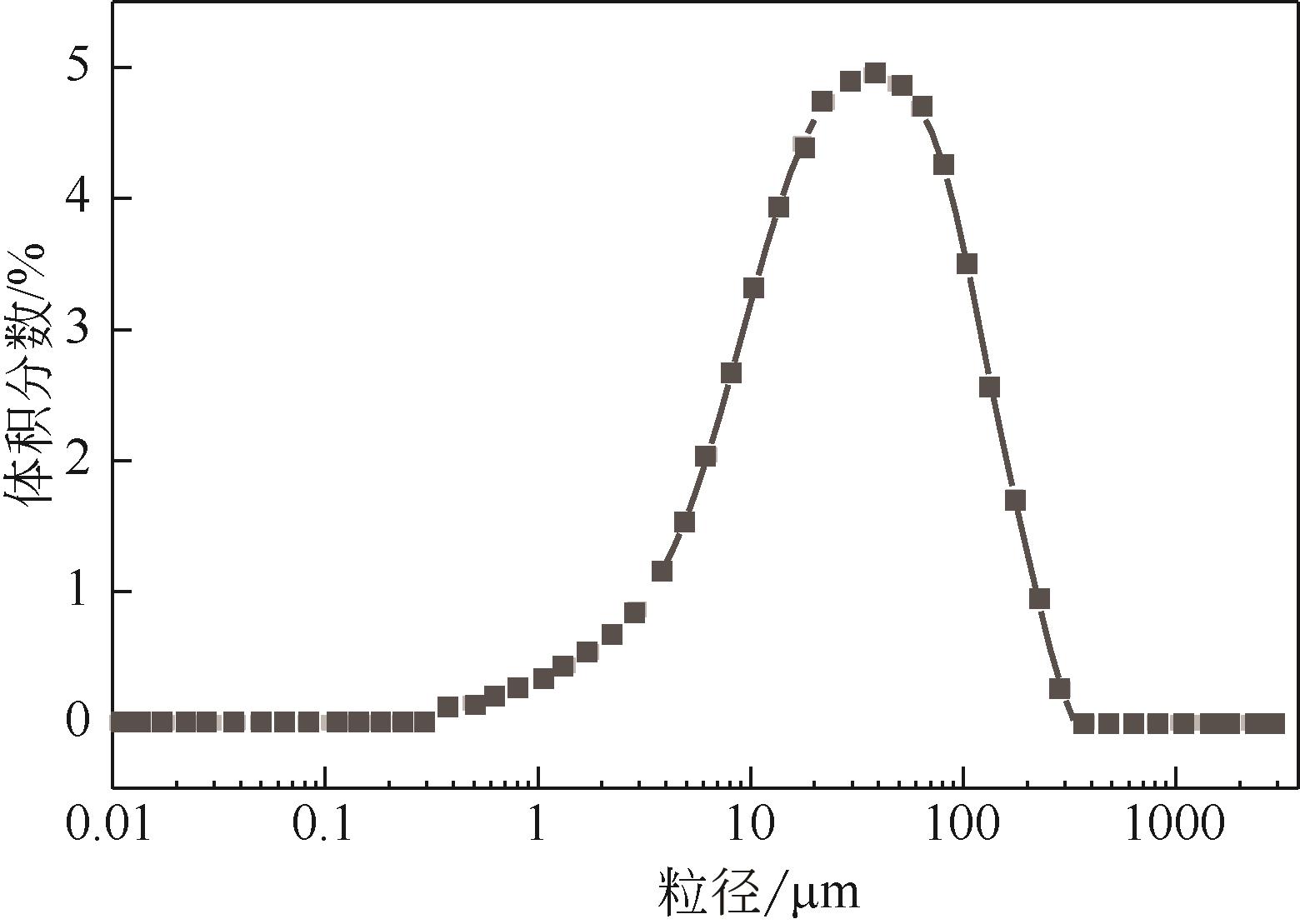
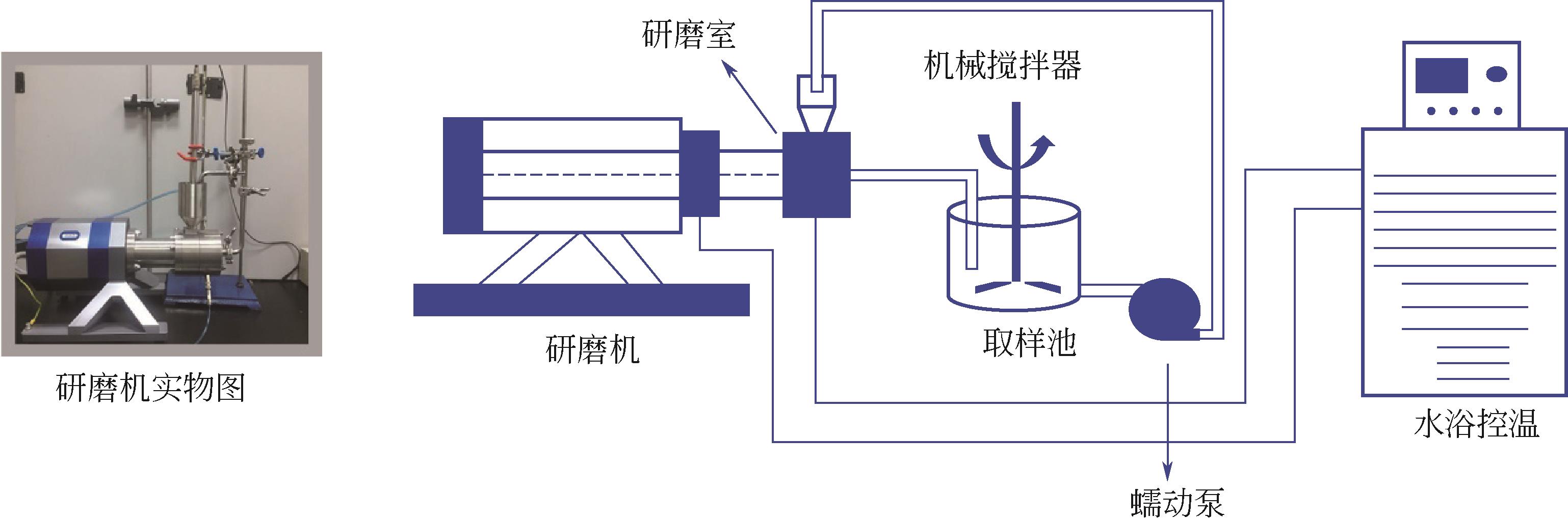
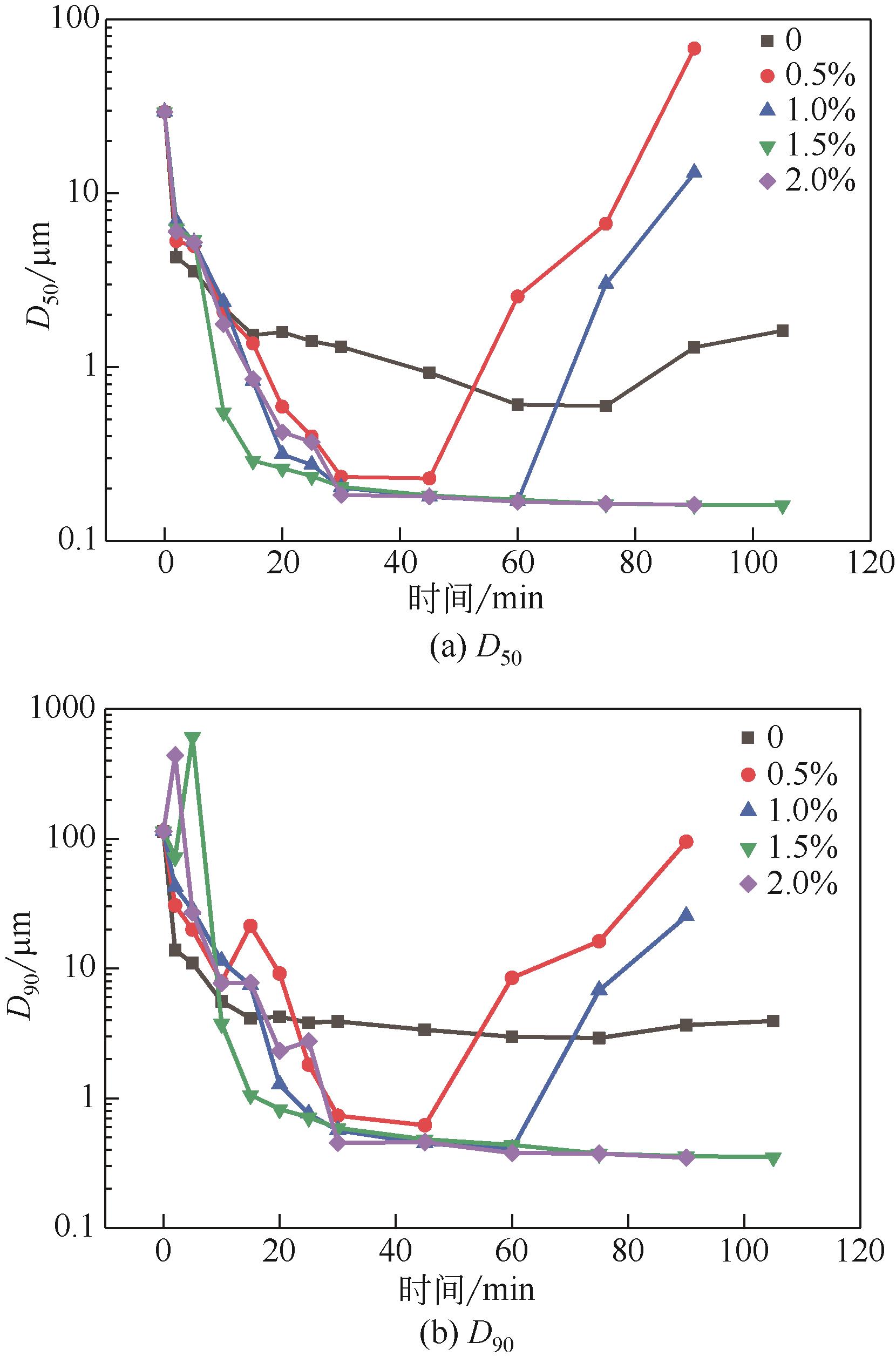
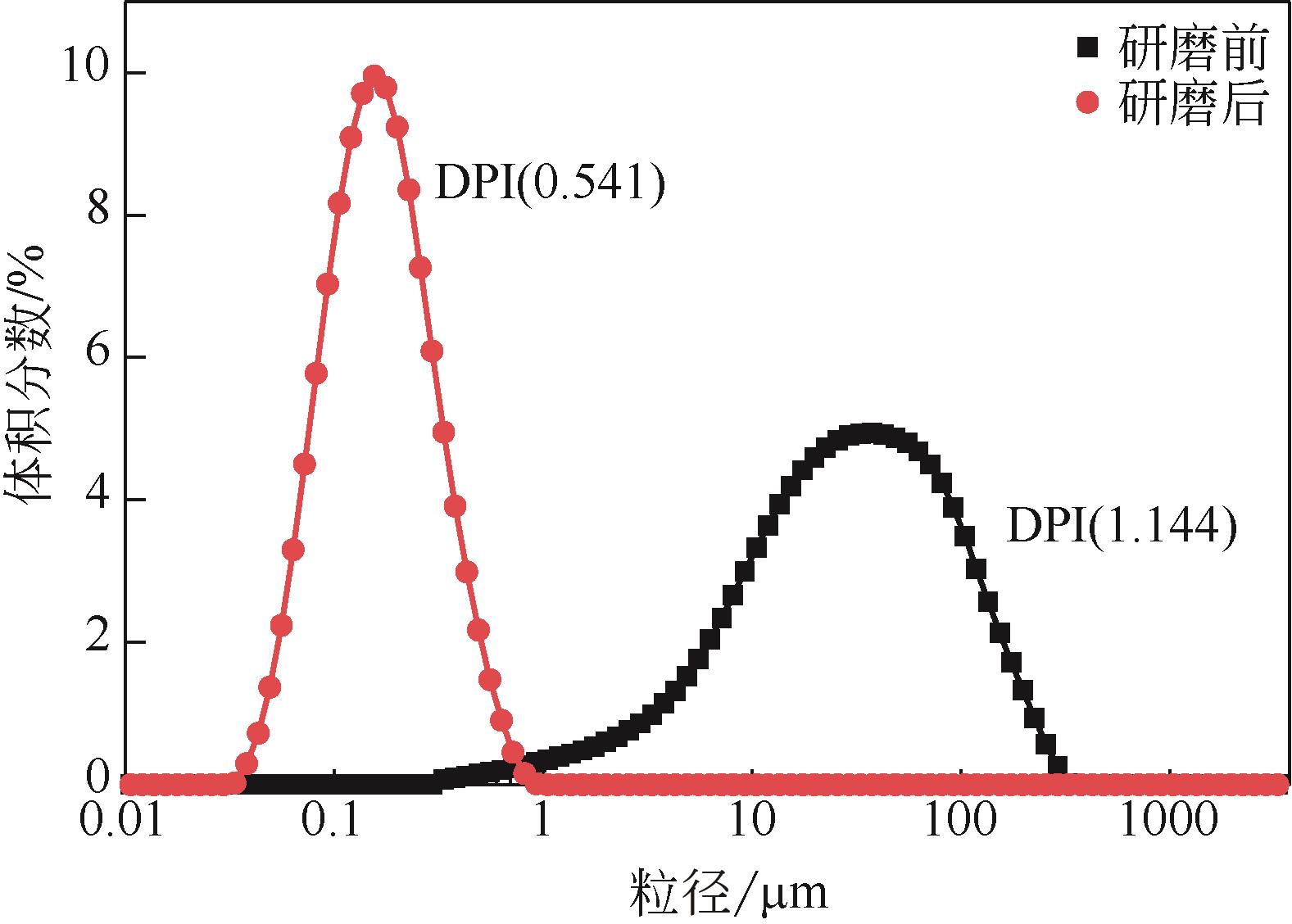
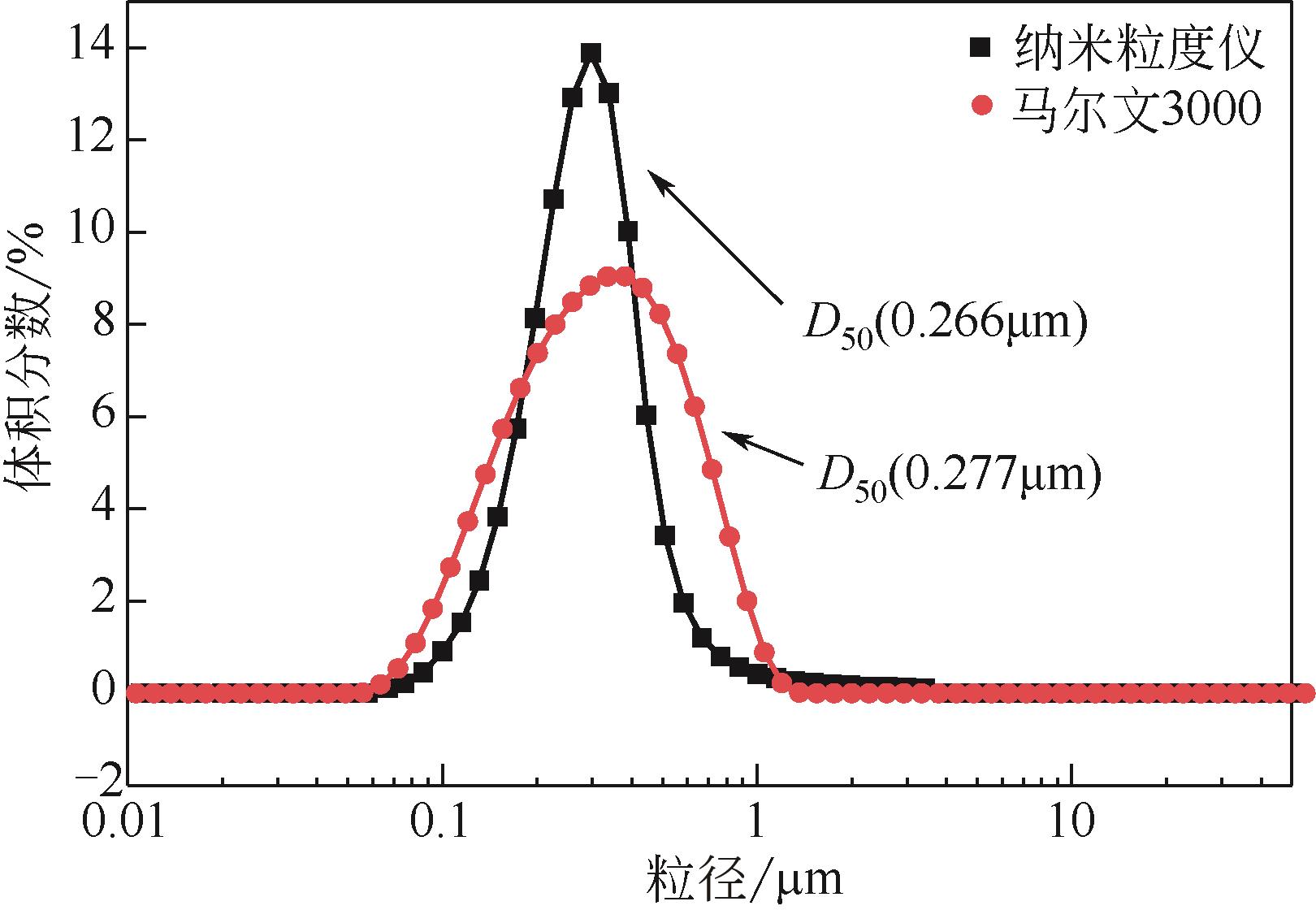
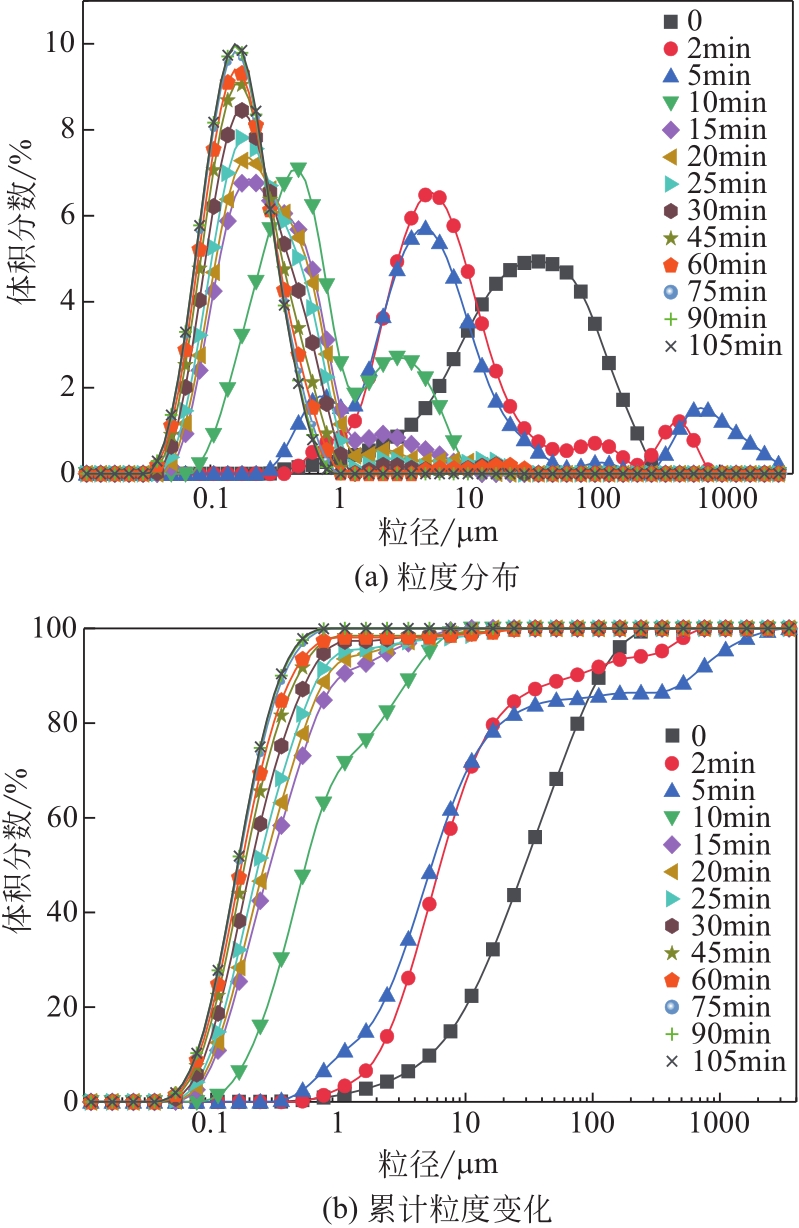
