Chemical Industry and Engineering Progress ›› 2021, Vol. 40 ›› Issue (5): 2747-2752.DOI: 10.16085/j.issn.1000-6613.2020-1073
• Resources and environmental engineering • Previous Articles Next Articles
Research progress of microwave-induced catalytic denitrification
HAO Runlong( ), QIAN Zhen, FU Le, YUAN Bo(
), QIAN Zhen, FU Le, YUAN Bo( )
)
- Hebei Key Lab of Power Plant Flue Gas Multi-Pollutants Control, North China Electric Power University, Baoding 071003, Hebei, China
-
Received:2020-06-15Online:2021-05-24Published:2021-05-06 -
Contact:YUAN Bo
微波诱导催化脱硝研究进展
- 华北电力大学燃煤电站烟气多污染物协同控制河北省重点实验室,河北 保定 071003
-
通讯作者:袁博 -
作者简介:郝润龙(1988—),男,教授,博士生导师,研究方向为基于高级氧化工艺的燃煤烟气多污染物深度协同控制方法与理论、燃煤电站超低排放工艺系统开发与理论研究和导电多功能复合膜合成与环境应用。E-mail:hrl-ncepu@hotmail.com 。 -
基金资助:国家自然科学基金(52000067);河北省自然科学基金(E2020502033);国家重点研发计划(2016YFC0203705);中央高校基本科研业务费面上项目(2020MS127)
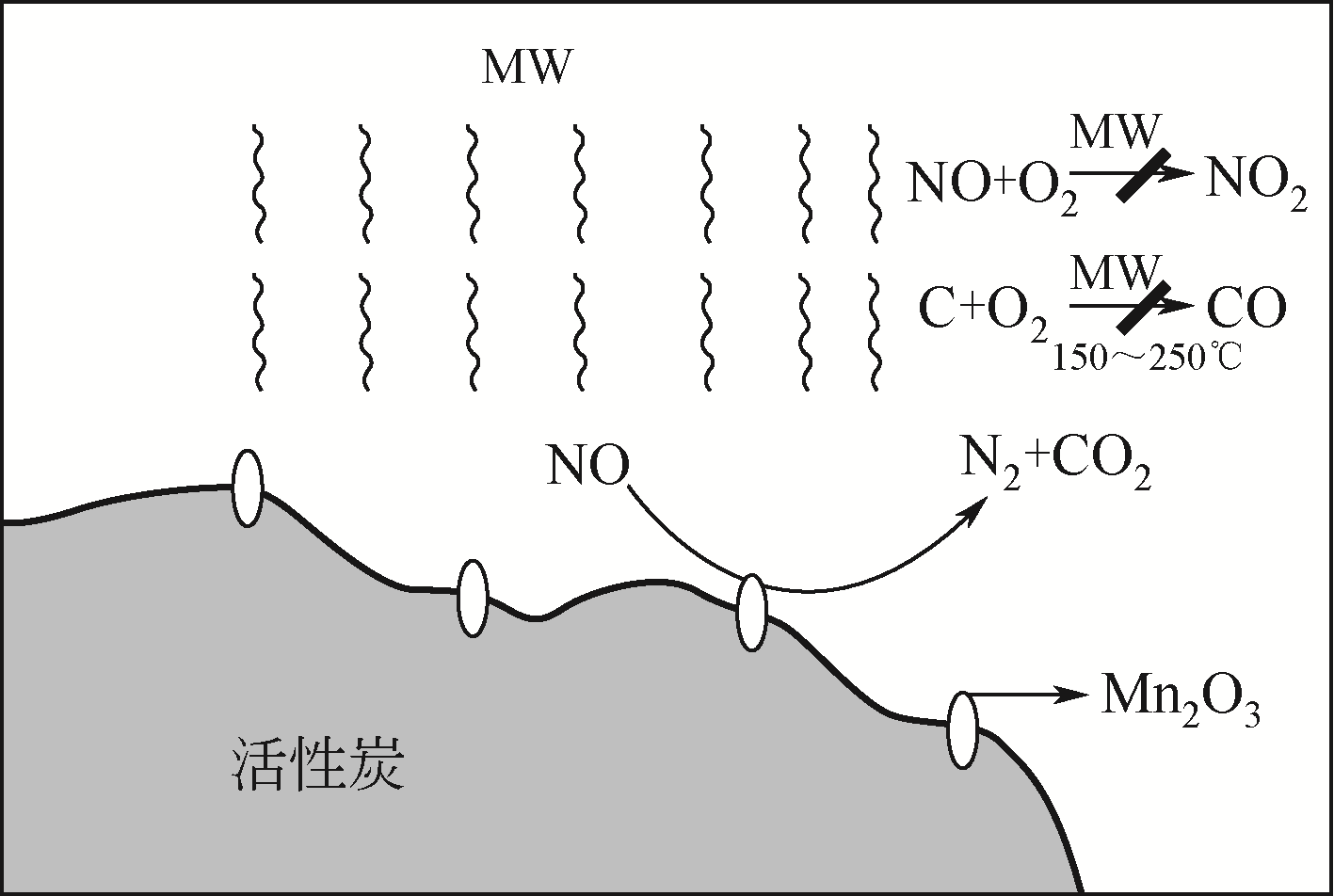
CLC Number:
Cite this article
HAO Runlong, QIAN Zhen, FU Le, YUAN Bo. Research progress of microwave-induced catalytic denitrification[J]. Chemical Industry and Engineering Progress, 2021, 40(5): 2747-2752.
