化工进展 ›› 2021, Vol. 40 ›› Issue (11): 6181-6194.DOI: 10.16085/j.issn.1000-6613.2020-2274
有机溶剂纳滤传递模型及最新膜材料研究进展
金业豪1,2( ), 冯孝权1,2, 朱军勇1,2(
), 冯孝权1,2, 朱军勇1,2( ), 张亚涛1,2(
), 张亚涛1,2( )
)
- 1.郑州大学化工学院,河南 郑州 450001
2.郑州市先进分离技术重点实验室,河南 郑州 450001
-
收稿日期:2020-11-16修回日期:2021-02-01出版日期:2021-11-05发布日期:2021-11-19 -
通讯作者:朱军勇,张亚涛 -
作者简介:金业豪(1997—),男,硕士研究生,研究方向为有机溶剂纳滤膜分离。E-mail:brucejinyehao@163.com 。 -
基金资助:国家自然科学基金(22178327)
Research progress in transfer models and membrane materials for organic solvent nanofiltration
JIN Yehao1,2( ), FENG Xiaoquan1,2, ZHU Junyong1,2(
), FENG Xiaoquan1,2, ZHU Junyong1,2( ), ZHANG Yatao1,2(
), ZHANG Yatao1,2( )
)
- 1.School of Chemical Engineering, Zhengzhou University, Zhengzhou 450001, Henan, China
2.Zhengzhou Key Laboratory of Advanced Separation Technology, Zhengzhou 450001, Henan, China
-
Received:2020-11-16Revised:2021-02-01Online:2021-11-05Published:2021-11-19 -
Contact:ZHU Junyong,ZHANG Yatao
摘要:
有机溶剂纳滤(organic solvent nanofiltration, OSN)是一种高效节能、操作简便的新型膜分离技术,在化工、制药、能源和环境等相关领域具有广阔的应用前景,因此受到了膜技术领域内研究者们的重点关注。本文首先简述了有机溶剂纳滤的应用背景,其次从有机溶剂纳滤传递模型与膜材料两个方面,总结归纳了近年来在有机溶剂纳滤领域取得的研究进展。总结了基于无机陶瓷、高分子聚合物、多孔有机聚合物、有机-无机杂化材料以及石墨烯类二维材料等用于制备新型OSN膜的研究进展,并结合传输模型讨论分析了有机溶剂分子在膜内的传输行为及膜的分离性能。最后简述了有机溶剂纳滤技术在化工及相关行业中的应用现状,并指出了这些关键有机溶剂纳滤膜材料在有机溶剂纳滤应用中存在的优势和挑战,提出了基于这些关键材料的特点进行设计和优化OSN膜性能的建议供参考,以期促进有机溶剂纳滤膜的研究和应用。
中图分类号:
引用本文
金业豪, 冯孝权, 朱军勇, 张亚涛. 有机溶剂纳滤传递模型及最新膜材料研究进展[J]. 化工进展, 2021, 40(11): 6181-6194.
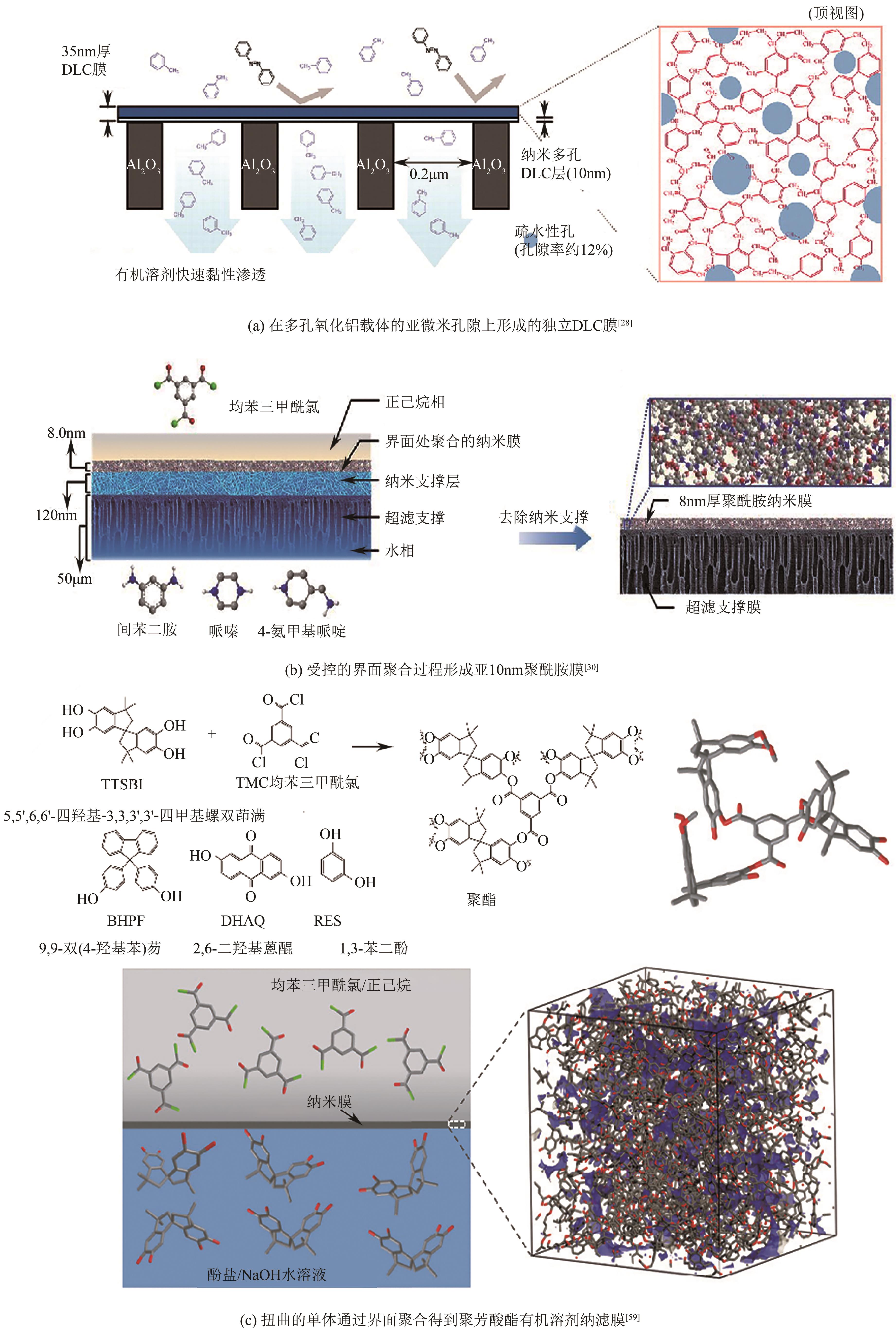
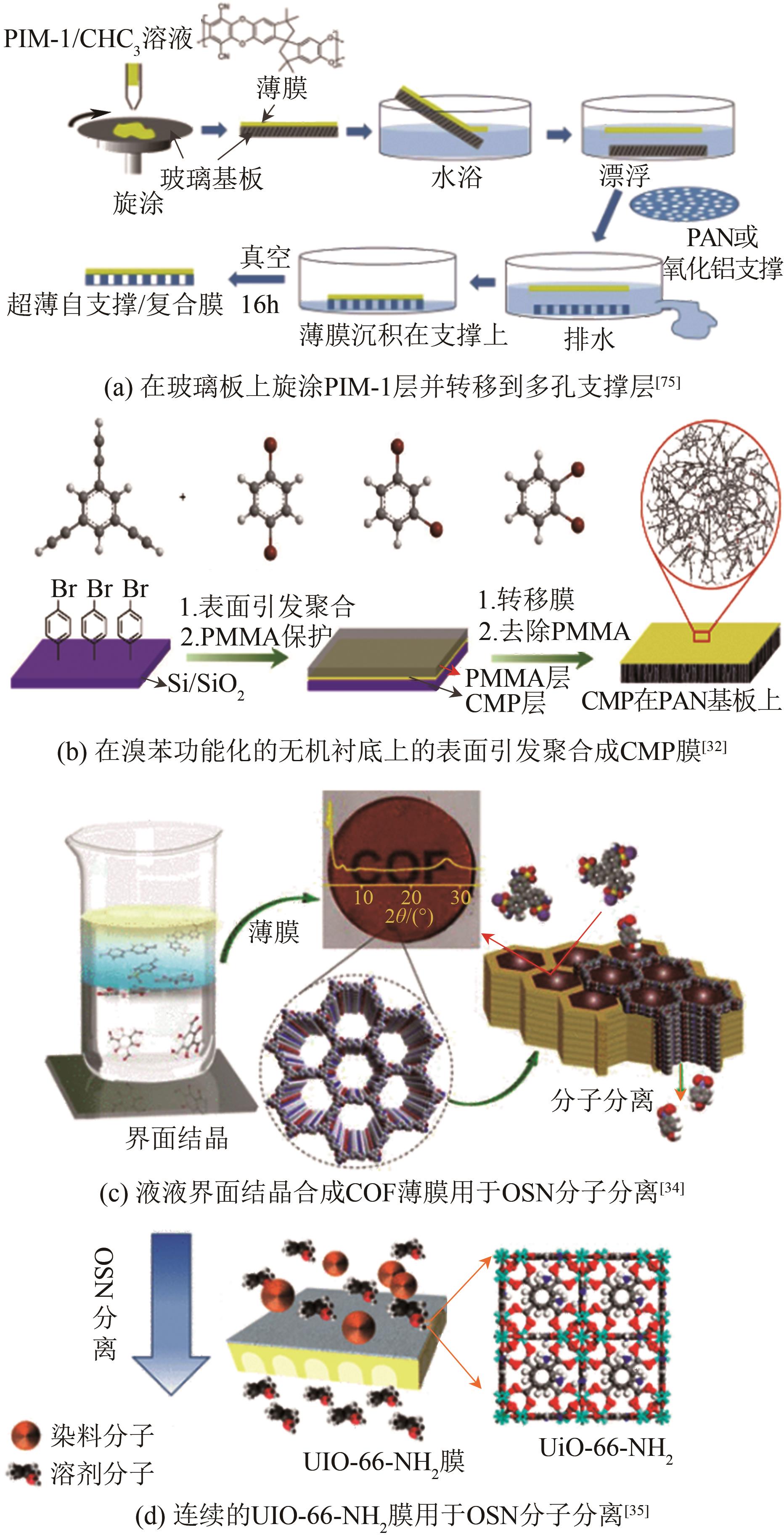
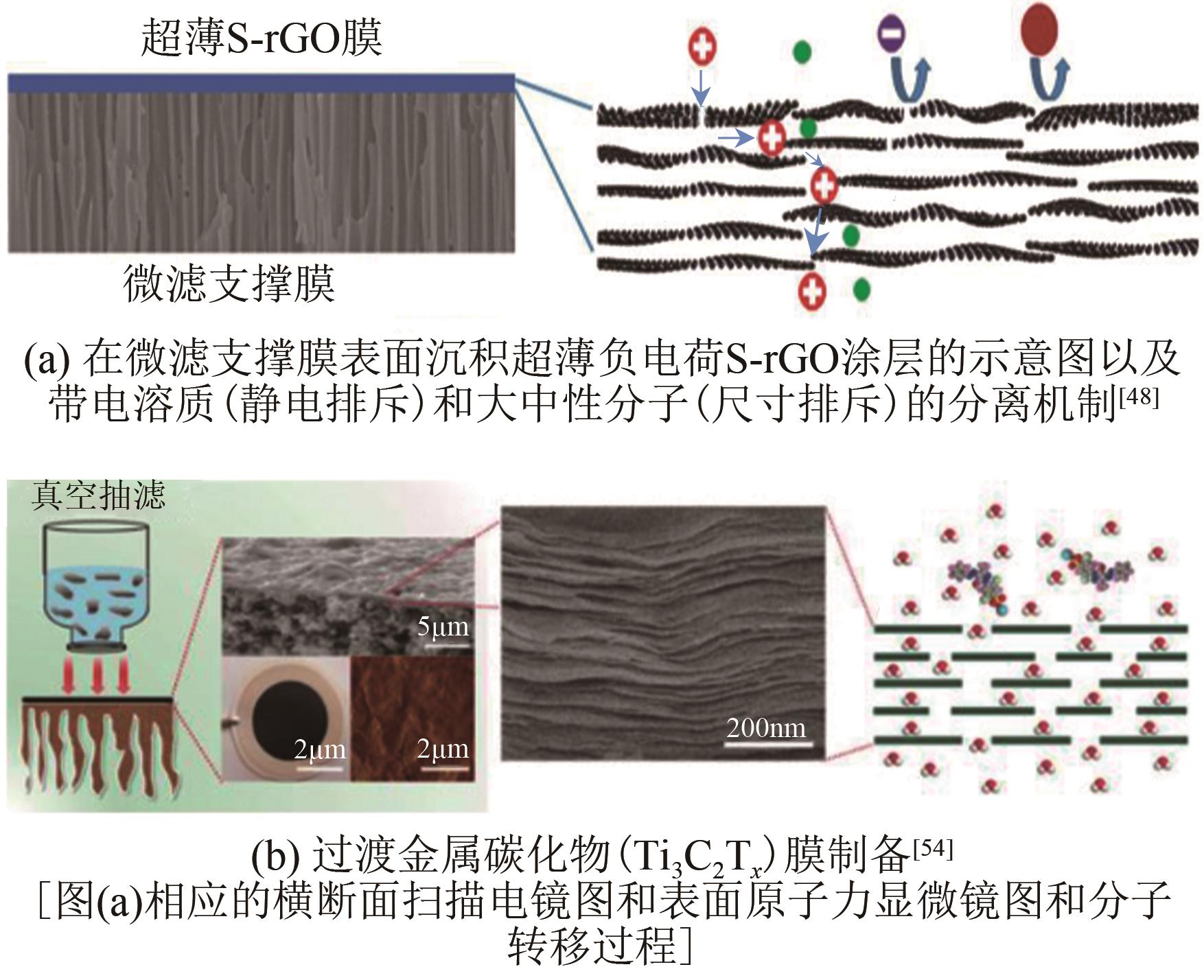
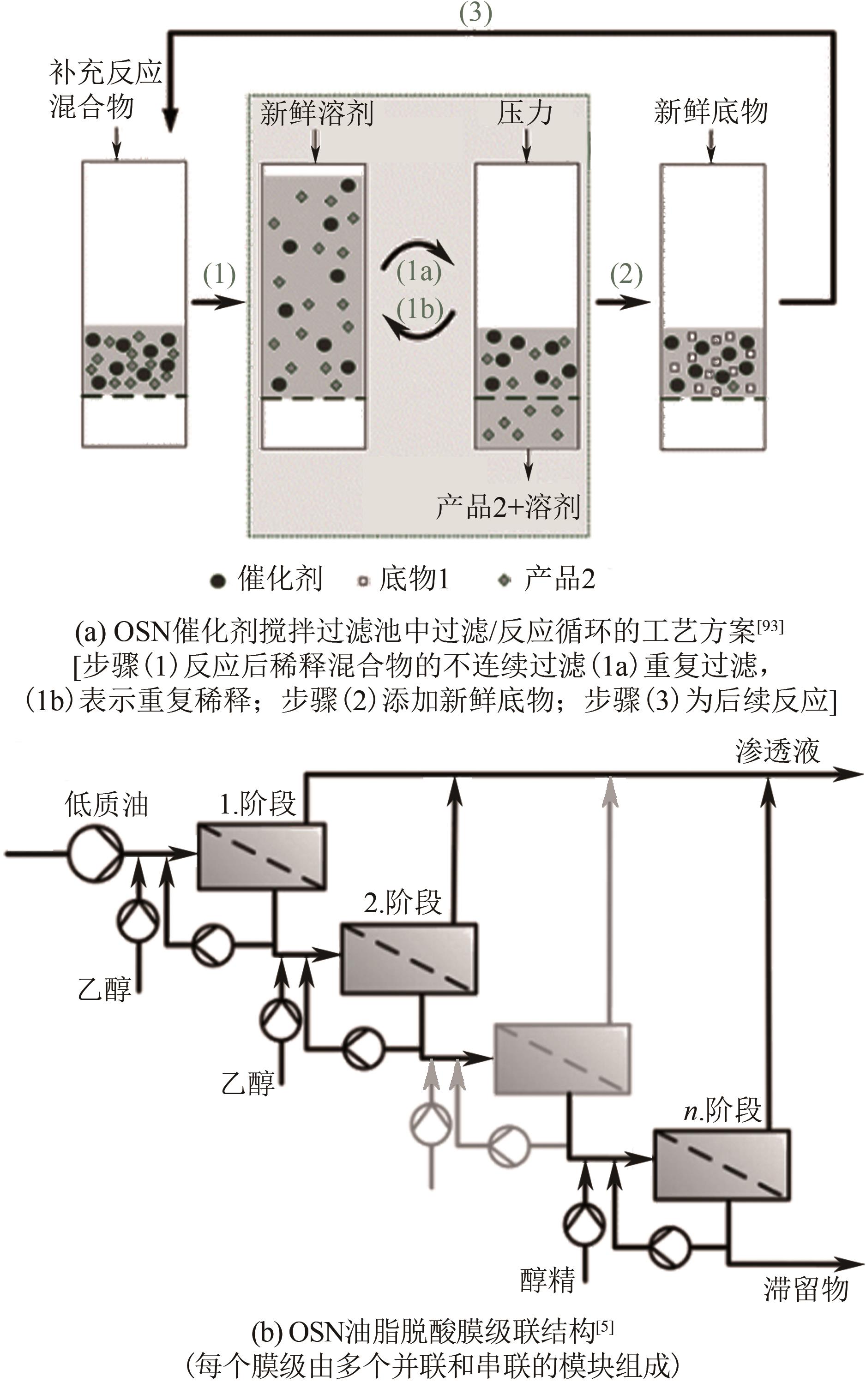
JIN Yehao, FENG Xiaoquan, ZHU Junyong, ZHANG Yatao. Research progress in transfer models and membrane materials for organic solvent nanofiltration[J]. Chemical Industry and Engineering Progress, 2021, 40(11): 6181-6194.
| 传输模型 | 传输机制 | 控制参数 |
|---|---|---|
| 不可逆热力学模型 | ||
| Kedem-Katchalsky模型 | 扩散+对流 | Pi,LV,σi |
| Spiegler-Kedemd模型 | 扩散+对流 | Pi,LV,σi |
| 溶解扩散模型 | ||
| 经典溶解扩散模型 | 扩散 | Pi,Pj |
| “简单”溶解扩散模型 | 扩散 | Pi,Pj |
| Maxwell-Stefan模型 | 多组分扩散 | Di,Ki |
| 孔流模型 | ||
| 哈根泊稷叶模型 | 对流 | rp,l,ε,τ |
| 道南空间孔流模型 | 扩散+对流+静电相互作用 | rp,l,ε,τ,Ψ |
| 修正的表面力孔流模型 | 扩散+对流+静电/亲和作用 | rp,l,ε,τ,φ |
表1 OSN的传输模型对应的传输机制、传输方程和模型参数
| 传输模型 | 传输机制 | 控制参数 |
|---|---|---|
| 不可逆热力学模型 | ||
| Kedem-Katchalsky模型 | 扩散+对流 | Pi,LV,σi |
| Spiegler-Kedemd模型 | 扩散+对流 | Pi,LV,σi |
| 溶解扩散模型 | ||
| 经典溶解扩散模型 | 扩散 | Pi,Pj |
| “简单”溶解扩散模型 | 扩散 | Pi,Pj |
| Maxwell-Stefan模型 | 多组分扩散 | Di,Ki |
| 孔流模型 | ||
| 哈根泊稷叶模型 | 对流 | rp,l,ε,τ |
| 道南空间孔流模型 | 扩散+对流+静电相互作用 | rp,l,ε,τ,Ψ |
| 修正的表面力孔流模型 | 扩散+对流+静电/亲和作用 | rp,l,ε,τ,φ |
| 溶剂 | 摩尔质量/g·mol-1 | 密度/g·mL-1 | 动力学半径/nm | 黏度/mPa·s | 相对极性 | Hansen溶解度参数 |
|---|---|---|---|---|---|---|
| 甲醇 | 32 | 0.791 | 0.38 | 0.544 | 0.762 | 29.7 |
| 乙醇 | 46 | 0.789 | 0.49 | 1.074 | 0.309 | 20.3 |
| 异丙醇 | 60 | 0.785 | 0.47 | 2.038 | 0.546 | 24.6 |
| 正丁醇 | 74 | 0.810 | 0.50 | 2.544 | 0.586 | 23.1 |
| 乙腈 | 41 | 0.786 | 0.34 | 0.369 | 0.46 | 24.4 |
| 四氢呋喃 | 72 | 0.866 | 0.48 | 0.456 | 0.207 | 19.4 |
| 丙酮 | 58 | 0.786 | 0.47 | 0.306 | 0.355 | 20.1 |
| 二甲基甲酰胺 | 73 | 0.944 | 0.50 | 0.816 | 0.386 | 24.8 |
| 水 | 18 | 1 | 0.27 | 0.89 | 1 | 47.8 |
| 正庚烷 | 100 | 0.684 | 0.75 | 0.4 | 0.012 | 15.3 |
| 正己烷 | 86 | 0.655 | 0.75 | 0.300 | 0.009 | 14.9 |
| 甲苯 | 92 | 0.867 | 0.55 | 0.555 | 0.099 | 18.2 |
| 二氯甲烷 | 85 | 1.326 | 0.49 | 0.414 | 0.309 | 20.3 |
| 环己烷 | 84 | 0.799 | 1.020 | — | — | 16.8 |
表2 常见有机溶剂的物理化学性质
| 溶剂 | 摩尔质量/g·mol-1 | 密度/g·mL-1 | 动力学半径/nm | 黏度/mPa·s | 相对极性 | Hansen溶解度参数 |
|---|---|---|---|---|---|---|
