Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (2): 1042-1052.DOI: 10.16085/j.issn.1000-6613.2024-0123
• Resources and environmental engineering • Previous Articles
Research progress and prospect on electrowinning recovery of lead from spent lead paste
LI Mingyang1( ), LIANG Jiangbei2, LIANG Sha2, XIE Weimin1(
), LIANG Jiangbei2, LIANG Sha2, XIE Weimin1( ), YANG Jiakuan2
), YANG Jiakuan2
- 1.Changjiang Basin Ecology and Environment Monitoring and Scientific Research Center, Changjiang Basin Ecology and Environment Administration, Ministry of Ecology and Environment, Wuhan 430010, Hubei, China
2.School of Environmental Science & Engineering, Huazhong University of Science and Technology, Wuhan 430074, Hubei, China
-
Received:2024-01-16Revised:2024-03-25Online:2025-03-10Published:2025-02-25 -
Contact:XIE Weimin
废铅膏电沉积回收铅技术研究进展与展望
李名扬1( ), 梁江北2, 梁莎2, 谢卫民1(
), 梁江北2, 梁莎2, 谢卫民1( ), 杨家宽2
), 杨家宽2
- 1.生态环境部长江流域生态环境监督管理局生态环境监测与科学研究中心,湖北 武汉 430010
2.华中科技大学环境科学与工程学院,湖北 武汉 430074
-
通讯作者:谢卫民 -
作者简介:李名扬(1991—),男,博士,高级工程师,研究方向为固废资源化。E-mail:limingyang0418@163.com。 -
基金资助:国家自然科学基金(52100153)
CLC Number:
Cite this article
LI Mingyang, LIANG Jiangbei, LIANG Sha, XIE Weimin, YANG Jiakuan. Research progress and prospect on electrowinning recovery of lead from spent lead paste[J]. Chemical Industry and Engineering Progress, 2025, 44(2): 1042-1052.
李名扬, 梁江北, 梁莎, 谢卫民, 杨家宽. 废铅膏电沉积回收铅技术研究进展与展望[J]. 化工进展, 2025, 44(2): 1042-1052.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-0123
| 方法 | 典型工艺 | 浸出溶液/电解液 | 阳极 | 阴极 | 添加剂 | 铅纯度/% | 铅回收率 /% | 电解能耗 /kWh·(t Pb)-1 | 电流 效率/% | 参考 文献 |
|---|---|---|---|---|---|---|---|---|---|---|
| 酸浸出- 电沉积工艺 | RSR工艺 | H2SiF6、HBF4 | 石墨基非导电 惰性网状材料, 表面沉积PbO2 | 铅 | 500mg/L 砷离子 | >99.99 | 96 | [ | ||
| USBM工艺 | H2SiF6 | 二氧化铅包覆的钛材料 | 铅 | 磷酸盐、动物骨胶、木质素磺酸盐 | 99.995~ 99.999 | 92 | 800 | 95~97 | [ | |
| CX-EW工艺(Engitec工艺) | HBF4 | 特殊设计的复合阳极材料 | 骨胶 | 800 | [ | |||||
| Placid工艺 | HCl-NaCl | 99.995 | 99.5 | 1300 | [ | |||||
| HClO4 | 石墨板 | 铜板 | 1.0g/L骨胶+1.0g/L木质素磺酸钠 | 99.9991 | 98.5 | 492 | >97 | [ | ||
| 甲磺酸(MSA) | 石墨板 | 钛板 | 磷酸 | 99.99 | 95.28 | 598.91 | 99.31 | [ | ||
| 碱浸出- 电沉积工艺 | AAS工艺 | NH3⋅H2O- (NH4)2SO4 | 低碳钢 | 铅 | 阳离子聚丙烯酰胺 | >95 | [ | |||
| NaOH-KNaC4H4O6 | 明胶 | 99.99 | 98.0 | 400~500 | ≥98 | [ | ||||
| NaOH | 多孔镍泡沫 | 铜箔 | 99.9992 | 99.9 | 317 | 99.85 | [ | |||
| NaOH-木糖醇 溶液 | 316不锈钢 | 316 不锈钢 | 381.71 | 99.68 | [ | |||||
| 其他溶剂浸出-电沉积工艺 | 氯化胆碱-尿素低共熔溶剂 | 低碳钢片 | 石墨片 | 754 | 85.77 | [ | ||||
