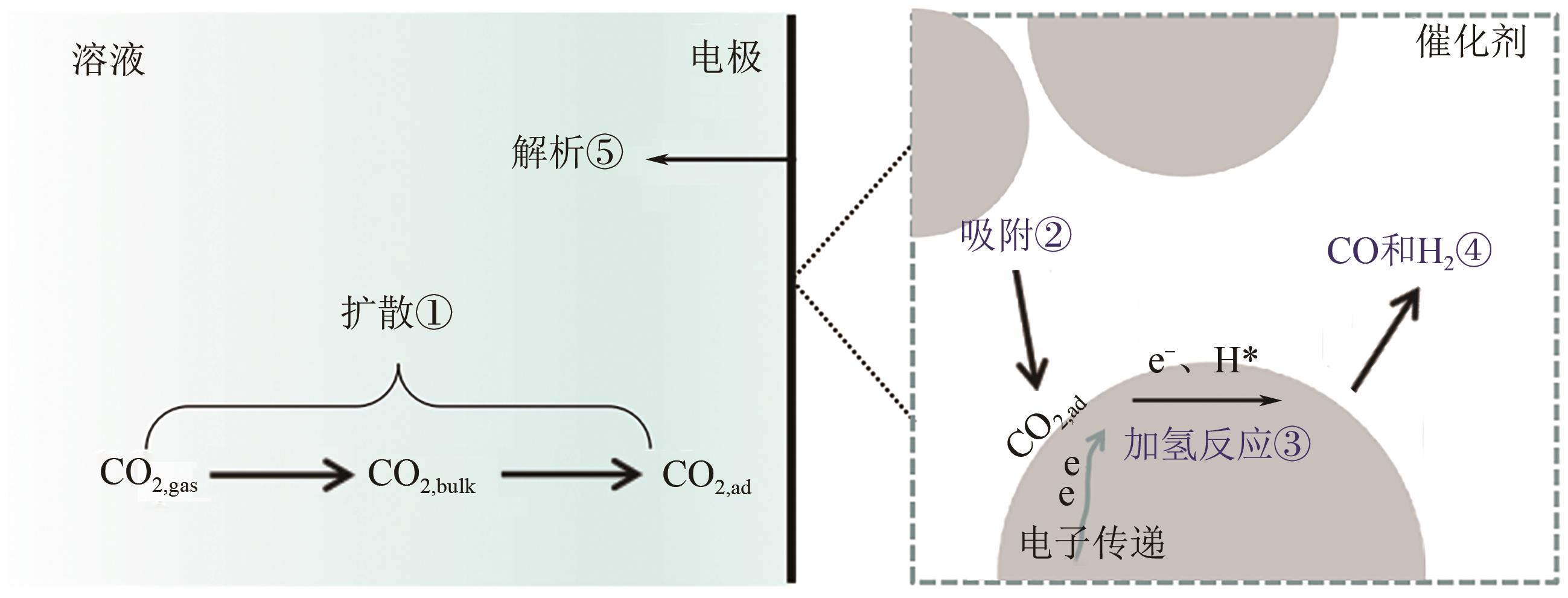
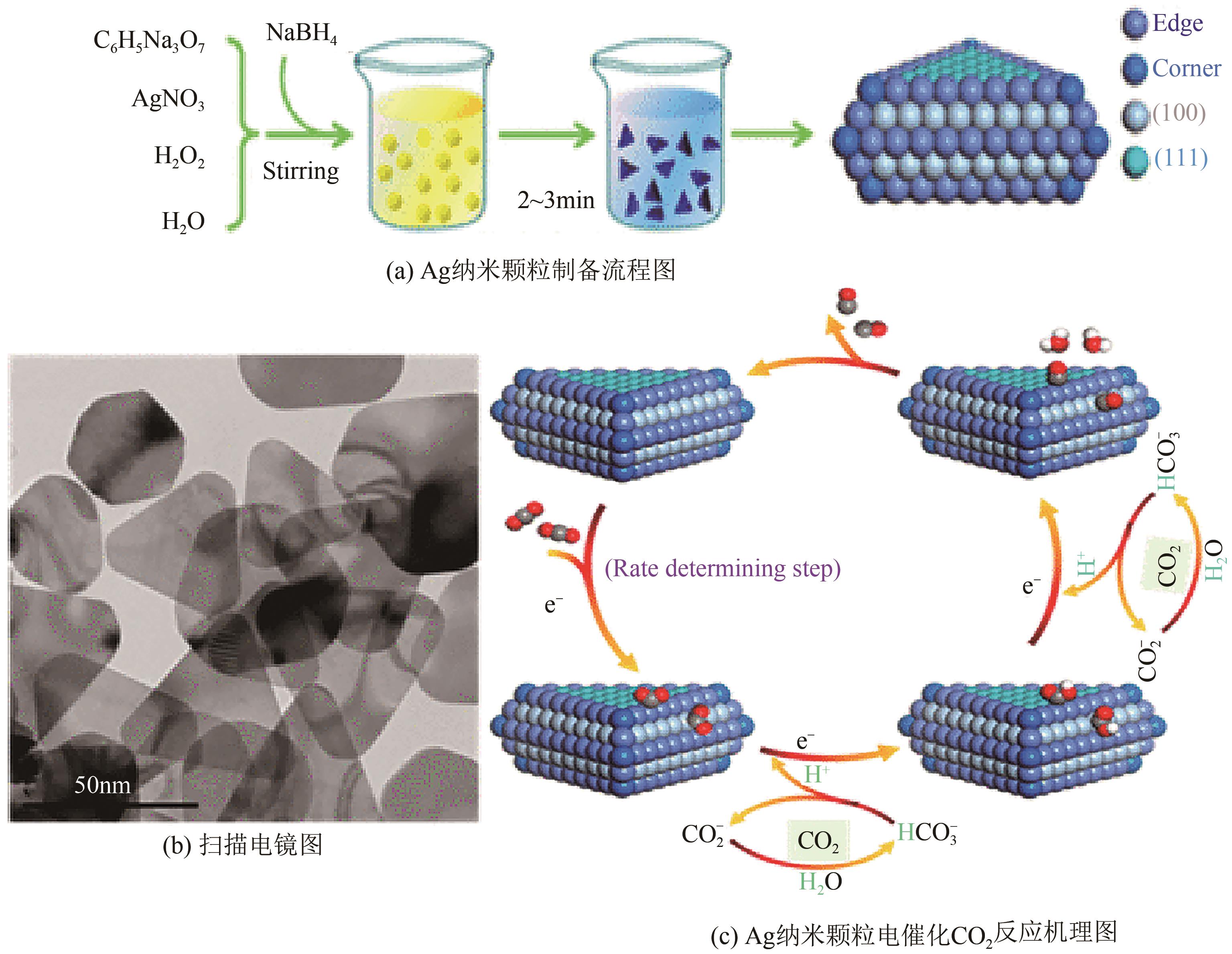
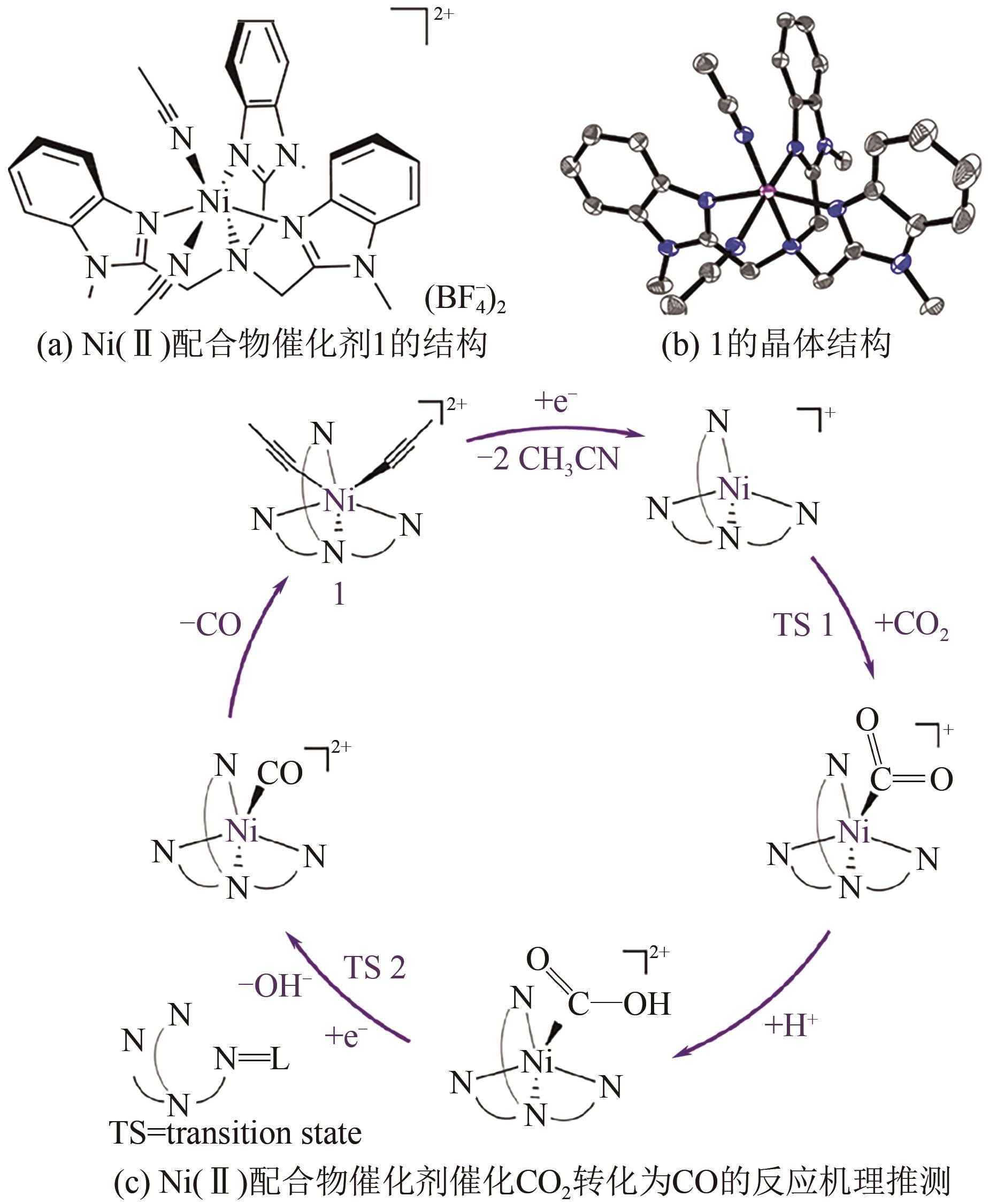
Chemical Industry and Engineering Progress ›› 2022, Vol. 41 ›› Issue (3): 1224-1240.DOI: 10.16085/j.issn.1000-6613.2021-2009
• Carbon dioxide capture, storage and utilization • Previous Articles Next Articles
Research progress of CO2 electrocatalytic reduction to syngas
HUA Yani1( ), FENG Shaoguang2(
), FENG Shaoguang2( ), DANG Xinyue1, HAO Wenbin1, ZHANG Baowen1, GAO Zhan1(
), DANG Xinyue1, HAO Wenbin1, ZHANG Baowen1, GAO Zhan1( )
)
- 1.School of Chemical Engineering and Technology, Xi’an Jiaotong University, Xi’an 710049, Shaanxi, China
2.Foran Energy Group Co. , Ltd. , Foshan 528000, Guangdong, China
-
Received:2021-09-23Revised:2021-11-08Online:2022-03-28Published:2022-03-23 -
Contact:GAO Zhan
CO2电催化还原产合成气研究进展
华亚妮1( ), 冯少广2(
), 冯少广2( ), 党欣悦1, 郝文斌1, 张保文1, 高展1(
), 党欣悦1, 郝文斌1, 张保文1, 高展1( )
)
- 1.西安交通大学化学工程与技术学院,陕西 西安 710049
2.佛燃能源集团股份有限公司,广东 佛山 528000
-
通讯作者:高展 -
作者简介:华亚妮(1986—),女,博士,助理教授,研究方向为CO2电催化还原。E-mail:huayani@xjtu.edu.cn |冯少广(1981—),男,博士,高级工程师,研究方向燃气及新能源、完整性管理等。E-mail:fengsg@fsgas.com 。 -
基金资助:国家引进海外高层次人才青年计划;电力系统国家重点实验室资助课题(SKLD21KM07)
CLC Number:
Cite this article
HUA Yani, FENG Shaoguang, DANG Xinyue, HAO Wenbin, ZHANG Baowen, GAO Zhan. Research progress of CO2 electrocatalytic reduction to syngas[J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1224-1240.
华亚妮, 冯少广, 党欣悦, 郝文斌, 张保文, 高展. CO2电催化还原产合成气研究进展[J]. 化工进展, 2022, 41(3): 1224-1240.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2021-2009
| 电化学半反应 | 电极电位(vs. SHE)/V |
|---|---|
| -0.42 | |
| -0.52 | |
| -0.61 | |
| -0.51 | |
| -0.38 | |
| -0.24 | |
| 0.064 | |
| 0.084 |
| 电化学半反应 | 电极电位(vs. SHE)/V |
|---|---|
| -0.42 | |
| -0.52 | |
| -0.61 | |
| -0.51 | |
| -0.38 | |
| -0.24 | |
| 0.064 | |
| 0.084 |
| 1 | JACKSON R B, LE QUÉRÉ C, ANDREW R M, et al. Global energy growth is outpacing decarbonization[J]. Environmental Research Letters, 2018, 13(12): 120401. |
| 2 | 王深, 吕连宏, 张保留, 等. 基于多目标模型的中国低成本碳达峰、碳中和路径[J]. 环境科学研究, 2021, 34(9): 2044-2055. |
| WANG S, LYU L H, ZHANG B L, et al. Multi objective programming model of low-cost path for China’s peaking carbon dioxide emissions and carbon neutrality[J]. Research of Environmental Sciences, 2021, 34(9): 2044-2055. | |
| 3 | RONG W F, ZOU H Y, ZANG W J, et al. Size-dependent activity and selectivity of atomic-level copper nanoclusters during CO/CO2 electroreduction[J]. Angewandte Chemie International Edition, 2021, 60(1): 466-472. |
| 4 | NAM D H, DE LUNA P, ROSAS-HERNÁNDEZ A, et al. Molecular enhancement of heterogeneous CO2 reduction[J]. Nature Materials, 2020, 19(3): 266-276. |
| 5 | PAN F P, YANG Y. Designing CO2 reduction electrode materials by morphology and interface engineering[J]. Energy & Environmental Science, 2020, 13(8): 2275-2309. |
| 6 | YAN C C, LIN L, WANG G X, et al. Transition metal-nitrogen sites for electrochemical carbon dioxide reduction reaction[J]. Chinese Journal of Catalysis, 2019, 40(1): 23-37. |
| 7 | SU X, YANG X F, HUANG Y Q, et al. Single-atom catalysis toward efficient CO2 conversion to CO and formate products[J]. Accounts of Chemical Research, 2019, 52(3): 656-664. |