| 甲醇 | 32 | 0.791 | 0.38 | 0.544 | 0.762 | 29.7 |
| 乙醇 | 46 | 0.789 | 0.49 | 1.074 | 0.309 | 20.3 |
| 异丙醇 | 60 | 0.785 | 0.47 | 2.038 | 0.546 | 24.6 |
| 正丁醇 | 74 | 0.810 | 0.50 | 2.544 | 0.586 | 23.1 |
| 乙腈 | 41 | 0.786 | 0.34 | 0.369 | 0.46 | 24.4 |
| 四氢呋喃 | 72 | 0.866 | 0.48 | 0.456 | 0.207 | 19.4 |
| 丙酮 | 58 | 0.786 | 0.47 | 0.306 | 0.355 | 20.1 |
| 二甲基甲酰胺 | 73 | 0.944 | 0.50 | 0.816 | 0.386 | 24.8 |
| 水 | 18 | 1 | 0.27 | 0.89 | 1 | 47.8 |
| 正庚烷 | 100 | 0.684 | 0.75 | 0.4 | 0.012 | 15.3 |
| 正己烷 | 86 | 0.655 | 0.75 | 0.300 | 0.009 | 14.9 |
| 甲苯 | 92 | 0.867 | 0.55 | 0.555 | 0.099 | 18.2 |
| 二氯甲烷 | 85 | 1.326 | 0.49 | 0.414 | 0.309 | 20.3 |
| 环己烷 | 84 | 0.799 | 1.020 | — | — | 16.8 |
| 膜材料 | 膜 | 溶剂渗透 | 溶质截留 | 使用或推荐使用 模型 | 参考 文献 | |||
|---|---|---|---|---|---|---|---|---|
| 分离层 | 支撑层 | 溶剂 | 渗透性 /L·m-2·h-1·bar-1 | 溶质 | 截留率 /% | |||
| 无机材料 | APTES接枝的γ-Al2O3 | α-Al2O3 | 甲苯 | 3.1 | 苏丹黑B | 72 | 不可逆热力学模型 | [ |
| MPTES接枝的γ-Al2O3 | α-Al2O3 | 异丙醇 | 0.78 | 苏丹黑B | 66 | 不可逆热力学模型 | [ | |
| 高分子聚合物材料 | 聚酰亚胺 | 异丙醇 | 2.7 | 玫瑰红 | 95 | 溶解扩散模型 | [ | |
| 类金刚石碳膜 | 聚丙烯腈 | 乙醇 | 84.1 | 偶氮苯 | 94.4 | 孔流模型 | [ | |
| 聚酰胺 | 聚丙烯腈 | 甲醇 | 13.3 | 甲基橙 | 97.7 | 溶解扩散模型 | [ | |
| 多孔有机材料 | 共轭微孔聚合物 | 聚丙烯腈 | 乙醇 | 13.8 | 玫瑰红 | 99 | 孔流模型 | [ |
| 自具微孔聚合物 | 聚丙烯腈 | 乙醇 | 4.3 | 甲基橙 | 93 | 溶解扩散模型 | [ | |
| Tp-BPY | 无纺布 | 甲醇 | 108 | 酸性品红 | 97 | 孔流模型 | [ | |
| M-TpBD | 乙醇 | 86.5 | 刚果红 | 96 | 孔流模型 | [ | ||
| 有机-无机杂化材料 | ZIF-8/PA | 聚酰亚胺 | 甲醇 | 2.5 | 聚苯乙烯 | 90 | 溶解扩散模型 | [ |
| UIO-66-NH2 | 聚酰亚胺 | 乙醇 | 0.88 | 玫瑰红 | 96.3 | 孔流模型 | [ | |
| 石墨烯类二维材料 | 还原氧化石墨烯 | 尼龙 | 甲醇 | 75.3 | 伊文思蓝/960 | 100 | 孔流模型 | [ |
| MXenes | 尼龙 | 异丙醇 | 983 | 酸性黄79 | 100 | 孔流模型 | [ | |
表3 有机溶剂纳滤膜材料及相应的渗透过滤性能和传输模型
| 膜材料 | 膜 | 溶剂渗透 | 溶质截留 | 使用或推荐使用 模型 | 参考 文献 | |||
|---|---|---|---|---|---|---|---|---|
| 分离层 | 支撑层 | 溶剂 | 渗透性 /L·m-2·h-1·bar-1 | 溶质 | 截留率 /% | |||
| 无机材料 | APTES接枝的γ-Al2O3 | α-Al2O3 | 甲苯 | 3.1 | 苏丹黑B | 72 | 不可逆热力学模型 | [ |
| MPTES接枝的γ-Al2O3 | α-Al2O3 | 异丙醇 | 0.78 | 苏丹黑B | 66 | 不可逆热力学模型 | [ | |
| 高分子聚合物材料 | 聚酰亚胺 | 异丙醇 | 2.7 | 玫瑰红 | 95 | 溶解扩散模型 | [ | |
| 类金刚石碳膜 | 聚丙烯腈 | 乙醇 | 84.1 | 偶氮苯 | 94.4 | 孔流模型 | [ | |
| 聚酰胺 | 聚丙烯腈 | 甲醇 | 13.3 | 甲基橙 | 97.7 | 溶解扩散模型 | [ | |
| 多孔有机材料 | 共轭微孔聚合物 | 聚丙烯腈 | 乙醇 | 13.8 | 玫瑰红 | 99 | 孔流模型 | [ |
| 自具微孔聚合物 | 聚丙烯腈 | 乙醇 | 4.3 | 甲基橙 | 93 | 溶解扩散模型 | [ | |
| Tp-BPY | 无纺布 | 甲醇 | 108 | 酸性品红 | 97 | 孔流模型 | [ | |
| M-TpBD | 乙醇 | 86.5 | 刚果红 | 96 | 孔流模型 | [ | ||
| 有机-无机杂化材料 | ZIF-8/PA | 聚酰亚胺 | 甲醇 | 2.5 | 聚苯乙烯 | 90 | 溶解扩散模型 | [ |
| UIO-66-NH2 | 聚酰亚胺 | 乙醇 | 0.88 | 玫瑰红 | 96.3 | 孔流模型 | [ | |
| 石墨烯类二维材料 | 还原氧化石墨烯 | 尼龙 | 甲醇 | 75.3 | 伊文思蓝/960 | 100 | 孔流模型 | [ |
| MXenes | 尼龙 | 异丙醇 | 983 | 酸性黄79 | 100 | 孔流模型 | [ | |
| 36 | HUANG L, CHEN J, GAO T, et al. Reduced graphene oxide membranes for ultrafast organic solvent nanofiltration[J]. Advanced Materials, 2016, 28(39): 8669-8674. |
| 37 | WANG J T, CHEN P P, SHI B B, et al. A regularly channeled lamellar membrane for unparalleled water and organics permeation[J]. Angewandte Chemie International Edition, 2018, 57(23): 6814-6818. |
| 38 | GESTEL T VAN, BRUGGEN B VAN DER, BUEKENHOUDT A, et al. Surface modification of γ-Al2O3/TiO2 multilayer membranes for applications in non-polar organic solvents[J]. Journal of Membrane Science, 2003, 224(1/2): 3-10. |
| 39 | MERLET R B, PIZZOCCARO-ZILAMY M A, NIJMEIJER A, et al. Hybrid ceramic membranes for organic solvent nanofiltration: state-of-the-art and challenges[J]. Journal of Membrane Science, 2020, 599: 117839. |
| 40 | XIA L L, REN J, WEYD M, et al. Ceramic-supported thin film composite membrane for organic solvent nanofiltration[J]. Journal of Membrane Science, 2018, 563: 857-863. |
