化工进展 ›› 2021, Vol. 40 ›› Issue (1): 205-220.DOI: 10.16085/j.issn.1000-6613.2020-0550
CO/CO2加氢制芳烃的研究进展
焦佳鹏( ), 田海锋(
), 田海锋( ), 何环环, 查飞(
), 何环环, 查飞( ), 郭效军, 唐小华
), 郭效军, 唐小华
- 西北师范大学化学化工学院,甘肃 兰州 730070
-
收稿日期:2020-04-09出版日期:2021-01-05发布日期:2021-01-12 -
通讯作者:田海锋,查飞 -
作者简介:焦佳鹏(1994—),男,硕士研究生,研究方向为CO2转化与利用。E-mail:jpjiao6420@126.com 。 -
基金资助:西北师范大学青年教师科研能力提升计划(NWNU-LKQN-18-21);甘肃省工业绿色低碳转型升级课题(GGLD-2019-062)
Recent advanced of CO/CO2 hydrogenation to aromatics
Jiapeng JIAO( ), Haifeng TIAN(
), Haifeng TIAN( ), Huanhuan HE, Fei ZHA(
), Huanhuan HE, Fei ZHA( ), Xiaojun GUO, Xiaohua TANG
), Xiaojun GUO, Xiaohua TANG
- College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou 730070, Gansu, China
-
Received:2020-04-09Online:2021-01-05Published:2021-01-12 -
Contact:Haifeng TIAN,Fei ZHA
摘要:
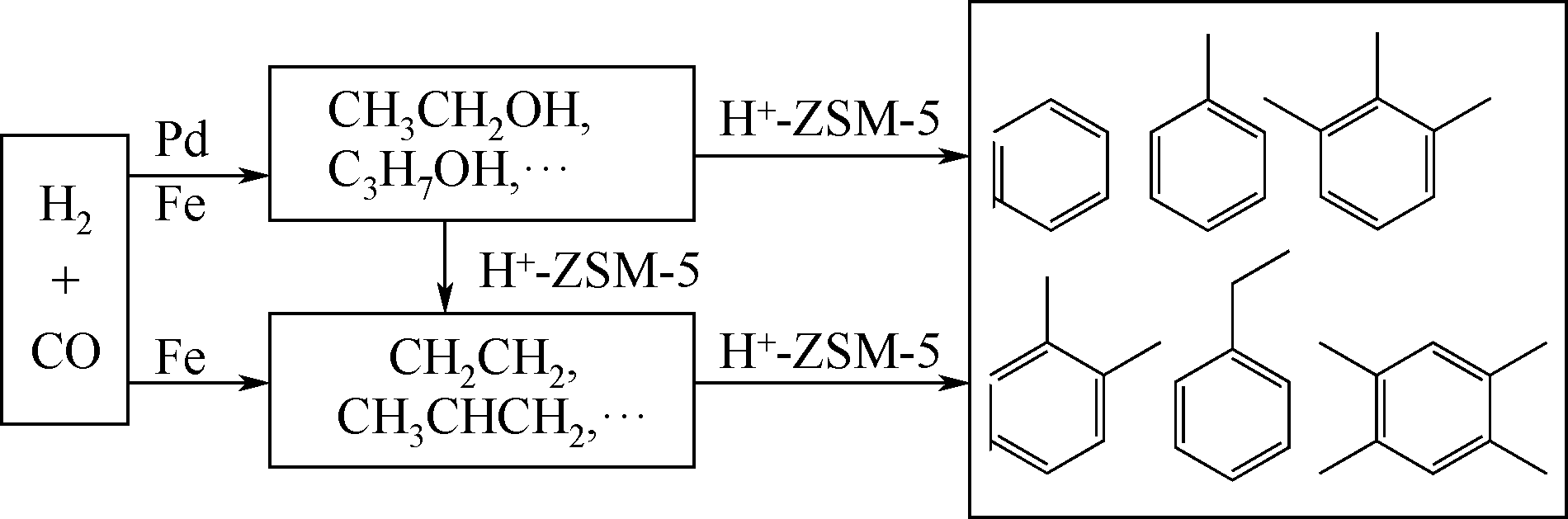
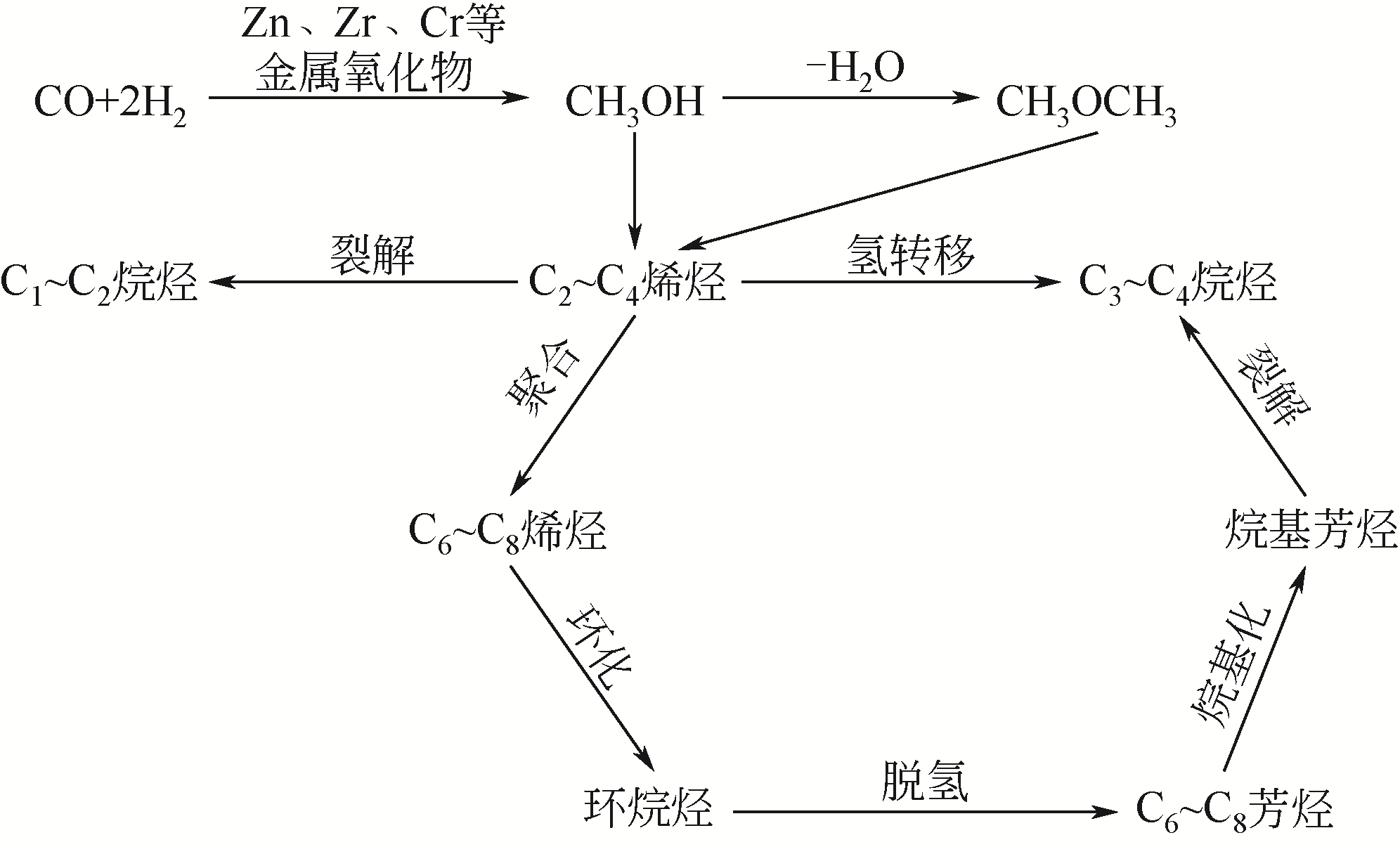
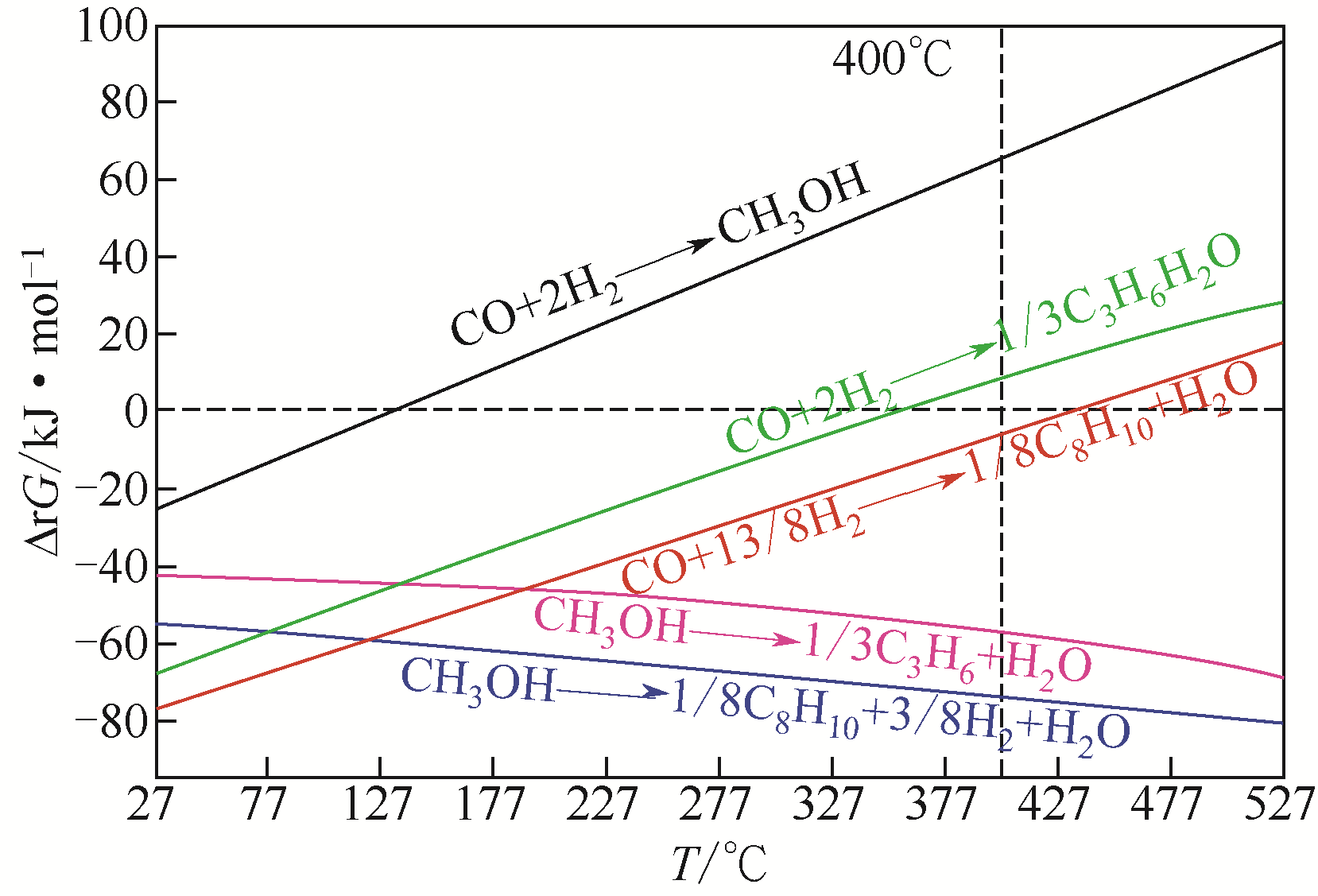
将CO/CO2直接转化为芳烃是一种极具挑战性的非石油路线合成途径。本文主要对CO/CO2通过不同反应途径制取芳烃过程中复合催化剂的开发和反应机理的研究进展进行了综述。阐述了利用反应耦合思想,构筑的复合催化剂在CO/CO2的高效转化和产物调控等方面取得了突破性的进展。重点介绍了复合催化剂用于CO加氢制芳烃主要的两种反应途径,活性金属的类别、分子筛的结构与酸性和活性组分的组装方式与接触度对CO2加氢制芳烃催化性能的影响。指出协同加氢与芳构化反应活性的匹配是影响催化剂性能的关键。提出开发高效稳定的催化剂用于提高CO/CO2的转化率和芳烃产物的产率以及反应机理的探索仍然是未来研究的重点。
中图分类号:
引用本文
焦佳鹏, 田海锋, 何环环, 查飞, 郭效军, 唐小华. CO/CO2加氢制芳烃的研究进展[J]. 化工进展, 2021, 40(1): 205-220.
