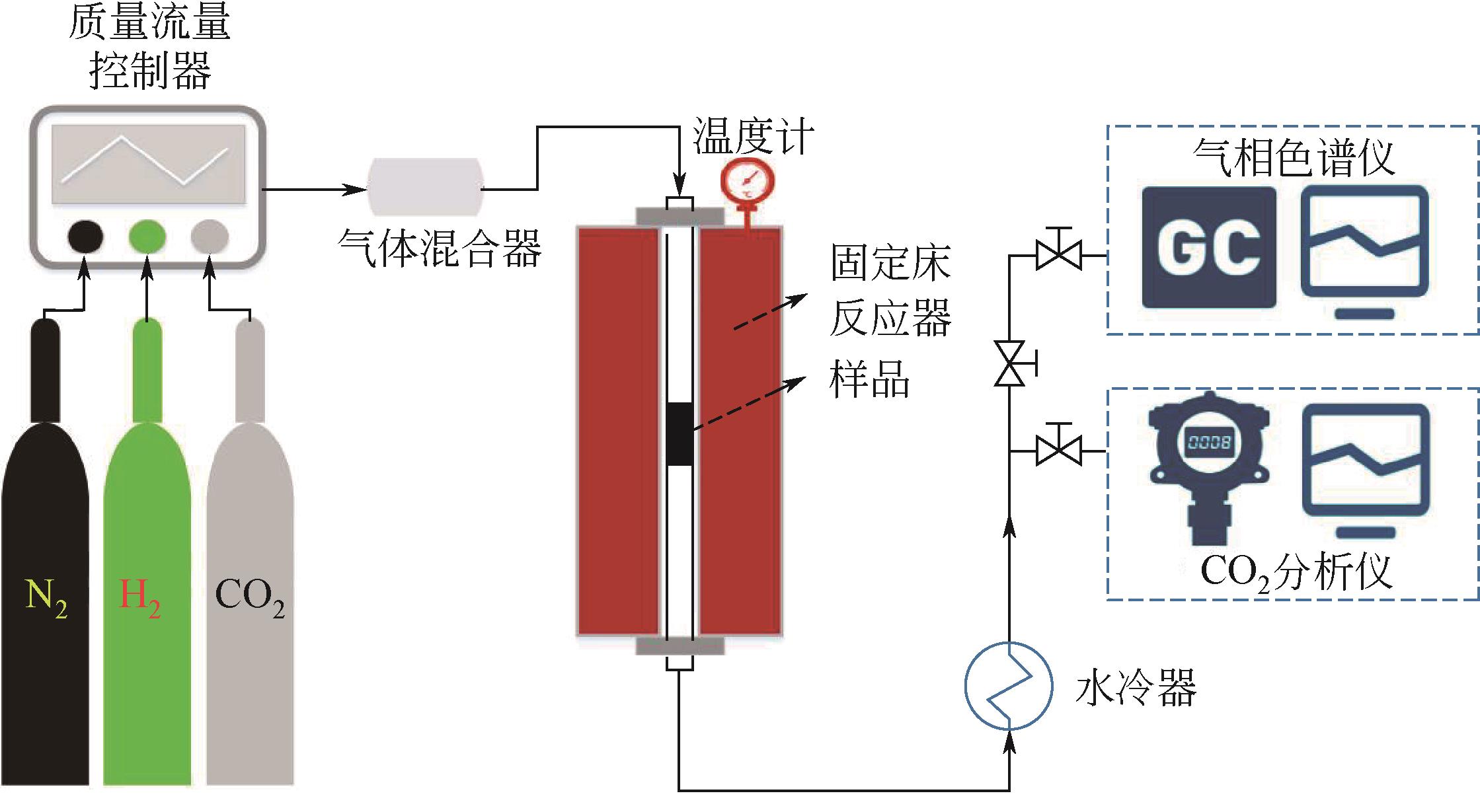
化工进展 ›› 2021, Vol. 40 ›› Issue (12): 6925-6933.DOI: 10.16085/j.issn.1000-6613.2020-2553
碱/碱土金属修饰Ni基催化剂的CO2吸附与甲烷化性能
王国栋( ), 郭亚飞(
), 郭亚飞( ), 李佳媛, 姚睿璇, 孙健, 赵传文(
), 李佳媛, 姚睿璇, 孙健, 赵传文( )
)
- 南京师范大学能源与机械工程学院,江苏 南京 210046
-
收稿日期:2020-12-23修回日期:2021-03-06出版日期:2021-12-05发布日期:2021-12-21 -
通讯作者:郭亚飞,赵传文 -
作者简介:王国栋(1995—),男,硕士研究生,研究方向为CO2捕集与转化利用。E-mail:1041890465@qq.com 。 -
基金资助:国家自然科学基金(51806108);能源清洁利用国家重点实验室开放基金(ZJUCEU2020009);江苏省研究生科研创新项目(KYCX21-1379)
CO2 adsorption and methanation performance of nickel-based catalysts modified with alkali/alkaline-earth metals
WANG Guodong( ), GUO Yafei(
), GUO Yafei( ), LI Jiayuan, YAO Ruixuan, SUN Jian, ZHAO Chuanwen(
), LI Jiayuan, YAO Ruixuan, SUN Jian, ZHAO Chuanwen( )
)
- School of Energy and Mechanical Engineering, Nanjing Normal University, Nanjing 210046, Jiangsu, China
-
Received:2020-12-23Revised:2021-03-06Online:2021-12-05Published:2021-12-21 -
Contact:GUO Yafei,ZHAO Chuanwen
摘要:
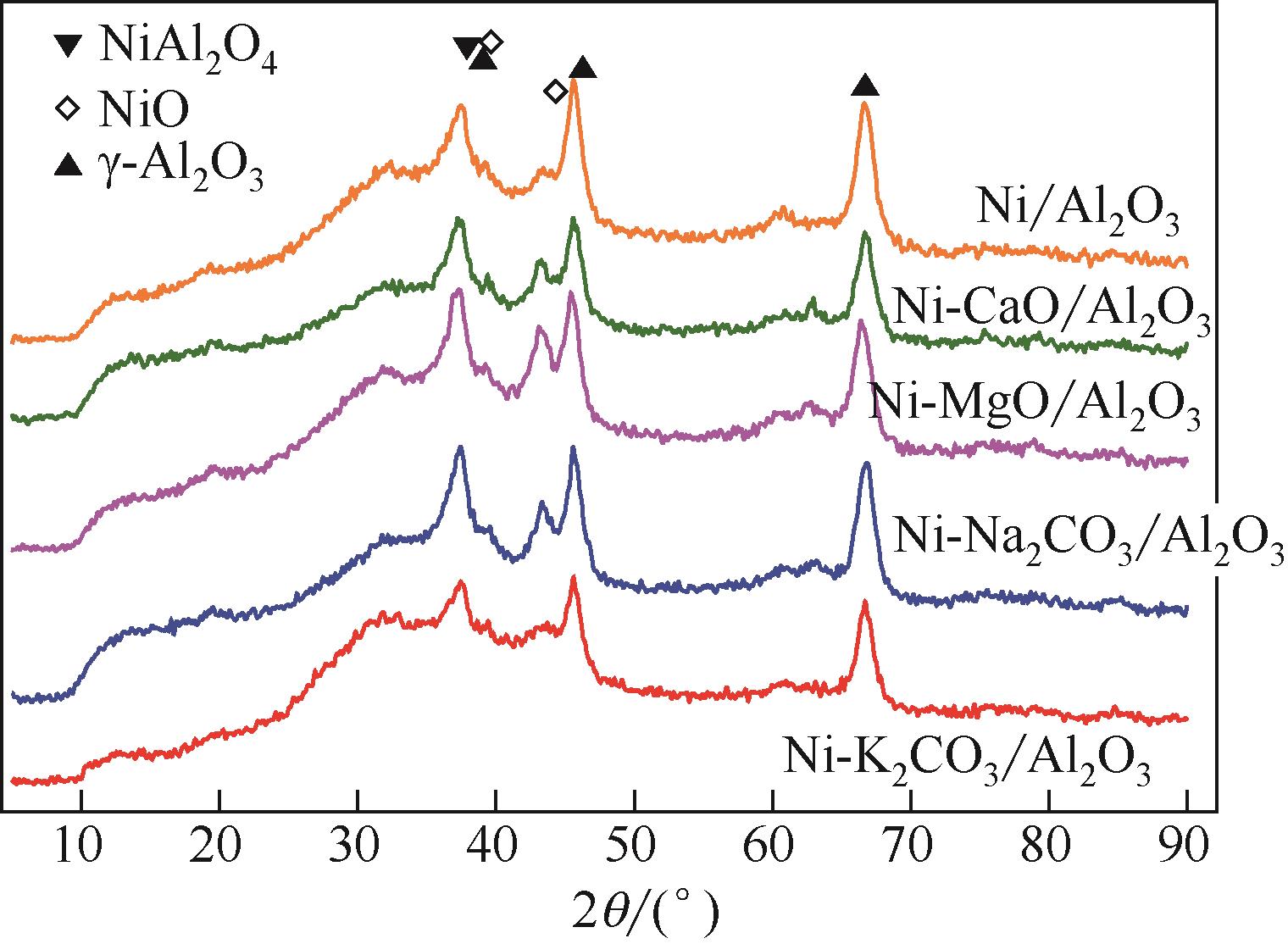
采用分步浸渍法制备了碱/碱土金属修饰Ni基催化剂Ni-M/Al2O3 (M=K2CO3, Na2CO3, MgO, CaO)。探究了碱/碱土金属的添加对改性Ni基催化剂CO2吸附和甲烷化性能的影响。研究发现,碱/碱土金属的添加提高了Ni/Al2O3催化剂表面的碱性活性位点密度,强化了其CO2吸附性能。碱/碱土金属类型影响Ni-M/Al2O3催化剂碱性活性位点的分布、NiO物相的转化及Ni的分散度,进而影响其甲烷化性能。MgO添加使NiO物相转化为与载体呈强相互作用的β型和γ型NiO,降低了催化剂表面的强碱性活性位点比例,有利于CO2吸附活化。Ni-MgO/Al2O3的CO2吸附容量最高为0.68mmolCO2/g,其CO2转化率和CH4选择性分别高达58.4%和95.4%,其在烟气CO2捕集与原位甲烷化中极具应用前景。
中图分类号:
引用本文
王国栋, 郭亚飞, 李佳媛, 姚睿璇, 孙健, 赵传文. 碱/碱土金属修饰Ni基催化剂的CO2吸附与甲烷化性能[J]. 化工进展, 2021, 40(12): 6925-6933.
WANG Guodong, GUO Yafei, LI Jiayuan, YAO Ruixuan, SUN Jian, ZHAO Chuanwen. CO2 adsorption and methanation performance of nickel-based catalysts modified with alkali/alkaline-earth metals[J]. Chemical Industry and Engineering Progress, 2021, 40(12): 6925-6933.
| 催化剂 | 比表面积/m2?g-1 | 孔体积/cm3?g-1 | 平均孔径/nm |
