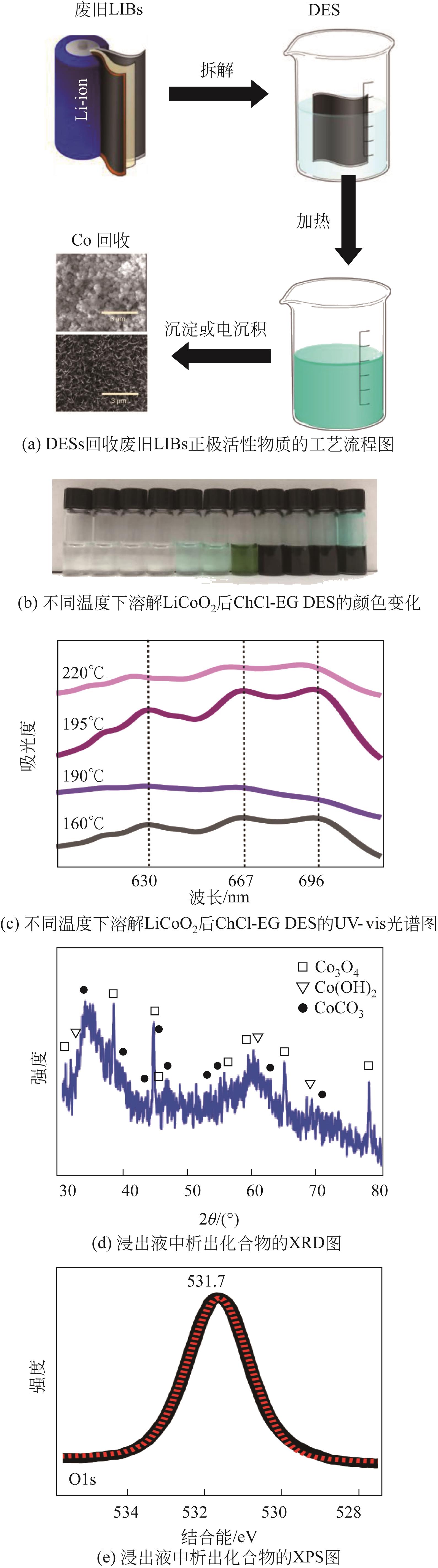
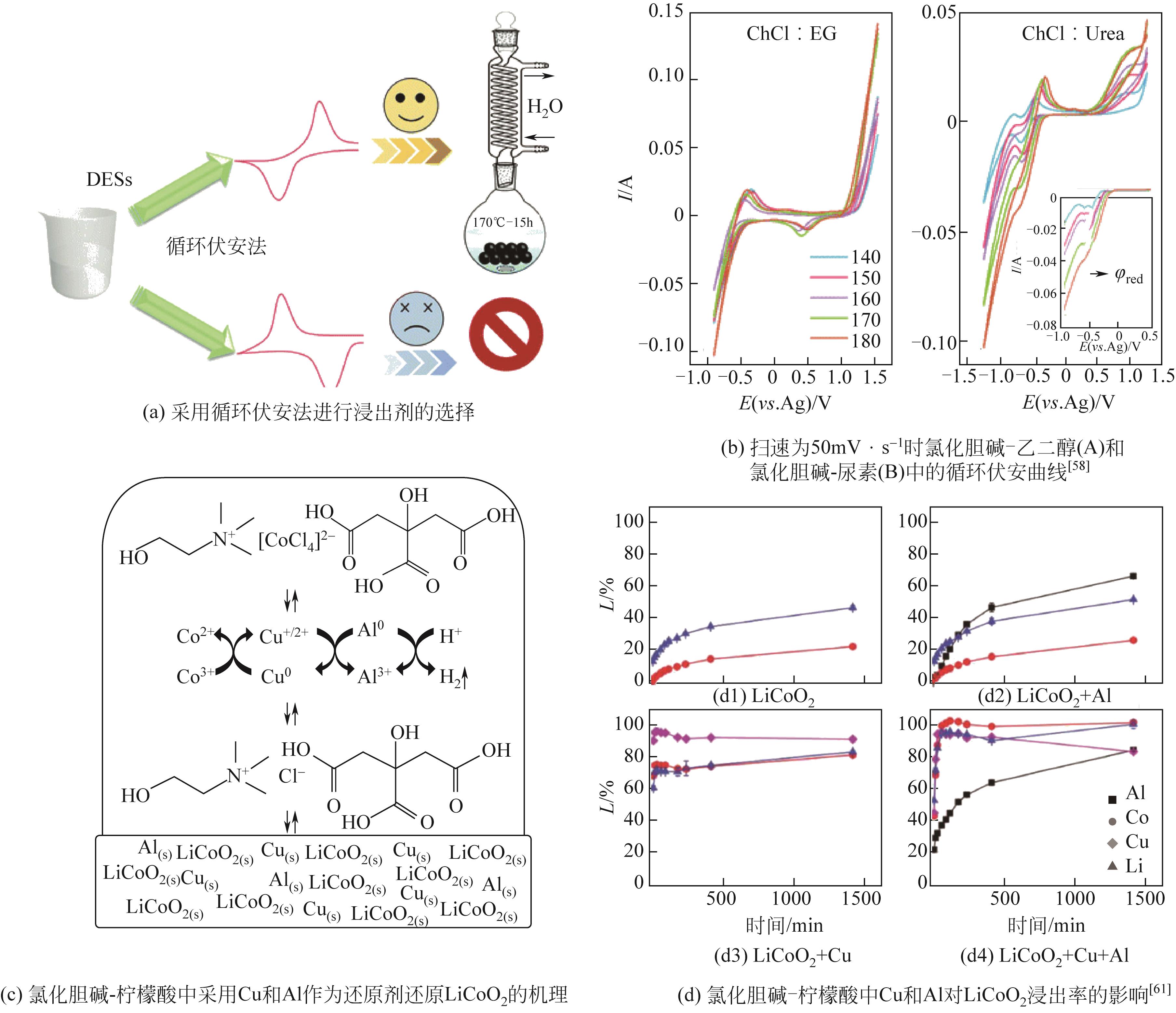
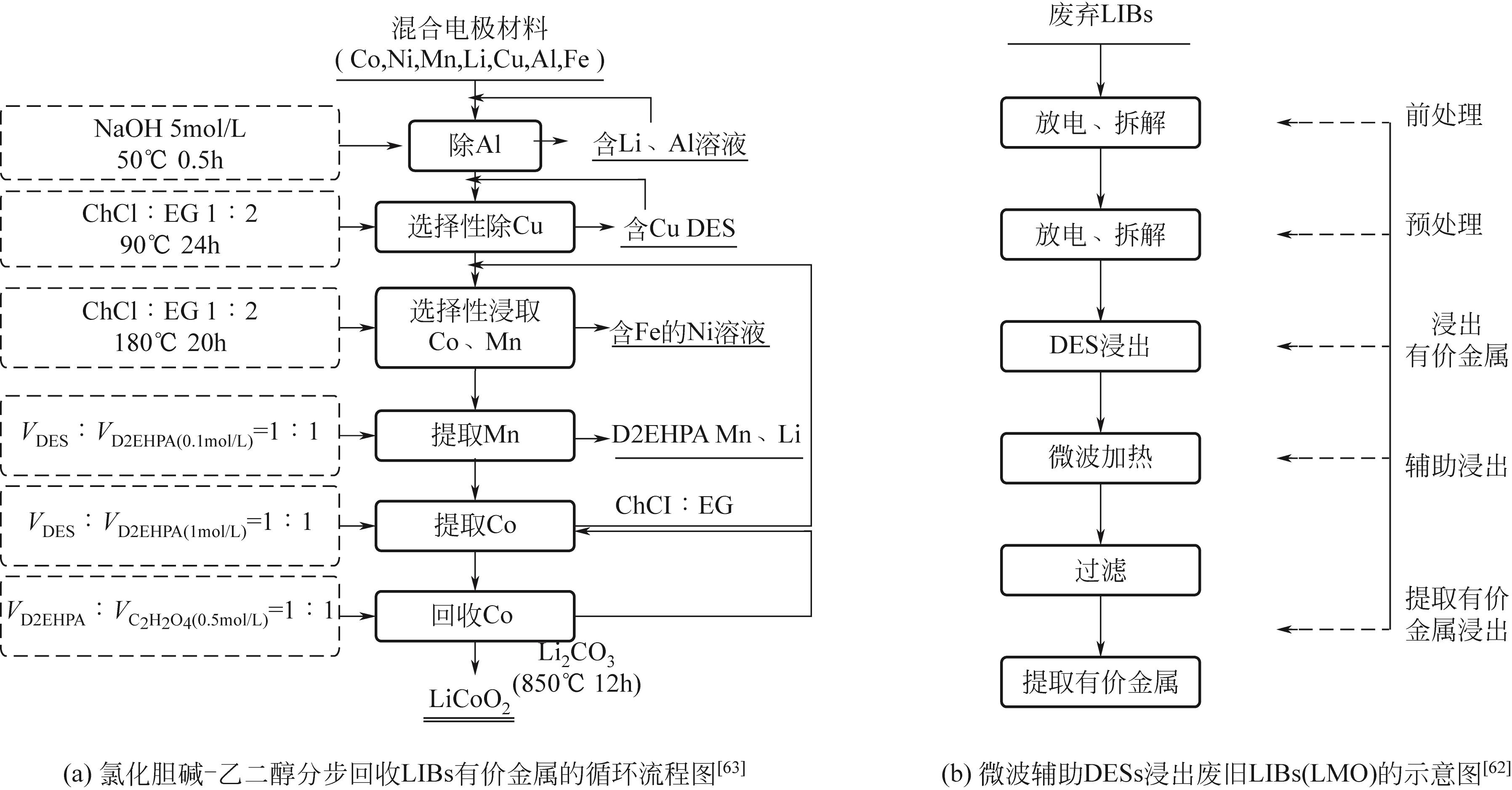
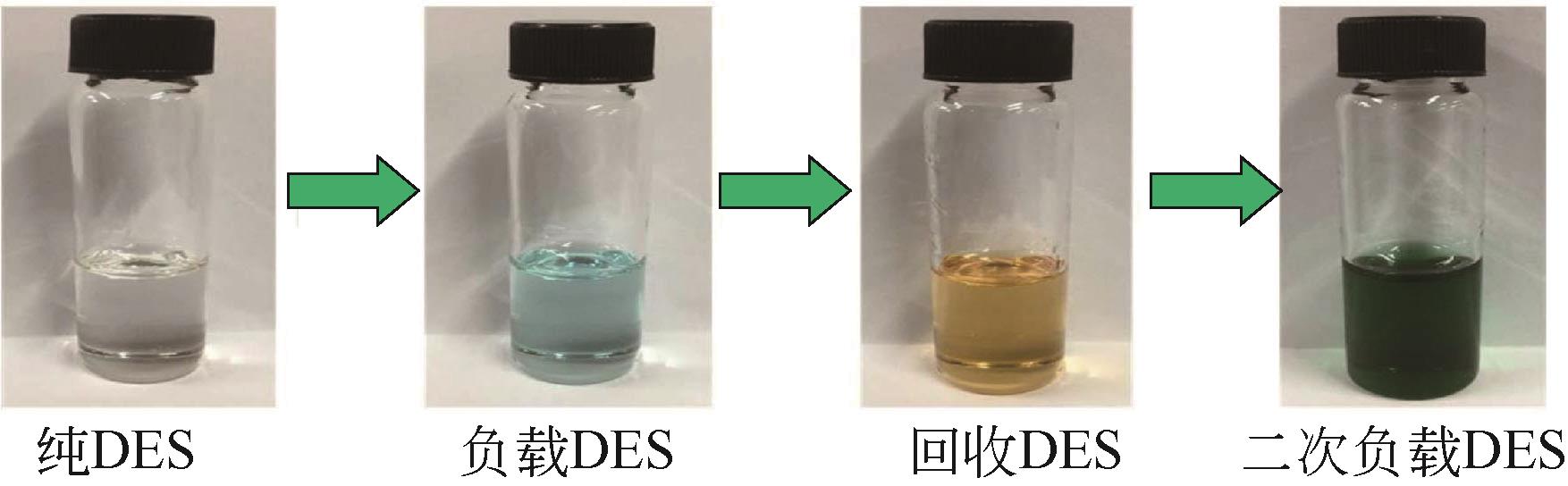
Chemical Industry and Engineering Progress ›› 2022, Vol. 41 ›› Issue (6): 3293-3305.DOI: 10.16085/j.issn.1000-6613.2021-1493
• Resources and environmental engineering • Previous Articles Next Articles
Progress of deep eutectic solvents in recovery of cathode materials from spent lithium ion batteries
CHENG Mingqiang( ), RU Juanjian(
), RU Juanjian( ), HUA Yixin, WANG Ding, GENG Xiao, ZHANG Wenwen, HUANG Haoming, WANG Daoxiang
), HUA Yixin, WANG Ding, GENG Xiao, ZHANG Wenwen, HUANG Haoming, WANG Daoxiang
- Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, Yunnan, China
-
Received:2021-07-15Revised:2021-10-20Online:2022-06-21Published:2022-06-10 -
Contact:RU Juanjian
低共熔溶剂在废旧锂离子电池正极材料回收中的研究进展
程明强( ), 汝娟坚(
), 汝娟坚( ), 华一新, 王丁, 耿笑, 张文文, 黄皓铭, 王道祥
), 华一新, 王丁, 耿笑, 张文文, 黄皓铭, 王道祥
- 昆明理工大学冶金与能源工程学院,云南 昆明 650093
-
通讯作者:汝娟坚 -
作者简介:程明强(1996—),男,硕士研究生,研究方向为锂离子电池材料回收。E-mail:1035754914@qq.com 。 -
基金资助:国家自然科学基金青年基金(51604136)
CLC Number:
Cite this article
CHENG Mingqiang, RU Juanjian, HUA Yixin, WANG Ding, GENG Xiao, ZHANG Wenwen, HUANG Haoming, WANG Daoxiang. Progress of deep eutectic solvents in recovery of cathode materials from spent lithium ion batteries[J]. Chemical Industry and Engineering Progress, 2022, 41(6): 3293-3305.
程明强, 汝娟坚, 华一新, 王丁, 耿笑, 张文文, 黄皓铭, 王道祥. 低共熔溶剂在废旧锂离子电池正极材料回收中的研究进展[J]. 化工进展, 2022, 41(6): 3293-3305.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2021-1493
| DESs | HBA/HBD摩尔比 | 密度/g?cm-3 | 黏度/mPa?s | 电导率/mS?cm-1 | 测量温度/℃ | 参考文献 |
|---|---|---|---|---|---|---|
| 氯化胆碱/乙二醇 | 1∶2 | 1.1139 | 25 | 9.73 | 30 | [ |
| 氯化胆碱/乙二醇 | 1∶2 | 1.12 | 36 | 7.61 | 20 | [ |
| 氯化胆碱/乙二醇 | 1∶3 | 1.12 | 19 | — | 20 | [ |
| 氯化胆碱/尿素 | 1∶2 | 1.1879 | 214 | 1.287 | 30 | [ |
| 氯化胆碱/尿素 | 1∶2 | 1.24 | 169 | 0.199 | 40 | [ |
| 氯化胆碱/草酸 | 1∶1 | 1.2371 | 89 | 2.35 | 30 | [ |
| 氯化胆碱/苹果酸 | 1∶1 | 1.2796 | 11475 | 0.041 | 30 | [ |
| 氯化胆碱/柠檬酸 | 1∶1 | 1.3313 | 45008 | 0.018 | 30 | [ |
| 氯化胆碱/丙三酸 | 1∶1 | — | 721 | 0.55 | 25 | [ |
| 氯化胆碱/对甲苯磺酸 | 1∶1 | 1.2074 | 183 | 1.138 | 30 | [ |
| 氯化胆碱/甘油 | 1∶2 | 1.1854 | 177 | 1.647 | 49 | [ |
| 氯化胆碱/甘油 | 1∶2 | 1.181 | 376 | 1.047 | — | [ |
| 氯化胆碱/乙酰胺 | 1∶2 | 1.8052 | 127 | 2.71 | 30 | [ |
| DESs | HBA/HBD摩尔比 | 密度/g?cm-3 | 黏度/mPa?s | 电导率/mS?cm-1 | 测量温度/℃ | 参考文献 |
|---|---|---|---|---|---|---|
| 氯化胆碱/乙二醇 | 1∶2 | 1.1139 | 25 | 9.73 | 30 | [ |
| 氯化胆碱/乙二醇 | 1∶2 | 1.12 | 36 | 7.61 | 20 | [ |
| 氯化胆碱/乙二醇 | 1∶3 | 1.12 | 19 | — | 20 | [ |
| 氯化胆碱/尿素 | 1∶2 | 1.1879 | 214 | 1.287 | 30 | [ |
| 氯化胆碱/尿素 | 1∶2 | 1.24 | 169 | 0.199 | 40 | [ |
| 氯化胆碱/草酸 | 1∶1 | 1.2371 | 89 | 2.35 | 30 | [ |
| 氯化胆碱/苹果酸 | 1∶1 | 1.2796 | 11475 | 0.041 | 30 | [ |
| 氯化胆碱/柠檬酸 | 1∶1 | 1.3313 | 45008 | 0.018 | 30 | [ |
| 氯化胆碱/丙三酸 | 1∶1 | — | 721 | 0.55 | 25 | [ |
| 氯化胆碱/对甲苯磺酸 | 1∶1 | 1.2074 | 183 | 1.138 | 30 | [ |
| 氯化胆碱/甘油 | 1∶2 | 1.1854 | 177 | 1.647 | 49 | [ |
| 氯化胆碱/甘油 | 1∶2 | 1.181 | 376 | 1.047 | — | [ |
| 氯化胆碱/乙酰胺 | 1∶2 | 1.8052 | 127 | 2.71 | 30 | [ |
| 正极活性物质 | DES | 温度/℃ | 时间/h | 固液比/mg·g-1 | 浸出效率/% | 参考文献 | |||
|---|---|---|---|---|---|---|---|---|---|
| Li | Co | Ni | Mn | ||||||
| LiCoO2 | ChCl-EG(1∶2) | 180 | 24 | 20 | 89.8 | 50.3 | — | — | [ |
| ChCl-EG(1∶2) | 220 | 24 | 20 | — | 94.1 | — | — | ||
| PTSA?1H2O?ChCl | 90 | 15 | 100 | 85 | 88 | — | — | [ | |