Jiapeng JIAO, Haifeng TIAN, Huanhuan HE, Fei ZHA, Xiaojun GUO, Xiaohua TANG. Recent advanced of CO/CO2 hydrogenation to aromatics[J]. Chemical Industry and Engineering Progress, 2021, 40(1): 205-220.
| 催化剂 | 反应条件 | CO转化率/% | 芳烃选择性/% | 参考文献 | ||
|---|---|---|---|---|---|---|
| 温度/℃ | 压强/MPa | 空速 | ||||
| FeMn-HZSM-5 | 320 | 1.0 | 2220mL·g-1·h-1 | 81.9 | 32.8① | [ |
| FeK-HZSM-5 | 320 | 1.0 | 2220mL·g-1·h-1 | 94.7 | 53.8① | [ |
| Co/ZSM-5 | 280 | 2.1 | 1000h-1 | 55.0 | 19.9① | [ |
| FeMnK/SiO2-HZSM-5 | 320 | 2.0 | 4000h-1 | 83.8 | 57.2① | [ |
| FeZrZn-Ni/ZSM-5 | 340 | 4.0 | 2000h-1 | 93.6 | 36.6① | [ |
| K/FeZrZn-Ni/ZSM-5 | 340 | 4.0 | 2000h-1 | 96.8 | 39.5① | [ |
| K-FeMnO/MoNi-ZSM-5 | 370 | 4.0 | 1395h-1 | 98.2 | 75.9① | [ |
| Fe-Pd/HZSM?5 | 310 | 8.6 | 3000h-1 | 75.7 | 32.0② | [ |
| FeZnNa@HZSM-5 | 340 | 2.0 | — | 88.8 | 50.6① | [ |
| FeMn-HZSM-5@Si | 320 | 1.0 | 3600mL·g-1·h-1 | 60.0 | 33.6①/93.0② | [ |
| Zn-ZrO2/H-ZSM-5 | 400 | 3 | — | 21.0 | 81.0① | [ |
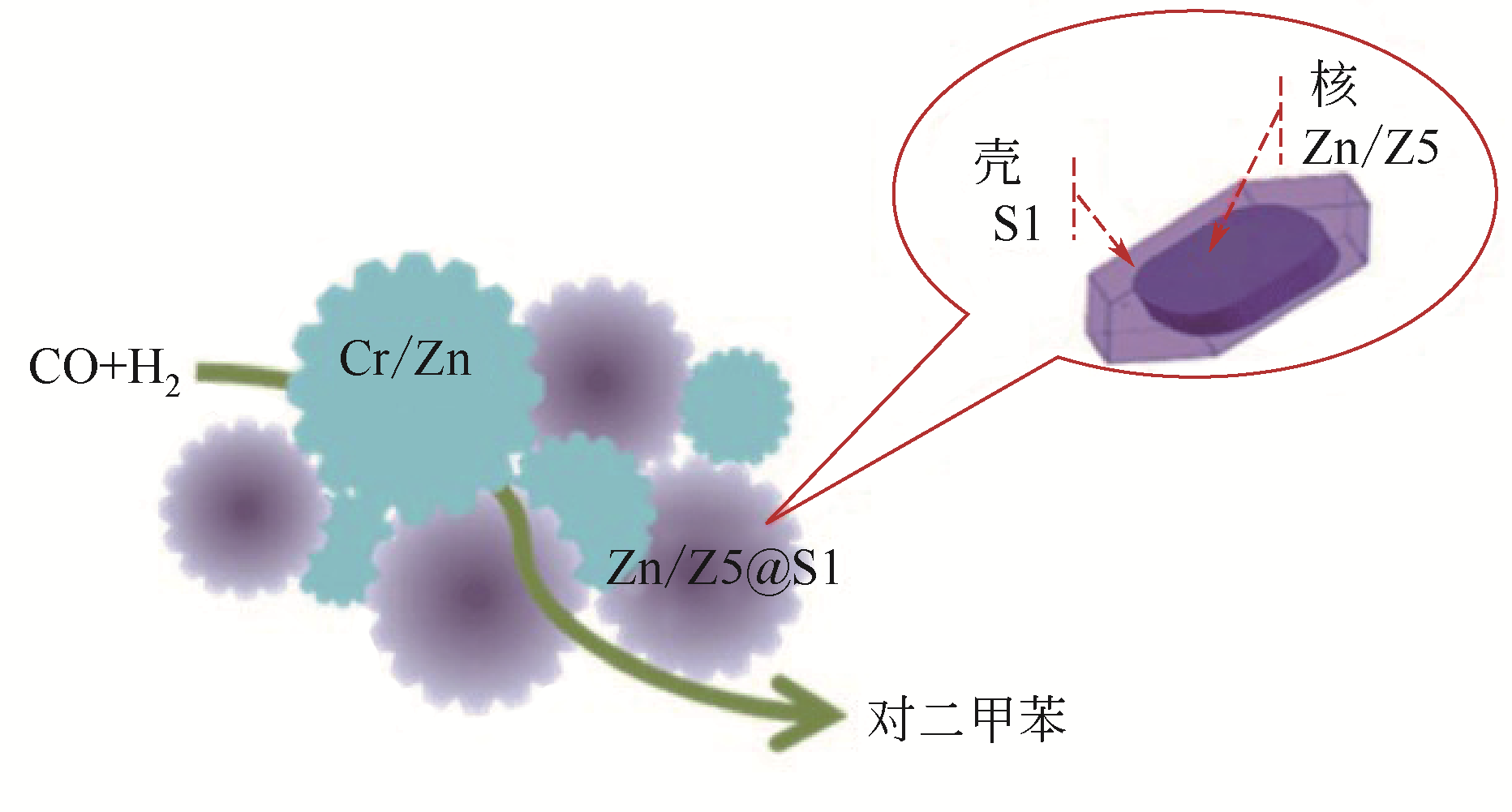
| Cr/Zn-Zn/Z5@S1 | 400 | 5 | 20.7g·h·mol-1 | 55.0 | 62.0① | [ |
| ZnCrOx-ZSM-5 | 350 | 4 | 1500mL·g-1·h-1 | 16.0 | 73.9① | [ |
| Mo/HZSM-5 | 380 | 6.9 | 3000h-1 | 64.4 | 97.6② | [ |
| PdZnAl + HZSM-5 | 310 | 6.9 | 700h-1 | 44.0 | 69.0② | [ |
| Mo-ZrO2/H-ZSM-5 | 400 | 4 | 3000mL·g-1·h-1 | 22.0 | 76.0① | [ |
| CeZrO2/HZSM-5 | 380 | 2 | 3500mL·g-1·h-1 | 8.1 | 83.1① | [ |
| Fe2O3-SiO2/Nb-ZSM-5 | 330 | 4 | 1813h-1 | 99.0 | 47.4① | [ |
| CuO-ZnO-Al2O3/Nb-Ni-ZSM-5 | 330 | 4 | 1813h-1 | 95.0 | 46.5① | [ |
| Fe-CuO-ZnO-Al2O3/Nb-Ni-ZSM-5 | 330 | 4 | 1813h-1 | 99.2 | 55.0① | [ |
| Zn-Cr/HZSM-5 | 395 | 2.0 | 4000h-1 | 11.1 | 72.4① | [ |
| m-ZrO2/HZSM-5 | 400 | 3.8 | — | 24.0 | 67.4① | [ |
| Mn-ZnO&H-ZSM-5 | 340 | 3.0 | 750mL·g-1·h-1 | 14.8 | 80.1① | [ |
表1 CO加氢制取芳烃的不同类型复合催化剂催化性能
| 催化剂 | 反应条件 | CO转化率/% | 芳烃选择性/% | 参考文献 | ||
|---|---|---|---|---|---|---|
| 温度/℃ | 压强/MPa | 空速 | ||||
| FeMn-HZSM-5 | 320 | 1.0 | 2220mL·g-1·h-1 | 81.9 | 32.8① | [ |
| FeK-HZSM-5 | 320 | 1.0 | 2220mL·g-1·h-1 | 94.7 | 53.8① | [ |
| Co/ZSM-5 | 280 | 2.1 | 1000h-1 | 55.0 | 19.9① | [ |
| FeMnK/SiO2-HZSM-5 | 320 | 2.0 | 4000h-1 | 83.8 | 57.2① | [ |
| FeZrZn-Ni/ZSM-5 | 340 | 4.0 | 2000h-1 | 93.6 | 36.6① | [ |
| K/FeZrZn-Ni/ZSM-5 | 340 | 4.0 | 2000h-1 | 96.8 | 39.5① | [ |
| K-FeMnO/MoNi-ZSM-5 | 370 | 4.0 | 1395h-1 | 98.2 | 75.9① | [ |
| Fe-Pd/HZSM?5 | 310 | 8.6 | 3000h-1 | 75.7 | 32.0② | [ |
| FeZnNa@HZSM-5 | 340 | 2.0 | — | 88.8 | 50.6① | [ |
| FeMn-HZSM-5@Si | 320 | 1.0 | 3600mL·g-1·h-1 | 60.0 | 33.6①/93.0② | [ |
| Zn-ZrO2/H-ZSM-5 | 400 | 3 | — | 21.0 | 81.0① | [ |
| Cr/Zn-Zn/Z5@S1 | 400 | 5 | 20.7g·h·mol-1 | 55.0 | 62.0① | [ |
| ZnCrOx-ZSM-5 | 350 | 4 | 1500mL·g-1·h-1 | 16.0 | 73.9① | [ |
| Mo/HZSM-5 | 380 | 6.9 | 3000h-1 | 64.4 | 97.6② | [ |
| PdZnAl + HZSM-5 | 310 | 6.9 | 700h-1 | 44.0 | 69.0② | [ |
| Mo-ZrO2/H-ZSM-5 | 400 | 4 | 3000mL·g-1·h-1 | 22.0 | 76.0① | [ |
| CeZrO2/HZSM-5 | 380 | 2 | 3500mL·g-1·h-1 | 8.1 | 83.1① | [ |
| Fe2O3-SiO2/Nb-ZSM-5 | 330 | 4 | 1813h-1 | 99.0 | 47.4① | [ |
| CuO-ZnO-Al2O3/Nb-Ni-ZSM-5 | 330 | 4 | 1813h-1 | 95.0 | 46.5① | [ |
| Fe-CuO-ZnO-Al2O3/Nb-Ni-ZSM-5 | 330 | 4 | 1813h-1 | 99.2 | 55.0① | [ |
| Zn-Cr/HZSM-5 | 395 | 2.0 | 4000h-1 | 11.1 | 72.4① | [ |
| m-ZrO2/HZSM-5 | 400 | 3.8 | — | 24.0 | 67.4① | [ |
| Mn-ZnO&H-ZSM-5 | 340 | 3.0 | 750mL·g-1·h-1 | 14.8 | 80.1① | [ |
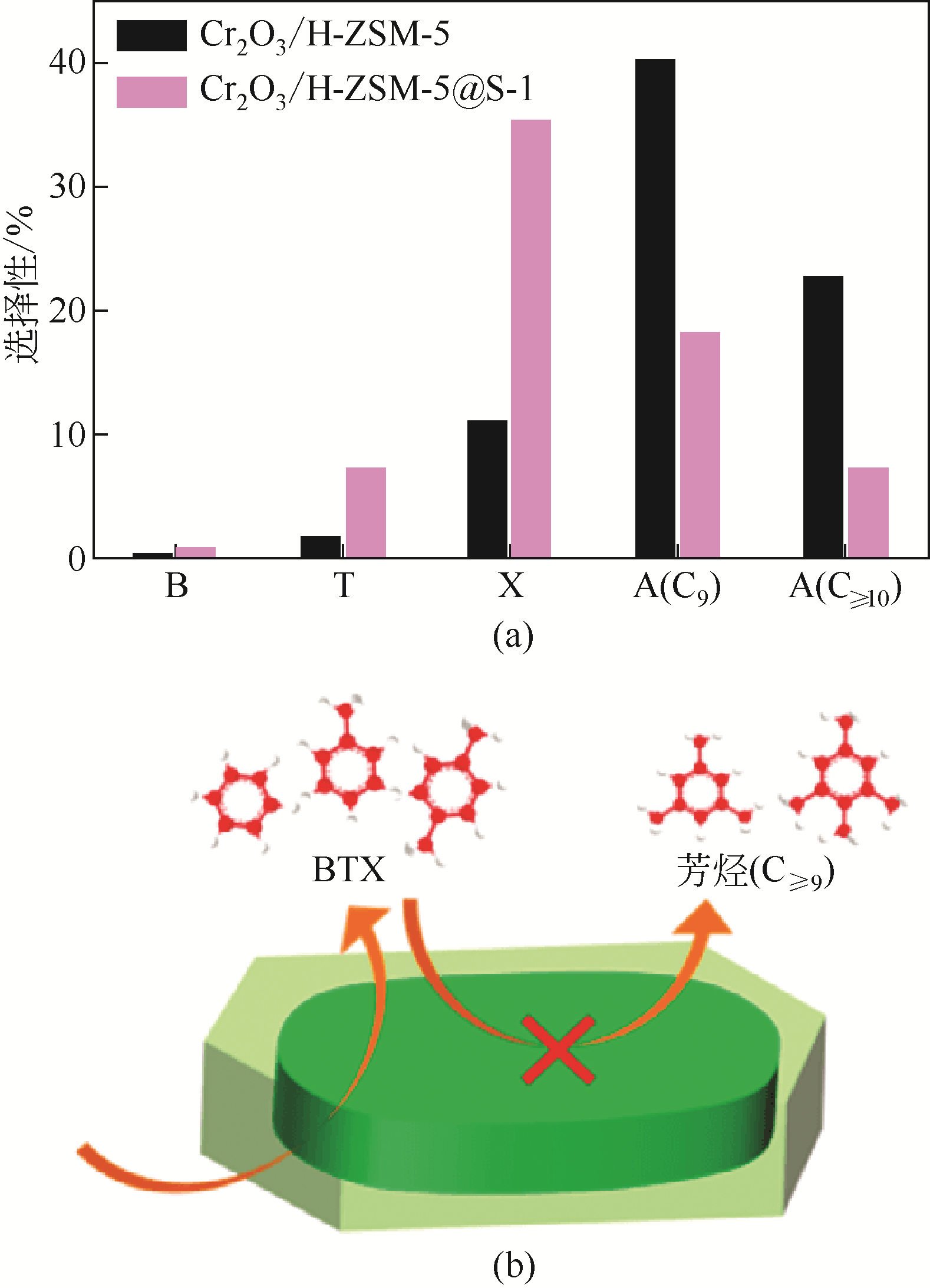
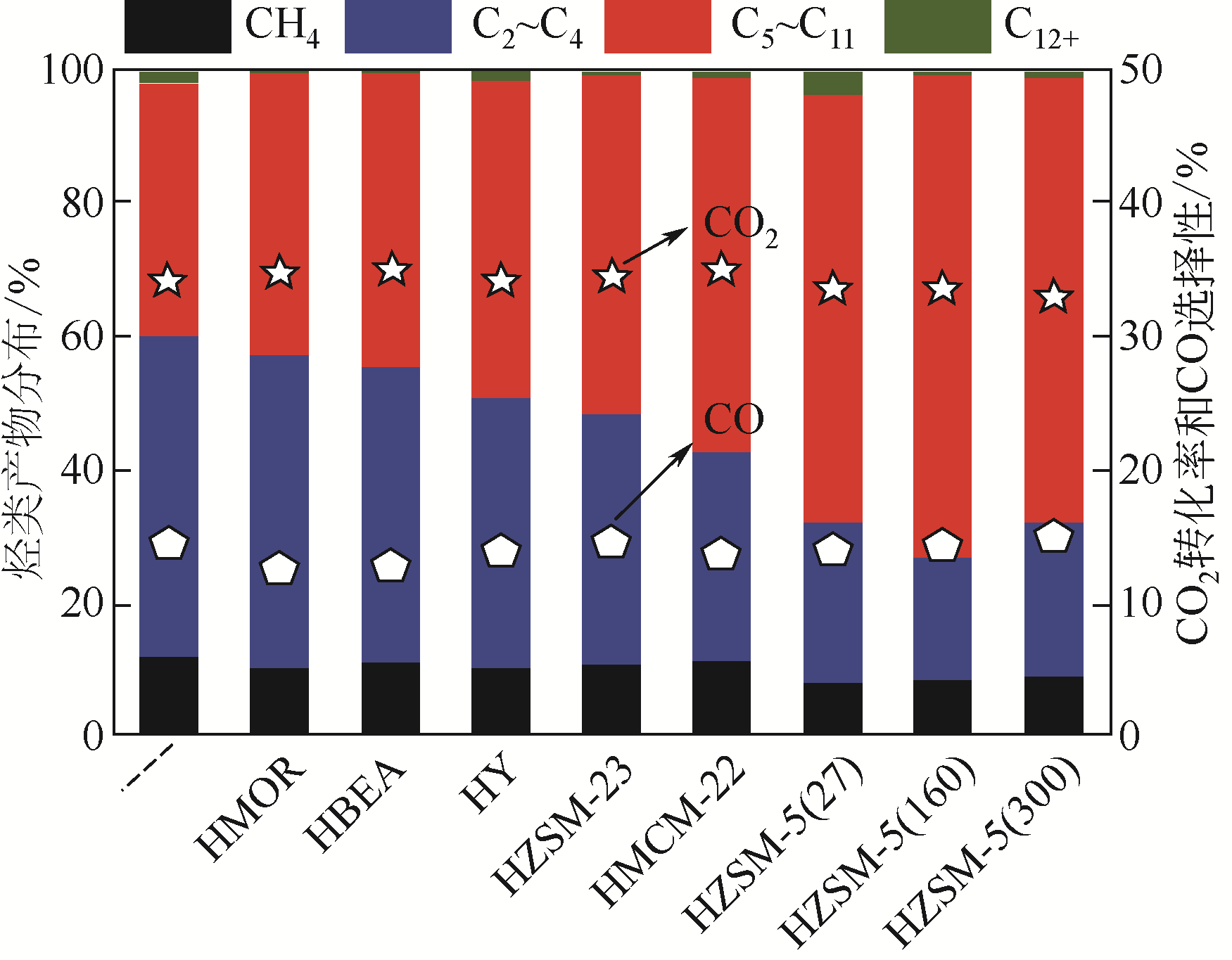
图17 Cr2O3/H-ZSM-5与Cr2O3/H-ZSM-5@S-1催化剂上CO2加氢制芳烃的产物选择性及在Cr2O3/H-ZSM-5@S-1上高选择性生产轻质芳烃的途径[112]
[T=350℃, P=3MPa, GHSV=1200mL·h-1·gcat-1, H2/CO2=3(5.42% CO)]
| 催化剂 | 反应条件 | CO2转化率/% | 芳烃选择性/% | 参考文献 | ||
|---|---|---|---|---|---|---|
| 温度/℃ | 压强/MPa | 空速/mL·g-1·h-1 | ||||
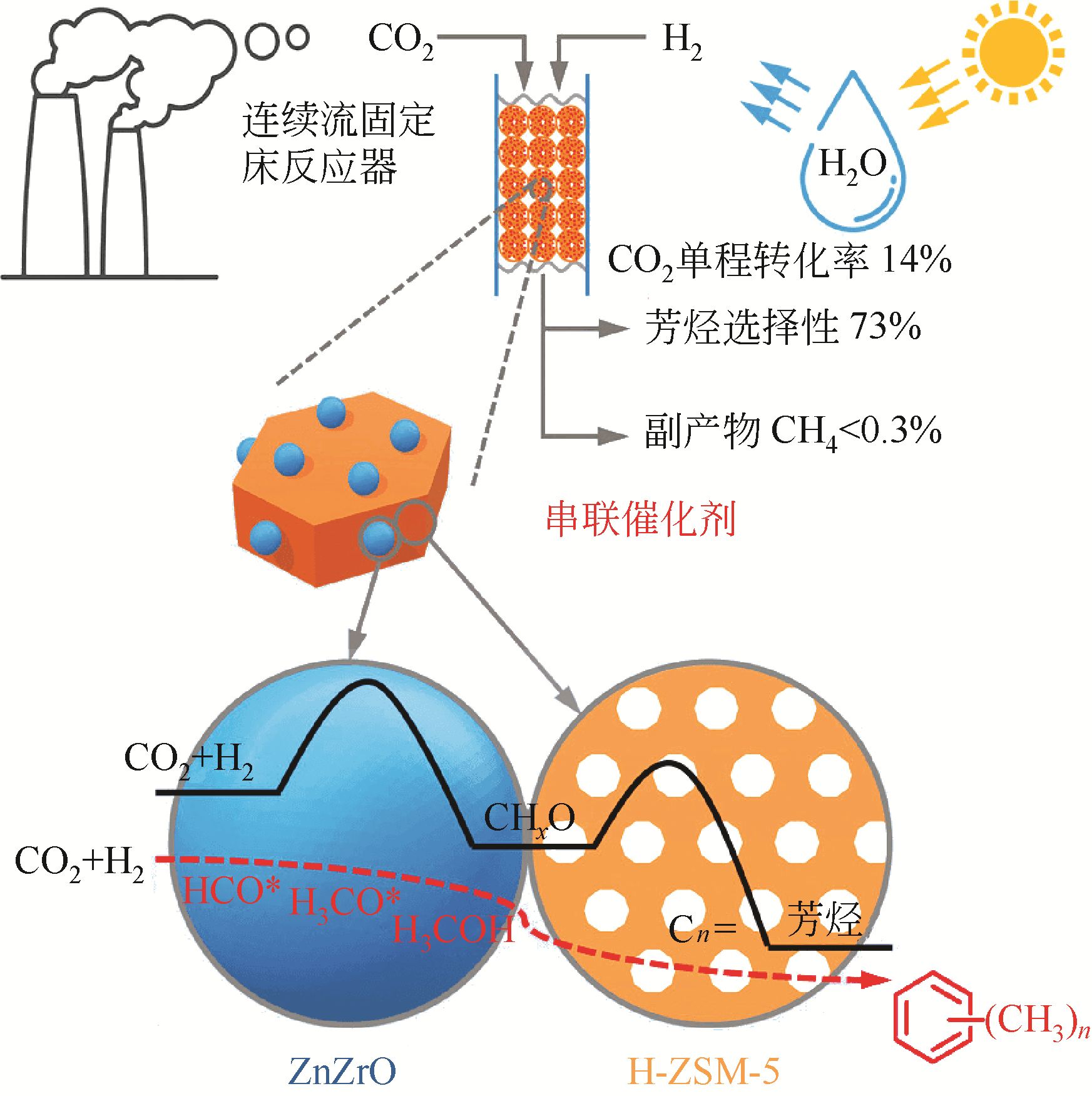
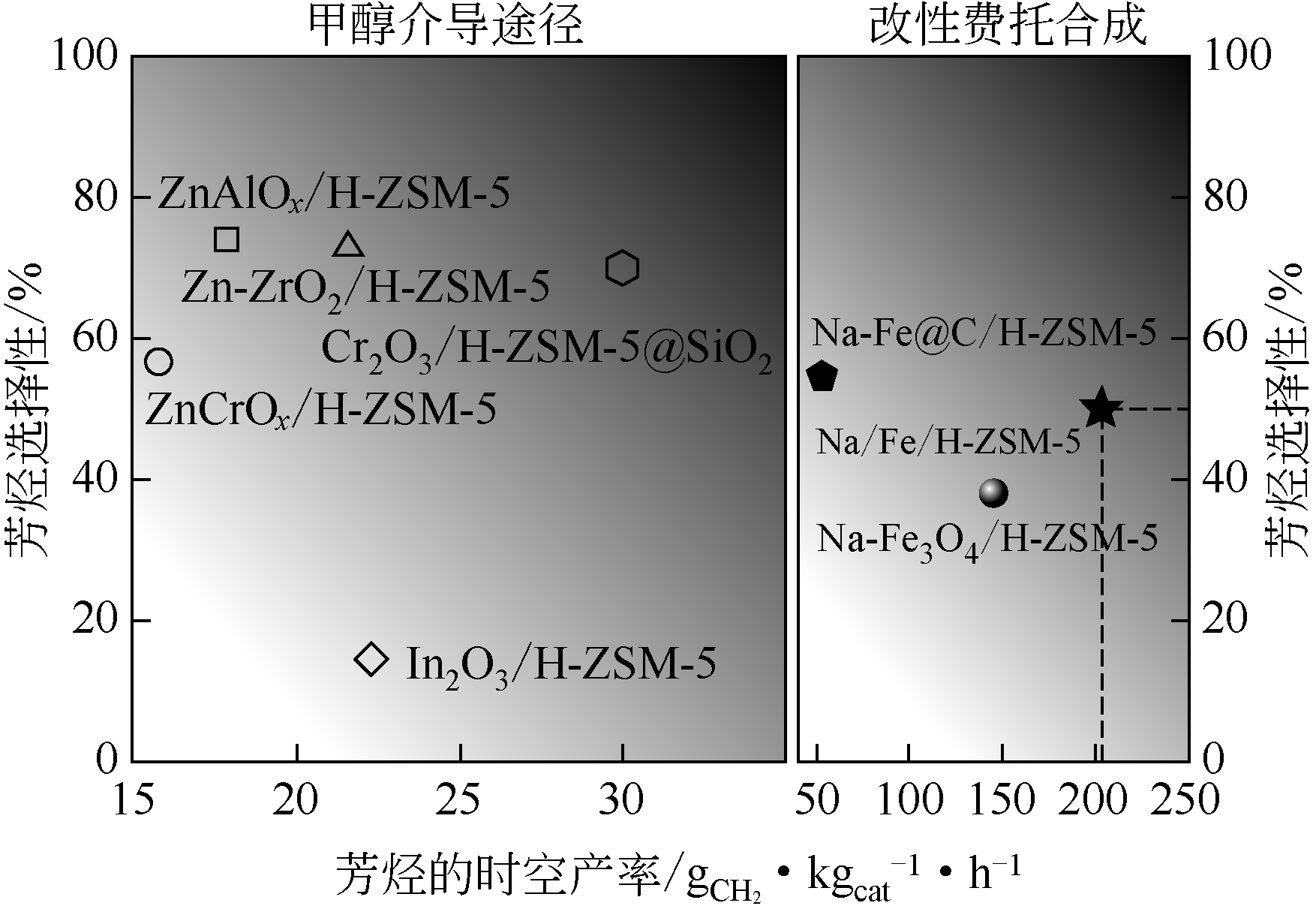
| ZnZrO/ZSM-5 | 320 | 4.0 | 1200 | 14.0 | 73.0① | [ |
| Na-Fe3O4/HZSM-5 | 320 | 3.0 | 4000 | 34.0 | 40.2① | [ |
| ZnAlOx&H-ZSM-5 | 320 | 3.0 | 6000 | 9.1 | 73.9① | [ |
| ae-ZnO-ZrO2/H-ZSM-5 | 340 | 4.0 | 7200 | 16.0 | 76.0① | [ |
| Cr2O3/H-ZSM-5 | 350 | 3.0 | 1200 | 34.5 | 75.9① | [ |
| Na-Fe@C/H-ZSM-5 | 320 | 3.0 | 9000 | 33.3 | 50.2① | [ |
| Na/Fe-HZSM-5 | 300 | 1.0 | 4800 | 21.8 | 54.7①/91.5② | [ |
| Fe2O3@KO2/ZSM-5 | 375 | 3.0 | 5000 | 47.4 | 23.4① | [ |
| FeK1.5/HSG|HZSM-5 | 340 | 2.0 | 26000 | 35.0 | 68.0① | [ |
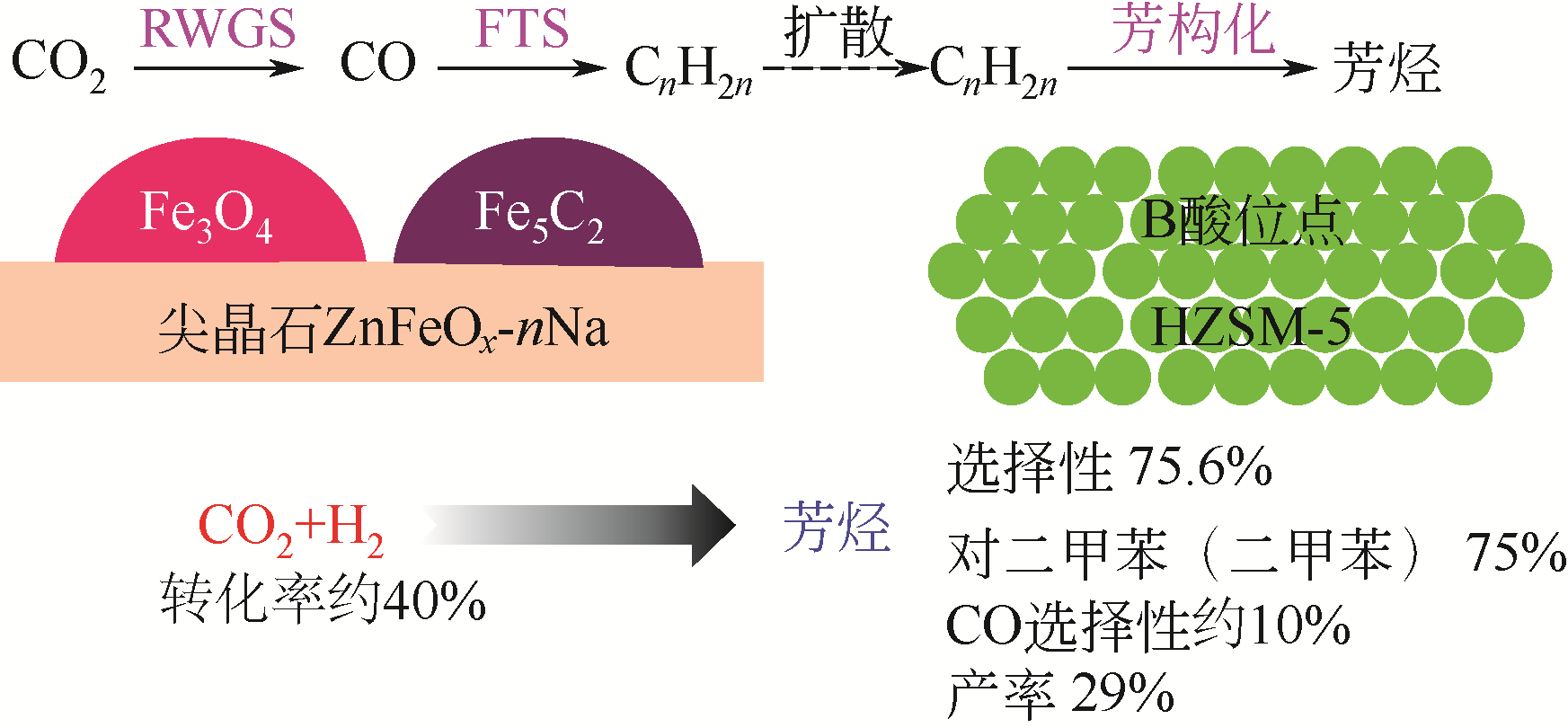
| ZnFeOx-nNa/HZSM-5 | 320 | 3.0 | 4000 | 41.2 | 75.6① | [ |