| 41 | TANARDI C R, CATANA R, BARBOIU M, et al. Polyethyleneglycol grafting of γ-alumina membranes for solvent resistant nanofiltration[J]. Microporous and Mesoporous Materials, 2016, 229: 106-116. |
| 42 | HOSSEINABADI S R, WYNS K, MEYNEN V, et al. Solvent-membrane-solute interactions in organic solvent nanofiltration (OSN) for Grignard functionalised ceramic membranes: explanation via Spiegler-Kedem theory[J]. Journal of Membrane Science, 2016, 513: 177-185. |
| 43 | AMIRILARGANI M, MERLET R B, CHU L Y, et al. Molecular separation using poly (styrene-co-maleic anhydride) grafted to γ-alumina: surface versus pore modification[J]. Journal of Membrane Science, 2019, 582: 298-306. |
| 44 | 卫旺, 相里粉娟, 金万勤, 等. 耐溶剂纳滤膜[J]. 化学进展, 2007, 19(10): 1592-1597. |
| WEI Wang, XIANGLI Fenjuan, JIN Wanqin, et al. Solvent resistant nanofiltration membranes[J]. Progress in Chemistry, 2007, 19(10): 1592-1597. | |
| 45 | VANDEZANDE P, LI X F, GEVERS L E M, et al. High throughput study of phase inversion parameters for polyimide-based SRNF membranes[J]. Journal of Membrane Science, 2009, 330(1/2): 307-318. |
| 46 | DAS S, HEASMAN P, BEN T, et al. Porous organic materials: strategic design and structure-function correlation[J]. Chemical Reviews, 2017, 117(3): 1515-1563. |
| 47 | HOU J, ZHANG H C, SIMON G P, et al. Polycrystalline advanced microporous framework membranes for efficient separation of small molecules and ions[J]. Advanced Materials, 2020, 32(18): 1902009. |
| 48 | YUAN S S, SWARTENBROEKX J, LI Y, et al. Facile synthesis of Kevlar nanofibrous membranes via regeneration of hydrogen bonds for organic solvent nanofiltration[J]. Journal of Membrane Science, 2019, 573: 612-620. |
| 1 | LIVELY R P, SHOLL D S. From water to organics in membrane separations[J]. Nature Materials, 2017, 16(3): 276-279. |
| 2 | MARCHETTI P, JIMENEZ SOLOMON M F, SZEKELY G, et al. Molecular separation with organic solvent nanofiltration: a critical review[J]. Chemical Reviews, 2014, 114(21): 10735-10806. |
| 49 | JIMENEZ-SOLOMON M F, SONG Q, JELFS K E, et al. Polymer nanofilms with enhanced microporosity by interfacial polymerization[J]. Nature Materials, 2016, 15(7): 760-767. |
| 50 | ZHU J Y, YUAN S S, WANG J, et al. Microporous organic polymer-based membranes for ultrafast molecular separations[J]. Progress in Polymer Science, 2020, 110: 101308. |
| 51 | WANG L H, SAHABUDEEN H, ZHANG T, et al. Liquid-interface-assisted synthesis of covalent-organic and metal-organic two-dimensional crystalline polymers[J]. npj 2D Materials and Applications, 2018, 2(1): 26. |
| 52 | BUDD P, ELABAS E, GHANEM B, et al. Solution-processed, organophilic membrane derived from a polymer of intrinsic microporosity[J]. Advanced Materials, 2004, 16(5): 456-459. |
| 53 | JIANG J X, SU F, TREWIN A, et al. Conjugated microporous poly(aryleneethynylene) networks[J]. Angewandte Chemie International Edition, 2007, 46(45): 8574-8578. |
| 54 | XU Y H, JIN S B, XU H, et al. Conjugated microporous polymers: design, synthesis and application[J]. Chemical Society Reviews, 2013, 42(20): 8012-8031. |
| 55 | AMIRILARGANI M, YOKOTA G N, VERMEIJ G H, et al. Melamine-based microporous organic framework thin films on an alumina membrane for high-flux organic solvent nanofiltration[J]. ChemSusChem, 2020, 13(1): 136-140. |
| 56 | CORCOS A, LEVATO G A, JIANG Z W, et al. Reducing the pore size of covalent organic frameworks in thin-film composite membranes enhances solute rejection[J]. ACS Materials Letters, 2019, 1(4): 440-446. |