|---|---|---|---|
| Ni-K2CO3/Al2O3 | 88.1 | 0.19 | 7.6 |
| Ni-Na2CO3/Al2O3 | 91.7 | 0.19 | 7.9 |
| Ni-MgO/Al2O3 | 52.0 | 0.14 | 9.4 |
| CaO-Ni/Al2O3 | 85.2 | 0.18 | 8.1 |
| Ni/Al2O3 | 121.8 | 0.26 | 7.4 |
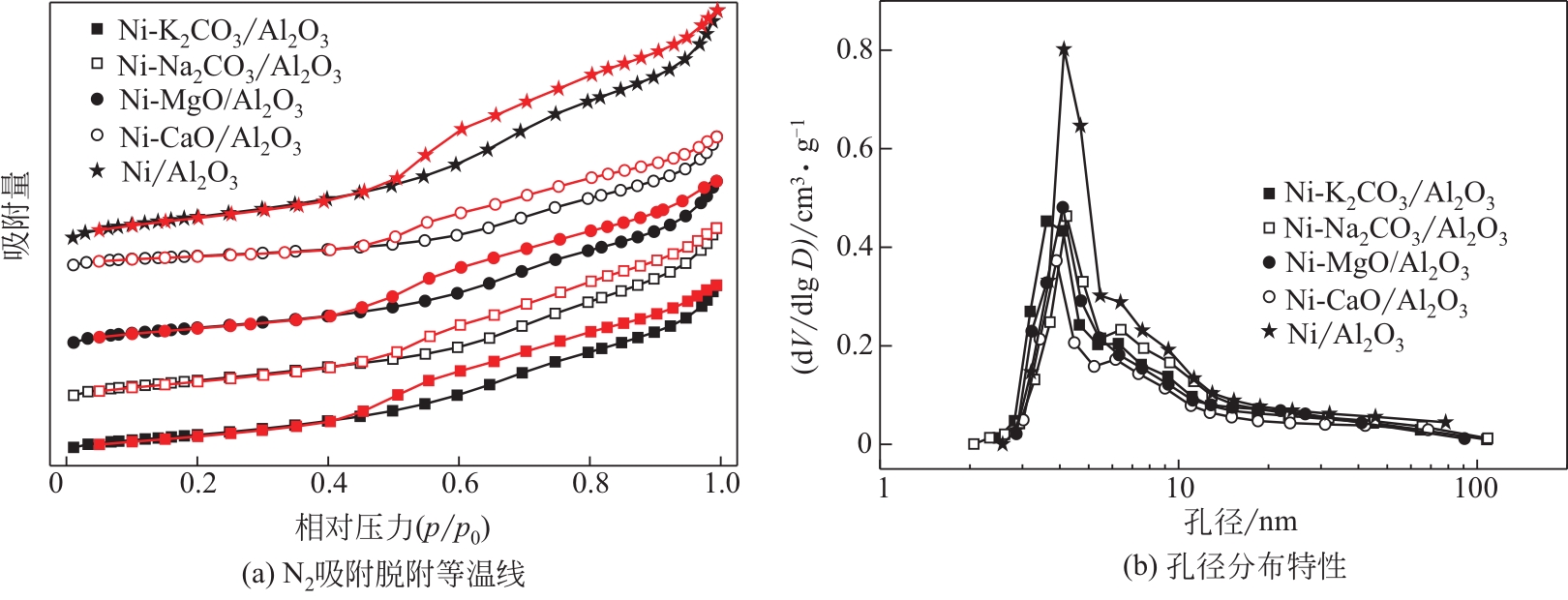
表1 不同催化剂的微观结构参数
| 催化剂 | 比表面积/m2?g-1 | 孔体积/cm3?g-1 | 平均孔径/nm |
|---|---|---|---|
| Ni-K2CO3/Al2O3 | 88.1 | 0.19 | 7.6 |
| Ni-Na2CO3/Al2O3 | 91.7 | 0.19 | 7.9 |
| Ni-MgO/Al2O3 | 52.0 | 0.14 | 9.4 |
| CaO-Ni/Al2O3 | 85.2 | 0.18 | 8.1 |
| Ni/Al2O3 | 121.8 | 0.26 | 7.4 |
| 催化剂 | 温度/℃ | 耗氢量/% | ||||
|---|---|---|---|---|---|---|
| α | β | γ | α | β | γ | |
| NiO/Al2O3 | 493 | 630 | — | 81.1 | 18.9 | — |
| Ni-CaO/Al2O3 | 501 | — | 746 | 81.7 | — | 18.3 |
| Ni-MgO/Al2O3 | — | 683 | 763 | — | 59.0 | 41.0 |
| Ni-Na2CO3/Al2O3 | 457 | 549/709 | — | 59.2 | 40.8 | — |
| Ni-K2CO3/Al2O3 | 433 | 709 | — | 81.1 | 18.9 | — |
表2 不同催化剂的H2-TPR定量分析结果
| 催化剂 | 温度/℃ | 耗氢量/% | ||||
|---|---|---|---|---|---|---|
| α | β | γ | α | β | γ | |
| NiO/Al2O3 | 493 | 630 | — | 81.1 | 18.9 | — |
| Ni-CaO/Al2O3 | 501 | — | 746 | 81.7 | — | 18.3 |
| Ni-MgO/Al2O3 | — | 683 | 763 | — | 59.0 | 41.0 |
| Ni-Na2CO3/Al2O3 | 457 | 549/709 | — | 59.2 | 40.8 | — |
| Ni-K2CO3/Al2O3 | 433 | 709 | — | 81.1 | 18.9 | — |
| 催化剂 | H2吸附量 /mL?g-1 | Ni分散度 /% | Ni活性比表面积 /m2?g-1 |
|---|---|---|---|
| Ni-K2CO3/Al2O3 | 0.59 | 3.1 | 20.7 |
| Ni-Na2CO3/Al2O3 | 0.64 | 3.3 | 22.2 |
| Ni-MgO/Al2O3 | 0.71 | 3.7 | 24.9 |
| Ni-CaO/Al2O3 | 0.68 | 3.5 | 23.8 |
| Ni/Al2O3 | 0.93 | 4.9 | 32.6 |
表3 动态脉冲H2化学吸附定量分析结果
| 催化剂 | H2吸附量 /mL?g-1 | Ni分散度 /% | Ni活性比表面积 /m2?g-1 |
|---|---|---|---|
| Ni-K2CO3/Al2O3 | 0.59 | 3.1 | 20.7 |
| Ni-Na2CO3/Al2O3 | 0.64 | 3.3 | 22.2 |
| Ni-MgO/Al2O3 | 0.71 | 3.7 | 24.9 |
| Ni-CaO/Al2O3 | 0.68 | 3.5 | 23.8 |
| Ni/Al2O3 | 0.93 | 4.9 | 32.6 |