| ZnCrOx-ZnZSM-5 | 320 | 5.0 | 2000 | 19.9 | 56.5①/81.1② | [ |
| Cr2O3/Zn-ZSM-5@SiO2 | 350 | 3.0 | 1200 | 22.1 | 70.1① | [ |
| Fe-Zn-Zr@HZSM-5 | 340 | 5.0 | 3000 | 23.5 | 92.2② | [ |
表2 CO2加氢制取芳烃的不同类型复合催化剂催化性能
| 催化剂 | 反应条件 | CO2转化率/% | 芳烃选择性/% | 参考文献 | ||
|---|---|---|---|---|---|---|
| 温度/℃ | 压强/MPa | 空速/mL·g-1·h-1 | ||||
| ZnZrO/ZSM-5 | 320 | 4.0 | 1200 | 14.0 | 73.0① | [ |
| Na-Fe3O4/HZSM-5 | 320 | 3.0 | 4000 | 34.0 | 40.2① | [ |
| ZnAlOx&H-ZSM-5 | 320 | 3.0 | 6000 | 9.1 | 73.9① | [ |
| ae-ZnO-ZrO2/H-ZSM-5 | 340 | 4.0 | 7200 | 16.0 | 76.0① | [ |
| Cr2O3/H-ZSM-5 | 350 | 3.0 | 1200 | 34.5 | 75.9① | [ |
| Na-Fe@C/H-ZSM-5 | 320 | 3.0 | 9000 | 33.3 | 50.2① | [ |
| Na/Fe-HZSM-5 | 300 | 1.0 | 4800 | 21.8 | 54.7①/91.5② | [ |
| Fe2O3@KO2/ZSM-5 | 375 | 3.0 | 5000 | 47.4 | 23.4① | [ |
| FeK1.5/HSG|HZSM-5 | 340 | 2.0 | 26000 | 35.0 | 68.0① | [ |
| ZnFeOx-nNa/HZSM-5 | 320 | 3.0 | 4000 | 41.2 | 75.6① | [ |
| ZnCrOx-ZnZSM-5 | 320 | 5.0 | 2000 | 19.9 | 56.5①/81.1② | [ |
| Cr2O3/Zn-ZSM-5@SiO2 | 350 | 3.0 | 1200 | 22.1 | 70.1① | [ |
| Fe-Zn-Zr@HZSM-5 | 340 | 5.0 | 3000 | 23.5 | 92.2② | [ |
| 1 | 梁兵连, 段洪敏, 侯宝林, 等. 二氧化碳加氢合成低碳烯烃的研究进展[J]. 化工进展, 2015, 34(10): 3746-3754. |
| LIANG B L, DUAN H M, HOU B L, et al. Progress in the catalytic hydrogenation of carbon dioxide to light olefins[J]. Chemical Industry and Engineering Progress, 2015, 34(10): 3746-3754. | |
| 2 | WANG W, WANG S P, MA X B, et al. Recent advances in catalytic hydrogenation of carbon dioxide[J]. Chemical Society Reviews, 2011, 40(7): 3703-3727. |
| 3 | IEA, International Energy Agency (2018). World Energy Outlook. [EB]2018.. |
| 4 | LACIS A A, SCHMIDT G A, RIND D, et al. Atmospheric CO2: Principal control knob governing Earth’s temperature[J]. Science, 2010, 330(6002): 356-359. |
| 5 | DONEY S C, FABRY V J, FEELY R A, et al. Ocean acidification: the other CO2 problem[J]. Annual review of marine science, 2009, 1: 169-192. |
| 6 | SKJÅNES K, LINDBLAD P, MULLER J. BioCO2-A multidisciplinary, biological approach using solar energy to capture CO2 while producing H2 and high value products[J]. Biomolecular Engineering, 2007, 24(4): 405-413. |
| 7 | 时璟丽, 高虎, 王红芳. 风电制氢经济性分析[J]. 中国能源, 2015, 37(2): 11-14. |
| SHI J L, GAO H, WANG H F. Economic analysis of wind power hydrogen production[J]. Energy of China, 2015, 37(2): 11-14. | |
| 8 | NI M, LEUNG D Y C, LEUNG M K H, et al. An overview of hydrogen production from biomass[J]. Fuel Processing Technology, 2006, 87(5): 461-472. |
| 9 | JANKE C, DUYAR M S, HOSKINS M, et al. Catalytic and adsorption studies for the hydrogenation of CO2 to methane[J]. Applied Catalysis B: Environmental, 2014, 152: 184-191. |
| 10 | RUNGTAWEEVORANIT B, BAEK J, ARAUJO J R, et al. Copper nanocrystals encapsulated in Zr-based metal-organic frameworks for highly selective CO2 hydrogenation to methanol[J]. Nano Letters, 2016, 16(12): 7645-7649. |
| 11 | ROHMANN K, KOTHE J, HAENEL M W, et al. Hydrogenation of CO2 to formic acid with a highly active ruthenium acriphos complex in DMSO and DMSO/water[J]. Angewandte Chemie International Edition, 2016, 55(31): 8966-8969. |
| 12 | OYOLA-RIVERA O, BALTANÁS M A, CARDONA-MARTĺNEZ N. CO2 hydrogenation to methanol and dimethyl ether by Pd-Pd2Ga catalysts supported over Ga2O3 polymorphs[J]. Journal of CO2 Utilization, 2015, 9: 8-15. |
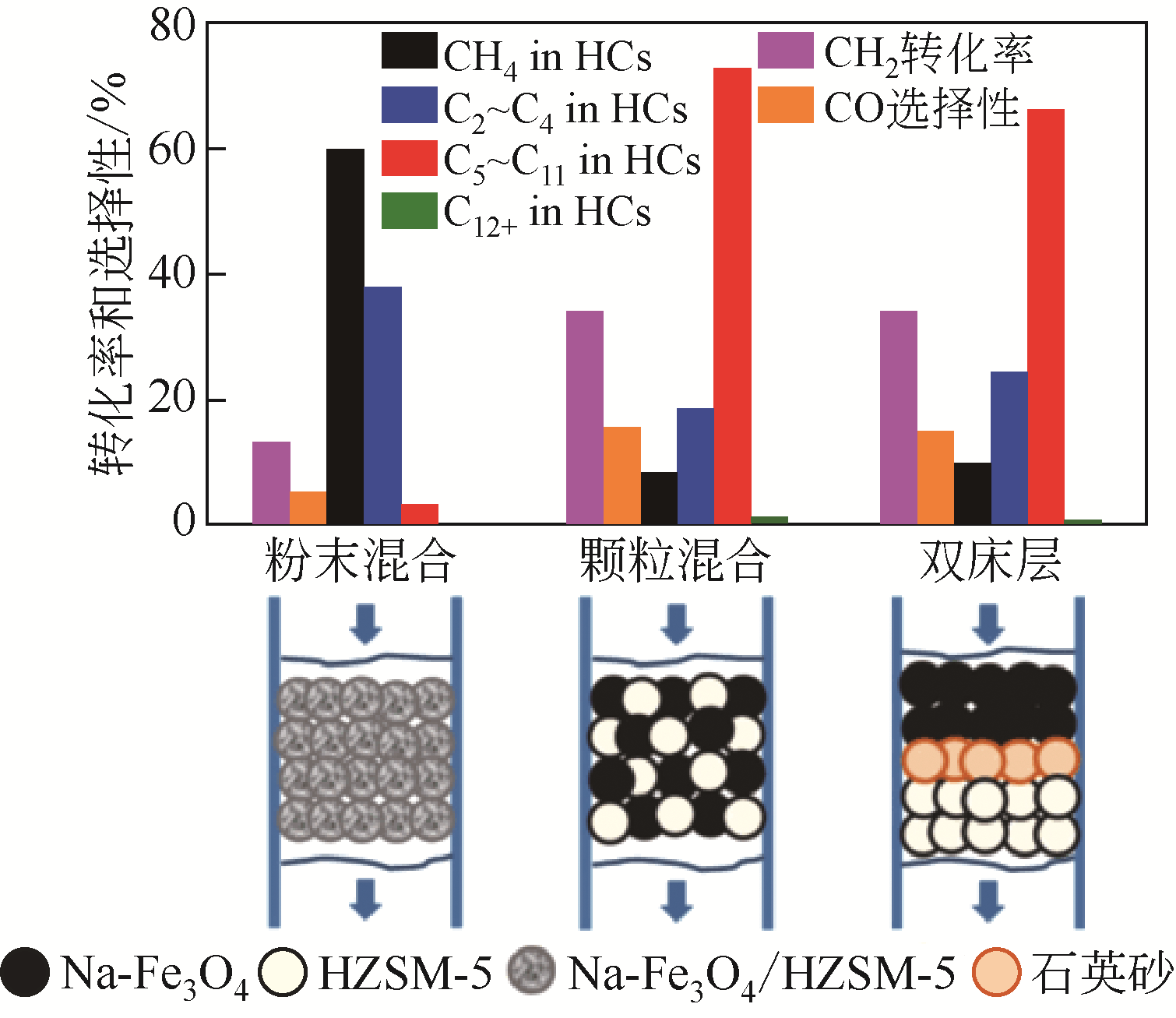
| 13 | LIU J H, ZHANG A F, JIANG X, et al. Overcoating the surface of Fe-based catalyst with ZnO and nitrogen-doped carbon toward high selectivity of light olefins in CO2 hydrogenation[J]. Industrial & Engineering Chemistry Research, 2019, 58(10): 4017-4023. |
| 14 | 许文娟, 马丽萍, 黄彬, 等. CO2催化加氢研究进展[J]. 化工进展, 2009, 28(S1): 284-289. |
| XU W J, MA L P, HUANG B,et al. Research progress of CO2 catalytic hydrogenation[J]. Chemical Industry and Engineering Progress, 2009, 28(S1): 284-289. | |
| 15 | SAEIDI S, AMIN N A S, RAHIMPOUR M R. Hydrogenation of CO2 to value-added products—A review and potential future developments[J]. Journal of CO2 Utilization, 2014, 5: 66-81. |
| 16 | CENTI G, QUADRELLI E A, PERATHONER S. Catalysis for CO2 conversion: a key technology for rapid introduction of renewable energy in the value chain of chemical industries[J]. Energy & Environmental Science, 2013, 6(6): 1711-1731. |
| 17 | KONDRATENKO E V, MUL G, BALTRUSAITIS J, et al. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes[J]. Energy & Environmental Science, 2013, 6(11): 3112-3135. |
