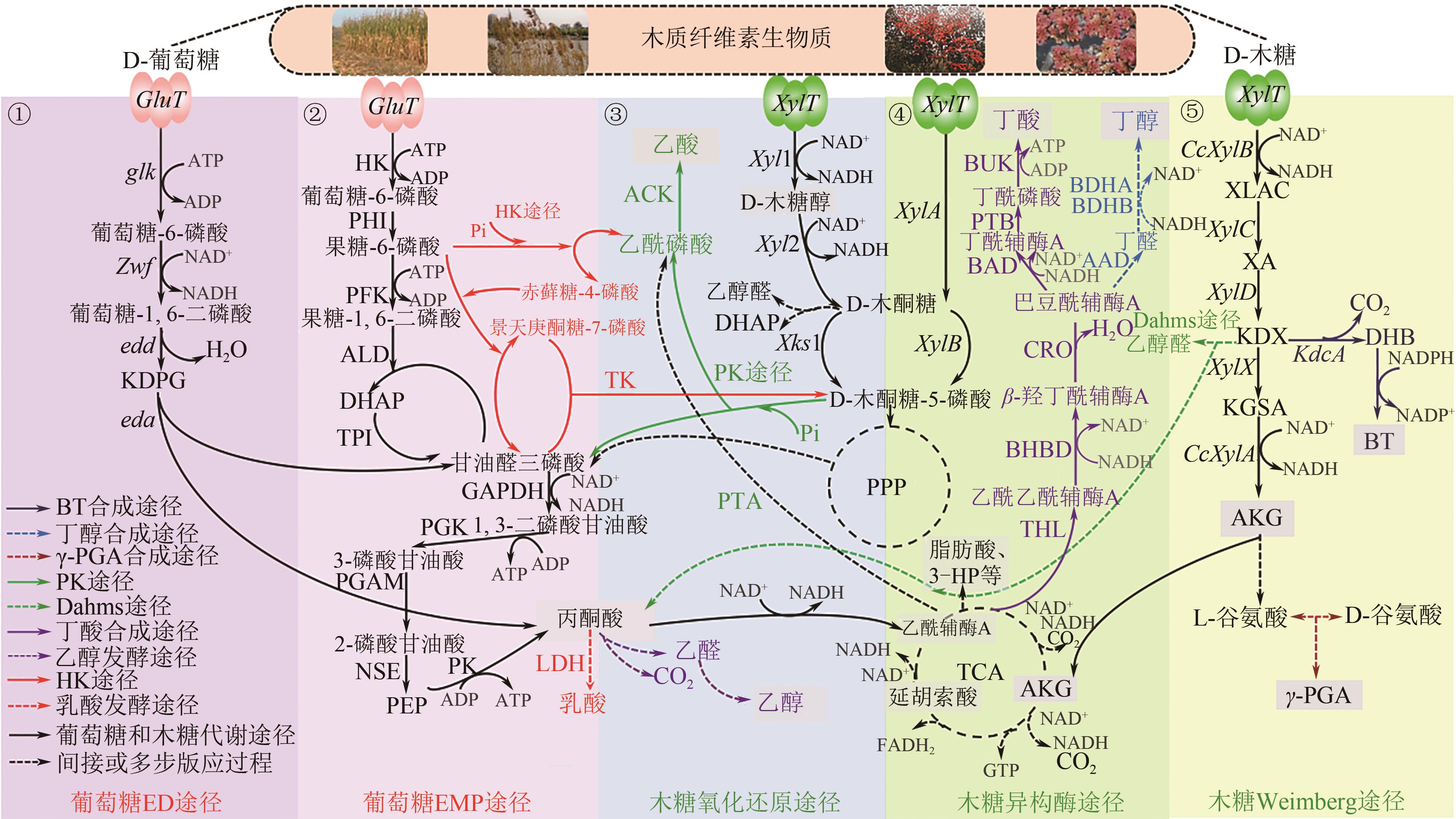
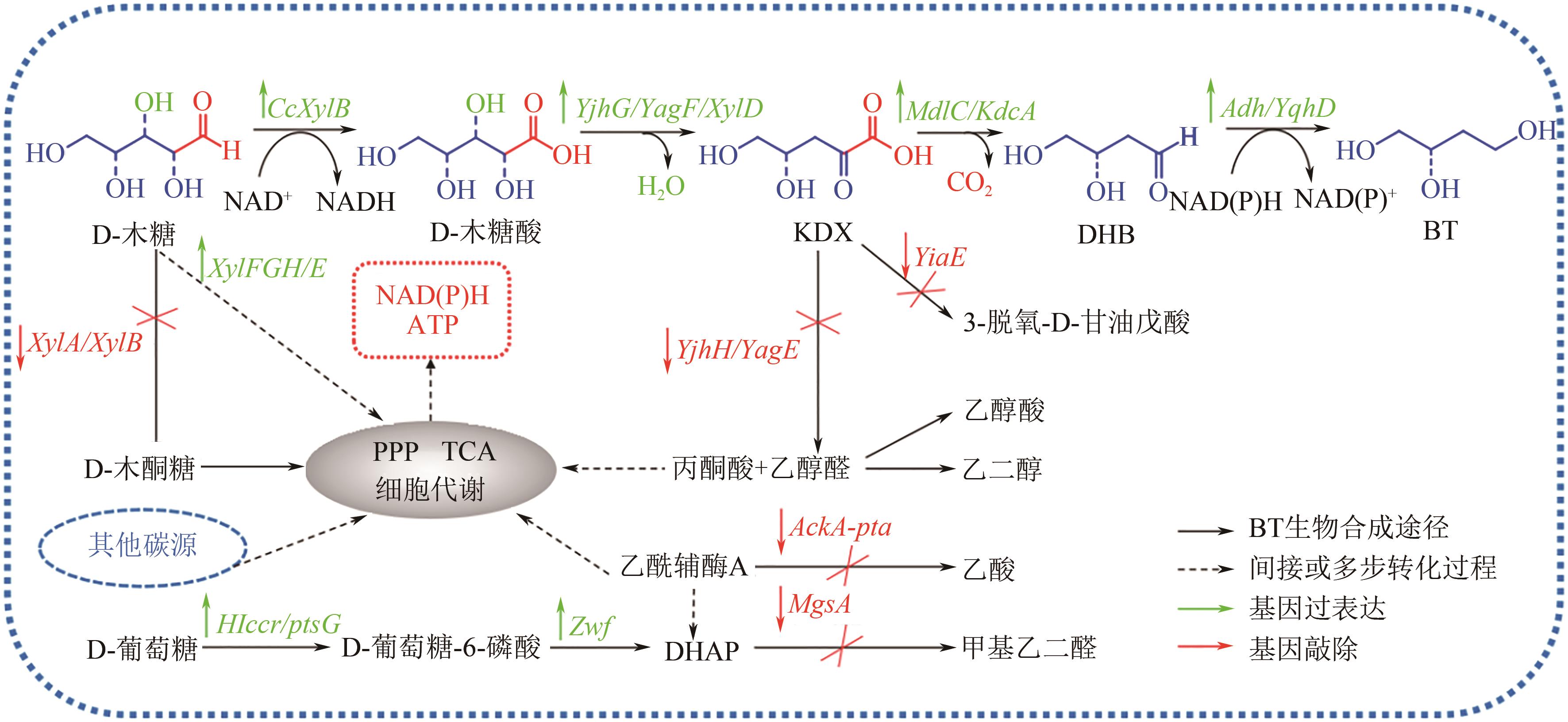
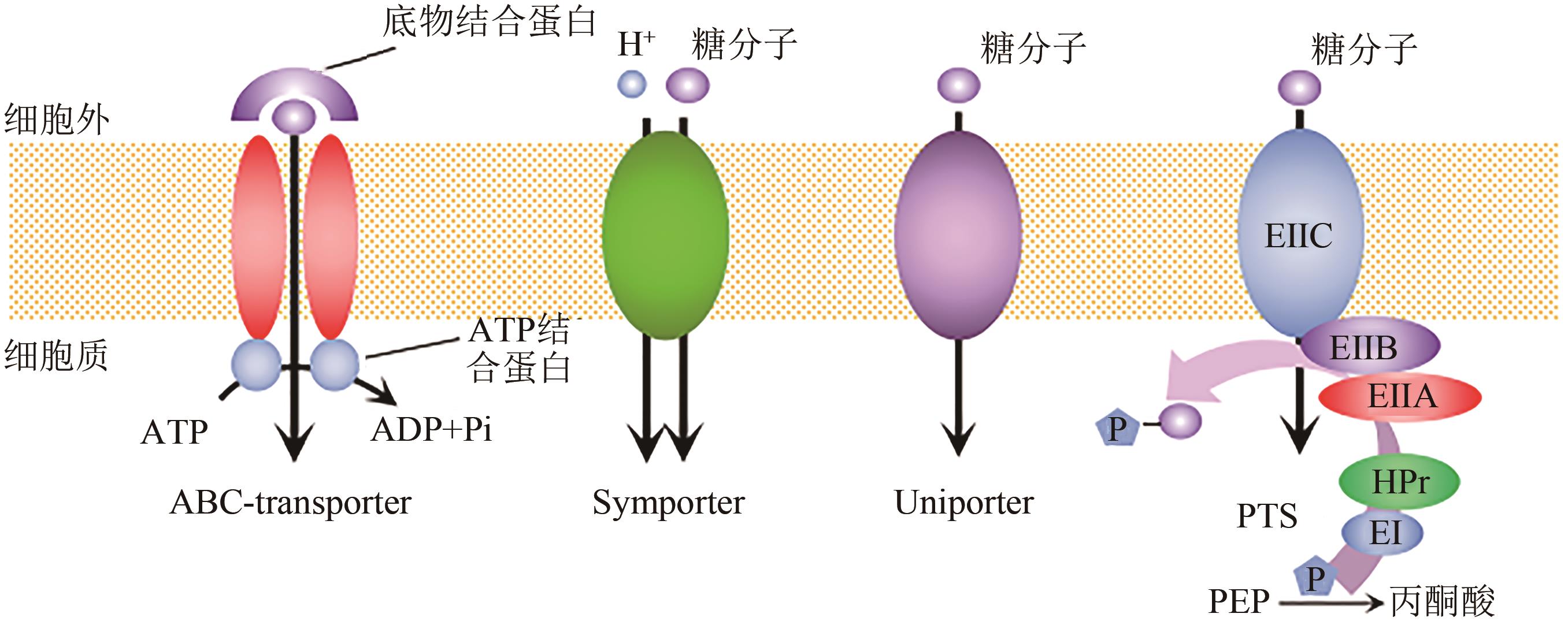
Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (1): 354-372.DOI: 10.16085/j.issn.1000-6613.2022-0573
• Biochemical and pharmaceutical engineering • Previous Articles Next Articles
Co-utilization of xylose and glucose to produce chemicals by microorganisms
WANG Chuandong1,2,3( ), ZHANG Junqi1,2(
), ZHANG Junqi1,2( ), LIU Dingyuan1,2, MA Yuanyuan4, LI Feng1,2(
), LIU Dingyuan1,2, MA Yuanyuan4, LI Feng1,2( ), SONG Hao1,2(
), SONG Hao1,2( )
)
- 1.School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China
2.Frontier Science Center for Synthetic Biology and Key Laboratory of Systems Bioengineering (Ministry of Education), Tianjin University, Tianjin 300072, China
3.Qingdao Institute of Ocean Engineering of Tianjin University Ltd. , Qingdao 266237, Shandong, China
4.School of Marine Science and Technology, Tianjin University, Tianjin 300072, China
-
Received:2022-04-06Revised:2022-06-11Online:2023-02-20Published:2023-01-25 -
Contact:LI Feng, SONG Hao
微生物共利用木糖和葡萄糖生产化学品研究进展
王川东1,2,3( ), 张君奇1,2(
), 张君奇1,2( ), 刘丁源1,2, 马媛媛4, 李锋1,2(
), 刘丁源1,2, 马媛媛4, 李锋1,2( ), 宋浩1,2(
), 宋浩1,2( )
)
- 1.天津大学化工学院,天津 300072
2.天津大学合成生物学前沿科学中心和系统生物工程教育部重点实验室,天津 300072
3.天津大学(青岛)海洋工程研究院有限公司,山东 青岛 266237
4.天津大学海洋科学与技术学院,天津 300072
-
通讯作者:李锋,宋浩 -
作者简介:王川东(1990—),男,硕士研究生,研究方向为合成生物学。E-mail:1137866329@qq.com
张君奇(1992—),男,博士研究生,研究方向为合成生物学。E-mail:1871898438@qq.com。 -
基金资助:国家重点研发计划(2018YFA0901300);国家自然科学基金(32071411)
CLC Number:
Cite this article
WANG Chuandong, ZHANG Junqi, LIU Dingyuan, MA Yuanyuan, LI Feng, SONG Hao. Co-utilization of xylose and glucose to produce chemicals by microorganisms[J]. Chemical Industry and Engineering Progress, 2023, 42(1): 354-372.
王川东, 张君奇, 刘丁源, 马媛媛, 李锋, 宋浩. 微生物共利用木糖和葡萄糖生产化学品研究进展[J]. 化工进展, 2023, 42(1): 354-372.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-0573
| 酶催化步骤 | Gθ/kJ·mol-1 | ∆Gθ/kJ·mol-1 |
|---|---|---|
| Step0:初始态 | 0 | — |
| Step1:XDH | -19.20 | -19.20 |
| Step2:XLA | -40.40 | -21.20 |
| Step3:XAD | -82.30 | -41.90 |
| Step4:KDXD | -121.50 | -39.20 |
| Step5:KGSADH | -156.80 | -35.30 |
| 酶催化步骤 | Gθ/kJ·mol-1 | ∆Gθ/kJ·mol-1 |
|---|---|---|
| Step0:初始态 | 0 | — |
| Step1:XDH | -19.20 | -19.20 |
| Step2:XLA | -40.40 | -21.20 |
| Step3:XAD | -82.30 | -41.90 |
| Step4:KDXD | -121.50 | -39.20 |
| Step5:KGSADH | -156.80 | -35.30 |
| 菌株 | 代谢途径 | 原料 | 技术方法 | 产物产量/g·L-1 | 收率/g·g-1 | 参考文献 |
|---|---|---|---|---|---|---|
| T. cutaneum | EMP-TCA途径 | 玉米秸秆 | 选择培养基筛选,3L发酵罐BF | 油脂 | 0.39 | [ |
| R. arrhizus | 磷酸酮酶途径 | 木糖、葡萄糖 | 选择培养基筛选,SF | FA 46.78 | 0.47 | [ |
| C. tyrobutyricum | ED-XR途径 | 大豆壳、玉米纤维、小麦秸秆、水稻秸秆、甘蔗渣 | 表达异源XylT、XylA、XylB基因,1L搅拌槽生物反应器 | 丁酸42.60 | 0.36 | [ |
| E. coli | XR-XI途径 | 空棕榈果束纤维 | 适应性进化,基因突变筛选,表达戊糖代谢基因,混糖,微型发酵罐FBF | 木糖醇4.80 | 0.99 | [ |
| K. marxianus | EMP途径 | 满江红 | 热酸水解、酶法糖化,半厌氧SF | 乙醇26.80 | 0.43 | [ |
| C. lusitaniae | EMP途径 | 满江红 | 热酸水解、酶法糖化,半厌氧SF | 乙醇23.20 | 0.37 | [ |
| S. stipitis | EMP途径 | 满江红 | 热酸水解、酶法糖化,半厌氧SF | 乙醇18.20 | 0.29 | [ |
| P. tannophilus | XR途径 | 玉米芯 | 稀酸热水解、木碳粉解毒处理、滤纸过滤,Luedeking-Piret模型分析,深层发酵 | 木糖醇0.81 | 0.81 | [ |
| D. hansenii varhanseni | XR途径 | 玉米芯 | 稀酸热水解、木碳粉解毒处理、滤纸过滤,Luedeking-Piret模型分析,深层发酵 | 木糖醇0.79 | 0.79 | [ |
| C. guillermondii | XR途径 | 稻草 | 稀酸热水解、木碳粉解毒处理、滤纸过滤,Luedeking-Piret模型分析,深层发酵 | 木糖醇0.76 | 0.76 | [ |
S. cerevisiaea C. utilis | EMP途径 | 芦苇秸秆 | 60目筛、热碱液处理,加纤维素酶,混菌有氧SF | 乙醇9.81 | 0.10 | [ |
| B. subtilis | 谷氨酸合成-WBG途径 | 木糖、葡萄糖 | 表达异源WBG途径基因,0.50L搅拌槽发酵罐BF | γ-PGA 5 | 0.26 | [ |
| CyanobacteriumSynechocystis | XI-EMP-TCA途径 | 木糖、葡萄糖、CO2 | 糖原合成突变体∆glgC中表达木糖分解代谢基因XylA、XylB,CO2固定,氮饥饿条件,SF | 丙酮0.61、 AKG 0.42 | 0.76、 0.32 | [ |
| C. glutamicum | EMP-XI途径 | 木糖、葡萄糖 | 结合甘油和3-HP合成基因,筛选活性醛脱氢酶,iolT1和glk取代PEP依赖的PTS,整合araE和XylA/B,5L发酵罐FBF | 3-HP 62.60 | 0.51 | [ |
| E. coli | XR-WBG途径 | 木糖、葡萄糖 | 表达异源合成基因Xdh、MdlC,敲除旁路基因XylA、YjhH和YagE,过表达Zwf,敲除MtfA,失活PntAB基因,SF | BT 7.23 | 0.55 | [ |
| K. pneumoniae | WBG途径 | 木糖、葡萄糖 | 表达Xdh、KivD及YjhG基因,敲除XylA基因,SF | BT 4.52 | 0.21 | [ |
| S. cerevisiae | WBG途径 | 木糖、葡萄糖 | XylB、XylD、KdcA、Adh基因编码的异源四酶反应,整合KdcA基因至酵母基因组,发酵罐FBF | BT 6.60 | 0.57 | [ |
| K. marxianus | XR-EMP | 木糖、葡萄糖 | 过表达异源XR、XD基因,敲除甘油、乙酸副产物基因,发酵罐FBF | 乙醇51.43 | 0.35 | [ |
| K. marxianus | XR-WBG途径 | 木糖、葡萄糖 | 敲除Xyl1、Xyl2,过表达异源Xyl1基因,选择培养基筛选,无灭菌FBF | 木糖醇 312.05 | 0.99 | [ |
| K. marxianus | XR途径 | 木糖、葡萄糖 | 过表达异源Xyl1基因,敲除木糖醇代谢基因,重构葡萄糖代谢,FBF | 木糖醇203.57 | 0.99 | [ |
| Y. lipolytica | EMP-XR途径 | 木糖、葡萄糖 | 表达外源XDH、XR和内源XK、GPD1和DGA2,敲除POX1-6和TGL4基因,5L发酵罐FBF | 油脂22.50、 柠檬酸67.20 | 0.06、 0.18 | [ |
| T. laichii | EMP-TCA | 木糖、葡萄糖 | 选择培养基筛选,FBF | 油脂 | 0.83 | [ |
| R. toruloides | EMP-TCA | 木糖、葡萄糖 | 选择培养基筛选,FBF | 油脂 | 0.65 | [ |
| L. starkeyi | 从头合成途径 | 玉米芯 | 稀酸处理,过碱化和活性炭吸附解毒,SF | 油脂 | 0.47 | [ |
| C. humicola | 从头合成途径 | 玉米秸秆 | 高温、高压、碱水解预处理,SF | 油脂 | 0.44 | [ |
| B. amyloliquefaciens | XI-不依赖谷氨酸的谷氨酸合成途径 | 玉米秸秆、豆粕 | 50L、150L曝气式连续搅拌固态生物反应器CF | γ-PGA 117、102* | — | [ |
| R. arrhizus | 磷酸酮酶途径 | 木糖、葡萄糖 | 定向进化、SF | FA 28.48 | 0.46 | [ |
| R. delemar | EMP-TCA途径 | 葡萄糖、玉米浆 | 粒径0.55mm球团固定、搅拌槽发酵罐BF | FA 39.56 | 0.40 | [ |
| R. oryzae | LA发酵 | 木糖、葡萄糖 | 诱变筛选,SF | 乳酸119.22 | 0.80 | [ |
| B. coagulans | LA发酵 | 玉米芯残渣 | SSF、FBF | 乳酸68 | 0.85 | [ |
| B. coagulans | LA发酵 | 麦秸 | 稀硫酸预处理,7.50L发酵罐SSF | 乳酸52.20 | 0.87 | [ |
