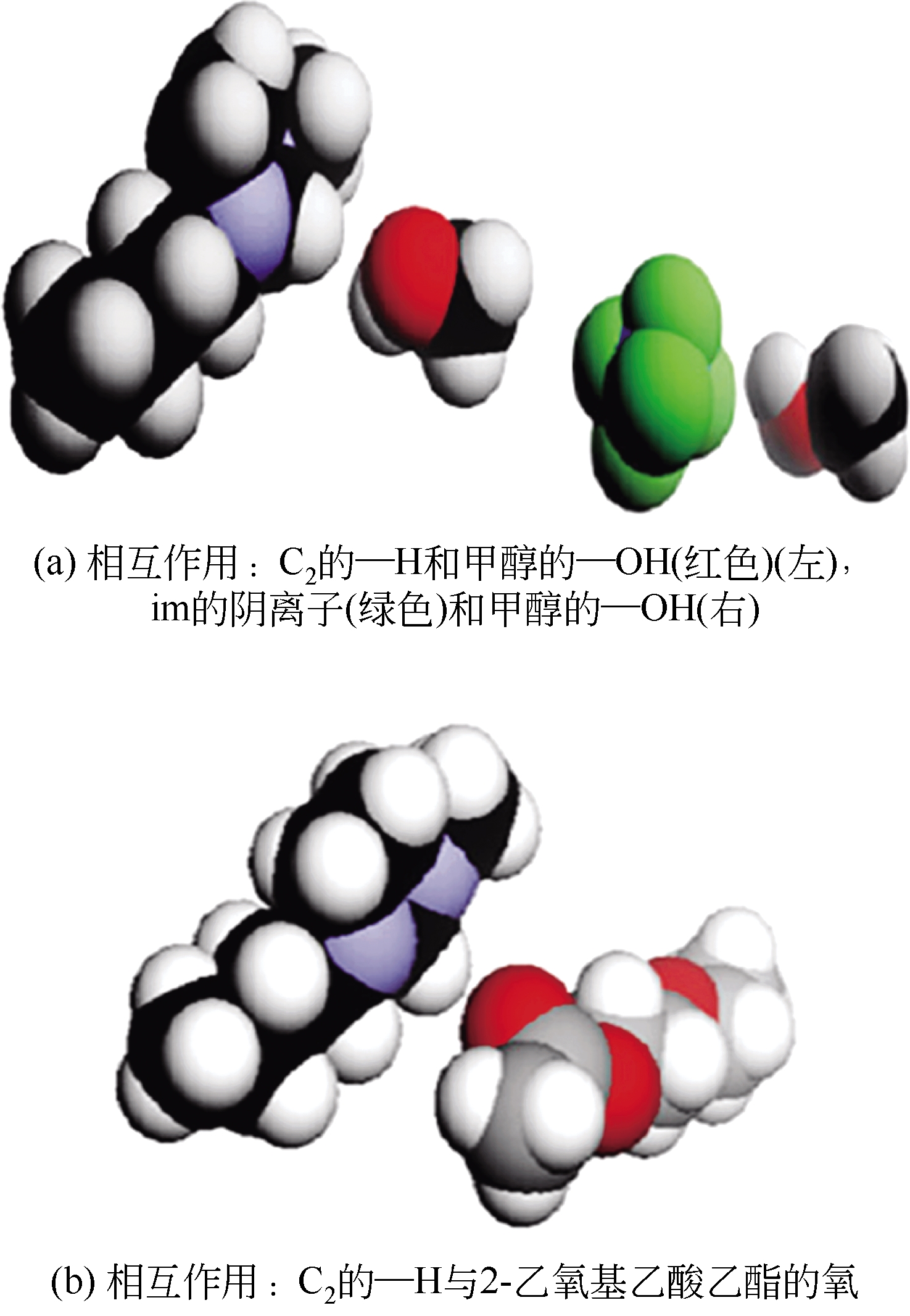
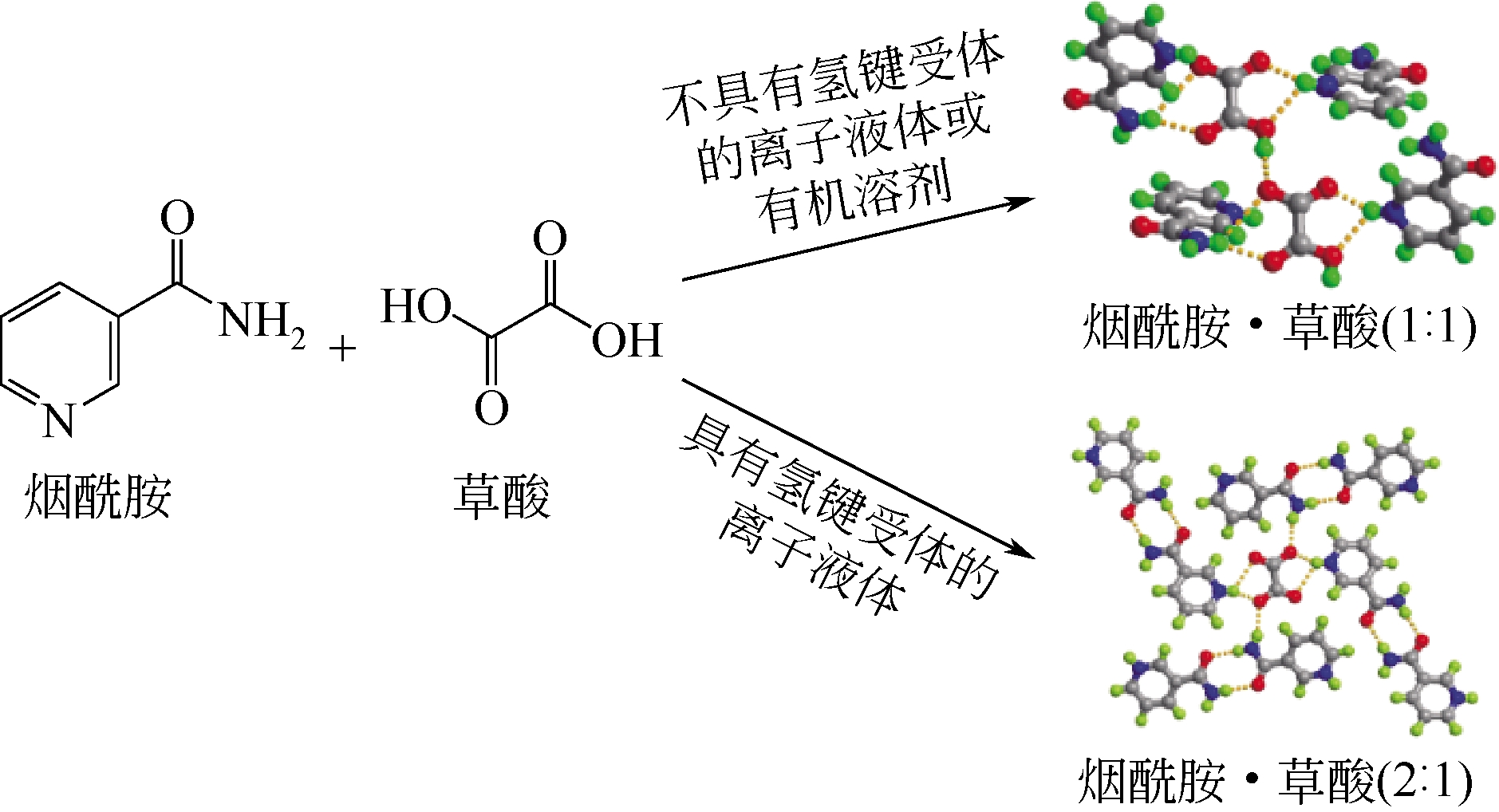
Chemical Industry and Engineering Progress ›› 2019, Vol. 38 ›› Issue (05): 2389-2401.DOI: 10.16085/j.issn.1000-6613.2018-1482
• Biochemical and pharmaceutical engineering • Previous Articles Next Articles
Application of ionic liquids in pharmaceutical crystal engineering
Zeren SHANG1,2,3( ),Weiguo HU1,2,3,4,Weiwei TANG1,2,3,Lina JIA1,2,3,Mingchen LI1,2,3,Yanxiao ZHAO1,2,3,Ning WEI1,2,3,Junbo GONG1,2,3(
),Weiguo HU1,2,3,4,Weiwei TANG1,2,3,Lina JIA1,2,3,Mingchen LI1,2,3,Yanxiao ZHAO1,2,3,Ning WEI1,2,3,Junbo GONG1,2,3( )
)
- 1. National Engineering Research Center of Industry Crystallization Technology,School of Chemical Engineering and Technology,Tianjin University,Tianjin 300072, China
2. Collaborative Innovation Center of Chemical Science and Engineering Tianjin,Tianjin 300072, China
3. Key Laboratory of Modern Drug Delivery and High Efficiency in Tianjin University,Tianjin 300072, China
4. North China Pharmaceutical Company Ltd. ,Shijiazhuang 050015,Hebei,China
-
Received:2018-07-17Revised:2019-01-03Online:2019-05-05Published:2019-05-05 -
Contact:Junbo GONG
离子液体在药物晶体工程中的应用
尚泽仁1,2,3( ),胡卫国1,2,3,4,汤伟伟1,2,3,贾丽娜1,2,3,李鸣晨1,2,3,赵燕晓1,2,3,魏宁1,2,3,龚俊波1,2,3(
),胡卫国1,2,3,4,汤伟伟1,2,3,贾丽娜1,2,3,李鸣晨1,2,3,赵燕晓1,2,3,魏宁1,2,3,龚俊波1,2,3( )
)
- 1. 天津大学化工学院国家工业结晶工程技术研究中心,天津 300072
2. 天津化学化工协同创新中心,天津 300072
3. 现代药物传递及功能高效化天津市重点实验室,天津 300072
4. 华北制药股份有限公司,河北 石家庄 050015
-
通讯作者:龚俊波 -
作者简介:<named-content content-type="corresp-name">尚泽仁</named-content>(1994—),男,硕士研究生,研究方向为药物晶体工程。E-mail:<email>shangzeren@tju.edu.cn</email>。 -
基金资助:国家自然科学基金(21676179、91634117、81361140344);国家科技重大专项(2017ZX07402003、2017ZX09101001);天津市科技计划(15JCZDJC33200)
CLC Number:
Cite this article
Zeren SHANG, Weiguo HU, Weiwei TANG, Lina JIA, Mingchen LI, Yanxiao ZHAO, Ning WEI, Junbo GONG. Application of ionic liquids in pharmaceutical crystal engineering[J]. Chemical Industry and Engineering Progress, 2019, 38(05): 2389-2401.
尚泽仁, 胡卫国, 汤伟伟, 贾丽娜, 李鸣晨, 赵燕晓, 魏宁, 龚俊波. 离子液体在药物晶体工程中的应用[J]. 化工进展, 2019, 38(05): 2389-2401.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2018-1482
| 药物名称 | 晶型 | 临床疗效 | 文献 |
|---|---|---|---|
| 甲苯达唑 | 晶型A,晶型B,晶型C | 晶型C > 晶型A | [ |
| 法莫替丁 | 晶型A,晶型B,晶型C,晶型D | 晶型B > 晶型A | [ |
| 棕榈氯霉素 | 晶型A,晶型B,晶型C | 晶型B和晶型C > 晶型A | [ |
| 尼群地平 | 晶型Ⅰ,晶型Ⅱ,晶型Ⅲ,晶型Ⅳ | 晶型Ⅳ > 晶型Ⅰ | [ |
| 利福定 | 晶型Ⅰ,晶型Ⅱ,晶型Ⅲ,晶型Ⅳ | 晶型Ⅳ > 晶型Ⅱ | [ |
| 药物名称 | 晶型 | 临床疗效 | 文献 |
|---|---|---|---|
| 甲苯达唑 | 晶型A,晶型B,晶型C | 晶型C > 晶型A | [ |
| 法莫替丁 | 晶型A,晶型B,晶型C,晶型D | 晶型B > 晶型A | [ |
| 棕榈氯霉素 | 晶型A,晶型B,晶型C | 晶型B和晶型C > 晶型A | [ |
| 尼群地平 | 晶型Ⅰ,晶型Ⅱ,晶型Ⅲ,晶型Ⅳ | 晶型Ⅳ > 晶型Ⅰ | [ |
| 利福定 | 晶型Ⅰ,晶型Ⅱ,晶型Ⅲ,晶型Ⅳ | 晶型Ⅳ > 晶型Ⅱ | [ |
| 名称 | 英文缩写 | CAS # | 结构 |
|---|---|---|---|
| 1-丁基-3-甲基咪唑双三氟甲磺酰亚胺盐 | [C4mim][N(CF3SO2)2] | 174899-83-3 |  |
| 1-己基-3-甲基咪唑双三氟甲磺酰亚胺盐 | [C6mim][N(CF3SO2)2] | 382150-50-7 |  |
| 1-丁基-3-甲基咪唑四氟硼酸盐 | [C4mim][BF4] | 174501-65-6 |  |
| 1-己基-3-甲基咪唑四氟硼酸盐 | [C6mim][BF4] | 244193-50-8 |  |
| 1-烯丙基-3-乙基咪唑四氟硼酸盐 | [aeim][BF4] | 945732-42-3 |  |
| 1-丁基-2,3-二甲基咪唑四氟硼酸盐 | [bdmim][BF4] | 402846-78-0 |  |
| 1,3-二烯丙基咪唑四氟硼酸盐 | [aaim][BF4] | 852699-06-0 |  |
| 1-乙基-3-甲基咪唑硫酸乙酯盐 | [emim][EtSO4] | 342573-75-5 |  |
| 1-丁基-3-甲基咪唑六氟磷酸盐 | [bmim][PF6] | 174501-64-5 |  |
| 1-乙基-3-甲基咪唑甲基磷酸酯盐 | [emim][CH3O(H)PO2] | 81994-80-1 |  |
| 1-己基-3-甲基咪唑六氟磷酸盐 | [hmim][PF6] | 304680-35-1 |  |
| 1-乙基-3-甲基咪唑乙酸盐 | [C2mim][OAc] | 143314-17-4 |  |
| 1-丁基-3-甲基咪唑溴盐 | [C4mim]Br | 85100-77-2 |  |
| 三己基(十四烷基)鏻双(草酸根)硼酸盐 | [P6,6,6,14][BOB] | 880497-87-0 |  |
| 名称 | 英文缩写 | CAS # | 结构 |
|---|---|---|---|