| 18 | WANG X X, YANG G H, ZHANG J F, et al. Synthesis of isoalkanes over a core (Fe-Zn-Zr)-shell (zeolite) catalyst by CO2 hydrogenation[J]. Chemical Communications, 2016, 52(46): 7352-7355. |
| 19 | CHOI Y H, JANG Y J, PARK H, et al. Carbon dioxide Fischer-Tropsch synthesis: a new path to carbon-neutral fuels[J]. Applied Catalysis B: Environmental, 2017, 202: 605-610. |
| 20 | NIZIOLEK A M, ONEL O, FLOUDAS C A. Production of benzene, toluene, and xylenes from natural gas via methanol: process synthesis and global optimization[J]. AIChE Journal, 2016, 62(5): 1531-1556. |
| 21 | NIZIOLEK A M, ONEL O, GUZMAN Y A, et al. Biomass-based production of benzene, toluene, and xylenes via methanol: process synthesis and deterministic global optimization[J]. Energy & Fuels, 2016, 30(6): 4970-4998. |
| 22 | SUN J, WANG Y. Recent advances in catalytic conversion of ethanol to chemicals[J]. ACS Catalysis, 2014, 4(4): 1078-1090. |
| 23 | 黄澎. 神府煤液化油石脑油馏分重整生产芳烃的研究[J]. 洁净煤技术, 2017, 23(2): 98-102. |
| HUANG P. Preparation of aromatic hydrocarbons by reforming naphtha fraction from Shenfu direct coal liquefaction oil[J]. Clean Coal Technology, 2017, 23(2): 98-102. | |
| 24 | BROSIUS R, CLAEYS M. Aromatics from syngas: CO taking control[J]. Chem., 2017, 3(2): 202-204. |
| 25 | SHEN X Q, KANG J C, NIU W, et al. Impact of hierarchical pore structure on the catalytic performances of MFI zeolites modified by ZnO for the conversion of methanol to aromatics[J]. Catalysis Science & Technology, 2017, 7(16): 3598-3612. |
| 26 | WANG J W, JIA Y M, ZHANG K, et al. Catalytic conversion of methanol to aromatics over nano-sized HZSM-5 zeolite modified by ZnSiF6·6H2O[J]. Catalysis Science & Technology, 2017, 7(8): 1776-1791. |
| 27 | JIA Y M, WANG J W, ZHANG K, et al. Nanocrystallite self-assembled hierarchical ZSM-5 zeolite microsphere for methanol to aromatics[J]. Microporous and Mesoporous Materials, 2017, 247: 103-115. |
| 28 | 王野. 二氧化碳直接高选择性合成液体燃料[J]. 物理化学学报, 2017, 33(12): 2319-2320. |
| WANG Y. Direct conversion of CO2 into liquid fuels with high selectivity[J]. Acta Physico-Chimica Sinica, 2017, 33(12): 2319-2320. | |
| 29 | CLAEYS M. Catalysis: cobalt gets in shape[J]. Nature, 2016, 538(7623): 44. |
| 30 | SABATIER P, SENDERENS J B. New methane synthesis[J]. Compte Rendu Acad. Sci. Paris, 1902, 134: 514-516. |
| 31 | FISCHER F, TROPSCH H. The preparation of synthetic oil mixtures (synthol) from carbon monoxide and hydrogen[J]. Brennstoff-Chem, 1923, 4: 276-285. |
| 32 | HENRICI-OLIVÉ G, OLIVE S. The Fischer-Tropsch synthesis: molecular weight distribution of primary products and reaction mechanism[J]. Angewandte Chemie International Edition in English, 1976, 15(3): 136-141. |
| 33 | LECKEL D. Diesel production from Fischer-Tropsch: the past, the present, and new concepts[J]. Energy Fuels, 2009, 23(5): 2342-2358. |
| 34 | ROFER-DEPOORTER C K. A comprehensive mechanism for the Fischer-Tropsch synthesis[J]. Chemical Reviews, 1981, 81(5): 447-474. |
| 35 | OJEDA M, NABAR R, NILEKAR A U, et al. CO activation pathways and the mechanism of Fischer-Tropsch synthesis[J]. Journal of Catalysis, 2010, 272(2): 287-297. |
| 36 | PEREGO C, BORTOLO R, ZENNARO R. Gas to liquids technologies for natural gas reserves valorization: the Eni experience[J]. Catalysis Today, 2009, 142(1-2): 9-16. |
| 37 | CHANG C D, LANG W H, SILVESTRI A J. Synthesis gas conversion to aromatic hydrocarbons[J]. Journal of Catalysis, 1979, 56(2): 268-273. |
| 38 | CAESAR P D, BRENNAN J A, GARWOOD W E, et al. Advances in Fischer-Tropsch chemistry[J]. Journal of Catalysis, 1979, 56(2): 274-278. |
| 39 | LI Z L, QU Y Z, WANG J J, et al. Highly selective conversion of carbon dioxide to aromatics over tandem catalysts[J]. Joule, 2019, 3(2): 570-583. |
| 40 | XIE C L, NIU Z Q, KIM D, et al. Surface and interface control in nanoparticle catalysis[J]. Chemical Reviews, 2020, 120(2): 1184-1249. |
| 41 | KASIPANDI S, BAE J W. Recent advances in direct synthesis of value-added aromatic chemicals from syngas by cascade reactions over bifunctional catalysts[J]. Advanced Materials, 2019, 31(34): 1803390. |
| 42 | WANG N, QIAN W Z, SHEN K, et al. Bayberry-like ZnO/MFI zeolite as high performance methanol-to-aromatics catalyst[J]. Chemical Communications, 2016, 52(10): 2011-2014. |