| PTSA?2H2O?ChCl | 90 | 15 | 100 | 100 | 100 | — | — | ||
| PTSA?3H2O?ChCl | 90 | 15 | 100 | 91 | 97 | — | — | ||
| ChCl-Urea(1∶2) | 180 | 12 | 20 | 94.7 | 97.9 | — | — | [ | |
| ChCl-甲酸(1∶2) | 90 | 12 | 20 | 99.8 | 99.1 | — | — | [ | |
| ChCl-乙酸(1∶2) | 90 | 12 | 20 | 63 | 18 | — | — | ||
| ChCl-丙酸(1∶2) | 90 | 12 | 20 | 39 | 15 | — | — | ||
| ChCl-正丁酸(1∶2) | 90 | 12 | 20 | 33 | 12 | — | — | ||
| PEG∶硫脲(1∶2) | 160 | 24 | 20 | — | 71.5 | — | — | [ | |
LiCoO2/Al/Cu Al∶LiCoO2=12%(质量分数) Cu∶LiCoO2=24%(质量分数) | ChCl-CA(1∶1) | 60 | 4 | 20 | — | 99.6 | — | — | [ |
| ChCl-EG(1∶1) | 60 | 4 | 20 | — | 2.1 | — | — | ||
| ChCl-丙酸(1∶1) | 60 | 4 | 20 | — | 81.2 | — | — | ||
| ChCl-丙酸脂(1∶1) | 60 | 4 | 20 | — | 24.4 | — | — | ||
| ChCl-OA(1∶1) | 60 | 4 | 20 | — | 19.6 | — | — | ||
| ChCl-CA(1∶2) | 40 | 1 | 20 | 93 | 98 | — | — | ||
| LiMn2O4 | ChCl-OA(1∶1) | 100 | 0.25 | 16.7 | 99 | 95 | [ | ||
| Li a Ni b Mn c Co d O2(a+b+c+d=2) | ChCl-EG(1∶2) | 180 | 24 | 4 | 71 | 32 | 7 | 60 | [ |
| LiNi1/3Mn1/3Co1/3O2 | ChCl-EG(1∶2) | 160 | 24 | 20 | — | 90 | — | 10 | [ |
| 正极活性物质 | DES | 温度/℃ | 时间/h | 固液比/mg·g-1 | 浸出效率/% | 参考文献 | |||
|---|---|---|---|---|---|---|---|---|---|
| Li | Co | Ni | Mn | ||||||
| LiCoO2 | ChCl-EG(1∶2) | 180 | 24 | 20 | 89.8 | 50.3 | — | — | [ |
| ChCl-EG(1∶2) | 220 | 24 | 20 | — | 94.1 | — | — | ||
| PTSA?1H2O?ChCl | 90 | 15 | 100 | 85 | 88 | — | — | [ | |
| PTSA?2H2O?ChCl | 90 | 15 | 100 | 100 | 100 | — | — | ||
| PTSA?3H2O?ChCl | 90 | 15 | 100 | 91 | 97 | — | — | ||
| ChCl-Urea(1∶2) | 180 | 12 | 20 | 94.7 | 97.9 | — | — | [ | |
| ChCl-甲酸(1∶2) | 90 | 12 | 20 | 99.8 | 99.1 | — | — | [ | |
| ChCl-乙酸(1∶2) | 90 | 12 | 20 | 63 | 18 | — | — | ||
| ChCl-丙酸(1∶2) | 90 | 12 | 20 | 39 | 15 | — | — | ||
| ChCl-正丁酸(1∶2) | 90 | 12 | 20 | 33 | 12 | — | — | ||
| PEG∶硫脲(1∶2) | 160 | 24 | 20 | — | 71.5 | — | — | [ | |
LiCoO2/Al/Cu Al∶LiCoO2=12%(质量分数) Cu∶LiCoO2=24%(质量分数) | ChCl-CA(1∶1) | 60 | 4 | 20 | — | 99.6 | — | — | [ |
| ChCl-EG(1∶1) | 60 | 4 | 20 | — | 2.1 | — | — | ||
| ChCl-丙酸(1∶1) | 60 | 4 | 20 | — | 81.2 | — | — | ||
| ChCl-丙酸脂(1∶1) | 60 | 4 | 20 | — | 24.4 | — | — | ||
| ChCl-OA(1∶1) | 60 | 4 | 20 | — | 19.6 | — | — | ||
| ChCl-CA(1∶2) | 40 | 1 | 20 | 93 | 98 | — | — | ||
| LiMn2O4 | ChCl-OA(1∶1) | 100 | 0.25 | 16.7 | 99 | 95 | [ | ||
| Li a Ni b Mn c Co d O2(a+b+c+d=2) | ChCl-EG(1∶2) | 180 | 24 | 4 | 71 | 32 | 7 | 60 | [ |
| LiNi1/3Mn1/3Co1/3O2 | ChCl-EG(1∶2) | 160 | 24 | 20 | — | 90 | — | 10 | [ |
| 1 | LI Jia, WANG Guangxu, XU Zhenming. Environmentally-friendly oxygen-free roasting/wet magnetic separation technology for in situ recycling cobalt, lithium carbonate and graphite from spent LiCoO2/graphite lithium batteries[J]. Journal of Hazardous Materials, 2016, 302: 97-104. |
| 2 | SCHIPPER Florian, AURBACH Doron. A brief review: past, present and future of lithium ion batteries[J]. Russian Journal of Electrochemistry, 2016, 52(12): 1095-1121. |
| 3 | WANG Mengmeng, ZHANG Congcong, ZHANG Fushen. An environmental benign process for cobalt and lithium recovery from spent lithium-ion batteries by mechanochemical approach[J]. Waste Management, 2016, 51: 239-244. |
| 4 | PALACÍN M R, DE GUIBERT A. Why do batteries fail? [J]. Science, 2016, 351(6273): 1253292. |
| 5 | NATARAJAN Subramanian, ARAVINDAN Vanchiappan. Burgeoning prospects of spent lithium-ion batteries in multifarious applications[J]. Advanced Energy Materials, 2018, 8(33): 1802303. |
