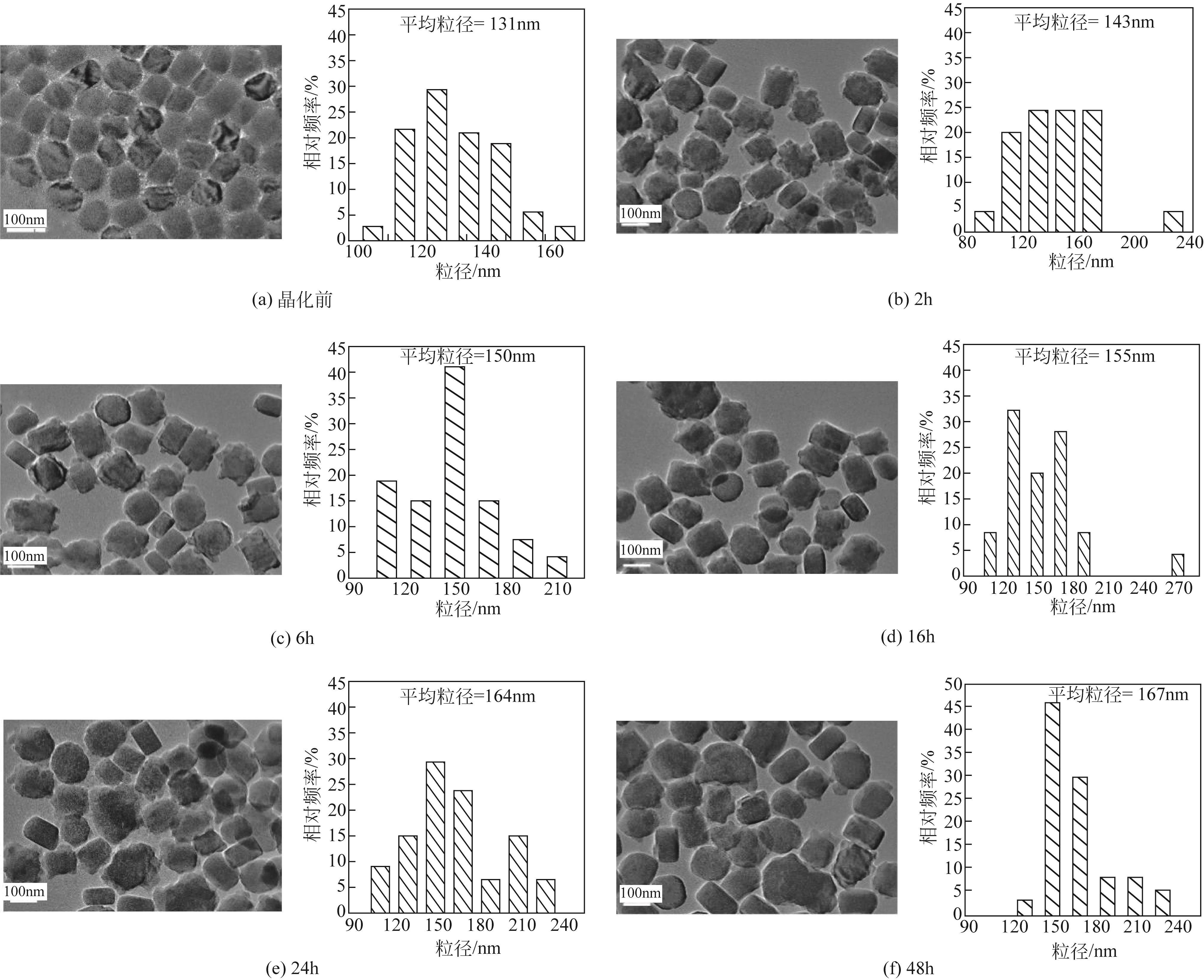
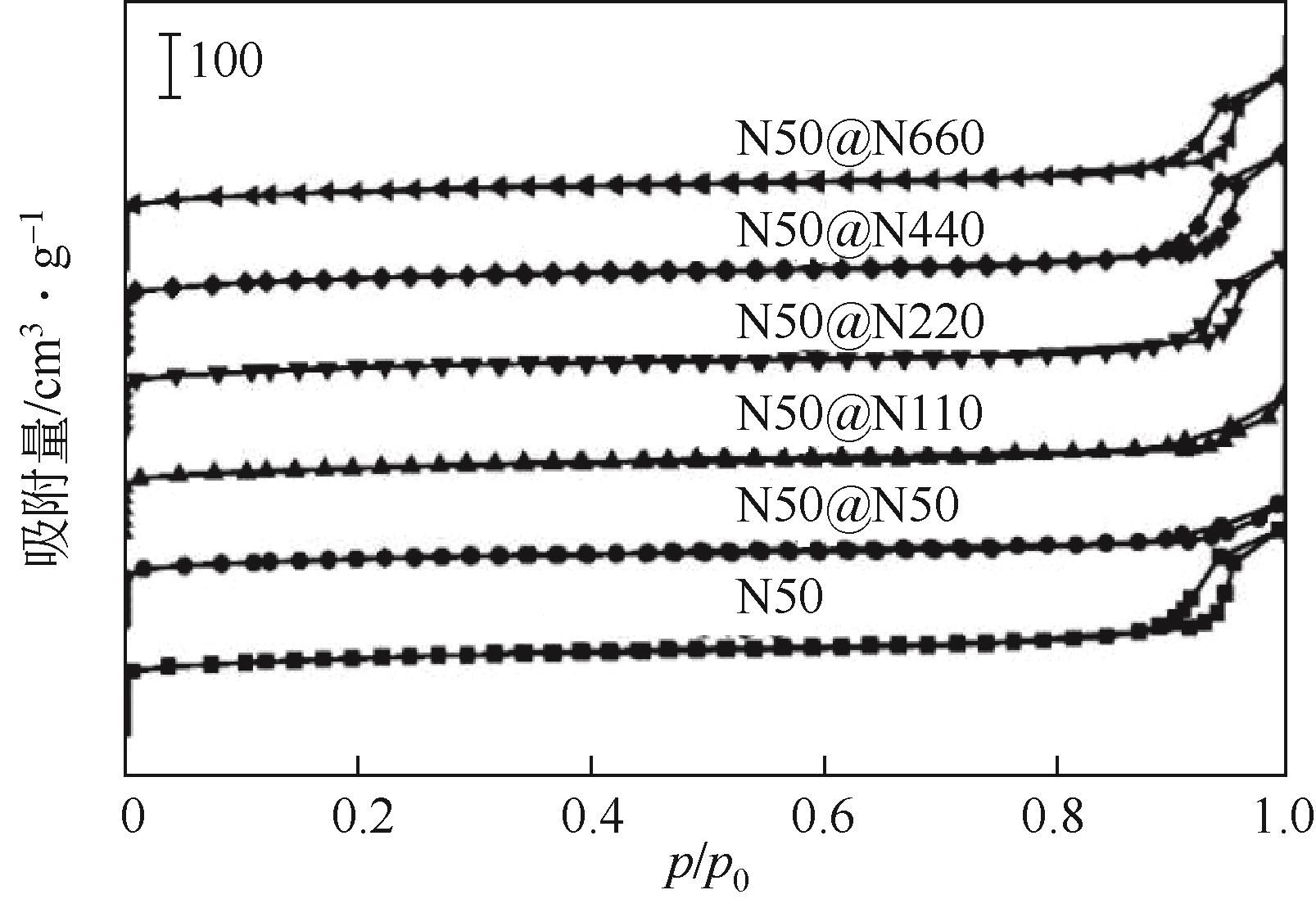
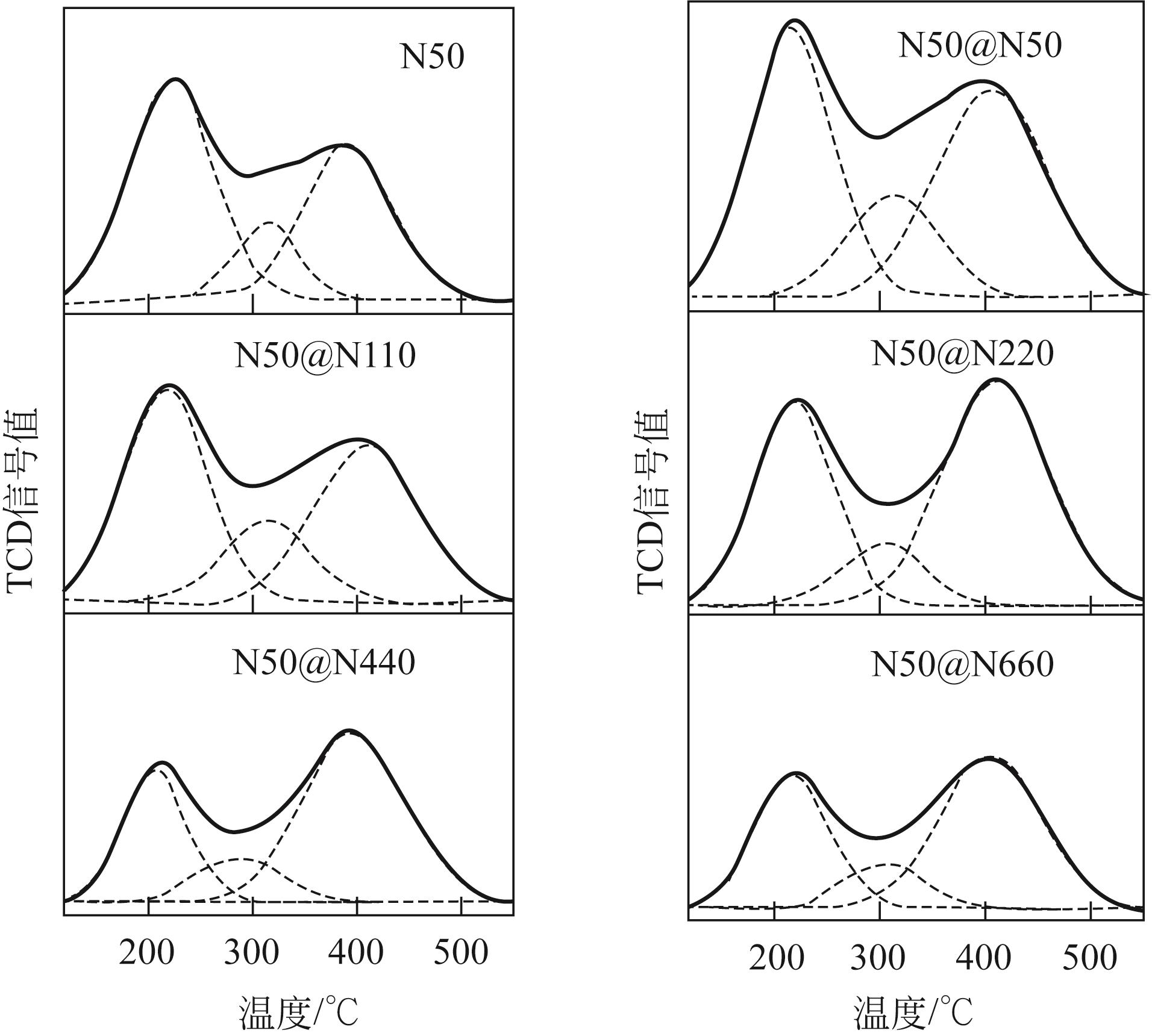
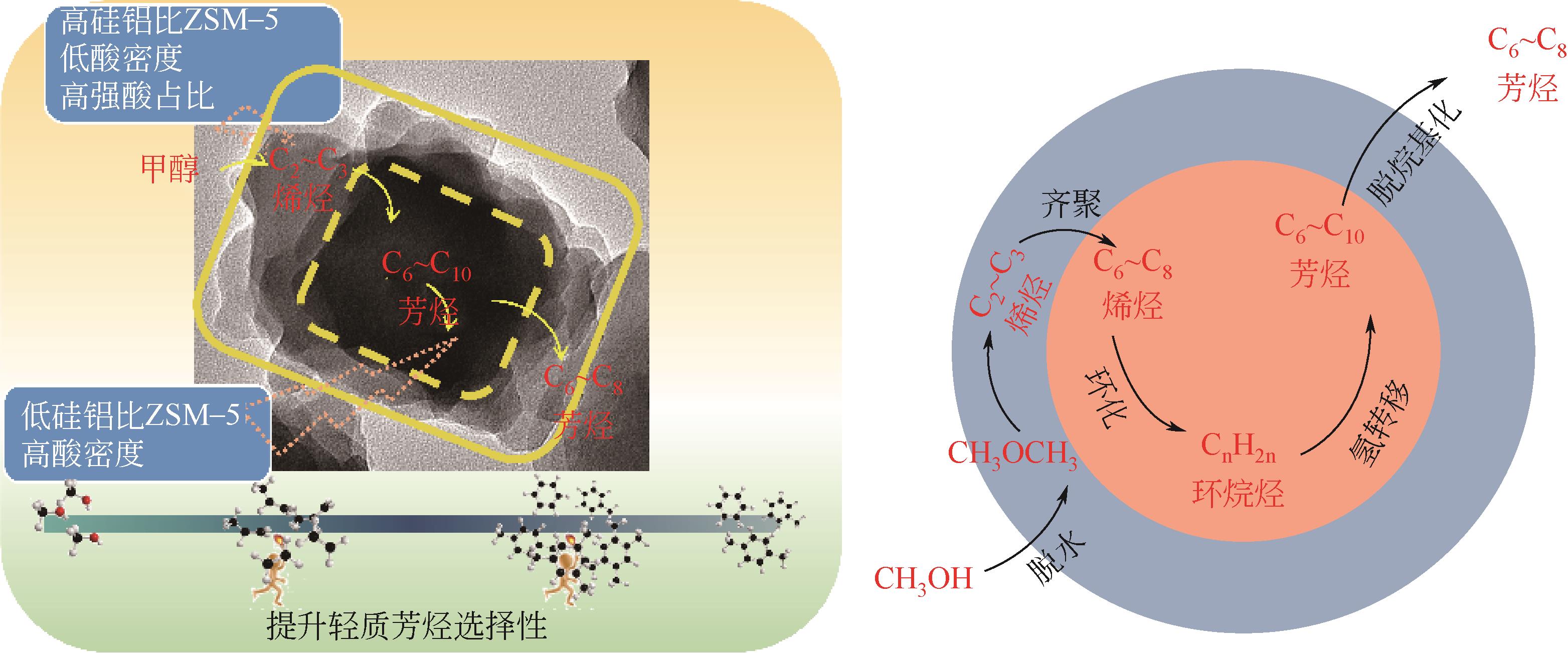
| 1 |
LI Teng, SHOINKHOROVA Tuiana, GASCON Jorge, et al. Aromatics production via methanol-mediated transformation routes[J]. ACS Catalysis, 2021, 11(13): 7780-7819.
|
| 2 |
陈嵩嵩, 张国帅, 霍锋, 等. 煤基大宗化学品市场及产业发展趋势[J]. 化工进展, 2020, 39(12): 5009-5020.
|
|
CHEN Songsong, ZHANG Guoshuai, HUO Feng, et al. Market and technology development trends of coal-based bulk chemicals[J]. Chemical Industry and Engineering Progress, 2020, 39(12): 5009-5020.
