Chemical Industry and Engineering Progress ›› 2021, Vol. 40 ›› Issue (6): 3181-3190.DOI: 10.16085/j.issn.1000-6613.2020-1434
• Industrial catalysis • Previous Articles Next Articles
Research progress of three-dimensional ordered macroporous perovskite metal oxides as highly efficient combustion catalysts
LI Cuicui( ), ZHANG Ting, AN Jing, ZENG Jianyou, MA Haixia(
), ZHANG Ting, AN Jing, ZENG Jianyou, MA Haixia( )
)
- School of Chemical Engineering, Northwest University, Xi’an 710069, Shaanxi, China
-
Received:2020-07-24Revised:2020-12-05Online:2021-06-22Published:2021-06-06 -
Contact:MA Haixia
三维有序大孔钙钛矿金属氧化物作为高效燃烧催化剂的研究进展
- 西北大学化工学院,陕西 西安 710069
-
通讯作者:马海霞 -
作者简介:李翠翠(1994—),女,硕士研究生,研究方向为纳米材料的制备。E-mail:lcc6560141@163.com 。 -
基金资助:国家自然科学基金(21373161)
CLC Number:
Cite this article
LI Cuicui, ZHANG Ting, AN Jing, ZENG Jianyou, MA Haixia. Research progress of three-dimensional ordered macroporous perovskite metal oxides as highly efficient combustion catalysts[J]. Chemical Industry and Engineering Progress, 2021, 40(6): 3181-3190.
李翠翠, 张婷, 安静, 曾见有, 马海霞. 三维有序大孔钙钛矿金属氧化物作为高效燃烧催化剂的研究进展[J]. 化工进展, 2021, 40(6): 3181-3190.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2020-1434
| 催化剂 | 反应物进料① | 加热速率/℃·min-1 | 碳烟和催化剂质量比② | T50或Tm/℃ | 参考文献 |
|---|---|---|---|---|---|
| LaFeO3 | 0.2%NO+5%O2+He | 2 | 1∶10 | 412 | [ |
| LaMn0.95Fe0.05O3 | 0.2%NO+5%O2+Ar | 2 | 1∶10 | 392④ | [ |
| La0.9K0.1CoO3 | 0.2%NO+5%O2+Ar | 2 | 1∶10 | 398 | [ |
| La0.95K0.05NiO3 | 0.2%NO+5%O2+N2 | 2 | 1∶10 | 338 | [ |
| Sr0.8K0.2TiO3 | 0.05%NO+20%O2+N2 | 5 | 1∶9 | 367 | [ |
| La0.5Sr0.5MnO3 | 0.05%NO+20%O2+N2 | 5 | 1∶9 | 385 | [ |
| KNO3/La0.8Ce0.2Mn0.6Fe0.4O3 | 20%O2+80%N2+N2 | 2 | 1∶9 | 379 | [ |
| Au0.04/ LaFeO3 | 0.2%NO+5%O2+Ar | 2 | 1∶10 | 368④ | [ |
| Au1.25/LaFeO3 | 0.2%NO+5%O2+Ar | 2 | 1∶10 | 387 | [ |
| NiCo2O4 | 0.1%NO+5%O2 | 2 | 1∶10 | 379 | [ |
| Co50Ce50③ | 0.25%NO+5%O2+N2 | 2 | 1∶10 | 406④ | [ |
| 催化剂 | 反应物进料① | 加热速率/℃·min-1 | 碳烟和催化剂质量比② | T50或Tm/℃ | 参考文献 |
|---|---|---|---|---|---|
| LaFeO3 | 0.2%NO+5%O2+He | 2 | 1∶10 | 412 | [ |
| LaMn0.95Fe0.05O3 | 0.2%NO+5%O2+Ar | 2 | 1∶10 | 392④ | [ |
| La0.9K0.1CoO3 | 0.2%NO+5%O2+Ar | 2 | 1∶10 | 398 | [ |
| La0.95K0.05NiO3 | 0.2%NO+5%O2+N2 | 2 | 1∶10 | 338 | [ |
| Sr0.8K0.2TiO3 | 0.05%NO+20%O2+N2 | 5 | 1∶9 | 367 | [ |
| La0.5Sr0.5MnO3 | 0.05%NO+20%O2+N2 | 5 | 1∶9 | 385 | [ |
| KNO3/La0.8Ce0.2Mn0.6Fe0.4O3 | 20%O2+80%N2+N2 | 2 | 1∶9 | 379 | [ |
| Au0.04/ LaFeO3 | 0.2%NO+5%O2+Ar | 2 | 1∶10 | 368④ | [ |
| Au1.25/LaFeO3 | 0.2%NO+5%O2+Ar | 2 | 1∶10 | 387 | [ |
| NiCo2O4 | 0.1%NO+5%O2 | 2 | 1∶10 | 379 | [ |
| Co50Ce50③ | 0.25%NO+5%O2+N2 | 2 | 1∶10 | 406④ | [ |
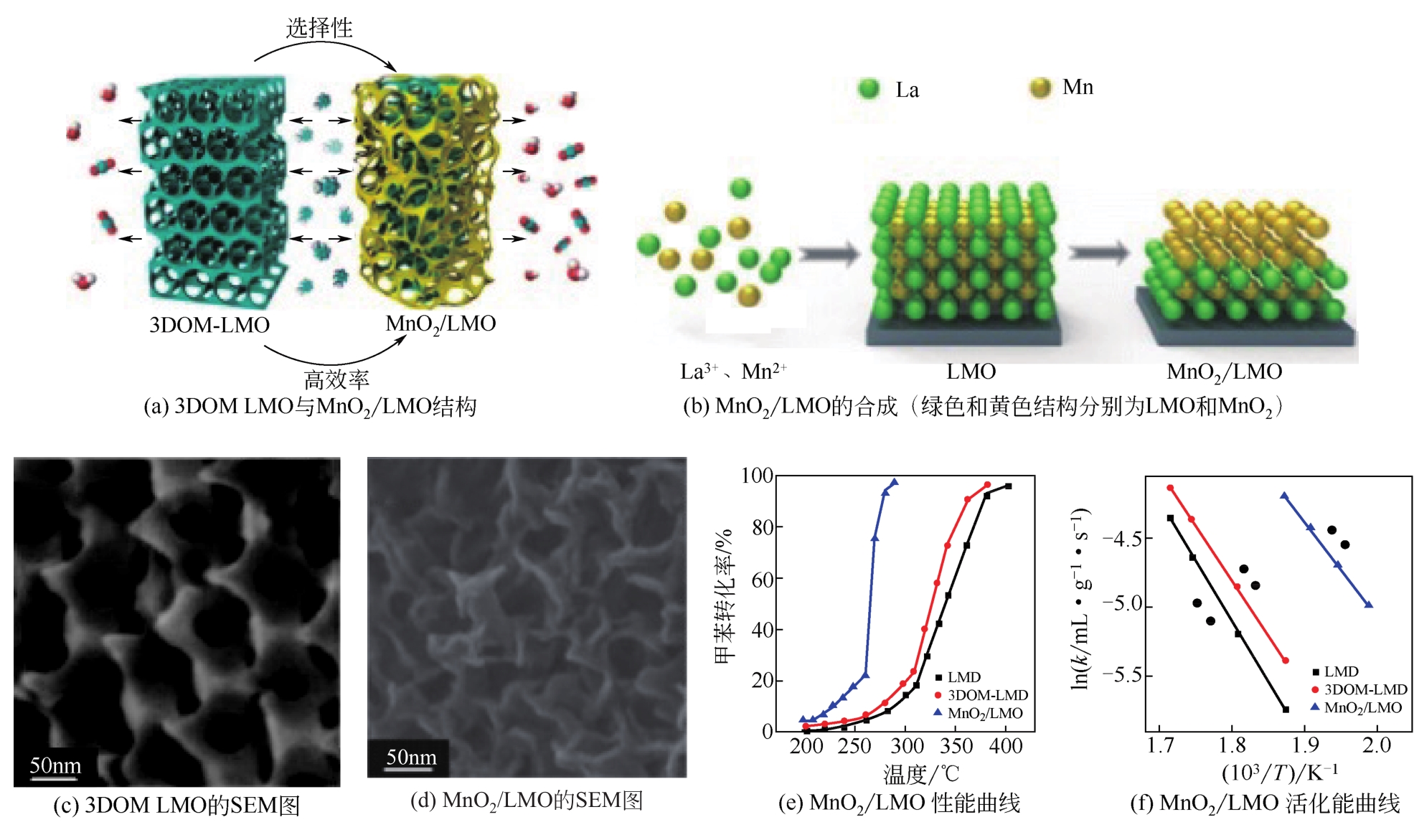
| 催化剂 | 质量/g | 反应物进料 | 空速/mL?g-1?h-1 | T50/℃① | T90/℃② | 活化能/kJ?mol-1 | 参考文献 |
|---|---|---|---|---|---|---|---|
| LaMnO3 | 0.1~0.2 | 0.1%甲苯+O2 | 20000 | 2223 | 243 | 58 | [ |
| SrFeO3-δ | 0.1 | 0.1%甲苯+O2 | 20000 | 292 | 340 | — | [ |
| 3Cox/ Eu0.6Sr0.4FeO3 | 0.1 | 0.1%甲苯+O2 | 20000 | 250 | 270 | 72 | [ |