| 1-丁基-3-甲基咪唑双三氟甲磺酰亚胺盐 | [C4mim][N(CF3SO2)2] | 174899-83-3 |  |
| 1-己基-3-甲基咪唑双三氟甲磺酰亚胺盐 | [C6mim][N(CF3SO2)2] | 382150-50-7 |  |
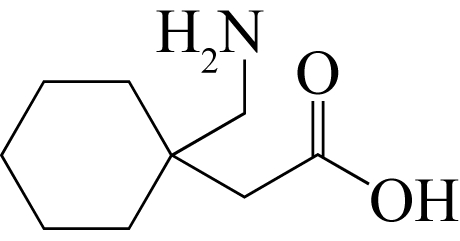
| 1-丁基-3-甲基咪唑四氟硼酸盐 | [C4mim][BF4] | 174501-65-6 |  |
| 1-己基-3-甲基咪唑四氟硼酸盐 | [C6mim][BF4] | 244193-50-8 |  |
| 1-烯丙基-3-乙基咪唑四氟硼酸盐 | [aeim][BF4] | 945732-42-3 |  |
| 1-丁基-2,3-二甲基咪唑四氟硼酸盐 | [bdmim][BF4] | 402846-78-0 |  |
| 1,3-二烯丙基咪唑四氟硼酸盐 | [aaim][BF4] | 852699-06-0 |  |
| 1-乙基-3-甲基咪唑硫酸乙酯盐 | [emim][EtSO4] | 342573-75-5 |  |
| 1-丁基-3-甲基咪唑六氟磷酸盐 | [bmim][PF6] | 174501-64-5 |  |
| 1-乙基-3-甲基咪唑甲基磷酸酯盐 | [emim][CH3O(H)PO2] | 81994-80-1 |  |
| 1-己基-3-甲基咪唑六氟磷酸盐 | [hmim][PF6] | 304680-35-1 |  |
| 1-乙基-3-甲基咪唑乙酸盐 | [C2mim][OAc] | 143314-17-4 |  |
| 1-丁基-3-甲基咪唑溴盐 | [C4mim]Br | 85100-77-2 |  |
| 三己基(十四烷基)鏻双(草酸根)硼酸盐 | [P6,6,6,14][BOB] | 880497-87-0 |  |
| 名称英文缩写 | 熔点/°C | 黏度25℃/Pa?s | 亲水/疏水 |
|---|---|---|---|
| [C4mim][N(CF3SO2)2] | -4.9 | 0.0511 | 亲水 |
| [C6mim][N(CF3SO2)2] | -10.5 | 0.0705 | 亲水 |
| [C4mim][BF4] | -17.0 | 0.0949 | 亲水 |
| [C6mim][BF4] | -81.0 | 0.1456 | 疏水 |
| [aeim][BF4] | — | — | 亲水 |
| [bdmim][BF4] | 39.7 | 0.6500 | 亲水 |
| [aaim][BF4] | 4.3 | — | 亲水 |
| [emim][EtSO4] | -65.0 | 0.0962 | 亲水 |
| [bmim][PF6] | 10.9 | 0.2740 | 疏水 |
| [emim][CH3O(H)PO2] | — | 0.1564 ① | 亲水 |
| [hmim][PF6] | -80.0 | 0.4830 | 疏水 |
| [C2mim][OAc] | < -20.0 | 0.1285 | 亲水 |
| [C4mim]Br | 72.9 | 0.0570 ② | 亲水 |
| [P6,6,6,14][BOB] | — | 0.6150 ① | 亲水 |
| 名称英文缩写 | 熔点/°C | 黏度25℃/Pa?s | 亲水/疏水 |
|---|---|---|---|
| [C4mim][N(CF3SO2)2] | -4.9 | 0.0511 | 亲水 |
| [C6mim][N(CF3SO2)2] | -10.5 | 0.0705 | 亲水 |
| [C4mim][BF4] | -17.0 | 0.0949 | 亲水 |
| [C6mim][BF4] | -81.0 | 0.1456 | 疏水 |
| [aeim][BF4] | — | — | 亲水 |
| [bdmim][BF4] | 39.7 | 0.6500 | 亲水 |
| [aaim][BF4] | 4.3 | — | 亲水 |
| [emim][EtSO4] | -65.0 | 0.0962 | 亲水 |
| [bmim][PF6] | 10.9 | 0.2740 | 疏水 |
| [emim][CH3O(H)PO2] | — | 0.1564 ① | 亲水 |
| [hmim][PF6] | -80.0 | 0.4830 | 疏水 |
| [C2mim][OAc] | < -20.0 | 0.1285 | 亲水 |
| [C4mim]Br | 72.9 | 0.0570 ② | 亲水 |
| [P6,6,6,14][BOB] | — | 0.6150 ① | 亲水 |
| 晶型 | 结晶方法 | 溶剂 | 文献 |
|---|---|---|---|
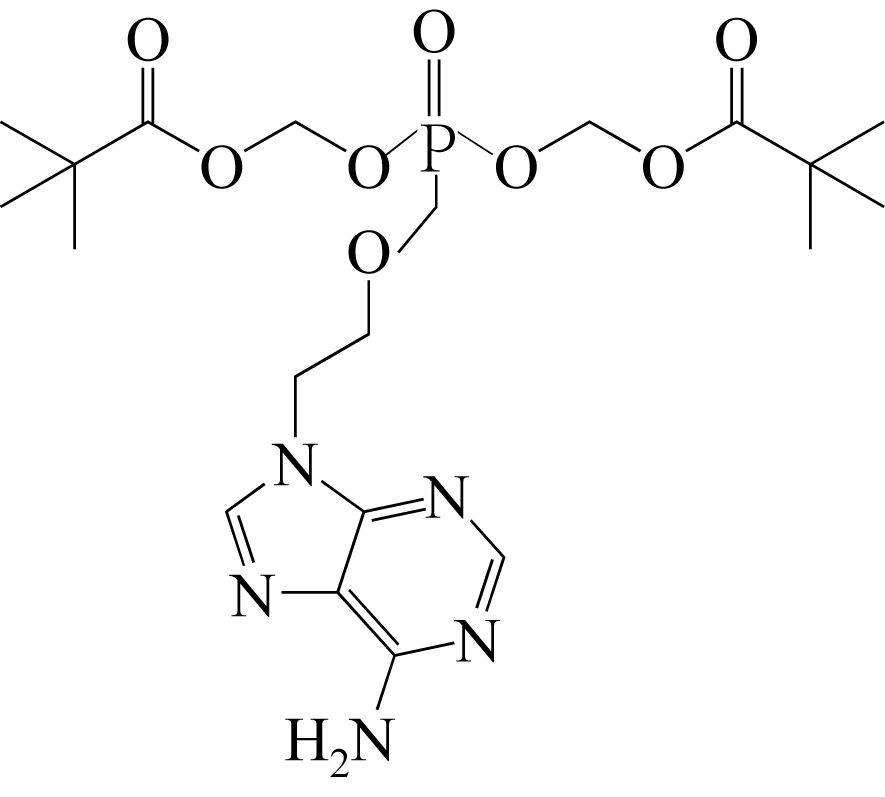
| 晶型Ⅰ无水物(C20H32N5O8P) | 冷却结晶 | 丙酮+二正丁醚 | [ |
| 晶型Ⅱ二水合物(C20H32N5O8P·2H2O) | 溶析结晶 | 甲醇/水 | [ |
| 晶型Ⅲ溶剂化物(C20H32N5O8P·CH3OH) | 蒸发结晶 | 甲醇 | [ |
| 晶型Ⅳ盐型(C20H32N5O8P·C4H4O4) | 冷却结晶 | 异丙醇+富马酸 | [ |
| 晶型Ⅴ无水物(C20H32N5O8P) | 喷雾干燥 | 乙醇 | [ |
| 晶型Ⅵ一水合物(C20H32N5O8P·H2O) | 蒸发结晶 | 二氯甲烷+甲醇+水蒸气 | [ |
| 晶型Ⅶ溶剂化物(C20H32N5O8P·C4H10O) | 溶析结晶 | 正丁醇/己烷 | [ |
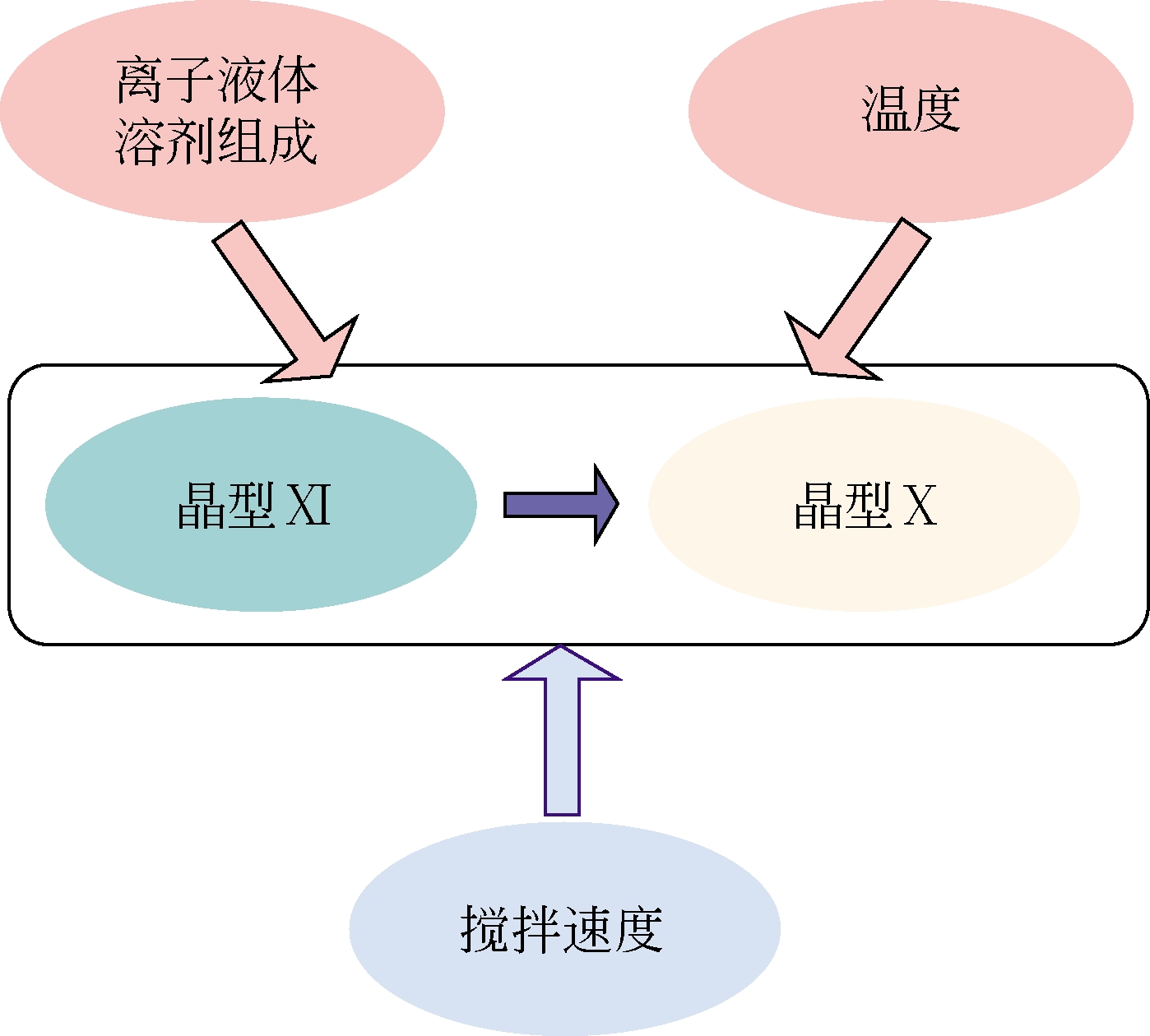
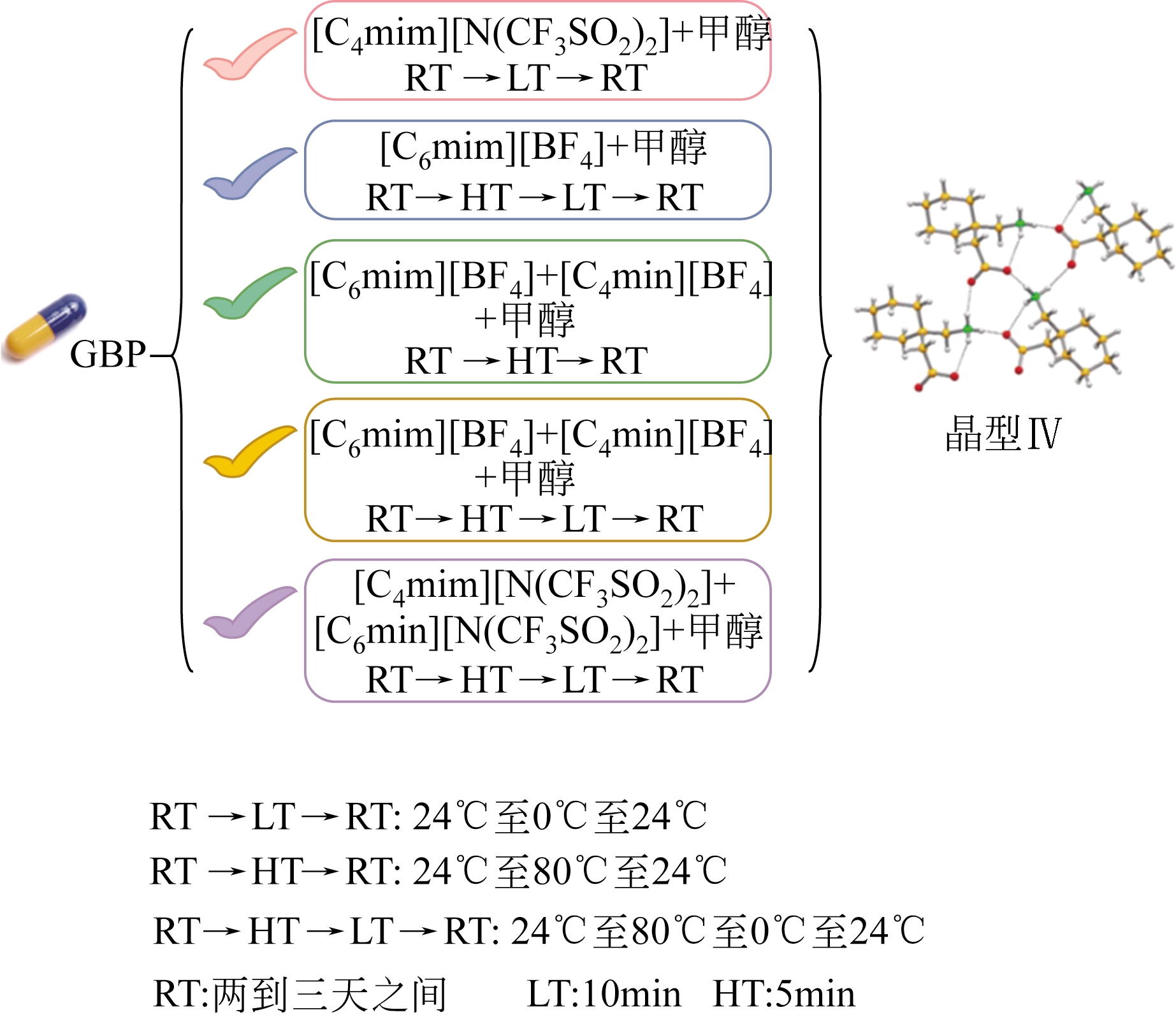
| 晶型Ⅷ无水物(C20H32N5O8P) | 溶析结晶 | [aeim][BF4]/水 | [ |
| 晶型Ⅸ半水合物(C20H32N5O8P·0.5H2O) | 溶析结晶 | [aeim][BF4]/水 | [ |
| 晶型Ⅹ无水物(C20H32N5O8P) | 溶析结晶 | 二氯甲烷/正己烷 | [ |
| 晶型Ⅺ无水物(C20H32N5O8P) | 溶析结晶 | [aeim][BF4]/[bdmim][BF4] | [ |
| 晶型 | 结晶方法 | 溶剂 | 文献 |
|---|---|---|---|
| 晶型Ⅰ无水物(C20H32N5O8P) | 冷却结晶 | 丙酮+二正丁醚 | [ |
| 晶型Ⅱ二水合物(C20H32N5O8P·2H2O) | 溶析结晶 | 甲醇/水 | [ |
| 晶型Ⅲ溶剂化物(C20H32N5O8P·CH3OH) | 蒸发结晶 | 甲醇 | [ |
| 晶型Ⅳ盐型(C20H32N5O8P·C4H4O4) | 冷却结晶 | 异丙醇+富马酸 | [ |
| 晶型Ⅴ无水物(C20H32N5O8P) | 喷雾干燥 | 乙醇 | [ |
| 晶型Ⅵ一水合物(C20H32N5O8P·H2O) | 蒸发结晶 | 二氯甲烷+甲醇+水蒸气 | [ |
| 晶型Ⅶ溶剂化物(C20H32N5O8P·C4H10O) | 溶析结晶 | 正丁醇/己烷 | [ |
| 晶型Ⅷ无水物(C20H32N5O8P) | 溶析结晶 | [aeim][BF4]/水 | [ |
| 晶型Ⅸ半水合物(C20H32N5O8P·0.5H2O) | 溶析结晶 | [aeim][BF4]/水 | [ |
| 晶型Ⅹ无水物(C20H32N5O8P) | 溶析结晶 | 二氯甲烷/正己烷 | [ |
| 晶型Ⅺ无水物(C20H32N5O8P) | 溶析结晶 | [aeim][BF4]/[bdmim][BF4] | [ |
| 1 | SCHMIDT G M J . Photodimerization in the solid state[J]. Pure and Applied Chemistry,1971, 27(4): 647-678. |
| 2 | BRAGA D . Crystal engineering, where from? where to?[J]. Chemical Communications, 2003, 39(22): 2751-2754. |