郝润龙, 钱真, 符乐, 袁博. 微波诱导催化脱硝研究进展[J]. 化工进展, 2021, 40(5): 2747-2752.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2020-1073
| 催化剂 | 还原剂 | 最佳反应条件 | NOx转化率 /% | SO2转化率 /% | 优缺点 | 参考 文献 | ||
|---|---|---|---|---|---|---|---|---|
| 反应温度/℃ | 微波功率/W | O2体积分数/% | ||||||
| Ni/HZSM-5 | CH4 | 225 | 200 | 2 | 96.0 | — | Ni/HZSM-5脱硝效率高;但稳定性差,反应过程中易失活 | [ |
| Co/HZSM-5 | 200 | 200 | 80.0 | — | ||||
| Ga-A型分子筛 | 尿素 | 104~113 | 259~280 | — | 92.0 | — | 在Ga-A型分子筛和尿素共同使用时微波脱硝效果好,但尿素作为还原剂会被消耗 | [ |
| Ga-A沸石 | NH4HCO3 | 80~120 | 259~280 | 14~19 | 95.45 | — | 沸石和NH4HCO3在微波辐射下脱硝效率高;但NH4HCO3受热易分解,产生CO2 | [ |
| 沸石 | NH4HCO3 | 200~250 | 211~280 | — | 86.5 | 99.1 | [ | |
| CuO | AC | 350 | 420 | — | 92.5 | <90.0 | 脱硝效率高,但脱硫效率不理想 | [ |
| Mn2O3 | — | 87.5 | — | |||||
| ZnO | — | 87.5 | — | |||||
| CuO | AC | 350 | 350 | 7.9 | 94.1 | 99.8 | 脱除效率高,但AC作为吸收剂会被消耗 | [ |
| Mn2O3 | AC | 300 | 400 | 5.8 | 98.7 | — | AC在微波作用下选择性反应,减少其消耗量 | [ |
| CeO2 | AC | 350 | 250 | 5 | 100.0 | — | 基本实现100%脱硝,但AC作为还原剂会被消耗 | [ |
| NiOx-CeO2 | 300 | 150 | 100.0 | — | ||||
| MnOx-CeO2 | 350 | 200 | 100.0 | — | ||||
| FeOx-CeO2 | 350 | 200 | 100.0 | — | ||||
| 催化剂 | 还原剂 | 最佳反应条件 | NOx转化率 /% | SO2转化率 /% | 优缺点 | 参考 文献 | ||
|---|---|---|---|---|---|---|---|---|
| 反应温度/℃ | 微波功率/W | O2体积分数/% | ||||||
| Ni/HZSM-5 | CH4 | 225 | 200 | 2 | 96.0 | — | Ni/HZSM-5脱硝效率高;但稳定性差,反应过程中易失活 | [ |
| Co/HZSM-5 | 200 | 200 | 80.0 | — | ||||
| Ga-A型分子筛 | 尿素 | 104~113 | 259~280 | — | 92.0 | — | 在Ga-A型分子筛和尿素共同使用时微波脱硝效果好,但尿素作为还原剂会被消耗 | [ |
| Ga-A沸石 | NH4HCO3 | 80~120 | 259~280 | 14~19 | 95.45 | — | 沸石和NH4HCO3在微波辐射下脱硝效率高;但NH4HCO3受热易分解,产生CO2 | [ |
| 沸石 | NH4HCO3 | 200~250 | 211~280 | — | 86.5 | 99.1 | [ | |
| CuO | AC | 350 | 420 | — | 92.5 | <90.0 | 脱硝效率高,但脱硫效率不理想 | [ |
| Mn2O3 | — | 87.5 | — | |||||
| ZnO | — | 87.5 | — | |||||
| CuO | AC | 350 | 350 | 7.9 | 94.1 | 99.8 | 脱除效率高,但AC作为吸收剂会被消耗 | [ |
| Mn2O3 | AC | 300 | 400 | 5.8 | 98.7 | — | AC在微波作用下选择性反应,减少其消耗量 | [ |
| CeO2 | AC | 350 | 250 | 5 | 100.0 | — | 基本实现100%脱硝,但AC作为还原剂会被消耗 | [ |
| NiOx-CeO2 | 300 | 150 | 100.0 | — | ||||
| MnOx-CeO2 | 350 | 200 | 100.0 | — | ||||
| FeOx-CeO2 | 350 | 200 | 100.0 | — | ||||