| B. coagulans | LA发酵 | 木薯、高粱粉 | 补加α-淀粉酶、糖化酶10L发酵罐SSF | 乳酸68.72 | 0.99 | [ |
| C. tyrobutyricum | ED-XR途径 | 木糖、葡萄糖 | 表达异源XylT、XylA、XylB基因,补加紫精,1L发酵罐BF | 丁酸46.40 | 0.43 | [ |
| K. marxianus | XR-EMP途径 | 玉米芯 | 稀酸处理、微曝气两段式,5L发酵罐SSF、FBF | 乙醇24.20 | 0.82 | [ |
| K. marxianus | XR-EMP途径 | 玉米芯 | 稀酸预处理,5L发酵罐微曝、两段式SSF、FBF | 木糖醇52 | 0.41 | [ |
| 菌株 | 代谢途径 | 原料 | 技术方法 | 产物产量/g·L-1 | 收率/g·g-1 | 参考文献 |
|---|---|---|---|---|---|---|
| T. cutaneum | EMP-TCA途径 | 玉米秸秆 | 选择培养基筛选,3L发酵罐BF | 油脂 | 0.39 | [ |
| R. arrhizus | 磷酸酮酶途径 | 木糖、葡萄糖 | 选择培养基筛选,SF | FA 46.78 | 0.47 | [ |
| C. tyrobutyricum | ED-XR途径 | 大豆壳、玉米纤维、小麦秸秆、水稻秸秆、甘蔗渣 | 表达异源XylT、XylA、XylB基因,1L搅拌槽生物反应器 | 丁酸42.60 | 0.36 | [ |
| E. coli | XR-XI途径 | 空棕榈果束纤维 | 适应性进化,基因突变筛选,表达戊糖代谢基因,混糖,微型发酵罐FBF | 木糖醇4.80 | 0.99 | [ |
| K. marxianus | EMP途径 | 满江红 | 热酸水解、酶法糖化,半厌氧SF | 乙醇26.80 | 0.43 | [ |
| C. lusitaniae | EMP途径 | 满江红 | 热酸水解、酶法糖化,半厌氧SF | 乙醇23.20 | 0.37 | [ |
| S. stipitis | EMP途径 | 满江红 | 热酸水解、酶法糖化,半厌氧SF | 乙醇18.20 | 0.29 | [ |
| P. tannophilus | XR途径 | 玉米芯 | 稀酸热水解、木碳粉解毒处理、滤纸过滤,Luedeking-Piret模型分析,深层发酵 | 木糖醇0.81 | 0.81 | [ |
| D. hansenii varhanseni | XR途径 | 玉米芯 | 稀酸热水解、木碳粉解毒处理、滤纸过滤,Luedeking-Piret模型分析,深层发酵 | 木糖醇0.79 | 0.79 | [ |
| C. guillermondii | XR途径 | 稻草 | 稀酸热水解、木碳粉解毒处理、滤纸过滤,Luedeking-Piret模型分析,深层发酵 | 木糖醇0.76 | 0.76 | [ |
S. cerevisiaea C. utilis | EMP途径 | 芦苇秸秆 | 60目筛、热碱液处理,加纤维素酶,混菌有氧SF | 乙醇9.81 | 0.10 | [ |
| B. subtilis | 谷氨酸合成-WBG途径 | 木糖、葡萄糖 | 表达异源WBG途径基因,0.50L搅拌槽发酵罐BF | γ-PGA 5 | 0.26 | [ |
| CyanobacteriumSynechocystis | XI-EMP-TCA途径 | 木糖、葡萄糖、CO2 | 糖原合成突变体∆glgC中表达木糖分解代谢基因XylA、XylB,CO2固定,氮饥饿条件,SF | 丙酮0.61、 AKG 0.42 | 0.76、 0.32 | [ |
| C. glutamicum | EMP-XI途径 | 木糖、葡萄糖 | 结合甘油和3-HP合成基因,筛选活性醛脱氢酶,iolT1和glk取代PEP依赖的PTS,整合araE和XylA/B,5L发酵罐FBF | 3-HP 62.60 | 0.51 | [ |
| E. coli | XR-WBG途径 | 木糖、葡萄糖 | 表达异源合成基因Xdh、MdlC,敲除旁路基因XylA、YjhH和YagE,过表达Zwf,敲除MtfA,失活PntAB基因,SF | BT 7.23 | 0.55 | [ |
| K. pneumoniae | WBG途径 | 木糖、葡萄糖 | 表达Xdh、KivD及YjhG基因,敲除XylA基因,SF | BT 4.52 | 0.21 | [ |
| S. cerevisiae | WBG途径 | 木糖、葡萄糖 | XylB、XylD、KdcA、Adh基因编码的异源四酶反应,整合KdcA基因至酵母基因组,发酵罐FBF | BT 6.60 | 0.57 | [ |
| K. marxianus | XR-EMP | 木糖、葡萄糖 | 过表达异源XR、XD基因,敲除甘油、乙酸副产物基因,发酵罐FBF | 乙醇51.43 | 0.35 | [ |
| K. marxianus | XR-WBG途径 | 木糖、葡萄糖 | 敲除Xyl1、Xyl2,过表达异源Xyl1基因,选择培养基筛选,无灭菌FBF | 木糖醇 312.05 | 0.99 | [ |
| K. marxianus | XR途径 | 木糖、葡萄糖 | 过表达异源Xyl1基因,敲除木糖醇代谢基因,重构葡萄糖代谢,FBF | 木糖醇203.57 | 0.99 | [ |
| Y. lipolytica | EMP-XR途径 | 木糖、葡萄糖 | 表达外源XDH、XR和内源XK、GPD1和DGA2,敲除POX1-6和TGL4基因,5L发酵罐FBF | 油脂22.50、 柠檬酸67.20 | 0.06、 0.18 | [ |
| T. laichii | EMP-TCA | 木糖、葡萄糖 | 选择培养基筛选,FBF | 油脂 | 0.83 | [ |
| R. toruloides | EMP-TCA | 木糖、葡萄糖 | 选择培养基筛选,FBF | 油脂 | 0.65 | [ |
| L. starkeyi | 从头合成途径 | 玉米芯 | 稀酸处理,过碱化和活性炭吸附解毒,SF | 油脂 | 0.47 | [ |
| C. humicola | 从头合成途径 | 玉米秸秆 | 高温、高压、碱水解预处理,SF | 油脂 | 0.44 | [ |
| B. amyloliquefaciens | XI-不依赖谷氨酸的谷氨酸合成途径 | 玉米秸秆、豆粕 | 50L、150L曝气式连续搅拌固态生物反应器CF | γ-PGA 117、102* | — | [ |
| R. arrhizus | 磷酸酮酶途径 | 木糖、葡萄糖 | 定向进化、SF | FA 28.48 | 0.46 | [ |
| R. delemar | EMP-TCA途径 | 葡萄糖、玉米浆 | 粒径0.55mm球团固定、搅拌槽发酵罐BF | FA 39.56 | 0.40 | [ |
| R. oryzae | LA发酵 | 木糖、葡萄糖 | 诱变筛选,SF | 乳酸119.22 | 0.80 | [ |
| B. coagulans | LA发酵 | 玉米芯残渣 | SSF、FBF | 乳酸68 | 0.85 | [ |
| B. coagulans | LA发酵 | 麦秸 | 稀硫酸预处理,7.50L发酵罐SSF | 乳酸52.20 | 0.87 | [ |
| B. coagulans | LA发酵 | 木薯、高粱粉 | 补加α-淀粉酶、糖化酶10L发酵罐SSF | 乳酸68.72 | 0.99 | [ |
| C. tyrobutyricum | ED-XR途径 | 木糖、葡萄糖 | 表达异源XylT、XylA、XylB基因,补加紫精,1L发酵罐BF | 丁酸46.40 | 0.43 | [ |
| K. marxianus | XR-EMP途径 | 玉米芯 | 稀酸处理、微曝气两段式,5L发酵罐SSF、FBF | 乙醇24.20 | 0.82 | [ |
| K. marxianus | XR-EMP途径 | 玉米芯 | 稀酸预处理,5L发酵罐微曝、两段式SSF、FBF | 木糖醇52 | 0.41 | [ |
| 1 | SANKARAN R, PARRA CRUZ R A, PAKALAPATI H, et al. Recent advances in the pretreatment of microalgal and lignocellulosic biomass: a comprehensive review[J]. Bioresource Technology, 2020, 298, 122476. |
| 2 | ZHENG Xiaojie, XIAN Xiaoling, HU Lei, et al. Efficient short-time hydrothermal depolymerization of sugarcane bagasse in one-pot for cellulosic ethanol production without solid-liquid separation, water washing, and detoxification[J]. Bioresource Technology, 2021, 339, 125575. |
| 3 | YU Hai, GUO Jian, CHEN Yefu, et al. Efficient utilization of hemicellulose and cellulose in alkali liquor-pretreated corncob for bioethanol production at high solid loading by Spathaspora passalidarum U1-58[J]. Bioresource Technology, 2017, 232: 168-175. |
| 4 | WANG Liang, YORK S W, INGRAM L O, et al. Simultaneous fermentation of biomass-derived sugars to ethanol by a co-culture of an engineered Escherichia coli and Saccharomyces cerevisiae [J]. Bioresource Technology, 2019, 273: 269-276. |
| 5 | HU Cuimin, WU Siguo, WANG Qian, et al. Simultaneous utilization of glucose and xylose for lipid production by Trichosporon cutaneum [J]. Biotechnology for Biofuels, 2011, 4(1): 25-33. |
| 6 | LIU Hua, HU Huirong, JIN Yuhan, et al. Co-fermentation of a mixture of glucose and xylose to fumaric acid by Rhizopus arrhizus RH7-13-9#[J]. Bioresource Technology, 2017, 233: 30-33. |
| 7 | FU Hongxin, YANG Shangtian, WANG Minqi, et al. Butyric acid production from lignocellulosic biomass hydrolysates by engineered Clostridium tyrobutyricum overexpressing xylose catabolism genes for glucose and xylose co-utilization[J]. Bioresource Technology, 2017, 234: 389-396. |
| 8 | KIM S M, CHOI B Y, RYU Y S, et al. Simultaneous utilization of glucose and xylose via novel mechanisms in engineered Escherichia coli [J]. Metabolic Engineering, 2015, 30: 141-148. |