|
| 3 |
米多, 王涛, 田佰和. 国内外芳烃主要产品市场分析[J]. 化学工业, 2020, 38(4): 57-67.
|
|
MI Duo, WANG Tao, TIAN Baihe. Analysis of the main aromatics market at home and abroad[J]. Chemical Industry, 2020, 38(4): 57-67.
|
| 4 |
ZHANG Dan, YANG Minbo, FENG Xiao, et al. Integration of methanol aromatization with light hydrocarbon aromatization toward increasing aromatic yields: conceptual process designs and comparative analysis[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(30): 11376-11388.
|
| 5 |
ZHANG Jingui, QIAN Weizhong, KONG Chuiyan, et al. Increasing para-xylene selectivity in making aromatics from methanol with a surface-modified Zn/P/ZSM-5 catalyst[J]. ACS Catalysis, 2015, 5(5): 2982-2988.
|
| 6 |
CHANG Clarence D, SILVESTRI Anthony J. The conversion of methanol and other O-compounds to hydrocarbons over zeolite catalysts[J]. Journal of Catalysis, 1977, 47(2): 249-259.
|
| 7 |
MA Yunhai, CAI Dali, LI Yiru, et al. The influence of straight pore blockage on the selectivity of methanol to aromatics in nanosized Zn/ZSM-5: an atomic Cs-corrected STEM analysis study[J]. RSC Advances, 2016, 6(78): 74797-74801.
|
| 8 |
YARULINA Irina, CHOWDHURY Abhishek Dutta, MEIRER Florian, et al. Recent trends and fundamental insights in the methanol-to-hydrocarbons process[J]. Nature Catalysis, 2018, 1(6): 398-411.
|
| 9 |
METZGER Kara E, MOYER Megan M, TREWYN Brian G. Tandem catalytic systems integrating biocatalysts and inorganic catalysts using functionalized porous materials[J]. ACS Catalysis, 2021, 11(1): 110-122.
|
| 10 |
YARULINA Irina, DE WISPELAERE Kristof, BAILLEUL Simon, et al. Structure-performance descriptors and the role of Lewis acidity in the methanol-to-propylene process[J]. Nature Chemistry, 2018, 10(8): 804-812.