| 57 | YUAN S S, LI X, ZHU J Y, et al. Covalent organic frameworks for membrane separation[J]. Chemical Society Reviews, 2019, 48(10): 2665-2681. |
| 58 | HALDER A, KARAK S, ADDICOAT M, et al. Ultrastable imine-based covalent organic frameworks for sulfuric acid recovery: an effect of interlayer hydrogen bonding[J]. Angewandte Chemie International Edition, 2018, 57(20): 5797-5802. |
| 59 | LI Y, WU Q, GUO X, et al. Laminated self-standing covalent organic framework membrane with uniformly distributed subnanopores for ionic and molecular sieving[J]. Nature Communications, 2020, 11(1): 599. |
| 60 | WU X W, HAN X, LIU Y H, et al. Control interlayer stacking and chemical stability of two-dimensional covalent organic frameworks via steric tuning[J]. Journal of the American Chemical Society, 2018, 140(47): 16124-16133. |
| 61 | DEY K, KUNJATTU H S, CHAHANDE A M, et al. Nanoparticle size-fractionation through self-standing porous covalent organic framework films[J]. Angewandte Chemie International Edition, 2020, 59(3): 1161-1165. |
| 62 | ZHANG K, HE Z J, GUPTA K M., et al. Computational design of 2D functional covalent-organic framework membranes for water desalination[J]. Environmental Science: Water Research & Technology, 2017, 3(4): 735-743. |
| 63 | TSARKOV S, KHOTIMSKIY V, BUDD P M, et al. Solvent nanofiltration through high permeability glassy polymers: effect of polymer and solute nature[J]. Journal of Membrane Science, 2012, 423/424: 65-72. |
| 64 | IGNACZ G, FEI F, SZEKELY G. Ion-stabilized membranes for demanding environments fabricated from polybenzimidazole and its blends with polymers of intrinsic microporosity[J]. ACS Applied Nano Materials, 2018, 1(11): 6349-6356. |
| 65 | GORGOJO Patricia, KARAN Santanu, WONG Himcheng, et al. Ultrathin polymer films with intrinsic microporosity: anomalous solvent permeation and high flux membranes[J]. Advanced Functional Materials, 2014, 24(30): 4729-4737. |
| 66 | GAO J, JAPIP S, CHUNG T S. Organic solvent resistant membranes made from a cross-linked functionalized polymer with intrinsic microporosity (PIM) containing thioamide groups[J]. Chemical Engineering Journal, 2018, 353: 689-698. |
| 67 | ZHOU S Y, ZHAO Y L, ZHENG J F, et al. High-performance functionalized polymer of intrinsic microporosity (PIM) composite membranes with thin and stable interconnected layer for organic solvent nanofiltration[J]. Journal of Membrane Science, 2019, 591: 117347. |
| 68 | XU Q S, JIANG J W. Effects of functionalization on the nanofiltration performance of PIM-1: molecular simulation investigation[J]. Journal of Membrane Science, 2019, 591: 117357. |
| 69 | HE X, SIN H, LIANG B, et al. Controlling the selectivity of conjugated microporous polymer membrane for efficient organic solvent nanofiltration[J]. Advanced Functional Materials, 2019, 29(32): 1900134. |
| 70 | LI C, LI S X, TIAN L, et al. Covalent organic frameworks (COFs)-incorporated thin film nanocomposite (TFN) membranes for high-flux organic solvent nanofiltration (OSN)[J]. Journal of Membrane Science, 2019, 572: 520-531. |
| 71 | DUAN K, WANG J, ZHANG Y T, et al. Covalent organic frameworks (COFs) functionalized mixed matrix membrane for effective CO2/N2 separation[J]. Journal of Membrane Science, 2019, 572: 588-595. |
| 72 | BURKE D W, SUN C, CASTANO I, et al. Acid exfoliation of imine-linked covalent organic frameworks enables solution processing into crystalline thin films[J]. Angewandte Chemie International Edition, 2020, 59(13): 5165-5171. |