| 6 | SUN Xin, HAO Han, ZHAO Fuquan, et al. The dynamic equilibrium mechanism of regional lithium flow for transportation electrification[J]. Environmental Science & Technology, 2019, 53(2): 743-751. |
| 7 | BARIK S P, PRABAHARAN G, KUMAR B. An innovative approach to recover the metal values from spent lithium-ion batteries[J]. Waste Management, 2016, 51: 222-226. |
| 8 | CHU Steven, CUI Yi, LIU Nian. The path towards sustainable energy[J]. Nature Materials, 2016, 16(1): 16-22. |
| 9 | HEELAN Joseph, GRATZ Eric, ZHENG Zhangfeng, et al. Current and prospective Li-ion battery recycling and recovery processes[J]. JOM, 2016, 68(10): 2632-2638. |
| 10 | LARCHER D, TARASCON J M. Towards greener and more sustainable batteries for electrical energy storage[J]. Nature Chemistry, 2015, 7(1): 19-29. |
| 11 | FAN Ersha, LI Li, WANG Zhenpo, et al. Sustainable recycling technology for Li-ion batteries and beyond: challenges and future prospects[J]. Chemical Reviews, 2020, 120(14): 7020-7063. |
| 12 | ORDOÑEZ J, GAGO E J, GIRARD A. Processes and technologies for the recycling and recovery of spent lithium-ion batteries[J]. Renewable and Sustainable Energy Reviews, 2016, 60: 195-205. |
| 13 | HARPER Gavin, SOMMERVILLE Roberto, KENDRICK Emma, et al. Recycling lithium-ion batteries from electric vehicles[J]. Nature, 2019, 575(7781): 75-86. |
| 14 | ZHENG Xiaohong, ZHU Zewen, LIN Xiao, et al. A mini-review on metal recycling from spent lithium ion batteries[J]. Engineering, 2018, 4(3): 361-370. |
| 15 | WANG Mengmeng, TAN Quanyin, LIU Lili, et al. A low-toxicity and high-efficiency deep eutectic solvent for the separation of aluminum foil and cathode materials from spent lithium-ion batteries[J]. Journal of Hazardous Materials, 2019, 380: 120846. |
| 16 | ZHANG Xiaoxiao, BIAN Yifan, XU Siwenyu, et al. Innovative application of acid leaching to regenerate Li(Ni1/3Co1/3Mn1/3)O2 cathodes from spent lithium-ion batteries[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(5): 5959-5968. |
| 17 | CHAGNES A. Lithium process chemistry: resources, extraction, batteries, and recycling[M]. Amsterdam: Elsevier, 2015. |
| 18 | CIEZ Rebecca E, WHITACRE J F. Examining different recycling processes for lithium-ion batteries[J]. Nature Sustainability, 2019, 2(2): 148-156. |
| 19 | WANG Rongchi, LIN Yuchuan, WU Shehuang. A novel recovery process of metal values from the cathode active materials of the lithium-ion secondary batteries[J]. Hydrometallurgy, 2009, 99(3/4): 194-201. |
| 20 | XU Jinqiu, THOMAS H R, FRANCIS Rob W, et al. A review of processes and technologies for the recycling of lithium-ion secondary batteries[J]. Journal of Power Sources, 2008, 177(2): 512-527. |
| 21 | YAO Yonglin, ZHU Meiying, ZHAO Zhuo, et al. Hydrometallurgical processes for recycling spent lithium-ion batteries: a critical review[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(11): 13611-13627. |
| 22 | ABBOTT Andrew P, BOOTHBY David, CAPPER Glen, et al. Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids[J]. Journal of the American Chemical Society, 2004, 126(29): 9142-9147. |
| 23 | 成洪业, 漆志文. 低共熔溶剂用于萃取分离的研究进展[J]. 化工进展, 2020, 39(12): 4896-4907. |
| CHENG Hongye, QI Zhiwen. Research progress of deep eutectic solvent for extractive separation[J]. Chemical Industry and Engineering Progress, 2020, 39(12): 4896-4907. | |