| 氯化胆碱-乙二醇低共熔溶剂 | 低碳钢片 | 石墨片 | 673.28 | 96.06 | [ | |||||
| 固相电解 工艺 | (NH4)2SO4- NH3·H2O | 不锈钢 | 不锈钢 | 99.89 | 96.15 | 493.89 | 91.68 | [ | ||
| H2SO4 | 不锈钢 | 铅板 | 99.7 | 1800 | [ | |||||
| 酒石酸钠 | 钛镀钌铱板材 | 316L不锈钢板 | 98.74 | 1068 | 91.60 | [ | ||||
| (NH4)2SO4 | 钛基锡锑锰钌 涂层阳极材料 | 铅板栅 | 95.80 | 99.46 | 597 | 87.63 | [ | |||
| NH3-NH4Cl | 铱钌涂覆的钛网 | 不锈钢 | 98.3 | 689.4 | 86.3 | [ | ||||
| Na2SO4 | SnO2-Sb2O5-MnO2-RuO2-钛涂层电极 | 方形铅板 | 97.29 | 97.3 | 818.1 | 98.5 | [ |
| 方法 | 典型工艺 | 浸出溶液/电解液 | 阳极 | 阴极 | 添加剂 | 铅纯度/% | 铅回收率 /% | 电解能耗 /kWh·(t Pb)-1 | 电流 效率/% | 参考 文献 |
|---|---|---|---|---|---|---|---|---|---|---|
| 酸浸出- 电沉积工艺 | RSR工艺 | H2SiF6、HBF4 | 石墨基非导电 惰性网状材料, 表面沉积PbO2 | 铅 | 500mg/L 砷离子 | >99.99 | 96 | [ | ||
| USBM工艺 | H2SiF6 | 二氧化铅包覆的钛材料 | 铅 | 磷酸盐、动物骨胶、木质素磺酸盐 | 99.995~ 99.999 | 92 | 800 | 95~97 | [ | |
| CX-EW工艺(Engitec工艺) | HBF4 | 特殊设计的复合阳极材料 | 骨胶 | 800 | [ | |||||
| Placid工艺 | HCl-NaCl | 99.995 | 99.5 | 1300 | [ | |||||
| HClO4 | 石墨板 | 铜板 | 1.0g/L骨胶+1.0g/L木质素磺酸钠 | 99.9991 | 98.5 | 492 | >97 | [ | ||
| 甲磺酸(MSA) | 石墨板 | 钛板 | 磷酸 | 99.99 | 95.28 | 598.91 | 99.31 | [ | ||
| 碱浸出- 电沉积工艺 | AAS工艺 | NH3⋅H2O- (NH4)2SO4 | 低碳钢 | 铅 | 阳离子聚丙烯酰胺 | >95 | [ | |||
| NaOH-KNaC4H4O6 | 明胶 | 99.99 | 98.0 | 400~500 | ≥98 | [ | ||||
| NaOH | 多孔镍泡沫 | 铜箔 | 99.9992 | 99.9 | 317 | 99.85 | [ | |||
| NaOH-木糖醇 溶液 | 316不锈钢 | 316 不锈钢 | 381.71 | 99.68 | [ | |||||
| 其他溶剂浸出-电沉积工艺 | 氯化胆碱-尿素低共熔溶剂 | 低碳钢片 | 石墨片 | 754 | 85.77 | [ | ||||
| 氯化胆碱-乙二醇低共熔溶剂 | 低碳钢片 | 石墨片 | 673.28 | 96.06 | [ | |||||
| 固相电解 工艺 | (NH4)2SO4- NH3·H2O | 不锈钢 | 不锈钢 | 99.89 | 96.15 | 493.89 | 91.68 | [ | ||
| H2SO4 | 不锈钢 | 铅板 | 99.7 | 1800 | [ | |||||
| 酒石酸钠 | 钛镀钌铱板材 | 316L不锈钢板 | 98.74 | 1068 | 91.60 | [ | ||||
| (NH4)2SO4 | 钛基锡锑锰钌 涂层阳极材料 | 铅板栅 | 95.80 | 99.46 | 597 | 87.63 | [ | |||
| NH3-NH4Cl | 铱钌涂覆的钛网 | 不锈钢 | 98.3 | 689.4 | 86.3 | [ | ||||
| Na2SO4 | SnO2-Sb2O5-MnO2-RuO2-钛涂层电极 | 方形铅板 | 97.29 | 97.3 | 818.1 | 98.5 | [ |
| 1 | ZHANG Wei, YANG Jiakuan, WU Xu, et al. A critical review on secondary lead recycling technology and its prospect[J]. Renewable and Sustainable Energy Reviews, 2016, 61: 108-122. |
| 2 | CHEN Kai, HUANG Lei, YAN Beizhan, et al. Effect of lead pollution control on environmental and childhood blood lead level in Nantong, China: An interventional study[J]. Environmental Science & Technology, 2014, 48(21): 12930-12936. |
| 3 | LIU Wei, CHEN Lujun, TIAN Jinping. Uncovering the evolution of lead in-use stocks in lead-acid batteries and the impact on future lead metabolism in China[J]. Environmental Science & Technology, 2016, 50(10): 5412-5419. |