| 3 | HILFIKER R . Polymorphism:in the pharmaceutical industry[M]. Hoboken:Wiley, 2006: 1-15. |
| 4 | CHEN J , SARMA B , EVANS J M B , et al . Pharmaceutical crystallization[J]. Crystal Growth & Design, 2011, 11(4): 887-895. |
| 5 | SARMA B , CHEN J , HIS H Y, et al . Solid forms of pharmaceuticals:polymorphs, salts and cocrystals[J]. Korean Journal of Chemical Engineering, 2011, 28(2): 315-322. |
| 6 | SUN C C , GRANT D J . Improved tableting properties of p-hydroxybenzoic acid by water of crystallization: a molecular insight[J]. Pharmaceutical Research, 2004, 21(2): 382-386. |
| 7 | CHANG S Y , SUN C C . Superior plasticity and tabletability of theophylline monohydrate[J]. Molecular Pharmaceutics, 2017, 14(6): 2047-2055. |
| 8 | CHAROENLARP P , WAIKAGUL J , MUENNOO C , et al . Efficacy of single-dose mebendazole, polymorphic forms A and C, in the treatment of hookworm and trichuris infections[J]. Southeast Asian Journal of Tropical Medicine & Public Health, 1993, 24(4): 712-716. |
| 9 | 唐素芳 . 药物多晶型的研究及其对药效和理化性质的影响[J]. 天津药学, 2002, 14(2): 12-14. |
| TANG S F . Research on pharmaceutical polymorphs and the influence of efficacy[J]. Tianjin Pharmacy, 2002, 14(2): 12-14. | |
| 10 | 张伟国, 刘昌孝 . 多晶型药物的生物利用度研究概况[J]. 天津药学, 2007, 19(2): 59-61. |
| ZHANG W G , LIU C X . The bioavailability research of pharmaceutical polymorphs[J]. Tianjin Pharmacy, 2007, 19(2): 59-61. | |
| 11 | DU G H , LV Y , ZHAO Y , et al . New nitrendipine crystal used for treating cardiovascular and cerebrovascular diseases:CN101544596[P]. 2009-09-30. |
| 12 | 马廷升, 朱兰翠 . 药物生物利用度影响因素的动态分析[J]. 怀化学院学报, 2006, 25(2): 76-77. |
| MA T S, ZHU L C . Study on the effective factors of bioavailability[J]. Journal of Huaihua University, 2006, 25(2): 76-77. | |
| 13 | DESIRAJU G R . Crystal engineering: a holistic view[J]. Angewandte Chemie: International Edition, 2007, 46(44): 8342-8356. |
| 14 | GAO Z , ROHANI S , GONG J , et al . Recent developments in the crystallization process: toward the pharmaceutical industry[J]. Engineering, 2017, 3(3): 343-353. |
| 15 | WU S , CHEN M , LI K , et al . Solvent penetration mediated phase transformation for the preparation of aggregated particles with well-defined shape [J]. Cryst. Eng. Comm., 2016, 18(48): 9223-9226. |
| 16 | TANG W , ZHANG M , MO H , et al . Higher-order self-assembly of benzoic acid in solution[J]. Crystal Growth & Design, 2017, 17(10): 5049-5053. |
| 17 | GAO Z , ALTIMIMI F , GONG J , et al . Ultrasonic irradiation and seeding to prevent metastable liquid-liquid phase separation and intensify crystallization [J]. Crystal Growth & Design, 2018, 18(4): 2628-2635. |
| 18 | REICHERT W M , HOLBREY J D , VIGOUR K B , et al . Approaches to crystallization from ionic liquids: complex solvents-complex results, or, a strategy for controlled formation of new supramolecular architectures?[J]. Chemical Communications, 2006, 42(46): 4767-4779. |
| 19 | HARDACRE C , BOWRON D T , HOLBREY J D , et al . Structure of molten 1, 3-dimethylimidazolium chloride using neutron diffraction[J]. The Journal of Chemical Physics, 2003, 118(1): 273-278. |
| 20 | HOUGH W L , SMIGLAK M , RODRIGUEZ H , et al . The third evolution of ionic liquids:active pharmaceutical ingredients[J]. New Journal of Chemistry, 2007, 31(8): 1429-1436. |
| 21 | WELTON T . Room-temperature ionic liquids: solvents for synthesis and catalysis[J]. Chemical Reviews, 1999, 99(8): 2071-2084. |