| 24 | 岳旭东, 袁冰, 朱国强, 等. 低共熔溶剂在有机合成和萃取分离中的应用进展[J]. 化工进展, 2018, 37(7): 2627-2634. |
| YUE Xudong, YUAN Bing, ZHU Guoqiang, et al. Development in the applications of deep eutectic solvents in organic synthesis and extraction separation[J]. Chemical Industry and Engineering Progress, 2018, 37(7): 2627-2634. | |
| 25 | Abbott A P, Capper G, Davies D L, et al. Novel solvent properties of choline chloride/urea mixtures[J]. Chemical Communications, 2003(1): 70-71. |
| 26 | LI Xiaoxia, Kyung Ho ROW. Development of deep eutectic solvents applied in extraction and separation[J]. Journal of Separation Science, 2016, 39(18): 3505-3520. |
| 27 | ABBOTT Andrew P, BARRON John C, RYDER Karl S, et al. Eutectic-based ionic liquids with metal-containing anions and cations[J]. Chemistry-A European Journal, 2007, 13(22): 6495-6501. |
| 28 | Abbott A P, Capper G, Davies D L, et al. Preparation of novel, moisture-stable, Lewis-acidic ionic liquids containing quaternary ammonium salts with functional side chains[J]. Chemical Communications, 2001(19): 2010-2011. |
| 29 | ABBOTT Andrew P, CAPPER Glen, DAVIES David L, et al. Solubility of metal oxides in deep eutectic solvents based on choline chloride[J]. Journal of Chemical & Engineering Data, 2006, 51(4): 1280-1282. |
| 30 | ABBOTT Andrew P, CAPPER Glen, DAVIES David L, et al. Ionic liquids based upon metal halide/substituted quaternary ammonium salt mixtures[J]. Inorganic Chemistry, 2004, 43(11): 3447-3452. |
| 31 | POPESCU Ana Maria, CONSTANTIN Virgil. Synthesis, characterization and thermophysical properties of three neoteric solvents-ionic liquids based on choline chloride[J]. Chemical Research in Chinese Universities, 2014, 30(1): 119-124. |
| 32 | KAYA Muammer. Recovery of metals and nonmetals from electronic waste by physical and chemical recycling processes[J]. Waste Management, 2016, 57: 64-90. |
| 33 | CHRISTOPHER Harris Robert. Physical properties of alcohol based deep eutectic solvents[D]. Leicester: University of Leicester, 2009. |
| 34 | SMITH Emma L, ABBOTT Andrew P, RYDER Karl S. Deep eutectic solvents (DESs) and their applications[J]. Chemical Reviews, 2014, 114(21): 11060-11082. |
| 35 | ZHAO Bingyi, XU Pei, YANG Fuxi, et al. Biocompatible deep eutectic solvents based on choline chloride: characterization and application to the extraction of rutin from Sophora japonica[J]. ACS Sustainable Chemistry & Engineering, 2015, 3(11): 2746-2755. |
| 36 | ABBOTT Andrew P, CAPPER Glen, GRAY Stephen. Design of improved deep eutectic solvents using hole theory[J]. ChemPhysChem, 2006, 7(4): 803-806. |
| 37 | ABBOTT Andrew P, HARRIS Robert C, RYDER Karl S. Application of hole theory to define ionic liquids by their transport properties[J]. The Journal of Physical Chemistry B, 2007, 111(18): 4910-4913. |
| 38 | Philipp ZÜRNER, FRISCH Gero. Leaching and selective extraction of indium and tin from zinc flue dust using an oxalic acid-based deep eutectic solvent[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(5): 5300-5308. |
| 39 | GHOBADI Roohollah, DIVSALAR Adeleh. Enzymatic behavior of bovine liver catalase in aqueous medium of sugar based deep eutectic solvents[J]. Journal of Molecular Liquids, 2020, 310: 113207. |