| 4 | 朱新锋, 杨丹妮, 胡红云, 等. 废铅酸蓄电池铅膏性质分析[J]. 环境工程学报, 2012, 6(9): 3259-3262. |
| ZHU Xinfeng, YANG Danni, HU Hongyun, et al. Properties of lead paste from spent lead-acid battery[J]. Chinese Journal of Environmental Engineering, 2012, 6(9): 3259-3262. | |
| 5 | LIU Kang, YANG Jiakuan, LIANG Sha, et al. An emission-free vacuum chlorinating process for simultaneous sulfur fixation and lead recovery from spent lead-acid batteries[J]. Environmental Science & Technology, 2018, 52(4): 2235-2241. |
| 6 | YU Wenhao, ZHANG Peiyuan, YANG Jiakuan, et al. A low-emission strategy to recover lead compound products directly from spent lead-acid battery paste: Key issue of impurities removal[J]. Journal of Cleaner Production, 2019, 210: 1534-1544. |
| 7 | YU Wenhao, LI Mingyang, LIANG Sha, et al. Novel PbO@C composite material directly derived from spent lead-acid batteries by one-step spray pyrolysis process[J]. Waste Management, 2023, 165: 51-58. |
| 8 | RAMUS K, HAWKINS P. Lead/acid battery recycling and the new Isasmelt process[J]. Journal of Power Sources, 1993, 42(1/2): 299-313. |
| 9 | ELLIS Timothy W, MIRZA Abbas H. The refining of secondary lead for use in advanced lead-acid batteries[J]. Journal of Power Sources, 2010, 195(14): 4525-4529. |
| 10 | TIAN Xi, WU Yufeng, HOU Ping, et al. Environmental impact and economic assessment of secondary lead production: Comparison of main spent lead-acid battery recycling processes in China[J]. Journal of Cleaner Production, 2017, 144: 142-148. |
| 11 | 彭露, 张伟, 喻文昊, 等. 废铅蓄电池火法冶炼环境影响分析[J]. 现代化工, 2016, 36(1): 17-20, 22. |
| PENG Lu, ZHANG Wei, YU Wenhao, et al. Environmental impact assessment of recycling spent lead-acid battery by using pyrometallurgical process[J]. Modern Chemical Industry, 2016, 36(1): 17-20, 22. | |
| 12 | ZHU Xinfeng, YANG Jiakuan, GAO Linxia, et al. Preparation of lead carbonate from spent lead paste via chemical conversion[J]. Hydrometallurgy, 2013, 134: 47-53. |
| 13 | MA Cheng, SHU Yuehong, CHEN Hongyu. Preparation of high-purity lead oxide from spent lead paste by low temperature burning and hydrometallurgical processing with ammonium acetate solution[J]. RSC Advances, 2016, 6(25): 21148-21155. |
| 14 | GAO Pengran, LIU Yi, Weixin LYU, et al. Methanothermal reduction of mixtures of PbSO4 and PbO2 to synthesize ultrafine α-PbO powders for lead acid batteries[J]. Journal of Power Sources, 2014, 265: 192-200. |