| 催化剂 | 最佳反应条件 | NOx 转化率/% | 优缺点 | 参考 文献 | ||
|---|---|---|---|---|---|---|
| 反应温度/℃ | 微波功率/W | O2体积分数/% | ||||
| Fe/NaZSM-5 | 300 | 16~18 | 2 | 70.0 | 20%Fe负载量的脱硝效果最佳,但总体脱硝效率偏低 | [ |
| Cu-ZSM-5 | 500~550 | 100 | 5 | — | 微波提高Cu-ZSM-5、Fe-ZSM-5稳定性和耐氧性,但其脱硝效率低 | [ |
| Fe-ZSM-5 | 400~600 | |||||
| MeOx/Al2O3(Me=Cu,Mn,Fe) | 250 | 100 | 10 | 94.8 | CeCuMnOx/Al2O3脱硝效率高,但其稳定性差且所含金属元素偏多 | [ |
| BaMnxMg1-xO3 | 250 | <150 | 10 | 99.8 | 微波显著提高BaMnxMg1-xO3脱硝率,但Mn和Mg之间比例难控制 | [ |
| BaMnO3 | 300 | <150 | 10 | 93.7 | 脱硝效率好,但Ba和A位之间比例难控制,催化剂制备难度大 | [ |
| Ba0.8Ca0.2MnO3 | 250 | 92.3 | ||||
| Ba0.8K0.2MnO3 | 250 | 99.9 | ||||
| Ba0.8La0.2MnO3 | 250 | 95.5 | ||||
| BaMoO3 | 300 | <150 | 10 | 93.7 | Fe取代Me位的催化剂方便回收利用,但其脱硝效率低 | [ |
| BaCoO3 | 250 | <150 | 99.8 | |||
| BaFeO3 | 250 | 1000 | 64.1 | |||
| MgCo2O4 | 300 | <100 | 10 | 69.7 | 脱除效率高,但复合催化剂制备成本高 | [ |
| MgCo2O4-BaCO3 | 250 | 99.6 | ||||
| 催化剂 | 最佳反应条件 | NOx 转化率/% | 优缺点 | 参考 文献 | ||
|---|---|---|---|---|---|---|
| 反应温度/℃ | 微波功率/W | O2体积分数/% | ||||
| Fe/NaZSM-5 | 300 | 16~18 | 2 | 70.0 | 20%Fe负载量的脱硝效果最佳,但总体脱硝效率偏低 | [ |
| Cu-ZSM-5 | 500~550 | 100 | 5 | — | 微波提高Cu-ZSM-5、Fe-ZSM-5稳定性和耐氧性,但其脱硝效率低 | [ |
| Fe-ZSM-5 | 400~600 | |||||
| MeOx/Al2O3(Me=Cu,Mn,Fe) | 250 | 100 | 10 | 94.8 | CeCuMnOx/Al2O3脱硝效率高,但其稳定性差且所含金属元素偏多 | [ |
| BaMnxMg1-xO3 | 250 | <150 | 10 | 99.8 | 微波显著提高BaMnxMg1-xO3脱硝率,但Mn和Mg之间比例难控制 | [ |
| BaMnO3 | 300 | <150 | 10 | 93.7 | 脱硝效率好,但Ba和A位之间比例难控制,催化剂制备难度大 | [ |
| Ba0.8Ca0.2MnO3 | 250 | 92.3 | ||||
| Ba0.8K0.2MnO3 | 250 | 99.9 | ||||
| Ba0.8La0.2MnO3 | 250 | 95.5 | ||||
| BaMoO3 | 300 | <150 | 10 | 93.7 | Fe取代Me位的催化剂方便回收利用,但其脱硝效率低 | [ |
| BaCoO3 | 250 | <150 | 99.8 | |||
| BaFeO3 | 250 | 1000 | 64.1 | |||
| MgCo2O4 | 300 | <100 | 10 | 69.7 | 脱除效率高,但复合催化剂制备成本高 | [ |
| MgCo2O4-BaCO3 | 250 | 99.6 | ||||
| 催化剂 | 氧化剂 | 最佳反应条件 | NOx转化率 /% | SO2转化率 /% | 优缺点 | 参考 文献 | ||
|---|---|---|---|---|---|---|---|---|
| 反应温度/℃ | 微波功率/W | O2质量分数/% | ||||||
| FeCu/沸石 | — | — | 280 | — | 91.7 | 79.6 | 脱硝效率好,但脱硫效率低 | [ |
| 沸石 | KMnO4 | — | 259 | — | 98.4 | 96.8 | 脱除效率高,但KMnO4具有强腐蚀性和毒性 | [ |
| — | O2/H2O/SO2 | 80 | 260 | 2~8 | 89.3 | 97.0 | 微波和光催化技术结合为烟气脱硝控制领域提供了新思路,但其反应成本和能耗偏高 | [ |