| 9 | DONZELLA L, VARELA J A, SOUSA M J, et al. Identification of novel pentose transporters in Kluyveromyces marxianus using a new screening platform[J]. FEMS Yeast Research, 2021, 21(4). |
| 10 | 杜仁鹏, 黄守峰, 裴芳艺, 等. 休哈塔假丝酵母木糖还原酶基因(xyl1)的克隆与序列分析[J]. 中国农学通报, 2015, 31(11): 124-129. |
| DU Renpeng, HUANG Shoufeng, PEI Fangyi, et al. Cloning and sequence analysis of the xylose reductase gene (xyl1) from Candida shehatae [J]. Chinese Agricultural Science Bulletin, 2015, 31(11): 124-129. | |
| 11 | CHUPAZA M H, PARK Y R, KIM S H, et al. Bioethanol production from Azolla filiculoides by Saccharomyces cerevisiae, Pichia stipitis, Candida lusitaniae, and Kluyveromyces marxianus [J]. Applied Biochemistry and Biotechnology, 2021, 193: 502-514. |
| 12 | RECH F R, FONTANA R C, ROSA C A, et al. Fermentation of hexoses and pentoses from sugarcane bagasse hydrolysates into ethanol by Spathaspora hagerdaliae [J]. Bioprocess and Biosystems Engineering, 2019, 42: 83-92. |
| 13 | SARAVANAN P, RAMESH S, JAYA N, et al. Prospective evaluation of xylitol production using Dabaryomyces hansenii var hansenii, Pachysolen tannophilus, and Candida guillermondii with sustainable agricultural residues[J]. Biomass Conversion and Biorefinery, 2021, 1-19. |
| 14 | 汤斌, 周逢云, 张庆庆, 等. Candida shehatae的纯化及其利用木糖和葡萄糖发酵的特性研究[J]. 食品科学, 2009, 30(3): 159-162. |
| TANG Bin, ZHOU Fengyun, ZHANG Qingqing, et al. Screening of Candida shehatae TZ8-13 converting xylose and glucose into ethanol efficiently and its fermentation characteristics[J]. Food Science, 2009, 30(3): 159-162. | |
| 15 | AGBOGBO F K, COWARD-KELLY G, TORRY-SMITH M, et al. Fermentation of glucose/xylose mixtures using Pichia stipitis [J]. Process Biochemistry, 2006, 41(11): 2333-2336. |
| 16 | SELIM K A, EASA S M, EL-DIWANY A I, et al. The xylose metabolizing yeast Spathaspora passalidarum is a promising genetic treasure for improving bioethanol production[J]. Fermentation, 2020, 6(1): 33-45. |
| 17 | SELIM K A, EL-GHWAS D E, EASA S M, et al. Bioethanol a microbial biofuel metabolite; new insights of yeasts metabolic engineering[J]. Fermentation, 2018, 4(1): 16-43. |
| 18 | SUN Lingling, WU Bo, ZHANG Zengqin, et al. Cellulosic ethanol production by consortia of Scheffersomyces stipitis and engineered Zymomonas mobilis [J]. Biotechnology for Biofuels, 2021, 14, 221. |
| 19 | LOPEZ P C, UDUGAMA I A, THOMSEN S T, et al. Promoting the co-utilisation of glucose and xylose in lignocellulosic ethanol fermentations using a data-driven feed-back controller[J]. Biotechnology for Biofuels, 2020, 13, 190. |
| 20 | 蔡世欣, 李敏, 侯余勇, 等. 芦苇秸秆混合发酵制备乙醇的工艺优化[J]. 化学与生物工程, 2021, 38(2): 26-31, 35. |
| CAI Shixin, LI Min, HOU Yuyong, et al. Optimization in preparation process of ethanol from reed straw by mixed fermentation of Saccharomyces cerevisiae and Candida utilis [J]. Chemistry & Bioengineering, 2021, 38(2): 26-31, 35. | |
| 21 | WANG Fengqin, DONG Yuheng, CHENG Xiang, et al. Effect of detoxification methods on ABE production from corn stover hydrolysate by Clostridium acetobutylicum CICC8016[J]. Biotechnology and Applied Biochemistry, 2020, 67(5): 790-798. |
| 22 | AGBOGBO F K, WENGER K S. Production of ethanol from corn stover hemicellulose hydrolyzate using Pichia stipitis [J]. Journal of Industrial Microbiology & Biotechnology, 2007, 34: 723-727. |
| 23 | LI Xiaowei, ZHAO Rui, LI Shan, et al. Global reprogramming of xylose metabolism in Saccharomyces cerevisiae efficiently produces ethanol from lignocellulose hydrolysates[J]. Industrial Crops & Products, 2022, 179, 114666. |
| 24 | STEPHENS C, CHRISTEN B, FUCHS T, et al. Genetic analysis of a novel pathway for D-xylose metabolism in Caulobacter crescentus [J]. Journal of Bacteriology, 2007, 189(5): 2181-2185. |
| 25 | ALMQVIST H, GLASER S J, TUFVEGREN C, et al. Characterization of the Weimberg pathway in Caulobacter crescentus [J]. Fermentation, 2018, 4(2): 44-55. |
| 26 | WASSERSTROM L, PORTUGAL-NUNES D, ALMQVIST H, et al. Exploring D-xylose oxidationin Saccharomyces cerevisiae through the Weimberg pathway[J]. AMB Express, 2018, 8: 33. |
| 27 | NDUKO J M, MATSUMOTO K, OOI T, et al. Effectiveness of xylose utilization for high yield production of lactate-enriched p(lactate-co-3-hydroxybutyrate) using a lactate-overproducing strain of Escherichia coli and an evolved lactate-polymerizing enzyme[J]. Metabolic Engineering, 2013, 15: 159-166. |
| 28 | SEKAR R, SHIN H D, DICHRISTINA T J. Activation of an otherwise silent xylose metabolic pathway in Shewanella oneidensis [J]. Applied and Environmental Microbiology, 2016, 82(13): 3996-4005. |
| 29 | LI Feng, LI Yuanxiu, SUN Liming, et al. Engineering Shewanella oneidensis enables xylose-fed microbial fuel cell[J]. Biotechnology for Biofuels, 2017, 196(10). |
| 30 | SHEN Lu, KOHLHAAS Martha, ENOKI Junichi, et al. A combined experimental and modelling approach for the Weimberg pathway optimisation[J]. Nature Communications, 2020, 11. |
| 31 | HALMSCHLAG B, HOFFMANN K, HANKE R, et al. Comparison of isomerase and weimberg pathway for γ-PGA production from xylose by engineered Bacillus subtilis [J]. Frontiers in Bioengineering and Biotechnology, 2020, 7: 476. |