| 催化剂 | 弱碱性位点(<200℃)/% | 中碱性位点(200~420℃)/% | 强碱性位点(>450℃)/% | 碱性位点密度/μmol?m-2 |
|---|---|---|---|---|
| Ni-K2CO3/Al2O3 | 39.8 | 26.7 | 33.5 | 3.7 |
| Ni-Na2CO3/Al2O3 | 15.5 | 54.5 | 30.0 | 3.7 |
| Ni-MgO/Al2O3 | 23.7 | 64.6 | 11.7 | 3.6 |
| Ni-CaO/Al2O3 | 12.5 | 63.4 | 24.1 | 3.3 |
| Ni/Al2O3 | 34.7 | 23.9 | 41.4 | 2.1 |
表4 催化剂的CO2-TPD定量计算结果
| 催化剂 | 弱碱性位点(<200℃)/% | 中碱性位点(200~420℃)/% | 强碱性位点(>450℃)/% | 碱性位点密度/μmol?m-2 |
|---|---|---|---|---|
| Ni-K2CO3/Al2O3 | 39.8 | 26.7 | 33.5 | 3.7 |
| Ni-Na2CO3/Al2O3 | 15.5 | 54.5 | 30.0 | 3.7 |
| Ni-MgO/Al2O3 | 23.7 | 64.6 | 11.7 | 3.6 |
| Ni-CaO/Al2O3 | 12.5 | 63.4 | 24.1 | 3.3 |
| Ni/Al2O3 | 34.7 | 23.9 | 41.4 | 2.1 |
| 1 | LEE S C, CHO M S, JUNG S Y, et al. Effects of alumina phases on CO2 sorption and regeneration properties of potassium-based alumina sorbents[J]. Adsorption, 2014, 20(2/3): 331-339. |
| 2 | MEUL S, DAMERIS M, LANGEMATZ U, et al. Impact of rising greenhouse gas concentrations on future tropical ozone and UV exposure[J]. Geophysical Research Letters, 2016, 43(6): 2919-2927. |
| 3 | DOWELL N MAC, FENNELL P S, SHAH N, et al. The role of CO2 capture and utilization in mitigating climate change[J]. Nature Climate Change, 2017, 7(4): 243-249. |
| 4 | GÖTZ M, LEFEBVRE J, MÖRS F, et al. Renewable power-to-gas: a technological and economic review[J]. Renewable Energy, 2016, 85: 1371-1390. |
| 5 | CIMINO S, BOCCIA F, LISI L. Effect of alkali promoters (Li, Na, K) on the performance of Ru/Al2O3 catalysts for CO2 capture and hydrogenation to methane[J]. Journal of CO2 Utilization, 2020, 37: 195-203. |
| 6 | AZIZ M A A, JALIL A A, TRIWAHYONO S, et al. CO2 methanation over heterogeneous catalysts: recent progress and future prospects[J]. Green Chemistry, 2015, 17(5): 2647-2663. |