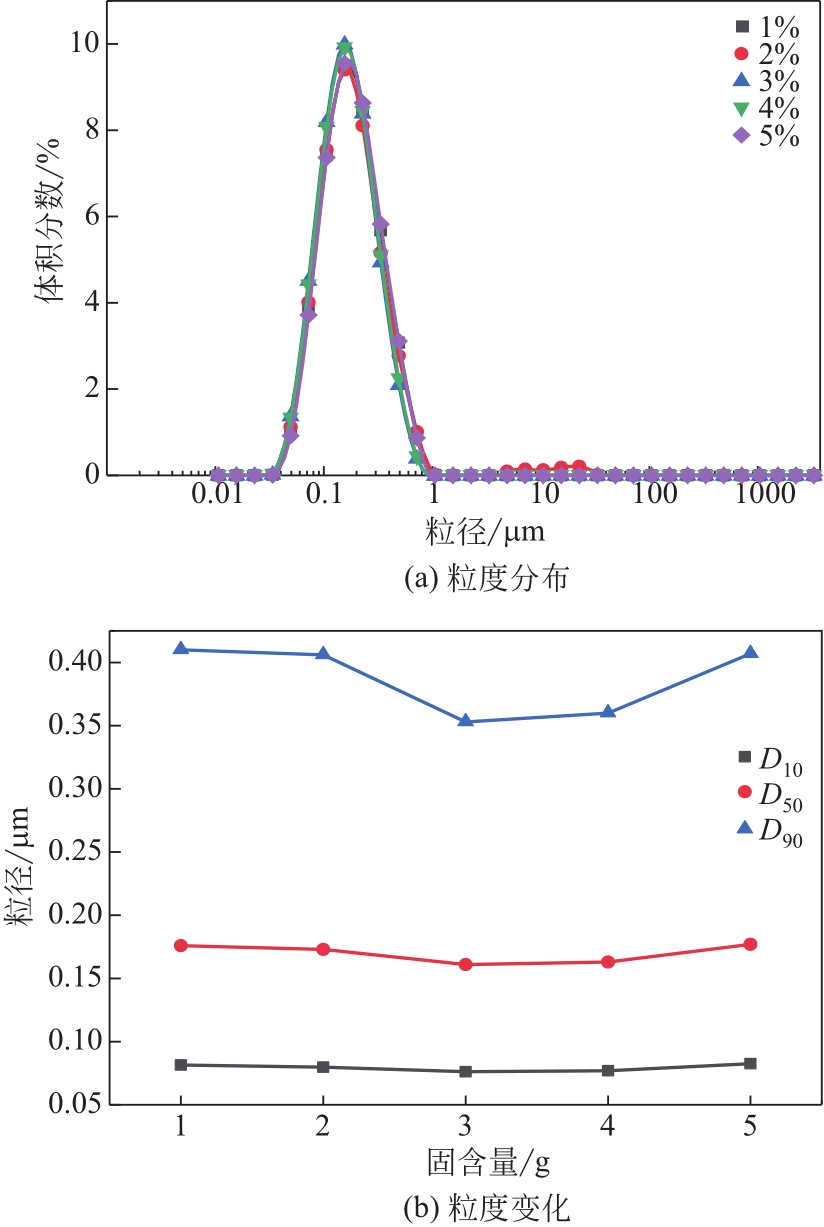
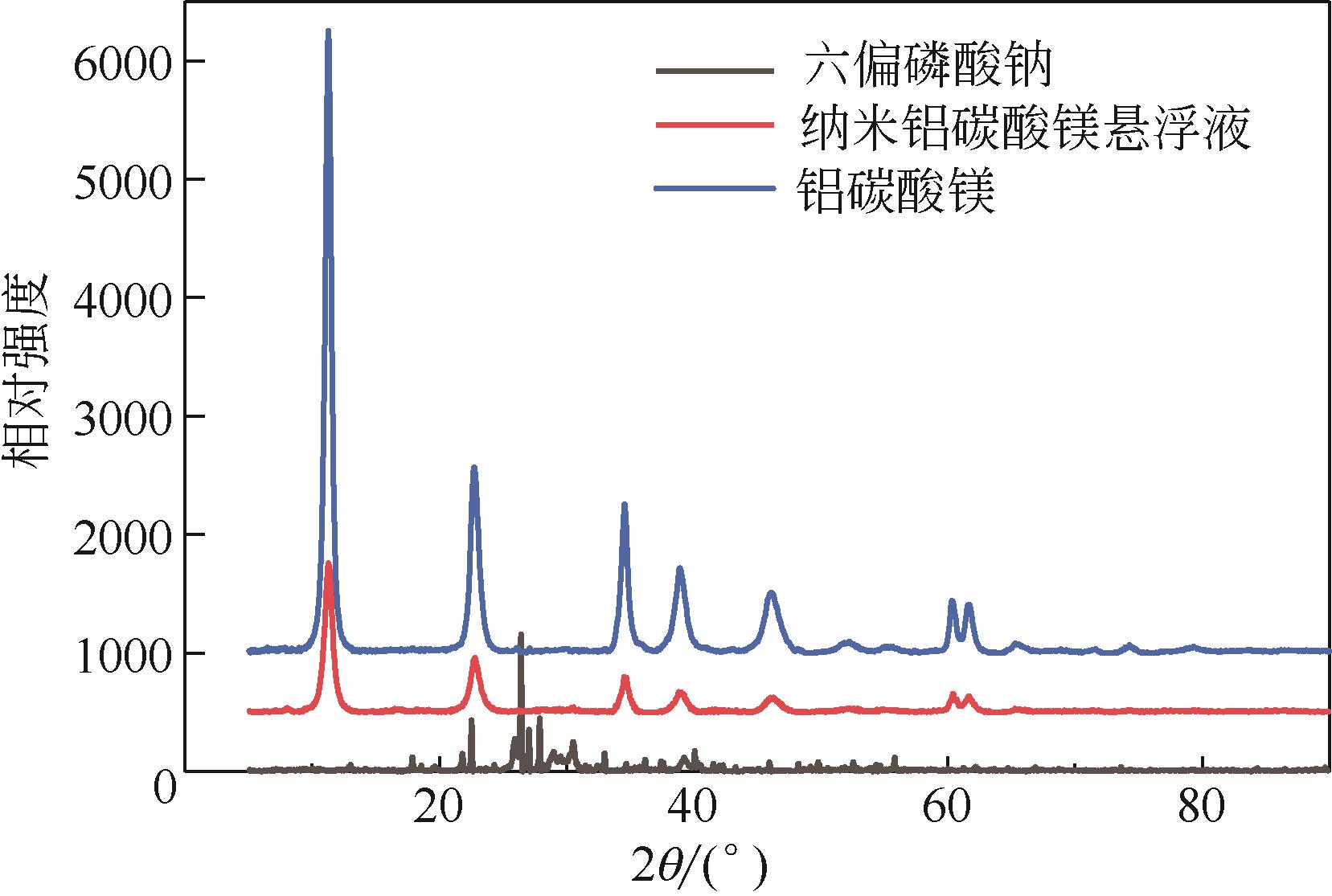
| 1 |
SHEGOKAR R, MÜLLER R H. Nanocrystals: industrially feasible multifunctional formulation technology for poorly soluble actives[J]. International Journal of Pharmaceutics, 2010, 399(1/2): 129-139.