| 催化剂 | 氧化剂 | 最佳反应条件 | NOx转化率 /% | SO2转化率 /% | 优缺点 | 参考 文献 | ||
|---|---|---|---|---|---|---|---|---|
| 反应温度/℃ | 微波功率/W | O2质量分数/% | ||||||
| FeCu/沸石 | — | — | 280 | — | 91.7 | 79.6 | 脱硝效率好,但脱硫效率低 | [ |
| 沸石 | KMnO4 | — | 259 | — | 98.4 | 96.8 | 脱除效率高,但KMnO4具有强腐蚀性和毒性 | [ |
| — | O2/H2O/SO2 | 80 | 260 | 2~8 | 89.3 | 97.0 | 微波和光催化技术结合为烟气脱硝控制领域提供了新思路,但其反应成本和能耗偏高 | [ |
| 1 | HAO Runlong, MAO Xinzhou, WANG Zheng, et al. A novel method of ultraviolet/NaClO2-NH4OH for NO removal: mechanism and kinetics[J]. Journal of Hazardous Materials, 2019, 368: 234-242. |
| 2 | 赵毅, 王涵, 王添灏. 同时脱硫脱硝固态反应剂研究进展[J]. 工业安全与环保, 2016, 42(4): 17-19. |
| ZHAO Yi, WANG Han, WANG Tianhao. Overview of solid sorbent for simultaneous desulfurization and denitrification[J]. Industrial Safety and Environmental Protection, 2016, 42(4): 17-19. | |
| 3 | HAO Runlong, WANG Xiaohui, ZHAO Xu, et al. A novel integrated method of vapor oxidation with dual absorption for simultaneous removal of SO2 and NO: feasibility and prospect[J]. Chemical Engineering Journal, 2018, 333: 583-593. |
| 4 | HAO Runlong, YANG Shuo, YUAN Bo, et al. Simultaneous desulfurization and denitrification through an integrative process utilizing NaClO2/Na2S2O8[J]. Fuel Processing Technology, 2017, 159: 145-152. |
| 5 | ZHAO Jing, WEI Xiaolin, LI Teng, et al. Effect of HCl and CO on nitrogen oxide formation mechanisms within the temperature window of SNCR[J]. Fuel, 2020, 267: 117231. |
| 6 | 山丕斌, 徐冰峰, 张关印, 等. 微波辐射处理高浓度氨氮废水研究进展[J]. 净水技术, 2019, 38(12): 55-60. |
| SHAN Pibin, XU Bingfeng, ZHANG Guanyin, et al. Research progress of microwave radiation process for high concentration ammonia nitrogen wastewater treatment[J]. Water Purification Technology, 2019, 38(12): 55-60. | |
| 7 | NUSHIRO Kazuya, KIKUCHI Satoshi, YAMADA Tohru. Microwave effect on catalytic enantioselective Claisen rearrangement[J]. Chemical Communications, 2013, 49(75): 8371-8373. |
| 8 | ZENG Bin, ZENG Wujun. Microwave-assisted ion-exchange synthesis and enhanced visible-light photoactivity of graphene-CdS/CuS nanoplates[J]. Journal of Nanoscience and Nanotechnology, 2020, 20(4): 2365-2371. |