| 8 | ZHANG W J, HU Y, MA L B, et al. Progress and perspective of electrocatalytic CO2 reduction for renewable carbonaceous fuels and chemicals[J]. Advanced Science, 2018, 5(1): 1700275. |
| 9 | 孙睿, 徐跃, 刘建芳, 等. CO2催化还原转化为高附加值化学品[J].中国科学:化学, 2018, 48(6): 547-561. |
| SUN Rui, XU Yue, LIU Jianfang, et al. Recent progress in CO2 catalytic reduction to high value-added chemicals[J]. Scientia Sinica (Chimica), 2018, 48(6): 547-561. | |
| 10 | CUI H J, GUO Y B, GUO L M, et al. Heteroatom-doped carbon materials and their composites as electrocatalysts for CO2 reduction[J]. Journal of Materials Chemistry A, 2018, 6(39): 18782-18793. |
| 11 | XIE H, WANG T Y, LIANG J S, et al. Cu-based nanocatalysts for electrochemical reduction of CO2 [J]. Nano Today, 2018, 21: 41-54. |
| 12 | FAN Q, ZHANG M L, JIA M W, et al. Electrochemical CO2 reduction to C2+ species: heterogeneous electrocatalysts, reaction pathways, and optimization strategies[J]. Materials Today Energy, 2018, 10: 280-301. |
| 13 | ABDULRASHEED A, JALILAB A A, GAMBO Y, et al. A review on catalyst development for dry reforming of methane to syngas: recent advances[J]. Renewable and Sustainable Energy Reviews, 2019, 108: 175-193. |
| 14 | JANG W J, SHIM J O, KIM H M, et al. A review on dry reforming of methane in aspect of catalytic properties[J]. Catalysis Today, 2019, 324: 15-26. |
| 15 | MESTERS C. A selection of recent advances in C1 chemistry[J]. Annual Review of Chemical and Biomolecular Engineering, 2016, 7: 223-238. |
| 16 | MA S C, HUANG S D, LIU Z P. Dynamic coordination of cations and catalytic selectivity on zinc-chromium oxide alloys during syngas conversion[J]. Nature Catalysis, 2019, 2(8): 671-677. |
| 17 | HE R, ZHANG A, DING Y L, et al. Achieving the widest range of syngas proportions at high current density over cadmium sulfoselenide nanorods in CO2 electroreduction[J]. Advanced Materials, 2018, 30(7): 1705872. |
| 18 | PAN F P, LI B Y, SARNELLO E, et al. Pore-edge tailoring of single-atom iron-nitrogen sites on graphene for enhanced CO2 reduction[J]. ACS Catalysis, 2020, 10(19): 10803-10811. |
| 19 | LU S S, SHI Y M, MENG N N, et al. Electrosynthesis of syngas via the co-reduction of CO2 and H2O[J]. Cell Reports Physical Science, 2020, 1(11): 100237. |
| 20 | DELAFONTAINE L, ASSET T, ATANASSOV P. Metal-nitrogen-carbon electrocatalysts for CO2 reduction towards syngas generation[J]. ChemSusChem, 2020, 13(7): 1688-1698. |
| 21 | QIAO J L, LIU Y Y, HONG F, et al. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels[J]. Chemical Society Reviews, 2014, 43(2): 631-675. |
| 22 | DAIYAN R, CHEN R, KUMAR P, et al. Tunable syngas production through CO2 electroreduction on cobalt-carbon composite electrocatalyst[J]. ACS Applied Materials & Interfaces, 2020, 12(8): 9307-9315. |
| 23 | REN W H, TAN X, YANG W F, Isolated diatomic Ni-Fe metal-nitrogen sites for synergistic electroreduction of CO 2[J]. Angewandte Chemie International Edition, 2019, 58(21): 6972-6976. |
| 24 | ZENG J Q, BEJTKA K, DI MARTINO G, et al. Microwave-assisted synthesis of copper-based electrocatalysts for converting carbon dioxide to tunable syngas[J]. ChemElectroChem, 2020, 7(1): 229-238. |
| 25 | QIN B H, ZHANG Q, LI Y H, et al. Formation of lattice-dislocated zinc oxide via anodic corrosion for electrocatalytic CO2 reduction to syngas with a potential-dependent CO:H2 ratio[J]. ACS Applied Materials & Interfaces, 2020, 12(27): 30466-30473. |