| 15 | SONMEZ M S, KUMAR R V. Leaching of waste battery paste components. Part 1: Lead citrate synthesis from PbO and PbO2 [J]. Hydrometallurgy, 2009, 95(1/2): 53-60. |
| 16 | SONMEZ M S, KUMAR R V. Leaching of waste battery paste components. Part 2: Leaching and desulphurisation of PbSO4 by citric acid and sodium citrate solution[J]. Hydrometallurgy, 2009, 95(1/2): 82-86. |
| 17 | LI Mingyang, YANG Jiakuan, LIANG Sha, et al. Review on clean recovery of discarded/spent lead-acid battery and trends of recycled products[J]. Journal of Power Sources, 2019, 436: 226853. |
| 18 | PAN Junqing, ZHANG Xuan, SUN Yanzhi, et al. Preparation of high purity lead oxide from spent lead acid batteries via desulfurization and recrystallization in sodium hydroxide[J]. Industrial & Engineering Chemistry Research, 2016, 55(7): 2059-2068. |
| 19 | 潘军青, 宋爽, 孙艳芝. 一种回收废旧铅酸电池直接生产氧化铅的方法: CN103014347A[P]. 2013-04-03. |
| PAN Junqing, SONG Shuang, SUN Yanzhi. Method for recycling waste lead-acid cells to directly produce lead oxide: CN103014347A[P]. 2013-04-03. | |
| 20 | HU Yuchen, YANG Jiakuan, HU Jingping, et al. Synthesis of nanostructured PbO@C composite derived from spent lead-acid battery for next-generation lead-carbon battery[J]. Advanced Functional Materials, 2018, 28(9): 1705294. |
| 21 | ZHU Xinfeng, HE Xiong, YANG Jiakuan, et al. Leaching of spent lead acid battery paste components by sodium citrate and acetic acid[J]. Journal of Hazardous Materials, 2013, 250/251: 387-396. |
| 22 | YANG Jiakuan, ZHU Xinfeng, LI Lei, et al. Leaching properties of lead paste in spent lead-acid battery with a hydrometallurgical process at room temperature[J]. Environmental Engineering and Management Journal, 2013, 12(11): 2175-2182. |
| 23 | DAVID PRENGAMAN R. Recovering lead from batteries[J]. JOM, 1995, 47(1): 31-33. |
| 24 | COLE E R, LEE A Y, PAULSON D L. Update on recovering lead from scrap batteries[J]. JOM, 1985. 37: 79-83. |
| 25 | 许文林, 聂文, 王雅琼. 废铅蓄电池铅资源化回收利用新工艺[J]. 电池工业, 2016, 20(1): 30-38. |
| XU Wenlin, NIE Wen, WANG Yaqiong. Lead recycling or recovery from waste lead storage batteries[J]. Chinese Battery Industry, 2016, 20(1): 30-38. | |
| 26 | Gustavo DÍAZ, ANDREWS David. Placid—A clean process for recycling lead from batteries[J]. JOM, 1996, 48(1): 29-31. |
| 27 | OLPER M, FRACCHIA P. Hydrometallurgical process for an overall recovery of the components of exhausted lead-acid batteries: US4769116A[P]. 1988-09-06. |