| 32 | BATOR I, WITTGENS A, ROSENAU F, et al. Comparison of three xylose pathways in Pseudomonas putida KT2440 for the synthesis of valuable products[J]. Frontiers in Bioengineering and Biotechnology, 2020, 7: 480. |
| 33 | CAO Yujin, NIU Wei, GUO Jiatao, et al. Biotechnological production of 1,2,4-butanetriol: an efficient process to synthesize energetic material precursor from renewable biomass[J]. Scientific Reports, 2015, 5: 18149. |
| 34 | LIU Min, DING Yamei, XIAN Mo, et al. Metabolic engineering of a xylose pathway for biotechnological production of glycolate in Escherichia coli [J]. Microbial Cell Factories, 2018, 17(1): 51-62. |
| 35 | CAO Yujin, XIAN Mo, ZOU Huibin, et al. Metabolic engineering of Escherichia coli for the production of xylonate[J]. PLoS ONE, 2013, 8(7): e67305. |
| 36 | BRÜSSELER C, SPATH A, SOKOLOWSKY S, et al. Alone at last! Heterologous expression of a single gene is sufficient for establishing the five-step Weimberg pathway in Corynebacterium glutamicum complete oxidation of xylose for bioelectricity generation by reconstructing a bacterial xylose utilization pathway in vitro[J]. Metabolic Engineering Communications, 2019, 9: e00090. |
| 37 | 高歌. 谷氨酸棒杆菌利用木糖合成L-鸟氨酸的代谢工程改造研究[D]. 上海: 华东理工大学, 2020. |
| GAO Ge. Research on metabolic engineering of using xylose to synthesize L-ornithine[D]. Shanghai: East China University of Science and Technology, 2020. | |
| 38 | CAM Y, ALKIM C, TRICHEZ D, et al. Engineering of a synthetic metabolic pathway for the assimilation of (D)-xylose into value-added chemicals[J]. ACS Synthetic Biology, 2015, 5(7): 607-618. |
| 39 | PEREIRA B, LI Zhengjun, DE MEY M, et al. Efficient utilization of pentoses for bioproduction of the renewable two-carbon compounds ethylene glycol and glycolate[J]. Metabolic Engineering, 2016, 34: 80-87. |
| 40 | URANUKUL B, WOOLSTON B M, FINK G R, et al. Biosynthesis of monoethylene glycol in Saccharomyces cerevisiae utilizing native glycolytic enzymes[J]. Metabolic Engineering, 2019, 51: 20-31. |
| 41 | CHOMVONG K, BAUER S, BENJAMIN D I, et al. Bypassing the pentose phosphate pathway: towards modular utilization of xylose[J]. PLoS ONE, 2016, 11(6): e0158111. |
| 42 | LI Qingyan, ZHAO Peng, YIN Hang, et al. CRISPR interference-guided modulation of glucose pathways to boost aconitic acid production in Escherichia coli [J]. Microbial Cell Factories, 2020, 19(1): 174-187. |
| 43 | KLINGNER A, BARTSCH A, DOGS M, et al. Large-scale 13C flux profiling reveals conservation of the entner-doudoroff pathway as a glycolytic strategy among marine bacteria that use glucose[J]. Applied and Environmental Microbiology, 2015, 81: 2408-2422. |
| 44 | LIU Hua, WANG Weinan, DENG Li, et al. High production of fumaric acid from xylose by newly selected strain Rhizopus arrhizus RH7-13-9#[J]. Bioresource Technology, 2015, 186: 348-350. |
| 45 | LEE T C, XIONG Wencheng, PADDOCK T, et al. Engineered xylose utilization enhances bio-products productivity in the Cyanobacterium Synechocystis sp. PCC6803[J]. Metabolic Engineering, 2015, 30: 179-189. |
| 46 | CHEN Zhen, HUANG Jinhai, WU Yao, et al. Metabolic engineering of Corynebacterium glutamicum for the production of 3-hydroxypropionic acid from glucose and xylose[J]. Metabolic Engineering, 2017, 39: 151-158. |
| 47 | 祖金珊, 徐世文, 陆信曜, 等. 整合型1, 2, 4-丁三醇重组菌的构建及共底物发酵[J]. 应用与环境生物学报, 2019, 25(4): 0966-0971. |
| ZU Jinshan, XU Shiwen, LU Xinyao, et al. Construction of a 1, 2, 4-butanetriol-integrated strain and fermentation of its co-substrate[J]. Chinese Journal of Applied and Environmental Biology, 2019, 25(4): 0966-0971. | |
| 48 | 陈雪松, 杨胜利. 高产木糖醇菌株的诱变选育及其发酵培养基优化[J]. 上海化工, 2021, 46(1): 11-15. |
| CHEN Xuesong, YANG Shengli. Mutagenesis breeding on high-productive strain of xylitol and optimization of its fermentation medium[J]. Shanghai Chemical Industry, 2021, 46(1): 11-15. | |
| 49 | CHIANG C J, LEE H M, GUO Hanjie, et al. Systematic approach to engineer Escherichia coli pathways for co-utilization of a glucose-xylose mixture[J]. Journal of Agricultural and Food Chemistry, 2013, 61: 7583-7590. |
| 50 | 张佳. 通过基因工程改造马克思克鲁维酵母实现高温高产木糖醇和乙醇[D]. 合肥: 中国科学技术大学, 2017. |
| ZHANG Jia. Xylitol or ethanol production at high temperature by engineered Kluyveromyces marxianus [D]. Hefei: University of Science and Technology of China, 2017. | |
| 51 | ZHANG Biao, REN Lili, ZHAO Zepeng, et al. High temperature xylitol production through simultaneous co-utilization of glucose and xylose by engineered Kluyveromyces marxianus [J]. Biochemical Engineering Journal, 2021, 165: 10782. |
| 52 | LEDESMA-AMARO R, LAZAR Z, Rakicka M, et al. Metabolic engineering of Yarrowia lipolytica to produce chemicals and fuels from xylose[J]. Metabolic Engineering, 2016, 38: 115-124. |
| 53 | 张艳芬. 产油酵母筛选及其利用葡萄糖和木糖的产油特性[D]. 呼和浩特: 内蒙古大学, 2014. |
| ZHANG Yanfen. The screening of olegious yeasts and the oil production characteristics in the using of glucose and xylose[D]. Hohhot: Inner Mongolia University, 2014. | |
| 54 | HUANG Chao, CHEN Xuefang, YANG Xiaoyan, et al. Bioconversion of corncob acid hydrolysate into microbial oil by the oleaginous yeast Lipomyces starkeyi [J]. Applied Biochemistry and Biotechnology, 2014, 172(4): 2197-2204. |
| 55 | FANG Junnan, HUAN Chenchen, LIU Yang, et al. Bioconversion of agricultural waste into poly-γ-glutamic acid in solid-state bioreactors at different scales[J]. Waste Management, 2020, 10(2): 939-948. |