| 3Co3O4/Eu0.6Sr0.4FeO3 | 0.1 | 0.1%甲苯+O2 | 20000 | 251 | 269 | 72.3 | [ |
| La0.6Sr0.4FeO3-δ | 0.1 | 0.1%甲苯+O2 | 20000 | 225 | 280 | — | [ |
| La0.6Sr0.4Fe0.8Bi0.2O3-δ | 0.1 | 0.1%甲苯+O2 | 20000 | 220 | 242 | 45.9 | [ |
| 12% MnOx/LaMnO3(质量分数) | 0.1 | 0.1%甲苯+O2 | 20000 | 193 | 215 | 61 | [ |
| MnO2/LaMnO3 | 0.05 | 0.2%甲苯+O2 | 120000 | 263 | 279 | 57 | [ |
| 7.63Au/LaCoO3 | 0.1 | 0.1%甲苯+O2 | 20000 | 188 | 202 | 31.4 | [ |
| 6.4Au/La0.6Sr0.4MnO3 | 0.05 | 0.1%甲苯+O2 | 20000 | 150 | 170 | 44 | [ |
| 1.67Mn3O4-2Au/La0.6Sr0.4CoO3 | 0.05 | 0.1%甲苯+O2 | 20000 | 214 | 230 | 41.7 | [ |
| 5.92Au/8MnOx/La0.6Sr0.4MnO3 | 0.05 | 0.1%甲苯+O2 | 20000 | 205 | 220 | 52.8 | [ |
| Au/Co3O4 | 0.05 | 0.1%甲苯+O2 | 20000 | 244 | 256 | 74 | [ |
| 1.99Au-Pd/Co3O4 | 0.05 | 0.1%甲苯+O2 | 40000 | 168 | 168 | 33 | [ |
| 催化剂 | 质量/g | 反应物进料 | 空速/mL?g-1?h-1 | T50/℃① | T90/℃② | 活化能/kJ?mol-1 | 参考文献 |
|---|---|---|---|---|---|---|---|
| LaMnO3 | 0.1~0.2 | 0.1%甲苯+O2 | 20000 | 2223 | 243 | 58 | [ |
| SrFeO3-δ | 0.1 | 0.1%甲苯+O2 | 20000 | 292 | 340 | — | [ |
| 3Cox/ Eu0.6Sr0.4FeO3 | 0.1 | 0.1%甲苯+O2 | 20000 | 250 | 270 | 72 | [ |
| 3Co3O4/Eu0.6Sr0.4FeO3 | 0.1 | 0.1%甲苯+O2 | 20000 | 251 | 269 | 72.3 | [ |
| La0.6Sr0.4FeO3-δ | 0.1 | 0.1%甲苯+O2 | 20000 | 225 | 280 | — | [ |
| La0.6Sr0.4Fe0.8Bi0.2O3-δ | 0.1 | 0.1%甲苯+O2 | 20000 | 220 | 242 | 45.9 | [ |
| 12% MnOx/LaMnO3(质量分数) | 0.1 | 0.1%甲苯+O2 | 20000 | 193 | 215 | 61 | [ |
| MnO2/LaMnO3 | 0.05 | 0.2%甲苯+O2 | 120000 | 263 | 279 | 57 | [ |
| 7.63Au/LaCoO3 | 0.1 | 0.1%甲苯+O2 | 20000 | 188 | 202 | 31.4 | [ |
| 6.4Au/La0.6Sr0.4MnO3 | 0.05 | 0.1%甲苯+O2 | 20000 | 150 | 170 | 44 | [ |
| 1.67Mn3O4-2Au/La0.6Sr0.4CoO3 | 0.05 | 0.1%甲苯+O2 | 20000 | 214 | 230 | 41.7 | [ |
| 5.92Au/8MnOx/La0.6Sr0.4MnO3 | 0.05 | 0.1%甲苯+O2 | 20000 | 205 | 220 | 52.8 | [ |
| Au/Co3O4 | 0.05 | 0.1%甲苯+O2 | 20000 | 244 | 256 | 74 | [ |
| 1.99Au-Pd/Co3O4 | 0.05 | 0.1%甲苯+O2 | 40000 | 168 | 168 | 33 | [ |
| 催化剂 | 质量/g | 反应物进料① | 空速/mL·g-1·h-1 | T50/℃ | T90/℃ | 活化能/kJ·mol-1 | 参考文献 |
|---|---|---|---|---|---|---|---|
| La2CuO4 | 0.05 | 2%CH4+20%O2 | 50000 | 560 | 672 | — | [ |
| La0.6Sr0.4MnO3 | 0.02 | 2%CH4+20%O2 | 30000 | 566 | 661 | 56.6 | [ |
| La0.7Ce0.3CoO3 | 0.02 | 2%CH4+20%O2 | 30000 | 479 | 555 | 53 | [ |
| LaMn0.97Pd0.03O3 | 0.05 | 1%CH4+17%O2 | 32000 | 412 | 504 | 51.5 | [ |
| 1.18% Pd/La0.6Sr0.4MnO3(质量分数) | 0.05 | 0.5%CH4+20%O2 | 40000 | 489 | 583 | — | [ |
| 3.63% Ag/La0.6Sr0.4MnO3(质量分数) | 0.02 | 2%CH4+20%O2 | 30000 | 454 | 524 | 37.5 | [ |
| 3% Au-Pd/La0.6Sr0.4MnO3(质量分数) | 0.02 | 5%CH4+30%O2 | 50000 | 331 | 354 | 46.3 | [ |
| 1.93% Au-Pd1.95/CoCr2O4(质量分数) | 0.05 | 2.5%CH4+20%O2 | 20000 | 353 | 394 | — | [ |
| Au-Pd-0.4% CoO/Co3O4(质量分数) | 0.05 | 2.5%CH4+20%O2 | 20000 | 312 | 341 | 63 | [ |
| 催化剂 | 质量/g | 反应物进料① | 空速/mL·g-1·h-1 | T50/℃ | T90/℃ | 活化能/kJ·mol-1 | 参考文献 |
|---|---|---|---|---|---|---|---|
| La2CuO4 | 0.05 | 2%CH4+20%O2 | 50000 | 560 | 672 | — | [ |