| 28 | 胡红云, 朱新锋, 杨家宽. 湿法回收废旧铅酸蓄电池中铅的研究进展[J]. 化工进展, 2009, 28(9): 1662-1666. |
| HU Hongyun, ZHU Xinfeng, YANG Jiakuan. Progress in the hydrometallurgical process for recovering lead from scrap lead-acid batteries[J]. Chemical Industry and Engineering Progress, 2009, 28(9): 1662-1666. | |
| 29 | ANDREWS D, RAYCHAUDHURI A, FRIAS C. Environmentally sound technologies for recycling secondary lead[J]. Journal of Power Sources, 2000, 88(1): 124-129. |
| 30 | EXPÓSITO E, INIESTA J, GONZÁLEZ-GARCÍA J, et al. Lead electrowinning in an acid chloride medium[J]. Journal of Power Sources, 2001, 92(1/2): 260-266. |
| 31 | ZHANG Xuan, SUN Yanzhi, PAN Junqing. A clean and highly efficient leaching-electrodeposition lead recovery route in HClO4 solution[J]. International Journal of Electrochemical Science, 2017, 12(8): 6966-6979. |
| 32 | 张轩. 废铅酸电池中回收高纯度金属铅和α-PbO新工艺及其电化学性能研究[D]. 北京: 北京化工大学, 2017. |
| ZHANG Xuan. Study on new recovery technology and electrochemical performance of high purity lead and α-PbO from the spent lead acid batteries[D]. Beijing: Beijing University of Chemical Technology, 2017. | |
| 33 | WU Zhenghui, DREISINGER David B, URCH Henning, et al. Fundamental study of lead recovery from cerussite concentrate with methanesulfonic acid (MSA)[J]. Hydrometallurgy, 2014, 142: 23-35. |
| 34 | 常聪, 李有刚, 陈永明, 等. 甲基磺酸体系铅电沉积工艺研究[J]. 矿冶工程, 2020, 40(1): 105-108, 113. |
| CHANG Cong, LI Yougang, CHEN Yongming, et al. Lead electrodeposition in methanesulfonic acid system[J]. Mining and Metallurgical Engineering, 2020, 40(1): 105-108, 113. | |
| 35 | CHANG Cong, YANG Shenghai, LI Yougang, et al. Green hydrometallurgical extraction of metallic lead from spent lead paste in the methanesulfonic acid system[J]. Separation and Purification Technology, 2023. 306: 122592. |
| 36 | 熊睿, 基于隔膜的铅电沉积及其机理研究[D]. 武汉: 华中科技大学, 2020. |
| XIONG Rui. Study on lead electrodeposition based on ion exchange membrane and its mechanism[D]. Wuhan: Huazhong University of Science and Technology, 2020. | |
| 37 | SCHWARTZ Lorne D, ETSELL Thomas H. The cementation of lead from ammoniacal ammonium sulphate solution[J]. Hydrometallurgy, 1998, 47(2/3): 273-279. |
| 38 | BRATT G C, PICKERING R W. Production of lead via ammoniacal ammonium sulfate leaching[J]. Metallurgical Transactions, 1970, 1(8): 2141-2149. |
| 39 | 陈维平, 田一庄, 杨霞, 等. 废铅蓄电池浆料回收技术研究[J]. 有色金属, 1997(4): 65-68, 58. |
| CHEN Weiping, TIAN Yizhuang, YANG Xia, et al. Study on slurry recovery technology of waste lead storage battery[J]. Nonferrous Metals Engineering, 1997(4): 65-68, 58. | |