|
| 11 |
GAO Yan, ZHENG Binghui, WU Guang, et al. Effect of the Si/Al ratio on the performance of hierarchical ZSM-5 zeolites for methanol aromatization[J]. RSC Advances, 2016, 6(87): 83581-83588.
|
| 12 |
MA Hao, SUN Yuan, YU Junping, et al. Theoretical study on the influence of ZSM-5 zeolite with different structures for methanol to aromatics[J]. Microporous and Mesoporous Materials, 2020, 294: 109838-109847.
|
| 13 |
MANDERSLOOT W G B, NICOLAIDES C P, SCURRELL M S. Comments on the effect of the acidic strength of silica-alumina in the conversion of methanol to hydrocarbons[J]. Applied Catalysis, 1986, 27(2): 393-396.
|
| 14 |
BI Yi, WANG Yingli, CHEN Xin, et al. Methanol aromatization over HZSM-5 catalysts modified with different zinc salts[J]. Chinese Journal of Catalysis, 2014, 35(10): 1740-1751.
|
| 15 |
LI Junhua, WANG Lina, ZHANG Dan, et al. Effect of ZSM-5 acid modification on aromatization performance of methanol[J]. Journal of Fuel Chemistry and Technology, 2019, 47(8): 957-963.
|
| 16 |
MIYAKE Koji, HIROTA Yuichiro, Kaito ONO, et al. Selective production of benzene, toluene and p-xylene (BTpX) from various C1-3 feedstocks over ZSM-5/silicalite-1 core-shell zeolite catalyst[J]. ChemistrySelect, 2016, 1(5): 967-969.
|
| 17 |
YANG Junhao, GONG Ke, MIAO Dengyun, et al. Enhanced aromatic selectivity by the sheet-like ZSM-5 in syngas conversion[J]. Journal of Energy Chemistry, 2019, 35: 44-48.
|
| 18 |
WANG Kai, DONG Mei, NIU Xianjun, et al. Highly active and stable Zn/ZSM-5 zeolite catalyst for the conversion of methanol to aromatics: effect of support morphology[J]. Catalysis Science & Technology, 2018, 8(21): 5646-5656.
|
| 19 |
董道敏, 刘宾, 柴永明, 等. 动态水热法制备silicalite-1分子筛膜包覆多孔缺陷Al2O3微球[J]. 化工进展, 2018, 37(10): 3943-3948.
|
|
DONG Daomin, LIU Bin, CHAI Yongming, et al. Dynamic hydrothermal synthesis of silicalite-1 zeolite membrane to encapsulate defective porous alumina spheres[J]. Chemical Industry and Engineering Progress, 2018, 37(10): 3943-3948.
|
| 20 |
XIE Chenlu, CHEN Chen, YU Yi, et al. Tandem catalysis for CO2 hydrogenation to C2—C4 hydrocarbons[J]. Nano Letters, 2017, 17(6): 3798-3802.
|
| 21 |
XU Yanfei, MA Guangyuan, BAI Jingyang, et al. Yolk@shell FeMn@hollow HZSM-5 nanoreactor for directly converting syngas to aromatics[J]. ACS Catalysis, 2021, 11(8): 4476-4485.
|
| 22 |
XU Guohao, ZHU Xuedong. A core-shell structured Zn/SiO2@ZSM-5 catalyst: preparation and enhanced catalytic properties in methane co-aromatization with propane[J]. Applied Catalysis B: Environmental, 2021, 293: 120241-120249.
|
| 23 |
WANG Xiaoxing, ZHANG Junfeng, ZHANG Tao, et al. Mesoporous ZnZSM-5 zeolites synthesized by one-step desilication and reassembly: a durable catalyst for methanol aromatization[J]. RSC Advances, 2016, 6(28): 23428-23437.
|
| 24 |
CUI Tianlu, LI Xinhao, Libing LYU, et al. Nanoscale Kirkendall growth of silicalite-1 zeolite mesocrystals with controlled mesoporosity and size[J]. Chemical Communications, 2015, 51(63): 12563-12566.
|
| 25 |
JIA Yanming, WANG Junwen, ZHANG Kan, et al. Hierarchical ZSM-5 zeolite synthesized via dry gel conversion-steam assisted crystallization process and its application in aromatization of methanol[J]. Powder Technology, 2018, 328: 415-429.
|
| 26 |
SUN Minghui, ZHOU Jian, HU Zhiyi, et al. Hierarchical zeolite single-crystal reactor for excellent catalytic efficiency[J]. Matter, 2020, 3(4): 1226-1245.