| La0.6Sr0.4MnO3 | 0.02 | 2%CH4+20%O2 | 30000 | 566 | 661 | 56.6 | [ |
| La0.7Ce0.3CoO3 | 0.02 | 2%CH4+20%O2 | 30000 | 479 | 555 | 53 | [ |
| LaMn0.97Pd0.03O3 | 0.05 | 1%CH4+17%O2 | 32000 | 412 | 504 | 51.5 | [ |
| 1.18% Pd/La0.6Sr0.4MnO3(质量分数) | 0.05 | 0.5%CH4+20%O2 | 40000 | 489 | 583 | — | [ |
| 3.63% Ag/La0.6Sr0.4MnO3(质量分数) | 0.02 | 2%CH4+20%O2 | 30000 | 454 | 524 | 37.5 | [ |
| 3% Au-Pd/La0.6Sr0.4MnO3(质量分数) | 0.02 | 5%CH4+30%O2 | 50000 | 331 | 354 | 46.3 | [ |
| 1.93% Au-Pd1.95/CoCr2O4(质量分数) | 0.05 | 2.5%CH4+20%O2 | 20000 | 353 | 394 | — | [ |
| Au-Pd-0.4% CoO/Co3O4(质量分数) | 0.05 | 2.5%CH4+20%O2 | 20000 | 312 | 341 | 63 | [ |
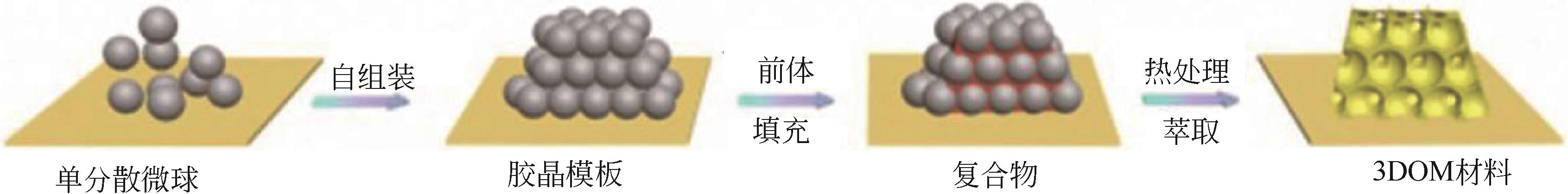
| 1 | YAN H, BLANFORD C F, LYTLE J C, et al. Influence of processing conditions on structures of 3D ordered macroporous metals prepared by colloidal crystal templating[J]. Chemistry of Materials, 2001, 13(11): 4314-4321. |
| 2 | 邬泉周, 李玉光. 三维有序大孔材料应用研究进展[J]. 化工进展, 2008, 27(3): 358-363. |
| WU Q Z, LI Y G. Progress of applications of three-dimensionally ordered macroporous materials[J]. Chemical Industry and Engineering Progress, 2008, 27(3): 358-363. | |
| 3 | VELEV O D, JEDE T A, LOBO R F, et al. Porous silica via colloidal crystallization[J]. Nature, 1997, 389(6650): 447-448. |
| 4 | TANAKA H, MISONO M. Advances in designing perovskite catalysts[J]. Current Opinion in Solid State and Materials Science, 2001, 5(5): 381-387. |
| 5 | CRESPIN M, HALL W K. The surface chemistry of some perovskite oxides[J]. Journal of Catalysis, 1981, 69(2): 359-370. |
| 6 | LI B, YANG Q, PENG Y, et al. Enhanced low-temperature activity of LaMnO3 for toluene oxidation: the effect of treatment with an acidic KMnO4[J]. Chemical Engineering Journal, 2019: 92-99. |
| 7 | YU Q, WANG C, LI X, et al. Engineering an effective MnO2 Catalyst from LaMnO3 for catalytic methane Combustion[J]. Fuel, 2019, 239: 1240-1245. |
| 8 | FANG F, FENG N, WANG L, et al. Fabrication of perovskite-typemacro/mesoporous La1-xKxFeO3-δnanotubes as an efficient catalyst for soot combustion[J]. Applied Catalysis B: Environmental, 2018, 236: 184-194. |
| 9 | PARRAVANO G. Catalytic activity of lanthanum and strontium manganite[J]. Journal of the American Chemical Society, 1953, 75(6): 1497-1498. |
| 10 | ROYER S, DUPREZ D, CAN F, et al. Perovskites as substitutes of noble metals for heterogeneous catalysis: dream or reality[J]. Chemical Reviews, 2014, 114(20): 10292-10368. |
| 11 | ZHU J J, LI H L, ZHONG L Y, et al. Perovskite oxides: preparation, characterizations, and applications in heterogeneous catalysis[J]. ACS Catalysis, 2014, 4(9): 2917-2940. |
| 12 | ZHANG G, LIU G, WANG L, et al. Inorganic perovskite photocatalysts for solar energy utilization[J]. Chemical Society Reviews, 2016, 45(21): 5951-5984. |
| 13 | ZHU Y, ZHOU W, SHAO Z P. Perovskite/carbon composites: applications in oxygen electrocatalysis[J]. Small, 2017, 13(12): 1603793. |