| 40 | 潘军青, 岳希红, 孙艳芝, 等. 一种清洁湿法固液两相电解还原回收铅的方法: CN101831668A[P]. 2010-09-15. |
| PAN Junqing, SUN Yanzhi, TANG Lu, et al. Clean wet-method solid-liquid two-phase electroreduction lead recovery method: CN101831668A[P]. 2010-09-15. | |
| 41 | PAN Junqing, ZHANG Chao, SUN Yanzhi, et al. A new process of lead recovery from waste lead-acid batteries by electrolysis of alkaline lead oxide solution[J]. Electrochemistry Communications, 2012, 19: 70-72. |
| 42 | 潘军青, 边亚茹. 铅酸蓄电池回收铅技术的发展现状[J]. 北京化工大学学报(自然科学版), 2014, 41(3): 1-14. |
| PAN Junqing, BIAN Yaru. Development and current situation of the recovery technology for lead acid batteries[J]. Journal of Beijing University of Chemical Technology (Natural Science Edition), 2014, 41(3): 1-14. | |
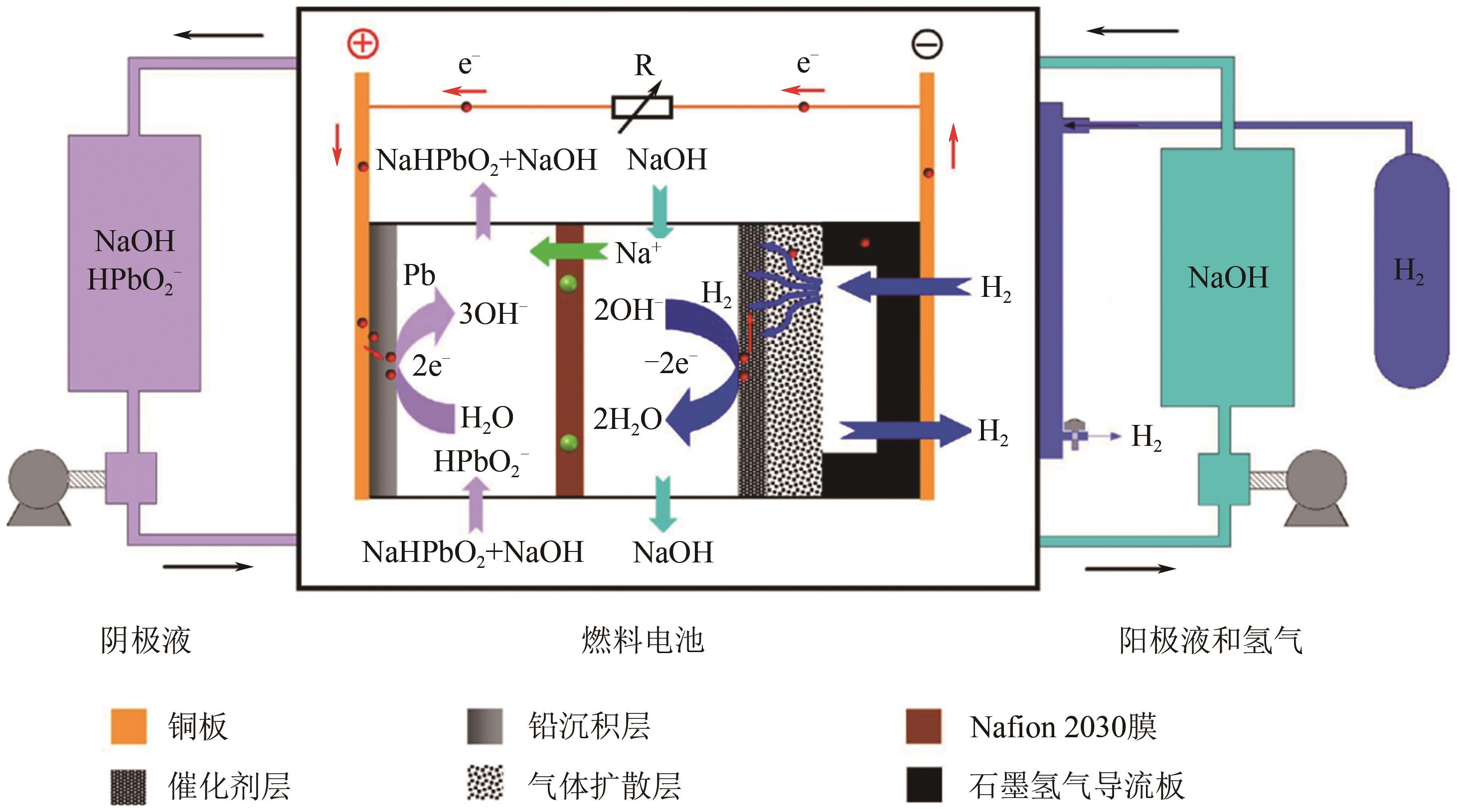
| 43 | PAN Junqing, SUN Yanzhi, LI Wei, et al. A green lead hydrometallurgical process based on a hydrogen-lead oxide fuel cell[J]. Nature Communications, 2013, 4: 2178. |
| 44 | 郭明宜. 碱性木糖醇体系回收废铅蓄电池铅膏的研究[D]. 洛阳: 河南科技大学, 2018. |
| GUO Mingyi. Recovery of lead from exhausted lead-acid battery paste in alkaline solution containing xylitol[D]. Luoyang: Henan University of Science and Technology, 2018. | |
| 45 | 耿笑. 氯化胆碱-尿素低共熔溶剂电解回收废铅膏制备铅粉的研究[D]. 昆明: 昆明理工大学, 2022. |
| GENG Xiao. Study on the preparation of lead powder by electrolytic recovery of waste lead paste using choline chloride urea low melting point solvent[D]. Kunming: Kunming University of Science and Technology, 2022. | |
| 46 | 耿笑, 汝娟坚, 华一新, 等. 低共熔溶剂电化学回收废铅膏可控制备铅粉的研究[J]. 有色金属科学与工程, 2021, 12(2): 8-13, 49. |
| GENG Xiao, RU Juanjian, HUA Yixin, et al. Electrochemical recovery of spent lead acid battery paste to controllably prepare lead powders in deep eutectic solvents[J]. Nonferrous Metals Science and Engineering, 2021, 12(2): 8-13, 49. | |
| 47 | 黄皓铭, 汝娟坚, 华一新, 等. 氯化胆碱-乙二醇低共熔溶剂中高效电解回收废铅膏制备铅粉[J]. 过程工程学报, 2023, 23(1): 107-114. |
| HUANG Haoming, RU Juanjian, HUA Yixin, et al. Recycling of scrap lead paste to prepare lead powder by high efficiency electrolysis in choline chloride-ethylene glycol deep eutectic solvent[J]. The Chinese Journal of Process Engineering, 2023, 23(1): 107-114. | |
| 48 | 贺山明, 吴鑫, 杜鹏, 等. 铵盐体系废铅膏固相电解还原直接制备金属铅的实验研究[J]. 蓄电池, 2020, 57(4): 151-155. |
| HE Shanming, WU Xin, DU Peng, et al. The study of direct preparation of lead from waste lead paste by solid-phase electroreduction in ammonium electrolyte system[J]. Chinese LABAT Man, 2020, 57(4): 151-155. | |