|
| 2 |
LIPINSKI C. Poor aqueous solubility: an industry wide problem in drug discovery[J]. American Pharmaceutical Review, 2002, 5: 82-85.
|
| 3 |
MALAMATARI M, TAYLOR K M G, MALAMATARIS S, et al. Pharmaceutical nanocrystals: production by wet milling and applications[J]. Drug Discovery Today, 2018, 23(3): 534-547.
|
| 4 |
BUCKTON G, BEEZER A E. The relationship between particle size and solubility[J]. International Journal of Pharmaceutics, 1992, 82(3): R7-R10.
|
| 5 |
KHADKA P, RO J, KIM H, et al. Pharmaceutical particle technologies: an approach to improve drug solubility, dissolution and bioavailability[J]. Asian Journal of Pharmaceutical Sciences, 2014, 9(6): 304-316.
|
| 6 |
JUNYAPRASERT V B, MORAKUL B. Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs[J]. Asian Journal of Pharmaceutical Sciences, 2015, 10(1): 13-23.
|
| 7 |
GAO L, ZHANG D R, CHEN M H. Drug nanocrystals for the formulation of poorly soluble drugs and its application as a potential drug delivery system[J]. Journal of Nanoparticle Research, 2008, 10(5): 845-862.
|
| 8 |
GULSUN T, GURSOY R N, ONER L. Nanocrystal technology for oral delivery of poorly water-soluble drugs[J]. FABAD Journal of Pharmaceutical Sciences, 2009, 34: 55-65.