| 22 | HALLETT J P , WELTON T . Room-temperature ionic liquids:solvents for synthesis and catalysis. 2 [J]. Chemical Reviews, 2011, 111(5): 3508-3576. |
| 23 | AHMED E , BRETERNITZ J , GROH M F , et al . Ionic liquids as crystallisation media for inorganic materials[J]. Cryst. Eng. Comm., 2012, 14(15): 4874-4885. |
| 24 | MEINDERSMA G W , HAAN A B DE . In ionic liquids uncoiled[M]. 8th ed. Hoboken:Wiley, 2013:119. |
| 25 | CHEN X , JI Y , WANG J . Improvement on the crystallization of lysozyme in the presence of hydrophilic ionic liquid[J]. Analyst, 2010, 135(9): 2241-2248. |
| 26 | JUDGE R A , TAKAHASHI S , LONGENECKER K L . The effect of ionic liquids on protein crystallization and X-ray diffraction resolution[J]. Crystal Growth & Design, 2009, 9(8): 3463-3469. |
| 27 | LI X , XU X , FENG Y D J , et al . The crystallization of lysozyme in the system of ionic liquid [BMIm][BF4]-water[J]. Crystal Research and Technology, 2008, 43(10): 1062-1068. |
| 28 | BARZEGAR M , HABIBI-YANGJEH A , BEHBOUDNIA M . Template-free preparation and characterization of nanocrystalline ZnO in aqueous solution of [EMIM][EtSO4] as a low-cost ionic liquid using ultrasonic irradiation and photocatalytic activity[J]. Journal of Physics and Chemistry of Solids, 2009, 70(10): 1353-1358. |
| 29 | BARZEGAR M , HABIBI-YANGJEH A , BEHBOUDNIA M . Ultrasonic-assisted preparation and characterization of CdS nanoparticles in the presence of a halide-free and low-cost ionic liquid and photocatalytic activity[J]. Journal of Physics and Chemistry of Solids, 2010, 71(9): 1393-1397. |
| 30 | ZHAO Y , CHEN Z , WANG H , et al . Crystallization control of CaCO3 by ionic liquids in aqueous solution[J]. Crystal Growth & Design, 2009, 9(11): 4984-4986. |
| 31 | AN J H , KIM J M, CHANG S M , et al . Application of ionic liquid to polymorphic design of pharmaceutical ingredients[J]. Crystal Growth & Design, 2010, 10(7): 3044-3050. |
| 32 | AN J H , KIM W S . Antisolvent crystallization using ionic liquids as solvent and antisolvent for polymorphic design of active pharmaceutical ingredient [J]. Crystal Growth & Design, 2013, 13(1): 31-39. |
| 33 | AN J H , JIN F , KIM H S, et al . Application of ionic liquid to polymorphic transformation of anti-viral/HIV drug adefovir dipivoxil[J]. Archives of Pharmacal Research, 2016, 39(5): 646-659. |
| 34 | MARTINS I C B , GOMES J R B , DUARTE M T , et al . Understanding polymorphic control of pharmaceuticals using imidazolium-based ionic liquid mixtures as crystallization directing agents[J]. Crystal Growth & Design, 2017, 17(2): 428-432. |
| 35 | AN J H , JIN F , KIM H S, et al . Investigation of the polymorphic transformation of the active pharmaceutical ingredient clopidogrel bisulfate using the ionic liquid AEImBF4 [J]. Crystal Growth & Design, 2016, 16(4): 1829-1836. |
| 36 | SMITH K B , BRIDSON R H , LEEKE G A . Crystallisation control of paracetamol from ionic liquids[J]. Cryst. Eng. Comm., 2014, 16(47): 10797-10803. |
| 37 | HA G S, KIM J H . Effect of an ionic liquid on vancomycin crystallization[J]. Korean Journal of Chemical Engineering, 2015, 32(4): 576-582. |
| 38 | KARTHIKA S , RADHAKRISHNAN T K , KALAICHELVI P . The role of hydrogen bonding propensity in tuning the morphology of crystals obtained from imidazolium based ionic liquids[J]. Journal of Crystal Growth, 2017, 463:168-175. |
| 39 | VICOSA A , LETOURNEAU J J , ESPITALIER F , et al . An innovative antisolvent precipitation process as a promising technique to prepare ultrafine rifampicin particles[J]. Journal of Crystal Growth, 2012, 342(1): 80-87. |