| 26 | HANSEN H A, VARLEY J B, PETERSON A A, et al. Understanding trends in the electrocatalytic activity of metals and enzymes for CO2 reduction to CO[J]. The Journal of Physical Chemistry Letters, 2013, 4(3): 388-392. |
| 27 | ZHU W L, MICHALSKY R, METIN Ö, et al. Monodisperse Au nanoparticles for selective electrocatalytic reduction of CO2 to CO[J]. Journal of the American Chemical Society, 2013, 135(45): 16833-16836. |
| 28 | FENG X F, JIANG K L, FAN S S, et al. Grain-boundary-dependent CO2 electroreduction activity[J]. Journal of the American Chemical Society, 2015, 137(14): 4606-4609. |
| 29 | ZHU W L, ZHANG Y J, ZHANG H Y, et al. Active and selective conversion of CO2 to CO on ultrathin Au nanowires[J]. Journal of the American Chemical Society, 2014, 136(46): 16132-16135. |
| 30 | LIU S B, TAO H B, ZENG L, et al. Shape-dependent electrocatalytic reduction of CO2 to CO on triangular silver nanoplates[J]. Journal of the American Chemical Society, 2017, 139(6): 2160-2163. |
| 31 | SHENG W C, KATTEL S, YAO S Y, et al. Electrochemical reduction of CO2 to synthesis gas with controlled CO/H2 ratios[J]. Energy & Environmental Science, 2017, 10(5): 1180-1185. |
| 32 | LIU Y M, TIAN D, BISWAS A N, et al. Transition metal nitrides as promising catalyst supports for tuning CO/H2 syngas production from electrochemical CO2 reduction[J]. Angewandte Chemie International Edition, 2020, 59(28): 11345-11348. |
| 33 | CHEN C J, SUN X F, YAN X P, et al. Boosting CO2 electroreduction on N,P-co-doped carbon aerogels[J]. Angewandte Chemie International Edition, 2020, 59(27): 11123-11129. |
| 34 | HOU C C, WANG H F, LI C X, et al. From metal-organic frameworks to single/dual-atom and cluster metal catalysts for energy applications[J]. Energy & Environmental Science, 2020, 13(6): 1658-1693. |
| 35 | QIN B H, LI Y H, FU H Q, et al. Electrochemical reduction of CO2 into tunable syngas production by regulating the crystal facets of earth-abundant Zn catalyst[J]. ACS Applied Materials & Interfaces, 2018, 10(24): 20530-20539. |
| 36 | JEON H S, SINEV I, SCHOLTEN F, et al. Operando evolution of the structure and oxidation state of size-controlled Zn nanoparticles during CO2 electroreduction[J]. Journal of the American Chemical Society, 2018, 140(30): 9383-9386. |
| 37 | LI X G, BI W T, CHEN M L, et al. Exclusive Ni-N4 sites realize near-unity CO selectivity for electrochemical CO2 reduction[J]. Journal of the American Chemical Society, 2017, 139(42): 14889-14892. |
| 38 | YAN C C, LI H B, YE Y F, et al. Coordinatively unsaturated nickel-nitrogen sites towards selective and high-rate CO2 electroreduction[J]. Energy & Environmental Science, 2018, 11(5): 1204-1210. |
| 39 | WATANABE M, SHIBATA M, KATO A, et al. Design of alloy electrocatalysts for CO2 reduction (Ⅲ): the selective and reversible reduction of on Cu alloy electrodes[J]. Journal of the Electrochemical Society, 1999, 138(11): 3382-3389. |
| 40 | ZHENG X L, JI Y F, TANG J, et al. Theory-guided Sn/Cu alloying for efficient CO2 electroreduction at low overpotentials[J]. Nature Catalysis, 2019, 2(1): 55-61. |