| 7 | GHAIB K, NITZ K, BEN-FARES F Z. Chemical methanation of CO2: a review[J]. ChemBioEng Reviews, 2016, 3(6): 266-275. |
| 8 | GAO J J, WANG Y L, PING Y, et al. A thermodynamic analysis of methanation reactions of carbon oxides for the production of synthetic natural gas[J]. RSC Advances, 2012, 2(6): 2358. |
| 9 | 孟凡会, 常慧蓉, 李忠. Ni-Mn/Al2O3催化剂在浆态床中CO甲烷化催化性能[J]. 化工学报, 2014, 65(8): 2997-3003. |
| MENG F H, CHANG H R, LI Z. Catalytic performance of Ni-Mn/Al2O3 catalyst for CO methanation in slurry-bed reactor[J]. CIESC Journal, 2014, 65(8): 2997-3003. | |
| 10 | GAO J J, LIU Q, GU F N, et al. Recent advances in methanation catalysts for the production of synthetic natural gas[J]. RSC Advances, 2015, 5(29): 22759-22776. |
| 11 | FRONTERA P, MACARIO A, FERRARO M, et al. Supported catalysts for CO2 methanation: a review[J]. Catalysts, 2017, 7(12): 59. |
| 12 | REN J, QIN X, YANG J Z, et al. Methanation of carbon dioxide over Ni-M/ZrO2 (M = Fe, Co, Cu) catalysts: effect of addition of a second metal[J]. Fuel Processing Technology, 2015, 137: 204-211. |
| 13 | ALDANA P A U, OCAMPO F, KOBL K, et al. Catalytic CO2 valorization into CH4 on Ni-based ceria-zirconia. Reaction mechanism by operando IR spectroscopy[J]. Catalysis Today, 2013, 215: 201-207. |
| 14 | WANG S D, PAN Q S, PENG J X, et al. In situ FTIR spectroscopic study of the CO2 methanation mechanism on Ni/Ce0.5Zr0.5O2[J]. Catalysis Science and Technology, 2014, 4(2): 502-509. |
| 15 | WANG W, GONG J L. Methanation of carbon dioxide: an overview[J]. Frontiers of Chemical Science and Engineering, 2011, 5(1): 2-10. |
| 16 | 王东旭, 肖显斌, 高静, 等. 助剂钾对镍基催化剂性能影响研究进展[J]. 化工进展, 2014, 33(3): 668-672. |
| WANG D X,XIAO X B,GAO J,et al. A review of potassium promoter effect on nickel-based catalyst performance[J]. Chemical Industry and Engineering Progress, 2014, 33(3): 668-672. | |