| 40 | LI Lu, LIU Juzhao, LUO Meng, et al. Efficient extraction and preparative separation of four main isoflavonoids from Dalbergia odorifera T. Chen leaves by deep eutectic solvents-based negative pressure cavitation extraction followed by macroporous resin column chromatography[J]. Journal of Chromatography B, 2016, 1033/1034: 40-48. |
| 41 | Elena ALAÑÓN M, Milena IVANOVIĆ, Sandra PIMENTEL-MORA, et al. A novel sustainable approach for the extraction of value-added compounds from Hibiscus sabdariffa L. calyces by natural deep eutectic solvents[J]. Food Research International, 2020, 137: 109646. |
| 42 | DUAN Ming, LUO Mengjuan, YANG Ziyi, et al. Application of choline-based deep eutectic solvent for the extraction of crude-oil contaminated soils[J]. Environmental Technology, 2021, 42(18): 2896-2901. |
| 43 | HOMAN Tom, SHAHBAZ Kaveh, FARID Mohammed M. Improving the production of propyl and butyl ester-based biodiesel by purification using deep eutectic solvents[J]. Separation and Purification Technology, 2017, 174: 570-576. |
| 44 | Sofía RIAÑO, PETRANIKOVA Martina, ONGHENA Bieke, et al. Separation of rare earths and other valuable metals from deep-eutectic solvents: a new alternative for the recycling of used NdFeB magnets[J]. RSC Advances, 2017, 7(51): 32100-32113. |
| 45 | CHEN Wang, LI Xiaowei, CHEN Linlin, et al. Tailoring hydrophobic deep eutectic solvent for selective lithium recovery from the mother liquor of Li2CO3 [J]. Chemical Engineering Journal, 2021, 420: 127648. |
| 46 | TRAN Mai K, RODRIGUES Marco Tulio F, KATO Keiko, et al. Deep eutectic solvents for cathode recycling of Li-ion batteries[J]. Nature Energy, 2019, 4(4): 339-345. |
| 47 | COSTA Ana Javorsky DA, MATOS José Fidel, BERNARDES Andréa Moura, et al. Beneficiation of cobalt, copper and aluminum from wasted lithium-ion batteries by mechanical processing[J]. International Journal of Mineral Processing, 2015, 145: 77-82. |
| 48 | DIEKMANN Jan, HANISCH Christian, Linus FROBÖSE, et al. Ecological recycling of lithium-ion batteries from electric vehicles with focus on mechanical processes[J]. Journal of the Electrochemical Society, 2016, 164(1): A6184-A6191. |
| 49 | PAGNANELLI Francesca, MOSCARDINI Emanuela, ALTIMARI Pietro, et al. Leaching of electrodic powders from lithium ion batteries: optimization of operating conditions and effect of physical pretreatment for waste fraction retrieval[J]. Waste Management, 2017, 60: 706-715. |
| 50 | ZHANG Tao, HE Yaqun, WANG Fangfang, et al. Chemical and process mineralogical characterizations of spent lithium-ion batteries: an approach by multi-analytical techniques[J]. Waste Management, 2014, 34(6): 1051-1058. |
| 51 | 黎华玲, 陈永珍, 宋文吉, 等. 湿法回收退役三元锂离子电池有价金属的研究进展[J]. 化工进展, 2019, 38(2): 921-932. |
| LI Hualing, CHEN Yongzhen, SONG Wenji, et al. Research progress on the recovery of valuable metals in retired LiNi x Co y Mn z O2 batteries by wet process[J]. Chemical Industry and Engineering Progress, 2019, 38(2): 921-932. | |
| 52 | ZENG Xianlai, LI Jinhui. Innovative application of ionic liquid to separate Al and cathode materials from spent high-power lithium-ion batteries[J]. Journal of Hazardous Materials, 2014, 271: 50-56. |
| 53 | LIU Fu, ABED M R M, LI K. Preparation and characterization of poly(vinylidene fluoride) (PVDF) based ultrafiltration membranes using nano γ-Al2O3 [J]. Journal of Membrane Science, 2011, 366(1/2): 97-103. |