| 41 | FAN M Y, ESLAMIBIDGOL M J, ZHU X W, et al. Understanding the improved activity of dendritic Sn1Pb3 alloy for the CO2 electrochemical reduction: a computational-experimental investigation[J]. ACS Catalysis, 2020, 10(18): 10726-10734. |
| 42 | ROSS M B, LI Y F, DE LUNA P, et al. Electrocatalytic rate alignment enhances syngas generation[J]. Joule, 2019, 3(1): 257-264. |
| 43 | XU J Q, LI X D, LIU W, et al. Carbon dioxide electroreduction into syngas boosted by a partially delocalized charge in molybdenum sulfide selenide alloy monolayers[J]. Angewandte Chemie International Edition, 2017, 56(31): 9121-9125. |
| 44 | COSTENTIN C, ROBERT M, SAVÉANT J M. Current issues in molecular catalysis illustrated by iron porphyrins as catalysts of the CO2-to-CO electrochemical conversion[J]. Accounts of Chemical Research, 2015, 48(12): 2996-3006. |
| 45 | FISHER B J, EISENBERG R. Electrocatalytic reduction of carbon dioxide by using macrocycles of nickel and cobalt[J]. Journal of the American Chemical Society, 1980, 102(24): 7361-7363. |
| 46 | WANG J W, HUANG H H, SUN J K, et al. Syngas production with a highly-robust nickel(Ⅱ) homogeneous electrocatalyst in a water-containing system[J]. ACS Catalysis, 2018, 8(8): 7612-7620. |
| 47 | WANG Y, GONELL S, MATHIYAZHAGAN U R, et al. Simultaneous electrosynthesis of syngas and an aldehyde from CO2 and an alcohol by molecular electrocatalysis[J]. ACS Applied Energy Materials, 2019, 2(1): 97-101. |
| 48 | MAO C L, WANG J X, ZOU Y J, et al. Hydrogen spillover to oxygen vacancy of TiO2- x H y /Fe: breaking the scaling relationship of ammonia synthesis[J]. Journal of the American Chemical Society, 2020, 142(41): 17403-17412. |
| 49 | QIN B H, LI Y H, WANG H J, et al. Efficient electrochemical reduction of CO2 into CO promoted by sulfur vacancies[J]. Nano Energy, 2019, 60: 43-51. |
| 50 | GENG Z G, KONG X D, CHEN W W, et al. Oxygen vacancies in ZnO nanosheets enhance CO2 electrochemical reduction to CO[J]. Angewandte Chemie International Edition, 2018, 57(21): 6054-6059. |
| 51 | ASADI M, KUMAR B, BEHRANGINIA A, et al. Robust carbon dioxide reduction on molybdenum disulphide edges[J]. Nature Communications, 2014, 5: 4470. |
| 52 | DAIYAN R, LOVELL E C, HUANG B S, et al. Uncovering atomic-scale stability and reactivity in engineered zinc oxide electrocatalysts for controllable syngas production[J]. Advanced Energy Materials, 2020, 10(28): 2001381. |
| 53 | PAN F P, ZHANG H G, LIU K X, et al. Unveiling active sites of CO2 reduction on nitrogen-coordinated and atomically dispersed iron and cobalt catalysts[J]. ACS Catalysis, 2018, 8(4): 3116-3122. |
| 54 | ZHANG L L, REN Y J, LIU W G, et al. Single-atom catalyst: a rising star for green synthesis of fine chemicals[J]. National Science Review, 2018, 5(5): 653-672. |
| 55 | ZHANG L L, ZHOU M X, WANG A Q, et al. Selective hydrogenation over supported metal catalysts: from nanoparticles to single atoms[J]. Chemical Reviews, 2020, 120(2): 683-733. |
| 56 | YANG X F, WANG A Q, QIAO B T, et al. Single-atom catalysts: a new frontier in heterogeneous catalysis[J]. Accounts of Chemical Research, 2013, 46(8): 1740-1748. |
| 57 | REN Y J, TANG Y, ZHANG L L, et al. Unraveling the coordination structure-performance relationship in Pt1/Fe2O3 single-atom catalyst[J]. Nature Communications, 2019, 10(1): 4500. |