| 40 | AZEVEDO JACQUELINE R DE , ESPITALIER F , LETOURNEAU J J , et al . Antisolvent crystallization of a cardiotonic drug in ionic liquids: effect of mixing on the crystal properties[J]. Journal of Crystal Growth, 2017, 472:29-34. |
| 41 | GOLOVANOV D G , LYSSENKO K A , ANTIPIN M Y , et al . Cocrystal of an ionic liquid with organic molecules as a mimic of ionic liquid solution[J]. Crystal Growth & Design, 2005, 5(1): 337-340. |
| 42 | TITI H M , KELLEY S P , EASTON M E , et al . Formation of ionic co-crystals of amphoteric azoles directed by the ionic liquid co-former 1-ethyl-3-methylimidazolium acetate[J]. Chemical Communications, 2017, 53(61): 8569-8572. |
| 43 | SHIMPI M R , VELAGA S P , SHAH F U , et al . Pharmaceutical crystal engineering using ionic liquid anion-solute interactions[J]. Crystal Growth & Design, 2017, 17(4): 1729-1734. |
| 44 | KANDULA R K , VEPURI S B , DEVARAJEGOWDA H C , et al . Crystallization of R-(+)-atenolol hydrochloride from racemic ionic liquid - a selective double decomposition green reaction[J]. Journal of Molecular Structure, 2018, 1169: 39-45. |
| 45 | BYRN S R , PFEIFFER R R , STOWELL J G . Solid-state chemistry of drugs[M]. 2nd ed. West Lafayette:SSCI, 1999: 233-247. |
| 46 | STOIMENOVSKI J , MACFARLANE D R , BICA K , et al . Crystalline vs. ionic liquid salt forms of active pharmaceutical ingredients: a position paper[J]. Pharmaceutical Research, 2010, 27(4): 521-526. |
| 47 | CHERUKUVADA S , NANGIA A . Polymorphism in an API ionic liquid:ethambutol dibenzoate trimorphs[J]. Cryst. Eng. Comm., 2012, 14(23): 7840-7843. |
| 48 | KUMAR V , PARMAR V S , MALHOTRA S V . Enhanced solubility and selective benzoylation of nucleosides in novel ionic liquid[J]. Tetrahedron Letters, 2007, 48(5): 809-812. |
| 49 | MONIRUZZAMAN M , TAHARA Y , TAMURA M , et al . Ionic liquid-assisted transdermal delivery of sparingly soluble drugs[J]. Chemical Communications, 2010, 46(9): 1452-1454. |
| 50 | JIANG Y , LIANG M , SVEJKAR D , et al . Albumin-micelles via a one-pot technology platform for the delivery of drugs[J]. Chemical Communications, 2014, 50(48): 6394-6397. |
| 51 | WINTER K DE , VERLINDEN K , KREN V , et al . Ionic liquids as cosolvents for glycosylation by sucrose phosphorylase: balancing acceptor solubility and enzyme stability[J]. Green Chemistry, 2013, 15(7): 1949-1955. |
| 52 | USUKI T , YASUDA N , YOSHIZAWA-FUJITA M , et al . Extraction and isolation of shikimic acid from ginkgo biloba leaves utilizing an ionic liquid that dissolves cellulose[J]. Chemical Communications, 2011, 47(38): 10560-10562. |
| 53 | DAI Y , SPRONSEN J VAN , WITKAMP G J , et al . Natural deep eutectic solvents as new potential media for green technology[J]. Analytica Chimica Acta, 2013, 766(5): 61-68. |
| 54 | SINTRA T E , SHIMIZU K , VENTURA S P M , et al . Enhanced dissolution of ibuprofen using ionic liquids as catanionic hydrotropes[J]. Physical Chemistry Chemical Physics, 2018, 20(3): 2094-2103. |
| 55 | SMITH K B , BRIDSON R H , LEEKE G A . Solubilities of pharmaceutical compounds in ionic liquids[J]. Journal of Chemical & Engineering Data, 2011, 56(5): 2039-2043. |
| 56 | JIN W , YANG Q , ZHANG Z , et al . Self-assembly induced solubilization of drug-like molecules in nanostructured ionic liquids[J]. Chemical Communications, 2015, 51(67): 13170-13173. |
| 57 | JIA L , XU S , LIU S , et al . Polymorphs of daidzein and intermolecular interaction effect on solution crystallization[J]. Cryst. Eng. Comm., 2017, 19(47): 7146-7153. |