| 49 | 胡彪, 陈龙, 王海北, 等. 废铅膏直接电解回收金属铅试验[J]. 环境工程, 2019, 37(10): 196-200. |
| HU Biao, CHEN Long, WANG Haibei, et al. Experimental study on recovering lead from waste lead paste by direct electrolysis[J]. Environmental Engineering, 2019, 37(10): 196-200. | |
| 50 | 李新颖, 王伟, 谢锋, 等. 废铅膏在酒石酸钠体系直接固相电解制备粗铅[J]. 有色金属(冶炼部分), 2022(8): 24-32. |
| LI Xinying, WANG Wei, XIE Feng, et al. Preparation of crude lead by direct solid phase electrolysis of spent lead paste in sodium tartrate system[J]. Nonferrous Metals (Extractive Metallurgy), 2022(8): 24-32. | |
| 51 | DAI Fushu, HUANG Hui, CHEN Buming, et al. Recovery of high purity lead from spent lead paste via direct electrolysis and process evaluation[J]. Separation and Purification Technology, 2019, 224: 237-246. |
| 52 | 戴富书. 固相电解法回收废铅酸蓄电池中的铅膏研究[D]. 昆明: 昆明理工大学, 2019. |
| DAI Fushu. Research on the recovery of lead paste from waste lead-acid batteries using solid-phase electrolysis method[D]. Kunming: Kunming University of Science and Technology, 2019. | |
| 53 | FAN Yangyang, LIU Yan, NIU Liping, et al. Preparation of metal lead from waste lead paste by direct electrochemical reduction in NH3-NH4Cl solution[J]. JOM, 2019, 71(12): 4518-4527. |
| 54 | WANG Lei, XIE Feng, WANG Wei, et al. Eco-friendly and efficient strategy for lead recovery from spent lead paste by bagged cathode solid-phase electroreduction[J]. Chemical Engineering Journal, 2023, 473: 145208. |
| [1] | GAO Yuli, WANG Hongqiu, HUANG Gexing, XIAN Nanying, SHI Xiaoyu. Research progress and the industrialization of all-solid-state battery [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 4767-4778. |
| [2] | LIANG Hongcheng, ZHAO Dongni, QUAN Yin, LI Jingni, HU Xinyi. Influence of SEI film morphology and structure on the performance of lithium-ion batteries [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5049-5062. |
| [3] | LI Haoran, WANG Yan, ZHANG Tao, LYU Li, TANG Wenxiang, TANG Shengwei. Controllable regulation of microdroplets size in W/O microemulsion with Cu(Ac)2-Zn(Ac)2 solution as aqueous phase [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5168-5176. |
| [4] | GUO Pei, CUI Cancan, KONG Dejie, HUANG Sheng. Development trend of sulfide solid electrolytes for solid-state lithium batteries in the context of “dual carbon goals” [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5193-5206. |