| 58 | CHENG Y, ZHAO S Y, LI H B, et al. Unsaturated edge-anchored Ni single atoms on porous microwave exfoliated graphene oxide for electrochemical CO2 [J]. Applied Catalysis B: Environmental, 2019, 243: 294-303. |
| 59 | YANG H B, HUNG S F, LIU S, et al. Atomically dispersed Ni(Ⅰ) as the active site for electrochemical CO2 reduction[J]. Nature Energy, 2018, 3(2): 140-147. |
| 60 | CHUNG H T, CULLEN D A, HIGGINS B D, et al. Direct atomic-level insight into the active sites of a high-performance PGM-free ORR catalyst[J]. Science, 2017, 357(6350): 479-484. |
| 61 | 张钰宁, 钮东方, 胡硕真, 等. 基于纳米金属的增强效应在CO2电还原反应中的应用进展[J]. 电化学, 2020, 26(4): 495-509. |
| ZHANG Yuning, NIU Dongfang, HU Shuozhen, et al. Recent progress on enhancing effect of nanosized metals for electrochemical CO2 reduction[J]. Journal of Electrochemistry, 2020, 26(4): 495-509. | |
| 62 | SONG X K, ZHANG H, YANG Y Q, et al. Bifunctional nitrogen and cobalt codoped hollow carbon for electrochemical syngas production[J]. Advance Science, 2018, 5(7): 1800177. |
| 63 | GU J, HSU C S, BAI L C, et al. Atomically dispersed Fe3+ sites catalyze efficient CO2 electroreduction to CO[J]. Science, 2019, 364(6445): 1091-1094. |
| 64 | LI J J, GUAN Q Q, WU H, et al. Highly active and stable metal single-atom catalysts achieved by strong electronic metal-support interactions[J]. Journal of the American Chemical Society, 2019, 141(37): 14515-14519. |
| 65 | SUN Z Y, MA T, TAO H C, et al. Fundamentals and challenges of electrochemical CO2 reduction using two-dimensional materials[J]. Chem, 2017, 3(4): 560-587. |
| 66 | LIU W G, ZHANG L L, LIU X, et al. Discriminating catalytically active FeN x species of atomically dispersed Fe-N-C catalyst for selective oxidation of the C-H bond[J]. Journal of the American Chemical Society, 2017, 139(31): 10790-10798. |
| 67 | LI T B, LIU F, TANG Y, et al. Maximizing the number of interfacial sites in single-atom catalysts for the highly selective, solvent-free oxidation of primary alcohols[J]. Angewandte Chemie International Edition, 2018, 57(26): 7795-7799. |
| 68 | WANG Q C, LEI Y P, WANG D S, et al. Defect engineering in earth-abundant electrocatalysts for CO2 and N2 reduction[J]. Energy & Environmental Science, 2019, 12(6): 1730-1750. |
| 69 | TERRONES M, BOTTELLO-MÉNDEZ A R, CAMPOS-DELGADO J, et al. Graphene and graphite nanoribbons: morphology, properties, synthesis, defects and applications[J]. Nano Today, 2010, 5(4): 351-372. |
| 70 | LUO J, WANG K J, HUA X, et al. Pyridinic-N protected synthesis of 3D nitrogen-doped porous carbon with increased mesoporous defects for oxygen reduction[J]. Small, 2019, 15(11): e1805325. |
| 71 | KUMAR B, ASADI M, PISASALE D, et al. Renewable and metal-free carbon nanofibre catalysts for carbon dioxide reduction[J]. Nature Communications, 2013, 4: 2819. |
| 72 | JI Y, SHI Y M, LIU C B, et al. Plasma-regulated N-doped carbon nanotube arrays for efficient electrosynthesis of syngas with a wide CO/H2 ratio[J]. Science China Materials, 2020, 63(11): 2351-2357. |
| 73 | XIE J F, ZHAO X T, WU M X, et al. Metal-free fluorine-doped carbon electrocatalyst for CO2 reduction outcompeting hydrogen evolution[J]. Angewandte Chemie International Edition, 2018, 57(31): 9640-9644. |
| 74 | 张少阳, 商阳阳, 赵瑞花, 等. 电催化还原二氧化碳制一氧化碳催化剂研究进展[J]. 化工进展. DOI: 10.16085/j.issn.1000-6613.2021-0804 . |
