO2 to higher alcohols [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1323-1337. |
| [13] | SHAN Xueying, LI Lingyu, ZHANG Meng, ZHANG Jiafu, LI Jinchun. Preparation and properties of flame retardant epoxy resin/low molecular weight polyphenylene ether materials [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1533-1541. |
| [14] | TAO Jinquan, JIA Yijing, BAI Tianyu, YAO Rongpeng, HUANG Wenbin, CUI Yan, ZHOU Yasong, WEI Qiang. Synthesis and catalytic MTP performance of Silicalite-1 zeolite with low cost [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1550-1558. |
| [15] | LIU Jiangtao, PENG Chong, ZHANG Yongchun. Low-carbon olefins from CO2 hydrogenation over Zn-modulated Fe-based catalysts [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1396-1405. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||