| 14 | HUANG X, ZHAO G, WANG G, et al. Synthesis and applications of nanoporous perovskite metal oxides[J]. Chemical Science, 2018, 9(15): 3623-3637. |
| 15 | ZHANG C X, ZHAO P Y, LIU S X, et al. Three-dimensionally ordered macroporous perovskite materials for environmental applications[J]. Chinese Journal of Catalysis, 2019, 40(9): 1324-1338. |
| 16 | ARANDIYAN H, WANG Y, SUN H Y, et al. Ordered meso- and macroporous perovskite oxide catalysts for emerging applications[J]. Chemical Communications, 2018, 54(50): 6484-6502. |
| 17 | LEE K C, LEE S Y. Preparation of highly cross-linked, monodisperse poly(methyl methacrylate) microspheres by dispersion polymerization; Part Ⅱ. Semi-continuous processes[J]. Macromolecular Research, 2008, 16(4): 293-302. |
| 18 | HONG J, HONG C K, SHIM S E. Synthesis of polystyrene microspheres by dispersion polymerization using poly(vinyl alcohol) as a steric stabilizer in aqueous alcohol media[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2007, 302: 225-233. |
| 19 | 佟斌,孙洪波,石建兵,等. 分散聚合法制备单分散交联PMMA微球[J]. 北京理工大学学报, 2007, 27(3): 270-273. |
| TONG B, SUN H B, SHI J B, et al. Preparation of monodisperse crosslinked poly(methyl methacrylate) microspheres by dispersion polymerization[J]. Transactions of Beijing Institute of Technology, 2007, 27(3): 270-273. | |
| 20 | KIM G, LIM S, LEE B H, et al. Effect of homogeneity of methanol/water/monomer mixture on the mode of polymerization of MMA: soap-free emulsion polymerization versus dispersion polymerization[J]. Polymer, 2010, 51(5): 1197-1205. |
| 21 | DEBNATH D, KHATUA B B. Preparation by suspension polymerization and characterization of polystyrene (PS)-poly(methyl methacrylate) (PMMA) core-shell nanocomposites[J]. Macromolecular Research, 2011, 19(6): 519-527. |
| 22 | 董殿权, 房超. PMMA纳米微球的制备与表征[J]. 化工新型材料, 2017, 45(9): 68-70. |
| DONG D Q, FANG C. Preparation and characterization of PMMA nanosphere[J]. New Chemical Materials, 2017, 45(9): 68-70. | |
| 23 | DENG J G, TAO X M, LI P, et al. A simple self-assembly method for colloidal photonic crystals with a large area[J]. Journal of Colloid and Interface Science, 2005, 286(2): 573-578. |
| 24 | DAI Z F, LI Y, DUAN G T, et al. Phase diagram, design of monolayer binary colloidal crystals, and their fabrication based on ethanol-assisted self-assembly at the air/water interface[J]. ACS Nano, 2012, 6(8): 6706-6716. |
| 25 | HOLGADO M, GARCÍASANTAMARÍA F, BLANCO A, et al. Electrophoretic deposition to control artificial opal growth[J]. Langmuir, 1999, 15(14): 4701-4704. |
| 26 | WONG S, KITAEV V, OZIN G A. Colloidal crystal films: advances in universality and perfection[J]. Journal of the American Chemical Society, 2003, 125(50): 15589-15598. |
| 27 | SUZUKI Y, SAWADA T, TAMURA K, et al. Colloidal crystallization by a centrifugation method[J]. Journal of Crystal Growth, 2011, 318(1): 780-783. |
| 28 | ISHIKAWA M, MORIMOTO H, OKUBO T, et al. Growth of colloidal crystals under microgravity[J]. International Journal of Modern Physics B, 2002, 16(1/2): 338-345. |