| ZHANG Shaoyang, SHANG Yangyang, ZHAO Ruihua, et al. Research progress on catalysts for electrocatalytic reduction of carbon dioxide to carbon monoxide[J]. Chemical Industry and Engineering Progress. DOI: 10.16085/j.issn.1000-6613.2021-0804 . | |
| 75 | MOHD ADLI N, SHAN W T, HWANG S, et al. Engineering atomically dispersed FeN4 active sites for CO2 electroreduction[J]. Angewandte Chemie International Edition, 2020, 60(2): 1022-1032. |
| 76 | HARA K, SAKATA T. Large current density CO2 reduction under high pressure using gas diffusion electrodes[J]. Bulletin of the Chemical Society of Japan, 1997, 70(3): 571-576. |
| 77 | XIANG H, RASUL S, HOU B, et al. Copper-indium binary catalyst on a gas diffusion electrode for high-performance CO2 electrochemical reduction with record CO production efficiency[J]. ACS Applied Materials & Interfaces, 2020, 12(1): 601-608. |
| 78 | XIE H, WAN Y Y, WANG X M, et al. Boosting Pd-catalysis for electrochemical CO2 reduction to CO on Bi-Pd single atom alloy nanodendrites[J]. Applied Catalysis B: Environmental, 2021, 289: 119783. |
| 79 | SONG Y F, ZHANG X M, XIE K, et al. High-temperature CO2 electrolysis in solid oxide electrolysis cells: developments, challenges, and prospects[J]. Advanced Materials, 2019, 31(50): 1902033. |
| 80 | HWANG J, AKKIRAJU K, CORCHADO-GARCÍA J, et al. A perovskite electronic structure descriptor for electrochemical CO2 reduction and the competing H2 evolution reaction[J]. The Journal of Physical Chemistry C, 2019, 123(40): 24469-24476. |
| 81 | ZHOU Y J, ZHOU Z W, SONG Y F, et al. Enhancing CO2 electrolysis performance with vanadium-doped perovskite cathode in solid oxide electrolysis cell[J]. Nano Energy, 2018, 50: 43-51. |
| 82 | GAUDILLERE C, NAVARRET L, SERRA J M. Syngas production at intermediate temperature through H2O and CO2 electrolysis with a Cu-based solid oxide electrolyzer cell[J].International Journal of Hydrogen Energy, 2014, 39(7): 3047-3054. |
| 83 | ZHU Y L, ZHOU W, RAN R, et al. Promotion of oxygen reduction by exsolved silver nanoparticles on a perovskite scaffold for low-temperature solid oxide fuel cells[J]. Nano Letters, 2016, 16(1): 512-518. |
| 84 | ZENG S, KAR P, THAKUR U K, et al. A review on photocatalytic CO2 reduction using perovskite oxide nanomaterials[J]. Nanotechnology, 2018, 29(5): 052001. |
| 85 | DELACOURT C, RIDGWAY P L, KERR J B, et al. Design of an electrochemical cell making syngas (CO+H2) from CO2 and H2O reduction at room temperature[J]. Journal of the Electrochemical Society, 2008, 155(1): B42. |
| 86 | MA M, CLARK E L, THERKILDSEN K T, et al. Insights into the carbon balance for CO2 electroreduction on Cu using gas diffusion electrode reactor designs[J]. Energy & Environmental Science, 2020, 13(3): 977-985. |
| 87 | GAO D F, WEI P F, LI H F, et al. Designing electrolyzers for electrocatalytic CO2 reduction[J]. Acta Physico Chimica Sinica, 2021, 37(5): 2009021. |
| 88 | JIANG K, SIAHROSTAMI S, ZHENG T T, et al. Isolated Ni single atoms in graphene nanosheets for high-performance CO2 reduction[J]. Energy & Environmental Science, 2018, 11(4): 893-903. |
| 89 | LI Y C, ZHOU D K, YAN Z F, et al. Electrolysis of CO2 to syngas in bipolar membrane-based electrochemical cells[J]. ACS Energy Letters, 2016, 1(6): 1149-1153. |