| 9 | 阎若萍, 王易芬, 涂桂飞, 等. 工业微波灭菌技术在食品加工领域的研究进展[J]. 食品工业科技, 2018, 39(8): 302-308. |
| YAN Ruoping, WANG Yifen, TU Guifei, et al. Development of industrial microwave sterilization technology in food processing[J]. Science and Technology of Food Industry, 2018, 39(8): 302-308. | |
| 10 | CHEN Jianan, XU Wentao, ZHU Jun, et al. Highly effective microwave catalytic direct decomposition of H2S over carbon encapsulated Mo2C-Co2C/SiC composite[J]. International Journal of Hydrogen Energy, 2019, 44(47): 25680-25694. |
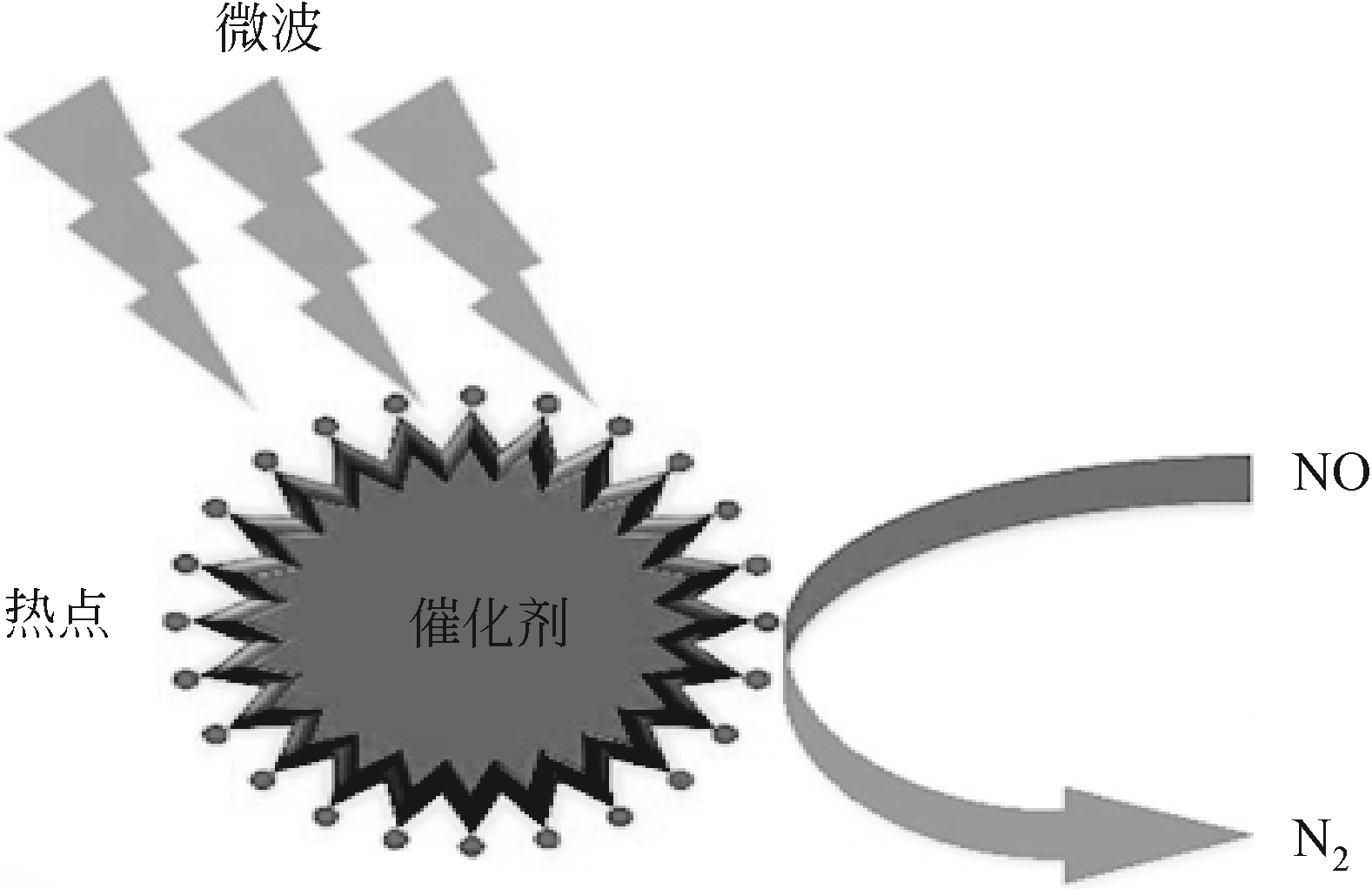
| 11 | WEI Rui, WANG Peng, ZHANG Guangshan, et al. Microwave-responsive catalysts for wastewater treatment: a review[J]. Chemical Engineering Journal, 2020, 382: 122781. |
| 12 | TANG Junwang, ZHANG Tao, MA Lei, et al. Microwave discharge-assisted NO reduction by CH4 over Co/HZSM-5 and Ni/HZSM-5 under O2 excess[J]. Catalysis Letters, 2001, 73(2/3/4): 193-197. |
| 13 | 魏在山, 林哲行, 仇荣亮, 等. 微波Ga-A型分子筛尿素催化还原烟气脱硝研究[J]. 中山大学学报(自然科学版), 2006, 45(1): 103-106. |
| WEI Zaishan, LIN Zhehang, QIU Rongliang, et al. Catalytic reduction of NOx with microwave urea over catalyst Ga-A zeolites[J]. Acta Scientiarum Naturalium Universitatis Sunyatseni, 2006, 45(1): 103-106. | |
| 14 | WEI Zaishan, DU Z Y, LIN Zhehang, et al. Removal of NOx by microwave reactor with ammonium bicarbonate and Ga-A zeolites at low temperature[J]. Energy, 2007, 32(8): 1455-1459. |
| 15 | WEI Zaishan, LIN Zhehang, NIU Hejingying, et al. Simultaneous desulfurization and denitrification by microwave reactor with ammonium bicarbonate and zeolite[J]. Journal of Hazardous Materials, 2009, 162(2/3): 837-841. |
| 16 | MA Shuangchen, JIN Xin, WANG Mengxuan, et al. Experimental study on removing NO from flue gas using microwave irradiation over activated carbon carried catalyst[J]. Science China: Technological Sciences, 2011, 54(12): 3431-3436. |
| 17 | MA Shuangchen, YAO Juanjuan, MA Xiaoying, et al. Removal of SO2 and NOx using microwave swing adsorption over activated carbon carried catalyst[J]. Chemical Engineering & Technology, 2013, 36(7): 1217-1224. |