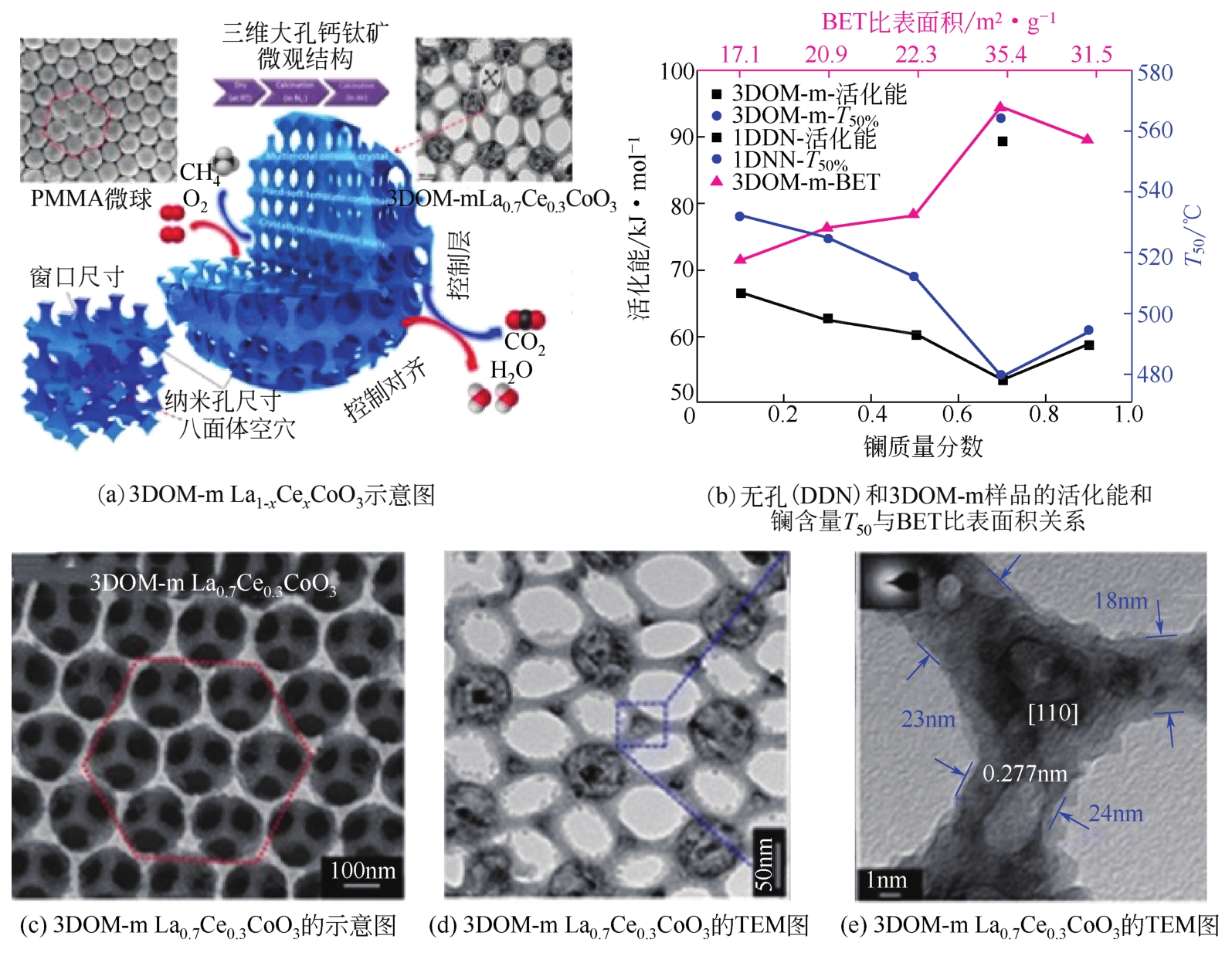
| 29 | 徐俊峰, 刘坚, 赵震, 等. 三维有序大孔钙钛矿LaFeO3催化剂的制备及其催化炭黑颗粒燃烧性能[J]. 催化学报, 2010, 31(2): 236-241. |
| XU J F, LIU J, ZHAO Z, et al. Preparation and catalytic performance of three-dimensionally ordered macroporous perovskite-type LaFeO3 catalyst for soot combustion[J]. Chinese Journal of Catalysis, 2010, 31(2): 236-241. | |
| 30 | SADAKANE M, SASAKI K, NAKAMURA H, et al. Important property of polymer spheres for the preparation of three-dimensionally ordered macroporous (3DOM) metal oxides by the ethylene glycol method: the glass-transition temperature[J]. Langmuir, 2012, 28(51): 17766-17770. |
| 31 | ZHENG J, LIU J, ZHAO Z, et al. The synthesis and catalytic performances of three-dimensionally ordered macroporous perovskite-type LaMn1-xFexO3 complex oxide catalysts with different pore diameters for diesel soot combustion[J]. Catalysis Today, 2012, 191(1): 146-153. |
| 32 | MEI X L, XIONG J, WEI Y C, et al. Three-dimensional ordered macroporous perovskite-type La1-xKxNiO3 catalysts with enhanced catalytic activity for soot combustion: the effect of K-substitution[J]. Chinese Journal of Catalysis, 2019, 40(5): 722-732. |
| 33 | ZHAO P, FENG N, FANG F, et al. Facile synthesis of three-dimensional ordered macroporous Sr1-xKxTiO3 perovskites with enhanced catalytic activity for soot combustion[J]. Catalysis Science & Technology, 2018, 8(21): 5462-5472. |
| 34 | ZHAO P, FANG F, FENG N, et al. Self-templating construction of mesopores on three-dimensionally ordered macroporous La0.5Sr0.5MnO3 perovskite with enhanced performance for soot combustion[J]. Catalysis Science & Technology, 2019, 9(8): 1835-1846. |
| 35 | FENG N, MENG J, WU Y, et al. KNO3 supported on three-dimensionally ordered macroporous La0.8Ce0.2Mn1-xFexO3 for soot removal[J]. Catalysis Science & Technology, 2016, 6(9): 2930-2941. |
| 36 | HARUTA M, KOBAYASHI T, SANO H, et al. Novel gold catalysts for the oxidation of carbon monoxide at a temperature far below 0℃[J]. Chemistry Letters, 1987, 16(2): 405-408. |
| 37 | HUTCHINGS G J. Vapor phase hydrochlorination of acetylene: correlation of catalytic activity of supported metal chloride catalysts[J]. Journal of Catalysis, 1985, 96(1): 292-295. |
| 38 | WEI Y, LIU J, ZHAO Z, et al. Highly Active catalysts of gold nanoparticles supported on three-dimensionally ordered macroporous LaFeO3 for soot oxidation[J]. Angewandte Chemie, 2011, 50(10): 2326-2329. |
| 39 | WEI Y, ZHAO Z, JIAO J, et al. Facile synthesis of three-dimensionally ordered macroporous LaFeO3-supported gold nanoparticle catalysts with high catalytic activity and stability for soot combustion[J]. Catalysis Today, 2015, 245: 37-45. |
| 40 | ZHAO M, DENG J, LIU J, et al. Roles of surface-active oxygen species on 3DOM cobalt-based spinel catalysts MxCo3–xO4 (M = Zn and Ni) for NOx-assisted soot oxidation[J]. ACS Catalysis, 2019, 9(8): 7548-7567. |