| 90 | SUN K, LIU R, CHEN Y K, et al. Solar-driven water splitting: a stabilized, intrinsically safe, 10% efficient, solar-driven water-splitting cell incorporating earth-abundant electrocatalysts with steady-state pH gradients and product separation enabled by a bipolar membrane[J]. Advanced Energy Materials, 2016, 6(13): 201670077. |
| 91 | MCDONALD M B, ARDO S, LEWIS N S, et al. Use of bipolar membranes for maintaining steady-state pH gradients in membrane-supported, solar-driven water splitting[J]. ChemSusChem, 2014, 7(11): 3021-3027. |
| 92 | HUANG Y Y, YANG R, ANANDHABABU G, et al. Cobalt/iron(oxides) heterostructures for efficient oxygen evolution and benzyl alcohol oxidation reactions[J]. ACS Energy Letters, 2018, 3(8): 1854-1860. |
| 93 | VERMA S, LU S, KENIS P J A. Co-electrolysis of CO2 and glycerol as a pathway to carbon chemicals with improved technoeconomics due to low electricity consumption[J]. Nature Energy, 2019, 4(6): 466-474. |
| 94 | LI T F, CAO Y, HE J F, et al. Electrolytic CO2 reduction in tandem with oxidative organic chemistry[J]. ACS Central Science, 2017, 3(7): 778-783. |
| 95 | WEI X F, LI Y, CHEN L S, et al. Formic acid electro-synthesis by concurrent cathodic CO2 reduction and anodic CH3OH oxidation[J]. Angewandte Chemie International Edition, 2021, 60(6): 3148-3155. |
| 96 | CHEN Z F, KANG P, ZHANG M T, et al. Cu(Ⅱ)/Cu(0) electrocatalyzed CO2 and H2O splitting[J]. Energy & Environmental Science, 2013, 6(3):813-817. |
| 97 | ZHANG X, WU Z S, ZHANG X, et al. Highly selective and active CO2 reduction electrocatalysts based on cobalt phthalocyanine/carbon nanotube hybrid structures[J]. Nature Communications, 2017, 8: 14675. |
| 98 | HE Q, LIU D B, LEE J H, et al. Electrochemical conversion of CO2 to syngas with controllable CO/H2 ratios over Co and Ni single-atom catalysts[J]. Angewandte Chemie International Edition, 2020, 59(8): 3033-3037. |
| 99 | LAN Y, CHEN J L, ZHANG H, et al. Fe/Fe3C nanoparticle-decorated N-doped carbon nanofibers for improving the nitrogen selectivity of electrocatalytic nitrate reduction[J]. Journal of Materials Chemistry A, 2020, 8(31): 15853-15863. |
| 100 | YAN X X, GU M Y, WANG Y, et al. In-situ growth of Ni nanoparticle-encapsulated N-doped carbon nanotubes on carbon nanorods for efficient hydrogen evolution electrocatalysis[J]. Nano Research, 2020, 13(4): 975-982. |
| 101 | SUN H, CHEN L, LIAN Y B, et al. Topotactically transformed polygonal mesopores on ternary layered double hydroxides exposing under-coordinated metal centers for accelerated water dissociation[J]. Advanced Materials, 2020, 32(52): e2006784. |
| 102 | BEHESHTI M, KAKOOEI S, ISMAIL M C, et al. Investigation of CO2 electrochemical reduction to syngas on Zn/Ni-based electrocatalysts using the cyclic voltammetry method[J]. Electrochimica Acta, 2020, 341: 135976. |
| 103 | PAN F P, LI B Y, SARNELLO E, et al. Atomically dispersed iron-nitrogen sites on hierarchically mesoporous carbon nanotube and graphene nanoribbon networks for CO2 reduction[J]. ACS Nano, 2020, 14(5): 5506-5516. |
| 104 | ZHENG Y, ZHENG S S, XUE H G, et al. Metal-organic frameworks/graphene-based materials: preparations and applications[J]. Advanced Functional Materials, 2018, 28(47): 1804950. |