| 58 | HILDEN J L , REYES C E , KELM M J , et al . Capillary precipitation of a highly polymorphic organic compound[J]. Crystal Growth & Design, 2003, 3(6): 921–926. |
| 59 | LANG M D , GRZESIAK A L , MATZGER A J . The use of polymer heteronuclei for crystalline polymorph selection[J]. Journal of the American Chemical Society, 2002, 124(50): 14834–14835. |
| 60 | ARIMILLI M N , LEE T T K, MANES L V , et al . Nucleotide analog compositions:WO9904774A2[P]. 1999-02-04. |
| 61 | WANG G , LU X , LIU Q , et al . A new crystal form of adefovir dipivoxil and its composition:WO2004043972A1[P]. 2004-05-27. |
| 62 | GALIMI S , VECCHIO E , PIZZOCZRO R . Adefovir dipivoxil crystalline monohydrate form:WO2009015892A1[P]. 2009-02-05. |
| 63 | MIN Y S , CHOUL L H . DH-type crystalline form of adefovir dipivoxil, preparing method thereof, and pharmaceutical composition for antiviral agent comprising the same:WO2010110506A1[P]. 2010-09-30. |
| 64 | AN J H , CHOI G J , KIM W S . Polymorphic and kinetic investigation of adefovir dipivoxil during phase transformation[J]. International Journal of Pharmaceutics, 2012, 422(1): 185-193. |
| 65 | DAVEY R J , DENT G , MUGHAL R K , et al . Concerning the relationship between structural and growth synthons in crystal nucleation:solution and crystal chemistry of carboxylic acids as revealed through IR spectroscopy[J]. Crystal Growth & Design, 2006, 6(8): 1788-1796. |
| 66 | IBERS J A . Gabapentin and gabapentin monohydrate[J]. Acta Crystallographica Section C, 2001, 57(5): 641-643. |
| 67 | REECE H A , LEVENDIS D C . Polymorphs of gabapentin[J]. Acta Crystallographica Section C, 2008, 64(3): o105-o108. |
| 68 | BRAGA D , GREPIONI F , MAINI L , et al . Polymorphic gabapentin:thermal behaviour, reactivity and interconversion of forms in solution and solid-state[J]. New Journal of Chemistry, 2008, 32(10): 1788-1795. |
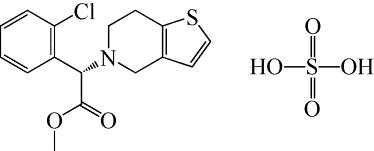
| 69 | KORADLA V , CHAWLA G , BANSAL A . Qualitative and quantitative analysis of clopidogrel bisulphate polymorphs[J]. Acta Pharmaceutica, 2004, 54(3): 193-204. |
| 70 | KIM H J, KIM K J . Quantitative study on polymorphic form in solution crystallization of clopidogrel hydrogen sulfate[J]. Industrial & Engineering Chemistry Research, 2009, 48(24): 11133-11139. |
| 71 | QU H M , MUNK T M , CORNETT C , et al . Influence of temperature on solvent-mediated anhydrate-to-hydrate transformation kinetics[J]. Pharmaceutical Research, 2011, 28(2): 364-373. |
| 72 | MCCABE W L , SMITH J C , HARRIOTT P . Unit operations of chemical engineering[M]. 7th ed. New York:McGraw Hill, 2005:11-15. |
| 73 | DONG Y , NG W K, SHEN S , et al . Preparation and characterization of spironolactone nanoparticles by antisolvent precipitation[J]. International Journal of Pharmaceutics, 2009, 375(1): 84-88. |
| 74 | FINNIE S D , RISTIC R I , SHERWOOD J N , et al . Morphological and growth rate distributions of small self-nucleated paracetamol crystals grown from pure aqueous solutions[J]. Journal of Crystal Growth, 1999, 207(4): 308-318. |
| 75 | YU Z Q , TAN R B H , CHOW P S . Effects of operating conditions on agglomeration and habit of paracetamol crystals in anti-solvent crystallization [J]. Journal of Crystal Growth, 2005, 279(3): 477-488. |
| 76 | LEE T, KUO C S, CHEN Y H . Solubility, polymorphism, crystallinity, and crystal habit of acetaminophen and ibuprofen:by initial solvent screening[J]. Pharmaceutical Technology, 2006, 30(10): 72-92. |
| 77 | SMITH K B . Crystallisation of active pharmaceutical ingredients using ionic liquids[D]. Birmingham:University of Birmingham, 2015. |