| 41 | ZHAI G J, WANG J G, CHEN Z M, et al. Highly enhanced soot oxidation activity over 3DOM Co3O4-CeO2 catalysts by synergistic promoting effect[J]. Journal of Hazardous Materials, 2019, 363: 214-226. |
| 42 | LIU Y, DAI H, DU Y, et al. Lysine-aided PMMA-templating preparation and high performance of three-dimensionally ordered macroporous LaMnO3 with mesoporous walls for the catalytic combustion of toluene[J]. Applied Catalysis B: Environmental, 2012, 119/120: 20-31. |
| 43 | LIU Y, DAI H, DU Y, et al. Controlled preparation and high catalytic performance of three-dimensionally ordered macroporous LaMnO3 with nanovoid skeletons for the combustion of toluene[J]. Journal of Catalysis, 2012, 287: 149-160. |
| 44 | JI K M, DAI H X, DENG J G, et al. Three-dimensionally ordered macroporous SrFeO3-δ with high surface area: active catalysts for the complete oxidation of toluene[J]. Applied Catalysis A: General, 2012, 425/426: 153-160. |
| 45 | JI K M, DAI H X, DENG J G, et al. Three-dimensionally ordered macroporous Eu0.6Sr0.4FeO3 supported cobalt oxides: highly active nanocatalysts for the combustion of toluene[J]. Applied Catalysis B: Environmental, 2013, 129: 539-548. |
| 46 | JI K, DAI H, DENG J, et al. A comparative study of bulk and 3DOM-structured Co3O4, Eu0.6Sr0.4FeO3, and Co3O4/Eu0.6Sr0.4FeO3: preparation, characterization, and catalytic activities for toluene combustion[J]. Applied Catalysis A: General, 2012, 447/448: 41-48. |
| 47 | ZHAO Z X, DAI H X, DENG J G, et al. Three-dimensionally ordered macroporous La0.6Sr0.4FeO3-δ: high-efficiency catalysts for the oxidative removal of toluene[J]. Microporous and Mesoporous Materials, 2012, 163: 131-139. |
| 48 | ZHAO Z X, DAI H X, DENG J G, et al. Preparation of three-dimensionally ordered macroporous La0.6Sr0.4Fe0.8Bi0.2O3-δ and their excellent catalytic performance for the combustion of toluene[J]. Journal of Molecular Catalysis A: Chemical, 2013, 336: 116-125. |
| 49 | LIU Y, DAI H, DENG J, et al. In situ poly(methyl methacrylate)-templating generation and excellent catalytic performance of MnOx/3DOM LaMnO3 for the combustion of toluene and methanol[J]. Applied Catalysis B: Environmental, 2013, 140/141: 493-505. |
| 50 | SI W Z, WANG Y, ZHAO S, et al. A facile method for in situ preparation of the MnO2/LaMnO3 catalyst for the removal of toluene[J]. Environmental Science and Technology, 2016, 50(8): 4572-4578. |
| 51 | LI X W, DAI H X, DENG J G, et al. Au/3DOM LaCoO3: high-performance catalysts for the oxidation of carbon monoxide and toluene[J]. Chemical Engineering Journal, 2013, 228: 965-975. |
| 52 | LIU Y X, DAI H X, DENG J G, et al. Au/3DOM La0.6Sr0.4MnO3: highly active nanocatalysts for the oxidation of carbon monoxide and toluene[J]. Journal of Catalysis, 2013, 305: 146-153. |
| 53 | OSHEA V A, ALVAREZGALVAN M C, REQUIES J, et al. Synergistic effect of Pd in methane combustion PdMnOx/Al2O3 catalysts[J]. Catalysis Communications, 2007, 8(8): 1287-1292. |