| [1] | SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298. |
| [2] | YANG Xiazhen, PENG Yifan, LIU Huazhang, HUO Chao. Regulation of active phase of fused iron catalyst and its catalytic performance of Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 310-318. |
| [3] | ZHENG Qian, GUAN Xiushuai, JIN Shanbiao, ZHANG Changming, ZHANG Xiaochao. Photothermal catalysis synthesis of DMC from CO2 and methanol over Ce0.25Zr0.75O2 solid solution [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 319-327. |
| [4] | ZHAO Wei, ZHAO Deyin, LI Shihan, LIU Hongda, SUN Jin, GUO Yanqiu. Synthesis and application of triazine drag reducing agent for nature gas pipeline [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 391-399. |
| [5] | ZHANG Jie, WANG Fangfang, XIA Zhonglin, ZHAO Guangjin, MA Shuangchen. Current SF6 emission, emission reduction and future prospects under “carbon peaking and carbon neutrality” [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 447-460. |
| [6] | WANG Lele, YANG Wanrong, YAO Yan, LIU Tao, HE Chuan, LIU Xiao, SU Sheng, KONG Fanhai, ZHU Canghai, XIANG Jun. Influence of spent SCR catalyst blending on the characteristics and deNO x performance for new SCR catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 489-497. |
| [7] | ZHAO Jingchao, TAN Ming. Effect of surfactants on the reduction of industrial saline wastewater by electrodialysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 529-535. |
| [8] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [9] | SUN Yuyu, CAI Xinlei, TANG Jihai, HUANG Jingjing, HUANG Yiping, LIU Jie. Optimization and energy-saving of a reactive distillation process for the synthesis of methyl methacrylate [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 56-63. |
| [10] | YANG Hanyue, KONG Lingzhen, CHEN Jiaqing, SUN Huan, SONG Jiakai, WANG Sicheng, KONG Biao. Decarbonization performance of downflow tubular gas-liquid contactor of microbubble-type [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 197-204. |
| [11] | SHU Bin, CHEN Jianhong, XIONG Jian, WU Qirong, YU Jiangtao, YANG Ping. Necessity analysis of promoting the development of green methanol under the goal of carbon neutrality [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4471-4478. |
| [12] | ZHANG Qi, ZHAO Hong, RONG Junfeng. Research progress of anti-toxicity electrocatalysts for oxygen reduction reaction in PEMFC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4677-4691. |
| [13] | GE Quanqian, XU Mai, LIANG Xian, WANG Fengwu. Research progress on the application of MOFs in photoelectrocatalysis [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4692-4705. |
| [14] | ZHU Chuanqiang, RU Jinbo, SUN Tingting, XIE Xingwang, LI Changming, GAO Shiqiu. Characteristics of selective non-catalytic reduction of NO x with solid polymer denitration agent [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4939-4946. |
| [15] | WANG Yaogang, HAN Zishan, GAO Jiachen, WANG Xinyu, LI Siqi, YANG Quanhong, WENG Zhe. Strategies for regulating product selectivity of copper-based catalysts in electrochemical CO2 reduction [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4043-4057. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||