| 17 | OSAKI T, MORI T. Role of potassium in carbon-free CO2 reforming of methane on K-promoted Ni/Al2O3 catalysts[J]. Journal of Catalysis, 2001, 204(1): 89-97. |
| 18 | ANG M L, OEMAR U, SAW E T, et al. Highly active Ni/xNa/CeO2 catalyst for the water-gas shift reaction: effect of sodium on methane suppression[J]. ACS Catalysis, 2014, 4(9): 3237-3248. |
| 19 | XU L L, WANG F G, CHEN M D, et al. Alkaline-promoted Ni based ordered mesoporous catalysts with enhanced low-temperature catalytic activity toward CO2 methanation[J]. RSC Advances, 2017, 7(30): 18199-18210. |
| 20 | TAN J J, WANG J M, ZHANG Z Y, et al. Highly dispersed and stable Ni nanoparticles confined by MgO on ZrO2 for CO2 methanation[J]. Applied Surface Science, 2019, 481: 1538-1548. |
| 21 | GUO M, LU G X. The difference of roles of alkaline-earth metal oxides on silica-supported nickel catalysts for CO2 methanation[J]. RSC Advances, 2014, 4(102): 58171-58177. |
| 22 | YANG W, FENG Y Y, CHU W. Promotion effect of CaO modification on mesoporous Al2O3-supported Ni catalysts for CO2 methanation[J]. International Journal of Chemical Engineering, 2016, 2016: 1-7. |
| 23 | CHENG C B, SHEN D K, XIAO R, et al. Methanation of syngas (H2/CO) over the different Ni-based catalysts[J]. Fuel, 2017, 189: 419-427. |
| 24 | ZHANG L J, BIAN L, ZHU Z T, et al. La-promoted Ni/Mg-Al catalysts with highly enhanced low-temperature CO2 methanation performance[J]. International Journal of Hydrogen Energy, 2018, 43(4): 2197-2206. |
| 25 | BERMEJO-LÓPEZ A, PEREDA-AYO B, GONZÁLEZ-MARCOS J A, et al. Ni loading effects on dual function materials for capture and in situ conversion of CO2 to CH4 using CaO or Na2CO3[J]. Journal of CO2 Utilization, 2019, 34: 576-587. |