| 56 | WEN Shuang, LIU Luo, NIE Kaili, et al. Enhanced fumaric acid production by fermentation of xylose using a modified strain of Rhizopusarrhizus [J]. BioResources, 2013, 8(2): 2186-2194. |
| 57 | ZHOU Zhengxiong, DU Guocheng, HUA Zhaozhe, et al. Optimization of fumaric acid production by Rhizopus delemar based on the morphology formation[J]. Bioresource Technology, 2011, 102(20): 9345-9349. |
| 58 | 潘丽军, 庞锐, 吴学凤, 等. 葡萄糖和木糖共发酵生产L-乳酸的培养基和培养条件响应面优化[J]. 食品科学, 2011, 32(9): 140-145. |
| PAN Lijun, PANG Rui, WU Xuefeng, et al. Optimization of co-fermentation of glucose and xylose for L-lactic acid production using response surface methodology[J]. Food Science, 2011, 32(9): 140-145. | |
| 59 | 姜珊. 利用木质纤维素高温生产乳酸的研究[D]. 上海: 上海交通大学, 2019. |
| JIANG Shan. High temperature production of lactic acid from lignocellulose [D]. Shanghai: Shanghai JiaoTong University, 2019. | |
| 60 | ZHANG Yuming, CHEN Xiangrong, LUO Jianquan, et al. An efficient process for lactic acid production from wheat straw by a newly isolated Bacillus coagulans strain IPE22[J]. Bioresource Technology, 2014(158): 396-399. |
| 61 | WANG Yujue, CAO Weifeng, LUO Jianquan, et al. One step open fermentation for lactic acid production from inedible starchy biomass by thermophilic Bacillus coagulans IPE22[J]. Bioresource Technology, 2019(272): 398-406. |
| 62 | FU Hongxin, YU Le, LIN Meng, et al. Metabolic engineering of Clostridium tyrobutyricum for enhanced butyric acid production from glucose and xylose[J]. Metabolic Engineering, 2017, 40: 50-58. |
| 63 | ABDEL-RAHMAN M A, XIAO Yaotian, TASHIRO Y, et al. Fed-batch fermentation for enhanced lactic acid production from glucose/xylose mixture without carbon catabolite repression[J]. Journal of Bioscience and Bioengineering, 2015, 119(2): 153-158. |
| 64 | JIN Yongsu, CATE J H. Metabolic engineering of yeast for lignocellulosic biofuel production[J]. Current Opinion in Chemical Biology, 2017, 41: 99-106. |
| 65 | AZHAR S H M, ABDULLA R, JAMBO S A, et al. Yeasts in sustainable bioethanol production: a review[J]. Biochemistry and Biophysics Reports, 2017, 10: 52-61. |
| 66 | WAHLBOM C F, VAN ZYL W H, JONSSON L J, et al. Generation of the improves recombinant xylose-utilizing S. cerevisiae TMB 3400 by random mutagenesis and physiological comparison with P. stipitis CBS6054[J]. FEMS Yeast Research, 2003, 3(3): 319-326. |
| 67 | SHI N Q, DAVIS B, SHERMAN F, et al. Disruption of the cytochrome c gene in xylose-utilizing yeast Pichia stipitis leads to higher ethanol production[J]. Yeast, 1999(15): 1021-1030. |
| 68 | WATANABE S, SAWAYAMA S. Pentose transporter: US 20120270290[P]. 2012-10-25. |
| 69 | LI Lulu, ZHANG Ling, WANG Dongmei, et al. Identification of a xylitol dehydrogenase gene from Kluyveromyces marxianus NBRC1777[J]. Molecular Biotechnology, 2013, 53(2): 159-169. |
| 70 | ZHANG Biao, ZHANG Ling, WANG Dongmei, et al. Identification of a xylose reductase gene in the xylose metabolic pathway of Kluyveromycesmarxianus NBRC1777[J]. Journal of Industrial Microbiology & Biotechnology, 2011, 38(12): 2001-2010. |
| 71 | WANG Rongliang, ZHANG Ling, WANG Dongmei, et al. Identification of a xylulokinase catalyzing xylulose phosphorylation in the xylose metabolic pathway of Kluyveromyces marxianus NBRC1777[J]. Journal of Industrial Microbiology & Biotechnology, 2011, 38(10): 1739-1746. |
| 72 | 韩锡铜, 高教琪, 陈丽杰, 等. Kluyveromyces marxianus 发酵己糖和戊糖生产乙醇[J]. 生物加工过程, 2015, 13(1): 6-11. |
| HAN Xitong, GAO Jiaoqi, CHEN lijie, et al. Ethanol production from hexose and pentose by Kluyveromyces marxianus [J]. Chinese Journal of Bioprocess Engineering, 2015,13(1): 6-11. | |
| 73 | 王金保. 重组大肠杆菌生物合成D-1, 2, 4-丁三醇的系统优化[D]. 北京: 北京理工大学, 2017. |
| WANG Jinbao. Systematic optimization of synthetic pathways for D-1,2,4-butanetriol production by recombinant E. coli[D]. Beijing: Beijing Institute of Technology, 2017. | |
| 74 | VALDEHUESA K N G, LIU Huaiwei, RAMOS K R M, et al. Direct bioconversion of D-xylose to 1,2,4-butanetriol in an engineered Escherichia coli [J]. Process Biochemistry, 2014, 49(1): 25-32. |
| 75 | 孙雷, 杨帆, 朱泰承, 等. 大肠杆菌合成1,2,4-丁三醇的途径优化[J]. 生物工程学报, 2016, 32(1): 51-63. |
| SUN Lei, YANG Fan, ZHU Taicheng, et al. Optimization of 1, 2, 4-butanetriol synthetic pathway in Escherichia coli [J]. Chinese Journal of Biotechnology, 2016, 32(1): 51-63. | |
| 76 | DIEN B S, NICHOLS N N, BOTHAST R J. Fermentation of sugar mixtures using Escherichia coli catabolite repression mutants engineered for production of L-lactic acid[J]. Journal of Industrial Microbiology & Biotechnology, 2002, 29(5): 221-227. |
| 77 | 何姝颖, 诸葛斌, 陆信曜, 等. 副产物途径的缺失对大肠杆菌合成D-1,2,4-丁三醇的影响[J]. 微生物学通报, 2017, 44(1): 30-37. |
| HE Shuying, ZHUGE Bin, LU Xinyao, et al. Influence of the deficiency of by-product pathways on biosynthesis of D-1,2,4-butanetriol in Escherichia coli [J]. Microbiology China, 2017, 44(1): 30-37. | |
| 78 | 李玉石, 刘郁青, 杨程雨, 等. 代谢改造克雷伯氏菌合成 D-1,2,4-丁三醇[J]. 微生物学通报, 2020, 47(8): 2505-2515. |
| LI Yushi, LIU Yuqing, YANG Chengyu, et al. Production of D-1, 2, 4-butanetriol by engineering Klebsiella pneumoniae [J]. Microbiology China, 2020, 47(8): 2505-2515. | |