| 18 | XU Wentao, ZHOU Jicheng, LI Hu, et al. Microwave-assisted catalytic reduction of NO into N2 by activated carbon supported Mn2O3 at low temperature under O2 excess[J]. Fuel Processing Technology, 2014, 127: 1-6. |
| 19 | PENG Kang, ZHOU Jicheng, XU Wentao, et al. Microwave irradiation-selective catalytic reduction of NO to N2 by activated carbon at low temperature[J]. Energy & Fuels, 2017, 31(7): 7344-7351. |
| 20 | TANG Junwang, ZHANG Tao, LIANG Dongbai, et al. Direct decomposition of NO by microwave heating over Fe/NaZSM-5[J]. Applied Catalysis B: Environmental, 2002, 36(1): 1-7. |
| 21 | OHNISHI Takeshi, KAWAKAMI Koutarou, NISHIOKA Masateru, et al. Direct decomposition of NO on metal-loaded zeolites with coexistence of oxygen and water vapor under unsteady-state conditions by NO concentration and microwave rapid heating[J]. Catalysis Today, 2017, 281(3): 566-574. |
| 22 | 周远松, 高风雨, 唐晓龙, 等. 金属氧化物催化CO还原NO的研究进展[J]. 化工进展, 2019, 38(11): 4941-4948. |
| ZHOU Yuansong, GAO Fengyu, TANG Xiaolong, et al. Research progress on NO reduction by CO over metal oxide catalysts[J]. Chemical Industry and Engineering Progress, 2019, 38(11): 4941-4948. | |
| 23 | XU Wentao, CAI Jinjun, ZHOU Jicheng, et al. Microwave irradiation coupled with MeOx/Al2O3 (Me=Cu, Mn, Ce) catalysts for nitrogen monoxide removal from flue gas at low temperatures[J]. Energy Technology, 2016, 4(7): 856-863. |
| 24 | 徐文涛. 微波催化转化NO及微波效应的研究[D]. 湖南:湘潭大学, 2016. |
| XU Wentao. Study on microwave catalytic conversion of nitric oxide and microwave effects[D]. Hunan: Xiangtan University, 2016. | |
| 25 | XU Wentao, ZHOU Jicheng, Yingpiao OU, et al. Microwave selective effect: a new approach towards oxygen inhibition removal for highly-effective NO decomposition by microwave catalysis over BaMnxMg1-xO3 mixed oxides at low temperature under excess oxygen[J]. Chemical Communications, 2015, 51(19): 4073-4076. |
| 26 | XU Wentao, SHI Ni, YOU Zhimin, et al. Low-temperature NO decomposition through microwave catalysis on BaMnO3-based catalysts under excess oxygen: effect of a-site substitution by Ca, K and La[J]. Fuel Processing Technology, 2017, 167: 205-214. |