| 26 | BERMEJO-LÓPEZ A, PEREDA-AYO B, GONZÁLEZ-MARCOS J A, et al. Mechanism of the CO2 storage and in situ hydrogenation to CH4. Temperature and adsorbent loading effects over Ru-CaO/Al2O3 and Ru-Na2CO3/Al2O3 catalysts[J]. Applied Catalysis B: Environmental, 2019, 256: 117845. |
| 27 | JIMÉNEZ-GONZÁLEZ C, BOUKHA Z, DE RIVAS B, et al. Behavior of coprecipitated NiAl2O4/Al2O3 catalysts for low-temperature methane steam reforming[J]. Energy & Fuels, 2014, 28(11): 7109-7121. |
| 28 | ZHOU L, LI L D, WEI N N, et al. Corrigendum: effect of NiAl2O4 formation on Ni/Al2O3 stability during dry reforming of methane[J]. ChemCatChem, 2015, 7(16): 2406. |
| 29 | ZHAO A M, YING W Y, ZHANG H T, et al. Ni-Al2O3 catalysts prepared by solution combustion method for syngas methanation[J]. Catalysis Communications, 2012, 17: 34-38. |
| 30 | HU D C, GAO J J, PING Y, et al. Enhanced investigation of CO methanation over Ni/Al2O3 catalysts for synthetic natural gas production[J]. Industrial & Engineering Chemistry Research, 2012, 51(13): 4875-4886. |
| 31 | CUI D M, LIU J, YU J, et al. Necessity of moderate metal-support interaction in Ni/Al2O3 for syngas methanation at high temperatures[J]. RSC Advances, 2015, 5(14): 10187-10196. |
| 32 | SONG K H, YAN X, KOH D J, et al. La effect on the long-term durability of Ni-Mg-Al2O3 catalysts for syngas methanation[J]. Applied Catalysis A: General, 2017, 530: 184-192. |
| 33 | XU L L, YANG H M, CHEN M D, et al. CO2 methanation over Ca doped ordered mesoporous Ni-Al composite oxide catalysts: the promoting effect of basic modifier[J]. Journal of CO2 Utilization, 2017, 21: 200-210. |
| 34 | SONG G, DING Y D, ZHU X, et al. Carbon dioxide adsorption characteristics of synthesized MgO with various porous structures achieved by varying calcination temperature[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2015, 470: 39-45. |