| 43 | NI Y M, PENG W Y, SUN A M, et al. High selective and stable performance of catalytic aromatization of alcohols and ethers over La/Zn/HZSM-5 catalysts[J]. Journal of Industrial and Engineering Chemistry, 2010, 16(4): 503-505. |
| 44 | KECSKEMÉTI A, BARTHOS R, SOLYMOSI F. Aromatization of dimethyl and diethyl ethers on Mo2C-promoted ZSM-5 catalysts[J]. Journal of Catalysis, 2008, 258(1): 111-120. |
| 45 | SONG Y Q, ZHU X X, XIE S J, et al. The effect of acidity on olefin aromatization over potassium modified ZSM-5 catalysts[J]. Catalysis Letters, 2004, 97(1/2): 31-36. |
| 46 | NIU X L, SONG Y Q, XIE S J, et al. Synthesis and catalytic reactivity of MCM-22/ZSM-35 composites for olefin aromatization[J]. Catalysis Letters, 2005, 103(3/4): 211-218. |
| 47 | LEE S, SARDESAI A. Liquid phase methanol and dimethyl ether synthesis from syngas[J]. Topics in Catalysis, 2005, 32(3/4): 197-207. |
| 48 | JIAO F, LI J J, PAN X L, et al. Selective conversion of syngas to light olefins[J]. Science, 2016, 351(6277): 1065-1068. |
| 49 | DANG S S, YANG H Y, GAO P, et al. A review of research progress on heterogeneous catalysts for methanol synthesis from carbon dioxide hydrogenation[J]. Catalysis Today, 2019, 330: 61-75. |
| 50 | LI Z L, WANG J J, QU Y Z, et al. Highly selective conversion of carbon dioxide to lower olefins[J]. ACS Catalysis, 2017, 7(12): 8544-8548. |
| 51 | FUJIMOTO K, KUDO Y, TOMINAGA H. Synthesis gas conversion utilizing mixed catalyst composed of CO reducing catalyst and solid acid: Ⅱ. Direct synthesis of aromatic hydrocarbons from synthesis gas[J]. Journal of Catalysis, 1984, 87(1): 136-143. |
| 52 | YANG X L, SU X, CHEN D, et al. Direct conversion of syngas to aromatics: a review of recent studies[J]. Chinese Journal of Catalysis, 2020, 41(4): 561-573. |
| 53 | RIEDEL T, SCHULZ H, SCHAUB G, et al. Fischer-Tropsch on iron with H2/CO and H2/CO2 as synthesis gases: the episodes of formation of the Fischer-Tropsch regime and construction of the catalyst[J]. Topics in Catalysis, 2003, 26(1/2/3/4): 41-54. |
| 54 | DRY M E. High quality diesel via the Fischer-Tropsch process—A review[J]. Journal of Chemical Technology and Biotechnology, 2002, 77(1): 43-50. |
| 55 | GALVIS H M T, BITTER J H, KHARE C B, et al. Supported iron nanoparticles as catalysts for sustainable production of lower olefins[J]. Science, 2012, 335(6070): 835-838. |
| 56 | XU Y B, LIU D P, LIU X H. Conversion of syngas toward aromatics over hybrid Fe-based Fischer-Tropsch catalysts and HZSM-5 zeolites[J]. Applied Catalysis A: General, 2018, 552: 168-183. |
| 57 | BOTES F G, BÖHRINGER W. The addition of HZSM-5 to the Fischer-Tropsch process for improved gasoline production[J]. Applied Catalysis A: General, 2004, 267(1/2): 217-225. |
| 58 | BÄURLE G, GUSE K, LOHRENGEL M, et al. Conversion of syngas to aromatic hydrocarbons on cobalt-manganese-zeolite catalysts[J].Studies in Surface Science and Catalysis, 1993, 75: 2789-2792. |
| 59 | STENCEL J M, RAO V U S, DIEHL J R, et al. Dual cobalt speciation in CoZSM-5 catalysts[J]. Journal of Catalysis, 1983, 84(1): 109-118. |
| 60 | GORMLEY R J, RAO V U S, ANDERSON R R, et al. Secondary reactions on metal-zeolite catalysts used in synthesis gas conversion[J]. Journal of Catalysis, 1988, 113(1): 193-205. |
| 61 | SHAMSI A, RAO V U S, GORMLEY R J, et al. Zeolite-supported cobalt catalysts for the conversion of synthesis gas to hydrocarbon products[J]. Industrial & Engineering Chemistry Product Research and Development, 1984, 23(4): 513-519. |
| 62 | LAPIDUS A L, KRYLOVA A Y. Catalytic synthesis of isoalkanes and aromatic hydrocarbons from CO and H2[J]. Russian Chemical Reviews, 1998, 67(11): 941. |
| 63 | RAO V U S, GORMLEY R J, SHAMSI A, et al. Promotion and characterization of zeolitic catalysts used in the synthesis of hydrocarbons from syngas[J]. Journal of Molecular Catalysis, 1985, 29(2): 271-283. |
| 64 | SCHULZ H. Short history and present trends of Fischer-Tropsch synthesis[J]. Applied Catalysis A: General, 1999, 186(1/2): 3-12. |
| 65 | XU Y F, LIU J G, MA G Y, et al. Synthesis of aromatics from syngas over FeMnK/SiO2 and HZSM-5 tandem catalysts[J]. Molecular Catalysis, 2018, 454: 104-113. |
| 66 | SHAMSI A, RAO V U S, GORMLEY R J, et al. Influence of preparative procedure on the activity and selectivity of FeZSM-5 catalysts in syngas conversion[J]. Applied Catalysis, 1986, 27(1): 55-68. |