| 79 | YUKAWA T, BAMBA T, GUIRIMAND G, et al. Optimization of 1,2,4-butanetriol production from xylose in Saccharomyces cerevisiae by metabolic engineering of NADH/NADPH balance[J]. Biotechnology and Bioengineering, 2020: 1-11. |
| 80 | 秦海青, 邱学良, 王成福, 等. 酵母发酵生产木糖醇的研究[J]. 中国食品添加剂, 2013(3): 152-157. |
| QIN Haiqing, QIU Xueliang, WANG Chengfu, et al. Research on xylitol production by yeast[J]. Chinese Food Additives, 2013(3): 152-157. | |
| 81 | SU Buli, WU Mianbin, LIN Jianping, et al. Metabolic engineering strategies for improving xylitol production from hemicellulosic sugars[J]. Biotechnology Letters, 2013, 35(11): 1781-1789. |
| 82 | GUMBI S T, KUMAR A, OLANIRAN A O. Lipid productivity and biosynthesis gene response of indigenous microalgae Chlorella sp.T4 strain for biodiesel production under different nitrogen and phosphorus load[J]. BioEnergy Research, 2022: 1-12. |
| 83 | CHO H U, PARK J M. Biodiesel production by various oleaginous microorganisms from organic wastes[J]. Bioresource Technology, 2018, 256: 502-508. |
| 84 | YANG Yan, HEIDARI F, HU Bo. Fungi(mold)-based lipid production[J]. Methods in Molecular Biology, 2019, 1995: 51-89. |
| 85 | SITEPU I R, GARAY L A, SESTRIC R, et al. Oleaginous yeasts for biodiesel: current and future trends in biology and production[J]. Biotechnology Advances, 2014, 32(7): 1336-1360. |
| 86 | GILL C, HALL M, RATLEDGE C. Lipid accumulation in an oleaginous yeast (Candida 107) growing on glucose in single-stage continuous culture[J]. Applied and Environmental Microbiology, 1977, 33(2): 231-239. |
| 87 | QIN Lei, LIU Lu, ZENG Anping, et al. From low-cost substrates to single cell oils synthesized by oleaginous yeasts[J]. Bioresource Technology, 2017, 245: 1507-1519. |
| 88 | 孔祥莉, 刘波, 赵宗保, 等. 斯达氏油脂酵母利用混合糖发酵产油脂[J]. 生物加工过程, 2007, 5(2): 36-41. |
| KONG Xiangli, LIU Bo, ZHAO Zongbao, et al. Microbial production of lipids by co-fermentation of glucose and xylose with Lipomyces starkeyi [J]. Chinese Journal of Bioprocess Engineering, 2007, 5(2): 36-41. | |
| 89 | SITEPU I R, JIN Mingjie, FERNANDEZ J E, et al. Identification of oleaginous yeast strains able to accumulate high intracellular lipids when cultivated in alkaline pretreated corn stover[J]. Applied Microbiology and Biotechnology, 2014, 98(17): 7645-7657. |
| 90 | QIU Yimin, WANG Qin, ZHU Chengjun, et al. Deciphering metabolic responses of biosurfactant lichenysin on biosynthesis of poly-γ- glutamic acid[J]. Applied Microbiology and Biotechnology, 2019, 103(5): 4003-4015. |
| 91 | FENG Jun, GU Yanyan, QUAN Yufen, et al. Construction of energy-conserving sucrose utilization pathways for improving poly-γ-glutamic acid production in Bacillus amyloliquefaciens [J]. Microbial Cell Factories, 2017, 16(98): 1-13. |
| 92 | FENG Jun, QUAN Yufen, GU Yanyan, et al. Enhancing poly-γ- glutamic acid production in Bacillus amyloliquefaciens by introducing the glutamate synthesis features from Corynebacterium glutamicum [J]. Microbial Cell Factories, 2017, 16(88): 1-12. |
| 93 | FENG Jun, GU Yanyan, QUAN Yufen, et al. Improved poly-γ- glutamic acid production in Bacillus amyloliquefaciens by modular pathway engineering[J]. Metabolic Engineering, 2015, 32(6): 106-115. |
| 94 | SAHA B C. Hemicellulose bioconversion[J]. Journal of Industrial Microbiology & Biotechnology, 2003, 30: 279-291. |
| 95 | ENGEL C A ROA, VAN GULIK W M, MARANG L, et al. Development of a low pH fermentation strategy for fumaric acid production by Rhizopus oryzae [J]. Enzyme and Microbial Technology, 2011, 48(1): 39-47. |
| 96 | ZHOU Yuqing, NIE Kaili, ZHANG Xin, et al. Production of fumaric acid from biodiesel-derived crude glycerol by Rhizopus arrhizus [J]. Bioresource Technology, 2014, 163: 48-53. |
| 97 | KAUTOLA H, LINKO Y Y. Fumaric acid production from xylose by immobilized Rhizopus arrhizus cells[J]. Applied Microbiology and Biotechnology, 1989, 31(5): 448-452. |
| 98 | YE Lidan, ZHOU Xingding, HUDARI M S B, et al. Highly efficient production of L-lactic acid from xylose by newly isolated Bacillus coagulans C106[J]. Bioresource Technology, 2013,132: 38-44. |
| 99 | LI Haibo, SCHMITZ O, ALPER H S. Enabling glucose/xylose co-transport in yeast through the directed evolution of a sugar transporter[J]. Applied Microbiology and Biotechnology, 2016, 100(23): 10215-10223. |
| 100 | TANG Ruiqi, WAGNER J M, ALPER H S, et al. Design, evolution, and characterization of a xylose biosensor in Escherichia coli using the XylR/xylO system with an expanded operating range[J]. ACS Synthetic Biology, 2020, 9(10): 2714-2722. |
| 101 | TRAN P H N, KO J K, GONG J, et al. Improved simultaneous co-fermentation of glucose and xylose by Saccharomyces cerevisiae for efficient lignocellulosic biorefinery[J]. Biotechnology for Biofuels, 2020, 13: 12. |
| 102 | DIAZ C A C, BENNETT R K, PAPOUTSAKIS E T, et al. Deletion of four genes in Escherichia coli enables preferential consumption of xylose and secretion of glucose[J]. Metabolic Engineering, 2019, 52: 168-177. |
| 103 | WU Youduo, XUE Chuang, CHEN Lijie, et al. Synergistic effect of calcium and zinc on glucose/xylose utilization and butanol tolerance of Clostridium acetobutylicum [J]. FEMS Microbiology Letters, 2016, 363(5): fnw023. |
| 104 | JOJIMA T, OMUMASABA C, INUI M, et al. Sugar transporters in efficient utilization of mixed sugar substrates: current knowledge and outlook[J]. Applied Microbiology and Biotechnology, 2010, 85(3): 471-480. |