| 27 | XU Wentao, CAI Jinjun, ZHOU Jicheng, et al. Highly effective direct decomposition of nitric oxide by microwave catalysis over BaMeO3(Me=Mn, Co, Fe) mixed oxides at low temperature under excess oxygen[J]. ChemCatChem, 2016, 8(2): 417-425. |
| 28 | XU Wentao, WANG Qige, PENG Kang, et al. Development of MgCo2O4-BaCO3 composites as microwave catalysts for the highly effective direct decomposition of NO under excess O2 at a low temperature[J]. Catalysis Science & Technology, 2019, 9(16): 4276-4285. |
| 29 | WEI Zaishan, ZENG Guihua, XIE Zhirong, et al. Microwave catalytic NOx and SO2 removal using FeCu/zeolite as catalyst[J]. Fuel, 2011, 90(4): 1599-1603. |
| 30 | WEI Zaishan, NIU Hejingying, JI Yongfeng. Simultaneous removal of SO2 and NOx by microwave with potassium permanganate over zeolite[J]. Fuel Processing Technology, 2009, 90(2): 324-329. |
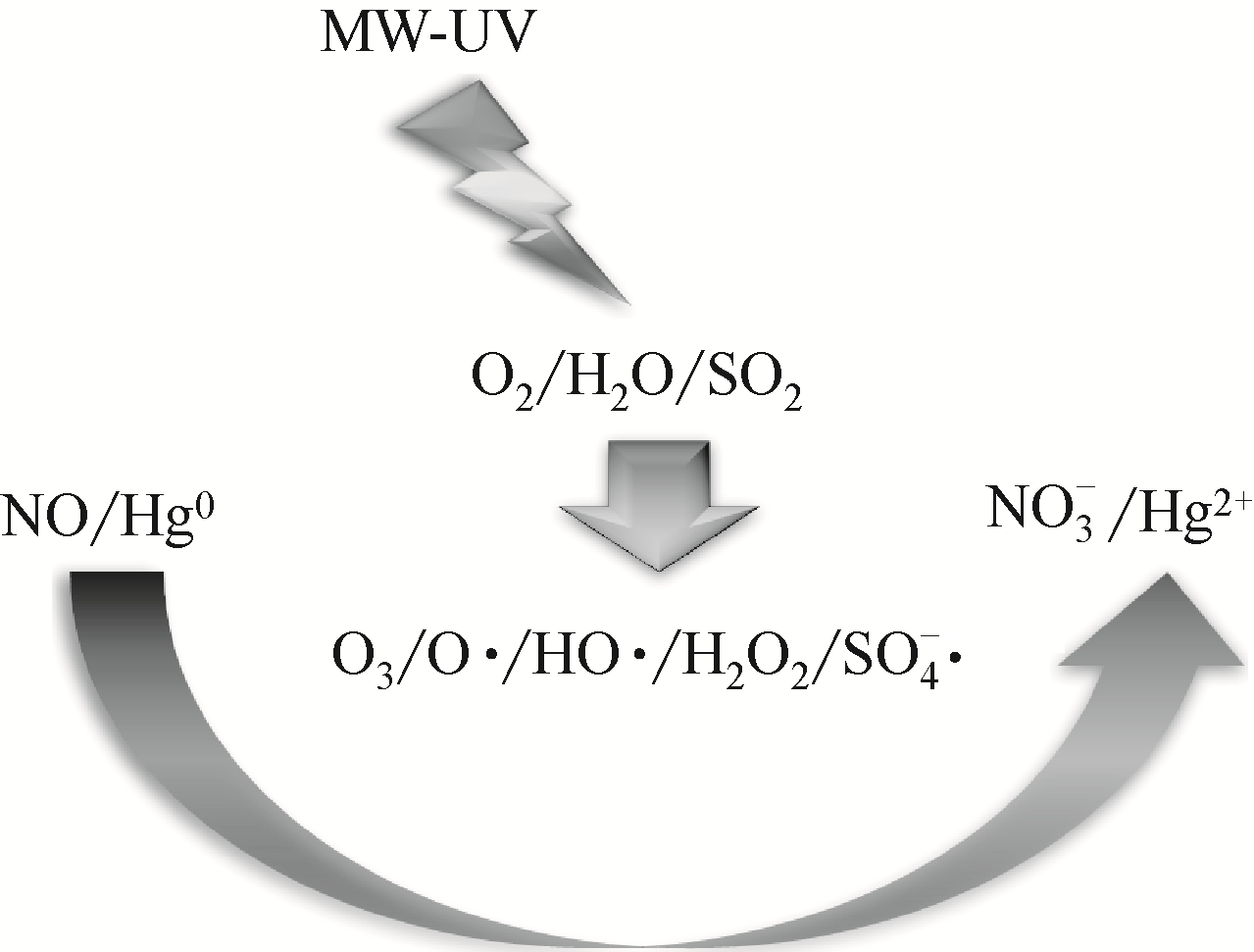
| 31 | HAO Runlong, WANG Zheng, GONG Yaping, et al. Photocatalytic removal of NO and Hg0 using microwave induced ultraviolet irradiating H2O/O2 mixture[J]. Journal of Hazardous Materials, 2020, 383: 121135. |
| 32 | 赵毅, 郝润龙, 齐萌, 等. 同时脱硫脱硝脱汞技术研究概述[J]. 中国电力, 2013, 46(10): 155-158. |
| ZHAO Yi, HAO Runlong, QI Meng, et al. Overview of simultaneous desulfurization, denitrification and demercuration technologies[J]. Electric Power, 2013, 46(10): 155-158. |
| [1] | ZHANG Mingyan, LIU Yan, ZHANG Xueting, LIU Yake, LI Congju, ZHANG Xiuling. Research progress of non-noble metal bifunctional catalysts in zinc-air batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 276-286. |
| [2] | SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298. |
| [3] | XIE Luyao, CHEN Songzhe, WANG Laijun, ZHANG Ping. Platinum-based catalysts for SO2 depolarized electrolysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 299-309. |
| [4] | YANG Xiazhen, PENG Yifan, LIU Huazhang, HUO Chao. Regulation of active phase of fused iron catalyst and its catalytic performance of Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 310-318. |
| [5] | ZHAO Wei, ZHAO Deyin, LI Shihan, LIU Hongda, SUN Jin, GUO Yanqiu. Synthesis and application of triazine drag reducing agent for nature gas pipeline [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 391-399. |