| 73 | WEI W, LIU J, JIANG J W. Computational design of 2D covalent-organic framework membranes for organic solvent nanofiltration[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(1): 1734-1744. |
| 74 | ZHAO Y Y, LIU Y L, WANG X M, et al. Impacts of metal-organic frameworks on structure and performance of polyamide thin-film nanocomposite membranes[J]. ACS Applied Materials & Interfaces, 2019, 11(14): 13724-13734. |
| 75 | JUE M L, KOH D Y, MCCOOL B A, et al. Enabling widespread use of microporous materials for challenging organic solvent separations[J]. Chemistry of Materials, 2017, 29(23): 9863-9876. |
| 76 | DENNY M S, MORETON J C, BENZ L, et al. Metal-organic frameworks for membrane-based separations[J]. Nature Reviews Materials, 2016, 1: 16078. |
| 77 | SORRIBAS S, GORGOJO P, TÉLLEZ C, et al. High flux thin film nanocomposite membranes based on metal-organic frameworks for organic solvent nanofiltration[J]. Journal of the American Chemical Society, 2013, 135(40): 15201-15208. |
| 78 | XU S J, SHEN Q, CHEN G E, et al. Novel β-CD@ZIF-8 nanoparticles-doped poly(m-phenylene isophthalamide) (PMIA) thin-film nanocomposite (TFN) membrane for organic solvent nanofiltration (OSN)[J]. ACS Omega, 2018, 3(9): 11770-11787. |
| 79 | WANG Naixin, LIU Tianjiao, SHEN Hongpan, et al. Ceramic tubular MOF hybrid membrane fabricated through in situ layer-by-layer self-assembly for nanofiltration[J]. AIChE Journal, 2016, 62(2): 538-546. |
| 80 | ZHANG R, JI S, WANG N, et al. Coordination-driven in situ self-assembly strategy for the preparation of metal-organic framework hybrid membranes[J]. Angewandte Chemie International Edition, 2014, 53(37): 9775-9779. |
| 81 | WEI W, GUPTA K M, LIU J, et al. Zeolitic imidazolate framework membranes for organic solvent nanofiltration: a molecular simulation exploration[J]. ACS Applied Materials & Interfaces, 2018, 10(39): 33135-33143. |
| 82 | RAN Jin, PAN Ting, WU Yuying, et al. Acid spacers endowing g-C3N4 membranes with superior permeability and stability[J]. Angewandte Chemie International Edition, 2019, 58(46): 16463-16468. |
| 83 | LI J, ZHOU X, WANG J, et al. Two-dimensional covalent organic frameworks (COFs) for membrane separation: a mini review[J]. Industrial & Engineering Chemistry Research, 2019, 58(34): 15394-15406. |
| 84 | LI Y Y, LI C, LI S X, et al. Graphene oxide (GO)-interlayered thin-film nanocomposite (TFN) membranes with high solvent resistance for organic solvent nanofiltration (OSN)[J]. Journal of Materials Chemistry A, 2019, 7(21): 13315-13330. |
| 85 | HUANG T F, PUSPASARI T, NUNES S P, et al. Ultrathin 2D-layered cyclodextrin membranes for high-performance organic solvent nanofiltration[J]. Advanced Functional Materials, 2019, 30(4): 1906797. |
| 86 | YANG Q, SU Y, CHI C, et al. Ultrathin graphene-based membrane with precise molecular sieving and ultrafast solvent permeation[J]. Nature Materials, 2017, 16(12): 1198-1202. |
| 87 | NIE L N, GOH K, WANG Y, et al. Realizing small-flake graphene oxide membranes for ultrafast size-dependent organic solvent nanofiltration[J]. Science Advances, 2020, 6(17): eaaz9184. |
| 88 | GUO B Y, JIANG S D, TANG M J, et al. MoS2 membranes for organic solvent nanofiltration: stability and structural control[J]. The Journal of Physical Chemistry Letters, 2019, 10(16): 4609-4617. |