| 105 | NIJLAND J G, VOS E, SHIN H Y, et al. Improving pentose fermentation by preventing ubiquitination of hexose transporters in Saccharomyces cerevisiae [J]. Biotechnology for Biofuels, 2016, 9: 158. |
| 106 | HAMACHER T, BECKER J, GÁRDONYI M, et al. Characterization of the xylose-transporting properties of yeast hexose transporters and their influence on xylose utilization[J]. Microbiology, 2002, 148(9): 2783-2788. |
| 107 | SEDLAK M, HO N W Y. Characterization of the effectiveness of hexose transporters for transporting xylose during glucose and xylose co-fermentation by a recombinant Saccharomyces yeast [J]. Yeast, 2004, 21(8): 671-684. |
| 108 | SALOHEIMO A, RAUTA J, STASYK O, et al. Xylose transport studies with xylose-utilizing Saccharomyces cerevisiae strains expressing heterologous and homologous permeases[J]. Applied Microbiology and Biotechnology, 2007, 74(5): 1041-1052. |
| 109 | YOUNG E, POUCHER A, COMER A, et al. Functional survey for heterologous sugar transport proteins, using Saccharomyces cerevisiae as a host[J]. Applied and Environmental Microbiology, 2011, 77(10): 3311-3319. |
| 110 | NADAI C, CROSATO G, GIACOMINI A, et al. Different gene expression patterns of hexose transporter genes modulate fermentation performance of four Saccharomyces cerevisiae strains[J]. Fermentation, 2021, 7(3): 164-181. |
| 111 | SHIN H Y, NIJLAND J G, DE WAAL P P, et al. An engineered cryptic Hxt11sugar transporter facilitates glucose-xylose co-consumption in Saccharomyces cerevisiae [J]. Biotechnology for Biofuels, 2015, 8: 176. |
| 112 | 蔡艳青, 齐显尼, 齐奇, 等. 敲除MIG1和SNF1基因对酿酒酵母共利用葡萄糖和木糖的影响[J]. 生物工程学报, 2018, 34(1): 54-67. |
| CAI Yanqing, QI Xianni, QI Qi, et al. Effect of MIG1 and SNF1 deletion on simultaneous utilization of glucose and xylose by Saccharomyces cerevisiae [J]. Chinese Journal of Biotechnology, 2018, 34(1): 54-67. | |
| 113 | GAO Weixia, HE Yulian, ZHANG Fang, et al. Metabolic engineering of Bacillus Amyloliquefaciens WLL3 for enhanced poly-γ-glutamic acid synthesis[J]. Microbial Biotechnology, 2019, 12(5): 932-945. |
| 114 | ZHANG Bobo, LU Liping, XU Ganrong. Why solid-state fermentation is more advantageous over submerged fermentation for converting high concentration of glycerol into Monacolin K by Monascus purpureus 9901: a mechanistic study[J]. Journal of Biotechnology, 2015, 206: 60-65. |
| 115 | MUKIYAT C, EVELYN E, BAHRI S, et al. A novel immobilization method of Saccharomyces cerevisiae on fermentation of nipa palm sap for fuel grade bioethanol production[J]. Key Engineering Materials, 2020, 849: 53-57. |
| 116 | GAO Hao, LU Jiasheng, JIANG Yujia, et al. Material-mediated cell immobilization technology in the biological fermentation process[J]. Biofuels Bioproducts & Biorefining, 2021, 15: 1160-1173. |
| 117 | XU Lei, TSCHIRNER U. Immobilized anaerobic fermentation for biofuel production by Clostridium co-culture[J]. Bioprocess and Biosystems Engineering, 2014, 37(8): 1551-1559. |
| 118 | EVRIM GÜNEŞ A. Ethanol production from starch by co-immobilized amyloglucosidase——Zymomonas Mobilis cells in a continuously-stirred bioreactor[J]. Biotechnology & Biotechnological Equipment, 2013, 27(1): 3506-3512. |
| 119 | VALDEZ-VAZQUEZA I, CASTILLO-RUBIO L G, PÉREZ-RANGEL M, et al. Enhanced hydrogen production from lignocellulosic substrates via bioaugmentation with Clostridium strains[J]. Industrial Crops & Products, 2019, 137: 105-111. |
| 120 | ABE A, FURUKAWA S, WATANABE S, et al. Yeasts and lactic acid bacteria mixed-specie biofilm formation is a promising cell immobilization technology for ethanol fermentation[J]. Applied Biochemistry and Biotechnology, 2013, 171: 72-79. |
| 121 | 江枫, 房伶晏, 王浩, 等. 固定化混合菌利用戊糖和己糖共发酵制备乙醇[J]. 林业工程学报, 2017, 2(4): 90-95. |
| JIANG Feng, FANG Lingyan, WANG Hao, et al. Co-fermentation of pentose and hexose to ethanol by immobilized mixed yeasts method[J]. Journal of Forestry Engineering, 2017, 2(4): 90-95. | |
| 122 | FERREIRA J, SANTOS V A Q, CRUZ C H G. Ethanol production by co-culture of Zymomonas mobilis and Pachysolen tannophilus using banana peels hydrolysate as substrate[J]. Acta Scientiarum Technology, 2018, 40(1): 35169. |
| 123 | LI Feng, YIN Changyi, SUN Liming, et al. Synthetic Klebsiella pneumoniae-Shewanella oneidensis consortium enables glycerol-fed high-performance microbial fuel cells[J]. Biotechnology Journal, 2018, 13(5): 1700491. |
| 124 | LI Feng, AN Xingjuan, WU Deguang, et al. Engineering microbial consortia for high-performance cellulosic hydrolyzates-fed microbial fuel cells [J]. Frontiers in Microbiology, 2019, 10: 409. |
| 125 | LIN Tong, BAI Xue, HU Yidan, et al. Synthetic Saccharomyces cerevisiae-Shewanella oneidensis consortium enables glucose-fed high-performance microbial fuel cell[J]. AIChE Journal, 2017, 63(6): 1830-1838. |
| 126 | ZHANG Yixing, VADLANI P V. Lactic acid production from biomass-derived sugars via co-fermentation of Lactobacillus brevis and Lactobacillus plantarum [J]. Journal of Bioscience and Bioengineering, 2015, 119(6): 694-699. |
| 127 | SAINI M, LIN Liju, CHIANG C J, et al. Synthetic consortium of Escherichia coli for n-butanol production by fermentation of the glucose-xylose mixture[J]. Journal of Agricultural and Food Chemistry, 2017, 65: 10040-10047. |