| 54 | Kristina RADOŠEVIĆ, CVJETKO BUBALO Marina, GAURINA SRČEK Višnje, et al. Evaluation of toxicity and biodegradability of choline chloride based deep eutectic solvents[J]. Ecotoxicology and Environmental Safety, 2015, 112: 46-53. |
| 55 | BAI Yaocai, MURALIDHARAN Nitin, LI Jianlin, et al. Sustainable direct recycling of lithium-ion batteries via solvent recovery of electrode materials[J]. ChemSusChem, 2020, 13(21): 5664-5670. |
| 56 | XIE Jing, LU Yichun. A retrospective on lithium-ion batteries[J]. Nature Communications, 2020, 11(1): 2499. |
| 57 | ROLDÁN-RUIZ María Jesús, FERRER María Luisa, GUTIÉRREZ María Concepción, et al. Highly efficient p-toluenesulfonic acid-based deep-eutectic solvents for cathode recycling of Li-ion batteries[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(14): 5437-5445. |
| 58 | WANG Shubin, ZHANG Zuotai, LU Zhouguang, et al. A novel method for screening deep eutectic solvent to recycle the cathode of Li-ion batteries[J]. Green Chemistry, 2020, 22(14): 4473-4482. |
| 59 | CHEN Linlin, CHAO Yanhong, LI Xiaowei, et al. Engineering a tandem leaching system for the highly selective recycling of valuable metals from spent Li-ion batteries[J]. Green Chemistry, 2021, 23(5): 2177-2184. |
| 60 | CHEN Yu, LU Yanhong, LIU Zhenghui, et al. Efficient dissolution of lithium-ion batteries cathode LiCoO2 by polyethylene glycol-based deep eutectic solvents at mild temperature[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(31): 11713-11720. |
| 61 | PEETERS Nand, BINNEMANS Koen, Sofía RIAÑO. Solvometallurgical recovery of cobalt from lithium-ion battery cathode materials using deep-eutectic solvents[J]. Green Chemistry, 2020, 22(13): 4210-4221. |
| 62 | XU Zhiwen, SHAO Huaishuang, ZHAO Qinxin, et al. Use of microwave-assisted deep eutectic solvents to recycle lithium manganese oxide from Li-ion batteries[J]. JOM, 2021, 73(7): 2104-2110. |
| 63 | SCHIAVI Pier Giorgio, ALTIMARI Pietro, BRANCHI Mario, et al. Selective recovery of cobalt from mixed lithium ion battery wastes using deep eutectic solvent[J]. Chemical Engineering Journal, 2021, 417: 129249. |
| 64 | Yingchun LYU, WU Xia, WANG Kai, et al. An overview on the advances of LiCoO2 cathodes for lithium-ion batteries[J]. Advanced Energy Materials, 2021, 11(2): 2000982. |
| 65 | CHEN Xiangping, XU Bao, ZHOU Tao, et al. Separation and recovery of metal values from leaching liquor of mixed-type of spent lithium-ion batteries[J]. Separation and Purification Technology, 2015, 144: 197-205. |
| 66 | JOULIÉ M, LAUCOURNET R, BILLY E. Hydrometallurgical process for the recovery of high value metals from spent lithium nickel cobalt aluminum oxide based lithium-ion batteries[J]. Journal of Power Sources, 2014, 247: 551-555. |
| 67 | WANG Shubin, WANG Chao, LAI Fengjiao, et al. Reduction-ammoniacal leaching to recycle lithium, cobalt, and nickel from spent lithium-ion batteries with a hydrothermal method: effect of reductants and ammonium salts[J]. Waste Management, 2020, 102: 122-130. |
| 68 | PENG Chao, LIU Fupeng, Arif T AJI, et al. Extraction of Li and Co from industrially produced Li-ion battery waste - using the reductive power of waste itself[J]. Waste Management, 2019, 95: 604-611. |
| 69 | ABBOTT Andrew P, CAPPER Glen, DAVIES David L, et al. Selective extraction of metals from mixed oxide matrixes using choline-based ionic liquids[J]. Inorganic Chemistry, 2005, 44(19): 6497-6499. |