| 67 | XU Y F, LIU J G, MA G Y, et al. Effect of iron loading on acidity and performance of Fe/HZSM-5 catalyst for direct synthesis of aromatics from syngas[J]. Fuel, 2018, 228: 1-9. |
| 68 | ZHANG Q H, KANG J C, WANG Y. Development of novel catalysts for Fischer-Tropsch synthesis: tuning the product selectivity[J]. ChemCatChem, 2010, 2(9): 1030-1058. |
| 69 | YANG Z Q, PAN X L, WANG J H, et al. FeN particles confined inside CNT for light olefin synthesis from syngas: effects of Mn and K additives[J]. Catalysis Today, 2012, 186(1): 121-127. |
| 70 | XU H, LI M Z, NAWAZ M A, et al. Doping of K and Zn elements in FeZr-Ni/ZSM-5: highly selective catalyst for syngas to aromatics[J]. Catalysis Communications, 2019, 121: 95-99. |
| 71 | CHENG L, MENG C, YANG T H, et al. One-step synthesis of aromatics from syngas over K-Modified FeMnO/MoNi-ZSM-5[J]. Energy & Fuels, 2018, 32(9): 9756-9762. |
| 72 | WEBER J L, DUGULAN I, DE JONGH P E, et al. Bifunctional catalysis for the conversion of synthesis gas to olefins and aromatics[J]. ChemCatChem, 2018, 10(5): 1107-1112. |
| 73 | YAN Q G, LU Y W, WAN C X, et al. Synthesis of aromatic-rich gasoline-range hydrocarbons from biomass-derived syngas over a Pd-promoted Fe/HZSM-5 catalyst[J]. Energy & Fuels, 2014, 28(3): 2027-2034. |
| 74 | ZHAO B, ZHAI P, WANG P F, et al. Direct transformation of syngas to aromatics over Na-Zn-Fe5C2 and hierarchical HZSM-5 tandem catalysts[J]. Chem., 2017, 3(2): 323-333. |
| 75 | YAN Q G, YU F, CAI Z Y, et al. Catalytic upgrading nitrogen-riched wood syngas to liquid hydrocarbon mixture over a Fe-Pd/ZSM-5 catalyst[J]. Biomass and Bioenergy, 2012, 47: 469-473. |
| 76 | MARTÍNEZ A, LOPEZ C. The influence of ZSM-5 zeolite composition and crystal size on the in situ conversion of Fischer-Tropsch products over hybrid catalysts[J]. Applied Catalysis A: General, 2005, 294(2): 251-259. |
| 77 | KANG S H, BAE J W, CHEON J Y, et al. Catalytic performance on iron-based Fischer-Tropsch catalyst in fixed-bed and bubbling fluidized-bed reactor[J]. Applied Catalysis B: Environmental, 2011, 103(1/2): 169-180. |
| 78 | WANG T, XU Y B, SHI C M, et al. Direct production of aromatics from syngas over a hybrid FeMn Fischer-Tropsch catalyst and HZSM-5 zeolite: local environment effect and mechanism-directed tuning of the aromatic selectivity[J]. Catalysis Science & Technology, 2019, 9(15): 3933-3946. |
| 79 | CHENG K, KANG J C, KING D L, et al. Advances in catalysis for syngas conversion to hydrocarbons[J]. Advances in Catalysis, 2017, 60: 125-208. |
| 80 | XU D Y, LI W Z, DUAN H M, et al. Reaction performance and characterization of Co/Al2O3 Fischer-Tropsch catalysts promoted with Pt, Pd and Ru[J]. Catalysis Letters, 2005, 102(3/4): 229-235. |
| 81 | YANG X L, SU X, LIANG B L, et al. The influence of alkali-treated zeolite on the oxide-zeolite syngas conversion process[J]. Catalysis Science & Technology, 2018, 8(17): 4338-4348. |
| 82 | CHENG K, ZHOU W, KANG J C, et al. Bifunctional catalysts for one-step conversion of syngas into aromatics with excellent selectivity and stability[J]. Chem., 2017, 3(2): 334-347. |
| 83 | ZHANG P P, TAN L, YANG G H, et al. One-pass selective conversion of syngas to para-xylene[J]. Chemical Science, 2017, 8(12): 7941-7946. |
| 84 | YANG J H, PAN X L, JIAO F, et al. Direct conversion of syngas to aromatics[J]. Chemical Communications, 2017, 53(81): 11146-11149. |
| 85 | YANG X L, SUN T, MA J G, et al. The influence of intimacy on the ‘iterative reactions’ during OX-ZEO process for aromatic production[J]. Journal of Energy Chemistry, 2019, 35: 60-65. |
| 86 | LIU S T, GUJAR A C, THOMAS P, et al. Synthesis of gasoline-range hydrocarbons over Mo/HZSM-5 catalysts[J]. Applied Catalysis A: General, 2009, 357(1): 18-25. |
| 87 | DAGLE R A, LIZARAZO-ADARME J A, DAGLE V L, et al. Syngas conversion to gasoline-range hydrocarbons over Pd/ZnO/Al2O3 and ZSM-5 composite catalyst system[J]. Fuel Processing Technology, 2014, 123: 65-74. |
| 88 | EREÑA J, ARANDES J M, BILBAO J, et al. Study of physical mixtures of Cr2O3-ZnO and ZSM-5 catalysts for the transformation of syngas into liquid hydrocarbons[J]. Industrial & Engineering Chemistry Research, 1998, 37(4): 1211-1219. |
| 89 | EREÑA J, ARANDES J M, BILBAO J, et al. Effect of the operating conditions on the conversion of syngas into liquid hydrocarbons over a Cr2O3-ZnO/ZSM5 bifunctional catalyst[J]. Journal of Chemical Technology and Biotechnology, 1998, 72(2): 190-196. |