| 128 | WEN Zhiqiang, MINTON N P, ZHANG Ying, et al. Enhanced solvent production by metabolic engineering of a twin-clostridial consortium[J]. Metabolic Engineering, 2017, 39: 38-48. |
| 129 | THEIRI M, CHADJAA H, MARINOVA M, et al. Development of sequential and simultaneous bacterial cultures to hydrolyse and detoxify wood pre-hydrolysate for enhanced acetone-butanol-ethanol (ABE) production[J]. Enzyme and Microbial Technology, 2020, 133: 109438. |
| 130 | SU Yikai, WILLIS L B, REHMANN L, et al. Spathaspora passalidarum selected for resistance to AFEX hydrolysate shows decreased cell yield[J]. FEMS Yeast Research, 2018, 18(6): 1-13. |
| 131 | 张影, 苟敏, 孙照勇, 等. 混合糖发酵条件下甲酸抑制木糖发酵的机制[J]. 应用和环境生物学报, 2017, 23(6): 0990-0998. |
| ZHANG Yin, GOU Min, SUN Zhaoyong, et al. The inhibitory mechanism of formic acid on xylose fermentation during mixed sugar fermentation[J]. Chinese Journal of Applied and Environmental Biology, 2017, 23(6): 0990-0998. | |
| 132 | JIN Xianchun, MA Jiangshan, SONG Jianing, et al. Promoted bioethanol production through fed-batch semisimultaneous saccharification and fermentation at a high biomass load of sodiumcarbonate-pretreated rice straw[J]. Energy, 2021, 226: 120353. |
| 133 | ZHANG Zhenting, LI Yanan, ZHANG Jianguo, et al. High-titer lactic acid production by Pediococcus acidilactici PA204 from corn stover through fed-batch simultaneous saccharification and fermentation[J]. Microorganisms, 2020, 8(10): 1491-1500. |
| 134 | DU Cong, LI Yimin, ZONG Han, et al. Production of bioethanol and xylitol from non-detoxified corn cob via a two-stage fermentation strategy[J]. Bioresource Technology, 2020, 310: 123427. |
| [1] | LI Mengyuan, GUO Fan, LI Qunsheng. Simulation and optimization of the third and fourth distillation columns in the recovery section of polyvinyl alcohol production [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 113-123. |
| [2] | SHENG Weiwu, CHENG Yongpan, CHEN Qiang, LI Xiaoting, WEI Jia, LI Linge, CHEN Xianfeng. Operating condition analysis of the microbubble and microdroplet dual-enhanced desulfurization reactor [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 142-147. |
| [3] | WANG Xueting, GU Xia, XU Xianbao, ZHAO Lei, XUE Gang, LI Xiang. Effectiveness of hydrothermal pretreatment on valeric acid production during food waste fermentation [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4994-5002. |
| [4] | HAN Hengwen, HAN Wei, LI Mingfeng. Research progress in olefin hydration process and the catalysts [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3489-3500. |
| [5] | QIN Kai, YANG Shilin, LI Jun, CHU Zhenyu, BO Cuimei. A Kalman filter algorithm-based high precision detection method for glucoamylase biosensors [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3177-3186. |
| [6] | HUANG Nengkun, WANG Ziwen, WANG Wengeng, WANG Xuefeng, TAN Jihuai, ZHU Xinbao. Design and synthesis of hydrogenated dimeric acid ethylene glycol ether esters as highly-efficient plasticizers for PVC [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2638-2646. |
| [7] | HUANG Yue, ZHAO Lixin, YAO Zonglu, YU Jiadong, LI Zaixing, SHEN Ruixia, AN Kemeng, HUANG Yali. Research progress in directed bioconversion of lactic acid and acetic acid from wood lignocellulosic wastes [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2691-2701. |
| [8] | TIAN Yuan, LOU Shujie, MENG Shanru, YAN Jingru, XIAO Haicheng. Recent progress of Co-based catalysts for higher alcohols synthesis form syngas [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 1869-1876. |
| [9] | XIAO Yaoxin, ZHANG Jun, HU Sheng, SHAN Rui, YUAN Haoran, CHEN Yong. Cu-Zn catalyzed hydrogenation of furfural with methanol as hydrogen donor [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1341-1352. |
| [10] | CHEN Hao, ZHANG Chuanhao, YU Feng, FAN Binbin, LI Ruifeng. Catalytic performance of zeolite Y in oligomerization of isobutyl alcohol [J]. Chemical Industry and Engineering Progress, 2023, 42(2): 794-802. |
| [11] | GUO Xiaoyu, LI Dongchen, ZHAO Wei, DU Zhenyi, LI Xiaoliang. Preparation of Au-Pd/MnO2 catalyst and its catalytic performance for benzyl alcohol oxidation [J]. Chemical Industry and Engineering Progress, 2023, 42(10): 5223-5231. |
| [12] | LI Wanqi, YANG Fengjuan, JIA Dechen, JIANG Weihong, GU Yang. Biological utilization and conversion of syngas [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 73-85. |
| [13] | XUE Machen, YANG Bolun, XIA Chungu, ZHU Gangli. Progress in heterogeneous catalyst for ethanol upgrading to higher (C6+) alcohols [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 194-203. |
| [14] | TIAN Yazhou, HU Yujing, LI Jiyou, REN Jiangyan, WANG Liwei, WANG Xiuli, DING Ying, CHENG Jue, ZHANG Junying. Synthesis, curing kinetics and properties of vanilla alcohol-based epoxy resin [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 477-484. |
| [15] | SUN Guoqi, WANG Wei, SONG Bing, WANG Liang, SHAO Ruiqi, XU Zhiwei, LUO Shigang, YAN Minjie, WANG Lijing, QIAN Xiaoming. Research progress of thermoplastic modification of polyvinyl alcohol [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 293-306. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||