| [6] | GAO Yufei, LU Jinfeng. Mechanism of heterogeneous catalytic ozone oxidation:A review [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 430-438. |
| [7] | ZHANG Jie, WANG Fangfang, XIA Zhonglin, ZHAO Guangjin, MA Shuangchen. Current SF6 emission, emission reduction and future prospects under “carbon peaking and carbon neutrality” [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 447-460. |
| [8] | WANG Lele, YANG Wanrong, YAO Yan, LIU Tao, HE Chuan, LIU Xiao, SU Sheng, KONG Fanhai, ZHU Canghai, XIANG Jun. Influence of spent SCR catalyst blending on the characteristics and deNO x performance for new SCR catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 489-497. |
| [9] | GU Yongzheng, ZHANG Yongsheng. Dynamic behavior and kinetic model of Hg0 adsorption by HBr-modified fly ash [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 498-509. |
| [10] | ZHAO Jingchao, TAN Ming. Effect of surfactants on the reduction of industrial saline wastewater by electrodialysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 529-535. |
| [11] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [12] | WANG Fu'an. Consumption and emission reduction of the reactor of 300kt/a propylene oxide process [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 213-218. |
| [13] | SHU Bin, CHEN Jianhong, XIONG Jian, WU Qirong, YU Jiangtao, YANG Ping. Necessity analysis of promoting the development of green methanol under the goal of carbon neutrality [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4471-4478. |
| [14] | CHENG Tao, CUI Ruili, SONG Junnan, ZHANG Tianqi, ZHANG Yunhe, LIANG Shijie, PU Shi. Analysis of impurity deposition and pressure drop increase mechanisms in residue hydrotreating unit [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4616-4627. |
| [15] | WANG Peng, SHI Huibing, ZHAO Deming, FENG Baolin, CHEN Qian, YANG Da. Recent advances on transition metal catalyzed carbonylation of chlorinated compounds [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4649-4666. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||