| [5] | SHAO Wei, MA Zhuang, ZHENG Hongwei, LIU Guangju, GAO Xiang, XIE Jian, HE Qinggang. Recent advances of organic materials for aqueous rechargeable batteries [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3872-3890. |
| [6] | WANG Qingtai, ZHANG Sai, WANG Jiemin. Numerical simulation for non-uniform compression of porous electrodes in vanadium flow batteries [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 2940-2949. |
| [7] | LIU Haodong, ZHANG Pengfei, HUANG Yuqi. Visualization and velocity field test of thermal runaway jet of ternary lithium battery [J]. Chemical Industry and Engineering Progress, 2024, 43(2): 703-712. |
| [8] | ZHANG Yixuan, HU Wei, LIU Mengyao, JU Jingge, ZHAO Yixia, KANG Weimin. Research progress of polymer electrolytes in zinc-ion batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1397-1410. |
| [9] | TIAN Xiaolu, YI Yikun, HAI Feng, WU Zhendi, ZHENG Shentuo, GUO Jingyu, LI Mingtao. Research progress in shear-thickening electrolytes for lithium-ion batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(11): 5786-5800. |
| [10] | CHEN Erjun, ZHANG Yuling, LU Shaolei, DUAN Haiyang, JIN Wenzhang. Stability and physicochemical properties of air nanobubbles [J]. Chemical Industry and Engineering Progress, 2022, 41(9): 4673-4681. |
| [11] | GUAN Haoran, ZHU Lina, ZHU Lingyue, YUAN Dandan, ZHANG Yuqing, WANG Baohui. Progress and challenges of electrochemical synthesis of ammonia from different hydrogen and nitrogen sources [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4098-4110. |
| [12] | HU Huakun, XUE Wendong, JIANG Peng, LI Yong. Research progress of safety additives for lithium ion batteries [J]. Chemical Industry and Engineering Progress, 2022, 41(10): 5441-5455. |
| [13] | GAO Chenxiang, ZHANG Ke, LIU Chunyuan, FENG Xin, HUO Pengfei. Research progress on the application of wood in electrochemistry [J]. Chemical Industry and Engineering Progress, 2021, 40(S2): 203-210. |
| [14] | PAN Di, KONG Jiangrong, LIU Xinnan, HUANG Meiqi, ZHOU Tao. Preparation Li7La3Zr2O12 garnet solid-state electrolyte by wet-chemical technique [J]. Chemical Industry and Engineering Progress, 2021, 40(S2): 334-339. |
| [15] | LUO Laiming, CHEN Si’an, WANG Haining, ZHANG Jin, LU Shanfu, XIANG Yan. Simulation and optimization of large-scale (200cm2) multiple-serpentine flow field for high temperature polymer electrolyte membrane fuel cells [J]. Chemical Industry and Engineering Progress, 2021, 40(9): 4975-4985. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||