| 89 | LIN H, DANGWAL S, LIU R C, et al. Reduced wrinkling in GO membrane by grafting basal-plane groups for improved gas and liquid separations[J]. Journal of Membrane Science, 2018, 563: 336-344. |
| 90 | MAHALINGAM D K, WANG S F, NUNES S P. Stable graphene oxide cross-linked membranes for organic solvent nanofiltration[J]. Industrial & Engineering Chemistry Research, 2019, 58(51): 23106-23113. |
| 91 | 汪林, 纪树兰, 王乃鑫, 等. 用于有机溶剂体系分离的氧化石墨烯基复合膜的构筑[J]. 膜科学与技术, 2020, 40(1): 352-359. |
| WANG Lin, JI Shulan, WANG Naixin, et al. Construction of graphene oxide membrane for the separation in organic solvent system[J]. Membrane Science and Technology, 2020, 40(1): 352-359. | |
| 92 | GAO J, ZHANG M Y, WANG J T, et al. Bioinspired modification of layer-stacked molybdenum disulfide (MoS2) membranes for enhanced nanofiltration performance[J]. ACS Omega, 2019, 4(2): 4012-4022. |
| 93 | CHEN C, WANG J, LIU D, et al. Functionalized boron nitride membranes with ultrafast solvent transport performance for molecular separation[J]. Nature Communications, 2018, 9(1): 1902. |
| 94 | WU X, CUI X, WU W, et al. Elucidating ultrafast molecular permeation through well-defined 2D nanochannels of lamellar membranes[J]. Angewandte Chemie International Edition, 2019, 58(51): 18524-18529. |
| 95 | KUMAR M, KHAN M A, ARAFAT H A. Recent developments in the rational fabrication of thin film nanocomposite membranes for water purification and desalination[J]. ACS Omega, 2020, 5(8): 3792-3800. |
| 96 | HOMAEIGOHAR S, ELBAHRI M. Graphene membranes for water desalination[J]. NPG Asia Materials, 2017, 9(8): e427. |
| 97 | DIDASKALOU C, KUPAI J, CSERI L, et al. Membrane-grafted asymmetric organocatalyst for an integrated synthesis-separation platform[J]. ACS Catalysis, 2018, 8(8): 7430-7438. |
| 98 | GROßEHEILMANN J, BÜTTNER H, KOHRT C, et al. Recycling of phosphorus-based organocatalysts by organic solvent nanofiltration[J]. ACS Sustainable Chemistry & Engineering, 2015, 3(11): 2817-2822. |
| 99 | PESHEV D, LIVINGSTON A G. OSN Designer, a tool for predicting organic solvent nanofiltration technology performance using Aspen One, MATLAB and CAPE OPEN[J]. Chemical Engineering Science, 2013, 104: 975-987. |
| 100 | VANNESTE J, ORMEROD D, THEYS G, et al. Towards high resolution membrane-based pharmaceutical separations[J]. Journal of Chemical Technology & Biotechnology, 2013, 88(1): 98-108. |
| 101 | FAHRENWALDT T, GROßEHEILMANN J, ERBEN F, et al. Organic solvent nanofiltration as a tool for separation of quinine-based organocatalysts[J]. Organic Process Research & Development, 2013, 17(9): 1131-1136. |
| 102 | KIM J F, GAFFNEY P R J, VALTCHEVA I B, et al. Organic solvent nanofiltration (OSN): a new technology platform for liquid-phase oligonucleotide synthesis (LPOS)[J]. Organic Process Research & Development, 2016, 20(8): 1439-1452. |
| 3 | ORMEROD D, NOTEN B, DORBEC M, et al. Cyclic peptide formation in reduced solvent volumes via in-line solvent recycling by organic solvent nanofiltration[J]. Organic Process Research & Development, 2015, 19(7): 841-848. |
| 4 | ANDRZEJ G, ANDRZEJ S. Intensification of biobased processes[M]. Croydon: CPI Group Ltd., 2018: 132-144. |
| 5 | WERTH K, KAUPENJOHANN P, SKIBOROWSKI M. The potential of organic solvent nanofiltration processes for oleochemical industry[J]. Separation and Purification Technology, 2017, 182: 185-196. |
| 6 | WANG J T, YUAN Z J, WU X L, et al. Beetle-inspired assembly of heterostructured lamellar membranes with polymer cluster-patterned surface for enhanced molecular permeation[J]. Advanced Functional Materials, 2019, 29(23): 1900819. |