| 70 | RODRIGUEZ RODRIGUEZ Nerea, MACHIELS Lieven, BINNEMANS Koen. p-Toluenesulfonic acid-based deep-eutectic solvents for solubilizing metal oxides[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(4): 3940-3948. |
| 71 | OJANEN Severi, Mari LUNDSTRÖM, Annukka SANTASALO-AARNIO, et al. Challenging the concept of electrochemical discharge using salt solutions for lithium-ion batteries recycling[J]. Waste Management, 2018, 76: 242-249. |
| 72 | MIAO Yu, HYNAN Patrick, JOUANNE Annette VON, et al. Current Li-ion battery technologies in electric vehicles and opportunities for advancements[J]. Energies, 2019, 12(6): 1074. |
| 73 | ZHANG Pingwei, YOKOYAMA Toshiro, ITABASHI Osamu, et al. Hydrometallurgical process for recovery of metal values from spent nickel-metal hydride secondary batteries[J]. Hydrometallurgy, 1998, 50(1): 61-75. |
| 74 | ZOU Haiyang, GRATZ Eric, APELIAN Diran, et al. A novel method to recycle mixed cathode materials for lithium ion batteries[J]. Green Chemistry, 2013, 15(5): 1183-1191. |
| 75 | 张英杰, 宁培超, 杨轩, 等. 废旧三元锂离子电池回收技术研究新进展[J]. 化工进展, 2020, 39(7): 2828-2840. |
| ZHANG Yingjie, NING Peichao, YANG Xuan, et al. Research progress on the recycling technology of spent ternary lithium ion battery[J]. Chemical Industry and Engineering Progress, 2020, 39(7): 2828-2840. | |
| 76 | JIANG Feng, CHEN Yuqian, JU Shaohua, et al. Ultrasound-assisted leaching of cobalt and lithium from spent lithium-ion batteries[J]. Ultrasonics Sonochemistry, 2018, 48: 88-95. |
| 77 | RU Juanjian, HUA Yixin, WANG Ding. Preparation and characterisation of TiN by microwave-assisted carbothermic reduction-nitridation in air atmosphere[J]. Advances in Applied Ceramics, 2017, 116(8): 468-476. |
| 78 | FU Yuanpeng, HE Yaqun, YANG Yong, et al. Microwave reduction enhanced leaching of valuable metals from spent lithium-ion batteries[J]. Journal of Alloys and Compounds, 2020, 832: 154920. |
| 79 | ABBOTT Andrew P, FRISCH Gero, HARTLEY Jennifer, et al. Processing of metals and metal oxides using ionic liquids[J]. Green Chemistry, 2011, 13(3): 471-481. |
| 80 | ABBOTT Andrew P, FRISCH Gero, RYDER Karl S. Metal complexation in ionic liquids[J]. Annual Reports Section A: Inorganic Chemistry, 2008, 104: 21-45. |
| 81 | ZANTE Guillaume, BRAUN Arthur, MASMOUDI Abderrazak, et al. Solvent extraction fractionation of manganese, cobalt, nickel and lithium using ionic liquids and deep eutectic solvents[J]. Minerals Engineering, 2020, 156: 106512. |
| 82 | OTHMAN Enas A, Aloijsius G J VAN DER HAM, MIEDEMA Henk, et al. Recovery of metals from spent lithium-ion batteries using ionic liquid[P8888][Oleate[J]. Separation and Purification Technology, 2020, 252: 117435. |
| 83 | YUE Duyuan, JIA Yongzhong, YAO Ying, et al. Structure and electrochemical behavior of ionic liquid analogue based on choline chloride and urea[J]. Electrochimica Acta, 2012, 65: 30-36. |
| 84 | ZHU Xiaolin, XU Cunying, TANG Jie, et al. Selective recovery of zinc from zinc oxide dust using choline chloride based deep eutectic solvents[J]. Transactions of Nonferrous Metals Society of China, 2019, 29(10): 2222-2228. |
| [1] | CUI Shoucheng, XU Hongbo, PENG Nan. Simulation analysis of two MOFs materials for O2/He adsorption separation [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 382-390. |