|
| 27 |
TAO Shuo, LI Xiaolei, WANG Xiaoge, et al. Facile synthesis of hierarchical nanosized single-crystal aluminophosphate molecular sieves from highly homogeneous and concentrated precursors[J]. Angewandte Chemie International Edition, 2020, 59(9): 3455-3459.
|
| 28 |
ZHU Jie, ZHU Yihan, ZHU Liangkui, et al. Highly mesoporous single-crystalline zeolite beta synthesized using a nonsurfactant cationic polymer as a dual-function template[J]. Journal of the American Chemical Society, 2014, 136(6): 2503-2510.
|
| 29 |
JAMIL Anas Karrar, MURAZA Oki, AL-AMER Adnan M. The role of alcohols and diols as co-solvents in fabrication of TON zeolite[J]. Journal of Industrial and Engineering Chemistry, 2015, 29: 112-119.
|
| 30 |
CHEN Xiaoxin, YAN Wenfu, CAO Xuejing, et al. Fabrication of silicalite-1 crystals with tunable aspect ratios by microwave-assisted solvothermal synthesis[J]. Microporous and Mesoporous Materials, 2009, 119(1/2/3): 217-222.
|
| 31 |
THOMMES Matthias, KANEKO Katsumi, NEIMARK Alexander V, et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report)[J]. Pure and Applied Chemistry, 2015, 87(9/10): 1051-1069.
|
| 32 |
GOODARZI Farnoosh, HERRERO Irene Pinilla, KALANTZOPOULOS Georgios N, et al. Synthesis of mesoporous ZSM-5 zeolite encapsulated in an ultrathin protective shell of silicalite-1 for MTH conversion[J]. Microporous and Mesoporous Materials, 2020, 292: 109730-109739.
|
| 33 |
JONES Andrew J, IGLESIA Enrique. The strength of Brønsted acid sites in microporous aluminosilicates[J]. ACS Catalysis, 2015, 5(10): 5741-5755.
|
| 34 |
JONES Andrew J, CARR Robert T, ZONES Stacey I, et al. Acid strength and solvation in catalysis by MFI zeolites and effects of the identity, concentration and location of framework heteroatoms[J]. Journal of Catalysis, 2014, 312: 58-68.
|
| 35 |
KATADA Naonobu, Hirofumi IGI, KIM Jong Ho. Determination of the acidic properties of zeolite by theoretical analysis of temperature-programmed desorption of ammonia based on adsorption equilibrium[J]. The Journal of Physical Chemistry B, 1997, 101(31): 5969-5977.
|
| 36 |
KALITA Pranjal, GUPTA Narendra M, KUMAR Rajiv. Synergistic role of acid sites in the Ce-enhanced activity of mesoporous Ce-Al-MCM-41 catalysts in alkylation reactions: FTIR and TPD-ammonia studies[J]. Journal of Catalysis, 2007, 245(2): 338-347.
|
| 37 |
WANG Chao, CHU Yueying, ZHENG Anmin, et al. New insight into the hydrocarbon-pool chemistry of the methanol-to-olefins conversion over zeolite H-ZSM-5 from GC-MS, solid-state NMR spectroscopy, and DFT calculations[J]. Chemistry—a European Journal, 2014, 20(39): 12432-12443.
|
| 38 |
Róbert BARTHOS, Tamás BÁNSÁGI, SÜLI ZAKAR Tímea, et al. Aromatization of methanol and methylation of benzene over Mo2C/ZSM-5 catalysts[J]. Journal of Catalysis, 2007, 247(2): 368-378.
|
| 39 |
FENG Chaoqun, SU Xiaofang, WANG Wei, et al. Facile synthesis of ultrafine nanosized ZSM-5 zeolite using a hydroxyl radical initiator for enhanced catalytic performance in the MTG reaction[J]. Microporous and Mesoporous Materials, 2021, 312: 110780-110790.
|
| 40 |
FU Tingjun, SHAO Juan, LI Zhong. Catalytic synergy between the low Si/Al ratio Zn/ZSM-5 and high Si/Al ratio HZSM-5 for high-performance methanol conversion to aromatics[J]. Applied Catalysis B: Environmental, 2021, 291: 120098-120113.
|
| 41 |
SCHULZ Hans. “Coking” of zeolites during methanol conversion: basic reactions of the MTO-, MTP- and MTG processes[J]. Catalysis Today, 2010, 154(3/4): 183-194.
|
 ), FU Tingjun(
), FU Tingjun( ), MA Qian, LI Zhong(
), MA Qian, LI Zhong( )
)