| 7 | VANDEZANDE P, GEVERS L E, VANKELECOM I F. Solvent resistant nanofiltration: separating on a molecular level[J]. Chemical Society Reviews, 2008, 37(2): 365-405. |
| 8 | WANG L, BOUTILIER M S H, KIDAMBI P R, et al. Fundamental transport mechanisms, fabrication and potential applications of nanoporous atomically thin membranes[J]. Nature Nanotechnology, 2017, 12(6): 509-522. |
| 9 | SHANNON M A, BOHN P W, ELIMELECH M, et al. Science and technology for water purification in the coming decades[J]. Nature, 2008, 452(7185): 301-310. |
| 10 | KOROS W J, ZHANG C. Materials for next-generation molecularly selective synthetic membranes[J]. Nature Materials, 2017, 16(3): 289-297. |
| 11 | 邢雅南, 苏保卫, 甄宏艳. 耐溶剂纳滤膜的制备与应用研究进展[J]. 化工进展, 2015, 34(11): 3832-3840. |
| XING Yanan, SU Baowei, ZHEN Hongyan. Research progress of solvent resistant nanofiltration membranes[J]. Chemical Industry and Engineering Progress, 2015, 34(11): 3832-3840. | |
| 12 | 周宗尧, 张朔, 王宁, 等. 有机溶剂分离膜技术研究进展[J]. 膜科学与技术, 2018, 38(1): 104-113. |
| ZHOU Zongyao, ZHANG Shuo, WANG Ning, et al. Progress in the technology of organic solvent separation membrane[J]. Membrane Science and Technology, 2018, 38(1): 104-113. | |
| 13 | ASADI TASHVIGH A, FENG Y N, WEBER M, et al. Selection of cross-linkers and cross-linking procedures for the fabrication of solvent-resistant nanofiltration membranes: a review[J]. Industrial & Engineering Chemistry Research, 2019, 58(25): 10678-10691. |
| 14 | LIANG B, HE X, HOU J, et al. Membrane separation in organic liquid: technologies, achievements, and opportunities[J]. Advanced Materials, 2019, 31(45): e1806090. |
| 15 | KEDEM O, KATCHALSKY A. Thermodynamic analysis of the permeability of biological membranes to non-electrolytes[J]. Biochimica et Biophysica Acta, 1958, 27: 229-246. |
| 16 | LONSDALE H K, MERTEN U, RILEY R L. Transport properties of cellulose acetate osmotic membranes[J]. Journal of Applied Polymer Science, 1965, 9(4): 1341-1362. |
| 17 | MASON E A, LONSDALE H K. Statistical-mechanical theory of membrane transport[J]. Journal of Membrane Science, 1990, 51(1/2): 1-81. |
| 18 | NIEMI H, PALOSAARI S. Flowsheet simulation of ultrafiltration and reverse osmosis processes[J]. Journal of Membrane Science, 1994, 91(1/2): 111-124. |
| 19 | WHU J A, BALTZIS B C, SIRKAR K K. Nanofiltration studies of larger organic microsolutes in methanol solutions[J]. Journal of Membrane Science, 2000, 170(2): 159-172. |
| 20 | BOWEN W R, WELFOOT J S. Modelling of membrane nanofiltration—pore size distribution effects[J]. Chemical Engineering Science, 2002, 57(8): 1393-1407. |
| 21 | MARCHETTI P, LIVINGSTON A G. Predictive membrane transport models for organic solvent nanofiltration: how complex do we need to be?[J]. Journal of Membrane Science, 2015, 476: 530-553. |
| 22 | 孙志娟, 张心亚, 黄洪, 等. 溶解度参数的发展及应用[J]. 橡胶工业, 2007, 54(1): 54-58. |
| SUN Zhijuan, ZHANG Xinya, HUANG Hong, et al. The development and application of solubility parameters[J]. China Rubber Industry, 2007, 54(1): 54-58. | |
| 23 | SUI X, YUAN Z, YU Y, et al. 2D material based advanced membranes for separations in organic solvents[J]. Small, 2020, 16(50): e2003400. |
| 24 | ZHENG S X, TU Q S, WANG M N, et al. Correlating interlayer spacing and separation capability of graphene oxide membranes in organic solvents[J]. ACS Nano, 2020, 14(5): 6013-6023. |
| 25 | CHUAH C Y, NIE L N, LEE J M, et al. Graphene-based advanced membrane applications in organic solvent nanofiltration[J]. Advanced Functional Materials, 2020, DOI: 10.1002/adfm.202006949. |