| 35 | ASHOK J, KATHIRASER Y, ANG M L, et al. Bi-functional hydrotalcite-derived NiO-CaO-Al2O3 catalysts for steam reforming of biomass and/or tar model compound at low steam-to-carbon conditions[J]. Applied Catalysis B: Environmental, 2015, 172/173: 116-128. |
| 36 | TAN C, GUO Y F, SUN J, et al. Structurally improved MgO adsorbents derived from magnesium oxalate precursor for enhanced CO2 capture[J]. Fuel, 2020, 278: 118379. |
| 37 | PAN Q S, PENG J X, SUN T J, et al. CO2 methanation on Ni/Ce0.5Zr0.5O2 catalysts for the production of synthetic natural gas[J]. Fuel Processing Technology, 2014, 123: 166-171. |
| 38 | SHOKROLLAHI Y M, RADFARNIA H R, ILIUTA M C. High temperature CO2 sorbents and their application for hydrogen production by sorption enhanced steam reforming process[J]. Chemical Engineering Journal, 2016, 283: 420-444. |
| 39 | ZHOU Z J, SUN N N, WANG B D, et al. 2D-layered Ni-MgO-Al2O3 nanosheets for integrated capture and methanation of CO2[J]. ChemSusChem, 2020, 13(2): 360-368. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [6] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [7] | 李化全, 王明华, 邱贵宝. 硫酸酸解钙钛矿相精矿的行为[J]. 化工进展, 2023, 42(S1): 536-541. |
| [8] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [9] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [10] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [11] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [12] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [13] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [14] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [15] | 朱传强, 茹晋波, 孙亭亭, 谢兴旺, 李长明, 高士秋. 固体高分子脱硝剂选择性非催化还原NO x 特性[J]. 化工进展, 2023, 42(9): 4939-4946. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||