|
| 9 |
JUNGHANNS J U, MÜLLER R H. Nanocrystal technology, drug delivery and clinical applications[J]. International Journal of Nanomedicine, 2008, 3(3): 295-309.
|
| 10 |
RAGHAVA SRIVALLI K M, MISHRA B. Drug nanocrystals: a way toward scale-up[J]. Saudi Pharmaceutical Journal, 2016, 24(4): 386-404.
|
| 11 |
LU Y, LI Y, WU W. Injected nanocrystals for targeted drug delivery[J]. Acta Pharmaceutica Sinica B, 2016, 6(2): 106-113.
|
| 12 |
SHEOKAND S, REDDY V, BANSAL A K. Pharmaceutical nanocrystals: from fundamentals to advances[J]. Pharma Times, 2018, 50: 20-25.
|
| 13 |
TUOMELA A, LAAKSONEN T, LARU J, et al. Solid formulations by a nanocrystal approach: critical process parameters regarding scale-ability of nanocrystals for tableting applications[J]. International Journal of Pharmaceutics, 2015, 485(1/2): 77-86.
|
| 14 |
AGIMELEN O S, SVOBODA V, AHMED B, et al. Multi-sensor inline measurements of crystal size and shape distributions during high shear wet milling of crystal slurries[J]. Advanced Powder Technology, 2018, 29(12): 2987-2995.
|
| 15 |
岳鹏飞, 刘阳, 谢锦, 等. 药物纳米晶体制备技术30年发展回顾与展望[J]. 药学学报, 2018, 53(4): 529-537.
|
|
YUE Pengfei, LIU Yang, XIE Jin, et al. Review and prospect on preparation technology of drug nanocrystals in the past thirty years[J]. Acta Pharmaceutica Sinica, 2018, 53(4): 529-537.
|
| 16 |
谢元彪, 许俊男, 陈颖翀, 等. 纳米晶体技术在难溶性药物中的应用进展与思考[J]. 世界科学技术: 中医药现代化, 2016, 18(10): 1788-1793.
|
|
XIE Yuanbiao, XU Junnan, CHEN Yingchong, et al. A progress and thinking on the application of nanocrystals technology to poorly soluble drugs[J]. Modernization of Traditional Chinese Medicine and Materia Medica—World Science and Technology, 2016, 18(10): 1788-1793.
|
| 17 |
STENGER F, GÖTZINGER M, JAKOB P, et al. Mechano-chemical changes of nano sized α-Al2O3 during wet dispersion in stirred ball Mills[J]. Particle & Particle Systems Characterization, 2004, 21(1): 31-38.
|
| 18 |
STENGER F, MENDE S, SCHWEDES J, et al. Nanomilling in stirred media Mills[J]. Chemical Engineering Science, 2005, 60(16): 4557-4565.
|
| 19 |
KWADE A. Wet comminution in stirred media Mills—Research and its practical application[J]. Powder Technology, 1999, 105(1/2/3): 14-20.
|
| 20 |
CERDEIRA A M, GANDER B, MAZZOTTI M. Role of milling parameters and particle stabilization on nanogrinding of drug substances of similar mechanical properties[J]. Chemical Engineering & Technology, 2011, 34(9): 1427-1438.
|
| 21 |
RAMACHANDRAN R, IMMANUEL C D, STEPANEK F, et al. A mechanistic model for breakage in population balances of granulation: theoretical kernel development and experimental validation[J]. Chemical Engineering Research and Design, 2009, 87(4): 598-614.
|
| 22 |
FALOLA A A, WANG X Z. On-line real-time characterisation of nanoparticle size distribution using a self-calibrating ultrasonic probe[C]//SRA Policy Forum Risk Governance for Key Enabling Technologies, Venice, Italy, 2017.
|
| 23 |
ZHANG L, CHAI G, ZENG X, et al. Preparation of fenofibrate immediate-release tablets involving wet grinding for improved bioavailability[J]. Drug Development and Industrial Pharmacy, 2010, 36(9): 1054-1063.
|
 ), ZHANG Yang2, FALOLA Akinola1, HE Yunliang1, WANG Xuezhong1(
), ZHANG Yang2, FALOLA Akinola1, HE Yunliang1, WANG Xuezhong1( )
)
 ), 张扬2, FALOLA Akinola1, 何运良1, 王学重1(
), 张扬2, FALOLA Akinola1, 何运良1, 王学重1( )
)