| 78 | HUNT PATRICIA A , KIRCHNER B , WELTON T . Characterising the electronic structure of ionic liquids:an examination of the 1-butyl-3-methylimidazolium chloride ion pair[J]. Chemistry:A European Journal, 2006, 12(26): 6762-6775. |
| 79 | HECQ J , DELEERS M , FANARA D , et al . Preparation and in vitro/in vivo evaluation of nano-sized crystals for dissolution rate enhancement of ucb-35440-3, a highly dosed poorly water-soluble weak base[J]. European Journal of Pharmaceutics and Biopharmaceutics, 2006, 64(3): 360-368. |
| 80 | KRAUSE K P , MULLER R H . Production and characterisation of highly concentrated nanosuspensions by high pressure homogenisation[J]. International Journal of Pharmaceutics, 2001, 214(1): 21-24. |
| 81 | WANG Z , CHEN J F , LE Y , et al . Preparation of ultrafine beclomethasone dipropionate drug powder by antisolvent precipitation[J]. Industrial & Engineering Chemistry Research, 2007, 46(14): 4839-4845. |
| 82 | RASENACK N , HARTENHAUER H , MULLER B W . Microcrystals for dissolution rate enhancement of poorly water-soluble drugs[J]. International Journal of Pharmaceutics, 2003, 254(2): 137-145. |
| 83 | ZHANG H X , WANG J X , ZHANG Z B , et al . Micronization of atorvastatin calcium by antisolvent precipitation process[J]. International Journal of Pharmaceutics, 2009, 374(1): 106-113. |
| 84 | MIZUUCHI H , JAITELY V , MURDAN S , et al . Room temperature ionic liquids and their mixtures:potential pharmaceutical solvents[J]. European Journal of Pharmaceutical Sciences, 2008, 33(4): 326-331. |
| 85 | DUGGIRALA N K , PERRY M L , ALMARSSON O , et al . Pharmaceutical cocrystals:along the path to improved medicines[J]. Chemical Communications, 2016, 52(4): 640-655. |
| 86 | AAKEROY C B , FASULO M E , DESPER J . Cocrystal or salt:does it really matter?[J]. Molecular Pharmaceutics, 2007, 4(3): 317-322. |
| 87 | ALMARSSON O , ZAWOROTKO M J . Crystal engineering of the composition of pharmaceutical phases. Do pharmaceutical co-crystals represent a new path to improved medicines?[J]. Chemical Communications, 2004, 40(17): 1889-1896. |
| 88 | CLARE SPEAKMAN J . Acid salts of carboxylic acids, crystals with some“very short”hydrogen bonds[C]// Structure and Bonding.Glasgow. 1972. |
| 89 | BRAGA D , GREPIONI F , MAINI L , et al . From unexpected reactions to a new family of ionic co-crystals:the case of barbituric acid with alkali bromides and caesium iodide[J]. Chemical Communications, 2010, 46(41): 7715-7717. |
| 90 | KELLEY S P , NARITA A , HOLBREY J D , et al . Understanding the effects of ionicity in salts, solvates, co-crystals, ionic co-crystals, and ionic liquids, rather than nomenclature, is critical to understanding their behavior[J]. Crystal Growth & Design, 2013, 13(3): 965-975. |
| 91 | ETTER M C . Hydrogen bonds as design elements in organic chemistry[J]. The Journal of Physical Chemistry, 1991, 95(12): 4601-4610. |
| 92 | WALSH R D B , BRADNER M W , FLEISCHMAN S , et al . Crystal engineering of the composition of pharmaceutical phases[J]. Chemical Communications, 2003, 39(2): 186-187. |
| 93 | JOHANSSON K M , IZGORODINA E I , FORSYTH M , et al . Protic ionic liquids based on the dimeric and oligomeric anions:[(AcO) x H x -1][J]. Physical Chemistry Chemical Physics, 2008, 10(20): 2972-2978. |
| 94 | BLAGDEN N , MATAS M DE , GAVAN P T , et al . Crystal engineering of active pharmaceutical ingredients to improve solubility and dissolution rates[J]. Advanced Drug Delivery Reviews, 2007, 59(7): 617-630. |