| 54 | JIANG Y, XIE S, YANG H, et al. Mn3O4-Au/3DOM La0.6Sr0.4CoO3: high-performance catalysts for toluene oxidation[J]. Catalysis Today, 2017, 281: 437-446. |
| 55 | JIANG Y, DENG J G, XIE S H, et al. Au/MnOx/3DOM La0.6Sr0.4MnO3: highly active nanocatalysts for the complete oxidation of toluene[J]. Industrial & Engineering Chemistry Research, 2015, 54(3): 900-910. |
| 56 | XIE S H, DAI H X, DENG J G, et al. Au/3DOM Co3O4: highly active nanocatalysts for the oxidation of carbon monoxide and toluene[J]. Nanoscale, 2013, 5(22): 11207-11219. |
| 57 | XIE S H, DENG J G, ZANG S M, et al. Au-Pd/3DOM Co3O4: highly active and stable nanocatalysts for toluene oxidation[J]. Journal of Catalysis, 2015, 322: 38-48. |
| 58 | YUAN J, DAI H, ZHANG L, et al. PMMA-templating preparation and catalytic properties of high-surface-area three-dimensional macroporous La2CuO4 for methane combustion[J]. Catalysis Today, 2011, 175(1): 209-215. |
| 59 | ARANDIYAN H, SCOTT J, WANG Y, et al. Meso-molding three-dimensional macroporous perovskites: a new approach to generate high-performance nanohybrid catalysts[J]. ACS Applied Materials & Interfaces, 2016, 8(4): 2457-2463. |
| 60 | LI J, SINGH U G, BENNETT J W, et al. BaCe1-xPdxO3-δ (0≤x≤0.1): redox controlled ingress and egress of palladium in a perovskite[J]. Chemistry of Materials, 2007, 19(6): 1418-1426. |
| 61 | GUO G, LIAN K, GU F, et al. Three dimensionally ordered macroporous Pd-LaMnO3 self-regeneration catalysts for methane combustion[J]. Chemical Communications, 2014, 50(88): 13575-13577. |
| 62 | ZHAO X T, ZHANG R X, LIU Y, et al. In-situ reduction-derived Pd/3DOM La0.6Sr0.4MnO3: good catalytic stability in methane combustion[J]. Applied Catalysis A: General, 2018, 568: 202-212. |
| 63 | ARANDIYAN H, DAI H, DENG J, et al. Three-dimensionally ordered macroporous La0.6Sr0.4MnO3 supported Ag nanoparticles for the combustion of methane[J]. Journal of Physical Chemistry C, 2014, 118(27): 14913-14928. |
| 64 | ARANDIYAN H, DAI H X, DENG J G, et al. Dual-templating synthesis of three-dimensionally ordered macroporous La0.6Sr0.4MnO3-supported Ag nanoparticles: controllable alignments and super performance for the catalytic combustion of methane[J]. Chemical Communications, 2013, 49(91): 10748. |
| 65 | WANG Y, ARANDIYAN H, SCOTT J, et al. High performance Au-Pd supported on 3D hybrid strontium-substituted lanthanum manganite perovskite catalyst for methane combustion[J]. ACS Catalysis, 2016, 6(10): 6935-6947. |
| 66 | WANG Z W, DENG J G, LIU Y X, et al. Three-dimensionally ordered macroporous CoCr2O4-supported Au-Pd alloy nanoparticles: highly active catalysts for methane combustion[J]. Catalysis Today, 2017, 281: 467-476. |
| 67 | XIE S H, LIU Y X, DENG J G, et al. Efficient removal of methane over cobalt-monoxide-doped AuPd nanocatalysts[J]. Environmental Science & Technology, 2017, 51(4): 2271-2279. |