| 90 | KIM D, LEE S, LEE J, et al. Syngas conversion beyond chemical equilibrium by in situ bimolecular reaction[J]. Research on Chemical Intermediates, 2016, 42(1): 249-267. |
| 91 | ARANDES J M, EREÑA J, GAYUBO A N A G, et al. Composition and quality of the gasoline obtained from syngas on Cr2O3-ZnO/ZSM5 catalysts[J]. Chemical Engineering Communications, 1999, 174(1): 1-19. |
| 92 | SIMARD F, MAHAY A, JEAN G, et al. Kinetic modelling of the catalytic conversion of synthesis gas[J]. The Canadian Journal of Chemical Engineering, 1991, 69(4): 898-906. |
| 93 | SIMARD F, SEDRAN U A, SEPULVEDA J, et al. ZnOCr2O3+ZSM-5 catalyst with very low Zn/Cr ratio for the transformation of synthesis gas to hydrocarbons[J]. Applied Catalysis A: General, 1995, 125(1): 81-98. |
| 94 | LU Y W, HU J, HAN J, et al. Synthesis of gasoline-range hydrocarbons from nitrogen-rich syngas over a Mo/HZSM-5 bi-functional catalyst[J]. Journal of the Energy Institute, 2016, 89(4): 782-792. |
| 95 | ZHANG Z X, BI P Y, JIANG P W, et al. Conversion of bio-syngas to liquid hydrocarbon over CuCoMn-Zeolite bifunctional catalysts[J]. Chinese Journal of Chemical Physics, 2014, 27(5): 573. |
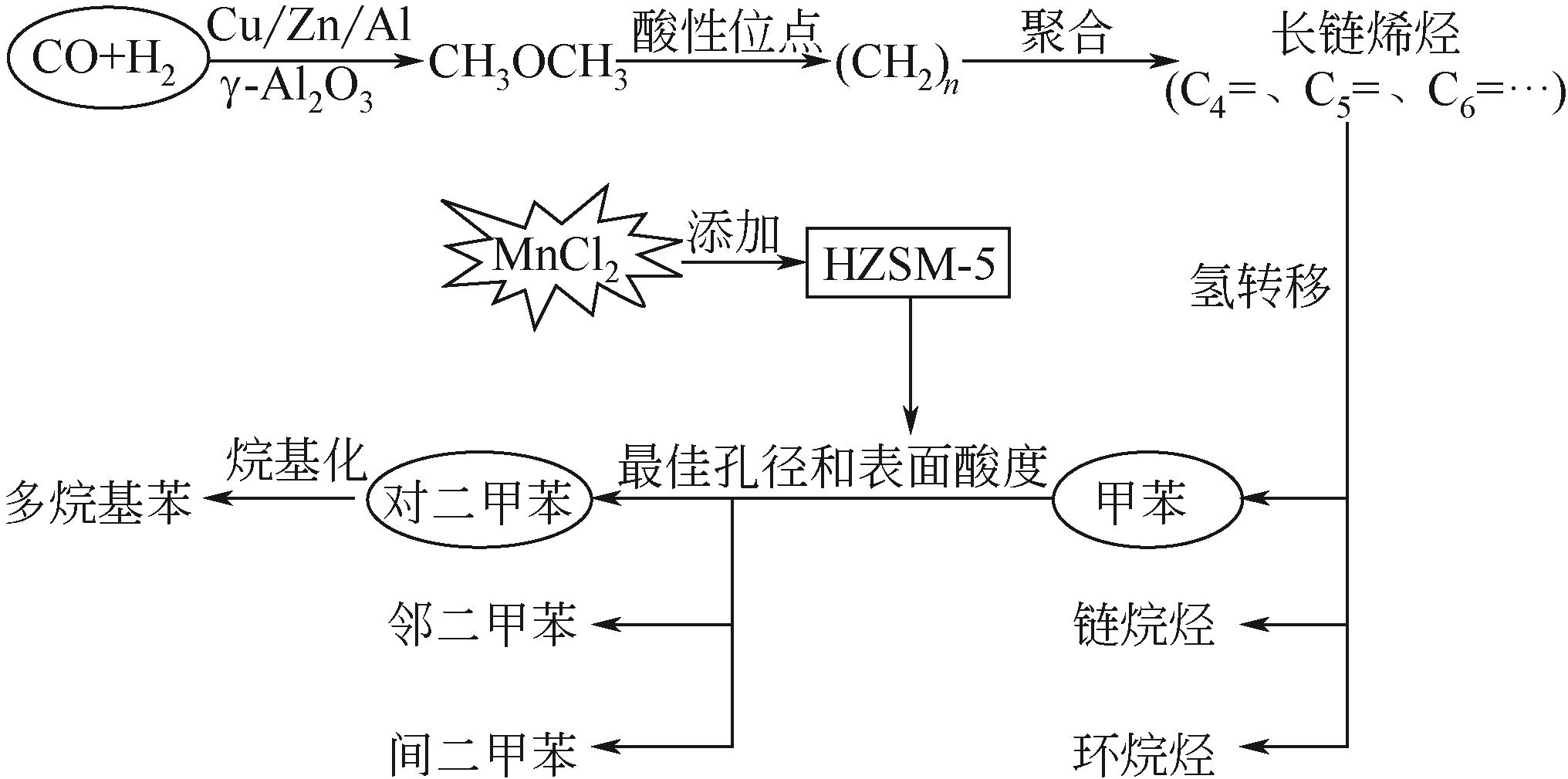
| 96 | ZHANG Q D, TAN Y S, YANG C H, et al. Characterization and catalytic application of MnCl2 modified HZSM-5 zeolites in synthesis of aromatics from syngas via dimethyl ether[J]. Journal of Industrial and Engineering Chemistry, 2013, 19(3): 975-980. |
| 97 | ZHOU W, SHI S L, WANG Y, et al. Selective conversion of syngas to aromatics over a Mo-ZrO2/H-ZSM-5 bifunctional catalyst[J]. ChemCatChem, 2019, 11(6): 1681-1688. |
| 98 | HUANG Z, WANG S, QIN F, et al. Ceria-Zirconia/Zeolite bifunctional catalyst for highly selective conversion of syngas into aromatics[J]. ChemCatChem, 2018, 10(20): 4519-4524. |
| 99 | YANG T H, CHENG L, LI N, et al. Effect of metal active sites on the product distribution over composite catalysts in the direct synthesis of aromatics from syngas[J]. Industrial & Engineering Chemistry Research, 2017, 56(41): 11763-11772. |
| 100 | LIU C, LIU S, ZHOU H B, et al. Selective conversion of syngas to aromatics over metal oxide/HZSM-5 catalyst by matching the activity between CO hydrogenation and aromatization[J]. Applied Catalysis A: General, 2019, 585: 117206. |
| 101 | WANG S, FANG Y, HUANG Z, et al. The effects of the crystalline phase of zirconia on C-O activation and C-C coupling in converting syngas into aromatics[J]. Catalysts, 2020, 10(2): 262. |
| 102 | FU Y, NI Y M, ZHU W L, et al. Enhancing syngas-to-aromatics performance of ZnO&H-ZSM-5 composite catalyst via Mn modulation[J]. Journal of Catalysis, 2020, 383: 97-102. |
| 103 | NEWSOME D S. The water-gas shift reaction[J]. Catalysis Reviews Science and Engineering, 1980, 21(2): 275-318. |
| 104 | CHANG C D, LANG W H, SILVESTRI A J, et al. Conversion of synthesis gas to hydrocarbon mixtures: US4096163[P]. 1978-06-20. |
| 105 | SAKAKURA T, CHOI J C, YASUDA H. Transformation of carbon dioxide[J]. Chemical Reviews, 2007, 107(6): 2365-2387. |
| 106 | OLAH G A, GOEPPERT A, PRAKASH G K S. Chemical recycling of carbon dioxide to methanol and dimethyl ether: from greenhouse gas to renewable, environmentally carbon neutral fuels and synthetic hydrocarbons[J]. The Journal of Organic Chemistry, 2009, 74(2): 487-498. |
| 107 | ARESTA M, DIBENEDETTO A, QUARANTA E. State of the art and perspectives in catalytic processes for CO2 conversion into chemicals and fuels: The distinctive contribution of chemical catalysis and biotechnology[J]. Journal of Catalysis, 2016, 343: 2-45. |
| 108 | LIU R, TIAN H F, YANG A M, et al. Preparation of HZSM-5 membrane packed CuO-ZnO-Al2O3 nanoparticles for catalysing carbon dioxide hydrogenation to dimethyl ether[J]. Applied Surface Science, 2015, 345: 1-9. |
| 109 | WEI J, GE Q J, YAO R W, et al. Directly converting CO2 into a gasoline fuel[J]. Nature Communications, 2017, 8(1): 1-9. |
| 110 | NI Y M, CHEN Z Y, FU Y, et al. Selective conversion of CO2 and H2 into aromatics[J]. Nature Communications, 2018, 9(1): 1-7. |
| 111 | ZHOU C, SHI J Q, ZHOU W, et al. Highly active ZnO-ZrO2 aerogels integrated with H-ZSM-5 for aromatics synthesis from carbon dioxide[J]. ACS Catalysis, 2020, 10(1): 302-310. |