| [1] | WU Ya, ZHAO Dan, FANG Rongmiao, LI Jingyao, CHANG Nana, DU Chunbao, WANG Wenzhen, SHI Jun. Research progress on highly efficient demulsifiers for complex crude oil emulsions and their applications [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4398-4413. |
| [2] | YANG Xuzhao, LI Qing, YUAN Kangkang, ZHANG Yingying, HAN Jingli, WU Shide. Thermodynamic properties of Gemini ionic liquid based deep eutectic solvents [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3123-3129. |
| [3] | LYU Chao, ZHANG Xiwen, JIN Lijian, YANG Linjun. Efficient capture of CO2 by a new biphasic solvent-ionic liquid system [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3226-3232. |
| [4] | CHEN Yu, LIU Chong, QIU Yuhui, BI Zixin, MU Tiancheng. Ionic liquids and deep eutectic solvents for green recycle of spent lithium-ion batteries [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 485-496. |
| [5] | HUA Qucheng, DUAN Qinghua. Research progress of ionic liquid as extreme pressure and anti-wear agent [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 331-339. |
| [6] | HAN Mingyang, QIAO Hui, FU Jiaming, MA Zewen, WANG Yan, OUYANG Jia. Research progress of non-aqueous solvents on the pretreatment of lignocellulose [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4086-4097. |
| [7] | SHAN Qingwen, ZHANG Juan, WANG Yajuan, LIU Wenqiang. Synthesis of polymeric ionic liquid and its performance on adsorption desulfurization [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4571-4579. |
| [8] | RUAN Jiawei, YE Xiangzhu, CHEN Lifang, QI Zhiwen. Recent progress in synthesis of organic carbonates from carbon dioxide catalyzed by ionic liquids and deep eutectic solvents [J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1176-1186. |
| [9] | WEI Tingting, WANG Xiankai, ZHAN Yong, CHEN Sisi, DONG Bin. Enhancement of cell lysis in activated sludge by catalytic ozonation of Mn2+ [J]. Chemical Industry and Engineering Progress, 2022, 41(2): 1009-1016. |
| [10] | NI Qing, LAI Jinbo, PENG Dongyue, GUAN Cuishi, LONG Jun. Progress in extraction separation of hydrocarbons by ionic liquids [J]. Chemical Industry and Engineering Progress, 2022, 41(2): 619-627. |
| [11] | LU Zeping, PEI Xinhua, XUE Yu, ZHANG Xiaoguang, HU Yi. Chemical modification of porcine pancreatic lipase with betaine ionic liquid to improve its enzymatic properties [J]. Chemical Industry and Engineering Progress, 2022, 41(11): 6045-6052. |
| [12] | XU Bo, JIANG Guobin, YU Jinlei, HU Jinyan, ZHAO Liang, XU Bingke. Impact of different surfactants on characteristics of single-phase microemulsions [J]. Chemical Industry and Engineering Progress, 2021, 40(S1): 350-356. |
| [13] | CHEN Yaju, REN Qinggang, ZHOU Xiantai, JI Hongbing. Recent advances in porous organic polymers for the synthesis of cyclic carbonates from carbon dioxide [J]. Chemical Industry and Engineering Progress, 2021, 40(7): 3564-3583. |
| [14] | BAI Ruibing, WANG Junfeng, WANG Daoguang, ZHANG Yanqiang. Research progress of ionic liquid-based extraction separation of lithium from brine [J]. Chemical Industry and Engineering Progress, 2021, 40(6): 3224-3238. |
| [15] | LIU Zheng, LIU Ran, HUA Er, JI Jianlong. Water effects on physicochemical properties of protic ionic liquid with N-hexylamine as cation and bis(trifluoromethylsulfonyl) imide as anion [J]. Chemical Industry and Engineering Progress, 2021, 40(4): 2270-2277. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||