| [1] | ZHANG Mingyan, LIU Yan, ZHANG Xueting, LIU Yake, LI Congju, ZHANG Xiuling. Research progress of non-noble metal bifunctional catalysts in zinc-air batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 276-286. |
| [2] | SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298. |
| [3] | XIE Luyao, CHEN Songzhe, WANG Laijun, ZHANG Ping. Platinum-based catalysts for SO2 depolarized electrolysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 299-309. |
| [4] | YANG Xiazhen, PENG Yifan, LIU Huazhang, HUO Chao. Regulation of active phase of fused iron catalyst and its catalytic performance of Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 310-318. |
| [5] | WANG Lele, YANG Wanrong, YAO Yan, LIU Tao, HE Chuan, LIU Xiao, SU Sheng, KONG Fanhai, ZHU Canghai, XIANG Jun. Influence of spent SCR catalyst blending on the characteristics and deNO x performance for new SCR catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 489-497. |
| [6] | LI Huaquan, WANG Minghua, QIU Guibao. Behavior of sulfuric acid acidolysis of perovskite concentrates [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 536-541. |
| [7] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [8] | CHENG Tao, CUI Ruili, SONG Junnan, ZHANG Tianqi, ZHANG Yunhe, LIANG Shijie, PU Shi. Analysis of impurity deposition and pressure drop increase mechanisms in residue hydrotreating unit [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4616-4627. |
| [9] | ZHU Jie, JIN Jing, DING Zhenghao, YANG Huipan, HOU Fengxiao. Modification of CaSO4 oxygen carrier by Zhundong coal ash in chemical looping gasification and its mechanism [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4628-4635. |
| [10] | WANG Peng, SHI Huibing, ZHAO Deming, FENG Baolin, CHEN Qian, YANG Da. Recent advances on transition metal catalyzed carbonylation of chlorinated compounds [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4649-4666. |
| [11] | ZHANG Qi, ZHAO Hong, RONG Junfeng. Research progress of anti-toxicity electrocatalysts for oxygen reduction reaction in PEMFC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4677-4691. |
| [12] | GE Quanqian, XU Mai, LIANG Xian, WANG Fengwu. Research progress on the application of MOFs in photoelectrocatalysis [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4692-4705. |
| [13] | WANG Weitao, BAO Tingyu, JIANG Xulu, HE Zhenhong, WANG Kuan, YANG Yang, LIU Zhaotie. Oxidation of benzene to phenol over aldehyde-ketone resin based metal-free catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4706-4715. |
| [14] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [15] | WANG Baoying, WANG Huangying, YAN Junying, WANG Yaoming, XU Tongwen. Research progress of polymer inclusion membrane in metal separation and recovery [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3990-4004. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||