| 26 | MERLET R B, TANARDI C R, VANKELECOM I F J, et al. Interpreting rejection in SRNF across grafted ceramic membranes through the Spiegler-Kedem model[J]. Journal of Membrane Science, 2017, 525: 359-367. |
| 27 | XU Y C, CHENG X Q, LONG J, et al. A novel monoamine modification strategy toward high-performance organic solvent nanofiltration (OSN) membrane for sustainable molecular separations[J]. Journal of Membrane Science, 2016, 497: 77-89. |
| 28 | KARAN S, SAMITSU S, PENG X, et al. Ultrafast viscous permeation of organic solvents through diamond-like carbon nanosheets[J]. Science, 2012, 335(6067): 444-447. |
| 29 | KARAN Santanu, JIANG Zhiwei, LIVINGSTON Andrew G. Sub-10 nm polyamide nanofilms with ultrafast solvent transport for molecular separation[J]. Science, 2015, 348: 1347-1351. |
| 30 | LIANG B, WANG H, SHI X H, et al. Microporous membranes comprising conjugated polymers with rigid backbones enable ultrafast organic-solvent nanofiltration[J]. Nature Chemistry, 2018, 10(9): 961-967. |
| 31 | LI J Q, ZHANG M X, FENG W L, et al. PIM-1 pore-filled thin film composite membranes for tunable organic solvent nanofiltration[J]. Journal of Membrane Science, 2020, 601: 117951. |
| 32 | DEY K, PAL M, ROUT K C, et al. Selective molecular separation by interfacially crystallized covalent organic framework thin films[J]. Journal of the American Chemical Society, 2017, 139(37): 13083-13091. |
| 33 | KANDAMBETH S, BISWAL B P, CHAUDHARI H D, et al. Selective molecular sieving in self-standing porous covalent-organic-framework membranes[J]. Advanced Materials, 2017, DOI:10.1002/adma.201603945. |
| 34 | QIAN H D, ZHENG J F, ZHANG S B. Preparation of microporous polyamide networks for carbon dioxide capture and nanofiltration[J]. Polymer, 2013, 54(2): 557-564. |
| 35 | MA D C, HAN G, GAO Z F, et al. Continuous UiO-66-type metal-organic framework thin film on polymeric support for organic solvent nanofiltration[J]. ACS Applied Materials & Interfaces, 2019, 11(48): 45290-45300. |
| [1] | 高逸飞, 易群, 齐凯, 高丽丽, 李雪莲. MOFs基膜材料的研究现状及其在H2/CH4分离中的应用[J]. 化工进展, 2022, 41(12): 6395-6407. |
| [2] | 孟祥伟, 吴晓莉, 高展鹏, 李文鹏, 王景涛. 蛭石层状膜的制备及有机溶剂纳滤性能[J]. 化工进展, 2022, 41(11): 5986-5995. |
| [3] | 高豪, 陆家声, 章文明, 董维亮, 方艳, 余子夷, 信丰学, 姜岷. 材料介导细胞固定化技术在生物发酵中的应用[J]. 化工进展, 2021, 40(7): 3923-3931. |
| [4] | 陈建松, 孙楠楠, 高强, 魏伟. 基于Aldol缩合反应的碳碳双键共价有机框架材料的设计合成[J]. 化工进展, 2021, 40(12): 6765-6776. |
| [5] | 朱本伟, 姚忠, 仲兆祥, 孙芸, 周明柱, 姜帅. 渗透汽化分离精油中挥发性芳香化合物的研究进展[J]. 化工进展, 2021, 40(11): 5875-5882. |
| [6] | 刘紫洋, 秦振平, 崔素萍, 贾萌萌, 安全福, 王乃鑫, 刘燕, 郭红霞. 有机溶剂纳滤膜的润湿性对渗透和分离性能的影响[J]. 化工进展, 2020, 39(7): 2715-2723. |
| [7] | 储李娜,敖先权,陈前林,郭妤,曹阳. 改性聚乙烯醇添加酒糟制备缓释肥包膜材料的表征及其性能[J]. 化工进展, 2020, 39(2): 627-634. |
| [8] | 孙亚伟, 谢美连, 刘庆岭, 马德刚, 纪娜, 宋春风. 膜法分离燃煤电厂烟气中CO2的研究现状及进展[J]. 化工进展, 2017, 36(05): 1880-1889. |
| [9] | 邢雅南, 苏保卫, 甄宏艳. 耐溶剂纳滤膜的制备与应用研究进展[J]. 化工进展, 2015, 34(11): 3832-3840. |
| [10] | 马岩红1,丁昀1,杨庆1,2,3,李鹏1. 正渗透膜材料的研究进展[J]. 化工进展, 2014, 33(12): 3299-3303. |
| [11] | 李杰,王乃鑫,纪树兰. 有机/无机杂化渗透汽化优先透醇膜研究进展[J]. 化工进展, 2014, 33(11): 2982-2990. |
| [12] | 王天雷,刘梅堂,马鸿文. 层层自组装技术制备类水滑石基新型薄膜材料的研究进展[J]. 化工进展, 2013, 32(07): 1584-1590. |
| [13] | 钟文锋1,杨敏林2,左远志2,黄斯珉2. 膜式液体除湿器研究进展[J]. 化工进展, 2013, 32(05): 971-977. |
| [14] | 成庆林,刘 扬,项新耀. 火用传递研究的形成脉络与发展现状[J]. 化工进展, 2012, 31(09): 1936-1941. |
| [15] | 黄 敏1,2,曾楚怡1,李继定2,陈 剑2. 面向环己烷氧化过程的渗透汽化膜材料及膜反应器(Ⅰ) 渗透汽化膜材料的初步选择 [J]. 化工进展, 2010, 29(4): 616-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||