| 112 | WANG Y, TAN L, TAN M H, et al. Rationally designing bifunctional catalysts as an efficient strategy to boost CO2 hydrogenation producing value-added aromatics[J]. ACS Catalysis, 2019, 9(2): 895-901. |
| 113 | GAO P, LI S G, BU X N, et al. Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst[J]. Nature Chemistry, 2017, 9(10): 1019-1024. |
| 114 | WANG Y, KAZUMI S, GAO W Z, et al. Direct conversion of CO2 to aromatics with high yield via a modified Fischer-Tropsch synthesis pathway[J]. Applied Catalysis B: Environmental, 2020, 269: 118792. |
| 115 | XU Y B, SHI C M, LIU B, et al. Selective production of aromatics from CO2[J]. Catalysis Science & Technology, 2019, 9(3): 593-610. |
| 116 | SU T M, QIN Z Z, HUANG G, et al. Density functional theory study on the interaction of CO2 with Fe3O4 (111) surface[J]. Applied Surface Science, 2016, 378: 270-276. |
| 117 | HAKIM A, MARLIZA T S, TAHARI N M ABU, et al. Studies on CO2 adsorption and desorption properties from various types of iron oxides (FeO, Fe2O3, and Fe3O4)[J]. Industrial & Engineering Chemistry Research, 2016, 55(29): 7888-7897. |
| 118 | RAMIREZ A, DUTTA CHOWDHURY A, DOKANIA A, et al. Effect of zeolite topology and reactor configuration on the direct conversion of CO2 to light olefins and aromatics[J]. ACS Catalysis, 2019, 9(7): 6320-6334. |
| 119 | WANG H W, HODGSON J, SHRESTHA T B, et al. Carbon dioxide hydrogenation to aromatic hydrocarbons by using an iron/iron oxide nanocatalyst[J]. Beilstein Journal of Nanotechnology, 2014, 5(1): 760-769. |
| 120 | WANG S W, WU T J, LIN J, et al. FeK-on-3D graphene-zeolite tandem catalyst with high efficiency and versatility in direct CO2 conversion to aromatics[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(21): 17825-17833. |
| 121 | CUI X, GAO P, LI S G, et al. Selective production of aromatics directly from carbon dioxide hydrogenation[J]. ACS Catalysis, 2019, 9(5): 3866-3876. |
| 122 | ZHU P F, SUN J, YANG G H, et al. Tandem catalytic synthesis of benzene from CO2 and H2[J]. Catalysis Science & Technology, 2017, 7(13): 2695-2699. |
| 123 | ZHANG J F, ZHANG M, CHEN S Y, et al. Hydrogenation of CO2 into aromatics over a ZnCrOx-zeolite composite catalyst[J]. Chemical Communications, 2019, 55(7): 973-976. |
| 124 | RAMASAMY K K, ZHANG H, SUN J M, et al. Conversion of ethanol to hydrocarbons on hierarchical HZSM-5 zeolites[J]. Catalysis Today, 2014, 238: 103-110. |
| 125 | 张贵泉. Zn改性HZSM-5分子筛催化剂上甲醇转化制轻质芳烃反应研究[D]. 西安: 西北大学, 2014. |
| ZHANG G Q. Study on the reaction of methanol conversion to light aromatic hydrocarbons over Zn modified HZSM-5 zeolite catalysts[D]. Xi’an: Northwest University, 2014. | |
| 126 | 田海锋, 杨爱梅, 查飞, 等. 咪唑改性HZSM-5分子筛的制备及其催化CO2加氢合成二甲醚[J]. 精细化工, 2014, 31(2):186-192. |
| TIAN H F, YANG A M, ZHA F. et al. Preparation of imidazole-modified HZSM-5 zeolite and its application in the catalytic synthesis of dimethyl ether from hydrogenation of carbon dioxide[J]. Fine Chemicals, 2014, 31(2):186-192. | |
| 127 | WANG Y, GAO W Z, KAZUMI S, et al. Direct and oriented conversion of CO2 to value-added aromatics[J]. Chemistry: A European Journal, 2019, 25(20): 5149-5153. |
| 128 | TIAN H F, LV J L, LIANG X H, et al. Tuning morphology of Zn/HZSM-5 on catalytic performance in methanol aromatization[J]. Energy Technolgy, 2018, 6(10): 1986-1993. |
| 129 | WANG X X, YANG G H, ZHANG J F, et al. Macroscopic assembly style of catalysts significantly determining their efficiency for converting CO2 to gasoline[J]. Catalysis Science & Technology, 2019, 9(19): 5401-5412. |
| [1] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [2] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [3] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [6] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [7] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [8] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [9] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [10] | 李东泽, 张祥, 田键, 胡攀, 姚杰, 朱林, 卜昌盛, 王昕晔. 基于水泥窑脱硝的碳基还原NO x 研究进展[J]. 化工进展, 2023, 42(9): 4882-4893. |
| [11] | 毛善俊, 王哲, 王勇. 基团辨识加氢:从概念到应用[J]. 化工进展, 2023, 42(8): 3917-3922. |
| [12] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [13] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| [14] | 王兰江, 梁瑜, 汤琼, 唐明兴, 李学宽, 刘雷, 董晋湘. 快速热解铂前体合成高分散的Pt/HY催化剂及其萘深度加氢性能[J]. 化工进展, 2023, 42(8): 4159-4166. |
| [15] | 黄玉飞, 李子怡, 黄杨强, 金波, 罗潇, 梁志武. 光催化CO2和CH4重整催化剂研究进展[J]. 化工进展, 2023, 42(8): 4247-4263. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||