| [2] | LI Shilin, HU Jingze, WANG Yilin, WANG Qingji, SHAO Lei. Research progress in separation and extraction of high value components by electrodialysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 420-429. |
| [3] | WANG Peng, ZHANG Yang, FAN Bingqiang, HE Dengbo, SHEN Changshuai, ZHANG Hedong, ZHENG Shili, ZOU Xing. Process and kinetics of hydrochloric acid leaching of high-carbon ferrochromium [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 510-517. |
| [4] | GUO Qiang, ZHAO Wenkai, XIAO Yonghou. Numerical simulation of enhancing fluid perturbation to improve separation of dimethyl sulfide/nitrogen via pressure swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 64-72. |
| [5] | MA Yi, CAO Shiwei, WANG Jiajun, LIN Liqun, XING Yan, CAO Tengliang, LU Feng, ZHAO Zhenlun, ZHANG Zhijun. Research progress in recovery of spent cathode materials for lithium-ion batteries using deep eutectic solvents [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 219-232. |
| [6] | HE Meijin. Application and development trend of molecular management in separation technology in petrochemical field [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 260-266. |
| [7] | LI Weihua, YU Qianwen, YIN Junquan, WU Yinkai, SUN Yingjie, WANG Yan, WANG Huawei, YANG Yufei, LONG Yuyang, HUANG Qifei, GE Yanchen, HE Yiyang, ZHAO Lingyan. Leaching behavior of heavy metals from broken ton bags filled with fly ash in acid rain environment [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4917-4928. |
| [8] | LIAO Zhixin, LUO Tao, WANG Hong, KONG Jiajun, SHEN Haiping, GUAN Cuishi, WANG Cuihong, SHE Yucheng. Application and progress of solvent deasphalting technology [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4573-4586. |
| [9] | PAN Yichang, ZHOU Rongfei, XING Weihong. Advanced microporous membranes for efficient separation of same-carbon-number hydrocarbon mixtures: State-of-the-art and challenges [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3926-3942. |
| [10] | WANG Baoying, WANG Huangying, YAN Junying, WANG Yaoming, XU Tongwen. Research progress of polymer inclusion membrane in metal separation and recovery [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3990-4004. |
| [11] | LYU Jie, HUANG Chong, FENG Ziping, HU Yafei, SONG Wenji. Performance and control system of gas engine heat pump based on waste heat recovery [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4182-4192. |
| [12] | HU Yafei, FENG Ziping, TIAN Jiayao, SONG Wenji. Waste heat recovery performance of an air-source gas engine-driven heat pump system in multi-heating operation modes [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4204-4211. |
| [13] | LOU Baohui, WU Xianhao, ZHANG Chi, CHEN Zhen, FENG Xiangdong. Advances in nanofluid for CO2 absorption and separation [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3802-3815. |
| [14] | LU Yang, ZHOU Jinsong, ZHOU Qixin, WANG Tang, LIU Zhuang, LI Bohao, ZHOU Lingtao. Leaching mechanism of Hg-absorption products on CeO2/TiO2 sorbentsin syngas [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3875-3883. |
| [15] | ZHOU Longda, ZHAO Lixin, XU Baorui, ZHANG Shuang, LIU Lin. Advances in electrostatic-cyclonic coupling enhanced multiphase media separation research [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3443-3456. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||